Effective Synthesis of High-Integrity mRNA Using In Vitro Transcription
Abstract
1. Introduction
2. Results and Discussion
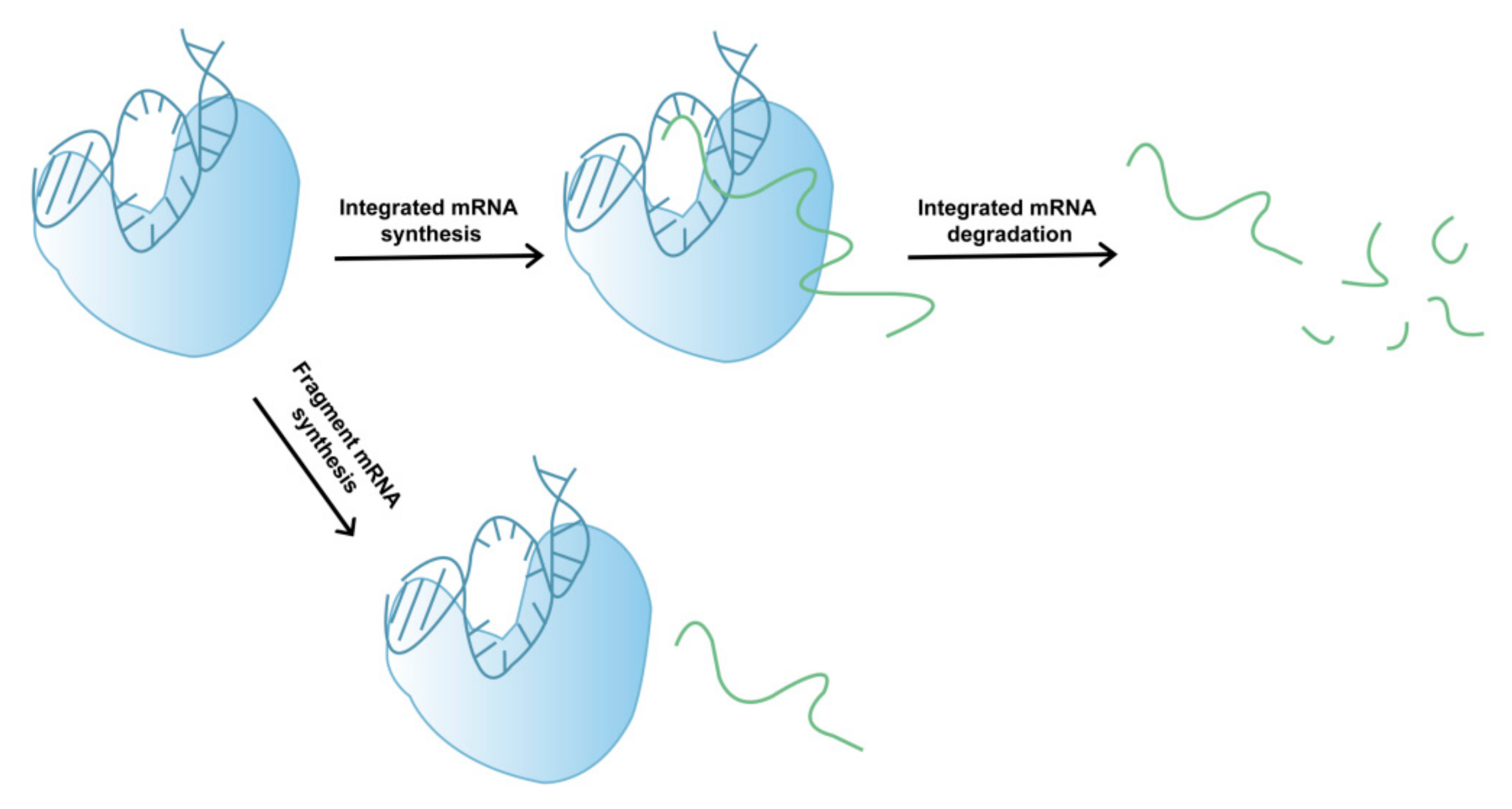
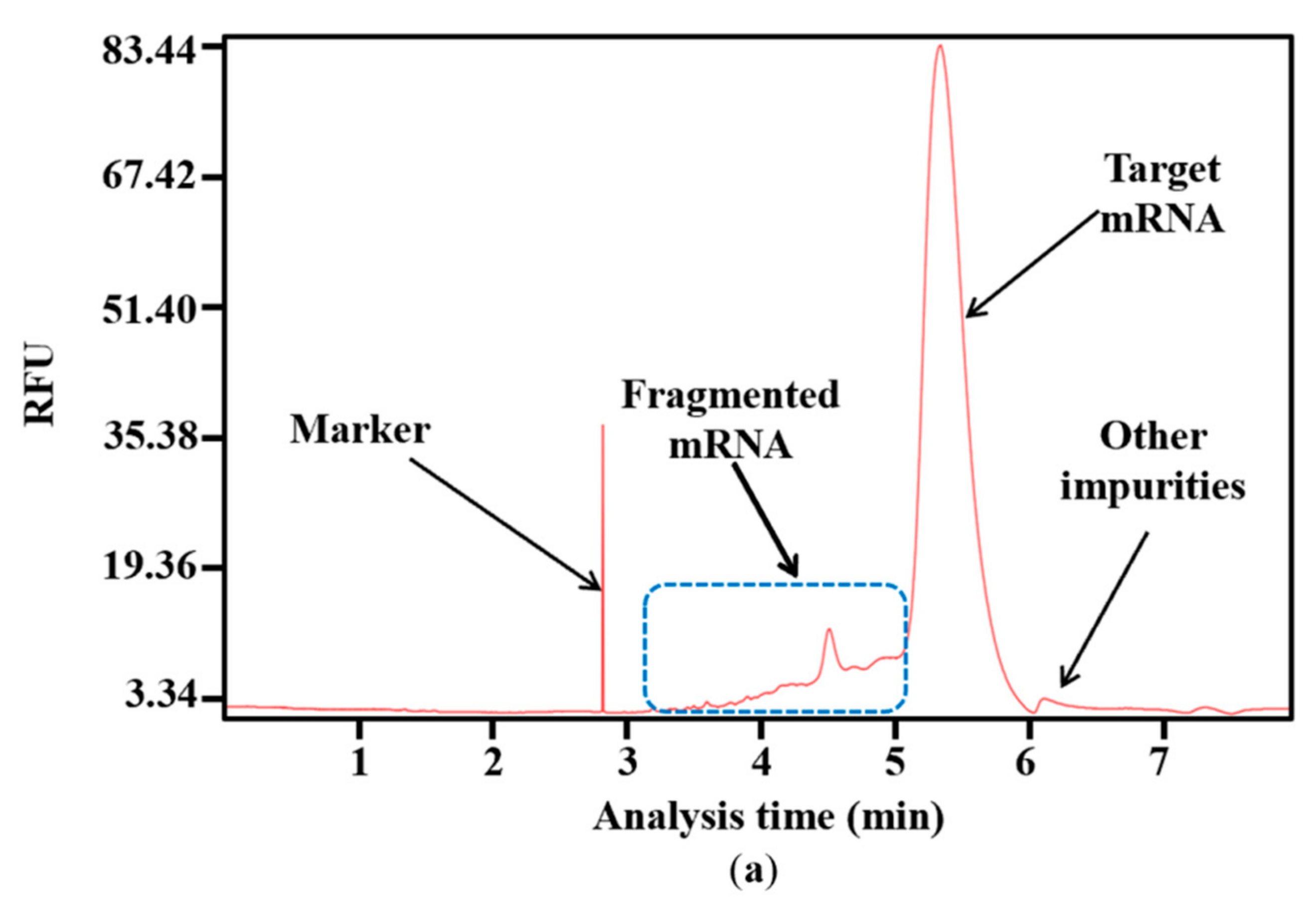
2.1. Fragmented mRNA Produced In Vitro Transcription
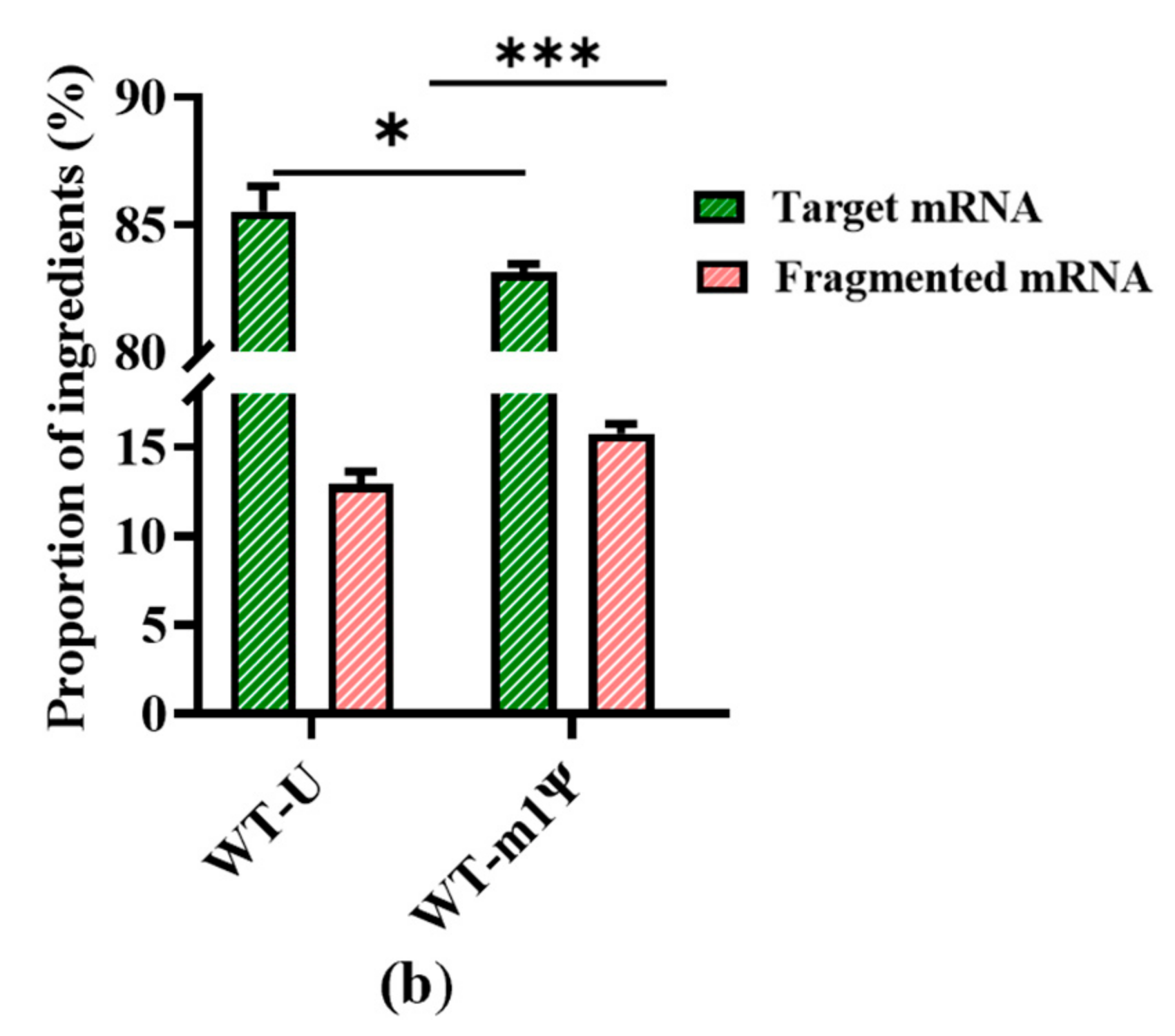
2.2. T7 RNAP Substitution Screening for Reducing Early Termination and the Formation of Fragmented mRNA
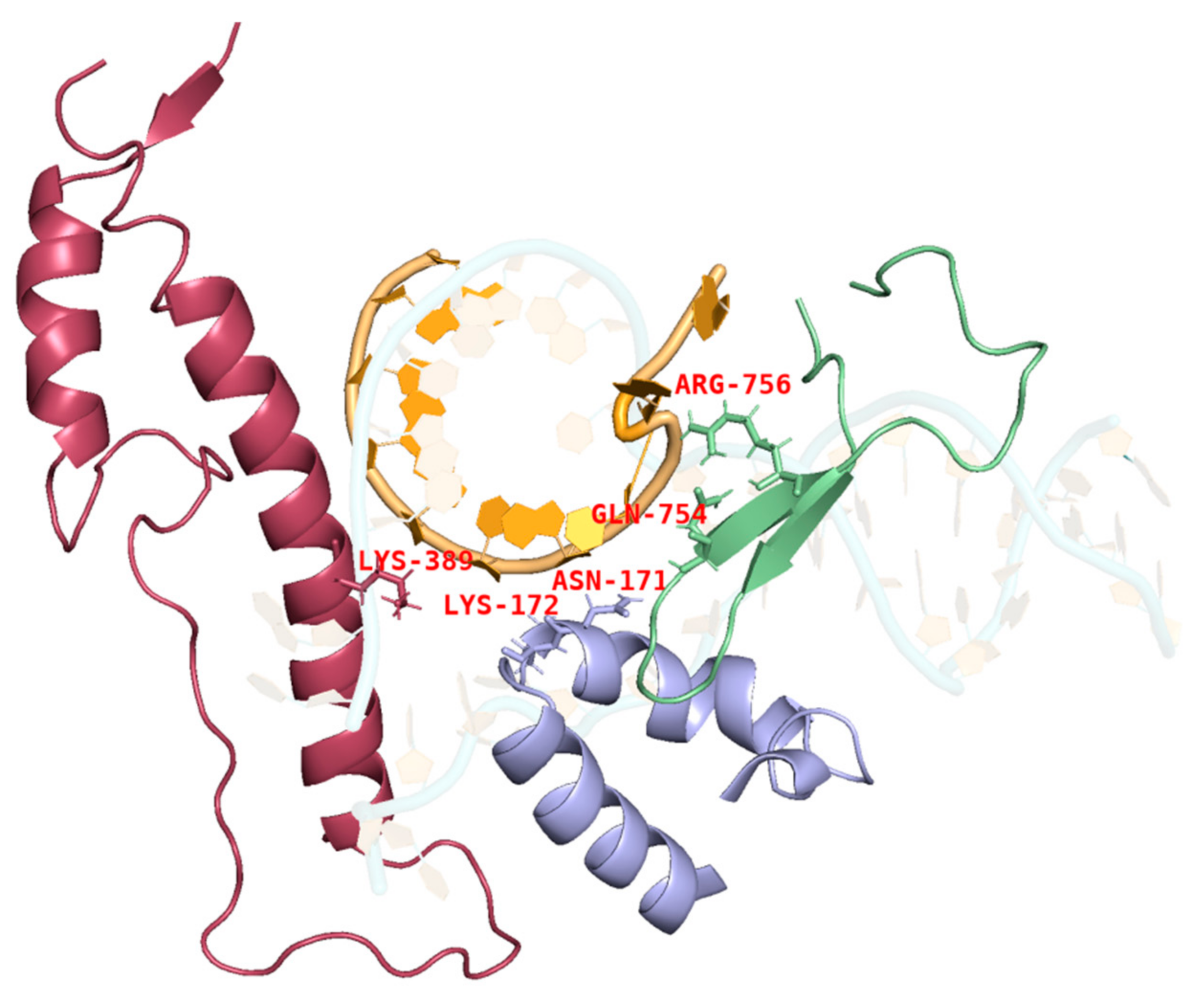
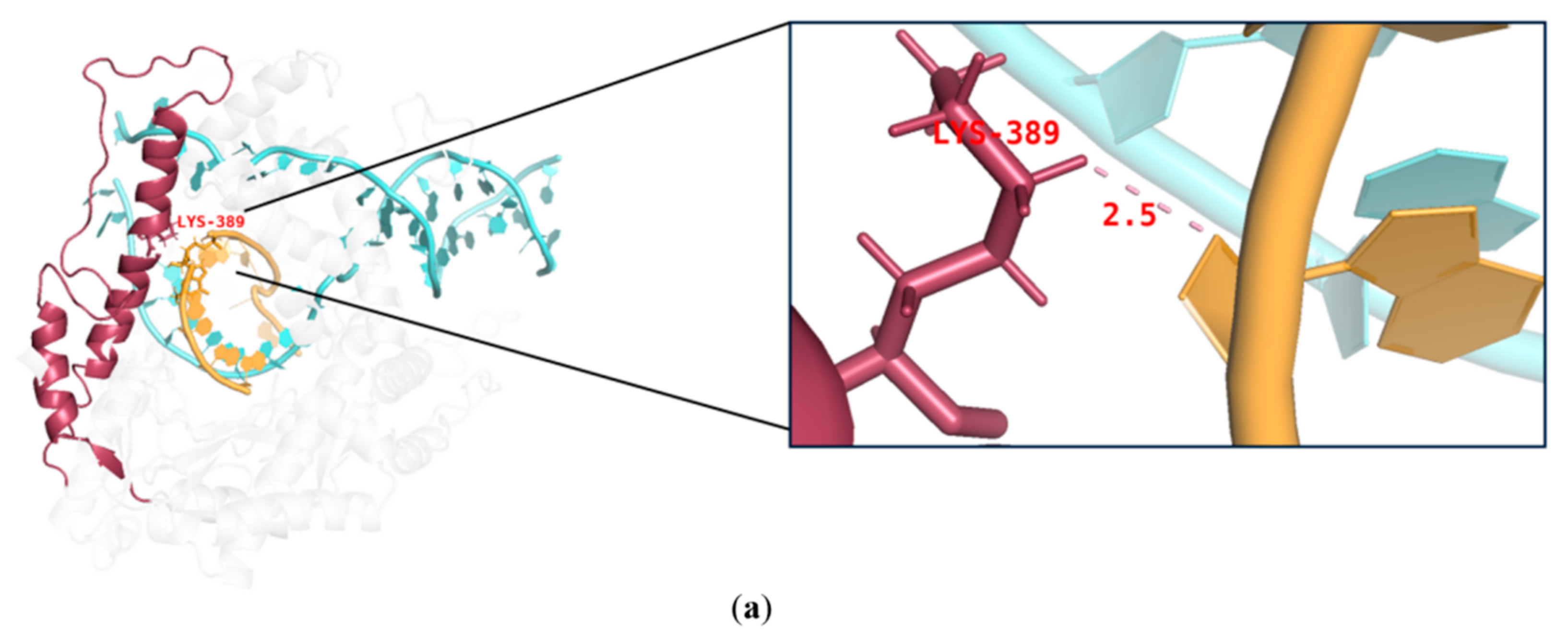
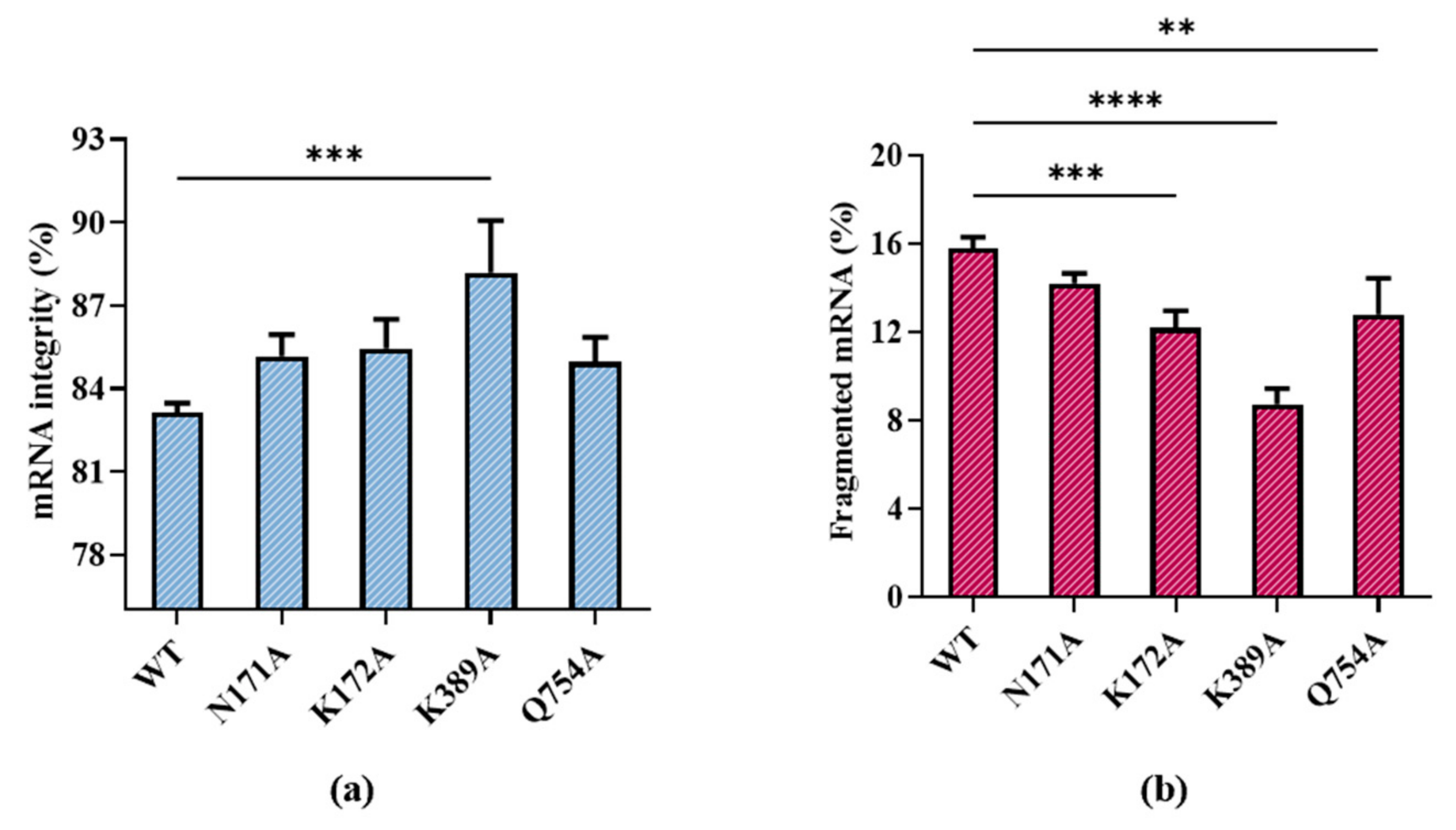
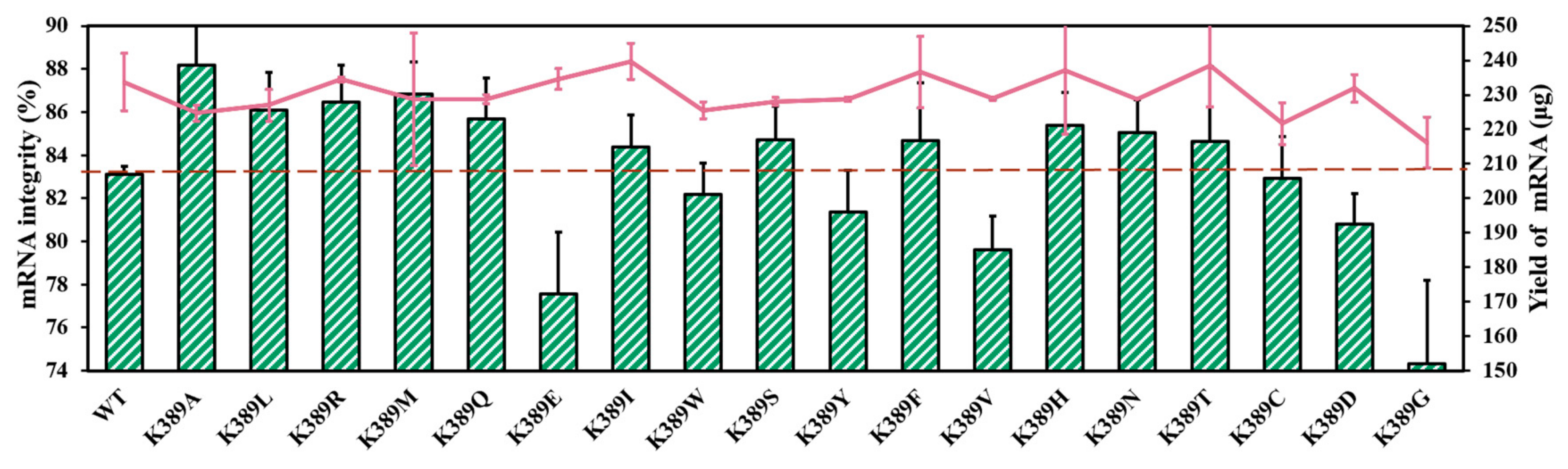
2.3. mRNA Integrity Correlated with Amino Acid α-Helix Propensity of Site K389
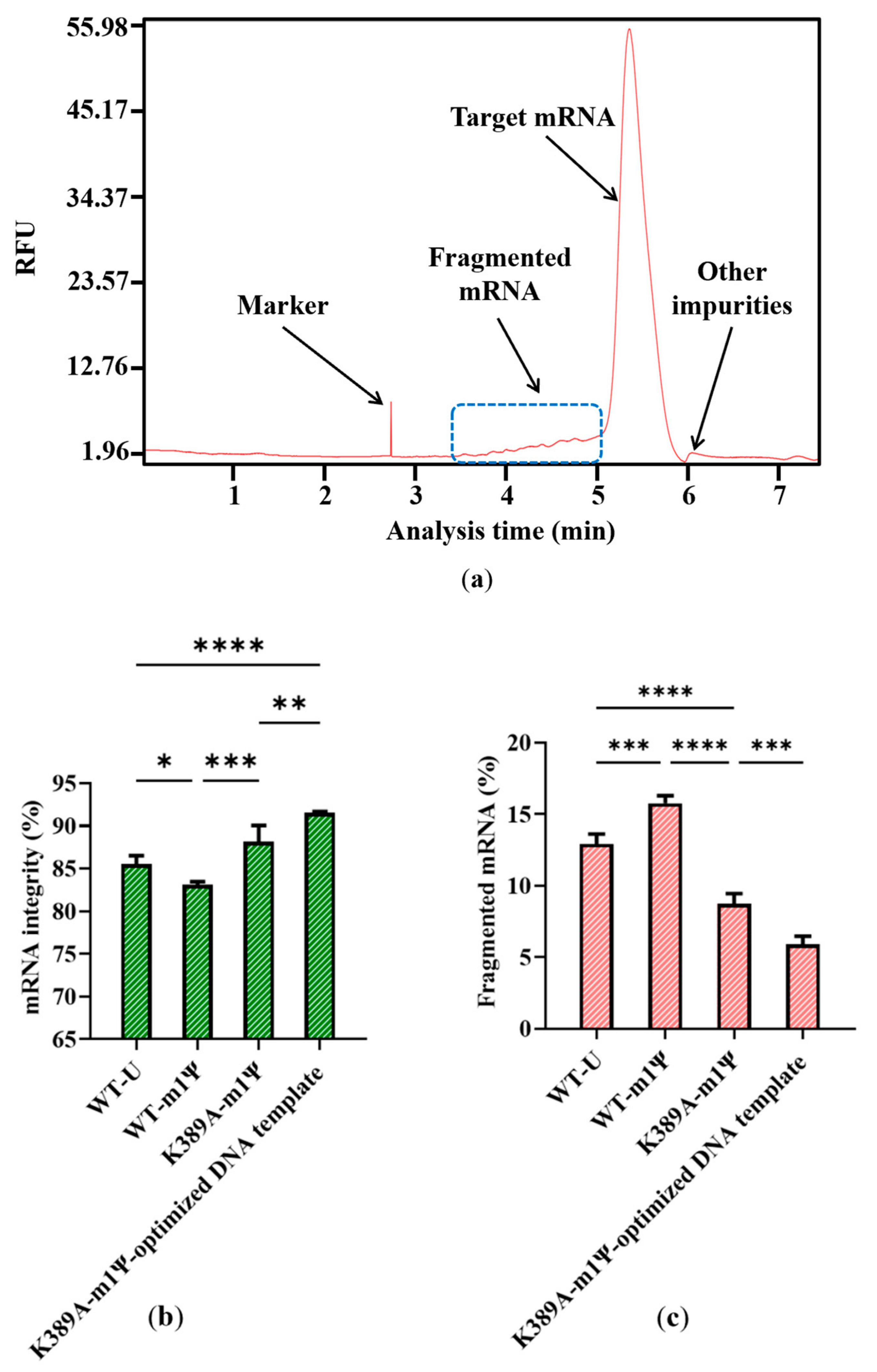
2.4. Optimized DNA Template Further Increase mRNA Integrity
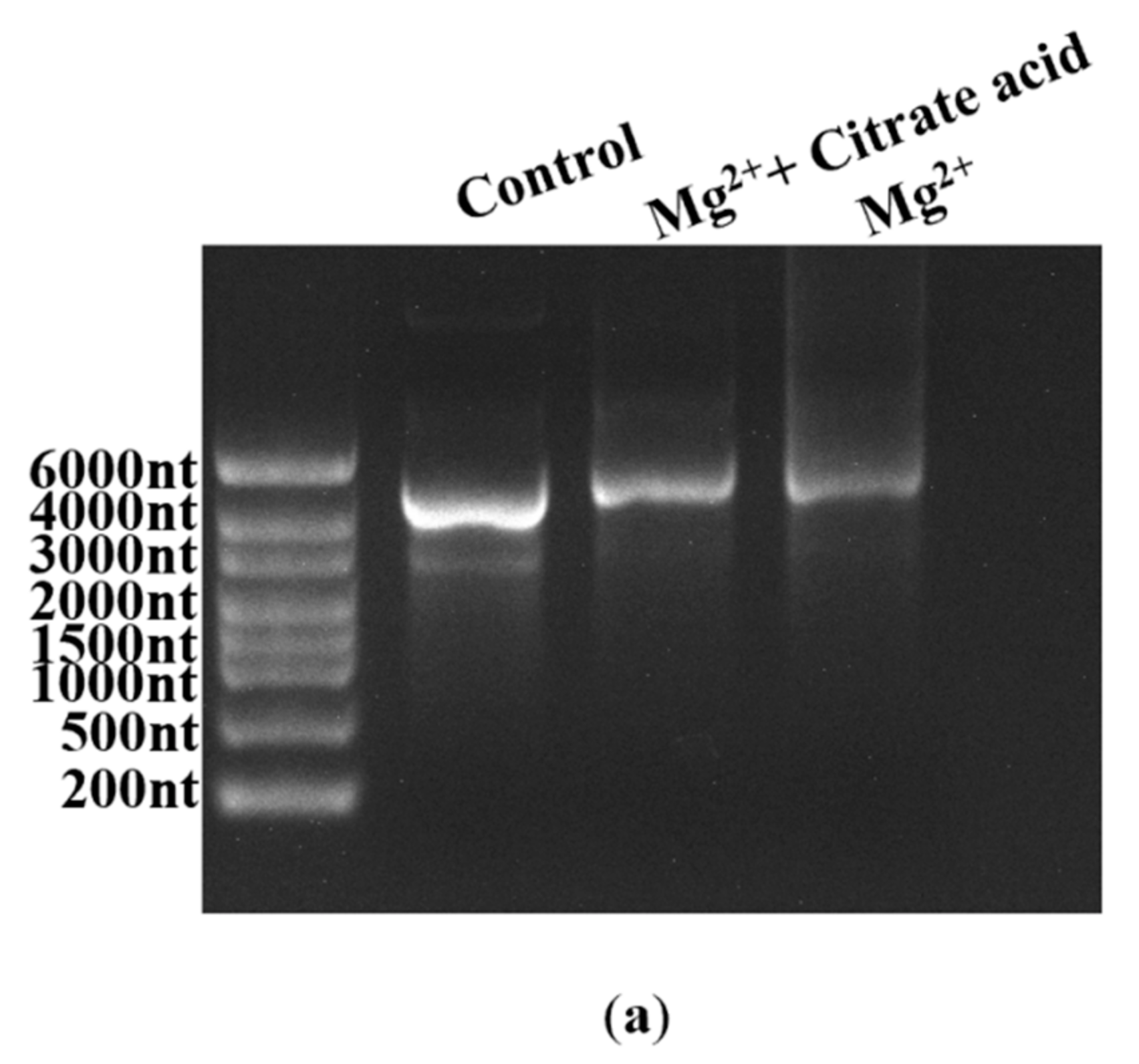
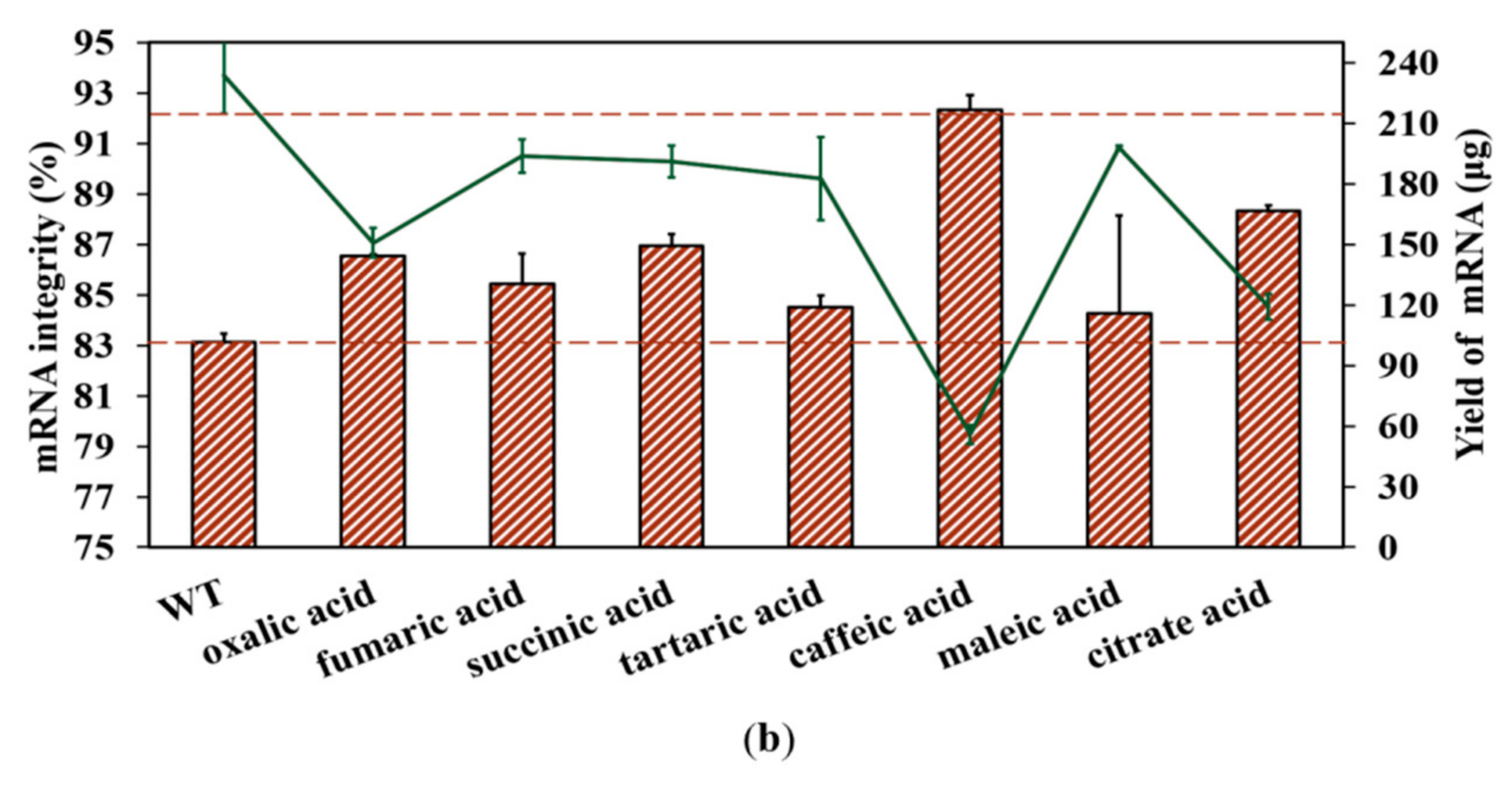
2.5. Increase in mRNA Integrity via Addition of Organic Acid
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Cloning and Heterologous Expression of T7 RNAP
3.3. Purification of T7 RNAP
3.4. Measurement of Specific Activity
3.5. In Vitro Transcription and Measurement of mRNA Integrity
3.6. Mg2+-Induced mRNA Degradation
3.7. Optimization of mRNA Integrity
3.8. Fragmented mRNA Measurement Using LC-MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.W.; Lagniton, P.N.P.; Liu, Y.; Xu, R.H. mRNA vaccines for COVID-19: What, why and how. Int. J. Biol. Sci. 2021, 17, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Nance, K.D.; Meier, J.L. Modifications in an Emergency: The Role of N1-Methylpseudouridine in COVID-19 Vaccines. ACS Cent. Sci. 2021, 7, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 mRNA vaccine development. Signal. Transduct. Target. Ther. 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Lamb, Y.N. BNT162b2 mRNA COVID-19 Vaccine: First Approval. Drugs 2021, 81, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Bolla, P.K.; Kommineni, N.; Butreddy, A.; Nukala, P.K.; Palakurthi, S.S.; Khan, W.A. Comprehensive Review of mRNA Vaccines. Int. J. Mol. Sci. 2023, 24, 2700. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Kwon, M.; Im, S.; Lee, K.; Lee, H. mRNA vaccines: The most recent clinical applications of synthetic mRNA. Arch. Pharmacal. Res. 2022, 45, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Zhang, Y.; Huang, L. mRNA vaccine for cancer immunotherapy. Mol. Cancer 2021, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Tian, Y.; Song, J.; An, G.; Yang, P. mRNA Vaccines: The Dawn of a New Era of Cancer Immunotherapy. Front. Immunol. 2022, 13, 887125. [Google Scholar] [CrossRef]
- Connolly, C.D.; Quinonez, S.C.; Ames, E.G. Rare disease therapeutics: The future of medical genetics in a changing landscape. Genet. Med. 2023, 25, 100339. [Google Scholar] [CrossRef]
- Khan, T.; Khan, A.; Ansari, J.K.; Najmi, M.H.; Wei, D.Q.; Muhammad, K.; Waheed, Y. Potential Immunogenic Activity of Computationally Designed mRNA- and Peptide-Based Prophylactic Vaccines against MERS, SARS-CoV, and SARS-CoV-2: A Reverse Vaccinology Approach. Molecules 2022, 27, 2375. [Google Scholar] [CrossRef] [PubMed]
- Henderson, J.M.; Ujita, A.; Hill, E.; Yousif-Rosales, S.; Smith, C.; Ko, N.; McReynolds, T.; Cabral, C.R.; Escamilla-Powers, J.R.; Houston, M.E. Cap 1 messenger RNA synthesis with Co-transcriptional cleancap® analog by in vitro transcription. Curr. Protoc. 2021, 1, e39. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Z.; Asahara, H.; Tzertzinis, G.; Roy, B. Synthesis of low immunogenicity RNA with high-temperature in vitro transcription. RNA 2020, 26, 345–360. [Google Scholar] [CrossRef]
- Kang, D.D.; Li, H.Y.; Dong, Y.Z. Advancements of in vitro transcribed mRNA (IVT mRNA) to enable translation into the clinics. Adv. Drug Deliv. Rev. 2023, 199, 114961. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.L.; Wilusz, J. In vitro transcription of modified RNAs. Methods Mol. Biol. 2012, 941, 171–180. [Google Scholar] [PubMed]
