3. Experimental Section
3.1. General
All reagents (Merck, Darmstadt, Germany) were used as supplied without prior purification. The progression of the reaction was monitored by analytical thin-layer chromatography using TLC sheets ALUGRAM-SIL G/UV254 (Macherey Nagel, Düren, Germany). Purification by flash chromatography was performed using silica gel (60 Å, 230–400 mesh, Merck, Darmstadt, Germany) with the indicated eluent.
3.2. Melting Point Determination
The melting points of the synthesised derivatives were determined using a StuartTM melting point apparatus SMP10 (Bibby Scientific Ltd., Staffordshire, UK).
3.3. NMR Spectroscopy
NMR spectra were acquired using a Varian VNMRS spectrometer (Palo Alto, CA, USA) operating at 599.87 MHz for 1H, 150.84 MHz for 13C, and Varian Mercury spectrometer (Palo Alto, CA, USA) operating at 400.13 MHz for 1H and 100.62 MHz for 13C. These experiments were conducted at a temperature of 299.15 K, and a 5 mm inverse-detection H-X probe with a z-gradient coil was used. Pulse programs from the Varian sequence library were employed. Chemical shifts (δ in ppm) were referenced to internal solvent standard CDCl3 77.0 ppm for 13C, while a partially deuterated signal of CHD2Cl 7.26 ppm was used for 1H referencing. MestReNova v. 15.0.1 (Mestrelab Research, Santiago de Compostela, Spain) was utilized for NMR spectra processing and analysis.
3.4. IR Spectroscopy
The infrared spectra of prepared compounds were recorded with Avatar FT−IR 6700 (Fourier transform infrared spectroscopy) spectrometer in the range from 400 to 4000 cm−1 with 64 repetitions for a single spectrum using the ATR (attenuated total reflectance) technique. All obtained data were analysed using Omnic 8.2.0.387 (2010) software, and the structure of all new compounds was confirmed by analysis of FT-IR spectrum by functional group identification.
3.5. Elemental Analysis
Elemental analysis of C, H, and N was performed using a CHNOS Elemental Analyzer vario MICRO from Elementar Analysensysteme GmbH (Langenselbold, Germany).
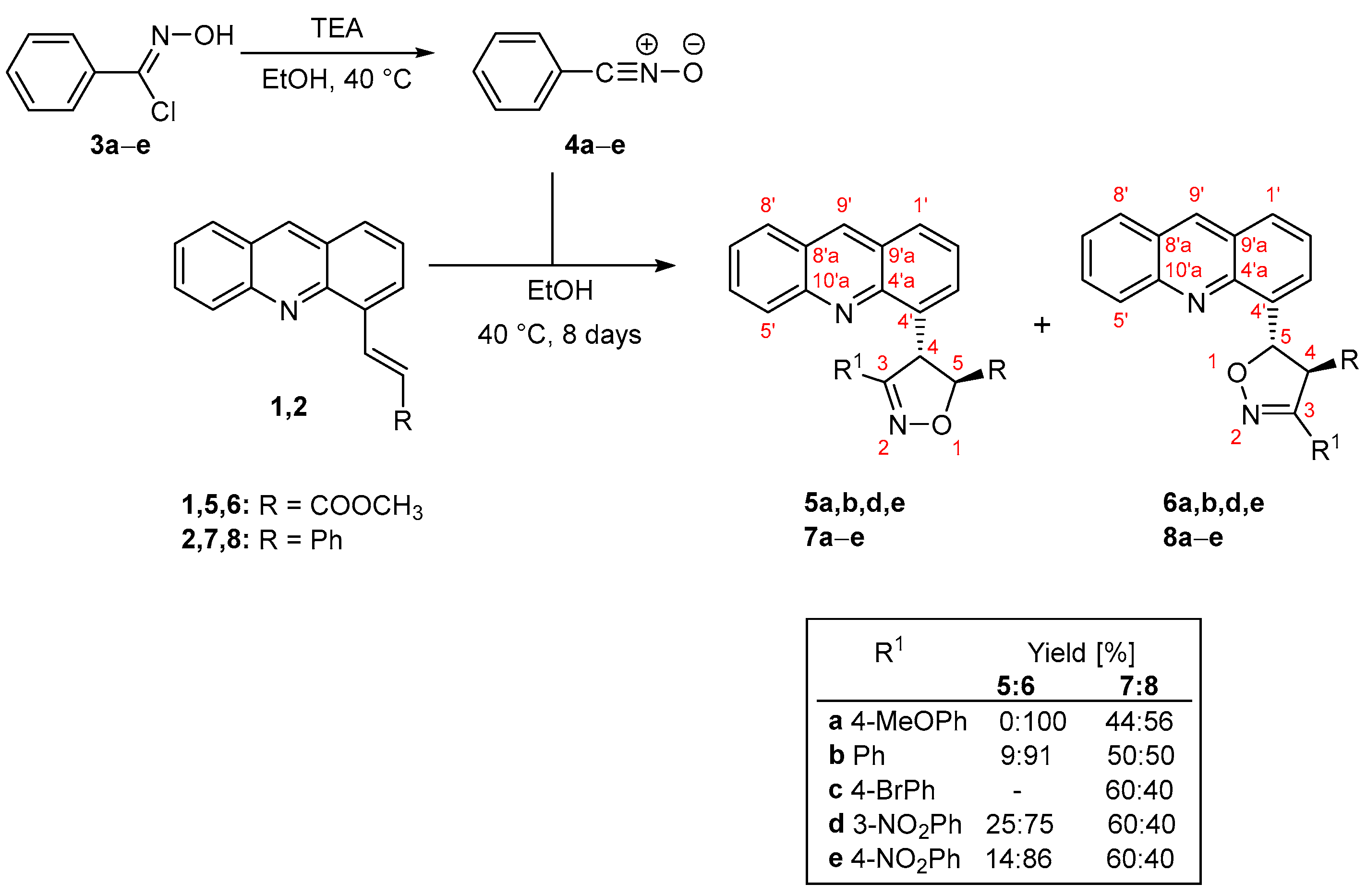
3.6. General Procedure for Preparation of Methyl 4-(Acridin-4-yl)-3-phenyl-4,5-dihydro-1,2-oxazole-5-carboxylates 5a,b,d,e and Methyl 5-(Acridin-4-yl)-3-phenyl-4,5-dihydro-1,2-oxazole-4-carboxylates 6a–e
The corresponding N-hydroxybenzenecarbonimidoyl chloride (3a: 423 mg; 3b: 354 mg; 3c: 533 mg; 3d: 457 mg; 3e: 457 mg, 2.28 mmol) was added to an ethanolic solution (5 mL) of methyl (2E)-3-(acridin-4-yl)-prop-2-enoate (1, 100 mg, 0.37 mmol), and the reaction mixture was heated to 40 °C. Triethylamine (230 mg, 0.317 mL, 2.28 mmol) was dissolved in ethanol (5 mL) and added to the reaction mixture over eight days. The reaction’s progress was tracked using 1H NMR spectra. Further purification by column chromatography (SiO2, n-Hex/EtOAc, 5:1) yielded compounds 5b and 6a,b,d,e.
Methyl-4-(acridin-4-yl)-3-phenyl-4,5-dihydro-1,2-oxazole-5-carboxylate (5b). Yield: 7.0 mg (5.0%). Mp. 114–116 °C. Yellow needles. For C24H18N2O3 (382.13) found: C 74.78, H 5.30, N 7.21%; calc.: C 75.38, H 4.74, N 7.33, O 12.55%. Rf (n-Hex/EtOAc, 5:1) 0.13. FT-IR: νmax 3104, 3069, 1782, 1647, 1218, 1167, 1025, 753 cm−1. 1H NMR (600 MHz CDCl3): δ 8.80 (1H, s, H-9′), 8.29 (1H, dd, J = 8.4, 1.2 Hz, H-5′), 8.04 (1H, d, J = 8.4 Hz, H-8′), 7.95 (1H, dd, J = 8.4, 1.2 Hz, H-1′), 7.84 (1H, ddd, J = 8.4, 6.6, 1.8 Hz, H-6′), 7.73 (2H, dd, J = 6.6, 1.2 Hz, H-2″,6″), 7.60 (1H, ddd, J = 8.4, 6.6, 1.2 Hz, H-7′), 7.54 (1H, dd, J = 6.6, 1.2 Hz, H-3′), 7.42 (1H, dd, J = 8.4, 7.2 Hz, H-2′), 7.27 (1H, t, J = 8.4 Hz, H-4″), 7.21 (2H, t, J = 7.8 Hz, H-3″,5″), 6.85 (1H, d, J = 4.2 Hz, H-4), 5.05 (1H, d, J = 4.2 Hz, H-5), 3.91 (3H, s, H-7) ppm. 13C NMR (150.1 MHz, CDCl3): δ 170.5 (C-6), 159.4 (C-3), 148.7 (C-10′a), 146.0 (C-4′a), 136.3 (C-9′), 136.2 (C-4′), 130.5 (C-6′), 130.1 (C-4″), 130.1 (C-5′), 128.6 (C-3″,5″), 128.5 (C-1′), 128.3 (C-1″), 128.0 (C-8′), 127.7 (C-2″,6″), 126.8 (C-3′,C-8′a), 126.7 (C-9′a), 126.2 (C-7′), 125.5 (C-2′), 86.7 (C-5), 52.7 (C-7), 52.0 (C-4) ppm.
