A Benzothiadiazole-Based Zn(II) Metal–Organic Framework with Visual Turn-On Sensing for Anthrax Biomarker and Theoretical Calculation
Abstract
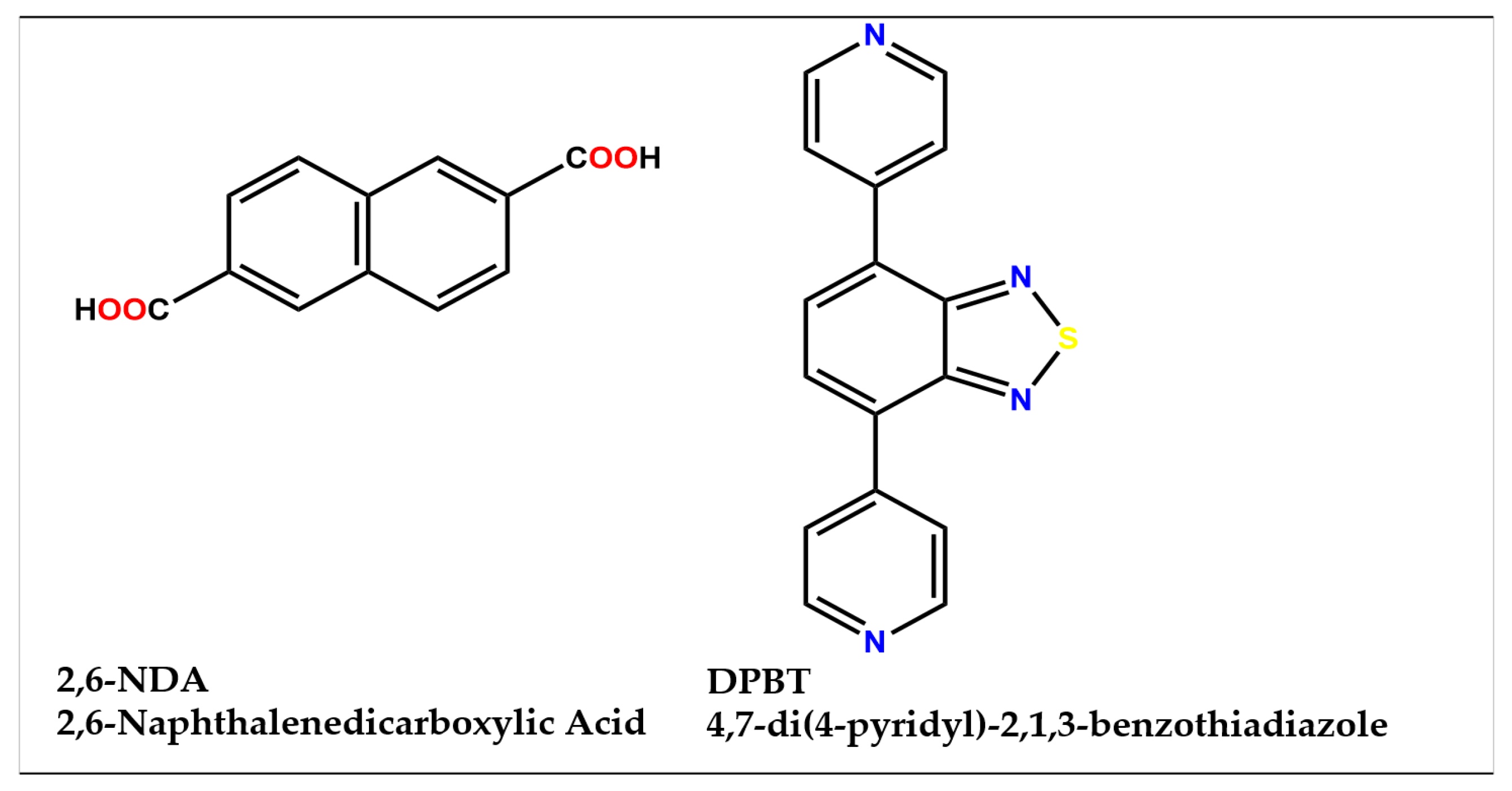
1. Introduction
2. Results
2.1. X-ray Structure Determination
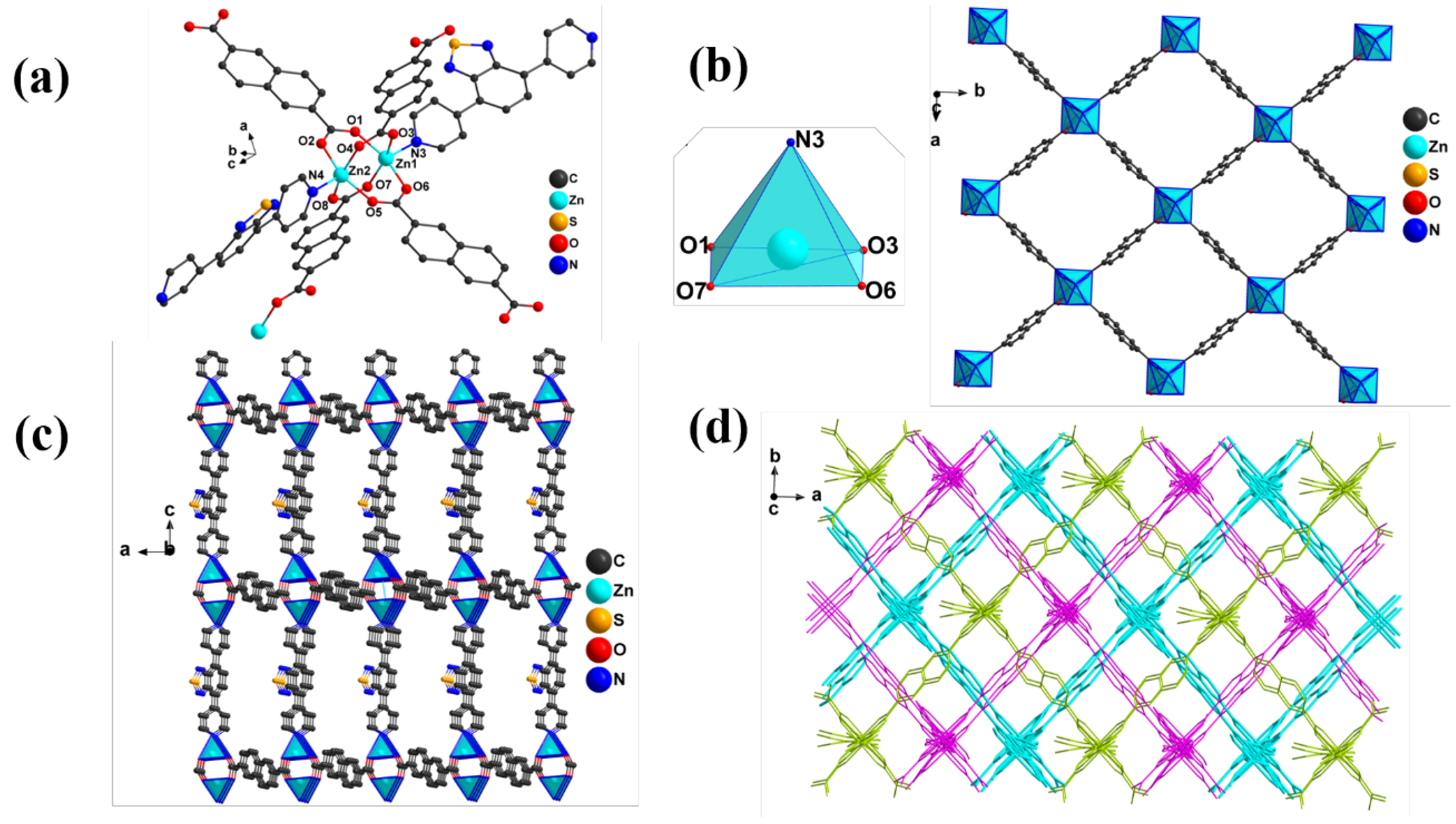
2.2. Crystal Structure of MOF-1
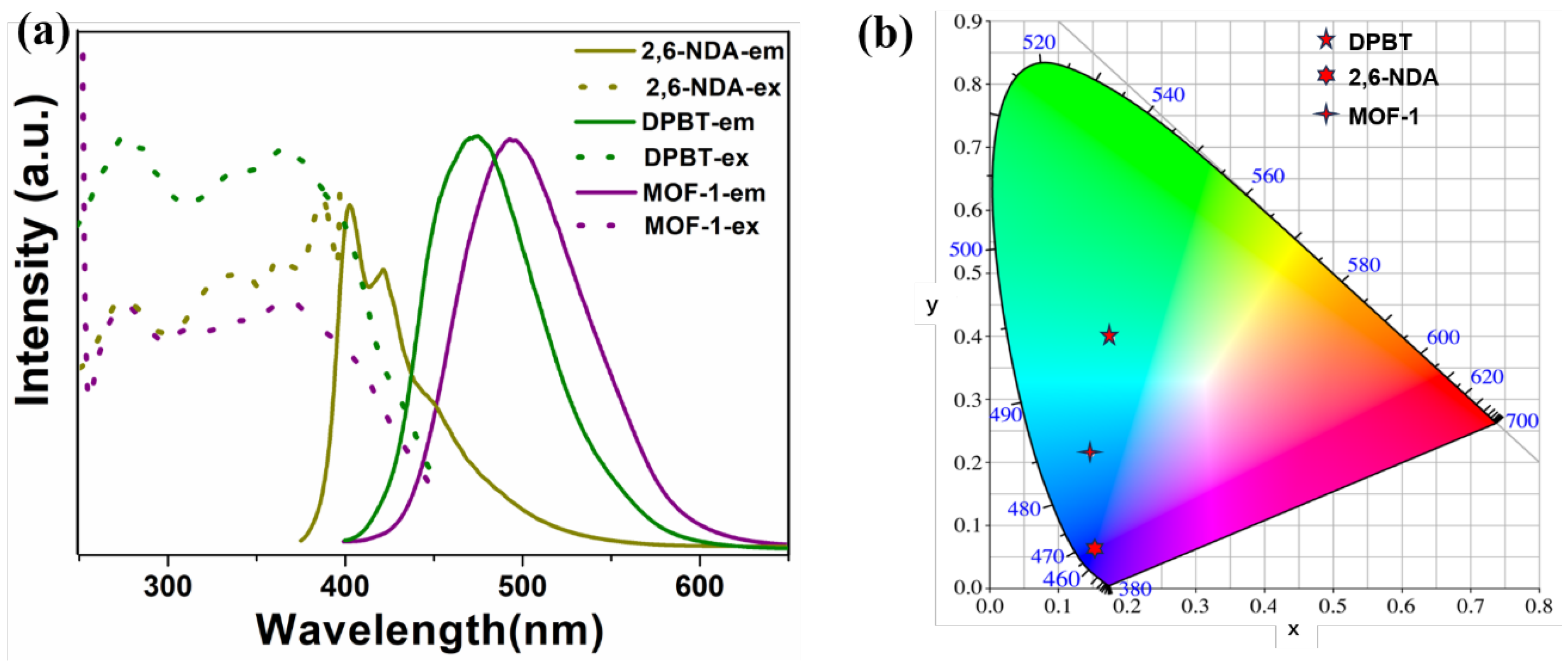
2.3. Photoluminescence Properties
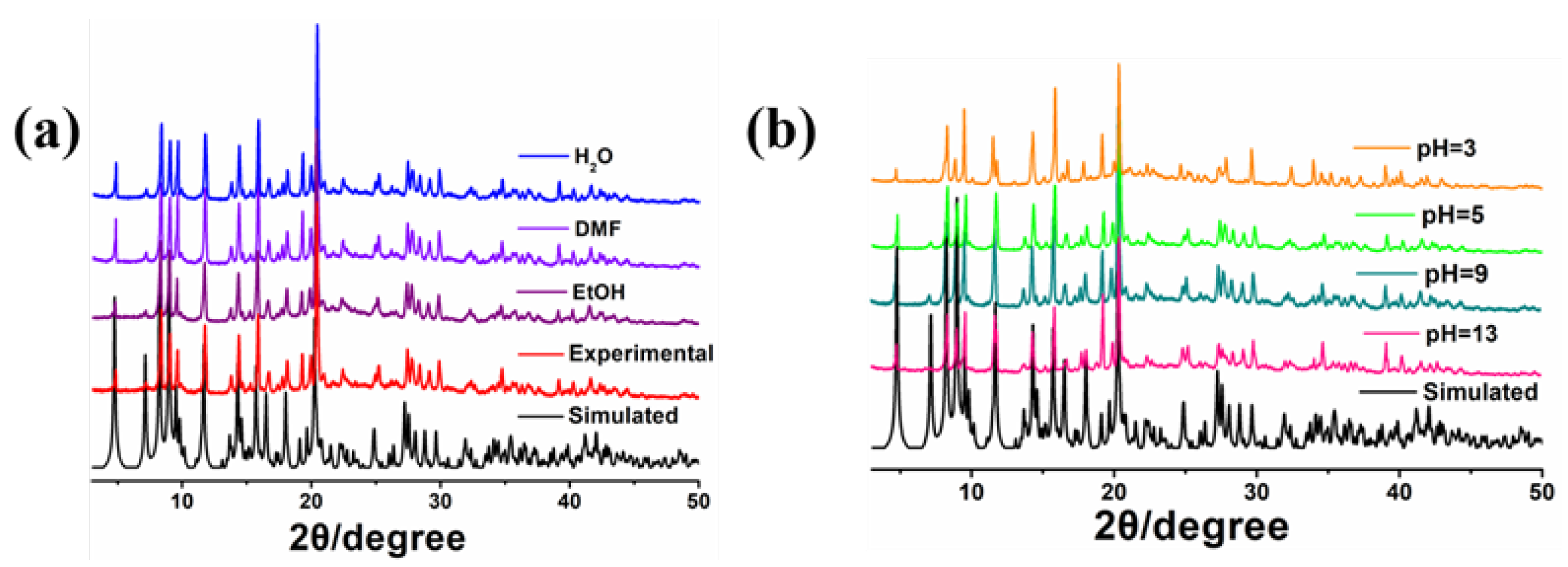
2.4. FT-IR, PXRD analysis and Stability of MOF-1
2.5. Fluorescence Detection of DPA
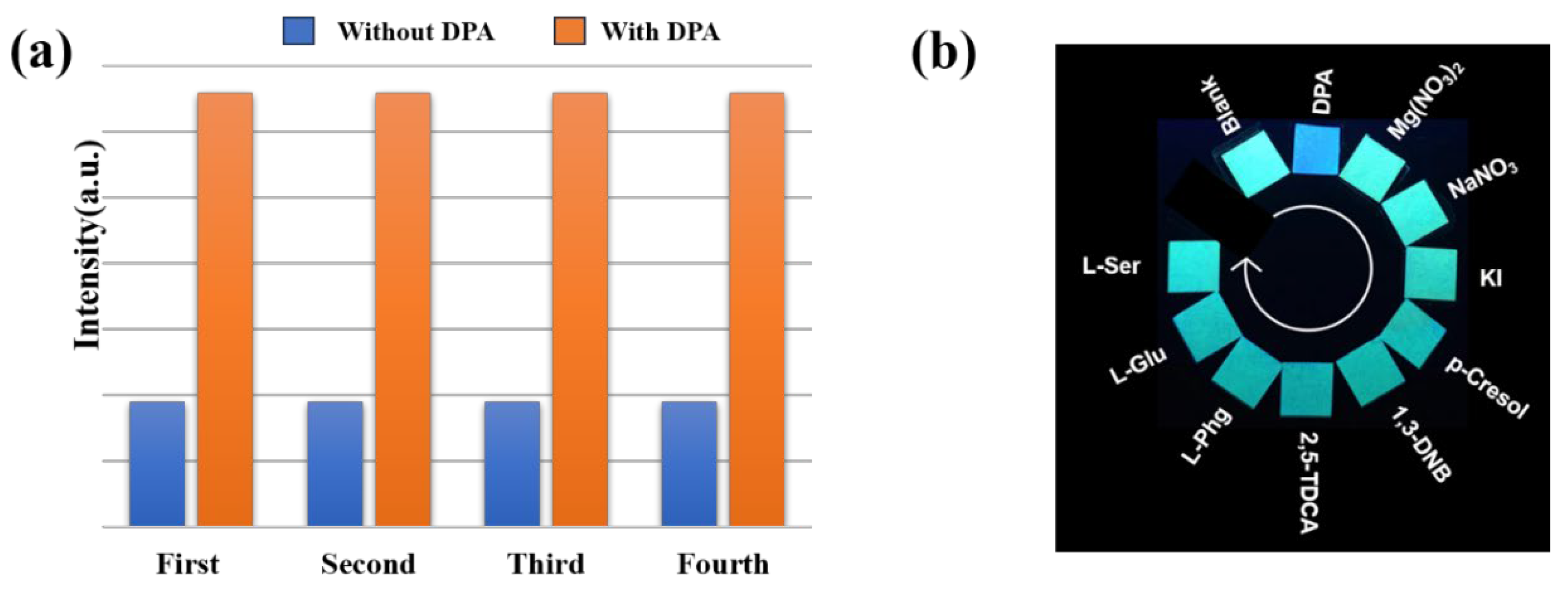
2.6. Recyclability and Visualizable Sensing
3. Discussion
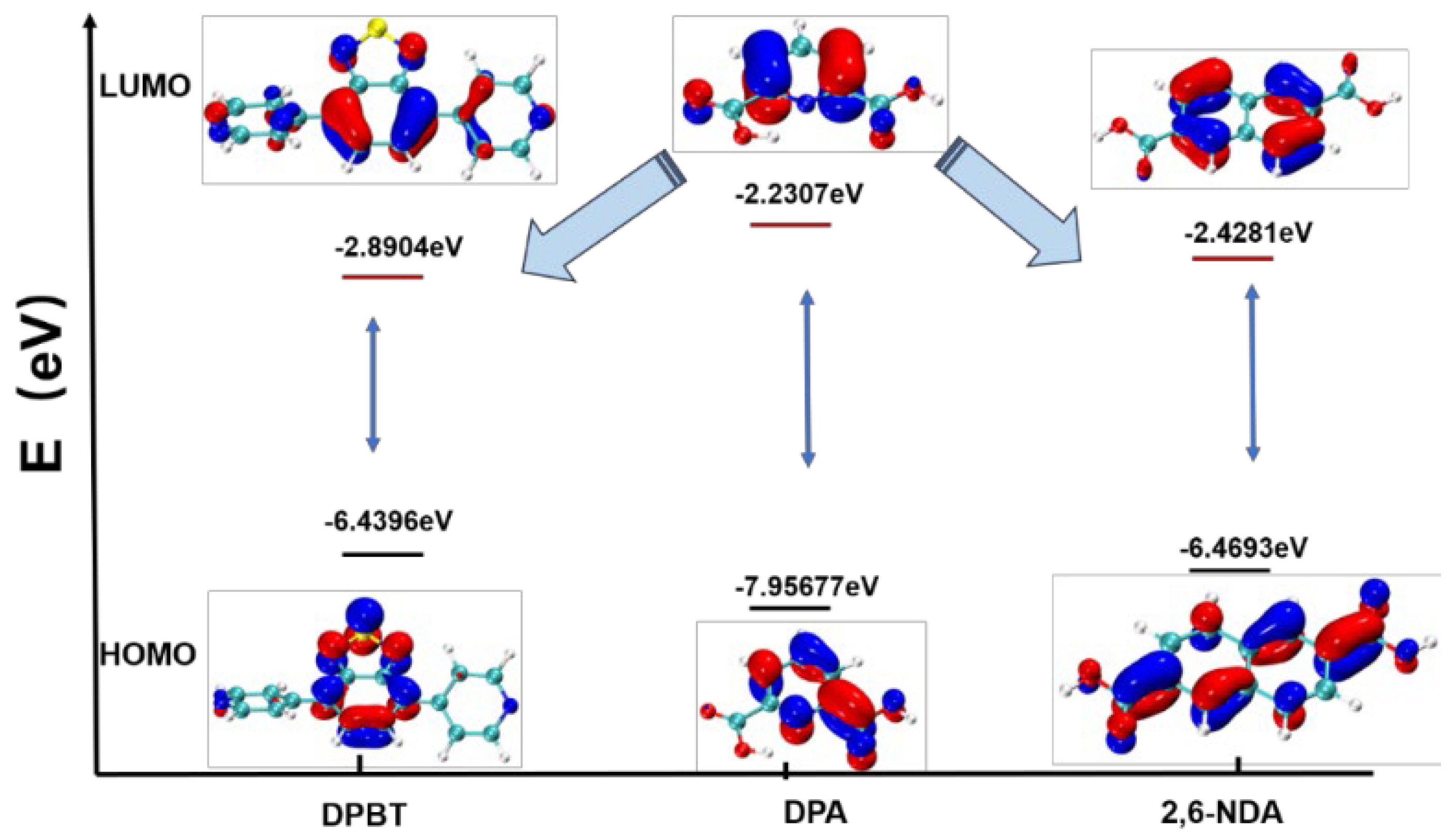
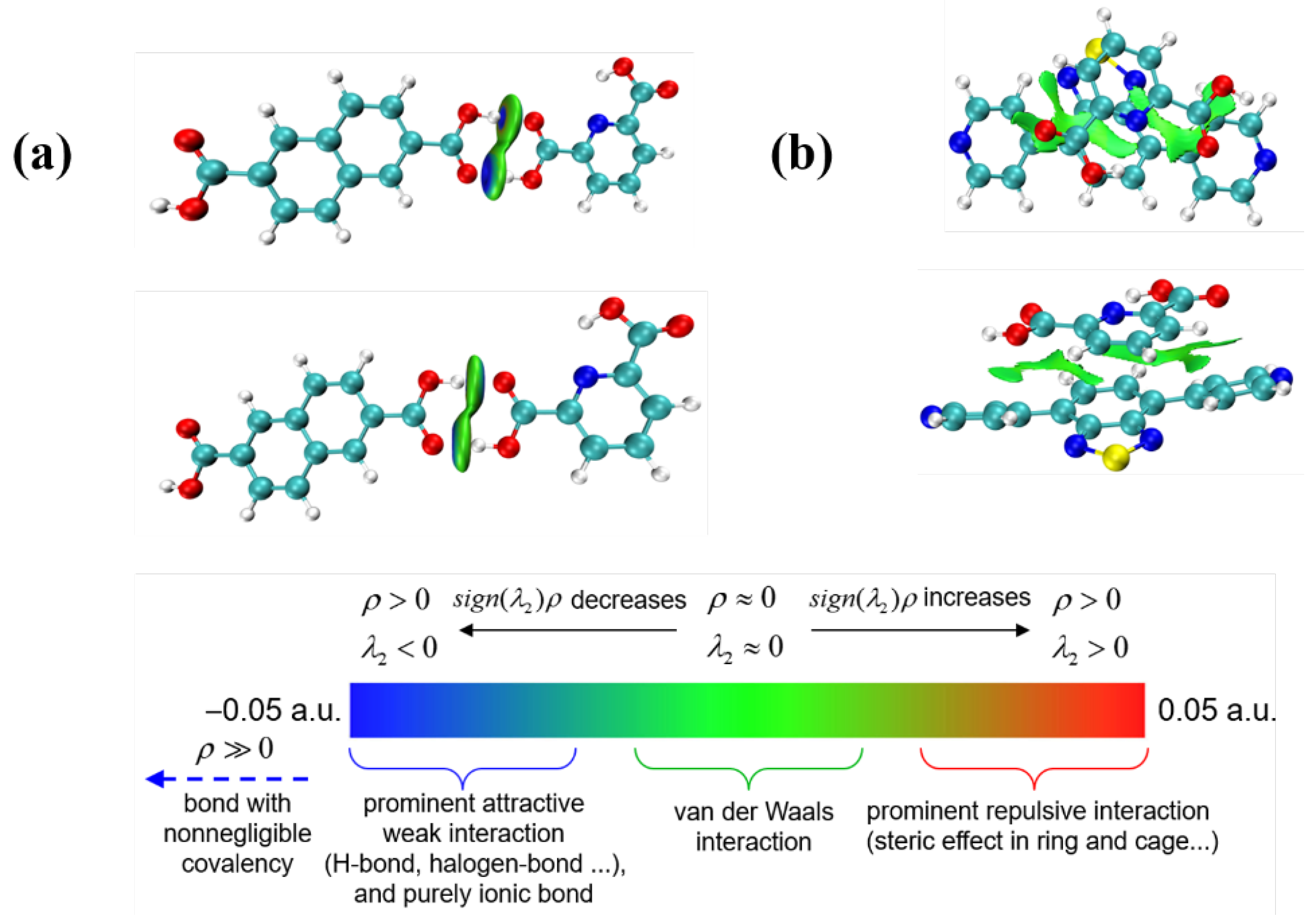
Mechanism of Luminescence Enhancing
4. Materials and Methods
4.1. Reagents and Methods
4.2. Synthesis of MOF-1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Liu, H.; Song, J.J.; Zhao, Z.Y.; Zhao, S.Q.; Tian, Z.Y.; Yan, F. Organic Electrochemical Transistors for Biomarker Detections. Adv. Sci. 2023, 10, 2305347. [Google Scholar] [CrossRef]
- Strimbu, K.; Tavel, J.A. What are biomarkers? Curr. Opin. HIV AIDS 2010, 5, 463. [Google Scholar] [CrossRef]
- Wang, Q.X.; Xue, S.F.; Chen, Z.H.; Ma, S.H.; Zhang, S.; Shi, G.; Zhang, M. Dual lanthanide-doped complexes: The development of a time-resolved ratiometric fluorescent probe for anthrax biomarker and a paper-based visual sensor. Biosens. Bioelectron. 2017, 94, 388–393. [Google Scholar] [CrossRef]
- Niu, X.; Wang, M.; Zhang, M.; Cao, R.; Liu, Z.; Hao, F.; Sheng, L.; Xu, H. Smart intercalation and coordination strategy to construct stable ratiometric fluorescence nanoprobes for the detection of anthrax biomarker. Inorg. Chem. Front. 2022, 9, 4582. [Google Scholar] [CrossRef]
