Enhanced CH4/N2 Separation Efficiency of UiO-66-Br2 through Hybridization with Mesoporous Silica
Abstract
1. Introduction
2. Results and Discussion
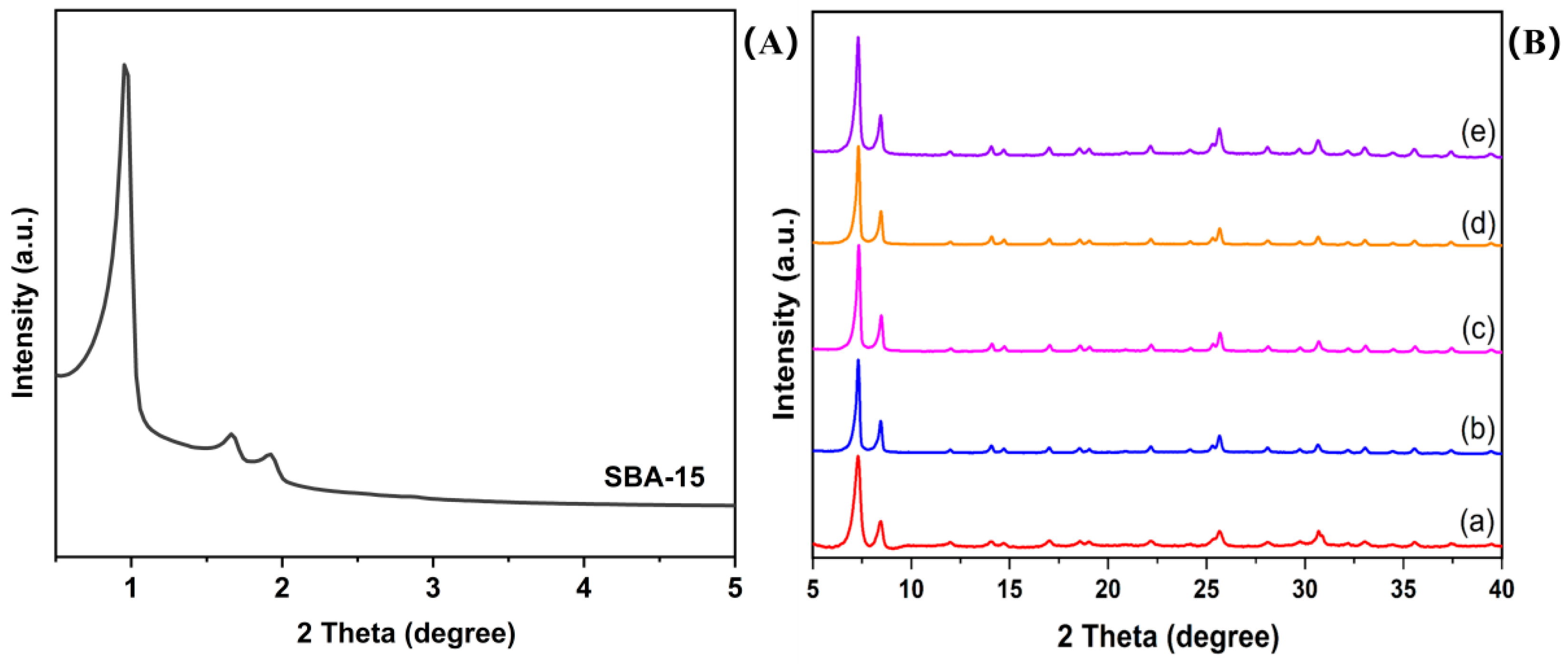
2.1. Powder X-ray Diffraction (PXRD) Patterns
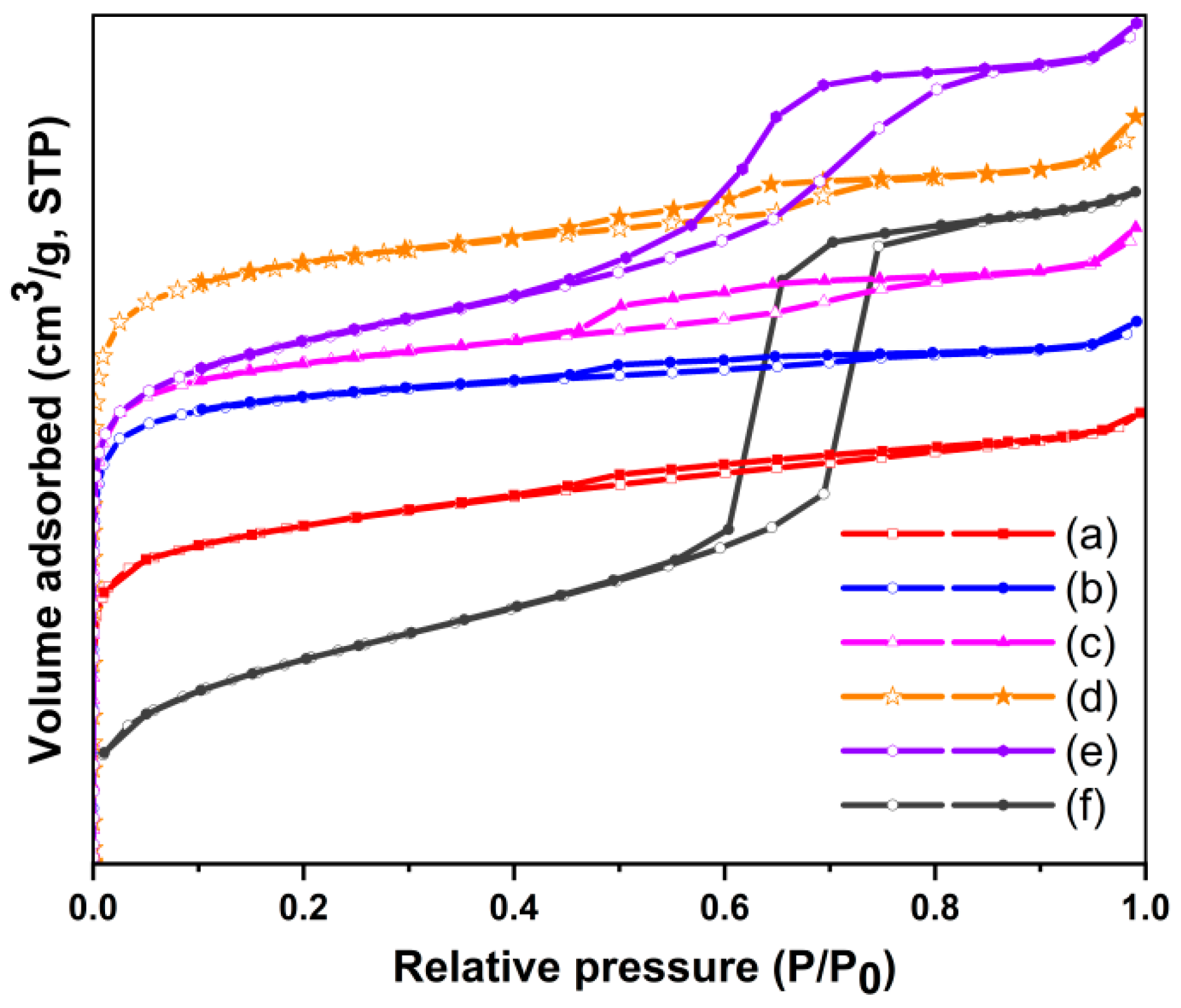
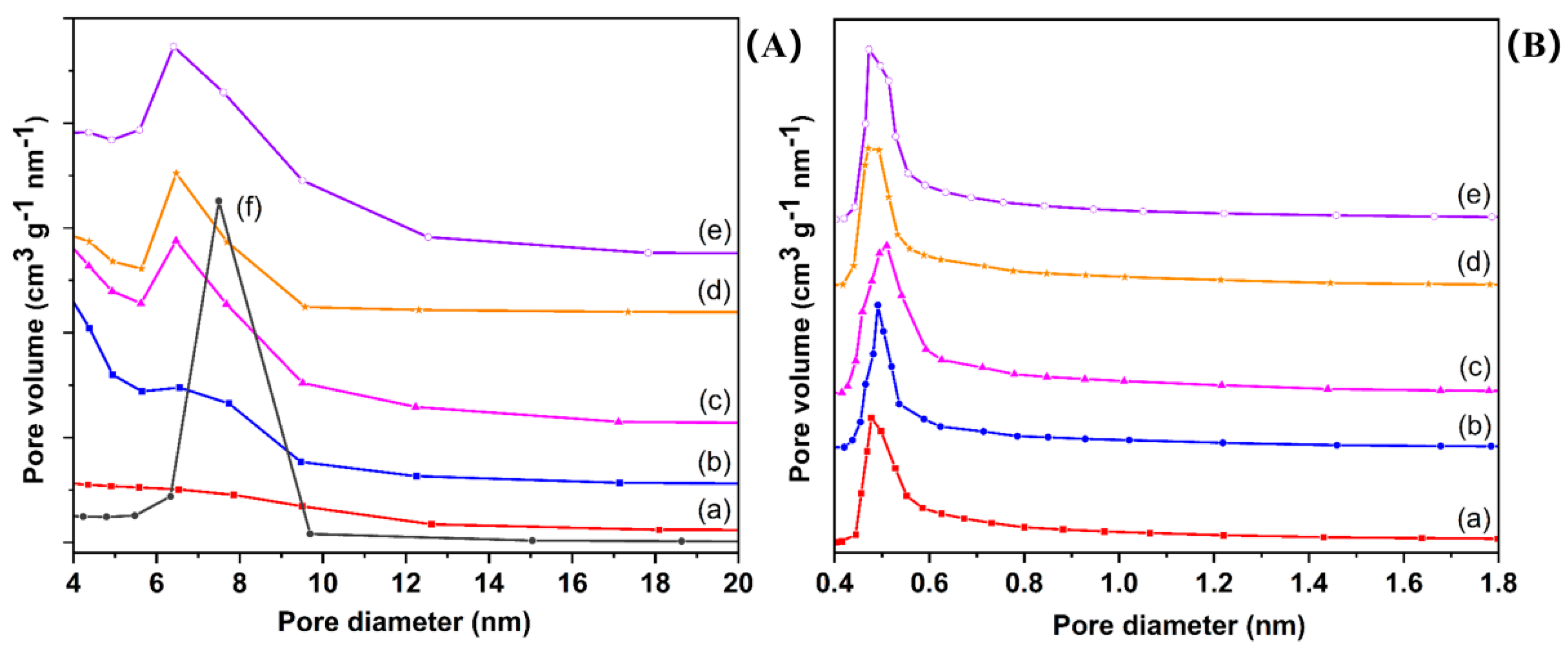
2.2. Nitrogen Adsorption–Desorption
2.3. SEM and TEM Images
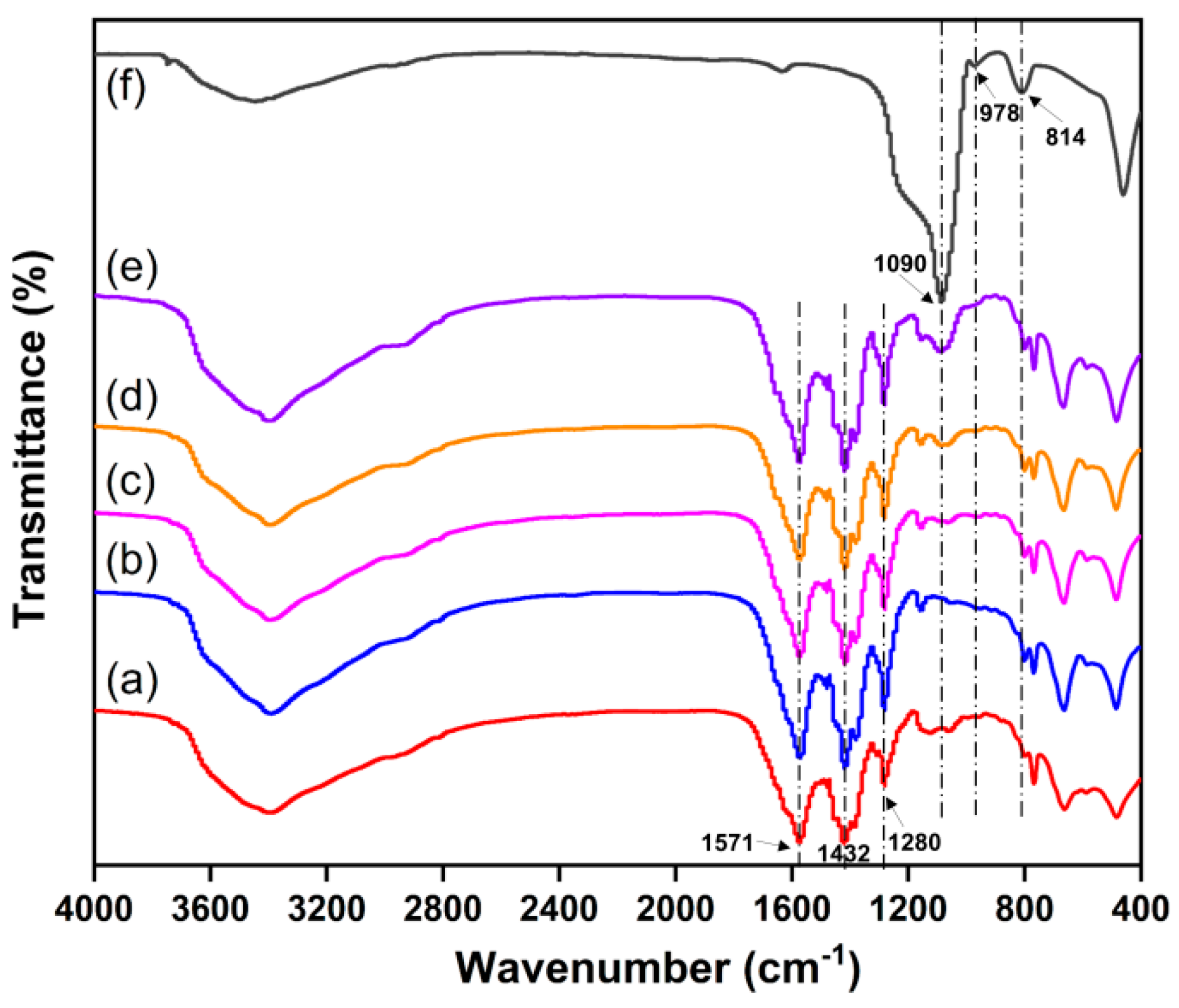
2.4. FT-IR Spectroscopy
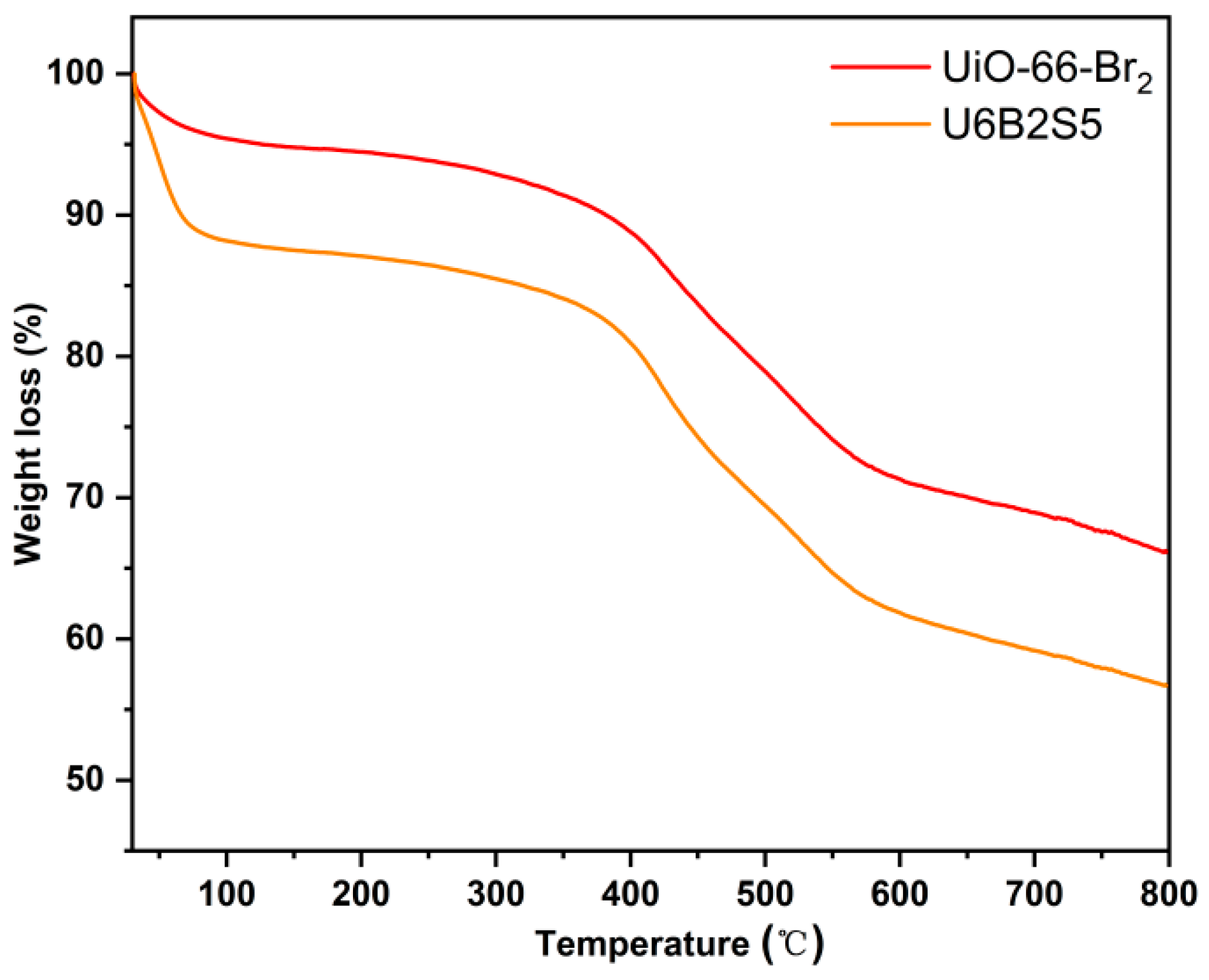
2.5. Thermogravimetric Analysis
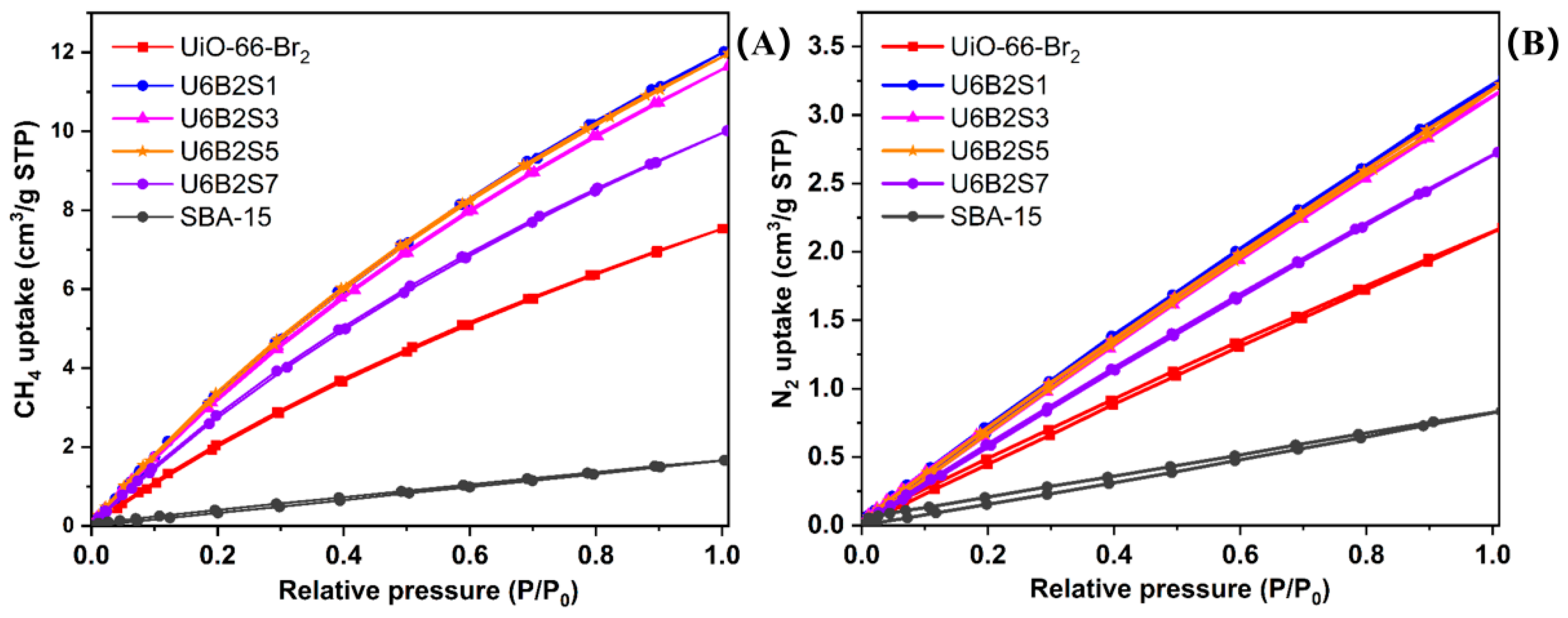
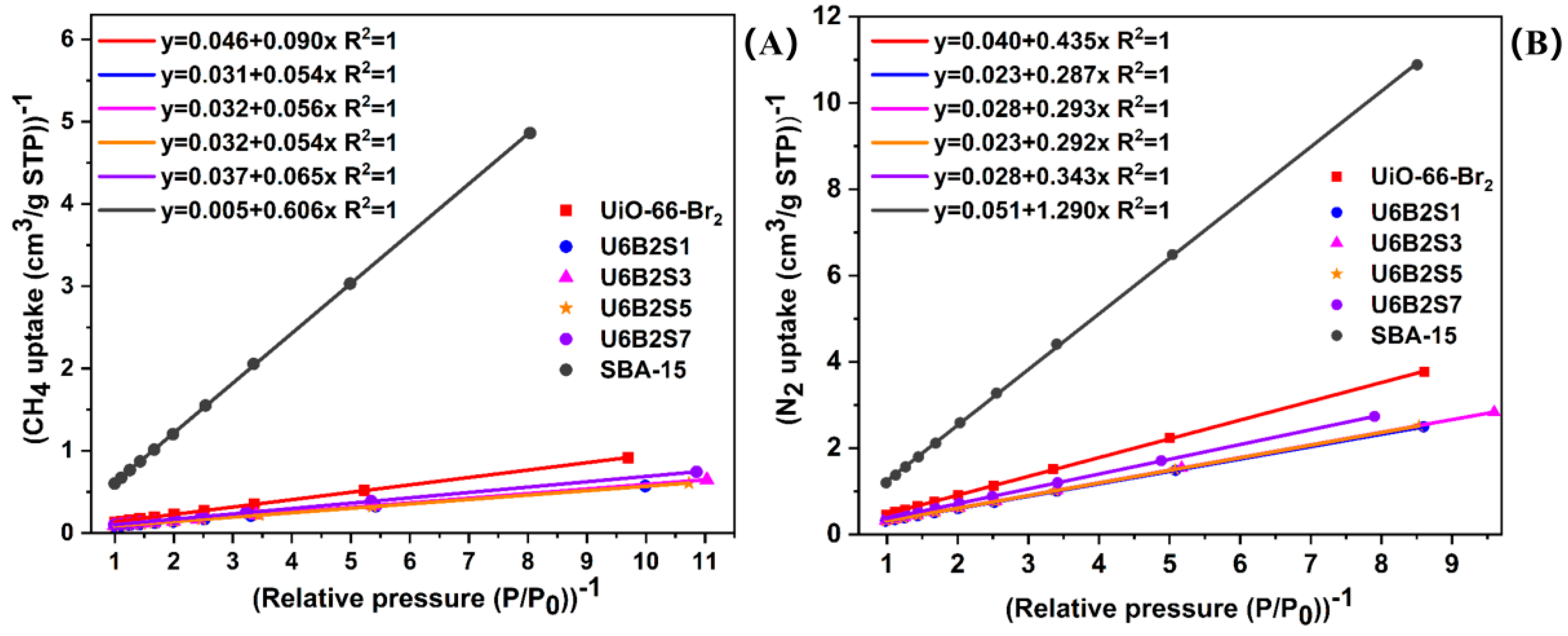
2.6. Adsorption and Separation Performance
3. Materials and Methods
3.1. Materials
3.2. Synthesis of SBA-15
3.3. Synthesis of UiO-66-Br2
3.4. Synthesis of UiO-66-Br2/SBA-15
3.5. Characterization
3.6. Adsorption Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wigley, T.M.L. The kyoto protocol: CO2, CH4 and climate implications. Geophys. Res. Lett. 1998, 25, 2285–2288. [Google Scholar] [CrossRef]
- Reilly, J.; Mayer, M.; Harnisch, J. The kyoto protocol and non-CO2 greenhouse gases and carbon sinks. Environ. Model. Assess. 2002, 7, 217–229. [Google Scholar] [CrossRef]
- Johansson, D.J.A.; Persson, U.M.; Azar, C. The cost of using global warming potentials: Analysing the trade off between CO2, CH4 and N2O. Clim. Chang. 2006, 77, 291–309. [Google Scholar] [CrossRef]
- Yacob, S.; Hassan, M.A.; Shirai, Y.; Wakisaka, M.; Subash, S. Baseline study of methane emission from open digesting tanks of palm oil mill effluent treatment. Chemosphere 2005, 59, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.L.; Kebreab, E.; Odongo, N.E.; McBride, B.W.; Okine, E.K.; France, J. Prediction of methane production from dairy and beef cattle. J. Dairy Sci. 2007, 90, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Liu, Y.; He, T.; Yin, L.; Shu, C.M.; Moon, I. System perspective on cleaner technologies for renewable methane production and utilisation towards carbon neutrality: Principles, techno-economics, and carbon footprints. Fuel 2022, 327, 125130. [Google Scholar] [CrossRef]
