Integrated Biorefinery for a Next-Generation Methanization Process Focusing on Volatile Fatty Acid Valorization: A Critical Review
Abstract
1. Introduction
2. Need for a Better Valorization of Global Waste Streams

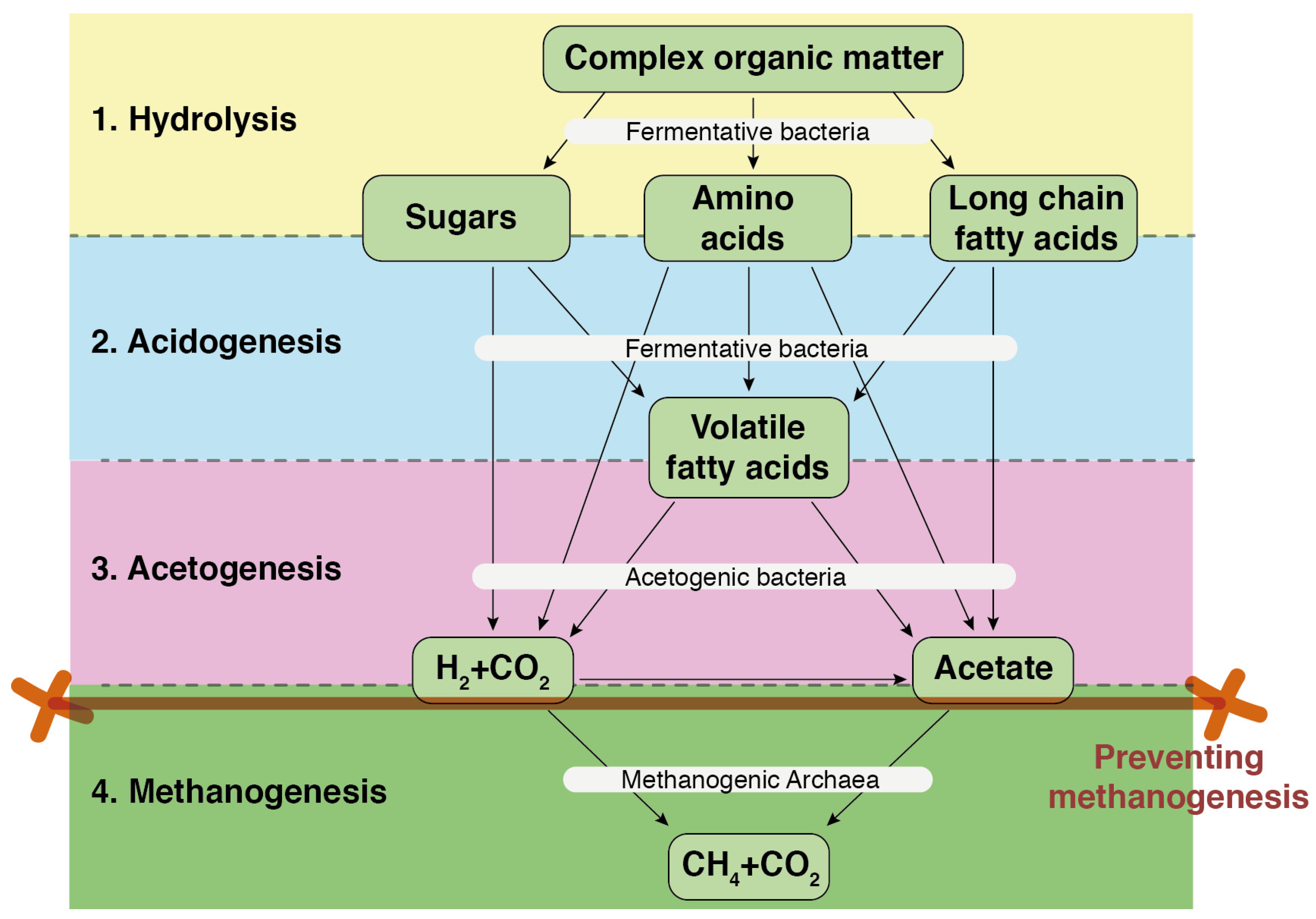
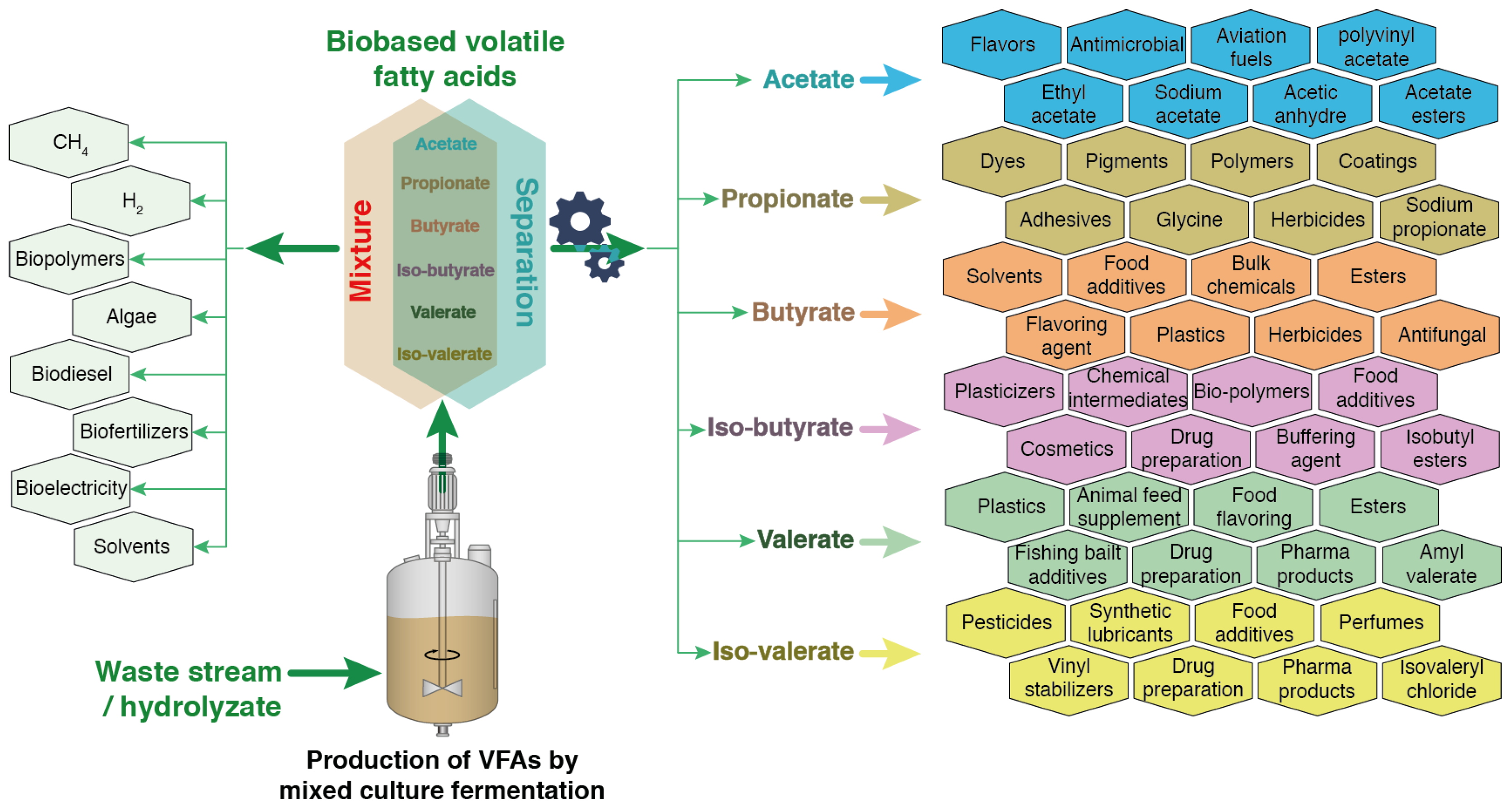
3. Optimizing VFA Production for a Next-Generation Methanization Process
4. Challenges in the Conversion of VFAs into Valuable Products: A Case Study on Microbial Lipids
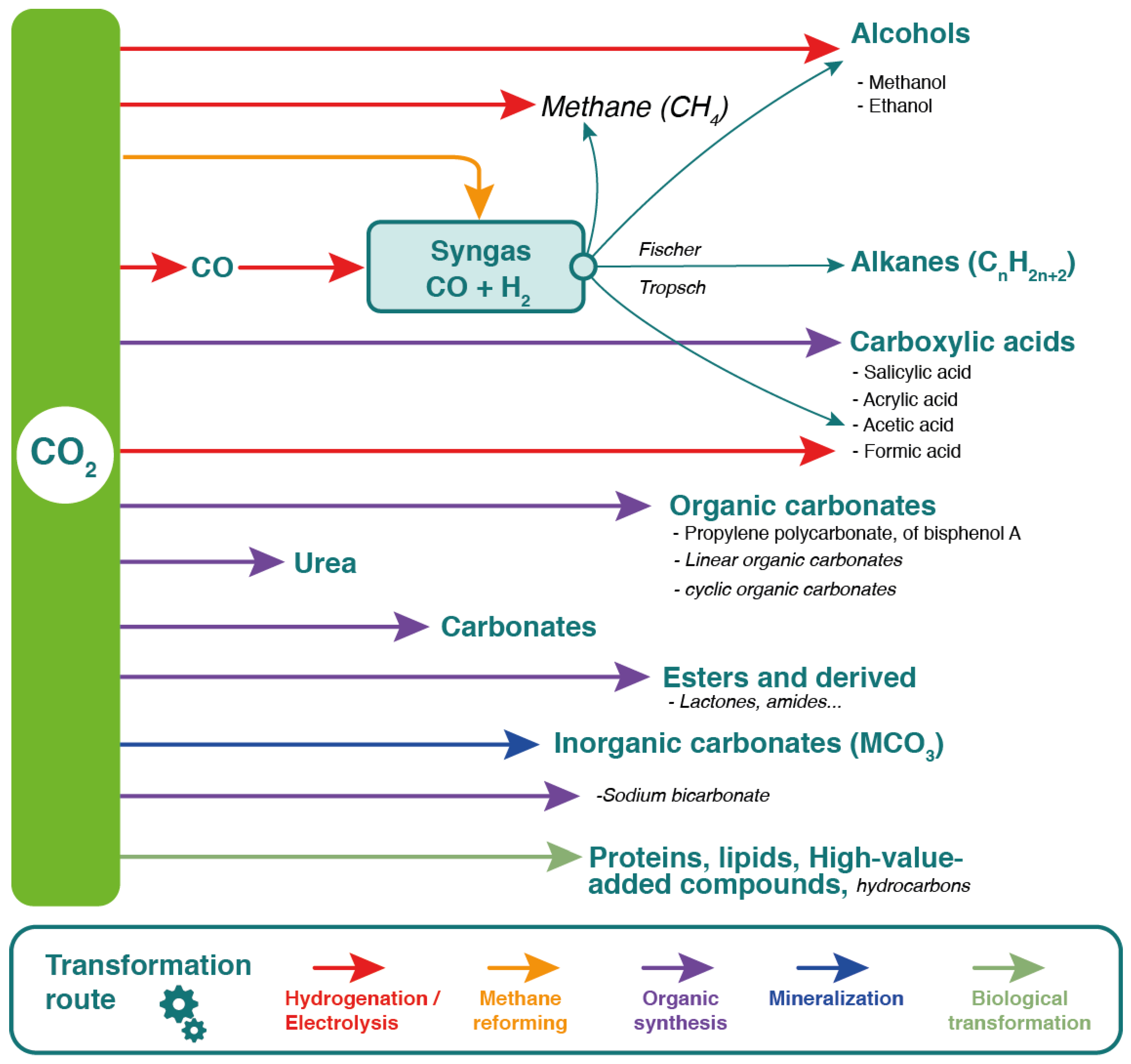
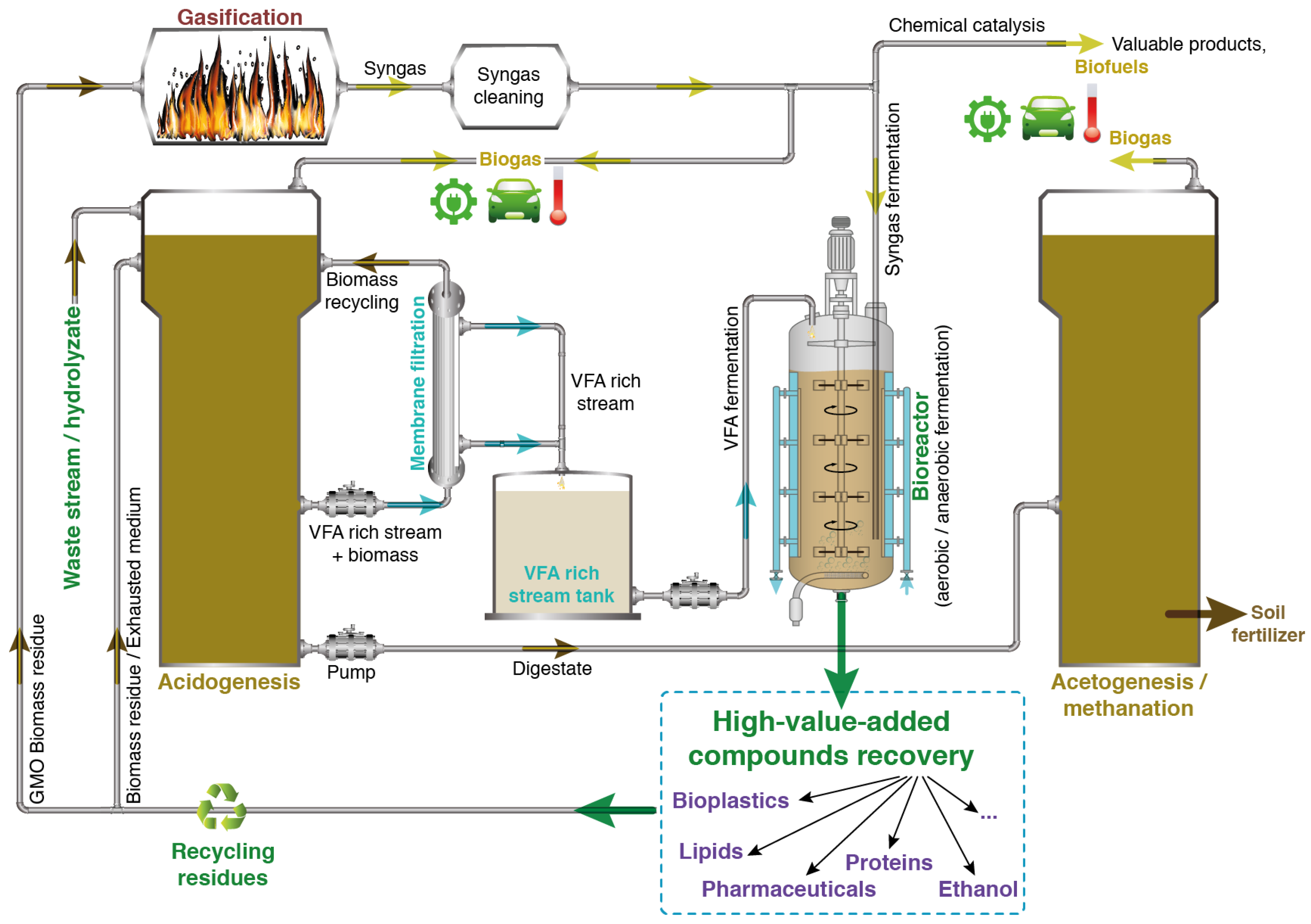
5. Integrated Biorefinery Approach for a Next-Generation Methanization Process
6. Techno-Economic Analysis and Life Cycle Assessment
7. Future Directions
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A Review of the Production and Applications of Waste-Derived Volatile Fatty Acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Shafiq, M.; Zhang, B.-B.; Zeb, L.; Ali, S.; Waqas, W.; Ayaz, M.; Ali, S.; Chi, Z. Integrated Biorefinery Approach for Bioconversion of Fish Manure to Volatile Fatty Acids and Its Valorization by Schizochytrium sp. for Docosahexaenoic Acid, Squalene, and Carotenoids Production. J. Water Process Eng. 2024, 58, 104749. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current Perspective on Pretreatment Technologies Using Lignocellulosic Biomass: An Emerging Biorefinery Concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- Priyadarshi, D.; Paul, K.K. Single Phase Blend: An Advanced Microwave Process for Improved Quality Low-Cost Biodiesel Production from Kitchen Food Waste. Biochem. Eng. J. 2018, 137, 273–283. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Sáez-Bastante, J.; Pinzi, S.; Dorado, M.P. Optimization of Solid Food Waste Oil Biodiesel by Ultrasound-Assisted Transesterification. Fuel 2019, 255, 115817. [Google Scholar] [CrossRef]
- Agarwal, M.; Tardio, J.; Mohan, S.V. Biohydrogen Production from Kitchen Based Vegetable Waste: Effect of Pyrolysis Temperature and Time on Catalysed and Non-Catalysed Operation. Bioresour. Technol. 2013, 130, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Greco, G.; Videgain, M.; Di Stasi, C.; Pires, E.; Manyà, J.J. Importance of Pyrolysis Temperature and Pressure in the Concentration of Polycyclic Aromatic Hydrocarbons in Wood Waste-Derived Biochars. J. Anal. Appl. Pyrolysis 2021, 159, 105337. [Google Scholar] [CrossRef]
- Robinson, J.; Dodds, C.; Stavrinides, A.; Kingman, S.; Katrib, J.; Wu, Z.; Medrano, J.; Overend, R. Microwave Pyrolysis of Biomass: Control of Process Parameters for High Pyrolysis Oil Yields and Enhanced Oil Quality. Energy Fuels 2015, 29, 1701–1709. [Google Scholar] [CrossRef]
- Beneroso, D.; Bermúdez, J.M.; Arenillas, A.; Menéndez, J.A. Integrated Microwave Drying, Pyrolysis and Gasification for Valorisation of Organic Wastes to Syngas. Fuel 2014, 132, 20–26. [Google Scholar] [CrossRef]
- Khelfa, A.; Rodrigues, F.A.; Koubaa, M.; Vorobiev, E. Microwave-Assisted Pyrolysis of Pine Wood Sawdust Mixed with Activated Carbon for Bio-Oil and Bio-Char Production. Processes 2020, 8, 1437. [Google Scholar] [CrossRef]