- Othong, J.; Boonmak, J.; Kielar, F.; Hadsadee, S.; Jungsuttiwong, S.; Youngme, S. Selfcalibrating sensor with logic gate operation for anthrax biomarker based on nanoscaled bimetallic lanthanoid MOF. Sens. Actuators B 2020, 316, 128156. [Google Scholar] [CrossRef]
- Wang, Z.X.; Hu, L.; Gao, Y.F.; Kong, F.Y.; Li, H.Y.; Zhu, J.; Fang, H.L.; Wang, W. Aggregation-Induced Emission Behavior of Dual-NIR-Emissive Zinc-Doped Carbon Nanosheets for Ratiometric Anthrax Biomarker Detection. ACS Appl. Bio Mater. 2020, 3, 9031–9042. [Google Scholar] [CrossRef]
- Han, H.M.; Dong, W.W.; Li, M.K.; Xu, D.D.; Hu, Z.; Zhao, J.; Li, D.S. Ratiometric fluorescence detection of an anthrax biomarker by modulating energy transfer in hetero Eu/Tb-MOFs. Inorg. Chem. Commun. 2023, 153, 110755. [Google Scholar] [CrossRef]
- He, L.; Deen, D.D.; Pagel, A.H.; Diez-Gonzalez, F.; Labuza, T.P. Concentration, detection and discrimination of Bacillus anthracis spores in orange juice using aptamer based surface enhanced Raman spectroscopy. Analyst 2013, 138, 1657. [Google Scholar] [CrossRef]
- Cong, Z.; Zhu, M.; Zhang, Y.; Yao, W.; Kosinova, M.; Fedin, V.P.; Wu, S.Y.; Gao, E. Three novel metal-organic frameworks with different coordination modes for trace detection of anthrax biomarkers. Dalton Trans. 2022, 51, 250–256. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, S.; Wang, L.; Guan, X. Nanopore back titration analysis of dipicolinic acid. Electrophoresis 2015, 36, 467–470. [Google Scholar] [CrossRef]
- Mawatari, K.; Atsumi, M.; Nakamura, F.; Yasuda, M.; Fukuuchi, T.; Yamaoka, N.; Kaneko, K.; Nakagomi, K.; Oku, N. Determination of Dipicolinic Acid in “Natto” by High-Performance Liquid Chromatography Coupled with Postcolumn Photoirradiation with Zinc Acetate. Int. J. Tryptophan Res. 2019, 12. [Google Scholar] [CrossRef]