- Esparza, Á.E.; Rowan, G.; Newhook, A.; Deglint, H.J.; Garrison, B.; Orth-Lashley, B.; Girard, M.; Shaw, W. Analysis of a tiered top-down approach using satellite and aircraft platforms to monitor oil and gas facilities in the permian basin. Renew. Sust. Energ. Rev. 2023, 178, 113265. [Google Scholar] [CrossRef]
- Chang, M.; Ren, J.; Wei, Y.; Yan, T.; Wang, J.X.; Liu, D.; Chen, J.F. Discovery of a scalable metal–organic framework with a switchable structure for efficient CH4/N2 separation. Chem. Mater. 2023, 35, 4286–4296. [Google Scholar] [CrossRef]
- Chen, R.; Li, J.; Zhou, F.; Sheng, B.; Sun, H.; Zheng, F.; Yang, Q.; Zhang, Z.; Ren, Q.; Bao, Z. Zr-based metal–organic framework with wall-shared dual ultramicroporous channels for effective CH4/N2 separation. Ind. Eng. Chem. Res. 2023, 62, 13144–13152. [Google Scholar] [CrossRef]
- Hu, G.; Guo, Y.; Zhao, Q.; Xiao, G.; Li, K.G.; May, E.F. Separation of methane and nitrogen using heavy reflux pressure swing adsorption: Experiments and modeling. Ind. Eng. Chem. Res. 2023, 62, 7114–7126. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Chen, G.; Yao, X.; Liu, G.; Xu, R.; Jin, W. Molecular design of two-dimensional graphdiyne membrane for selective transport of CO2 and H2 over CH4, N2, and CO. J. Membr. Sci. 2023, 675, 121557. [Google Scholar] [CrossRef]
- Fan, L.; Ma, N.; Zhang, W. Multi-stakeholder equilibrium-based subsidy allocation mechanism for promoting coalbed methane scale extraction-utilization. Energy 2023, 277, 127580. [Google Scholar] [CrossRef]
- Xu, C.; Yang, T.; Wang, K.; Fu, Q.; Ma, S. Gas extraction of coal seam roof fractured zone in china: A review. Fuel 2024, 357, 129930. [Google Scholar] [CrossRef]
- Xu, H.; Qin, Y.; Wu, F.; Zhang, F.; Liu, W.; Liu, J.; Guo, M. Numerical modeling of gas extraction from coal seam combined with a dual-porosity model: Finite difference solution and multi-factor analysis. Fuel 2022, 313, 122687. [Google Scholar] [CrossRef]
- Rufford, T.E.; Watson, G.C.Y.; Saleman, T.L.; Hofman, P.S.; Jensen, N.K.; May, E.F. Adsorption equilibria and kinetics of methane plus nitrogen mixtures on the activated carbon norit RB3. Ind. Eng. Chem. Res. 2013, 52, 14270–14281. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Meng, Z.; Li, Y. Adsorption separation of CH4/N2 on modified coal-based carbon molecular sieve. Sep. Purif. Technol. 2019, 218, 130–137. [Google Scholar] [CrossRef]