Methyl-5-(acridin-4-yl)-3-(4-methoxyphenyl)-4,5-dihydro-1,2-oxazole-4-carboxylate (6a). Yield: 133.1 mg (85.0%). M.p. 152–154 °C. Yellow powder. For C25H20N2O4 (412.45) found: C 72.75, H 4.73, N 6.88%; calc.: C 72.80, H 4.89, N 6.79, O 15.52%. Rf (5:1 v/v n-Hex/EtOAc) 0.16. FT-IR: νmax 3102, 3063, 2944, 2903, 1768, 1647, 1621, 1482, 1338, 1250, 1172, 1065, 1028 cm−1. 1H NMR (400 MHz CDCl3): δ 8.78 (1H, s, H-9′), 8.09 (1H, d, J = 8.8 Hz, H-5′), 8.01 (1H, d, J = 8.8 Hz, H-8′), 7.98 (1H, d, J = 6.8 Hz, H-3′), 7.96 (1H, d, J = 8.0 Hz, H-1′), 7.78 (1H, ddd, J = 8.4, 6.4, 1.2 Hz, H-6′), 7.71 (2H, d, J = 8.8 Hz, H-2″,6″), 7.56 (1H, ddd, J = 8.8, 6.4, 1.2 Hz, H-7′), 7.53 (1H, dd, J = 8.0, 6.8 Hz, H-2′), 7.12 (1H, d, J = 6.0 Hz, H-5), 6.85 (2H, d, J = 8.8 Hz, H-3″,5″), 4.59 (1H, d, J = 6.0 Hz, H-4), 3.95 (3H, s, H-7), 3.80 (3H, s, OCH3) ppm. 13C NMR (100.6 MHz, CDCl3): δ 170.6 (C-6), 161.2 (C-4″), 153.8 (C-3), 148.1 (C-10′a), 145.9 (C-4′a), 137.4 (C-4′), 136.0 (C-9′), 130.3 (C-6′), 129.7 (C-5′), 128.6 (C-2″,6″), 128.1 (C-8′), 128.2 (C-1′), 126.6 (C-8′a), 126.66 (C-9′a), 126.0 (C-7′), 125.9 (C-3′), 125.4 (C-2′), 121.1 (C-1″), 114.1 (C-3″,5″), 84.8 (C-5), 62.6 (C-4), 55.3 (OCH3), 52.9 (C-7) ppm.
Methyl-5-(acridin-4-yl)-3-phenyl-4,5-dihydro-1,2-oxazole-4-carboxylate (6b). Yield: 116.1 mg (80.0%). Mp. 177–179 °C. Yellow powder. For C24H18N2O3 (382.13) found: C 74.80, H 5.22, N 7.30%; calc.: C 75.38, H 4.74, N 7.33, O 12.55%; Rf (n-Hex/EtOAc, 5:1) 0.29. FT-IR: νmax 3096, 2910, 1770, 1646, 1604, 1536, 1328, 1217, 1045 cm−1. 1H NMR (400 MHz CDCl3): δ 8.78 (1H, s, H-9′), 8.08 (1H, d, J = 8.8 Hz, H-5′), 8.01 (1H, dd, J = 8.5, 0.7 Hz, H-8′), 7.97 (1H, m, H-1′), 7.97 (1H, m, H-3′), 7.78 (1H, m, H-6′), 7.78 (2H, m, H-2″,6″), 7.56 (1H, ddd, J = 8.5, 6.6, 1.2 Hz, H-7′), 7.53 (1H, dd, J = 8.5, 6.9 Hz, H-2′), 7.36 (3H, m, H-3″,5″, H-4″), 7.15 (1H, d, J = 6.5 Hz, H-5), 4.62 (1H, d, J = 6.5 Hz, H-4), 3.95 (3H, s, H-7) ppm. 13C NMR (100.6 MHz, CDCl3): δ 170.5 (C-6), 154.2 (C-3), 148.1 (C-10′a), 145.8 (C-4′a), 137.2 (C-4′), 136.0 (C-9′), 130.4 (C-6′), 130.2 (C-4″), 129.7 (C-5′), 128.7 (C-3″,5″), 128.1 (C-1′), 128.2 (C-8′), 128.6 (C-1″), 127.0 (C-2″,6″), 126.6 (C-8′a,9′a), 126.0 (C-3′), 126.0 (C-7′), 125.4 (C-2′), 85.1 (C-5), 62.3 (C-4), 52.9 (C-7) ppm.
Methyl-5-(acridin-4-yl)-3-(3-nitrophenyl)-4,5-dihydro-1,2-oxazole-4-carboxylate (6d). Yield: 102.3 mg (63.0%). Mp. 113–115 °C. Yellow needles. For C24H17N3O5 (427.42) found: C 67.24, H 4.08, N 9.80%; calc.: C 67.44, H 4.01, N 9.83, O 18.27%. Rf (5:1 v/v n-Hex/EtOAc) 0.16. FT-IR: νmax 3098, 2910, 1780, 1663, 1337, 1231, 1172, 1165, 1071, 1058 cm−1. 1H NMR (400 MHz CDCl3): δ 8.80 (1H, s, H-9′), 8.62 (1H, d, J = 2.0 Hz, H-2″), 8.23 (1H, d, J = 8.4 Hz, H-4″), 8.17 (1H, d, J = 8.4 Hz, H-6″), 8.05 (1H, d, J = 8.4 Hz, H-5′), 8.03 (1H, d, J = 8.4 Hz, H-8′), 7.99 (1H, d, J = 8.4 Hz, H-8′), 7.96 (1H, dd, J = 6.8, 1.3 Hz, H-3′), 7.79 (1H, m, H-6′), 7.56 (3H, m, H-2′, H-7′, H-5″), 7.21 (1H, d, J = 6.8 Hz, H-5), 4.69 (1H, d, J = 6.8 Hz, H-4), 3.98 (3H, s, H-7) ppm. 13C NMR (100.6 MHz, CDCl3): δ 170.0 (C-6), 152.6 (C-3), 148.5 (C-3″), 148.1 (C-10′a), 145.7 (C-4′a), 136.6 (C-4′), 136.2 (C-9′), 132.6 (C-6″), 130.7 (C-1″), 130.5 (C-6′), 129.8 (C-5″),129.6 (C-5′), 128.6 (C-1′), 128.2 (C-8′), 126.6 (C-8′a), 126.6 (C-9′a), 126.2 (C-7′), 126.0 (C-3′), 125.3 (C-2′), 124.6 (C-4″), 121.9 (C-2″), 86.1 (C-5), 61.6 (C-4), 53.1 (C-7) ppm.
Methyl-5-(acridin-4-yl)-3-(4-nitrophenyl)-4,5-dihydro-1,2-oxazole-4-carboxylate (6e). Yield: 115.3 mg (71.0%). Mp. 165–167 °C. Yellow needles. For C24H17N3O5 (427.42) found: C 67.34, H 4.11, N 9.73%; calc.: C 67.44, H 4.01, N 9.83, O 18.27%. Rf (CH2Cl2) 0.56. FT-IR: νmax 3124, 3102, 2980, 1771, 1652, 1629, 1621, 1640, 1566, 1344, 1157, 1067, 1039, 842 cm−1. 1H NMR (400 MHz CDCl3): δ 8.79 (1H, s, H-9′), 8.21 (2H, d, J = 8.9, Hz H-3″,5″), 8.02 (3H, m, H-1′, H-5′, H-8′), 7.96 (3H, m, H-3′, H-2″,6″), 7.78 (1H, ddd, J = 8.4, 6.8, 1.2 Hz, H-6′), 7.57 (1H, ddd, J = 8.4, 6.8, 1.2 Hz, H-7′), 7.54 (1H, dd, J = 8.4, 6.8 Hz, H-2′), 7.18 (1H, d, J = 6.8 Hz, H-5), 4.68 (1H, d, J = 6.8 Hz, H-4), 3.97 (3H, s, H-7) ppm. 13C NMR (100.6 MHz, CDCl3): δ 169.9 (C-6), 152.9 (C-3), 148.5 (C-4″), 148.1 (C-10′a), 145.6 (C-4′a), 136.5 (C-4′), 136.2 (C-9′), 134.9 (C-1″), 130.5 (C-6′), 129.5 (C-5′), 128.6 (C-1′), 128.2 (C-8′), 127.8 (C-2″,6″), 126.6 (C-8′a, C-9′a), 126.2 (C-3′, C-7′), 125.2 (C-3′), 123.9 (C-3″,5″), 86.4 (C-5), 61.5 (C-4), 53.2 (C-7) ppm.