- Chen, G.; Tu, X.; Homm, G.; Weidenkaff, A. Plasma Pyrolysis for a Sustainable Hydrogen Economy. Nat. Rev. Mater. 2022, 7, 333–334. [Google Scholar] [CrossRef]
- Hrycak, B.; Mizeraczyk, J.; Czylkowski, D.; Dors, M.; Budnarowska, M.; Jasiński, M. Hydrogen Production by the Steam Reforming of Synthetic Biogas in Atmospheric-Pressure Microwave (915 MHz) Plasma. Sci. Rep. 2023, 13, 2204. [Google Scholar] [CrossRef]
- Stigsson, C.; Furusjö, E.; Börjesson, P. A Model of an Integrated Hydrothermal Liquefaction, Gasification and Fischer-Tropsch Synthesis Process for Converting Lignocellulosic Forest Residues into Hydrocarbons. Bioresour. Technol. 2022, 353, 126070. [Google Scholar] [CrossRef] [PubMed]
- Taseska, T.; Yu, W.; Wilsey, M.K.; Cox, C.P.; Meng, Z.; Ngarnim, S.S.; Müller, A.M. Analysis of the Scale of Global Human Needs and Opportunities for Sustainable Catalytic Technologies. Top. Catal. 2023, 66, 338–374. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Zoetemeyer, R.J.; van Deursen, A.; van Andel, J.G. Anaerobic Digestion of Glucose with Separated Acid Production and Methane Formation. Water Res. 1979, 13, 571–580. [Google Scholar] [CrossRef]
- Weiland, P. Biogas Production: Current State and Perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef]
- ADEME; Thual, J.; Martin, S.; Mousset, J. La Méthanisation: Filière Majeure de Production de Gaz Renouvelable et Local, au Service D’une Agriculture Plus Durable et Résiliente; ADEME: Angers, France, 2023; pp. 1–12. Available online: https://librairie.ademe.fr/ged/8158/Avis_technique_M__thanisation.pdf (accessed on 21 May 2024).