- Yuan, B.; Wu, X.F.; Chen, Y.X.; Huang, J.H.; Luo, H.M.; Deng, S.G. Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: Approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Pan, Z.; Zhang, L.; Zhang, Z.E. Decarburization characteristics of coalbed methane by membrane separation technology. Fuel 2019, 242, 470–478. [Google Scholar] [CrossRef]
- Zhou, Y.Q.; Yuan, Y.; Cong, S.Z.; Liu, X.L.; Wang, Z. N2 selective adsorbents and membranes for natural gas purification. Sep. Purif. Technol. 2022, 300, 121808. [Google Scholar] [CrossRef]
- Shang, H.; Bai, H.H.; Li, X.M.; Li, J.P.; Yang, J.F. Site trials of methane capture from low-concentration coalbed methane drainage wells using a mobile skid-mounted vacuum pressure swing adsorption system. Sep. Purif. Technol. 2022, 295, 121271. [Google Scholar] [CrossRef]
- Hao, X.F.; Hu, H.J.; Li, Z.; Wu, L.M.; Liu, X.Q.; Zhang, Y.N. Adsorption properties of modified clinoptilolite for methane and nitrogen. Materials 2018, 11, 2024. [Google Scholar] [CrossRef] [PubMed]
- Mulgundmath, V.P.; Tezel, F.H.; Hou, F.; Golden, T.C. Binary adsorption behaviour of methane and nitrogen gases. J. Porous Mater. 2012, 19, 455–464. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, W.; Yong, J.Y.; Zhang, X.; Jiang, L. Solar-assisted temperature vacuum swing adsorption for direct air capture: Effect of relative humidity. Appl. Energy 2023, 348, 121493. [Google Scholar] [CrossRef]
- Subraveti, S.G.; Roussanaly, S.; Anantharaman, R.; Riboldi, L.; Rajendran, A. Techno-economic assessment of optimised vacuum swing adsorption for post-combustion CO2 capture from steam-methane reformer flue gas. Sep. Purif. Technol. 2021, 256, 117832. [Google Scholar] [CrossRef]
- Bae, J.S.; Yu, X.X.; Su, S. Enrichment of low-quality methane by various combinations of vacuum and temperature swing adsorption processes. Ind. Eng. Chem. Res. 2022, 61, 14298–14304. [Google Scholar] [CrossRef]
