A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents
Abstract
1. Introduction
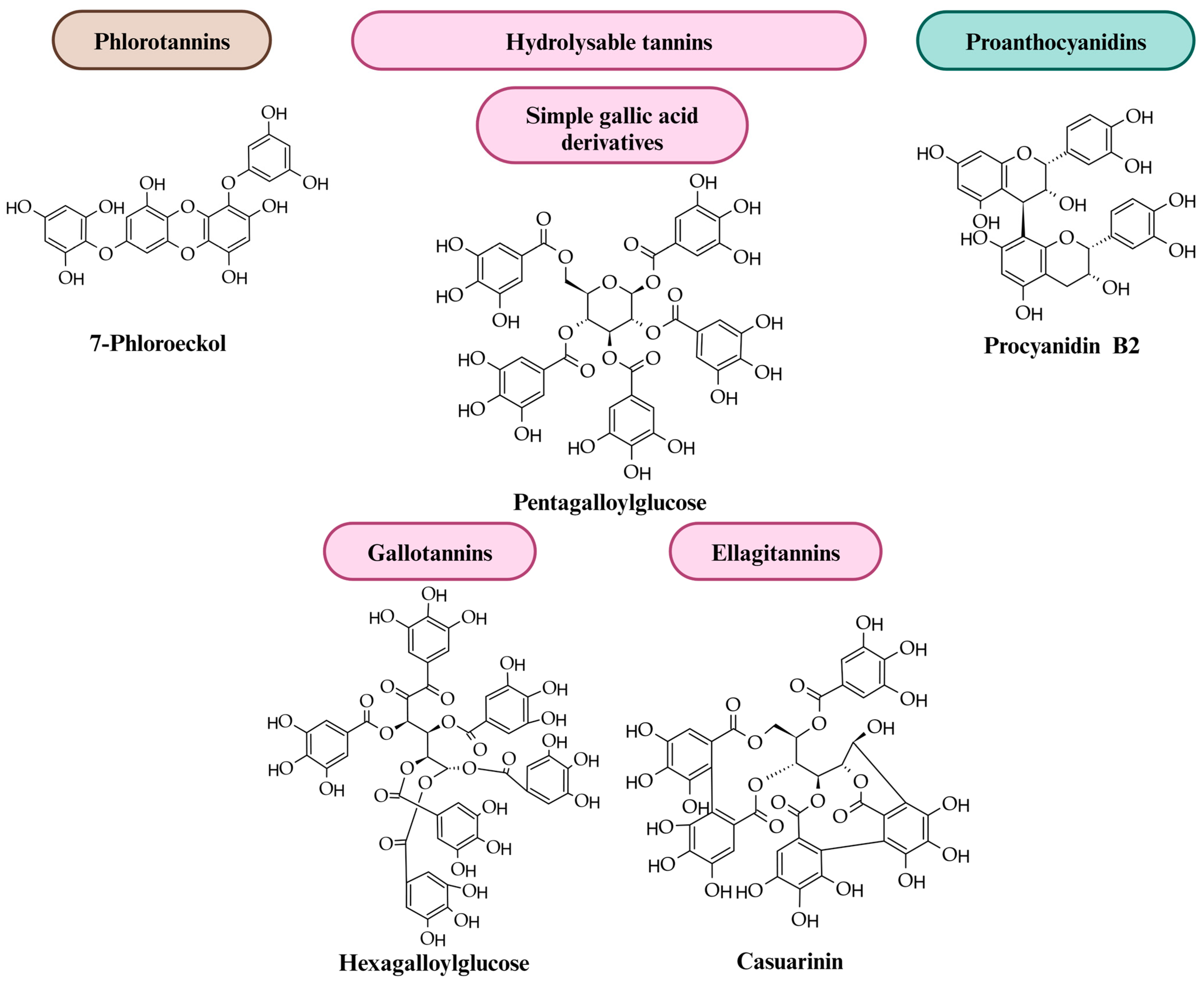
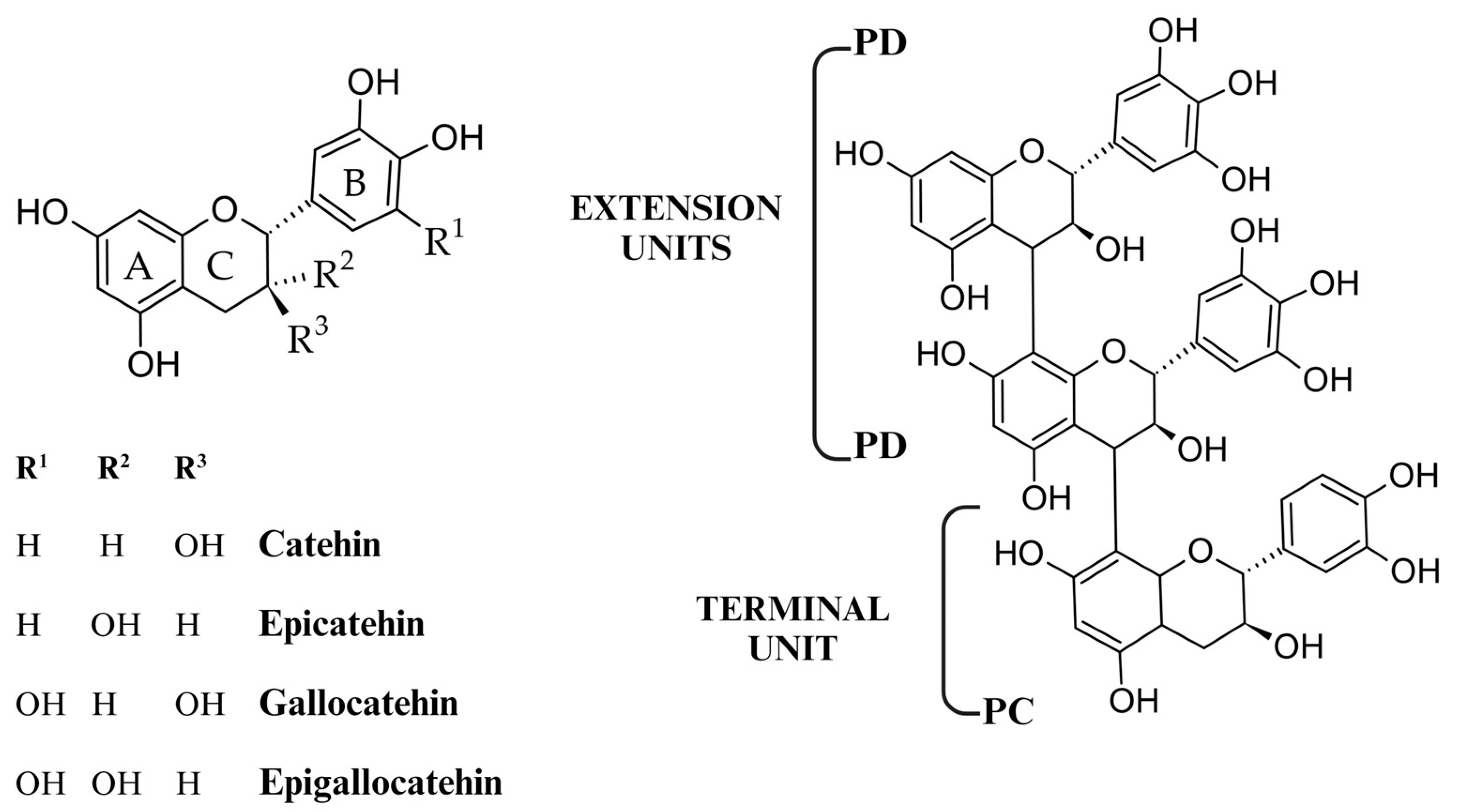
2. Tannins: Diversity, Structure and Distribution in Plants
| Plant Material | Compounds | Reference |
|---|---|---|
|
Brown alga
(Ecklonia kurome Okamura) | Eckol | [94] |
|
Paddle weed (marine brown alga)
(Ecklonia cava Kjellman) | Fucodiphlorethol G Phlorofucofuroeckol A Phloroglucinol-6,6′-bieckol | [95,96,97] |
| Walnut seeds (Juglans regia L.) | Pedunculagin Casuarictin Tellimagrandin I, II Glansreginin A, B Casuarinin | [98,99,100] |
| Mango peel (Magnifera indica L.) | Methyl gallate Maclurin 3-C-β-d-glucoside Iriflophenone 3-C-β-d-glucoside Tetra-O-galloyl-glucoside Penta-O-galloyl-glucoside Mangiferin Maclurin 3-C-(2-O-galloyl)-β-d-glucoside | [101] |
| Mango bark (Magnifera indica L.) | Gallic acid Methyl gallate Maclurin 3-C-β-d-glucoside Iriflophenone 3-C-β-d-glucoside Isomangiferin Iriflophenone 3-C-(2-O-galloyl)-β-d-glucoside Penta-O-galloyl-glucoside Mangiferin | [101] |
| Mango old leaves (Magnifera indica L.) | Gallic acid Methyl gallate Maclurin 3-C-β-d-glucoside Iriflophenone 3-C-β-d-glucoside Penta-O-galloyl-glucoside Iriflophenone 3-C-(2-O-Galloyl)-β-d-glucoside | [101] |
| Mango young leaves (Magnifera indica L.) | Gallic acid Methyl gallate Maclurin 3-C-β-d-glucoside Iriflophenone 3-C-β-d-glucoside Tetra-O-galloyl-glucoside Penta-O-galloyl-glucoside Iriflophenone 3-C-(2-O-galloyl)-β-d-glucoside | [101] |
| Evening Primrose (Oenothera erythrosepala Borbás) | Oenothein B | [102] |
| Garden Spurge (Euphorbia hirta L.) | Euphorbin A | [103] |
| Japanese cornelian cherry (Cornus officinalis Torr.) | Cornusiin A | [102] |
| Common Reaumuria (Reaumuria hirtella Jaub. and Spach.) | Hirtellin A | [104] |
| Hairy Agrimony (Agrimonia pilosa Ledeb.) | Agrimoniin | [105] |
| Autumn Olive (Elaeagnus umbellata Thunb.) | Casuglaunin A | [106] |
| Thiloa leaves (Thiloa glaucocarpa Eicher.) | Vescalagin | [107] |
| Blackberry fruits (Rubus fruticosus L.) | Sanguiin H-6 Lambertianin C | [108,109] |
| Raspberry fruits (Rubus idaeus L.) | Sanguiin H-6 Lambertianin C | [110] |
| Pomegranate fruits and peels (Punica granatum L.) | Punicalagin Punicalin Pedunculagin Vescalagin Castalagin Casuarin Granatin B Oenothein B Eucalbanin B Eucarpanin T1 Pomegraniins A, B | [111,112,113,114,115] |
| Jaboticaba seeds (Plinia cauliflora (Mart.) Kausel) | Pedunculagin | [116] |
3. Deep Eutectic Solvents
4. Extraction of Tannins Using Deep Eutectic Solvents
| Compounds | Yield | Plant Material | Parameters | Reference |
|---|---|---|---|---|
| Total proanthocyanidins | 189.6 mgCE/gDW | Chestnut shell | 5 g of DES:0.5 g of CSW, 65 °C, 24 h, Amberlite XAD-7 | [179] |
| Proanthocyanidins | 229.6 mgCE/gBM | Chestnut shell | Choline chloride:oxalic acid dihydrate, 1:10 (solid:liquid), MW, 60 min, 85 °C, Amberlite XAD-7 | [178] |
| Hydrolysable tannins | Alchemilla vulgaris L. | Choline chloride:urea (1:2), 50% water, 68.2 min, 30 °C | [213] | |
| Proanthocyanidins | 22.10 mg/g | Gingko biloba leaves | Choline chloride:malonic acid (1:2), 55% H2O, 65 °C, 53 min, 10.57:1 (V/w), macroporous resin D-101 | [188] |
| Proanthocyanidins | 75.25 mg/g | Cottonseed hulls | Choline chloride:levulinic acid (1:2), UAE, 33.21% water, 36.25 mL/g (liquid:solid ratio), 7.40 min | [186] |
| Ellagitannins | Pomegranate seed | HVED preatreatment, Choline chloride:citric aicd/acetic acid/lactic acid, 50 °C, 1:10 (liquid:solid), 60 min, 160 rpm | [194] | |
| Tannins | 50 mg/gDW | Pomegranate peel | US, choline chloride:fructose, 1:10 (solid:liquid), 50 °C, 90 min | [193] |
| Proanthocyanidins | 144.1 mgPAC/gBM | Grape pomace | Choline chloride:ethanol:water, 14.4% biomass, 102.8 °C, 5 h | [187] |
| Proanthocyanidins | 135 mg/g | Grape pomace | MAE, choline chloride:lactic acid:water (0.36:0.39:0.25), 99.2 °C, 3.56 min | [195] |
| Phlorotannins | Brown algae (Fucus vesiculosus L. and Ascophyllum nodosum (L.) Le Jolis) | Choline chloride:lactic acid, 20% H2O, maceration, 2h, 50 °C | [209] | |
| Phlorotannins | Fucus vesiculosus | UAE, lactic acid:choline chloride or lactic acid:glucose:H2O, 25 °C, 60 min, 1:10 (solid:liquid) | [211] | |
| Phlorotannins | 137.3 mgPGE/gDW | Fucus vesiculosus | UAE, choline chloride:lactic acid, 23 min, 30% water, 1:12 (solid:liquid) | [212] |
| Tannic acid | 1705.79 µg/g | Onion peel | UAE, Choline chloride:urea (1:1), H2O, 1:10 (solid:liquid), duty cycle of 10% | [206] |
| Ellagic acid | 5.21 mg/100 gextract | Raspberry seed | Citric acid:betaine:H2O (2:1:2), 85 °C, 147 min, 1:15.76 (solid:liquid) | [199] |
5. Biological Activity of Plant Tannins
6. Tannin-Based DESs
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miljković, V.M.; Nikolić, G.S.; Zvezdanović, J.; Mihajlov-Krstev, T.; Arsić, B.B.; Miljković, M.N. Phenolic Profile, Mineral Content and Antibacterial Activity of the Methanol Extract of Vaccinium myrtillus L. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 122–127. [Google Scholar] [CrossRef]
- Pinent, M.; González-Abuín, N.; Blay, M.; Ardévol, A. Chapter 16-Dietary Proanthocyanidin Modulation of Pancreatic β Cells: Molecular Aspects. In Molecular Nutrition and Diabetes; Mauricio, D., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 197–210. ISBN 978-0-12-801585-8. [Google Scholar]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and Ruminant Nutrition. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological Function of Plant Tannin and Its Application in Animal Health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Cuong, D.X.; Hoan, N.X.; Dong, D.H.; Thuy, L.T.M.; Thanh, N.V.; Ha, H.T.; Tuyen, D.T.T.; Chinh, D.X.; Cuong, D.X.; Hoan, N.X.; et al. Tannins: Extraction from Plants. In Tannins-Structural Properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019; ISBN 978-1-78984-797-0. [Google Scholar]
- Khatib, M.; Campo, M.; Bellumori, M.; Cecchi, L.; Vignolini, P.; Innocenti, M.; Mulinacci, N. Tannins from Different Parts of the Chestnut Trunk (Castanea sativa Mill.): A Green and Effective Extraction Method and Their Profiling by High-Performance Liquid Chromatography-Diode Array Detector-Mass Spectrometry. ACS Food Sci. Technol. 2023, 3, 1903–1912. [Google Scholar] [CrossRef]
- Bashir, I.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Fayaz, U.; Shams, R.; Srivastava, S.; Singh, R. Deep Eutectic Solvents for Extraction of Functional Components from Plant-Based Products: A Promising Approach. Sustain. Chem. Pharm. 2023, 33, 101102. [Google Scholar] [CrossRef]
- Oyoun, F.; Toncheva, A.; Henríquez, L.C.; Grougnet, R.; Laoutid, F.; Mignet, N.; Alhareth, K.; Corvis, Y. Deep Eutectic Solvents: An Eco-Friendly Design for Drug Engineering. ChemSusChem 2023, 16, e202300669. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T.; Ito, H. Ellagitannins Renewed the Concept of Tannins. In Chemistry and Biology of Ellagitannins; World Scientific Publishing Co.: Singapore, 2009; pp. 1–54. ISBN 978-981-279-740-7. [Google Scholar]
- Yoshida, T.; Hatano, T.; Ito, H.; Okuda, T. Structural Diversity and Antimicrobial Activities of Ellagitannins. In Chemistry and Biology of Ellagitannins; World Scientific: Singapore, 2009; pp. 55–93. ISBN 978-981-279-740-7. [Google Scholar]
- Ramos, S.; Alía, M.; Bravo, L.; Goya, L. Comparative Effects of Food-Derived Polyphenols on the Viability and Apoptosis of a Human Hepatoma Cell Line (HepG2). J. Agric. Food Chem. 2005, 53, 1271–1280. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Habibi, K.; Dehghanian, M.; Aliyar, S.; Asgari Lajayer, B.; Astatkie, T.; Minkina, T.; Keswani, C. Reinforcing the Bulwark: Unravelling the Efficient Applications of Plant Phenolics and Tannins against Environmental Stresses. Heliyon 2022, 8, e09094. [Google Scholar] [CrossRef]