- Lei, M.Y.; Ge, F.Y.; Ren, S.S.; Gao, X.J.; Zheng, H.G. A water-stable Cd-MOF and corresponding MOF@melamine foam composite for detection and removal of antibiotics, explosives, and anions. Sep. Purif. Technol. 2022, 286, 120433. [Google Scholar] [CrossRef]
- Wang, H.; Lustig, W.P.; Li, J. Sensing and capture of toxic and hazardous gases and vapors by metal-organic frameworks. Chem. Soc. Rev. 2018, 47, 4729. [Google Scholar] [CrossRef]
- Hu, M.L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal-organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Qian, G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord. Chem. Rev. 2014, 273–274, 76–86. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A. Metal ion detection using luminescent-MOFs: Principles, strategies and roadmap. Coord. Chem. Rev. 2020, 415, 213299. [Google Scholar] [CrossRef]
- Pal, T.K. Metal-organic framework (MOF)-based fluorescence “turn-on” sensors. Mater. Chem. Front. 2023, 7, 405. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, W.; Liu, S.Y.; Liu, W.S. Coumarin-encapsulated MOF luminescence sensor for detection of picric acid in water environment. Dyes Pigments 2021, 184, 108794. [Google Scholar] [CrossRef]
- Guo, X.R.; Zhou, L.Y.; Liu, X.Z.; Tan, G.J.; Yuan, F.; Alireza, N.E.; Qi, N.; Liu, J.Q.; Peng, Y.Q. Fluorescence detection platform of metal-organic frameworks for biomarkers. Colloids Surf. B Biointerfaces 2023, 229, 113455. [Google Scholar] [CrossRef]
- Dong, J.; Dao, X.Y.; Zhang, X.Y.; Zhang, X.D.; Sun, W.Y. Sensing Properties of NH2-MIL-101 Series for Specific Amino Acids via Turn-On Fluorescence. Molecules 2021, 26, 5336. [Google Scholar] [CrossRef]
