4-Vinyl Guaiacol: A Key Intermediate for Biobased Polymers
Abstract
1. Introduction
2. Results and Discussion
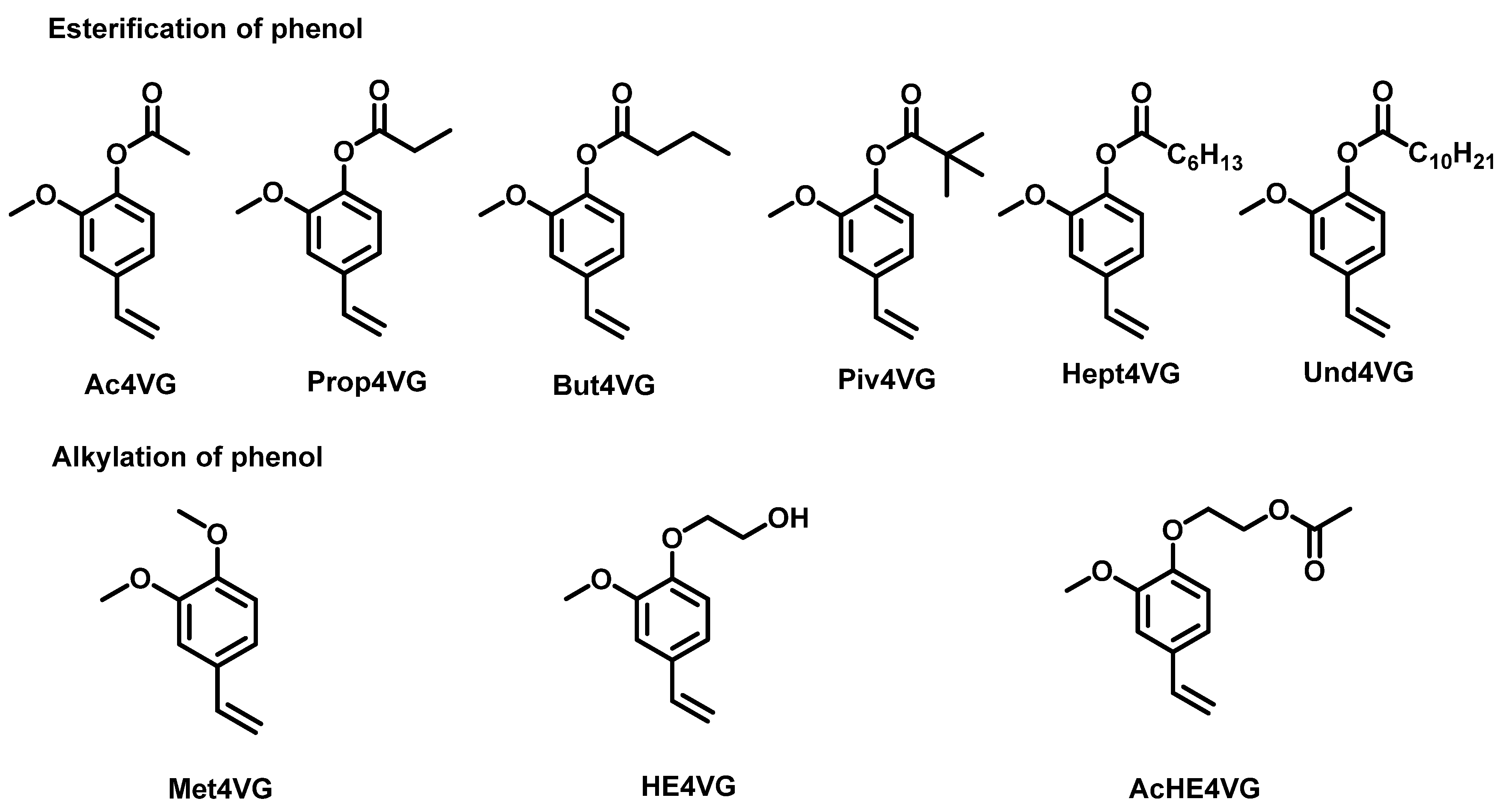
2.1. Synthesis of Biobased Monomers Derived from 4-Vinyl Guaiacol
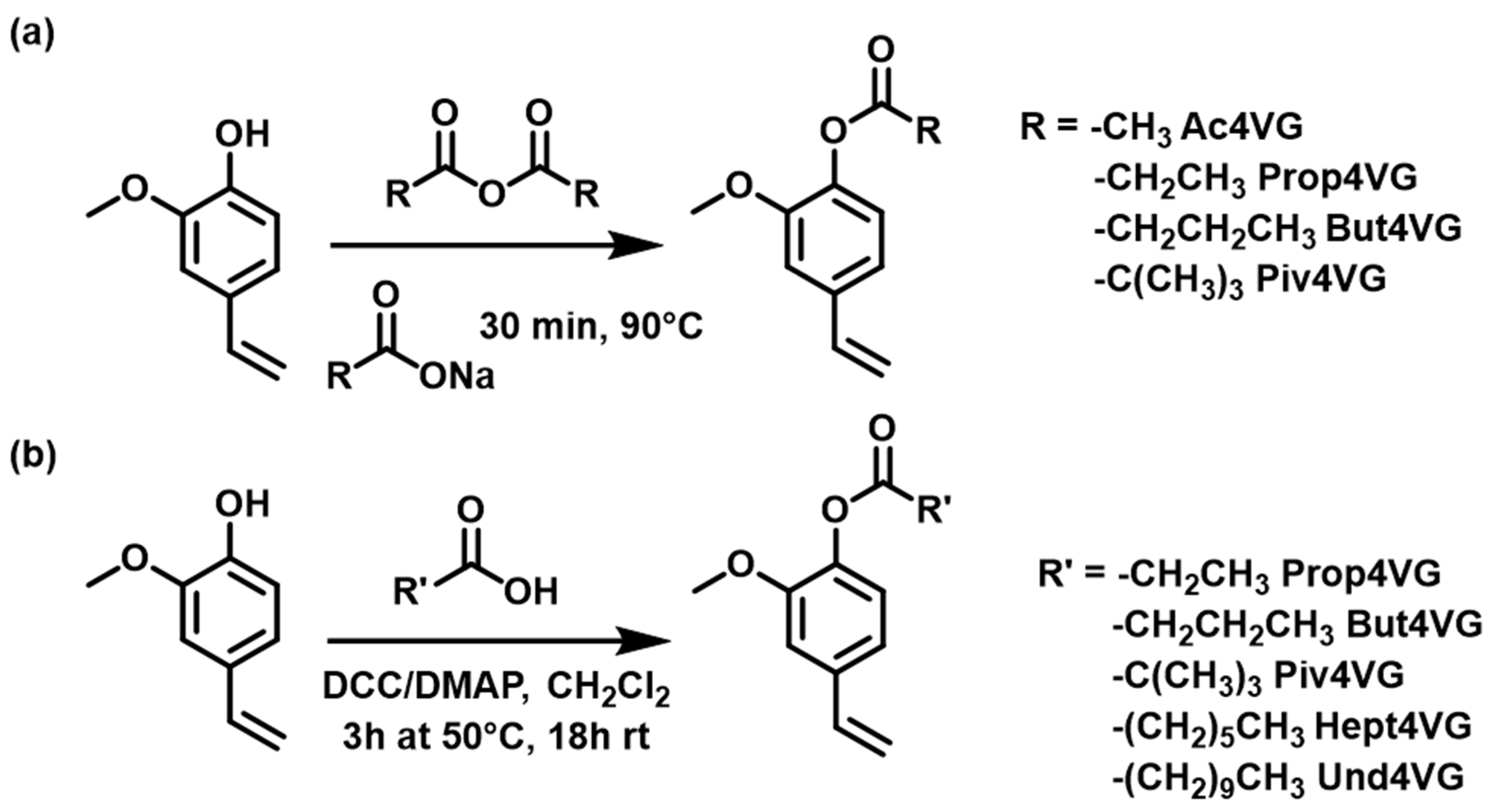
2.1.1. Esterification
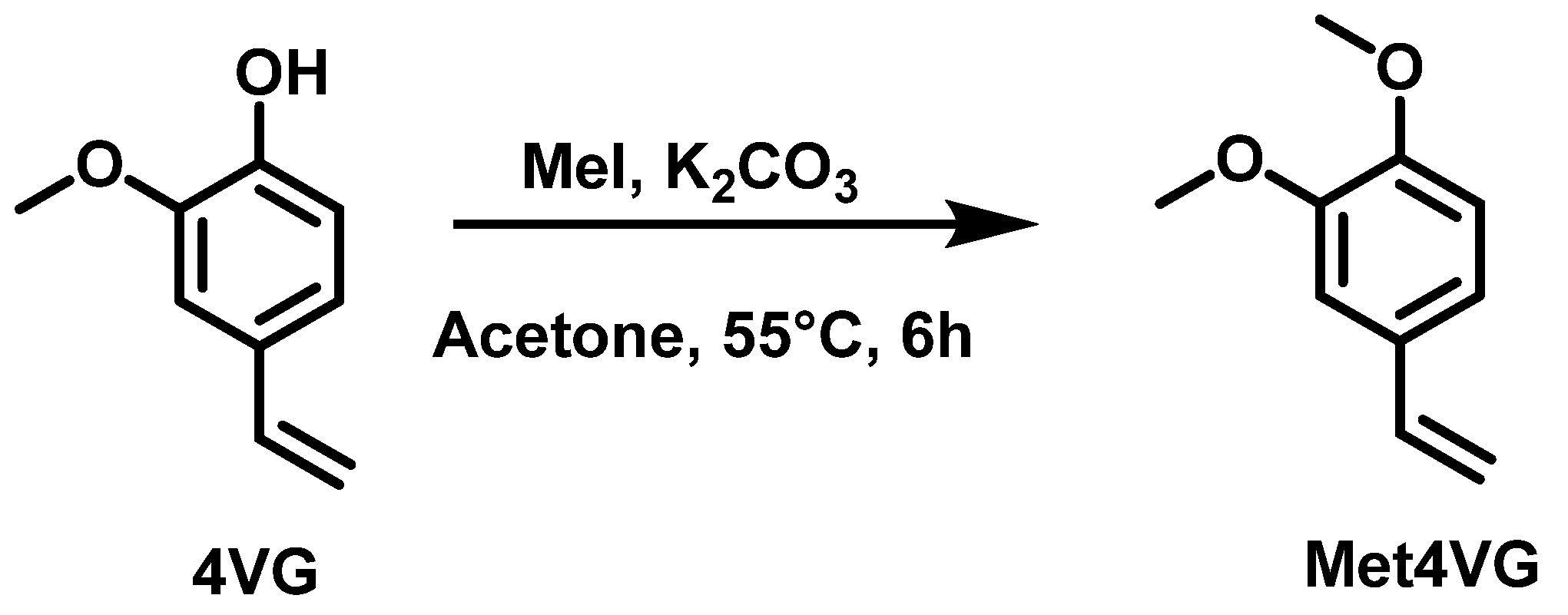
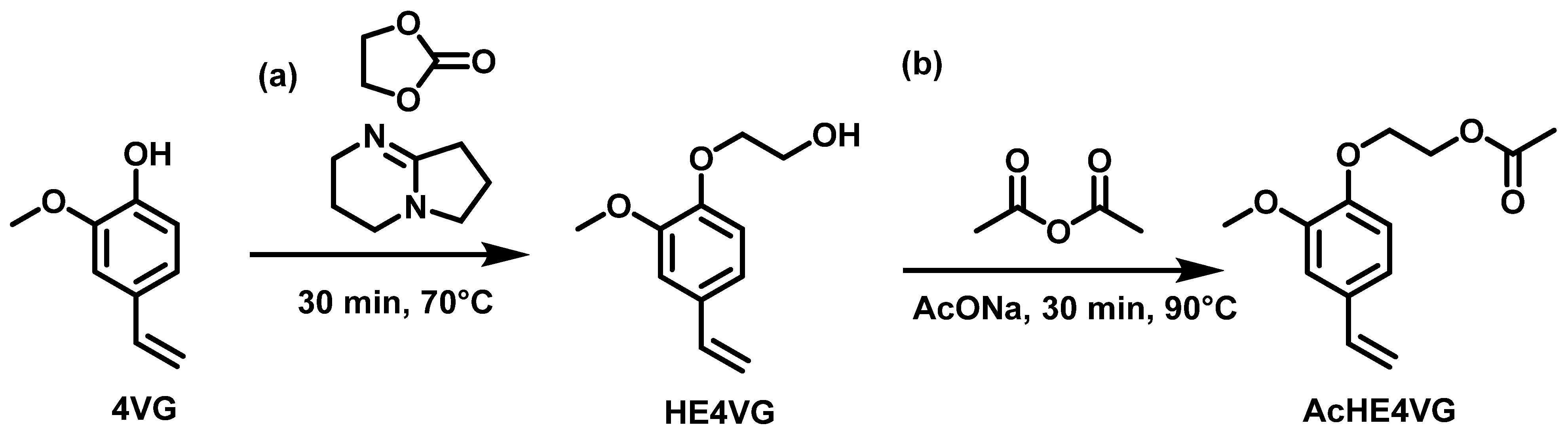
2.1.2. Alkylation
2.1.3. Green Metrics
2.2. Solution Homopolymerization of Biobased Monomers Derived from 4-Vinyl Guaiacol
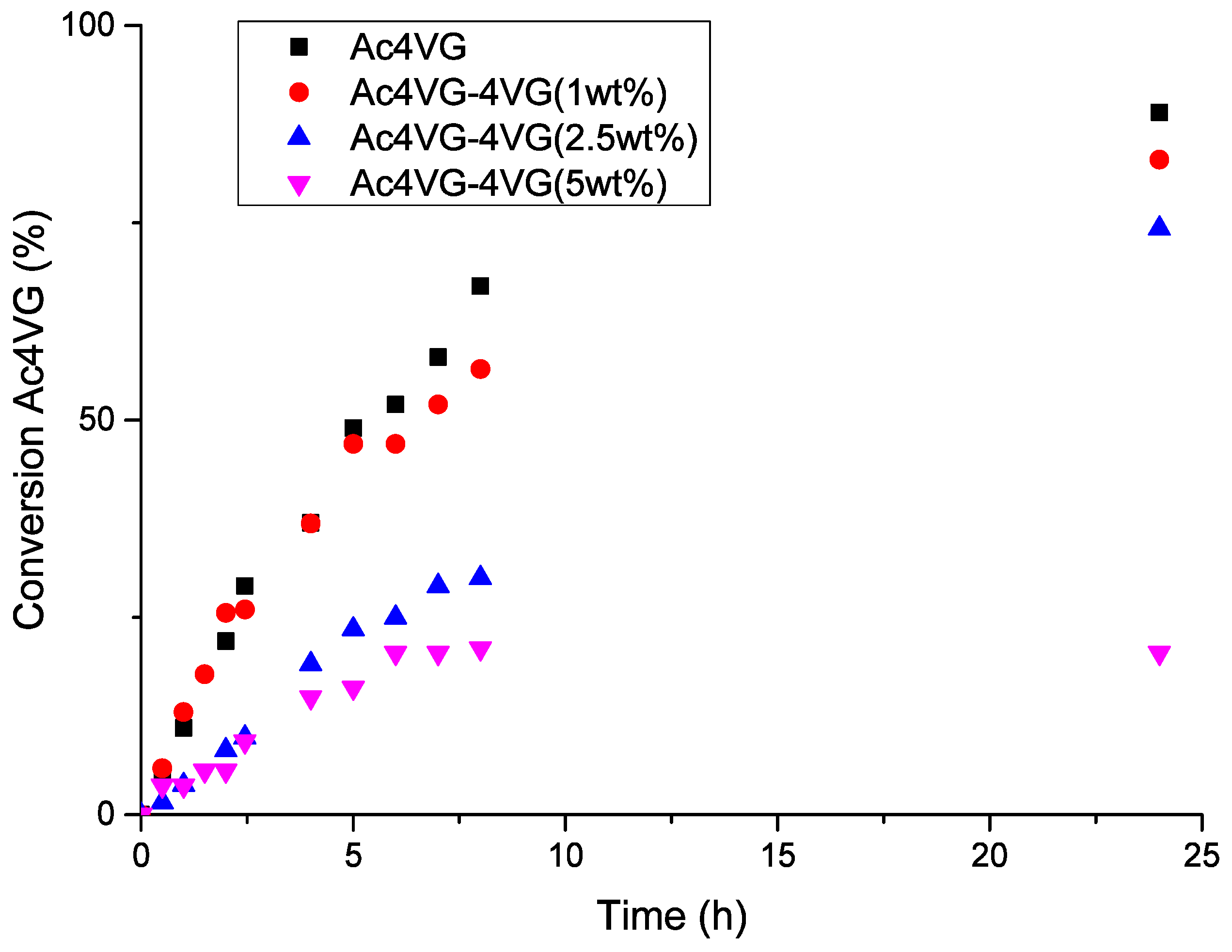
2.2.1. Homopolymerization of 4-Vinyl Guaiacol and Its Inhibitor Properties
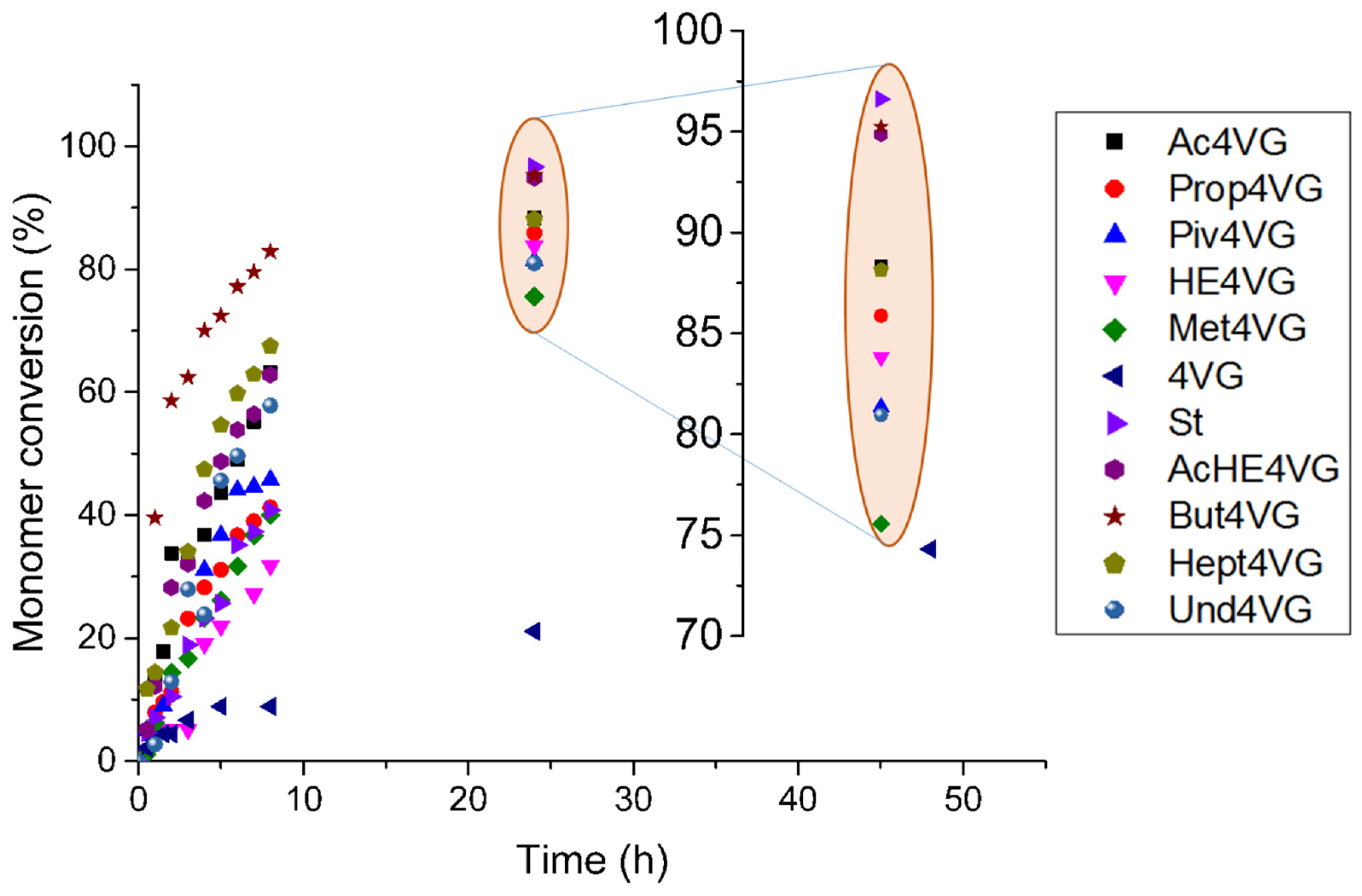
2.2.2. Homopolymerization of 4-Vinyl Guaiacol Derivatives
3. Experimental Section
3.1. Materials
3.2. Methods
3.2.1. Synthesis of 2-Methoxy-4-vinylphenyl Acetate (Ac4VG)
3.2.2. Synthesis of 2-Methoxy-4-vinylphenyl Propionate (Prop4VG)
3.2.3. Synthesis of 2-Methoxy-4-vinylphenyl Butyrate (But4VG)
3.2.4. Synthesis of 2-Methoxy-4-vinylphenyl Pivalate (Piv4VG)
3.2.5. Synthesis of 2-Methoxy-4-vinylphenyl Heptanoate (Hept4VG)
3.2.6. Synthesis of 2-Methoxy-4-vinylphenyl Undecanoate (Und4VG)
3.2.7. Synthesis of 2-(2-Methoxy-4-vinylphenoxy)ethan-1-ol (HE4VG)
3.2.8. Synthesis of 2-(2-Methoxy-4-vinylphenoxy)ethyl Acetate (AcHE4VG)
3.2.9. Synthesis of 1,2-Dimethoxy-4-vinylbenzene (Met4VG)
3.2.10. General Procedure for the Radical Homopolymerization of 4-vinyl Guaiacol Derivatives in Toluene
3.2.11. General Procedure for the Radical Homopolymerization of Styrene and 4-vinyl Guaiacol Derivatives in d8-toluene (Online 1H-NMR Measurements)
3.3. Characterization
3.3.1. Thermogravimetric Analysis
3.3.2. Differential Scanning Calorimetry
3.3.3. Size Exclusion Chromatography
3.3.4. Nuclear Magnetic Resonance Spectroscopy