3.7. General Procedure for Preparation of 4-[3-(4-Nitrophenyl)-5-phenyl-4,5-dihydro-1,2-oxazol-4-yl]acridines 7a–e and 4-(3-Phenyl-4-phenyl-4,5-dihydro-1,2-oxazol-5-yl)acridines 8a–e
A corresponding N-hydroxybenzenecarbonimidoyl chloride (3a: 395 m; 3b: 331 mg; 3c: 500 mg; 3d: 427 mg; 3e: 427 mg, 2.13 mmol) was added to a solution of methyl 4-[(1E)-2-phenylethenyl]acridine (2, 100 mg, 0.35 mmol) in ethanol (5 mL), and the reaction mixture was heated to 40 °C. Over eight days, a triethylamine solution (215 mg, 0.296 mL, 2.28 mmol) in ethanol (5 mL) was added. 1H NMR spectra of the reaction mixture were used to track the process. The solvent was evaporated under reduced pressure. Further purification by column chromatography (SiO2, c-Hex/EtOAc, 5:1) yielded compounds 7b and 8b, and (SiO2, n-Hex/EtOAc, 5:1) yielded compounds 8a,c and the mixture of isomers 7d/8d and 7e/8e.
4-(3,5-Diphenyl-4,5-dihydro-1,2-oxazole-4-yl)acridine (7b). Yield: 55.5 mg (39.0%). Mp. 177–179 °C. Yellow crystals. For C28H20N2O (400.48) found: C 84.01, H 5.16, N 7.13%; calc.: C 83.98, H 5.03, N 6.99, O 4.00%. Rf (5:1 v/v n-Hex/EtOAc) 0.35. FT-IR: νmax 3091, 2895, 1640, 1355, 1034, 1010, 874, 754 cm−1. 1H NMR (400 MHz CDCl3): δ 8.81 (1H, s, H-9′), 8.29 (1H, dd, J = 8.8, 1.0 Hz, H-5′), 8.05 (1H, dd, J = 8.4, 1.0 Hz, H-8′), 7.95 (1H, dd, J = 8.6, 1.4 Hz, H-1′), 7.84 (1H, ddd, J = 8.4, 6.6, 1.4 Hz, H-6′), 7.78 (2H, d, J = 7.8 Hz, H-2″,6″), 7.69 (2H, dd, J = 8.4, 1.5 Hz, H-2‴,6‴), 7.60 (2H, m, H-3′, H-7′), 7.44 (3H, m, H-2′, H-3″,5″), 7.35 (1H, td, J = 8.5, 1.2 Hz, H-4″), 7.20 (3H, m, H-3‴,5‴, H-4‴), 6.52 (1H, d, J = 3.4 Hz, H-4), 5.64 (1H, d, J = 3.4 Hz, H-5) ppm. 13C NMR (100.6 MHz, CDCl3): δ 159.0 (C-3), 148.6 (C-10′a), 146.4 (C-4′a), 141.6 (C-1‴), 137.1 (C-4′), 136.3 (C-9′), 130.4 (C-6′), 129.8 (C-4‴, C-5′), 129.1 (C-1‴), 128.6 (C-3′), 128.4 (C-3‴,5‴), 128.5 (C-3″,5″), 128.1 (C-8′), 128.1 (C-1′), 127.7 (C-1″, C-4″), 127.5 (C-2‴,6‴), 126.9 (C-9′a), 126.7 (C-8′a), 126.1 (C-7′), 125.8 (C-2′), 125.8 (C-2″,6″), 90.9 (C-5), 56.4 (C-4) ppm.
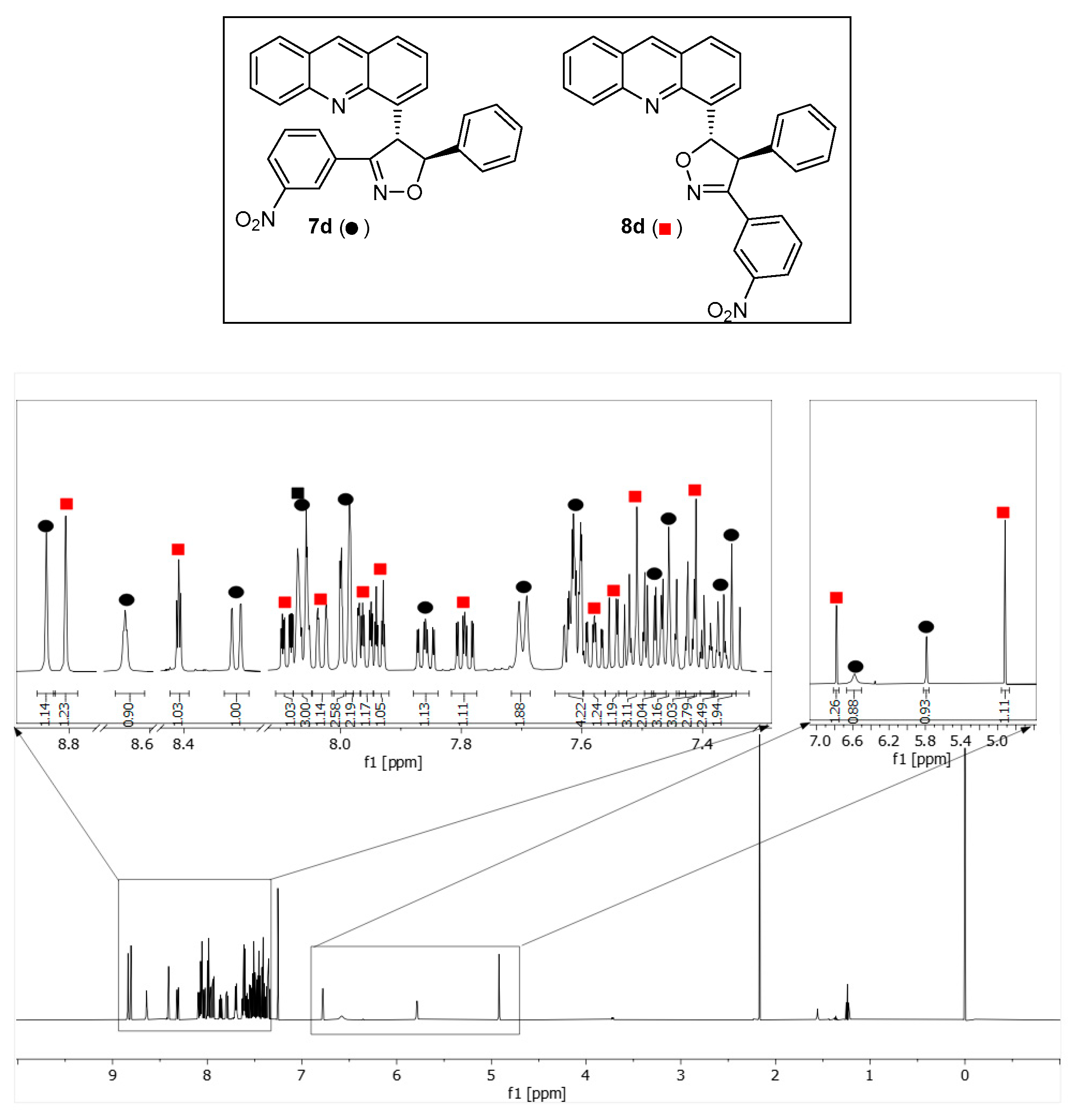
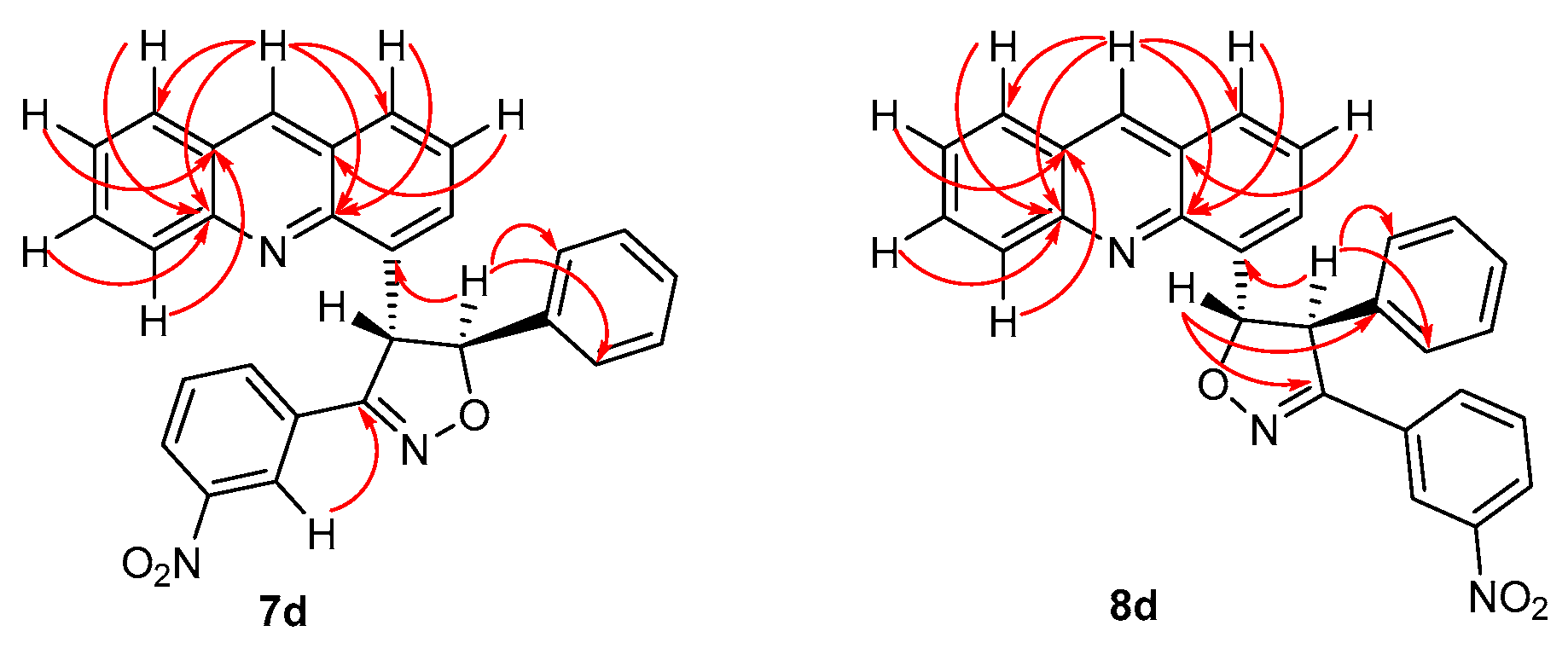
4-[3-(3-Nitrophenyl)-5-phenyl-4,5-dihydro-1,2-oxazole-4-yl]acridine (7d). The compound 7e was obtained as a mixture of 7d and 8e. Rf (5:1 v/v c-Hex/EtOAc) 0.32. 