- Mannino, A.M.; Micheli, C. Ecological Function of Phenolic Compounds from Mediterranean Fucoid Algae and Seagrasses: An Overview on the Genus Cystoseira Sensu Lato and Posidonia oceanica (L.) Delile. J. Mar. Sci. Eng. 2020, 8, 19. [Google Scholar] [CrossRef]
- Arapitsas, P. Hydrolyzable Tannin Analysis in Food. Food Chem. 2012, 135, 1708–1717. [Google Scholar] [CrossRef]
- Cheynier, V.; Souquet, J.-M.; Le Roux, E.; Guyot, S.; Rigaud, J. Size Separation of Condensed Tannins by Normal-Phase High-Performance Liquid Chromatography. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 178–184. [Google Scholar]
- Jordán, M.J.; Moñino, M.I.; Martínez, C.; Lafuente, A.; Sotomayor, J.A. Introduction of Distillate Rosemary Leaves into the Diet of the Murciano-Granadina Goat: Transfer of Polyphenolic Compounds to Goats’ Milk and the Plasma of Suckling Goat Kids. J. Agric. Food Chem. 2010, 58, 8265–8270. [Google Scholar] [CrossRef]
- Kiss, A.K.; Piwowarski, J.P. Ellagitannins, Gallotannins and Their Metabolites- The Contribution to the Anti-Inflammatory Effect of Food Products and Medicinal Plants. Curr. Med. Chem. 2018, 25, 4946–4967. [Google Scholar] [CrossRef]
- Haslam, E. Plant Polyphenols (Syn. Vegetable Tannins) and Chemical Defense-A Reappraisal. J. Chem. Ecol. 1988, 14, 1789–1805. [Google Scholar] [CrossRef]
- Haddock, E.A.; Gupta, R.K.; Haslam, E. The Metabolism of Gallic Acid and Hexahydroxydiphenic Acid in Plants. Part 3. Esters of (R)- and (S)-Hexahydroxydiphenic Acid and Dehydrohexahydroxydiphenic Acid with D-Glucopyranose (1C4 and Related Conformations). J. Chem. Soc. Perkin Trans. 1982, 1, 2535–2545. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and Hydrolysable Tannins: Occurrence, Dietary Intake and Pharmacological Effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Gross, G.G. Biosynthesis, Biodegradation, and Cellular Localization of Hydrolyzable Tannins. In Phytochemicals in Human Health Protection, Nutrition, and Plant Defense; Romeo, J.T., Ed.; Springer: Boston, MA, USA, 1999; pp. 185–213. ISBN 978-1-4615-4689-4. [Google Scholar]
- Barbehenn, R.V.; Peter Constabel, C. Tannins in Plant–Herbivore Interactions. Phytochemistry 2011, 72, 1551–1565. [Google Scholar] [CrossRef]
- Amarowicz, R.; Janiak, M. Hydrolysable Tannins. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 337–343. ISBN 978-0-12-814045-1. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.; Putnik, P.; Kovačević, D.B.; Muchenje, V.; Barba, F.J. Chapter 6-Sources, Chemistry, and Biological Potential of Ellagitannins and Ellagic Acid Derivatives. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 60, pp. 189–221. [Google Scholar]
- Jourdes, M.; Lefeuvre, D.; Quideau, S. C-Glycosidic Ellagitannins and Their Influence on Wine Chemistry. In Chemistry and Biology of Ellagitannins. An Underestimated Class of Bioactive Plant Polyphenols; World Scientific Publishing: Singapore, 2009; pp. 320–365. ISBN 978-981-279-740-7. [Google Scholar]
- Salminen, J.-P.; Karonen, M. Chemical Ecology of Tannins and Other Phenolics: We Need a Change in Approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- McLeod, M.N. Plant Tannins-Their Role in Forage Quality. Nutr. Abstr. Rev. 1974, 44, 803–815. [Google Scholar]
- Qu, S.; Chapman, N.; Xia, Z.; Feng, M.; Feng, S.; Wang, Z.; Liu, L. Ultramicroscopy Reveals a Layer of Multiply Folded Membranes around the Tannin-Accumulating Vacuole in Honeysuckle Petal Trichomes. Micron 2017, 99, 1–8. [Google Scholar] [CrossRef]
- Engström, M. Understanding the Bioactivity of Plant Tannins: Developments in Analysis Methods and Structure-Activity Studies. Ph.D. Thesis, University of Turku, Turku, Finland, 2016. [Google Scholar]
- Winkel-Shirley, B. Flavonoid Biosynthesis. A Colorful Model for Genetics, Biochemistry, Cell Biology, and Biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Dixon, R.A. Engineering of Plant Natural Product Pathways. Curr. Opin. Plant Biol. 2005, 8, 329–336. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, Z.; Maximova, S.; Payne, M.J.; Guiltinan, M.J. Proanthocyanidin Synthesis in Theobroma Cacao: Genes Encoding Anthocyanidin Synthase, Anthocyanidin Reductase, and Leucoanthocyanidin Reductase. BMC Plant Biol. 2013, 13, 202. [Google Scholar] [CrossRef]
- Yoshida, T.; Hatano, T.; Ito, H. Chapter Seven-High Molecular Weight Plant Poplyphenols (Tannins): Prospective Functions. In Recent Advances in Phytochemistry; Chemical Ecology and Phytochemistry of Forest Ecosystems; Romeo, J.T., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 39, pp. 163–190. [Google Scholar]
- Xie, D.-Y.; Sharma, S.B.; Paiva, N.L.; Ferreira, D.; Dixon, R.A. Role of Anthocyanidin Reductase, Encoded by BANYULS in Plant Flavonoid Biosynthesis. Science 2003, 299, 396–399. [Google Scholar] [CrossRef]
- Dixon, R.A.; Xie, D.-Y.; Sharma, S.B. Proanthocyanidins--a Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef]
- Goodwin, T.W.; Mercer, E.I. Introduction to Plant Biochemistry, 2nd ed.; Pergamon Press: Oxford, UK, 1983; ISBN 978-0-08-016223-2. [Google Scholar]
- Tharayil, N.; Suseela, V.; Triebwasser, D.J.; Preston, C.M.; Gerard, P.D.; Dukes, J.S. Changes in the Structural Composition and Reactivity of Acer Rubrum Leaf Litter Tannins Exposed to Warming and Altered Precipitation: Climatic Stress-Induced Tannins Are More Reactive. New Phytol. 2011, 191, 132–145. [Google Scholar] [CrossRef]
- Monagas, M.; Quintanilla-López, J.E.; Gómez-Cordovés, C.; Bartolomé, B.; Lebrón-Aguilar, R. MALDI-TOF MS Analysis of Plant Proanthocyanidins. J. Pharm. Biomed. Anal. 2010, 51, 358–372. [Google Scholar] [CrossRef]
- Porter, L.J. Flavans and Proanthocyanidins. In The Flavonoids, Advances in Research since 1980; Harborne, J.B., Ed.; Springer Science+Buisness Media: Dordrecht, The Netherlands, 1988; pp. 23–53. [Google Scholar]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of Proanthocyanidins in Common Foods and Estimations of Normal Consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jones, C.P.; Karonen, M.; Salminen, J.-P. Tannin Composition Affects the Oxidative Activities of Tree Leaves. J. Chem. Ecol. 2006, 32, 2235–2251. [Google Scholar] [CrossRef]
- Cheynier, V.; Fulcrand, H. Analysis of Polymeric Proanthocyanidins and Complex Polyphenols. In Methods in Polyphenol Analysis; Santos-Buelga, C., Williamson, G., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2003. [Google Scholar]
- Karonen, M.; Leikas, A.; Loponen, J.; Sinkkonen, J.; Ossipov, V.; Pihlaja, K. Reversed-Phase HPLC-ESI/MS Analysis of Birch Leaf Proanthocyanidins after Their Acidic Degradation in the Presence of Nucleophiles. Phytochem. Anal. 2007, 18, 378–386. [Google Scholar] [CrossRef]
- Grundhöfer, P.; Niemetz, R.; Schilling, G.; Gross, G.G. Biosynthesis and Subcellular Distribution of Hydrolyzable Tannins. Phytochemistry 2001, 57, 915–927. [Google Scholar] [CrossRef]
- He, M.; Tian, H.; Luo, X.; Qi, X.; Chen, X. Molecular Progress in Research on Fruit Astringency. Molecules 2015, 20, 1434–1451. [Google Scholar] [CrossRef]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins Are Polyphenolic Metabolites of Brown Algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Gümüş Yılmaz, G.; Gómez Pinchetti, J.L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Comparison of Extraction Techniques and Surfactants for the Isolation of Total Polyphenols and Phlorotannins from the Brown Algae Lobophora Variegata. Anal. Lett. 2019, 52, 2724–2740. [Google Scholar] [CrossRef]
- BioRender.Com. Available online: https://app.biorender.com/illustrations/65fb12682d08c19562fd5ba5 (accessed on 21 March 2024).