- Shukla, V.; Ahmad, M.; Siddiqui, K.A. Colorimetric recognition of a biomarker of trichloroethylene in human urine and photocatalytic dye degradation employing unprecedented Co(II) MOF luminescent probe. J. Mol. Struct. 2024, 1308, 138068. [Google Scholar] [CrossRef]
- Lei, N.N.; Li, W.C.; Zhao, D.S.; Li, W.Q.; Liu, X.; Liu, L.Y.; Yin, J.R.; Muddassir, M.; Wen, R.M.; Fan, L.M. A bifunctional luminescence sensor for biomarkers detection in serum and urine based on chemorobust Nickel(II) metal-organic framework. Spectrochim. Acta Part A 2024, 306, 123585. [Google Scholar] [CrossRef]
- Meng, Z.X.; Yang, F.N.; Wang, X.J.; Shan, W.L.; Liu, D.D.; Zhang, L.Y.; Yuan, G.Z. Trefoil-Shaped Metal–Organic Cages as Fluorescent Chemosensors for Multiple Detection of Fe3+, Cr2O72–, and Antibiotics. Inorg. Chem. 2023, 62, 1297. [Google Scholar] [CrossRef]
- Chaudhary, M.Y.; Kanzariya, D.B.; Das, A.; Pal, T.K. A fluorescent MOF and its synthesized MOF@cotton composite: Ratiometric sensing of vitamin B2 and antibiotic drug molecule. Spectrochim. Acta Part A 2024, 314, 124194. [Google Scholar] [CrossRef]
- Geng, J.; Li, Y.Y.; Lin, H.Y.; Liu, Q.Q.; Lu, J.J.; Wang, X.L. A new three-dimensional zinc(II) metal–organic framework as a fluorescence sensor for sensing the biomarker 3-nitrotyrosine. Dalton Trans. 2022, 51, 11390. [Google Scholar] [CrossRef]
- Li, W.Q.; Li, W.C.; Liu, X.; Wu, H.N.; Yang, J.Y.; Lu, F.Y.; Fan, L.M. A dual-responsive luminescent sensor for efficient detection of 3-nitrotyrosine and dipicolinic acid biomarkers based on copper(II) organic framework. Appl. Organomet. Chem. 2024, 38, e7452. [Google Scholar] [CrossRef]
- Cai, D.G.; Zheng, T.F.; Liu, S.J.; Wen, H.R. Fluorescence sensing and device fabrication with luminescent metal-organic frameworks. Dalton Trans. 2024, 53, 394. [Google Scholar] [CrossRef]
