In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L.
Abstract
1. Introduction
2. Results
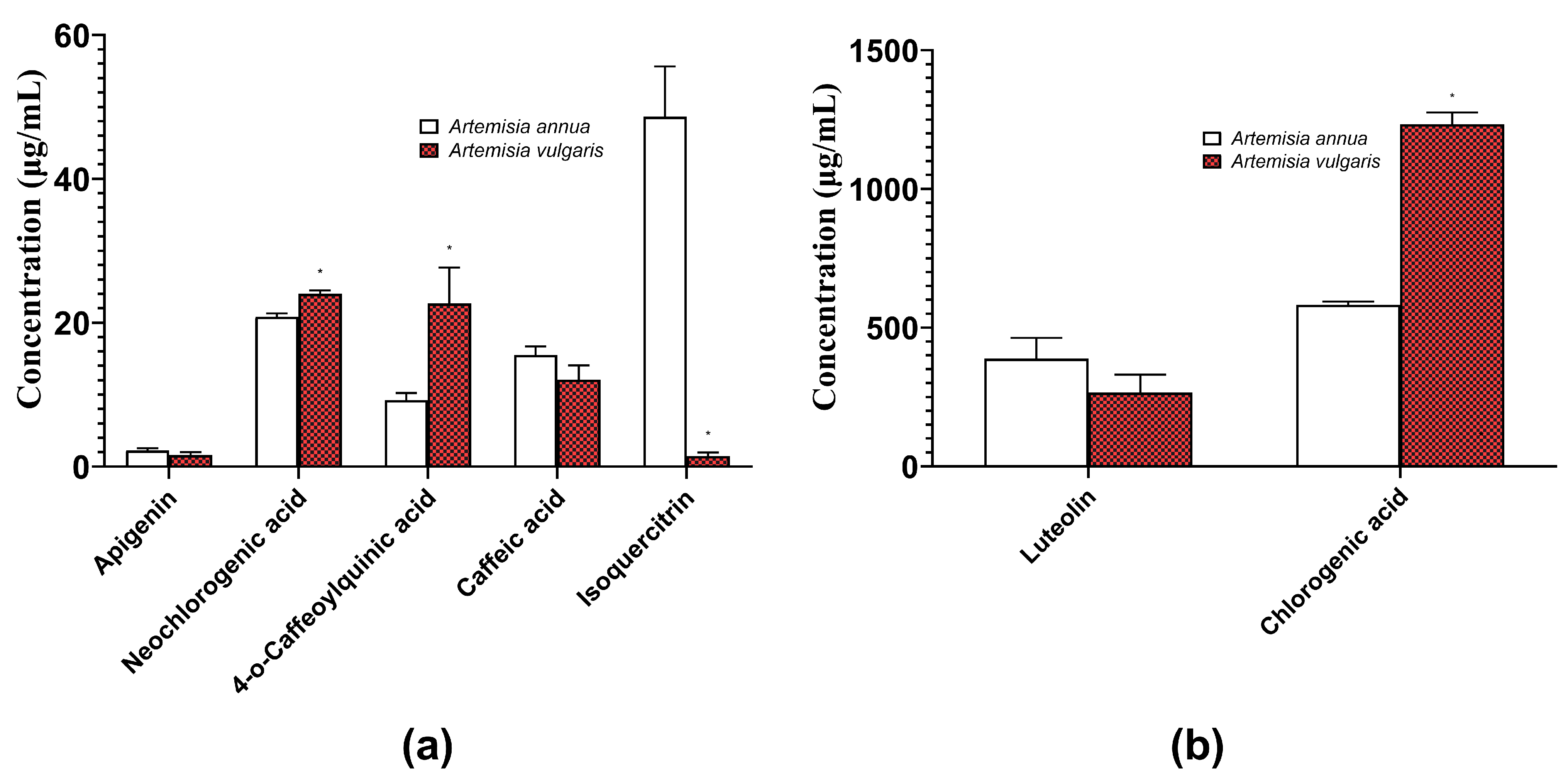
2.1. Quantification of Herbal Hydroalcoholic Extracts of A. annua and A. vulgaris