- Salminen, J.P.; Ossipov, V.; Haukioja, E.; Pihlaja, K. Seasonal Variation in the Content of Hydrolysable Tannins in Leaves of Betula Pubescens. Phytochemistry 2001, 57, 15–22. [Google Scholar] [CrossRef]
- Hofmann, A.S.; Gross, G.G. Biosynthesis of Gallotannins: Formation of Polygalloylglucoses by Enzymatic Acylation of 1,2,3,4,6-Penta-O-Galloylglucose. Arch. Biochem. Biophys. 1990, 283, 530–532. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Ellagitannin Biosynthesis: Laccase-Catalyzed Dimerization of Tellimagrandin II to Cornusiin E in Tellima Grandiflora. Phytochemistry 2003, 64, 1197–1201. [Google Scholar] [CrossRef]
- Gross, G.G. Enzymes in the Biosynthesis of Hydrolyzable Tannins. In Plant Polyphenols: Synthesis, Properties, Significance; Hemingway, R.W., Laks, P.E., Eds.; Springer: Boston, MA, USA, 1992; pp. 43–60. ISBN 978-1-4615-3476-1. [Google Scholar]
- Okuda, T.; Ito, H. Tannins of Constant Structure in Medicinal and Food Plants—Hydrolyzable Tannins and Polyphenols Related to Tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Gallotannin Biosynthesis: Beta-Glucogallin: Hexagalloyl 3-O-Galloyltransferase from Rhus Typhina Leaves. Phytochemistry 2001, 58, 657–661. [Google Scholar] [CrossRef]
- Haslam, E. Symmetry and Promiscuity in Procyanidin Biochemistry. Phytochemistry 1977, 16, 1625–1640. [Google Scholar] [CrossRef]
- Strack, D. 10—Phenolic Metabolism; Academic Press: London, UK, 1997; pp. 387–416. [Google Scholar]
- Biała, W.; Jasiński, M. The Phenylpropanoid Case—It Is Transport That Matters. Front. Plant Sci. 2018, 9, 1610. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Holton, T.; Cornish, E. Genetics and Biochemistry of Anthocyanin Biosynthesis. Plant Cell 1995, 7, 1071–1083. [Google Scholar] [CrossRef]
- Stafford, H.A. Flavonoid Evolution: An Enzymic Approach. Plant Physiol. 1991, 96, 680–685. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Dixon, R.A. Proanthocyanidin Biosynthesis--Still More Questions than Answers? Phytochemistry 2005, 66, 2127–2144. [Google Scholar] [CrossRef]
- Tian, L.; Pang, Y.; Dixon, R.A. Biosynthesis and Genetic Engineering of Proanthocyanidins and (Iso)Flavonoids. Phytochem. Rev. 2008, 3, 445–465. [Google Scholar] [CrossRef]
- Tanner, G.J.; Francki, K.T.; Abrahams, S.; Watson, J.M.; Larkin, P.J.; Ashton, A.R. Proanthocyanidin Biosynthesis in Plants: Purification of Legume Leucoanthocyanidin Reductase and Molecular Cloning of Its cDNA*. J. Biol. Chem. 2003, 278, 31647–31656. [Google Scholar] [CrossRef]
- Xie, D.-Y.; Sharma, S.B.; Dixon, R.A. Anthocyanidin Reductases from Medicago truncatula and Arabidopsis thaliana. Arch. Biochem. Biophys. 2004, 422, 91–102. [Google Scholar] [CrossRef]
- Zhao, J.; Pang, Y.; Dixon, R.A. The Mysteries of Proanthocyanidin Transport and Polymerization. Plant Physiol. 2010, 153, 437–443. [Google Scholar] [CrossRef]
- Ossipov, V.; Haukioja, E.; Ossipova, S.; Hanhimäki, S.; Pihlaja, K. Phenolic and Phenolic-Related Factors as Determinants of Suitability of Mountain Birch Leaves to an Herbivorous Insect. Biochem. Syst. Ecol. 2001, 29, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Riipi, M.; Ossipov, V.; Lempa, K.; Haukioja, E.; Koricheva, J.; Ossipova, S.; Pihlaja, K. Seasonal Changes in Birch Leaf Chemistry: Are There Trade-Offs between Leaf Growth and Accumulation of Phenolics? Oecologia 2002, 130, 380–390. [Google Scholar] [CrossRef] [PubMed]
- Schwechheimer, C.; Bevan, M. The Regulation of Transcription Factor Activity in Plants. Trends Plant Sci. 1998, 3, 378–383. [Google Scholar] [CrossRef]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY Superfamily of Plant Transcription Factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Stracke, R.; Werber, M.; Weisshaar, B. The R2R3-MYB Gene Family in Arabidopsis Thaliana. Curr. Opin. Plant Biol. 2001, 4, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Weisshaar, B.; Dröge-Laser, W.; Vicente-Carbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F.; bZIP Research Group. bZIP Transcription Factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Vom Endt, D.; Kijne, J.W.; Memelink, J. Transcription Factors Controlling Plant Secondary Metabolism: What Regulates the Regulators? Phytochemistry 2002, 61, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.M.; Schwinn, K.E. Transcriptional Regulation of Secondary Metabolism. Funct. Plant Biol. 2003, 30, 913–925. [Google Scholar] [CrossRef]
- Crifò, T.; Puglisi, I.; Petrone, G.; Recupero, G.R.; Lo Piero, A.R. Expression Analysis in Response to Low Temperature Stress in Blood Oranges: Implication of the Flavonoid Biosynthetic Pathway. Gene 2011, 476, 1–9. [Google Scholar] [CrossRef]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light Quality Affects Flavonoid Biosynthesis in Young Berries of Cabernet Sauvignon Grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef]
- Kyamuhangire, W.; Krekling, T.; Reed, E.; Pehrson, R. The Microstructure and Tannin Content of Banana Fruit and Their Likely Influence on Juice Extraction. J. Sci. Food Agric. 2006, 86, 1908–1915. [Google Scholar] [CrossRef]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.G.; Smith, P.A. Quantification of Condensed Tannins by Precipitation with Methyl Cellulose: Development and Validation of an Optimised Tool for Grape and Wine Analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Ortiz, J.; Marín-Arroyo, M.-R.; Noriega-Domínguez, M.-J.; Navarro, M.; Arozarena, I. Color, Phenolics, and Antioxidant Activity of Blackberry (Rubus glaucus Benth.), Blueberry (Vaccinium floribundum Kunth.), and Apple Wines from Ecuador. J. Food Sci. 2013, 78, C985–C993. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Espinoza, M.C.; Zafimahova, A.; Alvarado, P.G.M.; Dubreucq, E.; Poncet-Legrand, C. Grape Seed and Apple Tannins: Emulsifying and Antioxidant Properties. Food Chem. 2015, 178, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Chapter 8-Tannins: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. ISBN 978-0-08-045316-3. [Google Scholar]
- Paaver, U.; Matto, V.; Raal, A. Total Tannin Content in Distinct Quercus robur L. Galls. J. Med. Plants Res. 2010, 4, 702–705. [Google Scholar]
- Taper, M.L.; Case, T.J. Interactions between Oak Tannins and Parasite Community Structure: Unexpected Benefits of Tannins to Cynipid Gall-Wasps. Oecologia 1987, 71, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Singh, R. A Brief Note on Tannins. Res. Rev. J. Pharmacogn. Phytochem. 2022, 10, 1–2. [Google Scholar]
- Constabel, P.C.; Yoshida, K.; Walker, V. Diverse Ecological Roles of Plant Tannins: Plant Defense and Beyond. In Recent Advances in Polyphenol Research; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 115–142. ISBN 978-1-118-32963-4. [Google Scholar]
- Canuti, V.; Cecchi, L.; Khatib, M.; Guerrini, L.; Mulinacci, N.; Zanoni, B. A New Extract from Pomegranate (Punica granatum L.) By-Products as a Potential Oenological Tannin: Preliminary Characterization and Comparison with Existing Commercial Products. Molecules 2020, 25, 4460. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, H.; Matsuo, Y.; Omar, M.; Saito, Y.; Nishida, K.; Tanaka, T. Oxidation of the Oak Ellagitannin, Vescalagin. J. Nat. Prod. 2020, 83, 413–421. [Google Scholar] [CrossRef]
- Richard-Dazeur, C.; Jacolot, P.; Niquet-Léridon, C.; Goethals, L.; Barbezier, N.; Anton, P.M. HPLC-DAD Optimization of Quantification of Vescalagin, Gallic and Ellagic Acid in Chestnut Tannins. Heliyon 2023, 9, e18993. [Google Scholar] [CrossRef]
- Shuaibu, M.N.; Pandey, K.; Wuyep, P.A.; Yanagi, T.; Hirayama, K.; Ichinose, A.; Tanaka, T.; Kouno, I. Castalagin from Anogeissus Leiocarpus Mediates the Killing of Leishmania in Vitro. Parasitol. Res. 2008, 103, 1333–1338. [Google Scholar] [CrossRef]
- Mole, S. The Systematic Distribution of Tannins in the Leaves of Angiosperms: A Tool for Ecological Studies. Biochem. Syst. Ecol. 1993, 21, 833–846. [Google Scholar] [CrossRef]
- Iwashina, T. The Structure and Distribution of the Flavonoids in Plants. J. Plant Res. 2000, 113, 287–299. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-Ols: Nature, Occurrence and Biological Activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.; Ranalli, A.W.; Phillips, A.W. Cocoa Polyphenols, Changes in Cocoa Tannins during Processing. J. Agric. Food Chem. 1961, 9, 295–298. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Norton, E.L. Chemistry and Reactivity of Tannins in Vitis Spp.: A Review. Molecules 2020, 25, 2110. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Kodama, M.; Miura, I.; Kinzyo, Z.; Kido, M.; Mori, H.; Nakayama, Y.; Takahashi, M. Structure of an Anti-Plasmin Inhibitor, Eckol, Isolated from the Brown Alga Ecklonia Kurome OKAMURA and Inhibitory Activities of Its Derivatives on Plasma Plasmin Inhibitors. Chem. Pharm. Bull. 1989, 37, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Piao, M.J.; Zheng, J.; Yao, C.W.; Cha, J.W.; Kumara, M.H.S.R.; Han, X.; Kang, H.K.; Lee, N.H.; Hyun, J.W. Fucodiphlorethol G Purified from Ecklonia Cava Suppresses Ultraviolet B Radiation-Induced Oxidative Stress and Cellular Damage. Biomol. Ther. 2014, 22, 301–307. [Google Scholar] [CrossRef]
- Kirindage, K.G.I.S.; Jayasinghe, A.M.K.; Han, E.-J.; Han, H.-J.; Kim, K.-N.; Wang, L.; Heo, S.-J.; Jung, K.-S.; Ahn, G. Phlorofucofuroeckol-A Refined by Edible Brown Algae Ecklonia Cava Indicates Anti-Inflammatory Effects on TNF-α/IFN-γ-Stimulated HaCaT Keratinocytes and 12-O-Tetradecanoylphorbol 13-Acetate-Induced Ear Edema in BALB/c Mice. J. Funct. Foods 2023, 109, 105786. [Google Scholar] [CrossRef]
- Kang, S.-M.; Heo, S.-J.; Kim, K.-N.; Lee, S.-H.; Jeon, Y.-J. Isolation and Identification of New Compound, 2,7″-Phloroglucinol-6,6′-Bieckol from Brown Algae, Ecklonia Cava and Its Antioxidant Effect. J. Funct. Foods 2012, 4, 158–166. [Google Scholar] [CrossRef]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative Polyphenols from Walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef]
- Regueiro, J.; Sánchez-González, C.; Vallverdú-Queralt, A.; Simal-Gándara, J.; Lamuela-Raventós, R.; Izquierdo-Pulido, M. Comprehensive Identification of Walnut Polyphenols by Liquid Chromatography Coupled to Linear Ion Trap-Orbitrap Mass Spectrometry. Food Chem. 2014, 152, 340–348. [Google Scholar] [CrossRef]