- Aljohani, M.M.; Al-Qahtani, S.D.; Alshareef, M.; El-Desouky, M.G.; El-Bindary, A.A.; El-Metwaly, N.M.; El-Bindary, M.A. Highly efficient adsorption and removal bio-staining dye from industrial wastewater onto mesoporous Ag-MOFs. Process Saf. Environ. 2023, 172, 395–407. [Google Scholar] [CrossRef]
- Mohan, B.; Virender; Kadiyan, R.; Kumar, S.; Gupta, V.; Parshad, B.; Solovev, A.A.; Pombeiro, A.J.L.; Kumar, K.; Sharma, P.K. Carbon dioxide capturing activities of porous metal-organic frameworks (MOFs). Micropor. Mesopor. Mat. 2024, 366, 112932. [Google Scholar] [CrossRef]
- Wang, C.J.; Liu, X.L.; Yang, T.H.; Sridhar, D.; Algadi, H.; Bin Xu, B.; El-Bahy, Z.M.; Li, H.D.; Ma, Y.; Li, T.X.; et al. An overview of metal-organic frameworks and their magnetic composites for the removal of pollutants. Sep. Purif. Technol. 2023, 320, 124144. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhang, P.D.; Zhang, X.; Liu, J.H.; He, T.; Yu, J.M.; Li, J.R. Ethylene purification in a metal-organic framework over a wide temperature range via pore confinement. Green Energy Environ. 2023, 8, 1703–1710. [Google Scholar] [CrossRef]
- Yang, S.Q.; Hu, T.L.; Chen, B. Microporous metal-organic framework materials for efficient capture and separation of greenhouse gases. Sci. China Chem. 2023, 66, 2181–2203. [Google Scholar] [CrossRef]
- Yusuf, M.; Kumar, R.; Ali Khan, M.; Ahmed, M.J.; Otero, M.; Muthu Prabhu, S.; Son, M.; Hwang, J.H.; Hyoung Lee, W.; Jeon, B.H. Metal-organic framework-based composites for biogas and natural gas uptake: An overview of adsorption and storage mechanisms of gaseous fuels. Chem. Eng. J. 2023, 478, 147302. [Google Scholar] [CrossRef]
- Amaraweera, S.M.; Gunathilake, C.A.; Gunawardene, O.H.P.; Dassanayake, R.S.; Cho, E.B.; Du, Y.H. Carbon capture using porous silica materials. Nanomaterials 2023, 13, 2050. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Z.; Zhou, Y.; Wang, Z.; Du, M.; Wen, Z.; Yan, B.; Ma, Q.; Liu, N.; Xue, B. Phosphotungstic acid supported on Zr-SBA-15 as an efficient catalyst for one-pot conversion of furfural to γ-valerolactone. Fuel 2024, 356, 129631. [Google Scholar] [CrossRef]