1H NMR (600 MHz CDCl3): δ 8.84 (1H, s, H-9′), 8.64 (1H, t, J = 2.0 Hz, H-2‴), 8.31 (1H, dd, J = 8.8, 1.0 Hz, H-5′), 8.06 (1H, m, H-4‴, H-8′), 7.99 (2H, m, H-1′, H-6‴), 7.86 (1H, ddd, J = 8.8, 6.6, 1.4 Hz, H-6′), 7.70 (2H, d, J = 7.8 Hz, H-2″,6″), 7.61 (2H, m, H-3′, H-7′), 7.48 (1H, dd, J = 8.4, 7.0 Hz, H-2′), 7.46 (1H, t, J = 7.6 Hz, H-3″,5″), 7.38 (1H, td, J = 7.4, 1.8 Hz, H-4″), 7.35 (1H, t, J = 8.0 Hz, H-3‴,5‴), 6.58 (1H, br s, H-4), 5.78 (1H, d, J = 3.9 Hz, H-5) ppm. 13C NMR (150.1 MHz, CDCl3): δ 157.8 (C-3), 148.8 (C-10′a), 148.4 (C-3‴), 146.0 (C-4′a), 140.9 (C-1″), 136.6 (C-9′), 136.4 (C-4′), 131.0 (C-1‴), 132.9 (C-6″), 130.6 (C-6′), 129.9 (C-5′), 129.5 (C-5‴), 128.6 (C-3″,5″), 128.1 (C-4″), 128.6 (C-1′), 128.3 (C-3′), 128.0 (C-8′), 126.8 (C-9′a), 126.7 (C-8′a), 126.3 (C-7′), 125.6 (C-2′), 125.7 (C-2″,6″), 124.2 (C-4‴), 122.3 (C-2‴), 91.8 (C-5), 55.7 (C-4) ppm.
4-[3-(4-Nitrophenyl)-5-phenyl-4,5-dihydro-1,2-oxazole-4-yl]acridine (7e). The compound 7e was obtained as a mixture of 7e and 8e. Rf (5:1 v/v c-Hex/EtOAc) 0.32. 1H NMR (400 MHz CDCl3): δ 8.84 (1H, s, H-9′), 8.27 (2H, d, J = 9.0 Hz, H-3‴,5‴), 8.27 (1H, m, H-5′), 8.06 (1H, m, H-8′), 7.99 (1H, dd, J = 8.4, 1.0 Hz, H-8′), 7.85 (2H, d, J = 9.0 Hz, H-2‴,6‴), 7.85 (1H, m, H-6′), 7.71 (2H, m, H-2″,6″), 7.56 (1H, m, H-3′, H-7′), 7.46 (3H, m, H-2′, H-3″,5″), 7.38 (1H, m, H-4″), 6.55 (1H, br s, H-4), 5.76 (1H, d, J = 4.1 Hz, H-5) ppm. 13C NMR (100.6 MHz, CDCl3): δ 157.8 (C-3), 148.7 (C-10′a), 148.1 (C-4‴), 146.1 (C-4′a), 140.8 (C-1″), 136.4 (C-4′), 136.3 (C-9′), 135.4 (C-1‴), 130.7 (C-6′), 128.1 (C-4″), 129.7 (C-5′), 128.7 (C-3″,5″), 128.6 (C-1′), 128.2 (C-8′), 126.9 (C-9′a), 126.8 (C-8′a), 128.1 (C-2‴,6‴, C-3′), 126.3 (C-7′), 125.7 (C-2″,6″), 125.6 (C-2′), 123.5 (C-3‴,5‴), 92.0 (C-5), 55.7 (C-4) ppm.
4-[3-(4-Methoxyphenyl)-4-phenyl-4,5-dihydro-1,2-oxazole-5-yl]acridine (8a). Yield: 64.3 mg (42.0%). Mp. 225–227 °C. Yellow solid. For C29H22N2O2 (430.51) found: C 81.00, H 5.10, N 6.63%; calc.: C 80.91, H 5.15, N 6.51, O 7.43%. Rf (5:1 v/v c-Hex/EtOAc) 0.32. FT-IR: νmax 3085, 2930, 2915, 1638, 1608, 1564, 1480, 1353, 1264, 1205, 1056, 1019, 796, 754 cm−1. 1H NMR (400 MHz CDCl3): δ 8.77 (1H, s, H-9′), 8.12 (1H, d, J = 8.4 Hz, H-5′), 8.01 (1H, d, J = 8.4 Hz, H-8′), 7.96 (1H, dd, J = 6.8, 1.2 Hz, H-3′), 7.93 (1H, d, J = 8.5 Hz, H-1′), 7.79 (1H, ddd, J = 8.4, 6.8, 1.2 Hz, H-6′), 7.63 (2H, d, J = 8.2 Hz, H-2″,6″), 7.54 (1H, m, H-7′), 7.54 (3H, m, H-2‴,6‴, H-2′), 7.48 (2H, t, J = 7.6 Hz, H-3″,5″), 7.38 (1H, t, J = 7.6 Hz, H-4″), 6.72 (2H, d, J = 8.9 Hz, H-3‴,5‴), 6.68 (1H, d, J = 3.2 Hz, H-5), 4.84 (1H, d, J = 3.2 Hz, H-4), 3.70 (3H, s, OCH3) ppm. 13C NMR (100.6 MHz, CDCl3): δ 160.7 (C-4‴), 158.4 (C-3), 148.2 (C-10′a), 146.4 (C-4′a), 139.7 (C-1″), 138.2 (C-4′), 136.0 (C-9′), 130.2 (C-6′), 129.6 (C-5′), 128.8 (C-3″,5″, C-2‴,6‴), 128.3 (C-2″,6″), 128.2 (C-8′), 127.8 (C-1′), 127.5 (C-4″), 126.8 (C-8′a), 126.4 (C-9′a), 126.1 (C-3′), 125.8 (C-2′, C-7′), 121.6 (C-1‴), 114.0 (C-3‴,5‴), 88.6 (C-5), 63.1 (C-4), 55.2 (OCH3) ppm.