- Xi, Y.; Hu, M.; Gao, L.; Sun, Q.N.; Ma, E.H.; Hu, W.J.; Li, M.T.; Liu, W.; Sun, J.Y.; Zhang, C.L. A pyrazole-functional 3D cobalt-organic framework for fluorescence detection of Cu2+ and Hg2+. J. Mol. Struct. 2023, 1284, 135456. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Zhang, B.L.; Wang, T.; Chen, J.; Li, Z.Y.; Wang, R.H.; Li, S.Q.; Zhang, J.J. Two new Cd-based metal–organic frameworks for afterglow detection of Fe3+ and NH3. J. Mater. Chem. A 2024, 12, 7732–7741. [Google Scholar] [CrossRef]
- Wu, S.Y.; Lin, Y.N.; Liu, J.W.; Shi, W.; Yang, G.M.; Cheng, P. Rapid Detection of the Biomarkers for Carcinoid Tumors by a Water Stable Luminescent Lanthanide Metal-Organic Framework Sensor. Adv. Funct. Mater. 2018, 28, 1707169. [Google Scholar] [CrossRef]
- Wang, N.R.; Li, S.S.; Li, Z.H.; Gong, Y.Y.; Li, X. A Zn(II)-Metal-Organic Framework Based on 4-(4-Carboxy phenoxy) Phthalate Acid as Luminescent Sensor for Detection of Acetone and Tetracycline. Molecules 2023, 28, 999. [Google Scholar] [CrossRef]
- Pavlov, D.I.; Yu, X.L.; Ryadun, A.A.; Samsonenko, D.G.; Dorovatovskii, P.V.; Lazarenko, V.A.; Sun, N.; Sun, Y.; Fedin, V.P.; Potapov, A.S. Multiresponsive luminescent metal–organic framework for cooking oil adulteration detection and gallium(III) sensing. Food Chem. 2024, 445, 138747. [Google Scholar] [CrossRef]
- Tian, X.M.; Yao, S.L.; Qiu, C.Q.; Zheng, T.F.; Chen, Y.Q.; Huang, H.P.; Chen, J.L.; Liu, S.J.; Wen, H.R. Turn-on luminescent sensor toward Fe3+, Cr3+, and Al3+ based on a Co(II) metal-organic framework with open functional sites. Inorg. Chem. 2020, 59, 2803. [Google Scholar] [CrossRef]
- Ma, K.; Zhao, Y.N.; Han, X. Interesting pH-responsive behavior in benzothiadiazole-derived coordination polymer constructed via an in situ click synthesis. Cryst. Growth Des. 2018, 18, 7419–7425. [Google Scholar] [CrossRef]