- Clark, J.H. Green Biorefinery Technologies Based on Waste Biomass. Green Chem. 2019, 21, 1168–1170. [Google Scholar] [CrossRef]
- Karimi, B.; Sadet-Bourgeteau, S.; Cannavacciuolo, M.; Chauvin, C.; Flamin, C.; Haumont, A.; Jean-Baptiste, V.; Reibel, A.; Vrignaud, G.; Ranjard, L. Impact of Biogas Digestates on Soil Microbiota in Agriculture: A Review. Env. Chem. Lett. 2022, 20, 3265–3288. [Google Scholar] [CrossRef]
- Schievano, A.; Tenca, A.; Lonati, S.; Manzini, E.; Adani, F. Can Two-Stage Instead of One-Stage Anaerobic Digestion Really Increase Energy Recovery from Biomass? Appl. Energy 2014, 124, 335–342. [Google Scholar] [CrossRef]
- Schievano, A.; Tenca, A.; Scaglia, B.; Merlino, G.; Rizzi, A.; Daffonchio, D.; Oberti, R.; Adani, F. Two-Stage vs Single-Stage Thermophilic Anaerobic Digestion: Comparison of Energy Production and Biodegradation Efficiencies. Environ. Sci. Technol. 2012, 46, 8502–8510. [Google Scholar] [CrossRef]
- Jänisch, T.; Reinhardt, S.; Pohsner, U.; Böringer, S.; Bolduan, R.; Steinbrenner, J.; Oechsner, H. Separation of Volatile Fatty Acids from Biogas Plant Hydrolysates. Sep. Purif. Technol. 2019, 223, 264–273. [Google Scholar] [CrossRef]
- Garcia-Villalva, R.; Biset-Peiró, M.; Alarcón, A.; Bacariza, C.; Murcia-López, S.; Guilera, J. Comparison of Methane Reforming Routes for Hydrogen Production Using Dielectric Barrier Discharge Plasma-Catalysis. Int. J. Hydrogen Energy 2024, 59, 1367–1375. [Google Scholar] [CrossRef]
- Świrk Da Costa, K.; Summa, P.; Gopakumar, J.; van Valen, Y.; Da Costa, P.; Rønning, M. Excess-Methane CO2 Reforming over Reduced KIT-6-Ni-Y Mesoporous Silicas Monitored by In Situ XAS–XRD. Energy Fuels 2023, 37, 18952–18967. [Google Scholar] [CrossRef] [PubMed]
- Guilayn, F.; Jimenez, J.; Monlau, F.; Vaneeckhaute, C. Valorisation of Anaerobic Digestate: Towards Value-Added Products. In Renewable Energy Technologies for Energy Efficient Sustainable Development; Sinharoy, A., Lens, P.N.L., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 227–262. ISBN 978-3-030-87633-3. [Google Scholar]
- Lamolinara, B.; Pérez-Martínez, A.; Guardado-Yordi, E.; Guillén Fiallos, C.; Diéguez-Santana, K.; Ruiz-Mercado, G.J. Anaerobic Digestate Management, Environmental Impacts, and Techno-Economic Challenges. Waste Manag. 2022, 140, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Llamas, M.; Magdalena, J.A.; González-Fernández, C.; Tomás-Pejó, E. Volatile Fatty Acids as Novel Building Blocks for Oil-Based Chemistry via Oleaginous Yeast Fermentation. Biotechnol. Bioeng. 2020, 117, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Bedaso, B.; Laumeyer, C.; Pan, C.; Malovanyy, A.; Baresel, C.; Plaza, E.; Cetecioglu, Z. Volatile Fatty Acids Production from Municipal Waste Streams and Use as a Carbon Source for Denitrification: The Journey towards Full-Scale Application and Revealing Key Microbial Players. Renew. Sustain. Energy Rev. 2023, 175, 113163. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, Ö.; Cetecioglu, Z. Volatile Fatty Acid Production from Semi-Synthetic Milk Processing Wastewater under Alkali pH: The Pearls and Pitfalls of Microbial Culture. Bioresour. Technol. 2020, 297, 122415. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Zhang, S.; Phong Vo, H.N.; Bui, X.T.; Ngoc Hoang, B. Volatile Fatty Acids Production from Waste Streams by Anaerobic Digestion: A Critical Review of the Roles and Application of Enzymes. Bioresour. Technol. 2022, 359, 127420. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Hu, W.; Zhu, Z.; Zheng, X.; Chen, Y.; Chen, Y. Enhanced Volatile Fatty Acid Production from Food Waste Fermentation via Enzymatic Pretreatment: New Insights into the Depolymerization and Microbial Traits. ACS EST Eng. 2023, 3, 26–35. [Google Scholar] [CrossRef]
- IEA. Outlook for Biogas and Biomethane-Prospects for Organic Growth; IEA: Paris, France, 2020. [Google Scholar]
- Wilson, D.C.; Velis, C.A. Waste Management—Still a Global Challenge in the 21st Century: An Evidence-Based Call for Action. Waste Manag. Res. 2015, 33, 1049–1051. [Google Scholar] [CrossRef]
- United Nations Food Loss and Waste Reduction. Available online: https://www.un.org/en/observances/end-food-waste-day (accessed on 16 April 2024).