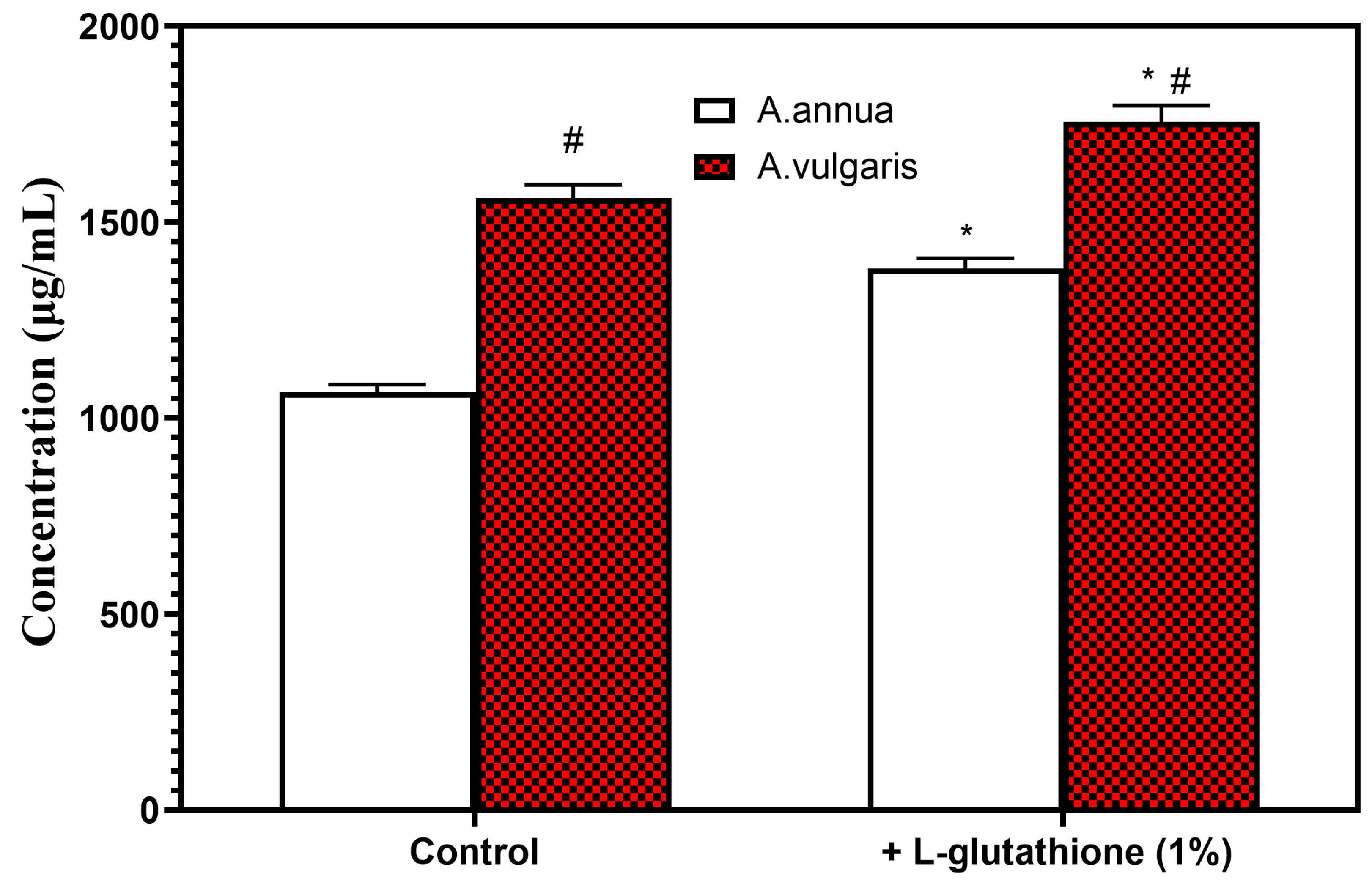
2.2. The Role of Excipients in the Optimization of Extraction Conditions of Bioactive Phenolic Compounds from Herbal Hydroalcoholic Extracts of A. annua and A. vulgaris