3.3.5. Gas Chromatography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ritchie, H. Sector by Sector: Where Do Global Greenhouse Gas Emissions Come From? Our World Date 2020. Available online: https://ourworldindata.org/ghg-emissions-by-sector (accessed on 14 December 2023).
- Speight, J.G. The Chemistry and Technology of Petroleum, 3rd ed.; Speight, J.G., Ed.; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 1999. [Google Scholar]
- Speight, J.G.; Ozum, B. Petroleum Refining Processes; Speight, J.G., Ozum, B., Eds.; CRC Press—Taylor & Francis Group: Boca Raton, FL, USA, 2001. [Google Scholar]
- Halttunen, K.; Slade, R.; Staffell, I. What If We Never Run out of Oil? From Certainty of “Peak Oil” to “Peak Demand”. Energy Res. Soc. Sci. 2022, 85, 102407. [Google Scholar] [CrossRef]
- van Asselt, H. Governing Fossil Fuel Production in the Age of Climate Disruption: Towards an International Law of ‘Leaving It in the Ground’. Earth Syst. Gov. 2021, 9, 100118. [Google Scholar] [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. CO2 Emissions by Fuel. Available online: https://ourworldindata.org/emissions-by-fuel (accessed on 14 December 2023).
- Williams, C.K.; Hillmyer, M.A. Polymers from Renewable Resources: A Perspective for a Special Issue of Polymer Reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Gallezot, P. Conversion of Biomass to Selected Chemical Products. Chem. Soc. Rev. 2012, 41, 1538–1558. [Google Scholar] [CrossRef] [PubMed]
- Stanzione, J.F., III; Sadler, J.M.; La Scala, J.J.; Reno, K.H.; Wool, R.P. Vanillin-Based Resin for Use in Composite Applications. Green Chem. 2012, 14, 2346–2352. [Google Scholar] [CrossRef]
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-Derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef]
- Rigo, E.; Ladmiral, V.; Caillol, S.; Lacroix-Desmazes, P. Recent Advances in Radical Polymerization of Bio-Based Monomers in Aqueous Dispersed Media. RSC Sustain. 2023, 1, 788–813. [Google Scholar] [CrossRef]