- Ito, H.; Okuda, T.; Fukuda, T.; Hatano, T.; Yoshida, T. Two Novel Dicarboxylic Acid Derivatives and a New Dimeric Hydrolyzable Tannin from Walnuts. J. Agric. Food Chem. 2007, 55, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Barreto, J.C.; Trevisan, M.T.S.; Hull, W.E.; Erben, G.; de Brito, E.S.; Pfundstein, B.; Würtele, G.; Spiegelhalder, B.; Owen, R.W. Characterization and Quantitation of Polyphenolic Compounds in Bark, Kernel, Leaves, and Peel of Mango (Mangifera indica L.). J. Agric. Food Chem. 2008, 56, 5599–5610. [Google Scholar] [CrossRef]
- Hatano, T.; Ogawa, N.; Kira, R.; Yasuhara, T.; Okuda, T. Tannins of Cornaceous Plants. I. Cornusiins A, B and C, Dimeric Monomeric and Trimeric Hydrolyzable Tannins from Cornus Officinalis, and Orientation of Valoneoyl Group in Related Tannins. Chem. Pharm. Bull. 1989, 37, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Amakura, Y.; Liu, Y.Z.; Okuda, T. Tannins and Related Polyphenols of Euphorbiaceous Plants. XI. Three New Hydrolyzable Tannins and a Polyphenol Glucoside from Euphorbia Humifusa. Chem. Pharm. Bull. 1994, 42, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.F.; Memon, M.U.; Yoshida, T.; Okuda, T. Tannins of Tamaricaceous Plants. VI. Four New Trimeric Hydrolyzable Tannins from Reaumuria Hirtella and Tamarix Pakistanica. Chem. Pharm. Bull. 1994, 42, 254–264. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Kuwahara, M.; Memon, M.U.; Shingu, T. Agrimoniin and Potentillin, an Ellagitannin Dimer and Monomer Having an α-Glucose Core. J. Chem. Soc. Chem. Commun. 1982, 163–164. [Google Scholar] [CrossRef]
- Ito, H.; Miki, K.; Yoshida, T. Elaeagnatins A-G, C-Glucosidic Ellagitannins from Elaeagnus Umbellata. Chem. Pharm. Bull. 1999, 47, 536–542. [Google Scholar] [CrossRef]
- Itakura, Y.; Habermehl, G.; Mebs, D. Tannins Occurring in the Toxic Brazilian Plant Thiloa Glaucocarpa. Toxicon 1987, 25, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Sangiovanni, E.; Vrhovsek, U.; Rossoni, G.; Colombo, E.; Brunelli, C.; Brembati, L.; Trivulzio, S.; Gasperotti, M.; Mattivi, F.; Bosisio, E.; et al. Ellagitannins from Rubus Berries for the Control of Gastric Inflammation: In Vitro and in Vivo Studies. PLoS ONE 2013, 8, e71762. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Characterization of Phenolic Compounds of Thorny and Thornless Blackberries. J. Agric. Food Chem. 2015, 63, 3012–3021. [Google Scholar] [CrossRef]
- Mullen, W.; Yokota, T.; Lean, M.E.J.; Crozier, A. Analysis of Ellagitannins and Conjugates of Ellagic Acid and Quercetin in Raspberry Fruits by LC-MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Song, B.; Li, J.; Li, J. Pomegranate Peel Extract Polyphenols Induced Apoptosis in Human Hepatoma Cells by Mitochondrial Pathway. Food Chem. Toxicol. 2016, 93, 158–166. [Google Scholar] [CrossRef]
- Gonzalez-Castillo, M.; de Jesus Loera, M.; Ascacio-Valdes, J.; Rodríguez-Herrera, R.; Zugasti-Cruz, A.; Salinas-Santander, M.; Cepeda-Nieto, A.C.; Vera-Reyes, I.; Ángel-Martínez, M.D.; Morlett-Chavez, A. Punicalin and Ellagic Acid from Pomegranate Peel Extract Facilitate Apoptotic Behaviour in the Hela Cell Line. Pak. J. Pharm. Sci. 2021, 34, 2181–2189. [Google Scholar] [PubMed]
- Ali, M.Y.; Jannat, S.; Chang, M.S. Discovery of Potent Angiotensin-Converting Enzyme Inhibitors in Pomegranate as a Treatment for Hypertension. J. Agric. Food Chem. 2023, 71, 11476–11490. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Aaby, K.; Alasalvar, C.; Heinonen, M.; Jacobs, G.; Voorspoels, S.; Koivumäki, T.; Kroon, P.A.; Pelvan, E.; et al. Validated Method for the Characterization and Quantification of Extractable and Nonextractable Ellagitannins after Acid Hydrolysis in Pomegranate Fruits, Juices, and Extracts. J. Agric. Food Chem. 2015, 63, 6555–6566. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Toselli, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Caboni, M.F. Determination of the Major Phenolic Compounds in Pomegranate Juices by HPLC−DAD−ESI-MS. J. Agric. Food Chem. 2013, 61, 5328–5337. [Google Scholar] [CrossRef]
- Silva Fernandes, A.; Hollanda Véras, J.; Silva, L.S.; Puga, S.C.; Luiz Cardoso Bailão, E.F.; de Oliveira, M.G.; Cardoso, C.G.; Carneiro, C.C.; Costa Santos, S.D.; Chen-Chen, L. Pedunculagin Isolated from Plinia Cauliflora Seeds Exhibits Genotoxic, Antigenotoxic and Cytotoxic Effects in Bacteria and Human Lymphocytes. J. Toxicol. Environ. Health A 2022, 85, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Terrill, T.H.; Rowan, A.M.; Douglas, G.B.; Barry, T.N. Determination of Extractable and Bound Condensed Tannin Concentrations in Forage Plants, Protein Concentrate Meals and Cereal Grains. J. Sci. Food Agric. 1992, 58, 321–329. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Iwaasa, A.D.; Xu, Z.; Schellenberg, M.P.; Zhang, Y.G.; Liu, X.L.; McAllister, T.A. Effect of Condensed Tannins on Ruminal Degradability of Purple Prairie Clover (Dalea purpurea Vent.) Harvested at Two Growth Stages. Anim. Feed Sci. Technol. 2012, 176, 17–25. [Google Scholar] [CrossRef]
- Li, Y.; Iwaasa, A.D.; Wang, Y.; Jin, L.; Han, G.; Zhao, M. Condensed Tannins Concentration of Selected Prairie Legume Forages as Affected by Phenological Stages during Two Consecutive Growth Seasons in Western Canada. Can. J. Plant Sci. 2014, 94, 817–826. [Google Scholar] [CrossRef]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of Bound Polyphenols from Carrot Dietary Fiber and Its in Vivo and in Vitro Antioxidant Activity. Food Chem. 2021, 339, 127879. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Chen, W.; Luo, M.; Xu, L.; Zhang, Q.; Luo, Z. Comparative Transcriptome Analysis Reveals Regulatory Network and Regulators Associated with Proanthocyanidin Accumulation in Persimmon. BMC Plant Biol. 2021, 21, 356. [Google Scholar] [CrossRef] [PubMed]
- Ristic, R.; Iland, P.G. Relationships between Seed and Berry Development of Vitis Vinifera L. Cv Shiraz: Developmental Changes in Seed Morphology and Phenolic Composition. Aust. J. Grape Wine Res. 2005, 11, 43–58. [Google Scholar] [CrossRef]
- Mueller, W.C.; Beckman, C.H. Ultrastructure and Development of Phenolic-Storing Cells in Cotton Roots. Can. J. Bot. 1976, 54, 2074–2082. [Google Scholar] [CrossRef]
- Parham, R.A.; Kaustinen, H.M. On the Site of Tannin Synthesis in Plant Cells. Bot. Gaz. 1977, 138, 465–467. [Google Scholar] [CrossRef]
- Hutzler, P.; Fischbach, R.; Heller, W.; Jungblut, T.P.; Reuber, S.; Schmitz, R.; Veit, M.; Weissenböck, G.; Schnitzler, J.-P. Tissue Localization of Phenolic Compounds in Plants by Confocal Laser Scanning Microscopy. J. Exp. Bot. 1998, 49, 953–965. [Google Scholar] [CrossRef]
- Brillouet, J.-M.; Romieu, C.; Schoefs, B.; Solymosi, K.; Cheynier, V.; Fulcrand, H.; Verdeil, J.-L.; Conéjéro, G. The Tannosome Is an Organelle Forming Condensed Tannins in the Chlorophyllous Organs of Tracheophyta. Ann. Bot. 2013, 112, 1003–1014. [Google Scholar] [CrossRef]
- Ribeiro, C.; Marinho, C.; Teixeira, S. Uncovering the Neglected Floral Secretory Structures of Rhamnaceae and Their Functional and Systematic Significance. Plants 2021, 10, 736. [Google Scholar] [CrossRef]
- Osier, T.L.; Lindroth, R.L. Effects of Genotype, Nutrient Availability, and Defoliation on Aspen Phytochemistry and Insect Performance. J. Chem. Ecol. 2001, 27, 1289–1313. [Google Scholar] [CrossRef]
- Beckman, C.H. Phenolic-Storing Cells: Keys to Programmed Cell Death and Periderm Formation in Wilt Disease Resistance and in General Defence Responses in Plants? Physiol. Mol. Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Kefeli, V.I.; Kalevitch, M.V.; Borsari, B. Phenolic Cycle in Plants and Environment. | Journal of Cell & Molecular Biology | EBSCOhost. J. Cell Mol. Biol. 2003, 2, 13. [Google Scholar]
- Forkner, R.E.; Marquis, R.J.; Lill, J.T. Feeny Revisited: Condensed Tannins as Anti-Herbivore Defences in Leaf-Chewing Herbivore Communities of Quercus. Ecol. Entomol. 2004, 29, 174–187. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and Challenges of Tannins as an Alternative to In-Feed Antibiotics for Farm Animal Production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Rubert-Nason, K.F.; Yang, P.; Morrow, C.J.; Lindroth, R.L. Environment and Genotype Influence Quantitative and Qualitative Variation in Condensed Tannins in Aspen. J. Chem. Ecol. 2023, 49, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R. Tannins: The New Natural Antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 549–551. [Google Scholar] [CrossRef]
- Lovelock, C.E.; Clough, B.F.; Woodrow, I.E. Distribution and Accumulation of Ultraviolet-Radiation-Absorbing Compounds in Leaves of Tropical Mangroves. Planta 1992, 188, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, K.; Sárvári, É.; Keresztes, Á. Protection of Thylakoids against Combined Light and Drought by a Lumenal Substance in the Resurrection Plant Haberlea Rhodopensis. Ann. Bot. 2010, 105, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Stefanowska, M.; Kuraś, M.; Kacperska, A. Low Temperature-Induced Modifications in Cell Ultrastructure and Localization of Phenolics in Winter Oilseed Rape (Brassica napus L. Var. oleifera L.) Leaves. Ann. Bot. 2002, 90, 637–645. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin Complexation with Metal Ions and Its Implication on Human Health, Environment and Industry: An Overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Lang, T.; Wei, P.; Chen, X.; Fu, Y.; Tam, N.F.; Hu, Z.; Chen, Z.; Li, F.; Zhou, H. Microcosm Study on Allelopathic Effects of Leaf Litter Leachates and Purified Condensed Tannins from Kandelia Obovata on Germination and Growth of Aegiceras Corniculatum. Forests 2021, 12, 1000. [Google Scholar] [CrossRef]
- Harris, H.B.; Burns, R.E. Influence of Tannin Content on Preharvest Seed Germination in Sorghum > 1. Agron. J. 1970, 62, 835–836. [Google Scholar] [CrossRef]
- Furlan, C.M.; Motta, L.; Santos, D. Tannins: What Do They Represent in Plant Life? In Tannins: Types, Foods Containing, and Nutrition; Petridis, G.K., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2011; pp. 251–263. ISBN 978-1-61761-127-8. [Google Scholar]