- Paschal, B.M.; McReynolds, L.A.; Noren, C.J.; Nichols, N.M. RNA polymerases. In Current Protocols in Molecular Biology; Wiley: Hoboken, NJ, USA, 2008; Chapter 3, Unit 3. [Google Scholar]
- Borkotoky, S.; Murali, A. The highly efficient T7 RNA polymerase: A wonder macromolecule in biological realm. Int. J. Biol. Macromol. 2018, 118, 49–56. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Zhang, X.; Zou, Y.; Li, J.; Chang, L.; He, Y.-C.; Jin, Q.; Ye, J. Effective synthesis of circRNA via a thermostable T7 RNA polymerase variant as the catalyst. Front. Bioeng. Biotechnol. 2024, 12, 1356354. [Google Scholar] [CrossRef]
- Bradley, C.C.; Gordon, A.J.E.; Wang, C.; Cooke, M.B.; Kohrn, B.F.; Kennedy, S.R.; Lichtarge, O.; Ronca, S.E.; Herman, C. RNA polymerase inaccuracy underlies SARS-CoV-2 variants and vaccine heterogeneity. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Boulain, J.C.; Dassa, J.; Mesta, L.; Savatier, A.; Costa, N.; Muller, B.H.; L’Hostis, G.; Stura, E.A.; Troesch, A.; Ducancel, F. Mutants with higher stability and specific activity from a single thermosensitive variant of T7 RNA polymerase. Protein Eng. Des. Sel. 2013, 26, 725–734. [Google Scholar]
- Arnaud-Barbe, N.; Cheynet-Sauvion, V.; Oriol, G.; Mandrand, B.; Mallet, F. Transcription of RNA templates by T7 RNA polymerase. Nucleic Acids Res. 1998, 26, 3550–3554. [Google Scholar] [CrossRef]
- Dousis, A.; Ravichandran, K.; Hobert, E.M.; Moore, M.J.; Rabideau, A.E. An engineered T7 RNA polymerase that produces mRNA free of immunostimulatory byproducts. Nat. Biotechnol. 2023, 41, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Schlee, M.; Deddouche, S.; Pruijssers, A.J.; Zillinger, T.; Goldeck, M.; Schuberth, C.; Van der Veen, A.G.; Fujimura, T.; Rehwinkel, J.; et al. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5’-diphosphates. Nature 2014, 514, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Takeuchi, O.; Sato, S.; Yoneyama, M.; Yamamoto, M.; Matsui, K.; Uematsu, S.; Jung, A.; Kawai, T.; Ishii, K.J.; et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 2006, 441, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Greenwald, E.; Ahmad, S.; Hur, S. An origin of the immunogenicity of in vitro transcribed RNA. Nucleic Acids Res. 2018, 46, 5239–5249. [Google Scholar] [CrossRef] [PubMed]
- Camperi, J.; Lippold, S.; Ayalew, L.; Roper, B.; Shao, S.; Freund, E.; Nissenbaum, A.; Galan, C.; Cao, Q.; Yang, F.; et al. Comprehensive Impurity Profiling of mRNA: Evaluating Current Technologies and Advanced Analytical Techniques. Anal. Chem. 2024, 96, 3886–3897. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Klein, L.J.; Ha, S.; Rustandi, R.R. High-Resolution capillary electrophoresis separation of large RNA under non-aqueous conditions. J. Chromatogr. A 2020, 1618, 460875. [Google Scholar] [CrossRef]
- Daniel, S.; Kis, Z.; Kontoravdi, C.; Shah, N. Quality by Design for enabling RNA platform production processes. Trends Biotechnol. 2022, 40, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Kis, Z. Stability Modelling of mRNA Vaccine Quality Based on Temperature Monitoring throughout the Distribution Chain. Pharmaceutics 2022, 14, 430. [Google Scholar] [CrossRef] [PubMed]
- Oivanen, M.; Kuusela, S.; Lönnberg, H. Kinetics and Mechanisms for the Cleavage and Isomerization of the Phosphodiester Bonds of RNA by Brønsted Acids and Bases. Chem. Rev. 1998, 98, 961–990. [Google Scholar] [CrossRef]
- Wayment-Steele, H.K.; Kim, D.S.; Choe, C.A.; Nicol, J.J.; Wellington-Oguri, R.; Watkins, A.M.; Parra Sperberg, R.A.; Huang, P.S.; Participants, E.; Das, R. Theoretical basis for stabilizing messenger RNA through secondary structure design. Nucleic Acids Res. 2021, 49, 10604–10617. [Google Scholar] [CrossRef]
- Forconi, M.; Herschlag, D. Metal Ion-Based RNA Cleavage as a Structural Probe. In Biophysical, Chemical, and Functional Probes of RNA Structure, Interactions and Folding: Part, A; Academic Press: Cambridge, MA, USA, 2009; Volume 468, pp. 91–106. [Google Scholar]