4-(3,4-Diphenyl-4,5-dihydro-1,2-oxazole-5-yl)acridine (8b). Yield: 59.8 mg (42.0%). Mp. 210–212 °C. Yellow powder. For C28H20N2O (400.48) found: C 83.82, H 5.11, N 7.06%; calc.: C 83.98, H 5.03, N 6.99, O 4.00%. Rf (5:1 v/v 5:1 v/v c-hex/EtOAc) 0.39. FT-IR: νmax 3105, 2918, 1641, 1479, 1332, 1060, 1056, 877, 764 cm−1. 1H NMR (400 MHz CDCl3): δ 8.79 (1H, s, H-9′), 8.12 (1H, d, J = 8.8 Hz, H-5′), 8.03 (1H, dd, J = 8.4, 1.0 Hz, H-8′), 7.95 (2H, m, H-1′, H-3′), 7.79 (1H, ddd, J = 8.4, 6.8, 1.2 Hz, H-6′), 7.61 (4H, m, H-2″,6″, H-2‴,6‴), 7.53 (2H, m, H-2′, H-7′), 7.49 (2H, t, J = 7.8 Hz, H-3″,5″), 7.39 (1H, t, J = 7.6 Hz, H-4″), 7.23 (3H, m, H-3″,5″, H-4″), 6.72 (1H, d, J = 3.2 Hz, H-5), 4.87 (1H, d, J = 3.2 Hz, H-4) ppm. 13C NMR (100.6 MHz, CDCl3): δ 159.0 (C-3), 148.3 (C-10′a), 146.5 (C-4′a), 139.7 (C-1″), 138.2 (C-4′), 136.2 (C-9′), 130.3 (C-6′), 129.9 (C-4‴), 129.8 (C-5′), 129.3 (C-1‴), 129.1 (C-3″,5″), 128.7 (C-3‴,5‴), 128.5 (C-2‴,6‴), 128.3 (C-8′), 128.1 (C-1′), 127.7 (C-4″), 127.5 (C-2″,6″), 126.9 (C-9′a), 126.6 (C-8′a), 126.2 (C-3′), 126.0 (C-7′), 125.7 (C-2′), 89.1 (C-5), 63.1 (C-4) ppm.
4-[3-(4-Bromophenyl)-4-phenyl-4,5-dihydro-1,2-oxazole-5-yl]acridine (8c). Yield: 39.2 mg (23.0%). Mp. 185–187 °C. Yellow powder. For C28H19BrN2O (479.38) found: C 70.03, H 3.88, N 5.71%; calc.: C 70.16, H 4.00, Br 16.67, N 5.84, O 3.34%. Rf (5:1 v/v c-Hex/EtOAc) 0.46. FT-IR: νmax 3105, 2920, 1634, 1330, 1060, 1025, 950, 917, 758 cm−1. 1H NMR (400 MHz CDCl3): δ 8.79 (1H, s, H-9′), 8.09 (1H, d, J = 8.8 Hz, H-5′), 8.02 (1H, d, J = 8.4 Hz, H-8′), 7.94 (2H, m, H-1′, H-3′), 7.79 (1H, ddd, J = 8.8, 6.6, 1.4 Hz, H-6′), 7.58 (2H, d, J = 8.0 Hz, H-2″,6″), 7.53 (1H, m, H-2′, H-7′), 7.49 (4H, m, H-3″,5″, H-2‴,6‴), 7.40 (1H, t, J = 7.3 Hz, H-4″), 7.34 (2H, d, J = 8.7 Hz, H-3‴,5‴), 6.71 (1H, d, J = 3.5 Hz, H-5), 4.84 (1H, d, J = 3.5 Hz, H-4) ppm. 13C NMR (100.6 MHz, CDCl3): δ 158.3 (C-3), 148.3 (C-10′a), 146.4 (C-4′a), 139.4 (C-1″), 138.0 (C-4′), 136.2 (C-9′), 131.9 (C-3‴,5‴), 130.4 (C-6′), 129.7 (C-5′), 129.2 (C-3″,5″), 128.9 (C-2‴,6‴), 128.4 (C-2″,6″), 128.3 (C-8′), 128.2 (C-1‴, C-1′), 127.9 (C-4″), 126.9 (C-8′a), 126.6 (C-9′a), 126.2(C-3′), 126.1 (C-7′), 125.6 (C-2′), 124.2 (C-4‴), 89.4 (C-5), 62.9 (C-4) ppm.
4-[3-(3-Nitrophenyl)-4-phenyl-4,5-dihydro-1,2-oxazole-5-yl]acridine (8d). The compound 8e was obtained as a mixture of 7d and 8d. Rf (5:1 v/v c-Hex/EtOAc) 0.32. 1H NMR (600 MHz CDCl3): δ 8.81 (1H, s, H-9′), 8.41 (1H, t, J = 2.0 Hz, H-2‴), 8.09 (1H, ddd, J = 8.2, 2.3, 1.1 Hz, H-4‴), 8.06 (1H, m, H-5′), 8.03 (1H, ddt, J = 8.4, 1.4, 0.6 Hz, H-8′), 7.97 (1H, m, H-1′), 7.96 (1H, ddd, J = 8.0, 1.7, 1.1 Hz, H-6‴), 7.94 (dt, J = 6.9, 1.3 Hz, H-3′), 7.79 (1H, ddd, J = 8.8, 6.6, 1.4 Hz, H-6′), 7.61 (2H, m, H-2″,6″), 7.58 (1H, ddd, J = 8.4, 6.6, 1.1 Hz, H-7′), 7.54 (1H, dd, J = 8.4, 6.9 Hz, H-2′), 7.51 (2H, m, H-3″,5″), 7.42 (1H, m, H-4″), 7.41 (2H, t, J = 8.0 Hz, H-3‴,5‴) ppm. 13C NMR (150.1 MHz, CDCl3): δ 157.5 (C-3), 148.3 (C-3‴), 148.2 (C-10′a), 146.2 (C-4′a), 138.7 (C-1″), 137.5 (C-4′), 136.1 (C-9′), 132.8 (C-6‴), 131.0 (C-1‴), 130.4 (C-6′), 129.6 (C-5‴), 129.5 (C-5′), 129.2 (C-3″,5″), 128.6 (C-1′), 128.3 (C-2″,6″), 128.2 (C-8′), 128.1 (C-4″), 126.8 (C-9′a), 126.5 (C-8′a), 126.0 (C-7′), 125.9 (C-3′), 125.4 (C-2′), 124.2 (C-4‴), 122.1 (C-2‴), 89.9 (C-5), 62.5 (C-4) ppm.
4-[3-(4-Nitrophenyl)-4-phenyl-4,5-dihydro-1,2-oxazole-5-yl]acridine (8e). The compound 8d was obtained as a mixture of 7e and 8e. Rf (5:1 v/v c-Hex/EtOAc) 0.32. 1H NMR (400 MHz CDCl3): δ 8.78 (1H, s, H-9′), 8.06 (2H, m, H-3‴,5‴), 8.04 (1H, m, H-5′), 8.01 (1H, m, H-8′), 7.96 (1H, dd, J = 8.4, 0.8 Hz, H-1′), 7.91 (1H, dt, J = 6.9, 1.3 Hz, H-2′), 7.78 (1H, ddd, J = 8.8, 6.7, 1.5 Hz, H-6′), 7.76 (2H, d, J = 9.2 Hz, H-2‴,6‴), 7.62 (1H, m, H-7′), 7.56 (2H, m, H-2″,6″), 7.53 (1H, m, H-2′), 7.50 (2H, m, H-3″,5″), 7.41 (1H, m, H-4″), 6.76 (1H, d, J = 3.7 Hz, H-5), 4.91 (1H, d, J = 3.7 Hz, H-4) ppm. 13C NMR (100.6 MHz, CDCl3): δ 157.6 (C-3), 148.2 (C-10′a), 148.1 (C-4‴), 146.2 (C-4′a), 138.8 (C-1″), 137.4 (C-4′), 136.2 (C-9′), 135.3 (C-1‴), 130.4 (C-6′), 129.5 (C-5′), 128.6 (C-3″,5″), 128.3 (C-1′), 128.2 (C-2″,6″), 128.1 (C-4″, C-8′, C-2‴,6‴), 126.8 (C-9′a), 126.5 (C-8′a), 126.0 (C-3′, C-7′), 125.6 (C-2′), 123.8 (C-3‴,5‴), 90.2 (C-5), 62.0 (C-4) ppm.