- Demonsais, L.; Utz-Pugin, A.; Loubéry, S.; Lopez-Molina, L. Identification of Tannic Cell Walls at the Outer Surface of the Endosperm upon Arabidopsis Seed Coat Rupture. Plant J. 2020, 104, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Nierop, K.G.J.; Preston, C.M.; Verstraten, J.M. Linking the B Ring Hydroxylation Pattern of Condensed Tannins to C, N and P Mineralization. A Case Study Using Four Tannins. Soil Biol. Biochem. 2006, 38, 2794–2802. [Google Scholar] [CrossRef]
- Triebwasser, D.J.; Tharayil, N.; Preston, C.M.; Gerard, P.D. The Susceptibility of Soil Enzymes to Inhibition by Leaf Litter Tannins Is Dependent on the Tannin Chemistry, Enzyme Class and Vegetation History. New Phytol. 2012, 196, 1122–1132. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Kobatake, M.; Tanikawa, N.; Nakaji, T.; Ohashi, M.; Makita, N. Anatomical Patterns of Condensed Tannin in Fine Roots of Tree Species from a Cool-Temperate Forest. Ann. Bot. 2021, 128, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.P.; Evans, T.E.; Morris, P. The Effect of Plant Growth Regulators on Growth, Morphology and Condensed Tannin Accumulation in Transformed Root Cultures of Lotus Corniculatus. Plant Cell Tiss. Organ Cult. 1996, 44, 219–227. [Google Scholar] [CrossRef]
- Cabrera, A.; Martin, A. Genetics of Tannin Content and Its Relationship with Flower and Testa Colours in Vicia Faba. J. Agric. Sci. 1989, 113, 93–98. [Google Scholar] [CrossRef]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, J. Polyphenols: A First Evidence in the Synergism and Bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A Comparison between Pure Active Pharmaceutical Ingredients and Therapeutic Deep Eutectic Solvents: Solubility and Permeability Studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef]
- ElAchkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and Properties of Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Dietz, C.H.J.T.; Van Spronsen, J.; Kroon, M.C.; Gallucci, F.; Van Sint Annaland, M.; Tuinier, R. A Search for Natural Hydrophobic Deep Eutectic Solvents Based on Natural Components. ACS Sustain. Chem. Eng. 2019, 7, 2933–2942. [Google Scholar] [CrossRef]
- Florindo, C.; McIntosh, A.J.S.; Welton, T.; Branco, L.C.; Marrucho, I.M. A Closer Look into Deep Eutectic Solvents: Exploring Intermolecular Interactions Using Solvatochromic Probes. Phys. Chem. Chem. Phys. 2017, 20, 206–213. [Google Scholar] [CrossRef]
- Kovács, A.; Neyts, E.C.; Cornet, I.; Wijnants, M.; Billen, P. Modeling the Physicochemical Properties of Natural Deep Eutectic Solvents. ChemSusChem 2020, 13, 3789–3804. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Alonso, D.A.; Baeza, A.; Chinchilla, R.; Guillena, G.; Pastor, I.M.; Ramón, D.J. Deep Eutectic Solvents: The Organic Reaction Medium of the Century. Eur. J. Org. Chem. 2016, 2016, 612–632. [Google Scholar] [CrossRef]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Intensification of Biotransformations Using Deep Eutectic Solvents: Overview and Outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Pinho, S.P.; Coutinho, J.A.P. Insights into the Nature of Eutectic and Deep Eutectic Mixtures. J. Solut. Chem. 2019, 48, 962–982. [Google Scholar] [CrossRef]
- Mao, S.; Li, K.; Hou, Y.; Liu, Y.; Ji, S.; Qin, H.; Lu, F. Synergistic Effects of Components in Deep Eutectic Solvents Relieve Toxicity and Improve the Performance of Steroid Biotransformation Catalyzed by Arthrobacter Simplex. J. Chem. Technol. Biotechnol. 2018, 93, 2729–2736. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, J.-X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the Toxicity and Biodegradability of Deep Eutectic Solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and Biodegradability of Pure and Aqueous Mixtures of Deep Eutectic Solvents: Glyceline, Ethaline, and Reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Perrone, S.; Messa, F.; Troisi, L.; Salomone, A. N-, O- and S-Heterocycles Synthesis in Deep Eutectic Solvents. Molecules 2023, 28, 3459. [Google Scholar] [CrossRef]
- Rushell, E.; Kumar Tailor, Y.; Khandewal, S.; Verma, K.; Agarwal, M.; Kumar, M. Deep Eutectic Solvent Promoted Synthesis of Structurally Diverse Hybrid Molecules with Privileged Heterocyclic Substructures. New J. Chem. 2019, 43, 12462–12467. [Google Scholar] [CrossRef]
- Zwain, A.A.; Ahmad, I.; Khalaf Jebur Ali, R.; Kahtan, M.; Khdyair Hamad, A.; Abdulgader Hassan, E.; Asiri, M.; Ridha, B.M.; Alsalamy, A. Synthesis, Antioxidant, and Antimicrobial Evaluation of Novel 2,3-Dihydro-1H-Pyrazole-4-Carbonitrile Derivatives Using K2CO3/Glycerol as a Green Deep Eutectic Solvent. Front. Mater. 2023, 10, 1–10. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Application of Deep Eutectic Solvents in Biomass Pretreatment and Conversion. Green Energy Environ. 2019, 4, 95–115. [Google Scholar] [CrossRef]
- Vigier, K.D.O.; Chatel, G.; Jérôme, F. Contribution of Deep Eutectic Solvents for Biomass Processing: Opportunities, Challenges, and Limitations. ChemCatChem 2015, 7, 1250–1260. [Google Scholar] [CrossRef]
- Amesho, K.T.T.; Lin, Y.-C.; Mohan, S.V.; Halder, S.; Ponnusamy, V.K.; Jhang, S.-R. Deep Eutectic Solvents in the Transformation of Biomass into Biofuels and Fine Chemicals: A Review. Environ. Chem. Lett. 2023, 21, 183–230. [Google Scholar] [CrossRef]
- Cunha, S.C.; Fernandes, J.O. Extraction Techniques with Deep Eutectic Solvents. TrAC Trends Anal. Chem. 2018, 105, 225–239. [Google Scholar] [CrossRef]
- Duan, L.; Dou, L.-L.; Guo, L.; Li, P.; Liu, E.-H. Comprehensive Evaluation of Deep Eutectic Solvents in Extraction of Bioactive Natural Products. ACS Sustain. Chem. Eng. 2016, 4, 2405–2411. [Google Scholar] [CrossRef]
- Mitar, A.; Kučić Grgić, D.; Prlić Kardum, J. Ekstrakcija i Ispitivanje Stabilnosti Polifenola Komine Masline u Prirodnim Eutektičkim Otapalima. Kemija u industriji Časopis kemičara i kemijskih inženjera Hrvatske 2019, 68, 407–414. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; McKenzie, K.J.; Ryder, K.S. Electrodeposition of Zinc–Tin Alloys from Deep Eutectic Solvents Based on Choline Chloride. J. Electroanal. Chem. 2007, 599, 288–294. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ballantyne, A.; Harris, R.C.; Juma, J.A.; Ryder, K.S.; Forrest, G. A Comparative Study of Nickel Electrodeposition Using Deep Eutectic Solvents and Aqueous Solutions. Electrochim. Acta 2015, 176, 718–726. [Google Scholar] [CrossRef]
- Vukmirovic, M.B.; Adzic, R.R.; Akolkar, R. Copper Electrodeposition from Deep Eutectic Solvents—Voltammetric Studies Providing Insights into the Role of Substrate: Platinum vs Glassy Carbon. J. Phys. Chem. B 2020, 124, 5465–5475. [Google Scholar] [CrossRef]
- Qadr, G.; Awad, M.I.; Haji, K.; Jumaa, J.A.; Abdallah, H.H. Nickel Electrodeposition from Deep Eutectic Solvents Containing Copper Ions at a High Temperature. J. Mol. Liq. 2023, 378, 121584. [Google Scholar] [CrossRef]
- González-Rivera, J.; Mero, A.; Husanu, E.; Mezzetta, A.; Ferrari, C.; D’Andrea, F.; Bramanti, E.; Pomelli, C.S.; Guazzelli, L. Combining Acid-Based Deep Eutectic Solvents and Microwave Irradiation for Improved Chestnut Shell Waste Valorization. Green Chem. 2021, 23, 10101–10115. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Gonzalez, J.; Mezzetta, A.; Cabrera Ruiz, J.; D’Andrea, F.; Pomelli, C.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Tomasi, I.T.; Santos, S.C.R.; Boaventura, R.A.R.; Botelho, C.M.S. Optimization of Microwave-Assisted Extraction of Phenolic Compounds from Chestnut Processing Waste Using Response Surface Methodology. J. Clean. Prod. 2023, 395, 136452. [Google Scholar] [CrossRef]
- Živković, J.; Mujić, I.; Nikolić, G.; Vidović, S.; Mujić, A. Extraction and analysis of condensed tannins in castanea sativa mill. J. Cent. Eur. Agric. 2009, 10, 283–288. [Google Scholar]
- Aimone, C.; Grillo, G.; Boffa, L.; Giovando, S.; Cravotto, G. Tannin Extraction from Chestnut Wood Waste: From Lab Scale to Semi-Industrial Plant. Appl. Sci. 2023, 13, 2494. [Google Scholar] [CrossRef]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of Solid Wastes from Chestnut Industry Processing: Extraction and Optimization of Polyphenols, Tannins and Ellagitannins and Its Potential for Adhesives, Cosmetic and Pharmaceutical Industry. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef]
- Moccia, F.; Gallucci, N.; Giovando, S.; Zuorro, A.; Lavecchia, R.; D’Errico, G.; Panzella, L.; Napolitano, A. A Tunable Deep Eutectic Solvent-Based Processing for Valorization of Chestnut Wood Fiber as a Source of Ellagic Acid and Lignin. J. Environ. Chem. Eng. 2022, 10, 107773. [Google Scholar] [CrossRef]
- Smink, D.; Kersten, S.R.A.; Schuur, B. Process Development for Biomass Delignification Using Deep Eutectic Solvents. Conceptual Design Supported by Experiments. Chem. Eng. Res. Des. 2020, 164, 86–101. [Google Scholar] [CrossRef]
- Cui, J.; Fang, D.; Tian, X.; Peng, J.; Chen, D.; Xu, S.; Ma, L. Sustainable Conversion of Cottonseed Hulls to Valuable Proanthocyanidins through Ultrasound-Assisted Deep Eutectic Solvent Extraction. Ultrason. Sonochem. 2023, 100, 106605. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Tuning of Proanthocyanidin Extract’s Composition through Quaternary Eutectic Solvents Extraction. Antioxidants 2020, 9, 1124. [Google Scholar] [CrossRef]
- Cao, J.; Chen, L.; Li, M.; Cao, F.; Zhao, L.; Su, E. Efficient Extraction of Proanthocyanidin from Ginkgo Biloba Leaves Employing Rationally Designed Deep Eutectic Solvent-Water Mixture and Evaluation of the Antioxidant Activity. J. Pharm. Biomed. Anal. 2018, 158, 317–326. [Google Scholar] [CrossRef]
- Qa’dan, F.; Nahrstedt, A.; Schmidt, M.; Mansoor, K. Polyphenols from Ginkgo Biloba. Sci. Pharm. 2010, 78, 897–907. [Google Scholar] [CrossRef]
- Thilakarathna, W.P.D.W.; Rupasinghe, H.P.V. Optimization of the Extraction of Proanthocyanidins from Grape Seeds Using Ultrasonication-Assisted Aqueous Ethanol and Evaluation of Anti-Steatosis Activity In Vitro. Molecules 2022, 27, 1363. [Google Scholar] [CrossRef]