- Mallick, A.; El-Zohry, A.M.; Shekhah, O.; Yin, J.; Jia, J.; Aggarwal, H.; Emwas, A.-H.; Mohammed, O.F.; Eddaoudi, M. Unprecedented ultralow detection limit of amines using a thiadiazole-functionalized Zr(IV)-based metal-organic framework. J. Am. Chem. Soc. 2019, 141, 7245–7249. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXTL, version 6.14; Bruker AXS, Inc.: Madison, WI, USA, 2000–2003. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sarkisov, L.; Harrison, A. Computational structure characterisation tools in application to ordered and disordered porous materials. Mol. Simul. 2011, 37, 1248–1257. [Google Scholar] [CrossRef]
- Spek, A.L.J. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Rani, P.; Husain, A.; Bhasin, K.K.; Kumar, G. Zinc(II)-MOF: A Versatile Luminescent Sensor for Selective Molecular Recognition of Flame Retardants and Antibiotics. Inorg. Chem. 2024, 63, 3486. [Google Scholar] [CrossRef]
- Ju, Z.M.; Yan, W.; Gao, X.J.; Shi, Z.Z.; Wang, T.; Zheng, H.G. Syntheses, Characterization, and Luminescence Properties of Four Metal−Organic Frameworks Based on a Linear-Shaped Rigid Pyridine Ligand. Cryst. Growth Des. 2016, 16, 2496–2503. [Google Scholar] [CrossRef]
- Wang, L.Y.; Liu, T.T.; Cheng, J.W.; Zou, H.Q.; Lu, J.; Liu, H.T.; Li, Y.W.; Dou, J.M.; Wang, S.N. Turn-off luminescence sensing activities of amide-functionalized Zn-MOF toward biomarker (2-methoxyethoxy)acetic acid and antibiotic tetracycline. J. Mol. Struct. 2024, 1296, 136815. [Google Scholar] [CrossRef]
- Ru, J.; Shi, Y.X.; Li, T.; Cao, F.; Guo, Q.; Wang, Y.L. A water-stable 2D Y-MOF decorated with tris(3′-F-4′-carboxybiphenyl) amine for multi-responsive fluorescence sensing of CrVI and nitrofuran antibiotics. J. Mol. Struct. 2024, 1297, 136978. [Google Scholar] [CrossRef]