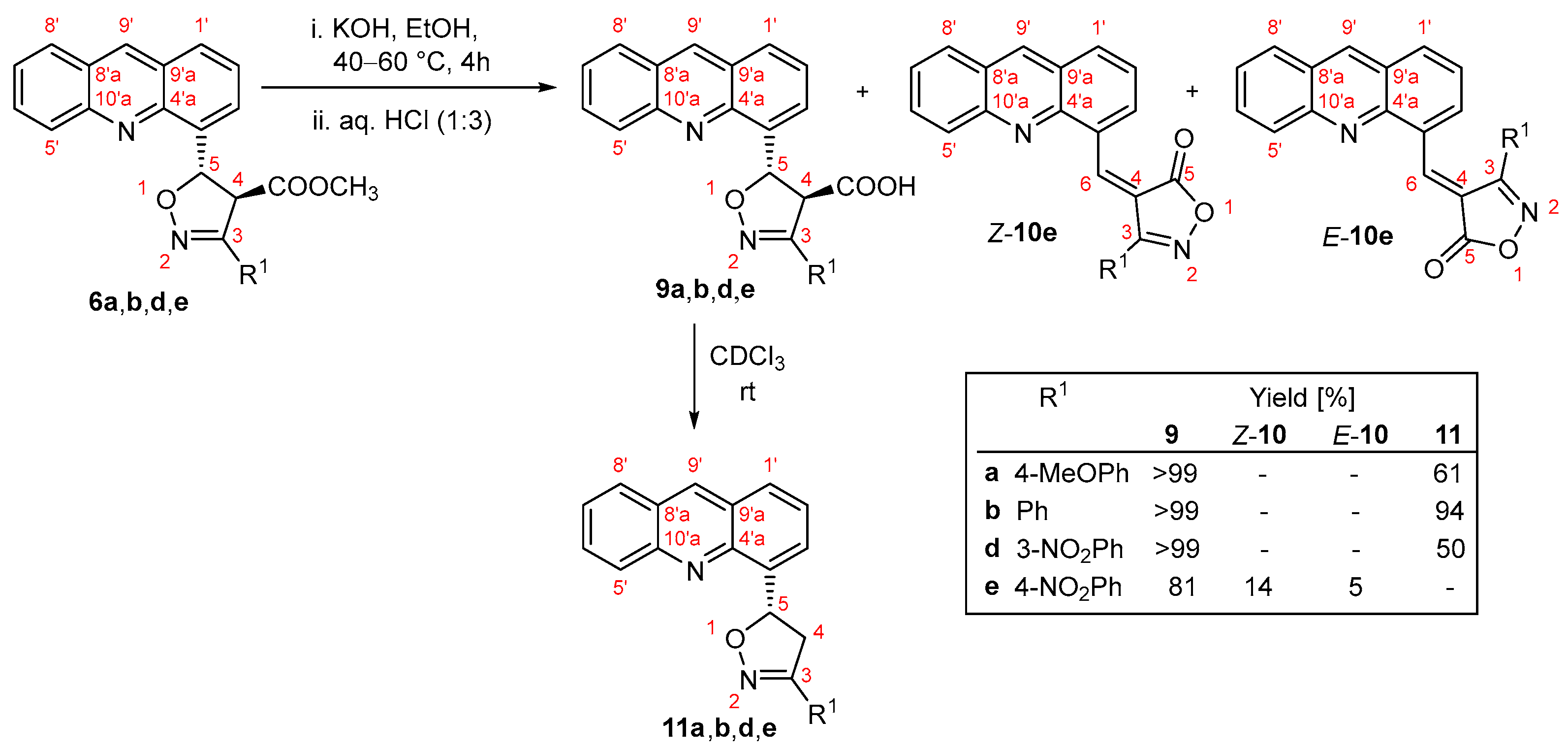
3.8. Synthesis of 5-(Acridin-4-yl)-3-phenyl-4,5-dihydro-1,2-oxazole-4-carboxylic Acids 9a,b,d,e, (4Z)-4-[(Acridin-4-yl)Methylidene)]-3-(4-Nitrophenyl)-4,5-Dihydro-1,2-Oxazol-5-One Z-10e and (4E)-4-[(Acridin-4-yl)Methylidene)]-3-(4-Nitrophenyl)-4,5-Dihydro-1,2-Oxazol-5-One E-10e
To a solution of methylester (6a: 100 mg; 6b: 100 mg, 0.261 mmol, 0.24 mmol; 6d: 100 mg, 0.23 mmol; 6e: 100 mg, 0.23 mmol) in ethanol (5 mL) heated to 40 °C, KOH (6a: 136 mg, 2.40 mmol; 6b: 146 mg, 2.61 mmol; 6e: 146 mg, 2.30 mmol; 6d: 146 mg, 2.30 mmol) was added. The reaction mixture was stirred at 60 °C and monitored using TLC (SiO2, c-Hex/EtOAc, 1:1). Upon complexion of the reaction, the solvent was evaporated, and water (10 mL) was added. The resulting aqueous solution was acidified (HCl, 3:1), and the precipitate was extracted with diethyl ether (2 × 10 mL). The organic layer was dried, and the solvent was subsequently evaporated to yield the crude products. These products were further purified by column chromatography (SiO2, c-Hex/EtOAc, 1:1).
The compounds Z-10e and E-10e were separated from the mixture of 6e and 9e using column chromatography (SiO2, CHCl3/MeOH, 4:1). The ratio of isomers Z-10e and E-10e was determined to be 1.00:0.14 based on 1H NMR spectra.
5-(Acridin-4-yl)-3-(4-methoxyphenyl)-4,5-dihydro-1,2-oxazole-4-carboxylic acid (9a). Yield: 76.3 mg (79.0%). Mp. 190–192 °C. Yellow powder. For C24H18N2O4 (398.42) found: C 72.15, H 4.75, N 7.14%; calc.: C 72.35, H 4.55, N 7.03, O 16.06%. Rf (c-Hex/EtOAc, 1:1) 0.31. FT-IR: νmax 3443, 3117, 2929, 1788, 1643, 1622, 1525, 1353, 1243, 1209, 1115, 1066, 1038, 711, 617 cm−1. 1H NMR (600 MHz CDCl3): δ 9.05 (1H, s, H-9′), 8.40 (1H, d, J = 8.4 Hz, H-5′), 8.16 (1H, dt, J = 7.3, 1.5 Hz, H-3′), 8.13 (1H, d, J = 8.6 Hz, H-8′), 8.06 (1H, d, J = 8.4 Hz, H-1′), 7.96 (1H, ddd, J = 8.4, 6.7, 1.4 Hz, H-6′), 7.70 (1H, ddd, J = 8.6, 6.7, 1.0 Hz, H-7′), 7.64 (1H, m, H-2′), 7.62 (2H, d, J = 9.1 Hz, H-2″,6″), 6.85 (2H, d, J = 9.1 Hz, H-3″,5″), 6.83 (1H, d, J = 5.5 Hz, H-5), 4.76 (1H, d, J = 5.5 Hz, H-4), 3.79 (3H, s, OCH3) ppm. 13C NMR (150.1 MHz, CDCl3): δ 169.5 (C-6), 161.3 (C-4″), 153.8 (C-3), 147.1 (C-10′a), 144.2 (C-4′a), 140.0 (C-9′), 135.6 (C-4′), 132.9 (C-6′), 129.0 (C-2″,6″), 128.8 (C-1′), 128.7 (C-8′), 127.7 (C-3′), 127.3 (C-9′a), 127.0 (C-7′), 126.8 (C-8′a), 126.5 (C-5′), 126.1 (C-2′), 121.5 (C-1″), 114.2 (C-3″,5″), 82.3 (C-5), 62.4 (C-4), 55.3 (OCH3) ppm.
5-(Acridin-4-yl)-3-phenyl-4,5-dihydro-1,2-oxazole-4-carboxylic acid (9b). Yield: 94.3 mg (84.0%). Mp. 190–192 °C. Yellow powder. For C23H16N2O3 (368.40) found: C 74.85, H 4.22, N 7.68%; calc.: C 74.99, H 4.38, N 7.60, O 13.03%; Rf (c-Hex/EtOAc, 1:1) 0.34. FT-IR: νmax 3428, 3112, 2906, 1799, 1231, 1198, 1040, 508 cm−1. 1H NMR (600 MHz CDCl3): δ 9.06 (1H, s, H-9′), 8.41 (1H, d, J = 8.4 Hz, H-5′), 8.16 (1H, dd, J = 7.0, 1.4 Hz, H-3′), 8.14 (1H, d, J = 8.0 Hz, H-8′), 8.07 (1H, d, J = 8.4 Hz, H-1′), 7.97 (1H, ddd, J = 8.4, 6.8, 1.5 Hz, H-6′), 7.70 (3H, m, H-7′, H-2″,6″), 7.65 (1H, dd, J = 8.4, 7.0 Hz, H-2′), 7.35 (3H, m, H-3″,5″, H-4″), 6.87 (1H, d, J = 5.5 Hz, H-5), 4.80 (1H, d, J = 5.5 Hz, H-4) ppm. 13C NMR (150.1 MHz, CDCl3): δ 169.4 (C-6), 154.3 (C-3), 147.0 (C-10′a), 144.2 (C-4′a), 140.0 (C-9′), 135.4 (C-4′), 132.8 (C-6′), 130.4 (C-4″), 128.9 (C-1′, C-8′), 128.8 (C-3″,5″), 128.4 (C-1″), 127.7 (C-3′), 127.5 (C-2″,6″), 127.1 (C-7′), 126.9 (C-8′a), 126.6 (C-9′a), 126.5 (C-5′), 126.2 (C-2′), 82.6 (C-5), 62.1 (C-4) ppm.