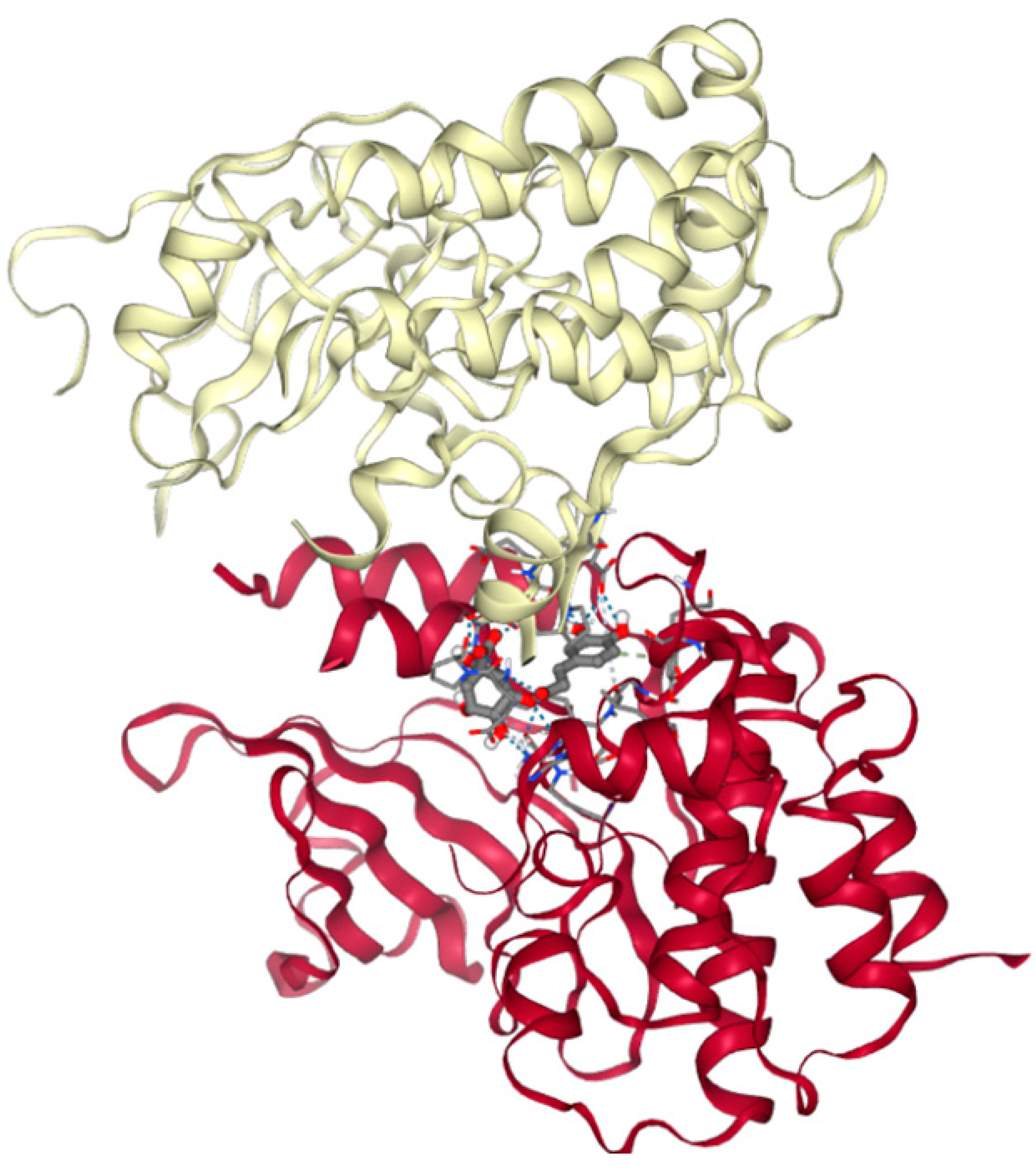
2.3. In Silico Studies of Anticancer Activity of Main Bioactive Phenolic Compounds from Herbal Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L.