- Markham, R.; Smith, J.D. The structure of ribonucleic acid. 1. Cyclic nucleotides produced by ribonuclease and by alkaline hydrolysis. Biochem. J. 1952, 52, 552–557. [Google Scholar] [CrossRef]
- Leppek, K.; Byeon, G.W.; Kladwang, W.; Wayment-Steele, H.K.; Kerr, C.H.; Xu, A.F.; Kim, D.S.; Topkar, V.V.; Choe, C.; Rothschild, D.; et al. Combinatorial optimization of mRNA structure, stability, and translation for RNA-based therapeutics. Nat. Commun. 2022, 13, 1536. [Google Scholar] [CrossRef] [PubMed]
- Schoenmaker, L.; Witzigmann, D.; Kulkarni, J.A.; Verbeke, R.; Kersten, G.; Jiskoot, W.; Crommelin, D.J.A. mRNA-lipid nanoparticle COVID-19 vaccines: Structure and stability. Int. J. Pharm. 2021, 601, 120586. [Google Scholar] [CrossRef]
- Martínez, J.; Lampaya, V.; Larraga, A.; Magallón, H.; Casabona, D. Purification of linearized template plasmid DNA decreases double-stranded RNA formation during IVT reaction. Front. Mol. Biosci. 2023, 10, 1248511. [Google Scholar] [CrossRef]
- Piao, X.; Yadav, V.; Wang, E.; Chang, W.; Tau, L.; Lindenmuth, B.E.; Wang, S.X. Double-stranded RNA reduction by chaotropic agents during in vitro transcription of messenger RNA. Mol. Ther. Nucleic Acids 2022, 29, 618–624. [Google Scholar] [CrossRef]
- Bonner, G.; Lafer, E.M.; Sousa, R. The thumb subdomain of T7 RNA polymerase functions to stabilize the ternary complex during processive transcription. J. Biol. Chem. 1994, 269, 25129–25136. [Google Scholar] [CrossRef]
- Gopal, V.; Brieba, L.G.; Guajardo, R.; McAllister, W.T.; Sousa, R. Characterization of structural features important for T7 RNAP elongation complex stability reveals competing complex conformations and a role for the non-template strand in RNA displacement. J. Mol. Biol. 1999, 290, 411–431. [Google Scholar] [CrossRef]