- Zhang, R.J.; Wang, J.J.; Xu, H.; Zhu, Z.H.; Zheng, T.F.; Peng, Y.; Chen, J.L.; Liu, S.J.; Wen, H.R. Stable Cd(II)-Based Metal-Organic Framework as a Multiresponsive Luminescent Sensor for Acetylacetone, Salicylaldehyde, and Benzaldehyde with High Sensitivity and Selectivity. Cryst. Growth Des. 2023, 23, 5564. [Google Scholar] [CrossRef]
- Hong, C.; Li, L.; Zou, J.Y.; Zhang, L.; You, S.Y. A turn-on fluorescent Zn(II) metal-organic framework sensor for quantitative anthrax biomarker detection. Dalton Trans. 2023, 52, 6067–6076. [Google Scholar] [CrossRef]
- Yang, S.L.; Song, D.X.; Li, K.S.; Wang, L.; Zhang, Y.; Sun, Y.G.; Zhu, M.C.; Wu, S.Y. Synthesis of a lanthanide-based bimetallic-metal-organic framework for luminescence sensing anthrax biomarker. Dyes Pigments 2023, 220, 111673. [Google Scholar]
- Wang, R.N.; Zhang, H.; Sun, J.; Su, Z.M. Eu3+-MOF fluorescence sensor based on a dual-ligand strategy for visualised detection of an anthrax biomarker 2,6-pyridine dicarboxylic acid. Inorg. Chem. Front. 2024, 11, 269. [Google Scholar] [CrossRef]
- Wang, L.X.; Li, A.J.; Wang, Z.H.; Wang, W.Z.; Zhou, H.F.; Liu, B. A layered Y(III)-viologen framework for efficient detection of nitrofurazone. J. Solid State Chem. 2022, 316, 123617. [Google Scholar]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Weigenda, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T.; Chen, Q.X. Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J. Comput. Chem. 2022, 43, 539. [Google Scholar] [CrossRef]


| MOF-1 | |
|---|---|
| Empirical formula | C120H64N12O24S3Zn6 |
| Formula weight | 2546.23 |
| Crystal system | monoclinic |