- Calvo-Flores, F.G.; Martin-Martinez, F.J. Biorefineries: Achievements and Challenges for a Bio-Based Economy. Front. Chem. 2022, 10, 973417. [Google Scholar] [CrossRef]
- Biermann, U.; Bornscheuer, U.; Meier, M.A.R.; Metzger, J.O.; Schäfer, H.J. Oils and Fats as Renewable Raw Materials in Chemistry. Angew. Chemie Int. Ed. 2011, 50, 3854–3871. [Google Scholar] [CrossRef]
- Montero de Espinosa, L.; Meier, M.A.R. Plant Oils: The Perfect Renewable Resource for Polymer Science?! Eur. Polym. J. 2011, 47, 837–852. [Google Scholar] [CrossRef]
- Lu, Y.; Larock, R.C. Novel Polymeric Materials from Vegetable Oils and Vinyl Monomers: Preparation, Properties, and Applications. ChemSusChem 2009, 2, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.M.B.; Imbrogno, S.; Bloembergen, S. Carbohydrate Functionalized Hybrid Latex Particles. Carbohydr. Polym. 2017, 173, 233–252. [Google Scholar] [CrossRef] [PubMed]
- Spekreijse, J.; Le Nôtre, J.; van Haveren, J.; Scott, E.L.; Sanders, J.P.M. Simultaneous Production of Biobased Styrene and Acrylates Using Ethenolysis. Green Chem. 2012, 14, 2747–2751. [Google Scholar] [CrossRef]
- Hayes, G.; Laurel, M.; MacKinnon, D.; Zhao, T.; Houck, H.A.; Becer, C.R. Polymers without Petrochemicals: Sustainable Routes to Conventional Monomers. Chem. Rev. 2023, 123, 2609–2734. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; June, S.M.; Long, T.E. Principles of Step-Growth Polymerization (Polycondensation and Polyaddition). In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 7–47. [Google Scholar] [CrossRef]
- Stewart, D. Lignin as a Base Material for Materials Applications: Chemistry, Application and Economics. Ind. Crop. Prod. 2008, 27, 202–207. [Google Scholar] [CrossRef]
- Guadix-Montero, S.; Sankar, M. Review on Catalytic Cleavage of C–C Inter-Unit Linkages in Lignin Model Compounds: Towards Lignin Depolymerisation. Top. Catal. 2018, 61, 183–198. [Google Scholar] [CrossRef]