- Brieba, L.G.; Gopal, V.; Sousa, R. Scanning mutagenesis reveals roles for helix n of the bacteriophage T7 RNA polymerase thumb subdomain in transcription complex stability, pausing, and termination. J. Biol. Chem. 2001, 276, 10306–10313. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.W.; Steitz, T.A. Structural basis for the transition from initiation to elongation transcription in T7 RNA polymerase. Science 2002, 298, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Tahirov, T.H.; Temiakov, D.; Anikin, M.; Patlan, V.; McAllister, W.T.; Vassylyev, D.G.; Yokoyama, S. Structure of a T7 RNA polymerase elongation complex at 2.9 Å resolution. Nature 2002, 420, 43–50. [Google Scholar] [CrossRef]
- Temiakov, D.; Tahirov, T.H.; Anikin, M.; McAllister, W.T.; Vassylyev, D.G.; Yokoyama, S. Crystallization and preliminary crystallographic analysis of T7 RNA polymerase elongation complex. Acta Crystallogr. Sect. D Biol. Crystallogr. 2003, 59, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Bandwar, R.P.; Ma, N.; Emanuel, S.A.; Anikin, M.; Vassylyev, D.G.; Patel, S.S.; McAllister, W.T. The transition to an elongation complex by T7 RNA polymerase is a multistep process. J. Biol. Chem. 2007, 282, 22879–22886. [Google Scholar] [CrossRef] [PubMed]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A. From rejection to the Nobel Prize: Karikó and Weissman’s pioneering work on mRNA vaccines, and the need for diversity and inclusion in translational immunology. Front. Immunol. 2023, 14, 1306025. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.R.; Muramatsu, H.; Nallagatla, S.R.; Bevilacqua, P.C.; Sansing, L.H.; Weissman, D.; Karikó, K. Incorporation of pseudouridine into mRNA enhances translation by diminishing PKR activation. Nucleic Acids Res. 2010, 38, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Weissman, D. mRNA transcript therapy. Expert Rev. Vaccines 2015, 14, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Yadav, T.; Kumar, S.; Mishra, G.; Saxena, S.K. Tracking the COVID-19 vaccines: The global landscape. Hum. Vaccines Immunother. 2023, 19, 2191577. [Google Scholar] [CrossRef]