- Puértolas, E.; Koubaa, M.; Barba, F.J. An Overview of the Impact of Electrotechnologies for the Recovery of Oil and High-Value Compounds from Vegetable Oil Industry: Energy and Economic Cost Implications. Food Res. Int. 2016, 80, 19–26. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-Conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2015, 8, 214–234. [Google Scholar] [CrossRef]
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and New Insights in the Sustainable and Green Recovery of Nutritionally Valuable Compounds from Stevia rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef]
- Zhu, Z.; He, J.; Liu, G.; Barba, F.J.; Koubaa, M.; Ding, L.; Bals, O.; Grimi, N.; Vorobiev, E. Recent Insights for the Green Recovery of Inulin from Plant Food Materials Using Non-Conventional Extraction Technologies: A Review. Innov. Food Sci. Emerg. Technol. 2016, 33, 1–9. [Google Scholar] [CrossRef]
- Conde-Mejía, C.; Jiménez-Gutiérrez, A.; El-Halwagi, M. A Comparison of Pretreatment Methods for Bioethanol Production from Lignocellulosic Materials. Process Saf. Environ. Prot. 2012, 90, 189–202. [Google Scholar] [CrossRef]
- FAO. Food Loss/Waste; FAO: Rome, Italy, 2016; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/bc83ceb7-e00e-4322-98ae-9148d0ef1b87/content (accessed on 21 May 2024).
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical Conversion of Sewage Sludge: A Critical Review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical Conversion of Sewage Sludge for Energy and Resource Recovery: Technical Challenges and Prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Bezirgiannidis, A.; Chatzopoulos, P.; Tsakali, A.; Ntougias, S.; Melidis, P. Renewable Energy Recovery from Sewage Sludge Derived from Chemically Enhanced Precipitation. Renew. Energy 2020, 162, 1811–1818. [Google Scholar] [CrossRef]
- Valipour, A.; Raman, V.K.; Ahn, Y.-H. Effectiveness of Domestic Wastewater Treatment Using a Bio-Hedge Water Hyacinth Wetland System. Water 2015, 7, 329–347. [Google Scholar] [CrossRef]
- Bagheri, M.; Bauer, T.; Burgman, L.E.; Wetterlund, E. Fifty Years of Sewage Sludge Management Research: Mapping Researchers’ Motivations and Concerns. J. Environ. Manag. 2023, 325, 116412. [Google Scholar] [CrossRef]
- Gottardo, M.; Crognale, S.; Tonanzi, B.; Rossetti, S.; D’Annibale, L.; Dosta, J.; Valentino, F. Volatile Fatty Acid Production from Hydrolyzed Sewage Sludge: Effect of Hydraulic Retention Time and Insight into Thermophilic Microbial Community. Biomass Conv. Bioref. 2022, 1–12. [Google Scholar] [CrossRef]
- Raheem, A.; Sikarwar, V.S.; He, J.; Dastyar, W.; Dionysiou, D.D.; Wang, W.; Zhao, M. Opportunities and Challenges in Sustainable Treatment and Resource Reuse of Sewage Sludge: A Review. Chem. Eng. J. 2018, 337, 616–641. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Liang, S.; Guan, R.; Shi, Y.; Yu, W.; Zhu, S.; Fan, W.; Hou, H.; Hu, J.; et al. Synergistic Effect of Water Content and Composite Conditioner of Fenton’s Reagent Combined with Red Mud on the Enhanced Hydrogen Production from Sludge Pyrolysis. Water Res. 2017, 123, 378–387. [Google Scholar] [CrossRef]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of Sewage Sludge in a Screw-Feeding Reactor: Products Characterization and Ecological Risk Assessment of Heavy Metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Enebe, N.L.; Chigor, C.B.; Obileke, K.; Lawal, M.S.; Enebe, M.C. Biogas and Syngas Production from Sewage Sludge: A Sustainable Source of Energy Generation. Methane 2023, 2, 192–217. [Google Scholar] [CrossRef]
- IEA. Tracking Clean Energy Progress; IEA: Paris, France, 2023. [Google Scholar]
- IEA. Energy Technology Perspectives 2017: Catalysing Energy Technology Transformations; Organisation for Economic Co-Operation and Development: Paris, France, 2017. [Google Scholar]
- Aldenius, M.; Mullen, C.; Pettersson-Löfstedt, F. Electric Buses in England and Sweden—Overcoming Barriers to Introduction. Transp. Res. Part D Transp. Environ. 2022, 104, 103204. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Sreekrishnan, T.R. Anaerobic Digestion from the Viewpoint of Microbiological, Chemical, and Operational Aspects—A Review. Environ. Rev. 2010, 18, 255–278. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile Fatty Acids Production from Food Waste: Effects of pH, Temperature, and Organic Loading Rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Senthil Kumar, P.; Varjani, S. Valorization of Agro-Industrial Wastes for Biorefinery Process and Circular Bioeconomy: A Critical Review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar] [CrossRef]
- Patel, A.; Sarkar, O.; Rova, U.; Christakopoulos, P.; Matsakas, L. Valorization of Volatile Fatty Acids Derived from Low-Cost Organic Waste for Lipogenesis in Oleaginous Microorganisms—A Review. Bioresour. Technol. 2021, 321, 124457. [Google Scholar] [CrossRef]
- Lin, M.; Feng, L.; Cheng, Z.; Wang, K. Effect of Ethanol or Lactic Acid on Volatile Fatty Acid Profile and Microbial Community in Short-Term Sequentially Transfers by Ruminal Fermented with Wheat Straw In Vitro. Process Biochem. 2021, 102, 369–375. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. The Effects of pH on the Production of Volatile Fatty Acids and Microbial Dynamics in Long-Term Reactor Operation. J. Environ. Manag. 2022, 319, 115700. [Google Scholar] [CrossRef]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile Fatty Acid Production from Mesophilic Acidogenic Fermentation of Organic Fraction of Municipal Solid Waste and Food Waste under Acidic and Alkaline pH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and Retention Time on Volatile Fatty Acids Production during Mixed Culture Fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef]