- Kreye, O.; Oelmann, S.; Meier, M.A.R. Renewable Aromatic–Aliphatic Copolyesters Derived from Rapeseed. Macromol. Chem. Phys. 2013, 214, 1452–1464. [Google Scholar] [CrossRef]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Arnhold, K.; Brünig, H.; Scheibner, H.; Müller, M.T.; Voit, B. Polyesters with Bio-Based Ferulic Acid Units: Crosslinking Paves the Way to Property Consolidation. Polym. Chem. 2021, 12, 5139–5148. [Google Scholar] [CrossRef]
- Bazin, A.; Avérous, L.; Pollet, E. Ferulic Acid as Building Block for the Lipase-Catalyzed Synthesis of Biobased Aromatic Polyesters. Polymers 2021, 13, 3693. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, S.; Manseri, A.; Ladmiral, V.; Bongiovanni, R.; Caillol, S.; Lacroix-Desmazes, P. Eugenol: A Promising Building Block for Synthesis of Radically Polymerizable Monomers. Macromol. Chem. Phys. 2019, 220, 1900179. [Google Scholar] [CrossRef]
- Ladmiral, V.; Jeannin, R.; Fernandes Lizarazu, K.; Lai-Kee-Him, J.; Bron, P.; Lacroix-Desmazes, P.; Caillol, S. Aromatic Biobased Polymer Latex from Cardanol. Eur. Polym. J. 2017, 93, 785–794. [Google Scholar] [CrossRef]
- Zago, E.; Dubreucq, E.; Lecomte, J.; Villeneuve, P.; Fine, F.; Fulcrand, H.; Aouf, C. Synthesis of Bio-Based Epoxy Monomers from Natural Allyl- and Vinyl Phenols and the Estimation of Their Affinity to the Estrogen Receptor α by Molecular Docking. New J. Chem. 2016, 40, 7701–7710. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Bio-Based Functional Styrene Monomers Derived from Naturally Occurring Ferulic Acid for Poly(Vinylcatechol) and Poly(Vinylguaiacol) via Controlled Radical Polymerization. Macromolecules 2017, 50, 4206–4216. [Google Scholar] [CrossRef]