- Zhan, Y.; Yang, F.; Ye, Z.; Zhang, J.; Mao, Y.; Li, L. Extraction of Proanthocyanidins from Grape Seeds and Their Protective Effect on Spinal Cord Injury in Rats. Food Sci. Technol. 2022, 42, e44821. [Google Scholar] [CrossRef]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary Metabolites, Anthocyanins, and Hydrolyzable Tannins in the Pomegranate Fruit. Front. Plant Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Rajha, H.N.; Mhanna, T.; El Kantar, S.; El Khoury, A.; Louka, N.; Maroun, R.G. Innovative Process of Polyphenol Recovery from Pomegranate Peels by Combining Green Deep Eutectic Solvents and a New Infrared Technology. LWT 2019, 111, 138–146. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Boussetta, N.; Marina, M.L.; García, M.C.; Vorobiev, E. High Voltage Electrical Discharges Followed by Deep Eutectic Solvents Extraction for the Valorization of Pomegranate Seeds (Punica granatum L.). Innov. Food Sci. Emerg. Technol. 2022, 79, 103055. [Google Scholar] [CrossRef]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Impact of Eutectic Solvents Utilization in the Microwave Assisted Extraction of Proanthocyanidins from Grape Pomace. Molecules 2022, 27, 246. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.R.; van den Bruinhorst, A.; Kollau, L.J.B.M.; Kroon, M.C.; Binnemans, K. Degradation of Deep-Eutectic Solvents Based on Choline Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2019, 7, 13. [Google Scholar] [CrossRef]
- Vo, T.P.; Tran, T.Q.D.; Vo, L.T.V.; Nguyen, T.H.P.; Nguyen, V.K.; Dang, T.C.T.; Nguyen, L.G.K.; Chung, T.Q.; Nguyen, D.Q. Optimization of Ultrasonic-Assisted Extraction to Attain Tannins and Flavonoids from Spent Tea Leaves Using Natural Deep Eutectic Solvents. Chem. Eng. Commun. 2024, 211, 974–985. [Google Scholar] [CrossRef]
- Vo, T.P.; Tran, T.Q.D.; Phan, T.H.; Huynh, H.D.; Vo, T.T.Y.; Vo, N.M.K.; Ha, M.P.; Le, T.N.; Nguyen, D.Q. Ultrasonic-Assisted and Enzymatic-Assisted Extraction to Recover Tannins, Flavonoids, and Terpenoids from Used Tea Leaves Using Natural Deep Eutectic Solvents. Int. J. Food Sci. Technol. 2023, 58, 5855–5864. [Google Scholar] [CrossRef]
- Teslić, N.; Santos, F.; Oliveira, F.; Stupar, A.; Pojić, M.; Mandić, A.; Pavlić, B.; Kljakić, A.C.; Duarte, A.R.C.; Paiva, A.; et al. Simultaneous Hydrolysis of Ellagitannins and Extraction of Ellagic Acid from Defatted Raspberry Seeds Using Natural Deep Eutectic Solvents (NADES). Antioxidants 2022, 11, 254. [Google Scholar] [CrossRef]
- Pal, C.B.T.; Jadeja, G.C. Deep Eutectic Solvent-Based Extraction of Polyphenolic Antioxidants from Onion (Allium cepa L.) Peel. J. Sci. Food Agric. 2019, 99, 1969–1979. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Dhumal, S.; Singh, S.; Pandiselvam, R.; Rais, N.; Natta, S.; Senapathy, M.; Sinha, N.; et al. Onion (Allium cepa L.) Peel: A Review on the Extraction of Bioactive Compounds, Its Antioxidant Potential, and Its Application as a Functional Food Ingredient. J. Food Sci. 2022, 87, 4289–4311. [Google Scholar] [CrossRef] [PubMed]
- Ciardi, M.; Ianni, F.; Sardella, R.; Di Bona, S.; Cossignani, L.; Germani, R.; Tiecco, M.; Clementi, C. Effective and Selective Extraction of Quercetin from Onion (Allium cepa L.) Skin Waste Using Water Dilutions of Acid-Based Deep Eutectic Solvents. Materials 2021, 14, 6465. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Zhang, Y.; Zheng, Y.; Li, Y. Temperature-Responsive Deep Eutectic Solvents as Eco-Friendly and Recyclable Media for Microwave Extraction of Flavonoid Compounds from Waste Onion (Allium cepa L.) Skins. Biomass Conv. Bioref. 2024, 14, 3729–3738. [Google Scholar] [CrossRef]
- Riyamol; Jeevitha, G.C. Microwave and Ultrasound-Assisted Natural Deep Eutectic Solvents-Based Extraction of Pectin from Onion Peel Wastes. CyTA-J. Food 2024, 22, 2311215. [Google Scholar] [CrossRef]
- Bozinou, E.; Palaiogiannis, D.; Athanasiadis, V.; Chatzilazarou, A.; Lalas, S.I.; Makris, D.P. Glycerol-Based Deep Eutectic Solvents for Simultaneous Organosolv Treatment/Extraction: High-Performance Recovery of Antioxidant Polyphenols from Onion Solid Wastes. Sustainability 2022, 14, 15715. [Google Scholar] [CrossRef]
- Sukor, N.F.; Selvam, V.P.; Jusoh, R.; Kamarudin, N.S.; Rahim, S.A. Intensified DES Mediated Ultrasound Extraction of Tannic Acid from Onion Peel. J. Food Eng. 2021, 296, 110437. [Google Scholar] [CrossRef]
- Zengin, G.; de la Luz Cádiz-Gurrea, M.; Fernández-Ochoa, Á.; Leyva-Jiménez, F.J.; Carretero, A.S.; Momotko, M.; Yildiztugay, E.; Karatas, R.; Jugreet, S.; Mahomoodally, M.F.; et al. Selectivity Tuning by Natural Deep Eutectic Solvents (NADESs) for Extraction of Bioactive Compounds from Cytinus Hypocistis—Studies of Antioxidative, Enzyme-Inhibitive Properties and LC-MS Profiles. Molecules 2022, 27, 5788. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-Bioactivity and Extraction Perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Daurtseva, A.V.; Pozharitskaya, O.N.; Flisyuk, E.V.; Shikov, A.N. Natural Deep Eutectic Solvents as Alternatives for Extracting Phlorotannins from Brown Algae. Pharm. Chem. J. 2019, 53, 243–247. [Google Scholar] [CrossRef]
- Schuh, L.; Reginato, M.; Florêncio, I.; Falcao, L.; Boron, L.; Gris, E.F.; Mello, V.; Báo, S.N. From Nature to Innovation: The Uncharted Potential of Natural Deep Eutectic Solvents. Molecules 2023, 28, 7653. [Google Scholar] [CrossRef]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus Vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Shevyrin, V.A.; Kovaleva, E.G.; Flisyuk, E.V.; Shikov, A.N. Optimization of Extraction of Phlorotannins from the Arctic Fucus Vesiculosus Using Natural Deep Eutectic Solvents and Their HPLC Profiling with Tandem High-Resolution Mass Spectrometry. Mar. Drugs 2023, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.J.; Jokić, S.; Jerković, I.; Molnar, M. Optimization of Deep Eutectic Solvent Extraction of Phenolic Acids and Tannins from Alchemilla vulgaris L. Plants 2022, 11, 11040474. [Google Scholar] [CrossRef] [PubMed]
- Sieniawska, E. Activities of Tannins—From In Vitro Studies to Clinical Trials. Nat. Prod. Commun. 2015, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Chou, I.-W.; Hung, M.-C. Natural Tannins as Anti-SARS-CoV-2 Compounds. Int. J. Biol. Sci. 2022, 18, 4669–4676. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.G. Antinutritional Effects of Condensed and Hydrolyzable Tannins. Basic Life Sci 1992, 59, 693–698. [Google Scholar] [CrossRef] [PubMed]
- Farha, A.K.; Yang, Q.-Q.; Kim, G.; Li, H.-B.; Zhu, F.; Liu, H.-Y.; Gan, R.-Y.; Corke, H. Tannins as an Alternative to Antibiotics. Food Biosci. 2020, 38, 100751. [Google Scholar] [CrossRef]
- Horn, R.C.; Vargas, V.M.F. Antimutagenic Activity of Extracts of Natural Substances in the Salmonella/Microsome Assay. Mutagenesis 2003, 18, 113–118. [Google Scholar] [CrossRef]
- Masota, N.E.; Ohlsen, K.; Schollmayer, C.; Meinel, L.; Holzgrabe, U. Isolation and Characterization of Galloylglucoses Effective against Multidrug-Resistant Strains of Escherichia Coli and Klebsiella Pneumoniae. Molecules 2022, 27, 5045. [Google Scholar] [CrossRef]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin Profile, Antioxidant Properties, and Antimicrobial Activity of Extracts from Two Mediterranean Species of Parasitic Plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Hatano, T.; Edamatsu, R.; Hiramatsu, M.; Mori, A.; Fujita, Y.; Yasuhara, T.; Yoshida, T.; Okuda, T. Effects of the Interaction of Tannins with Co-Existing Substances. VI.: Effects of Tannins and Related Polyphenols on Superoxide Anion Radical, and on 1, 1-Diphenyl-2-Picrylhydrazyl Radical. Chem. Pharm. Bull. 1989, 37, 2016–2021. [Google Scholar] [CrossRef]
- Chen, R.H.; Yang, L.J.; Hamdoun, S.; Chung, S.K.; Lam, C.W.; Zhang, K.X.; Guo, X.; Xia, C.; Law, B.Y.K.; Wong, V.K.W. 1,2,3,4,6-Pentagalloyl Glucose, a RBD-ACE2 Binding Inhibitor to Prevent SARS-CoV-2 Infection. Front. Pharmacol. 2021, 12, 634176. [Google Scholar] [CrossRef] [PubMed]
- Son, S.-Y.; Choi, J.-H.; Kim, E.-B.; Yin, J.; Seonu, S.-Y.; Jin, S.-Y.; Oh, J.-Y.; Lee, M.-W. Chemopreventive Activity of Ellagitannins from Acer Pseudosieboldianum (Pax) Komarov Leaves on Prostate Cancer Cells. Plants 2023, 12, 1047. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Yoon, K.H.; Yin, J.; Le, T.T.; Ahn, H.S.; Yoon, S.H.; Lee, M.W. Antioxidative and Anti-Inflammatory Activities of Galloyl Derivatives and Antidiabetic Activities of Acer Ginnala. Evid.-Based Complement. Altern. Med. Ecam 2017, 2017, 6945912. [Google Scholar] [CrossRef] [PubMed]
- Honma, A.; Koyama, T.; Yazawa, K. Anti-Hyperglycaemic Effects of the Japanese Red Maple Acer Pycnanthum and Its Constituents the Ginnalins B and C. J. Enzym. Inhib. Med. Chem. 2011, 26, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase Inhibitors Isolated from Medicinal Plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Amen, Y.M.; Marzouk, A.M.; Zaghloul, M.G.; Afifi, M.S. A New Acylated Flavonoid Tetraglycoside with Anti-Inflammatory Activity from Tipuana Tipu Leaves. Nat. Prod. Res. 2015, 29, 511–517. [Google Scholar] [CrossRef]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and Anti-Biofilm Activity of Tannic Acid against Staphylococcus Aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Reyes, A.W.B.; Hong, T.G.; Hop, H.T.; Arayan, L.T.; Huy, T.X.N.; Min, W.; Lee, H.J.; Lee, K.S.; Kim, S. The in Vitro and in Vivo Protective Effects of Tannin Derivatives against Salmonella Enterica Serovar Typhimurium Infection. Microb. Pathog. 2017, 109, 86–93. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Kim, D.H.; Yoo, J.-S.; Jo, E.S.; Rugamba, A.; Jang, K.-J.; Yang, Y.M. Tannic Acid Inhibits Non-Small Cell Lung Cancer (NSCLC) Stemness by Inducing G0/G1 Cell Cycle Arrest and Intrinsic Apoptosis. Anticancer Res. 2020, 40, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Liang, Y.; Jiang, B.; Li, X.; Xun, H.; He, W.; Lau, H.T.; Ma, X. Apoptotic Effect of Tannic Acid on Fatty Acid Synthase Over-Expressed Human Breast Cancer Cells. Tumor Biol. 2016, 37, 2137–2143. [Google Scholar] [CrossRef]
- Geng, N.; Zheng, X.; Wu, M.; Yang, L.; Li, X.; Chen, J. Tannic Acid Synergistically Enhances the Anticancer Efficacy of Cisplatin on Liver Cancer Cells through Mitochondria-mediated Apoptosis. Oncol. Rep. 2019, 42, 2108–2116. [Google Scholar] [CrossRef] [PubMed]
- Nagesh, P.K.B.; Hatami, E.; Chowdhury, P.; Kashyap, V.K.; Khan, S.; Hafeez, B.B.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic Acid Induces Endoplasmic Reticulum Stress-Mediated Apoptosis in Prostate Cancer. Cancers 2018, 10, 68. [Google Scholar] [CrossRef] [PubMed]