- Perumal, S.K.; Lee, S.; Yu, H.; Heo, J.; Kang, M.J.; Kim, Y.; Park, M.; Lee, H.; Kim, H.S. Synergistic interaction between ruthenium catalysts and grafted niobium on SBA-15 for 2,5-furandicarboxylic acid production using 5-hydroxymethylfurfural. ACS. Appl. Mater. Interfaces 2024, 16, 7353–7363. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.F.; Gao, W.W.; Yun, D.; Xu, C.Z.; Li, Z.; Xia, C.G. A reflux system for SBA-15 synthesis for the selective hydrogenation of cinnamyl aldehyde. New. J. Chem. 2023, 47, 12314–12319. [Google Scholar] [CrossRef]
- Wang, X.; Song, C. Developing high-capacity solid “molecular basket” sorbents for selective CO2 capture and separation. Acc. Chem. Res. 2023, 56, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Li, S.; Liu, C.; He, H. The capture and catalytic conversion of CO2 by dendritic mesoporous silica-based nanoparticles. Energy Environ. Mater. 2024, 7, e12593. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, S.; Xu, R.; Lee, J.W.; Kang, Y.T. Structural modified metal-organic frameworks by hierarchical layer-by-layer method for efficient CO2 capture enhancement. J. CO2 Util. 2023, 77, 102603. [Google Scholar] [CrossRef]
- Belmoujahid, Y.; Bonne, M.; Scudeller, Y.; Schleich, D.; Grohens, Y.; Lebeau, B. Thermal conductivity of monolithic assemblies of sba-15 ordered mesoporous silica particles. Micropor. Mesopor. Mat. 2015, 201, 124–133. [Google Scholar] [CrossRef]
- Zhou, Y.P.; Wang, J.; Yang, J.W.; Duan, L.H.; Liu, H.B.; Wu, J.F.; Gao, L.A. Mesoporous silica-confined MOF-525 for stable adsorption of tetracycline over a wide ph application range. ACS Appl. Nano Mater. 2024, 7, 3806–3816. [Google Scholar] [CrossRef]
- Li, M.; Xiao, J.; Chen, L.; Ren, B.; Liu, Z.J.; Guo, Y.X.; Wang, Y.L. A study of the optimal diffusion distance of ibuprofen through the synthesis of different sizes of mesoporous silica. J. Solid State Chem. 2023, 321, 123911. [Google Scholar] [CrossRef]