- Zhang, X.; Carter, M.C.D.; Belowich, M.E.; Wan, G.; Crimmins, M.; Laughlin, K.B.; Even, R.C.; Kalantar, T.H. Catechol-Functionalized Latex Polymers Display Improved Adhesion to Low-Surface-Energy Thermoplastic Polyolefin Substrates. ACS Appl. Polym. Mater. 2019, 1, 1317–1325. [Google Scholar] [CrossRef]
- Boarino, A.; Wang, H.; Olgiati, F.; Artusio, F.; Özkan, M.; Bertella, S.; Razza, N.; Cagno, V.; Luterbacher, J.S.; Klok, H.-A.; et al. Lignin: A Sustainable Antiviral Coating Material. ACS Sustain. Chem. Eng. 2022, 10, 14001–14010. [Google Scholar] [CrossRef]
- Fujisawa, S.; Kadoma, Y. Effect of Phenolic Compounds on the Polymerization of Methyl Methacrylate. Dent. Mater. 1992, 8, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Kodaira, K.; Ōnishi, Y.; Ito, K. An Oligomerization of 2-methoxy-4-vinylphenol. Makromol. Chem. Rapid Commun. 1980, 1, 427–431. [Google Scholar] [CrossRef]
- Leisch, H.; Grosse, S.; Morley, K.; Abokitse, K.; Perrin, F.; Denault, J.; Lau, P.C.K. Chemicals from Agricultural Biomass: Chemoenzymatic Approach for Production of Vinylphenols and Polyvinylphenols from Phenolic Acids. Green Process. Synth. 2013, 2, 4206–4216. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Nakamura, K.; Hatakeyama, H. Differential Thermal Analysis of Styrene Derivatives Related to Lignin. Polymer 1978, 19, 593–594. [Google Scholar] [CrossRef]
- Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Effect of Substituent Groups on Hydrogen Bonding of Polyhydroxystyrene Derivatives. Polym. J. 1983, 15, 361–366. [Google Scholar] [CrossRef]
- van Schijndel, J.; Molendijk, D.; van Beurden, K.; Canalle, L.A.; Noël, T.; Meuldijk, J. Preparation of Bio-Based Styrene Alternatives and Their Free Radical Polymerization. Eur. Polym. J. 2020, 125, 109534. [Google Scholar] [CrossRef]
- Alexakis, A.E.; Ayyachi, T.; Mousa, M.; Olsén, P.; Malmström, E. 2-Methoxy-4-Vinylphenol as a Biobased Monomer Precursor for Thermoplastics and Thermoset Polymers. Polymers 2023, 15, 2168. [Google Scholar] [CrossRef]