- Soomro, A.F.; Abbasi, I.A.; Ni, Z.; Ying, L.; Liu, J. Influence of Temperature on Enhancement of Volatile Fatty Acids Fermentation from Organic Fraction of Municipal Solid Waste: Synergism between Food and Paper Components. Bioresour. Technol. 2020, 304, 122980. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, Y.M.; Choi, Y.-N.; Kim, H.G.; Park, J.M. Influence of Temperature on Volatile Fatty Acid Production and Microbial Community Structure during Anaerobic Fermentation of Microalgae. Bioresour. Technol. 2015, 191, 475–480. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-Based Volatile Fatty Acid Production and Recovery from Waste Streams: Current Status and Future Challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhang, Y.; Pan, Y.-R.; Li, L.; Liu, J.; Butler, D. Stepwise pH Control to Promote Synergy of Chemical and Biological Processes for Augmenting Short-Chain Fatty Acid Production from Anaerobic Sludge Fermentation. Water Res. 2019, 155, 193–203. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.; Liu, Y.; Ngo, H.H.; Guo, W.; Yang, Q.; Li, X. Novel Stepwise pH Control Strategy to Improve Short Chain Fatty Acid Production from Sludge Anaerobic Fermentation. Bioresour. Technol. 2018, 249, 431–438. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors Influencing Volatile Fatty Acids Production from Food Wastes via Anaerobic Digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Ueno, Y.; Haruta, S.; Ishii, M.; Igarashi, Y. Microbial Community in Anaerobic Hydrogen-Producing Microflora Enriched from Sludge Compost. Appl. Microbiol. Biotechnol. 2001, 57, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zheng, X.-J.; Yu, H.-Q.; Zhu, R.-F. Biological Hydrogen Production by Anaerobic Sludge at Various Temperatures. Int. J. Hydrogen Energy 2006, 31, 780–785. [Google Scholar] [CrossRef]
- Lim, S.-J.; Kim, B.J.; Jeong, C.-M.; Choi, J.; Ahn, Y.H.; Chang, H.N. Anaerobic Organic Acid Production of Food Waste in Once-a-Day Feeding and Drawing-off Bioreactor. Bioresour. Technol. 2008, 99, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, H.; Waki, M.; Moriya, N.; Yasuda, T.; Tanaka, Y.; Haga, K. Effect of Fermentation Temperature on Hydrogen Production from Cow Waste Slurry by Using Anaerobic Microflora within the Slurry. Appl. Microbiol. Biotechnol. 2007, 74, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Banu, J.R.; Kavitha, S.; Kannah, R.Y.; Usman, T.M.M.; Kumar, G. Application of Chemo Thermal Coupled Sonic Homogenization of Marine Macroalgal Biomass for Energy Efficient Volatile Fatty Acid Recovery. Bioresour. Technol. 2020, 303, 122951. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of Anaerobic Digestion for Volatile Fatty Acids, Hydrogen or Methane Production: A Critical Review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-Q. A Microbial Polyhydroxyalkanoates (PHA) Based Bio- and Materials Industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef] [PubMed]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent Advances and Challenges towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic Fermentation of Food Waste for Volatile Fatty Acid Production with Co-Generation of Biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Kucek, L.A.; Nguyen, M.; Angenent, L.T. Conversion of L-Lactate into n-Caproate by a Continuously Fed Reactor Microbiome. Water Res. 2016, 93, 163–171. [Google Scholar] [CrossRef]
- Clark, J.H.; Budarin, V.; Deswarte, F.E.I.; Hardy, J.J.E.; Kerton, F.M.; Hunt, A.J.; Luque, R.; Macquarrie, D.J.; Milkowski, K.; Rodriguez, A.; et al. Green Chemistry and the Biorefinery: A Partnership for a Sustainable Future. Green Chem. 2006, 8, 853–860. [Google Scholar] [CrossRef]
- Parchami, M.; Wainaina, S.; Mahboubi, A.; I’Ons, D.; Taherzadeh, M.J. MBR-Assisted VFAs Production from Excess Sewage Sludge and Food Waste Slurry for Sustainable Wastewater Treatment. Appl. Sci. 2020, 10, 2921. [Google Scholar] [CrossRef]
- Yesil, H.; Taner, H.; Ugur Nigiz, F.; Hilmioglu, N.; Tugtas, A.E. Pervaporative Separation of Mixed Volatile Fatty Acids: A Study Towards Integrated VFA Production and Separation. Waste Biomass Valor. 2020, 11, 1737–1753. [Google Scholar] [CrossRef]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of Membrane Processes for the Recovery and Purification of Bio-Based Volatile Fatty Acids: A Comprehensive Review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Fufachev, E.; van Munster, K.D.; van Beek, M.B.M.; Sprakel, L.M.J.; Edelijn, C.N.; Weckhuysen, B.M.; Kersten, S.R.A.; Bruijnincx, P.C.A.; Schuur, B. Recovery and Conversion of Acetic Acid from a Phosphonium Phosphinate Ionic Liquid to Enable Valorization of Fermented Wastewater. Green Chem. 2019, 21, 2023–2034. [Google Scholar] [CrossRef]
- Eregowda, T.; Rene, E.R.; Rintala, J.; Lens, P.N.L. Volatile Fatty Acid Adsorption on Anion Exchange Resins: Kinetics and Selective Recovery of Acetic Acid. Sep. Sci. Technol. 2020, 55, 1449–1461. [Google Scholar] [CrossRef]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Azan Tajarudin, H. Selective Adsorption and Recovery of Volatile Fatty Acids from Fermented Landfill Leachate by Activated Carbon Process. Sci. Total Environ. 2020, 707, 134533. [Google Scholar] [CrossRef]
- Aydin, S.; Yesil, H.; Tugtas, A.E. Recovery of Mixed Volatile Fatty Acids from Anaerobically Fermented Organic Wastes by Vapor Permeation Membrane Contactors. Bioresour. Technol. 2018, 250, 548–555. [Google Scholar] [CrossRef]