- Zienkiewicz-Strzalka, M.; Blachnio, M.; Derylo-Marczewska, A.; Winter, S.; Maciejewska, M. Mesoporous carbons and highly cross-linking polymers for removal of cationic dyes from aqueous solutions-studies on adsorption equilibrium and kinetics. Materials 2024, 17, 1374. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, S.Y.; Yoon, T.U.; Kim, M.B.; Park, W.; Han, H.H.; Kong, C.I.; Park, C.Y.; Kim, J.H.; Bae, Y.S. Improved methane/nitrogen separation properties of zirconium-based metal-organic framework by incorporating highly polarizable bromine atoms. Chem. Eng. J. 2020, 399, 125717. [Google Scholar] [CrossRef]
- Yang, F.; Huang, H.L.; Wang, X.Y.; Li, F.; Gong, Y.H.; Zhong, C.L.; Li, J.R. Proton conductivities in functionalized uio-66: Tuned properties, thermogravimetry mass, and molecular simulation analyses. Cryst. Growth. Des. 2015, 15, 5827–5833. [Google Scholar] [CrossRef]
- Huang, R.Q.; Liu, Z.; Wang, S.; Yu, C.L.; Wei, R.Z.; Tang, Q. Synthesis, crystal structure, and properties of manganese/cobalt complexes based on 2,5-dibromoterephthalic acid ligands. Chin. J. Inorg. Chem. 2023, 39, 159–167. [Google Scholar]
- Dang, V.L.; Kieu, T.T.; Nguyen, T.T.T.; Truong, T.T.T.; Hoang, D.T.; Vu, T.L.C.; Nguyen, T.M.T.; Le, T.S.; Doan, T.H.Y.; Pham, T.D. Surface modification of zeolite by cationic surfactant and the application on adsorptive removal of azo dye ponceau 4R. J. Mol. Struct. 2024, 1304, 137619. [Google Scholar] [CrossRef]
- Li, C.; Ye, F.; Chahine, R.; Yang, T.; Xiao, J. Genetic algorithm optimized artificial neural network models of single- and multi-component gas adsorption isotherms for hydrogen purification. Int. J. Hydrog. Energy 2024, 52, 1127–1142. [Google Scholar] [CrossRef]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef]
- Liu, H.; Ding, W.; Lei, S.H.; Tian, X.P.; Zhou, F.B. Selective adsorption of CH4/N2 on Ni-based MOF/SBA-15 composite materials. Nanomaterials 2019, 9, 149. [Google Scholar] [CrossRef]
- Zhang, L.; Cui, Y.; Zhang, C.; Wang, L.; Wan, H.; Guan, G. Biodiesel production by esterification of oleic acid over brønsted acidic ionic liquid supported onto Fe-incorporated SBA-15. Ind. Eng. Chem. Res. 2012, 51, 16590–16596. [Google Scholar] [CrossRef]

| Samples | BET Surface Area (m2/g) | Total Pore Volume (cm3/g) | Micropore Volume (cm3/g) |
|---|---|---|---|
| UiO-66-Br2 | 515.9 | 0.28 | 0.21 |
| U6B2S1 | 626.2 | 0.29 | 0.23 |
| U6B2S3 | 610.2 | 0.31 | 0.24 |