| Space group | C2/c |
| a, Å | 17.6526 (17) |
| b, Å | 19.6903 (19) |
| c, Å | 37.142 (3) |
| α, deg | 90 |
| β, deg | 90.106 (2) |
| γ, deg | 90 |
| V, Å3 | 12,910 (2) |
| Z | 4 |
| ρcalcd, g/cm−3 | 1.310 |
| T/K | 298.15 |
| μ, mm−1 | 1.214 |
| 2θ, deg | 3.794 to 50.038 |
| F (000) | 5152.0 |
| Index ranges | −20 ≤ h ≤ 20, −23 ≤ k ≤ 23, −26 ≤ l ≤ 44 |
| Data/restraints/parameters | 11,387/2403/779 |
| GOF (F2) | 1.067 |
| R1a,wR2b (I > 2σ(I)) | 0.1070, 0.2790 |
| R1a, wR2b (all data) | 0.1591, 0.3075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ru, J.; Shi, Y.-X.; Yang, Q.-Y.; Li, T.; Wang, H.-Y.; Cao, F.; Guo, Q.; Wang, Y.-L. A Benzothiadiazole-Based Zn(II) Metal–Organic Framework with Visual Turn-On Sensing for Anthrax Biomarker and Theoretical Calculation. Molecules 2024, 29, 2755. https://doi.org/10.3390/molecules29122755
Ru J, Shi Y-X, Yang Q-Y, Li T, Wang H-Y, Cao F, Guo Q, Wang Y-L. A Benzothiadiazole-Based Zn(II) Metal–Organic Framework with Visual Turn-On Sensing for Anthrax Biomarker and Theoretical Calculation. Molecules. 2024; 29(12):2755. https://doi.org/10.3390/molecules29122755
Chicago/Turabian StyleRu, Jing, Yi-Xuan Shi, Qing-Yun Yang, Teng Li, Hai-Ying Wang, Fan Cao, Qiang Guo, and Yan-Lan Wang. 2024. "A Benzothiadiazole-Based Zn(II) Metal–Organic Framework with Visual Turn-On Sensing for Anthrax Biomarker and Theoretical Calculation" Molecules 29, no. 12: 2755. https://doi.org/10.3390/molecules29122755
APA StyleRu, J., Shi, Y.-X., Yang, Q.-Y., Li, T., Wang, H.-Y., Cao, F., Guo, Q., & Wang, Y.-L. (2024). A Benzothiadiazole-Based Zn(II) Metal–Organic Framework with Visual Turn-On Sensing for Anthrax Biomarker and Theoretical Calculation. Molecules, 29(12), 2755. https://doi.org/10.3390/molecules29122755