- Xia, X. Detailed dissection and critical evaluation of the Pfizer/BioNTech and Moderna mRNA vaccines. Vaccines 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Corbett, K.S.; Flynn, B.; Foulds, K.E.; Francica, J.R.; Boyoglu-Barnum, S.; Werner, A.P.; Flach, B.; O’Connell, S.; Bock, K.W.; Minai, M.; et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020, 383, 1544–1555. [Google Scholar] [CrossRef]
- Pace, C.N.; Scholtz, J.M. A helix propensity scale based on experimental studies of peptides and proteins. Biophys. J. 1998, 75, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Calvopina-Chavez, D.G.; Gardner, M.A.; Griffitts, J.S. Engineering efficient termination of bacteriophage T7 RNA polymerase transcription. G3 2022, 12, jkac070. [Google Scholar] [CrossRef] [PubMed]
- Conrad, T.; Plumbom, I.; Alcobendas, M.; Vidal, R.; Sauer, S. Maximizing transcription of nucleic acids with efficient T7 promoters. Commun. Biol. 2020, 3, 439. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Dang, Y.; Zhou, M.; Yuan, H.; Liu, Y. Codon usage biases co-evolve with transcription termination machinery to suppress premature cleavage and polyadenylation. Elife 2018, 7, e33569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhou, Z.; Dang, Y.; Na, H.; Adam, C.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Liu, Y. Genome-wide role of codon usage on transcription and identification of potential regulators. Proc. Natl. Acad. Sci. USA 2021, 118, e2022590118. [Google Scholar] [CrossRef]
- Lyakhov, D.L.; He, B.; Zhang, X.; Studier, F.W.; Dunn, J.J.; McAllister, W.T. Mutant bacteriophage T7 RNA polymerases with altered termination properties. J. Mol. Biol. 1997, 269, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Patiño-Guillén, G.; Pešović, J.; Panić, M.; Savić-Pavićević, D.; Bošković, F.; Keyser, U.F. Single-molecule RNA sizing enables quantitative analysis of alternative transcription termination. Nat. Commun. 2024, 15, 1699. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Chen, T.; Zhang, Y.; Zhang, Y.; Simair, A.; Rujie, C.; Sadaf Zaidi, N.U.S.; Guo, X.; Wei, X.; Siegel, G.; et al. Large-Scale in Vitro Transcription, RNA Purification and Chemical Probing Analysis. Cell. Physiol. Biochem. 2018, 48, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Benyamin, Y.P.; Aniela, W.; Tilmann, R.; Andeas, F.; Martin, K. Method of RNA in Vitro Transcription Using a Buffer Containing a Dicarboxylic Acid or Tricarboxylic Acid or a Salt Thereof. WO2017109161A1, 26 June 2017. [Google Scholar]
- Chen, T.H.; Potapov, V.; Dai, N.; Ong, J.L.; Roy, B. N1-methyl-pseudouridine is incorporated with higher fidelity than pseudouridine in synthetic RNAs. Sci. Rep. 2022, 12, 13017. [Google Scholar] [CrossRef]
- Miller, M.; Alvizo, O.; Chng, C.; Jenne, S.; Mayo, M.; Mukherjee, A.; Sundseth, S.; Chintala, A.; Penfield, J.; Riggins, J.; et al. An Engineered T7 RNA polymerase for Efficient co-transcriptional capping with reduced dsRNA byproducts in mRNA synthesis. bioRxiv 2022. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Zhang, X.; Zou, Y.; Li, J.; Wang, C.; He, Y.; Jin, Q.; Ye, J. Effective Synthesis of High-Integrity mRNA Using In Vitro Transcription. Molecules 2024, 29, 2461. https://doi.org/10.3390/molecules29112461
He W, Zhang X, Zou Y, Li J, Wang C, He Y, Jin Q, Ye J. Effective Synthesis of High-Integrity mRNA Using In Vitro Transcription. Molecules. 2024; 29(11):2461. https://doi.org/10.3390/molecules29112461
Chicago/Turabian StyleHe, Wei, Xinya Zhang, Yangxiaoyu Zou, Ji Li, Chong Wang, Yucai He, Qiuheng Jin, and Jianren Ye. 2024. "Effective Synthesis of High-Integrity mRNA Using In Vitro Transcription" Molecules 29, no. 11: 2461. https://doi.org/10.3390/molecules29112461
APA StyleHe, W., Zhang, X., Zou, Y., Li, J., Wang, C., He, Y., Jin, Q., & Ye, J. (2024). Effective Synthesis of High-Integrity mRNA Using In Vitro Transcription. Molecules, 29(11), 2461. https://doi.org/10.3390/molecules29112461