- McGuire, T.M.; Miyajima, M.; Uchiyama, M.; Buchard, A.; Kamigaito, M. Epoxy-Functionalised 4-Vinylguaiacol for the Synthesis of Bio-Based, Degradable Star Polymers via a RAFT/ROCOP Strategy. Polym. Chem. 2020, 11, 5844–5850. [Google Scholar] [CrossRef]
- Ewing, T.A.; Nouse, N.; van Lint, M.; van Haveren, J.; Hugenholtz, J.; van Es, D.S. Fermentation for the Production of Biobased Chemicals in a Circular Economy: A Perspective for the Period 2022–2050. Green Chem. 2022, 24, 6373–6405. [Google Scholar] [CrossRef]
- Nomura, K.; Binti Awang, N.W. Synthesis of Bio-Based Aliphatic Polyesters from Plant Oils by Efficient Molecular Catalysis: A Selected Survey from Recent Reports. ACS Sustain. Chem. Eng. 2021, 9, 5486–5505. [Google Scholar] [CrossRef]
- Jordan, A.; Whymark, K.D.; Sydenham, J.; Sneddon, H.F. A Solvent-Reagent Selection Guide for Steglich-Type Esterification of Carboxylic Acids. Green Chem. 2021, 23, 6405–6413. [Google Scholar] [CrossRef]
- Wang, F.; Finnin, J.; Tait, C.; Quirk, S.; Chekhtman, I.; Donohue, A.C.; Ng, S.; D’Souza, A.; Tait, R.; Prankerd, R. The Hydrolysis of Diclofenac Esters: Synthetic Prodrug Building Blocks for Biodegradable Drug-Polymer Conjugates. J. Pharm. Sci. 2016, 105, 773–785. [Google Scholar] [CrossRef]
- Fadlallah, S.; Sinha Roy, P.; Garnier, G.; Saito, K.; Allais, F. Are Lignin-Derived Monomers and Polymers Truly Sustainable? An in-Depth Green Metrics Calculations Approach. Green Chem. 2021, 23, 1495–1535. [Google Scholar] [CrossRef]
- Sheldon, R.A. Fundamentals of Green Chemistry: Efficiency in Reaction Design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef]
- Martínez, J.; Cortés, J.F.; Miranda, R. Green Chemistry Metrics, A Review. Processes 2022, 10, 1274. [Google Scholar] [CrossRef]