5-(Acridin-4-yl)-3-(3-nitrophenyl)-4,5-dihydro-1,2-oxazole-4-carboxylic acid (9d). Yield: 95.7 mg (99.0%). Mp. 183–185 °C. For C23H15N3O5 (413.39) found: C 66.71, H 3.56, N 10.22%; calc.: C 66.83, H 3.66, N 10.16, O 19.35%. Rf (1:1 v/v c-Hex/EtOAc) 0.11. FT-IR: νmax 3445, 3107, 1792, 1659, 1634, 1354, 1149, 1136, 1082, 1054, 733, 695, 617 cm−1. 1H NMR (600 MHz CDCl3): δ 9.09 (1H, s, H-9′), 8.45 (1H, dd, J = 2.1, 1.7 Hz, H-2″), 8.43 (1H, dd, J = 8.8, 1.0 Hz, H-5′), 8.22 (1H, ddd, J = 8.2, 2.2, 1.1 Hz, H-4″), 8.16 (2H, m, H-3′, H-3′), 8.11 (2H, m, H-1′, H-6″), 8.00 (1H, ddd, J = 8.8, 6.6, 1.4 Hz, H-6′), 7.73 (1H, ddd, J = 8.4, 6.6, 1.0 Hz, H-7′), 7.67 (1H, dd, J = 8.4, 7.0 Hz, H-2′), 7.55 (1H, m, H-5″), 6.94 (1H, d, J = 5.9 Hz, H-5), 4.85 (1H, d, J = 5.9 Hz, H-4) ppm. 13C NMR (150.1 MHz, CDCl3): δ 169.4 (C-4), 154.3 (C-3), 148.3 (C-3″), 147.0 (C-10′a), 144.2 (C-4′a), 140.0 (C-9′), 135.4 (C-4′), 133.0 (C-6″), 132.8 (C-6′), 130.9 (C-1″), 128.9 (C-1′, C-8′), 128.8 (C-5″), 127.6 (C-3′), 127.1 (C-7′), 126.9 (C-8′a), 126.6 (C-9′a), 126.5 (C-5′), 126.2 (C-2′), 124.6 (C-4″), 122.2 (C-2″), 82.6 (C-5), 62.1 (C-4) ppm.
5-(Acridin-4-yl)-3-(4-nitrophenyl)-4,5-dihydro-1,2-oxazole-4-carboxylic acid (9e). Yield: 58.7 mg (63.0%). Mp. 250–253 °C. Yellow powder. For C23H15N3O5 (413.39) found: C 66.79, H 3.45, N 10.14%; calc.: C 66.83, H 3.66, N 10.17, O 19.35%. Rf (c-Hex/EtOAc, 1:1) 0.16. FT-IR: νmax 3486, 3102, 2946, 2916, 1798, 1655, 1570, 1333, 1205, 1171, 1080, 1057, 831, 460 cm−1. 1H NMR (600 MHz CDCl3): δ 9.09 (1H, s, H-9′), 8.42 (1H, d, J = 8.4 Hz, H-5′), 8.21 (2H, d, J = 9.2 Hz, H-3″,5″), 8.16 (2H, m, H-3′, H-8′), 8.11 (1H, dd, J = 8.4, 1.4 Hz, H-1′), 7.99 (1H, ddd, J = 8.4, 6.4, 1.2 Hz, H-6′), 7.87 (2H, d, J = 9.1 Hz, H-2″,6″), 7.73 (1H, ddd, J = 8.4, 6.4, 1.2 Hz, H-7′), 7.67 (1H, t, J = 7.7 Hz, H-2′), 6.94 (1H, d, J = 6.0 Hz, H-5), 4.82 (1H, d, J = 6.0 Hz, H-4) ppm. 13C NMR (150.1 MHz, CDCl3): δ 167.8 (C-6), 140.2 (C-9′), 133.2 (C-6′), 129.1 (C-1′), 128.6 (C-8′), 128.2 (C-2″,6″), 127.6 (C-3′), 127.1 (C-7′), 126.0 (C-5′), 125.9 (C-2′), 123.8 (C-3″,5″), 83.3 (C-5), 61.4 (C-4) ppm. The resonance lines for carbons C-3, C-4′, C-4′a, C-10′a, C-8′a, C-9′a, C-1″, C-4″ were not detected.
(4Z)-4-[(Acridin-4-ylmethylidene)]-3-(4-nitrophenyl)-4,5-dihydro-1,2-oxazole-5-one (Z-10e). The compound was obtained as a mixture of Z-10e and E-10e. Rf (4:1 v/v CHCl3/MeOH) 0.80. 1H NMR (600 MHz CDCl3): δ 9.85 (1H, s, H-6), 9.73 (1H, dd, J = 7.3, 1.4 Hz, H-3′), 8.87 (1H, s, H-9′), 8.54 (2H, d, J = 8.9 Hz, H-3″,5″), 8.33 (1H, dd, J = 8.4, 0.6 Hz, H-1′), 8.08 (2H, d, J = 8.9 Hz, H-2″,6″), 8.08 (1H, m, H-5′), 8.06 (1H, dd, J = 8.4, 1.2 Hz, H-8′), 7.86 (1H, ddd, J = 8.4, 6.6, 1.2 Hz, H-6′), 7.77 (1H, dd, J = 8.4, 7.3 Hz, H-2′), 7.63 (1H, ddd, J = 8.4, 6.6, 1.2 Hz, H-7′) ppm. 13C NMR (150.1 MHz, CDCl3): δ 168.5 (C-5), 162.7 (C-3), 149.6 (C-4″), 149.3 (C-6), 148.8 (C-10′a), 147.0 (C-4′a), 137.3 (C-9′), 137.2 (C-3′), 136.0 (C-1′), 134.1 (C-1″), 131.6 (C-6′), 130.1 (C-2″,6″), 129.7 (C-5′), 129.3 (C-4′), 128.2 (C-8′), 126.9 (C-8′a), 126.8 (C-7′), 126.2 (C-9′a), 125.6 (C-2′), 124.4 (C-3″,5″), 121.4 (C-4) ppm.
3.9. Synthesis of 4-(3-Phenyl-4,5-dihydro-1,2-oxazol-5-yl)acridines 11a,b,d,e
Carboxylic acids 9a,b,d,e (20 mg) were dissolved in deuterated chloroform (0.6 mL) and allowed to stand at room temperature for 3 weeks. The reactions were monitored by 1H NMR spectroscopy.