| U6B2S5 | 607.2 | 0.32 | 0.26 |
| U6B2S7 | 521.7 | 0.34 | 0.16 |
| SBA-15 | 550.2 | 0.77 | - |
| CH4 Adsorption | N2 Adsorption | Sample | CH4 Adsorption | N2 Adsorption | |||||
|---|---|---|---|---|---|---|---|---|---|
| P/P0 | VCH4 (cm3/g) | P/P0 | VN2 (cm3/g) | P/P0 | VCH4 (cm3/g) | P/P0 | VN2 (cm3/g) | ||
| UiO-66-Br2 | 0.10 | 1.09 | 0.12 | 0.27 | U6B2S5 | 0.09 | 1.65 | 0.12 | 0.40 |
| 0.19 | 1.93 | 0.20 | 0.45 | 0.19 | 3.12 | 0.20 | 0.66 | ||
| 0.30 | 2.85 | 0.30 | 0.66 | 0.29 | 4.59 | 0.30 | 0.99 | ||
| 0.40 | 3.66 | 0.40 | 0.88 | 0.40 | 6.03 | 0.39 | 1.31 | ||
| 0.50 | 4.41 | 0.50 | 1.09 | 0.50 | 7.12 | 0.49 | 1.62 | ||
| 0.60 | 5.09 | 0.60 | 1.31 | 0.60 | 8.23 | 0.59 | 1.93 | ||
| 0.70 | 5.76 | 0.70 | 1.51 | 0.69 | 9.13 | 0.69 | 2.25 | ||
| 0.80 | 6.36 | 0.80 | 1.72 | 0.82 | 10.35 | 0.81 | 2.60 | ||
| 0.90 | 6.94 | 0.90 | 1.93 | 0.90 | 11.05 | 0.90 | 2.86 | ||
| 1.00 | 7.53 | 1.02 | 2.18 | 1.01 | 11.97 | 1.01 | 3.22 | ||
| U6B2S1 | 0.10 | 1.75 | 0.12 | 0.40 | U6B2S7 | 0.09 | 1.35 | 0.13 | 0.37 |
| 0.18 | 3.08 | 0.20 | 0.67 | 0.19 | 2.59 | 0.20 | 0.59 | ||
| 0.30 | 4.74 | 0.29 | 1.00 | 0.31 | 4.02 | 0.29 | 0.83 | ||
| 0.40 | 5.93 | 0.40 | 1.34 | 0.40 | 4.99 | 0.40 | 1.14 | ||
| 0.50 | 7.17 | 0.50 | 1.67 | 0.50 | 5.91 | 0.49 | 1.39 | ||
| 0.59 | 8.14 | 0.60 | 1.99 | 0.59 | 6.80 | 0.59 | 1.65 | ||
| 0.71 | 9.31 | 0.70 | 2.30 | 0.70 | 7.69 | 0.69 | 1.92 | ||
| 0.80 | 10.16 | 0.80 | 2.60 | 0.80 | 8.49 | 0.79 | 2.18 | ||
| 0.90 | 11.13 | 0.90 | 2.91 | 0.90 | 9.21 | 0.89 | 2.44 | ||
| 1.00 | 12.01 | 1.02 | 3.28 | 1.01 | 10.01 | 1.01 | 2.73 | ||
| U6B2S3 | 0.09 | 1.54 | 0.10 | 0.35 | SBA-15 | 0.12 | 0.21 | 0.12 | 0.09 |
| 0.19 | 2.99 | 0.19 | 0.65 | 0.20 | 0.33 | 0.20 | 0.15 | ||
| 0.29 | 4.48 | 0.29 | 0.98 | 0.30 | 0.49 | 0.29 | 0.23 | ||
| 0.42 | 5.98 | 0.39 | 1.29 | 0.39 | 0.65 | 0.39 | 0.31 | ||
| 0.50 | 6.92 | 0.49 | 1.61 | 0.50 | 0.83 | 0.49 | 0.39 | ||
| 0.60 | 7.99 | 0.60 | 1.94 | 0.60 | 0.99 | 0.59 | 0.47 | ||
| 0.70 | 8.96 | 0.70 | 2.24 | 0.70 | 1.15 | 0.69 | 0.56 | ||
| 0.80 | 9.88 | 0.80 | 2.54 | 0.80 | 1.31 | 0.79 | 0.64 | ||
| 0.90 | 10.72 | 0.90 | 2.83 | 0.90 | 1.49 | 0.89 | 0.73 | ||
| 1.01 | 11.64 | 1.02 | 3.20 | 1.00 | 1.66 | 1.01 | 0.84 | ||