- Andraos, J. Unification of Reaction Metrics for Green Chemistry: Applications to Reaction Analysis. Org. Process Res. Dev. 2005, 9, 149–163. [Google Scholar] [CrossRef]
- Barton, S.C.; Bird, R.A.; Russell, K.E. The Effect of Phenols and Aromatic Thiols on the Polymerization of Methyl Methacrylate. Can. J. Chem. 1963, 41, 2737–2742. [Google Scholar] [CrossRef]
- Van Herk, A.M. Pulsed Initiation Polymerization as a Means of Obtaining Propagation Rate Coefficients in Free-Radical Polymerizations. II Review up to 2000. Macromol. Theory Simul. 2000, 9, 433–441. [Google Scholar] [CrossRef]
- Buback, M.; Kuchta, F. Termination Kinetics of Free-radical Polymerization of Styrene over an Extended Temperature and Pressure Range. Macromol. Chem. Phys. 1997, 198, 1455–1480. [Google Scholar] [CrossRef]
- Takeshima, H.; Satoh, K.; Kamigaito, M. Naturally-Derived Amphiphilic Polystyrenes Prepared by Aqueous Controlled/Living Cationic Polymerization and Copolymerization of Vinylguaiacol with R-OH/BF3 OEt2. Polymers 2018, 10, 1404. [Google Scholar] [CrossRef] [PubMed]
- Van Krevelen, D.W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; ISBN 9780080548197. [Google Scholar]
- Bauer, M.; Bertario, A.; Boccardi, G.; Fontaine, X.; Rao, R.; Verrier, D. Reproducibility of 1H-NMR Integrals: A Collaborative Study. J. Pharm. Biomed. Anal. 1998, 17, 419–425. [Google Scholar] [CrossRef]
- Patnode, W.; Scheiber, W.J. The Density, Thermal Expansion, Vapor Pressure, and Refractive Index of Styrene, and the Density and Thermal Expansion of Polystyrene. J. Am. Chem. Soc. 1939, 61, 3449–3451. [Google Scholar] [CrossRef]
- Killian, W.G.; Norfleet, A.T.; Lira, C.T. Densities of Selected Deuterated Solvents. J. Chem. Eng. Data 2022, 67, 893–901. [Google Scholar] [CrossRef]
- 1,4-Bis(Trimethylsilyl)Benzene. Available online: www.chemspider.com/Chemical-Structure.24010.html (accessed on 16 April 2023).