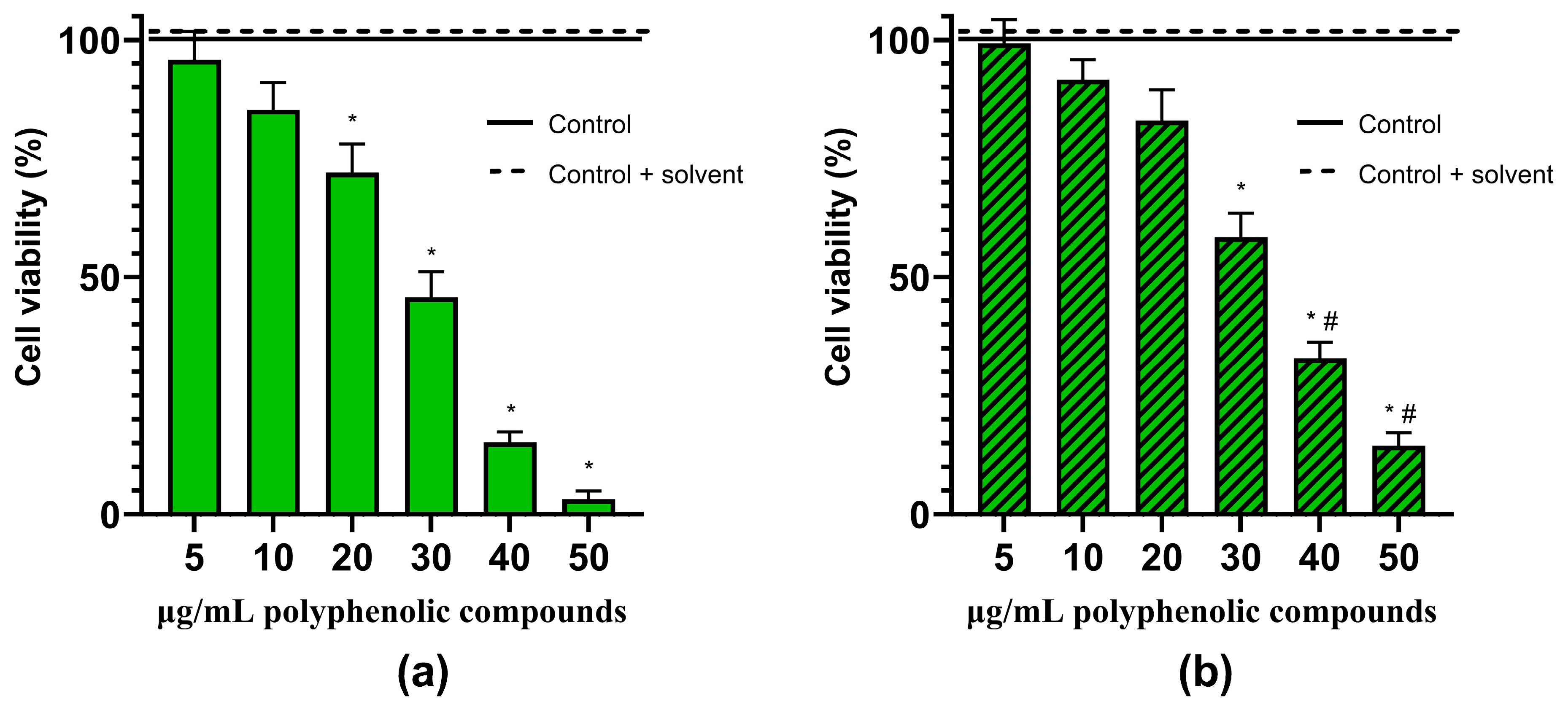
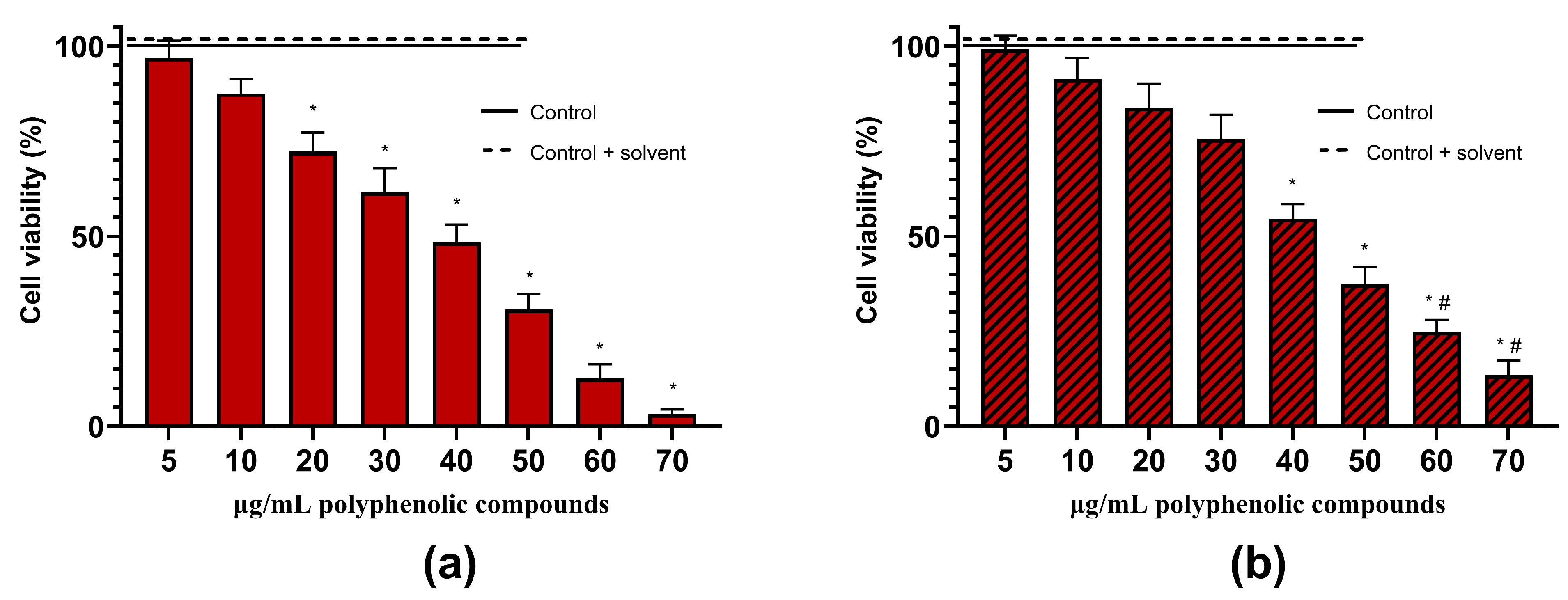
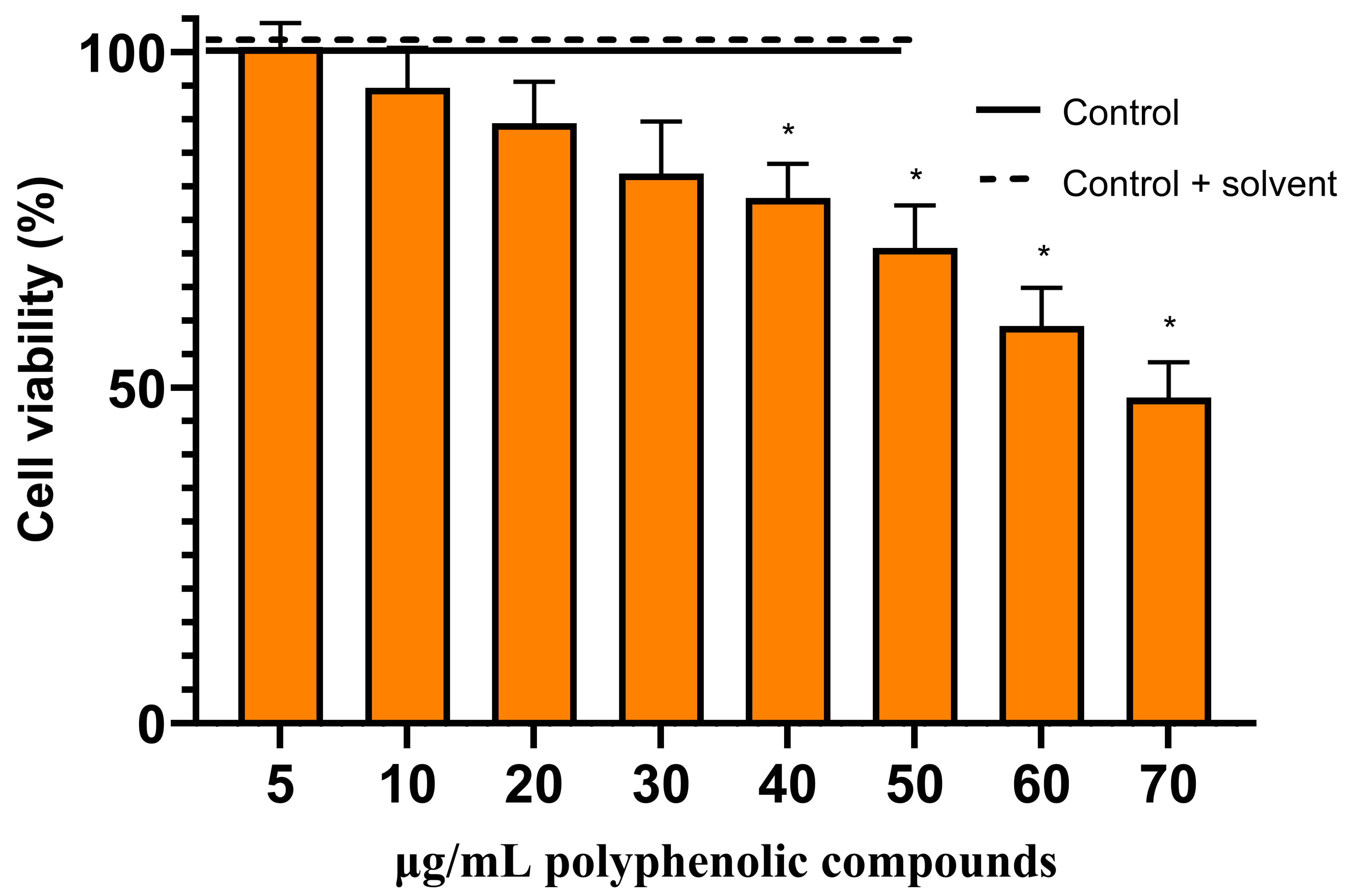
2.4. Anticancer Activity of Herbal Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L. and its Main Bioactive Phenolic Compound—Chlorogenic Acid
3. Discussion
3.1. Optimization of Extraction Conditions of Phenolic Compounds from Herbal Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L.
3.2. Molecular Docking of Main Bioactive Phenolic Compounds from Herbal Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L. with Proteins from Apoptosis and Necroptosis Pathways