| Sample | Adsorption Equilibrium Amount (cm3/g) | |||
|---|---|---|---|---|
| Adsorption Pressure of 1.0 bar | Desorption Pressure of 0.1 bar | |||
| VCH4 (cm3/g) | VN2 (cm3/g) | VCH4 (cm3/g) | VN2 (cm3/g) | |
| UiO-66-Br2 | 7.53 | 2.18 | 1.33 | 0.30 |
| U6B2S1 | 12.01 | 3.28 | 2.14 | 0.44 |
| U6B2S3 | 11.64 | 3.20 | 1.71 | 0.39 |
| U6B2S5 | 11.97 | 3.22 | 1.53 | 0.39 |
| U6B2S7 | 10.01 | 2.73 | 1.46 | 0.34 |
| SBA-15 | 1.66 | 0.84 | 0.25 | 0.14 |
| Sample | Parameters Related to the Adsorption Selectivity | |||
|---|---|---|---|---|
| αCH4/N2 | WCH4/N2 | SCH4/N2 | Reference | |
| SBA-15 | 2.13 | 2.02 | 4.30 | this work |
| UiO-66-Br2 | 4.84 | 3.31 | 16.02 | this work |
| U6B2S1 | 5.30 | 3.46 | 18.34 | this work |
| U6B2S3 | 5.24 | 3.54 | 18.55 | this work |
| U6B2S5 | 5.45 | 3.68 | 20.06 | this work |
| U6B2S7 | 5.27 | 3.58 | 18.86 | this work |
| 5A Zeolite | 0.94 | - | 0.81 | [49] |
| MOF-5 | 1.13 | 0.67 | [49] | |
| MOF-177 | 4.00 | 8.45 | [49] | |
| MOF-1/SBA-15 | 2.17 | 2.19 | 4.75 | [50] |
| MOF-2/SBA-15 | 3.44 | 3.24 | 11.1 | [50] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zong, Z.; Zhou, Y.; Yin, C.; Lei, Y.; Wang, R.; Deng, Y.; Wu, T. Enhanced CH4/N2 Separation Efficiency of UiO-66-Br2 through Hybridization with Mesoporous Silica. Molecules 2024, 29, 2750. https://doi.org/10.3390/molecules29122750
Wang H, Zong Z, Zhou Y, Yin C, Lei Y, Wang R, Deng Y, Wu T. Enhanced CH4/N2 Separation Efficiency of UiO-66-Br2 through Hybridization with Mesoporous Silica. Molecules. 2024; 29(12):2750. https://doi.org/10.3390/molecules29122750
Chicago/Turabian StyleWang, Hu, Ziao Zong, Yadong Zhou, Chaochuang Yin, Yizhu Lei, Renshu Wang, Yuheng Deng, and Tingting Wu. 2024. "Enhanced CH4/N2 Separation Efficiency of UiO-66-Br2 through Hybridization with Mesoporous Silica" Molecules 29, no. 12: 2750. https://doi.org/10.3390/molecules29122750
APA StyleWang, H., Zong, Z., Zhou, Y., Yin, C., Lei, Y., Wang, R., Deng, Y., & Wu, T. (2024). Enhanced CH4/N2 Separation Efficiency of UiO-66-Br2 through Hybridization with Mesoporous Silica. Molecules, 29(12), 2750. https://doi.org/10.3390/molecules29122750