- Roduit, B.; Hartmann, M.; Folly, P.; Sarbach, A.; Brodard, P.; Baltensperger, R. Determination of Thermal Hazard from DSC Measurements. Investigation of Self-Accelerating Decomposition Temperature (SADT) of AIBN. J. Therm. Anal. Calorim. 2014, 117, 1017–1026. [Google Scholar] [CrossRef]
- Franks, F.; Ives, D.J.G. The Structural Properties of Alcohol–Water Mixtures. Q. Rev. Chem. Soc. 1966, 20, 1–44. [Google Scholar] [CrossRef]
- Van Hook, J.P.; Tobolsky, A.V. Tobolsky The Thermal Decomposition of 2-2-Azo-Bis-Isobutyronitrile. J. Am. Chem. Soc. 1958, 80, 779–782. [Google Scholar]
- Lacroix-Desmazes, P.; Severac, R.; Boutevin, B. Reverse Iodine Transfer Polymerization of Methyl Acrylate and N-Butyl Acrylate. Macromolecules 2005, 38, 6299–6309. [Google Scholar]

| Esterification | Etherification | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | Ac4VG | Prop4VG | But4VG | Piv4VG | Hept4VG | Und4VG | AcHE4VG | HE4VG | Met4VG | |||
| Route 1 | Route 2 | Route 1 | Route 2 | Route 1 | Route 2 | |||||||
| AE (%) | 76 | 73 | 91 | 71 | 92 | 69 | 92 | 93 | 95 | 80 | 81 | 56 |
| E-factor (kg waste/kg product) | 0.5 | 1.9 | 19.9 | 0.8 | 15.8 | 1.2 | 30.2 | 17.6 | 11.4 | 2.9 | 1.2 | 44.4 |
| C-efficiency (%) | 74 | 40 | 36 | 61 | 45 | 55 | 24 | 38 | 56 | 28 | 51 | 40 |
| RME (%) | 66 | 35 | 36 | 56 | 44 | 45 | 23 | 37 | 54 | 26 | 46 | 10 |
| Biobased carbon (%) | 82 | 75 | 100 | 69 | 100 | 64 | 64 | 100 | 100 | 69 | 82 | 90 |
| Monomer | Monomer Conversion (%) (24 h) | Td,5% (°C) a | Tg,exp (°C) a | Tg,calc (°C) b | Mn (g/mol) (24 h) c | Đ (24 h) c | |
|---|---|---|---|---|---|---|---|
| Styrene | 97 | 0.056 | 365 | 104 | 100 | 7,500 | 1.7 |
| 4VG | 21 | n.m. | 202 | 83 | n.d. | 4,500 | 2.4 |
| Ac4VG | 88 | 0.123 | 317 | 117 | 110 | 14,700 | 2.3 |
| Prop4VG | 86 | n.m. | 341 | 81 | 84 | 13,900 | 2.3 |
| But4VG | 95 | 0.106 | 346 | 71 | 62 | 15,200 | 2.4 |
| Piv4VG | 81 | n.m. | 346 | 96 | 102 | 32,000 | 1.9 |
| Hept4VG | 88 | 0.153 | 344 | 16 | 11 | 23,700 | 2.3 |
| Und4VG | 81 | n.m. | 332 | 5 | −35 | 7900 | 2.4 |
| HE4VG | 84 | n.m. | n.m. d | n.m. | n.d. | n.m. | n.m. |
| AcHE4VG | 95 | n.m. | 334 | 37 | 38 | 11,600 and 7300 (bimodal) | 1.4 and 1.9 |
| Met4VG | 76 | n.m. | 357 | 71 | 78 | 11,900 | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigo, E.; Totée, C.; Ladmiral, V.; Caillol, S.; Lacroix-Desmazes, P. 4-Vinyl Guaiacol: A Key Intermediate for Biobased Polymers. Molecules 2024, 29, 2507. https://doi.org/10.3390/molecules29112507
Rigo E, Totée C, Ladmiral V, Caillol S, Lacroix-Desmazes P. 4-Vinyl Guaiacol: A Key Intermediate for Biobased Polymers. Molecules. 2024; 29(11):2507. https://doi.org/10.3390/molecules29112507
Chicago/Turabian StyleRigo, Elena, Cédric Totée, Vincent Ladmiral, Sylvain Caillol, and Patrick Lacroix-Desmazes. 2024. "4-Vinyl Guaiacol: A Key Intermediate for Biobased Polymers" Molecules 29, no. 11: 2507. https://doi.org/10.3390/molecules29112507
APA StyleRigo, E., Totée, C., Ladmiral, V., Caillol, S., & Lacroix-Desmazes, P. (2024). 4-Vinyl Guaiacol: A Key Intermediate for Biobased Polymers. Molecules, 29(11), 2507. https://doi.org/10.3390/molecules29112507