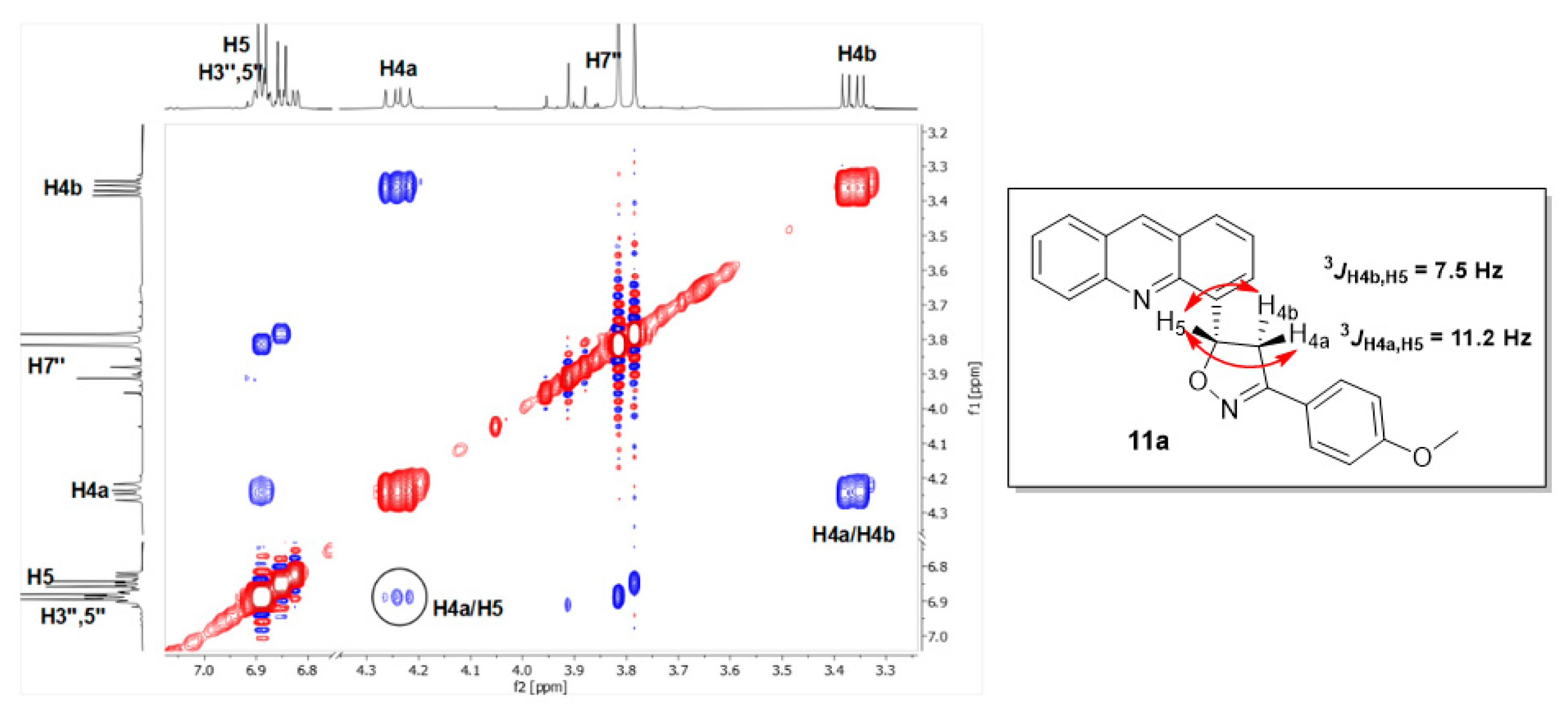
4-[3-(4-Methoxyphenyl)-4,5-dihydro-1,2-oxazol-5-yl]acridine (11a). The compound was obtained as a mixture of 9a and 11a. Yield: 61.0% (from 1H NMR). Rf (3:1 v/v toluene/EtOAc) 0.68. 1H NMR (600 MHz CDCl3): δ 8.77 (1H, s, H-9′), 8.23 (1H, d, J = 8.4 Hz, H-5′), 8.01 (1H, dd, J = 8.4, 1.5 Hz, H-8′), 7.98 (1H, dd, J = 6.9, 1.3 Hz, H-3′), 7.94 (1H, dd, J = 8.4, 1.4 Hz, H-1′), 7.79 (1H, ddd, J = 8.4, 6.6, 1.4 Hz, H-6′), 7.65 (2H, d, J = 9.0 Hz, H-2″,6″), 7.56 (1H, ddd, J = 8.4, 6.6, 1.1 Hz, H-7′), 7.53 (1H, dd, J = 8.4, 6.9 Hz, H-2′), 6.89 (2H, d, J = 9.0 Hz, H-3″,5″), 6.89 (1H, m, H-5), 4.24 (1H, dd, J = 17.0, 11.2 Hz, H-4a), 3.81 (3H, s, OCH3), 3.36 (1H, dd, J = 17.0, 7.5 Hz, H-4b) ppm. 13C NMR (150.1 MHz, CDCl3): δ 161.0 (C-4″), 156.6 (C-3), 148.2 (C-10′a), 146.2 (C-4′a), 139.2 (C-4′), 136.0 (C-9′), 130.1 (C-6′), 129.9 (C-5′), 128.3 (C-2″,6″), 128.1 (C-8′), 127.8 (C-1′), 126.6 (C-9′a), 126.4 (C-8′a), 126.0 (C-3′), 125.9 (C-7′), 125.6 (C-2′), 122.4 (C-1″), 114.1 (C-3″,5″), 79.5 (C-5), 55.3 (OCH3), 43.9 (C-4) ppm.
4-(3-Phenyl-4,5-dihydro-1,2-oxazol-5-yl)acridine (11b). The compound was obtained as a mixture of 9b and 11b. Yield: 94.0% (from 1H NMR). Rf (5:1 v/v n-Hex/EtOAc) 0.29. 1H NMR (400 MHz CDCl3): δ 8.78 (1H, s, H-9′), 8.23 (1H, d, J = 8.4 Hz, H-5′), 8.02 (1H, d, J = 8.4 Hz, H-8′), 7.98 (1H, dd, J = 6.9, 1.3 Hz, H-3′), 7.95 (1H, d, J = 8.4 Hz, H-1′), 7.79 (1H, ddd, J = 8.4, 6.6, 1.4 Hz, H-6′), 7.72 (2H, m, H-2″,6″), 7.55 (2H, m, H-2′, H-7′), 7.38 (3H, m, H-3″,5″, H-4″), 6.93 (1H, dd, J = 11.2, 7.5 Hz, H-5), 4.28 (1H, dd, J = 17.1, 11.2 Hz, H-4a), 3.41 (1H, dd, J = 17.1, 7.5 Hz, H-4b) ppm. 13C NMR (100.6 MHz, CDCl3): δ 158.1 (C-3), 149.3 (C-10′a), 147.2 (C-4′a), 140.1 (C-4′), 137.0 (C-9′), 131.2 (C-6′), 131.0 (C-5′, C-4″), 129.7 (C-1″, C-3″,5″), 129.1 (C-8′), 128.9 (C-1′), 127.8 (C-2″,6″), 127.7 (C-9′a), 127.5 (C-8′a), 126.9 (C-3′, C-7′), 126.6 (C-2′), 80.9 (C-5), 44.7 (C-4) ppm.
4-[3-(3-Nitrophenyl)-4,5-dihydro-1,2-oxazol-5-yl]acridine (11d). Yield: 50.0% (from 1H NMR). Rf (1:1 v/v c-Hex/EtOAc) 0.82. 1H NMR (600 MHz, CDCl3): δ 8.80 (1H, s, H-9′), 8.46 (1H, dd, J = 2.3, 1.2 Hz, H-2″), 8.24 (1H, ddd, J = 8.2, 2.3, 1.1 Hz, H-4″), 8.22 (1H, d, J = 8.4 Hz, H-5′), 8.14 (1H, m, H-6″), 8.03 (1H, dd, J = 8.4, 1.4 Hz, H-8′), 7.98 (1H, m, H-1′), 7.81 (1H, ddd, J = 8.4, 6.6, 1.4 Hz, H-6′), 7.59 (1H, m, H-7′, H-5″), 7.55 (1H, m, H-2′), 7.56 (2H, m, H-2′,), 6.98 (1H, dd, J = 11.5, 7.9 Hz, H-5), 4.32 (1H, dd, J = 17.1, 11.4 Hz, H-4a), 3.46 (1H, dd, J = 17.1, 7.8 Hz, H-4b) ppm. 13C NMR (150.1 MHz, CDCl3): δ 155.5 (C-3), 148.5 (C-3″), 148.3 (C-10′a), 146.1 (C-4′a), 138.4 (C-4′), 136.1 (C-9′), 132.3 (C-6″), 131.8 (C-1″), 130.3 (C-6′), 129.9 (C-5′), 129.7 (C-5″), 128.2 (C-8′), 128.1 (C-1′), 126.8 (C-9′a), 126.5 (C-8′a), 126.0 (C-3′, C-7′), 125.4 (C-2′), 124.4 (C-4″), 121.6 (C-2″), 80.9 (C-5), 43.2 (C-4) ppm.
4-[3-(4-Nitrophenyl)-4,5-dihydro-1,2-oxazol-5-yl]acridine (11e). Yield: 36.0% (from 1H NMR). Rf (1:1 v/v c-Hex/EtOAc) 0.83. 1H NMR (600 MHz CDCl3): δ 8.80 (1H, s, H-9′), 8.25 (1H, d, J = 9.0 Hz, H-5″), 8.20 (1H, dd, J = 8.7, 1.0 Hz, H-5′), 8.03 (1H, dd, J = 8.4, 1.4 Hz, H-8′), 7.98 (1H, dd, J = 8.4, 1.4 Hz, H-1′), 7.96 (1H, dd, J = 6.8, 1.3 Hz, H-3′), 7.89 (2H, d, J = 9.0 Hz, H-2″,6″), 7.80 (1H, ddd, J = 8.7, 6.6, 1.4 Hz, H-6′), 7.58 (1H, ddd, J = 8.4, 6.6, 1.2 Hz, H-7′), 7.55 (1H, dd, J = 8.4, 7.2 Hz, H-2′), 6.97 (1H, dd, J = 11.5, 7.9 Hz, H-5), 4.30 (1H, dd, J = 17.1, 11.5 Hz, H-4a), 3.45 (1H, dd, J = 17.1, 7.9 Hz, H-4b) ppm. 13C NMR (150.1 MHz, CDCl3): δ 155.7 (C-3), 148.4 (C-4″), 148.3 (C-10′a), 146.1 (C-4′a), 138.3 (C-4′), 136.1 (C-9′, C-1″), 130.3 (C-6′), 129.9 (C-5′), 128.2 (C-1′, C-8′), 127.5 (C-2″,6″), 126.7 (C-8′a), 126.6 (C-9′a), 126.0 (C-3′), 125.9 (C-7′), 125.4 (C-2′), 124.0 (C-3″,5″), 81.2 (C-5), 43.0 (C-4) ppm.