- Hassan, G.K.; Massanet-Nicolau, J.; Dinsdale, R.; Jones, R.J.; Abo-Aly, M.M.; El-Gohary, F.A.; Guwy, A. A Novel Method for Increasing Biohydrogen Production from Food Waste Using Electrodialysis. Int. J. Hydrogen Energy 2019, 44, 14715–14720. [Google Scholar] [CrossRef]
- Chalmers Brown, R.; Tuffou, R.; Massanet Nicolau, J.; Dinsdale, R.; Guwy, A. Overcoming Nutrient Loss during Volatile Fatty Acid Recovery from Fermentation Media by Addition of Electrodialysis to a Polytetrafluoroethylene Membrane Stack. Bioresour. Technol. 2020, 301, 122543. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Park, Y.-K.; González-Fernández, C.; Robles-Iglesias, R.; Vidal, L.; Fontanille, P.; Kennes, C.; Tomás Pejó, E.; Nicaud, J.-M.; Fickers, P. Bioproducts Generation from Carboxylate Platforms by the Non-Conventional Yeast Yarrowia lipolytica. FEMS Yeast Res. 2021, 21, foab047. [Google Scholar] [CrossRef] [PubMed]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J.; Kim, N.; Kang, J. The Effect of Volatile Fatty Acids as a Sole Carbon Source on Lipid Accumulation by Cryptococcus albidus for Biodiesel Production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of Volatile Fatty Acids into Lipids by the Oleaginous Yeast Yarrowia Lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef]
- Huang, X.-F.; Liu, J.-N.; Lu, L.-J.; Peng, K.-M.; Yang, G.-X.; Liu, J. Culture Strategies for Lipid Production Using Acetic Acid as Sole Carbon Source by Rhodosporidium toruloides. Bioresour. Technol. 2016, 206, 141–149. [Google Scholar] [CrossRef]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous Yeast Yarrowia lipolytica Culture with Synthetic and Food Waste-Derived Volatile Fatty Acids for Lipid Production. Biotechnol. Biofuels 2017, 10, 247. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, X.; Chen, R.; Yuan, M.; Liu, J. Efficient Bioconversion of High-Content Volatile Fatty Acids into Microbial Lipids by Cryptococcus Curvatus ATCC 20509. Bioresour Technol 2017, 239, 394–401. [Google Scholar] [CrossRef]
- El Kantar, S.; Koubaa, M. Valorization of Low-Cost Substrates for the Production of Odd Chain Fatty Acids by the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 284. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of Oleaginous Yeasts for Lipid Production Using Volatile Fatty Acids as Substrate. Biomass Bioenergy 2020, 138, 105553. [Google Scholar] [CrossRef]
- Morales-Palomo, S.; González-Fernández, C.; Tomás-Pejó, E. Prevailing Acid Determines the Efficiency of Oleaginous Fermentation from Volatile Fatty Acids. J. Environ. Chem. Eng. 2022, 10, 107354. [Google Scholar] [CrossRef]
- Krikigianni, E.; Matsakas, L.; Rova, U.; Christakopoulos, P.; Patel, A. Investigating the Bioconversion Potential of Volatile Fatty Acids: Use of Oleaginous Yeasts Rhodosporidium toruloides and Cryptococcus curvatus towards the Sustainable Production of Biodiesel and Odd-Chain Fatty Acids. Appl. Sci. 2022, 12, 6541. [Google Scholar] [CrossRef]
- Al Sahyouni, W.; El Kantar, S.; Khelfa, A.; Park, Y.-K.; Nicaud, J.-M.; Louka, N.; Koubaa, M. Optimization of Cis-9-Heptadecenoic Acid Production from the Oleaginous Yeast Yarrowia lipolytica. Fermentation 2022, 8, 245. [Google Scholar] [CrossRef]
- Park, Y.-K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.-M. Optimization of Odd Chain Fatty Acid Production by Yarrowia lipolytica. Biotechnol. Biofuels 2018, 11, 158. [Google Scholar] [CrossRef] [PubMed]
- Park, G.W.; Son, S.; Moon, M.; Sin, S.; Min, K.; Lee, J.-S.; Chang, H.N. Volatile Fatty Acids from Lipid-Extracted Yeast Provide Additional Feedstock for Microbial Lipid Production. Catalysts 2021, 11, 1009. [Google Scholar] [CrossRef]
- Liew, F.; Martin, M.E.; Tappel, R.C.; Heijstra, B.D.; Mihalcea, C.; Köpke, M. Gas Fermentation—A Flexible Platform for Commercial Scale Production of Low-Carbon-Fuels and Chemicals from Waste and Renewable Feedstocks. Front. Microbiol. 2016, 7, 694. [Google Scholar] [CrossRef] [PubMed]
- Liakakou, E.T.; Infantes, A.; Neumann, A.; Vreugdenhil, B.J. Connecting Gasification with Syngas Fermentation: Comparison of the Performance of Lignin and Beech Wood. Fuel 2021, 290, 120054. [Google Scholar] [CrossRef]
- Rückel, A.; Hannemann, J.; Maierhofer, C.; Fuchs, A.; Weuster-Botz, D. Studies on Syngas Fermentation With Clostridium Carboxidivorans in Stirred-Tank Reactors with Defined Gas Impurities. Front. Microbiol. 2021, 12, 655390. [Google Scholar] [CrossRef]
- Phillips, J.R.; Huhnke, R.L.; Atiyeh, H.K. Syngas Fermentation: A Microbial Conversion Process of Gaseous Substrates to Various Products. Fermentation 2017, 3, 28. [Google Scholar] [CrossRef]
- Dhakal, N.; Acharya, B. Syngas Fermentation for the Production of Bio-Based Polymers: A Review. Polymers 2021, 13, 3917. [Google Scholar] [CrossRef]
- Ellacuriaga, M.; Gil, M.V.; Gómez, X. Syngas Fermentation: Cleaning of Syngas as a Critical Stage in Fermentation Performance. Fermentation 2023, 9, 898. [Google Scholar] [CrossRef]
- Chawl, S.K.; George, M.; Patel, F.; Patel, S. Production of Synthesis Gas by Carbon Dioxide Reforming of Methane over Nickel Based and Perovskite Catalysts. Procedia Eng. 2013, 51, 461–466. [Google Scholar] [CrossRef]
- Kurdi, A.N.; Ibrahim, A.A.; Al-Fatesh, A.S.; Alquraini, A.A.; Abasaeed, A.E.; Fakeeha, A.H. Hydrogen Production from CO2 Reforming of Methane Using Zirconia Supported Nickel Catalyst. RSC Adv. 2022, 12, 10846–10854. [Google Scholar] [CrossRef] [PubMed]
- RECORD, Réseau Coopératif de Recherche sur les Déchets et L’environnement. Les Filières de Valorisation Du CO2—État de L’art et Avis D’experts. France, 2014, 246p, 12-0237/1A. Available online: https://record-net.org/storage/etudes/12-0237-1A/rapport/Rapport_record12-0237_1A.pdf (accessed on 21 May 2024).