- Darvin, P.; Baeg, S.J.; Joung, Y.H.; Sp, N.; Kang, D.Y.; Byun, H.J.; Park, J.U.; Yang, Y.M. Tannic Acid Inhibits the Jak2/STAT3 Pathway and Induces G1/S Arrest and Mitochondrial Apoptosis in YD-38 Gingival Cancer Cells. Int. J. Oncol. 2015, 47, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mori, K.; Hatano, T.; Okumura, T.; Uehara, I.; Komagoe, K.; Fujita, Y.; Okuda, T. Studies on Inhibition Mechanism of Autoxidation by Tannins and Flavonoids. V. Radical-Scavenging Effects of Tannins and Related Polyphenols on 1, 1-Diphenyl-2-Picrylhydrazyl Radical. Chem. Pharm. Bull. 1989, 37, 1919–1921. [Google Scholar] [CrossRef]
- Okuda, T.; Kimura, Y.; Yoshida, T.; Hatano, T.; Okuda, H.; Arichi, S. Studies on the Activities of Tannins and Related Compounds from Medicinal Plants and Drugs. I. Inhibitory Effects on Lipid Peroxidation in Mitochondria and Microsomes of Liver. Chem. Pharm. Bull. 1983, 31, 1625–1631. [Google Scholar] [CrossRef]
- Mun, S.-H.; Kang, O.-H.; Kong, R.; Zhou, T.; Kim, S.-A.; Shin, D.-W.; Kwon, D.-Y. Punicalagin Suppresses Methicillin Resistance of Staphylococcus Aureus to Oxacillin. J. Pharmacol. Sci. 2018, 137, 317–323. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Wu, Q.; Zheng, Z.; Liu, P.; Li, G.; Peng, X.; Xia, X. Antimicrobial Activity of Punicalagin Against Staphylococcus Aureus and Its Effect on Biofilm Formation. Foodborne Pathog. Dis. 2017, 14, 282–287. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial Activity of 10 Different Plant Polyphenols against Bacteria Causing Food-Borne Disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Gu, H.-M.; Li, X.-Z.; Xu, Z.-N.; Chen, Y.-S.; Li, Y. Anti-Helicobacter Pylori Compounds from the Ethanol Extracts of Geranium Wilfordii. J. Ethnopharmacol. 2013, 147, 204–207. [Google Scholar] [CrossRef]
- Salih, E.Y.A.; Julkunen-Tiitto, R.; Lampi, A.-M.; Kanninen, M.; Luukkanen, O.; Sipi, M.; Lehtonen, M.; Vuorela, H.; Fyhrquist, P. Terminalia Laxiflora and Terminalia Brownii Contain a Broad Spectrum of Antimycobacterial Compounds Including Ellagitannins, Ellagic Acid Derivatives, Triterpenes, Fatty Acids and Fatty Alcohols. J. Ethnopharmacol. 2018, 227, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Kolodzeij, H.; Kayser, O.; Latté, K.P.; Ferreira, D. Evaluation of the Antimicrobial Potency of Tannins and Related Compounds Using the Microdilution Broth Method. Planta Med. 2007, 65, 444–446. [Google Scholar] [CrossRef]
- Fogliani, B.; Raharivelomanana, P.; Bianchini, J.-P.; Bouraı¨ma-Madjèbi, S.; Hnawia, E. Bioactive Ellagitannins from Cunonia Macrophylla, an Endemic Cunoniaceae from New Caledonia. Phytochemistry 2005, 66, 241–247. [Google Scholar] [CrossRef]
- Miyasaki, Y.; Rabenstein, J.D.; Rhea, J.; Crouch, M.-L.; Mocek, U.M.; Kittell, P.E.; Morgan, M.A.; Nichols, W.S.; Van Benschoten, M.M.; Hardy, W.D.; et al. Isolation and Characterization of Antimicrobial Compounds in Plant Extracts against Multidrug-Resistant Acinetobacter Baumannii. PLoS ONE 2013, 8, 1–8. [Google Scholar] [CrossRef]
- Vu, T.T.; Kim, H.; Tran, V.K.; Vu, H.D.; Hoang, T.X.; Han, J.W.; Choi, Y.H.; Jang, K.S.; Choi, G.J.; Kim, J.-C. Antibacterial Activity of Tannins Isolated from Sapium Baccatum Extract and Use for Control of Tomato Bacterial Wilt. PLoS ONE 2017, 12, 1–12. [Google Scholar] [CrossRef]
- Štumpf, S.; Hostnik, G.; Langerholc, T.; Pintarič, M.; Kolenc, Z.; Bren, U. The Influence of Chestnut Extract and Its Components on Antibacterial Activity against Staphylococcus Aureus. Plants 2023, 12, 2043. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T.; Koshiyama, I.; Fukushima, D. Antioxidative Properties of Procyanidins B-1 and B-3 from Azuki Beans in Aqueous Systems. Agric. Biol. Chem. 1988, 52, 2717–2722. [Google Scholar] [CrossRef]
- Gao, W.; Yu, T.; Li, G.; Shu, W.; Jin, Y.; Zhang, M.; Yu, X. Antioxidant Activity and Anti-Apoptotic Effect of the Small Molecule Procyanidin B1 in Early Mouse Embryonic Development Produced by Somatic Cell Nuclear Transfer. Molecules 2021, 26, 6150. [Google Scholar] [CrossRef]
- Fujii, W.; Toda, K.; Kawaguchi, K.; Kawahara, S.; Katoh, M.; Hattori, Y.; Fujii, H.; Makabe, H. Syntheses of Prodelphinidin B3 and C2, and Their Antitumor Activities through Cell Cycle Arrest and Caspase-3 Activation. Tetrahedron 2013, 69, 3543–3550. [Google Scholar] [CrossRef]
- Dinis, L.-T.; Oliveira, M.M.; Almeida, J.; Costa, R.; Gomes-Laranjo, J.; Peixoto, F. Antioxidant Activities of Chestnut Nut of Castanea Sativa Mill. (Cultivar ‘Judia’) as Function of Origin Ecosystem. Food Chem. 2012, 132, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; De Bruyne, T.; Hermans, N.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in Health Care: Current and New Trends. Curr. Med. Chem. 2004, 11, 1345–1359. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Wang, G.; Wang, Y.; Chen, B.; Sun, Y.; Mo, W.; Li, G.; Huang, Y. Condensed Tannins Enhanced Antioxidant Capacity and Hypoxic Stress Survivability but Not Growth Performance and Fatty Acid Profile of Juvenile Japanese Seabass (Lateolabrax japonicus). Anim. Feed Sci. Technol. 2020, 269, 114671. [Google Scholar] [CrossRef]
- Iglesias, J.; Pazos, M.; Torres, J.L.; Medina, I. Antioxidant Mechanism of Grape Procyanidins in Muscle Tissues: Redox Interactions with Endogenous Ascorbic Acid and α-Tocopherol. Food Chem. 2012, 134, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Borisova, M.P.; Kataev, A.A.; Sivozhelezov, V.S. Action of Tannin on Cellular Membranes: Novel Insights from Concerted Studies on Lipid Bilayers and Native Cells. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Zarin, M.A.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, Antimicrobial and Cytotoxic Potential of Condensed Tannins from Leucaena Leucocephala Hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar] [CrossRef]
- Ambreen, M.; Mirza, S.A. Evaluation of Anti-Inflammatory and Wound Healing Potential of Tannins Isolated from Leaf Callus Cultures of Achyranthes Aspera and Ocimum Basilicum. Pak. J. Pharm. Sci. 2020, 33, 361–369. [Google Scholar] [PubMed]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.-W.; Kang, M.-C.; Kim, E.-A.; Jeon, Y.-J. Anti-Inflammatory Activity of Phlorotannin-Rich Fermented Ecklonia Cava Processing by-Product Extract in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Pallarès, V.; Cedó, L.; Castell-Auví, A.; Pinent, M.; Ardévol, A.; Arola, L.; Blay, M. Effects of Grape Seed Procyanidin Extract over Low-Grade Chronic Inflammation of Obese Zucker Fa/Fa Rats. Food Res. Int. 2013, 53, 319–324. [Google Scholar] [CrossRef]
- Stefanović, O.D. Synergistic Activity of Antibiotics and Bioactive Plant Extracts: A Study Against Gram-Positive and Gram-Negative Bacteria. In Bacterial Pathogenesis and Antibacterial Control; IntechOpen: London, UK, 2018; p. 154. ISBN 978-1-78923-161-8. [Google Scholar]
- Marrone, G.; Di Lauro, M.; Izzo, F.; Cornali, K.; Masci, C.; Vita, C.; Occhiuto, F.; Di Daniele, N.; De Lorenzo, A.; Noce, A. Possible Beneficial Effects of Hydrolyzable Tannins Deriving from Castanea sativa L. in Internal Medicine. Nutrients 2024, 16, 45. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and Antiviral Activity of Hydrolysable Tannins. Mini Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Cha, S.-H.; Cho, S.; Park, Y. Tannic Acid-Mediated Green Synthesis of Antibacterial Silver Nanoparticles. Arch. Pharmacal Res. 2016, 39, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Kamel, R.; Elkasabgy, N.A.; Shao, P.; Farag, M.A. Recent Advances in Tannic Acid (Gallotannin) Anticancer Activities and Drug Delivery Systems for Efficacy Improvement; A Comprehensive Review. Molecules 2021, 26, 1486. [Google Scholar] [CrossRef] [PubMed]
- Dakheel, M.M.; Alkandari, F.A.H.; Mueller-Harvey, I.; Woodward, M.J.; Rymer, C. Antimicrobial in Vitro Activities of Condensed Tannin Extracts on Avian Pathogenic Escherichia coli. Lett. Appl. Microbiol. 2020, 70, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Kleszcz, R.; Majchrzak-Celińska, A.; Baer-Dubowska, W. Tannins in Cancer Prevention and Therapy. Br. J. Pharmacol. 2023. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-A.; Choi, H.S.; Ryu, E.-S.; Ko, J.; Shin, H.-S.; Lee, J.-M.; Chung, H.; Jun, E.; Oh, E.-S.; Kang, D.-H. Tannic Acid Attenuates the Formation of Cancer Stem Cells by Inhibiting NF-κB-Mediated Phenotype Transition of Breast Cancer Cells. Am. J. Cancer Res. 2019, 9, 1664–1681. [Google Scholar] [PubMed]
- Phiwchai, I.; Yuensook, W.; Sawaengsiriphon, N.; Krungchanuchat, S.; Pilapong, C. Tannic Acid (TA): A Molecular Tool for Chelating and Imaging Labile Iron. Eur. J. Pharm. Sci. 2018, 114, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Hartomo, T.B.; Pham, T.V.H.; Lee, M.J.; Yamamoto, T.; Morikawa, S.; Hasegawa, D.; Takeda, H.; Kawasaki, K.; Kosaka, Y.; et al. Epigallocatechin Gallate Inhibits Sphere Formation of Neuroblastoma BE(2)-C Cells. Environ. Health Prev. Med. 2012, 17, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, X.-Q.; Zhang, Q.; Zhu, J.-Y.; Li, Y.; Xie, C.-F.; Li, X.-T.; Wu, J.-S.; Geng, S.-S.; Zhong, C.-Y.; et al. (−)-Epigallocatechin-3-Gallate Inhibits Colorectal Cancer Stem Cells by Suppressing Wnt/β-Catenin Pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Li, J.; Zhang, Q.; Zhong, P.; Teng, T.; Chen, M.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-Gallate Inhibits the Growth and Increases the Apoptosis of Human Thyroid Carcinoma Cells through Suppression of EGFR/RAS/RAF/MEK/ERK Signaling Pathway. Cancer Cell Int. 2019, 19, 43. [Google Scholar] [CrossRef]
- Li, T.-M.; Chen, G.-W.; Su, C.-C.; Lin, J.-G.; Yeh, C.-C.; Cheng, K.-C.; Chung, J.-G. Ellagic Acid Induced P53/P21 Expression, G1 Arrest and Apoptosis in Human Bladder Cancer T24 Cells. Anticancer Res. 2005, 25, 971–979. [Google Scholar] [PubMed]
- Jensen, J.D.; Dunn, J.H.; Luo, Y.; Liu, W.; Fujita, M.; Dellavalle, R.P. Ellagic Acid Inhibits Melanoma Growth In Vitro. Dermatol. Rep. 2011, 3, e36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, G.; Wei, J.; Dang, S.; Yu, X.; Ding, L.; Shang, C.; Zhang, H.; Zhang, Z.; Chen, H.; et al. Ellagic Acid Induces Cell Cycle Arrest and Apoptosis via the TGF-β1/Smad3 Signaling Pathway in Human Colon Cancer HCT-116 Cells. Oncol. Rep. 2020, 44, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Buchholz, T.A.; Aggarwal, B.B. Chemosensitization and Radiosensitization of Tumors by Plant Polyphenols. Antioxid. Redox Signal. 2005, 7, 1630–1647. [Google Scholar] [CrossRef] [PubMed]
- Xue, P.; Zhang, G.; Zhang, J.; Ren, L. Synergism of Ellagic Acid in Combination with Radiotherapy and Chemotherapy for Cancer Treatment. Phytomedicine 2022, 99, 153998. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhang, J.; Huang, J.; Yu, X.; Cheng, J.; Shang, Q.; Hu, Y.; Liu, C.; Zhang, M.; Hu, L.; et al. Self-Healing, Antibacterial, and 3D-Printable Polymerizable Deep Eutectic Solvents Derived from Tannic Acid. ACS Sustain. Chem. Eng. 2022, 10, 7954–7964. [Google Scholar] [CrossRef]
- Picchio, M.L.; Minudri, D.; Mantione, D.; Criado-Gonzalez, M.; Guzmán-González, G.; Schmarsow, R.; Müller, A.J.; Tomé, L.C.; Minari, R.J.; Mecerreyes, D. Natural Deep Eutectic Solvents Based on Choline Chloride and Phenolic Compounds as Efficient Bioadhesives and Corrosion Protectors. ACS Sustain. Chem. Eng. 2022, 10, 8135–8142. [Google Scholar] [CrossRef]
- de Lacalle, J.L.; Gallastegui, A.; Olmedo-Martínez, J.L.; Moya, M.; Lopez-Larrea, N.; Picchio, M.L.; Mecerreyes, D. Multifunctional Ionic Polymers from Deep Eutectic Monomers Based on Polyphenols. ACS Macro Lett. 2023, 12, 125–132. [Google Scholar] [CrossRef]

| Compounds | Activity | Reference |
|---|---|---|
| Hydrolysable Tannins | ||
| Simple Gallic Acid Derivatives | ||
| 1,2,3,4,6-penta-galloyl-d-glucopyranoside | Antibacterial activity against Escherichia coli (MIC = 32 μg/mL) and Klebsiella pneumoniae (MIC = 32 μg/mL) | [220] |
| Antioxidant activity determined (TEAC-ABTS = 11.2 ± 0.8; FRAP = 8.4 ± 0.4; DPPH-scavenging = 6.2 ± 0.6; ORAC-PYR = 9.1 ± 1.4), results expressed as Trolox Equivalents (mM TE/g). | [221] | |
| Antioxidant activity by inhibiting superoxide anion radical in the hypoxanthine-xanthine oxidase system (IC50 = 3.4 μM) | [222] | |
| Antiviral activity against the COVID-19 by blockade the fusion of SARS-CoV-2 spike-RBD to ACE2 receptors (IC50 = 46.9 μM) | [223] | |
| Chemopreventive activity as anti-proliferative activity in androgen-independent prostate cancer PC-3 cells (IC50 = 242 µM) and in androgen-dependent LNCaP cells (IC50 = 0.81 µM) | [224] | |
| Ginnalin B | Antioxidant activity by DPPH radical scavenging (IC50 = 12.14 µM), superoxide scavenging (IC50 = 20.80 µM) and inhibition of nitric oxide production (IC50 = 100 µM) | [225] |
| Anti-inflammatory activity as α-glucosidase inhibition (IC50 = 38.5 µM) | [226] | |
| Acertannin | Antioxidant activity by DPPH radical scavenging (IC50 = 6.87 µM), superoxide scavenging (IC50 = 2.96 µM) and inhibition of nitric oxide production (IC50 = 100 µM) | [225] |
| Anti-inflammatory activity as α-glucosidase inhibition (IC50 = 88.42 µM) | [227] | |
| Maplexin D | Antioxidant activity by DPPH radical scavenging (IC50 = 6.92 µM), superoxide scavenging (IC50 = 3.01 µM), and inhibition of nitric oxide production (IC50 = 100 µM) | [225] |
| Maplexin E | Antioxidant activity by DPPH radical scavenging (IC50 = 5.72 µM), superoxide scavenging (IC50 = 2.83 µM) and inhibition of nitric oxide production (IC50 = 36.08 µM) | [225] |
| Anti-inflammatory activity as α-glucosidase inhibition (IC50 = 8.26 μM) | [227] | |
| Anti-inflammatory activity in rat paw oedema (35.3% of inhibition of oedema) | [228] | |
| Gallotannins | ||
| Tannic acid | Antibacterial activity against 50 methicillin-sensitive S. aureus and 50 methicillin-resistant S. aureus, (MIC from 40–160 μg/mL) | [229] |
| Antibacterial activity against Salmonella enterica serovar Typhimurium, 40 µg/mL showed complete inhibition of bacterial growth | [230] | |
| Anticancer activity in non-small-cell lung carcinoma (NSCLC) category with no significant toxicity effects on human bronchial epithelial cells (IC50 = 40–60 μM at 24 h, 20–40 μM at 48 h) | [231] | |
| Anticancer activity in breast cancer MDA-MB-231 cells (IC50 = 2.5 μM) and in MCF-7 cells (IC50 = 4.0 μM) | [232] | |
| Anticancer activity in preventing liver cancer progression in vitro through inducing the mitochondrial-mediated apoptosis in HepG2 cells (IC50 = 360 μM) | [233] | |
| Anticancer activity in reducing cellular growth, clonogenic, invasive, and migratory capacities of pancreatic cancer cells C4-2 (IC50 = 2.92 μM), DU 145 (IC50 = 8.95 μM) and PC-3 cells (IC50 = 8.53 μM) | [234] | |
| Anticancer activity in gingival squamous cell carcinoma (GSCC) cellular proliferation in vitro (IC50 = 50 μM) | [235] | |
| Octagalloylglucose | Anthelmintic activity tested in vitro against the egg hatching of Haemonchus contortus | [29] |
| Ellagitannins | ||
| Pedunculagin | Antioxidant activity by DPPH radical scavenging (IC50 = 56 μM) | [236] |
| Antioxidant activity by inhibiting superoxide anion radical in the hypoxanthine-xanthine oxidase system (IC50 = 2.8 μM) | [222] | |
| Most potent antioxidant activity by inhibiting lipid peroxidation in rat liver mitochondria and in rat liver microsomes (IC50 = 1.2 μM) | [237] | |
| Punicalagin | Antibacterial activity against the six MRSA strains (MIC from 31.25–62.5 μg/mL) | [238] |
| Antibacterial activity against Staphylococcus aureus (MIC = 250 μg/mL) | [239] | |
| Antibacterial activity against Vibrio vulnificus (MIC = 71 µg/mL) | [240] | |
| Antiviral effect as inhibitory effect on enveloped viruses known to use glycosaminoglycans for entry, including HCMV (IC50 = 16.76 µM), HCV (IC50 = 16.72 µM), DENV-2 (IC50 = 7.86 µM), MV (IC50 = 25.49 µM), and RSV (IC50 = 0.54 µM) | ||
| Corilagin | Antibacterial activity against Helicobacter pylori, (MIC = 8 μg/mL) | [241] |
| Antibacterial activity against Mycobacterium smegmatis, (MIC = 500 µg/mL) | [242] | |
| Weak antibacterial activities against Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa and Proteus mirabilis (MICs from 1000–2000 µg/ mL) | [243] | |
| Chebulagic acid | Antimicrobial activity against plant pathogen Erwinia carotovora (19 mm), human pathogens Staphylococcus aureus (11 mm) and Corynebacterium accolans (10 mm) and human pathogenic yeast Candida albicans (12 mm), expressed as inhibition zone diameter (mm) for 100 µg of compound | [244] |
| Anti-inflammatory activity by xanthine oxidase inhibition (IC50 = 48 µM) | [244] | |
| Weak antibacterial activity against multidrug-resistant Acinetobacter baumannii (MIC = 1000 µg/mL) | [245] | |
| Antibacterial activities against Ralstonia solanacearum and Xanthomonas arboricola pv. pruni (MIC = 52 μg/mL) | [246] | |
| Antiviral effect as inhibitory effect on enveloped viruses known to use glycosaminoglycans for entry, including HCMV (IC50 = 25 µM), HCV (IC50 = 12 µM), DENV-2 (IC50 = 13.11 µM), MV (IC50 = 34 µM), and RSV (IC50 = 0.38 µM) | ||
| Geraniin | Antibacterial activity against 20 strains of Staphylococcus aureus (MIC = 190 µg/mL), 26 strains of the genus Salmonella (MIC = 1085 µg/mL), Vibrio vulnificus (MIC = 120 µg/mL) | [240] |
| Chemopreventive activity as anti-proliferative activity in androgen-independent prostate cancer PC-3 cells (IC50 = 271 µM) and in androgen-dependent LNCaP cells (IC50 = 0.36 µM) | [224] | |
| Antioxidant activity by inhibiting superoxide anion radical in the hypoxanthine-xanthine oxidase system (IC50 = 3.0 μM) | [222] | |
| Vescalin | Antibacterial activity against Staphylococcus aureus (MIC = 300 µg/mL) | [247] |
| Vescalagin | Antibacterial activity against Staphylococcus aureus (MIC = 400 µg/mL) | [247] |
| Castalin | Antibacterial activity against Staphylococcus aureus (MIC = 533 µg/mL) | [247] |
| Castalagin | Strong antibacterial activity against 20 strains of Staphylococcus aureus (MIC = 114 µg/mL), 26 strains of the genus Salmonella (MIC = 322 µg/mL) and Vibrio vulnificus (MIC = 83 µg/mL) | [240] |
| Komaniin | Chemopreventive activity as anti-proliferative activity in androgen-independent prostate cancer PC-3 cells (IC50 = 179.2 µM) and in androgen-dependent LNCaP cells (IC50 = 0.91 µM) | [224] |
| Punicafolin | Chemopreventive activity as anti-proliferative activity in androgen-independent prostate cancer PC-3 cells (IC50 = 314 µM) and in androgen-dependent LNCaP cells (IC50 = 0.68 µM) | [224] |
| Granatin B | Chemopreventive activity as anti-proliferative activity in androgen-independent prostate cancer PC-3 cells (IC50 = 157 µM) and in androgen-dependent LNCaP cells (IC50 = 2.06 µM) | [224] |
| Mallotusinic acid | Chemopreventive activity as anti-proliferative activity in androgen-independent prostate cancer PC-3 cells (IC50 = 308 µM) and in androgen-dependent LNCaP cells (IC50 = 3.48 µM) | [224] |
| Proanthocyanidins | ||
| Procyanidin B1 | Strong antioxidant activity by β-carotene destruction (0.126 mol/min) | [248] |
| DNA damage repairability at a concentration of 50 μM promotes the development of mouse embryos in vitro by increasing OGG1 mRNA and protein expression in blastocysts | [249] | |
| Procyanidin B3 | Strong antioxidant activity by β-carotene destruction (0.126 mol/min) | [248] |
| Prodelphinidin B3 and C2 | Anticancer activity through cell cycle arrest and caspase-3 activation in PC-3 prostate cancer cells (IC50 ˂ 50 µM) | [250] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molnar, M.; Jakovljević Kovač, M.; Pavić, V. A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents. Molecules 2024, 29, 2615. https://doi.org/10.3390/molecules29112615
Molnar M, Jakovljević Kovač M, Pavić V. A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents. Molecules. 2024; 29(11):2615. https://doi.org/10.3390/molecules29112615
Chicago/Turabian StyleMolnar, Maja, Martina Jakovljević Kovač, and Valentina Pavić. 2024. "A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents" Molecules 29, no. 11: 2615. https://doi.org/10.3390/molecules29112615
APA StyleMolnar, M., Jakovljević Kovač, M., & Pavić, V. (2024). A Comprehensive Analysis of Diversity, Structure, Biosynthesis and Extraction of Biologically Active Tannins from Various Plant-Based Materials Using Deep Eutectic Solvents. Molecules, 29(11), 2615. https://doi.org/10.3390/molecules29112615