- Das, T.; Al-Waili, I.; Balasubramanian, V.; Appleby, G.; Kaparaju, P.; Parthasarathy, R.; Eshtiaghi, N. Process Modelling and Techno-Economic Analysis of Anaerobic Digestion of Sewage Sludge Integrated with Wet Oxidation Using a Gravity Pressure Vessel and Thermal Hydrolysis. Sci. Total Environ. 2024, 912, 169024. [Google Scholar] [CrossRef] [PubMed]
- Veluswamy, G.K.; Shah, K.; Ball, A.S.; Guwy, A.J.; Dinsdale, R.M. A Techno-Economic Case for Volatile Fatty Acid Production for Increased Sustainability in the Wastewater Treatment Industry. Environ. Sci. Water Res. Technol. 2021, 7, 927–941. [Google Scholar] [CrossRef]
- Ps, R.; Yd, Y.; Sh, K.; Cs, P. Techno-Economic Analysis of Power to Gas (P2G) Process for the Development of Optimum Business Model: Part 1 Methane Production. Clean Technol. 2022, 28, 182–192. [Google Scholar] [CrossRef]
- Böhm, H.; Lehner, M.; Kienberger, T. Techno-Economic Assessment of Thermally Integrated Co-Electrolysis and Methanation for Industrial Closed Carbon Cycles. Front. Sustain. 2021, 2, 726332. [Google Scholar] [CrossRef]
- Park, S.; Choi, K.; Lee, C.; Kim, S.; Yoo, Y.; Chang, D. Techno-Economic Analysis of Adiabatic Four-Stage CO2 Methanation Process for Optimization and Evaluation of Power-to-Gas Technology. Int. J. Hydrogen Energy 2021, 46, 21303–21317. [Google Scholar] [CrossRef]
- Gracia, J.; Cabeza, I.; Acevedo, P. Life Cycle Analysis for the Production of Volatile Fatty Acids from Wastewater Treatment Plant Sludge. Cogent Eng. 2024, 11, 2335846. [Google Scholar] [CrossRef]
- Pinto, A.S.S.; McDonald, L.J.; Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.; McManus, M. Production of Volatile Fatty Acids by Anaerobic Digestion of Biowastes: Techno-Economic and Life Cycle Assessments. Bioresour. Technol. 2023, 388, 129726. [Google Scholar] [CrossRef]
- Bartek, L.; Strid, I.; Henryson, K.; Junne, S.; Rasi, S.; Eriksson, M. Life Cycle Assessment of Fish Oil Substitute Produced by Microalgae Using Food Waste. Sustain. Prod. Consum. 2021, 27, 2002–2021. [Google Scholar] [CrossRef]
- Pati, S.; De, S.; Chowdhury, R. Integrated Techno-Economic, Investment Risk and Life Cycle Analysis of Indian Lignocellulosic Biomass Valorisation via Co-Gasification and Syngas Fermentation. J. Clean. Prod. 2023, 423, 138744. [Google Scholar] [CrossRef]
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol Fuel Production, Utilization, and Techno-Economy: A Review. Env. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Muthudineshkumar, R.; Anand, R. Life Cycle Assessment on Biofuel Production from Biomass Gasification and Syngas Fermentation. IOP Conf. Ser. Earth Environ. Sci. 2019, 312, 012016. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Conversion of Biomass to Biofuels and Life Cycle Assessment: A Review. Environ. Chem. Lett. 2021, 19, 4075–4118. [Google Scholar] [CrossRef]
- Moncada, J.; Posada, J.A.; Ramírez, A. Early Sustainability Assessment for Potential Configurations of Integrated Biorefineries. Screening of Bio-Based Derivatives from Platform Chemicals. Biofuels Bioprod. Biorefining 2015, 9, 722–748. [Google Scholar] [CrossRef]
- Aboudi, K.; Fernández-Güelfo, L.A.; Álvarez-Gallego, C.J.; Romero-García, L.I. Biogas, Biohydrogen, and Polyhydroxyalkanoates Production from Organic Waste in the Circular Economy Context. In Sustainable Biofuels; Ray, R.C., Ed.; Applied Biotechnology Reviews; Academic Press: Cambridge, MA, USA, 2021; pp. 305–343. ISBN 978-0-12-820297-5. [Google Scholar]
- Bakkaloglu, S.; Cooper, J.; Hawkes, A. Life Cycle Environmental Impact Assessment of Methane Emissions from the Biowaste Management Strategy of the United Kingdom: Towards Net Zero Emissions. J. Clean. Prod. 2022, 376, 134229. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koubaa, M. Integrated Biorefinery for a Next-Generation Methanization Process Focusing on Volatile Fatty Acid Valorization: A Critical Review. Molecules 2024, 29, 2477. https://doi.org/10.3390/molecules29112477
Koubaa M. Integrated Biorefinery for a Next-Generation Methanization Process Focusing on Volatile Fatty Acid Valorization: A Critical Review. Molecules. 2024; 29(11):2477. https://doi.org/10.3390/molecules29112477
Chicago/Turabian StyleKoubaa, Mohamed. 2024. "Integrated Biorefinery for a Next-Generation Methanization Process Focusing on Volatile Fatty Acid Valorization: A Critical Review" Molecules 29, no. 11: 2477. https://doi.org/10.3390/molecules29112477
APA StyleKoubaa, M. (2024). Integrated Biorefinery for a Next-Generation Methanization Process Focusing on Volatile Fatty Acid Valorization: A Critical Review. Molecules, 29(11), 2477. https://doi.org/10.3390/molecules29112477