3.3. Effects of Artemisia annua L. and Artemisia vulgaris L. Extracts and Chlorogenic Acid on the Viability of the Rat C6 Glioma Cells
4. Materials and Methods
4.1. Plant Materials, Chemicals, and Equipment
4.2. Preparation of Artemisia Plant Extracts
4.3. High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD) Conditions for the Extract Analysis
4.4. Molecular Docking
4.5. Cell Viability Assessment In Vitro
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shahcheraghi, S.H.; Alimardani, M.; Lotfi, M.; Lotfi, M.; Uversky, V.N.; Guetchueng, S.T.; Palakurthi, S.S.; Charbe, N.B.; Hromić-Jahjefendić, A.; Aljabali, A.A.A.; et al. Advances in glioblastoma multiforme: Integrating therapy and pathology perspectives. Pathol. Res. Pract. 2024, 257, 155285. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Faujdar, C.; Sharma, R.; Sharma, S.; Malik, B.; Nepali, K.; Liou, J.P. Glioblastoma: Current Status, Emerging Targets, and Recent Advances. J. Med. Chem. 2022, 65, 8596–8685. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Colamaria, A.; Fochi, N.P.; Sacco, M.; Landriscina, M.; Parbonetti, G.; de Notaris, M.; Coppola, G.; De Santis, E.; Giordano, G.; et al. Recurrent Glioblastoma Treatment: State of the Art and Future Perspectives in the Precision Medicine Era. Biomedicines 2022, 10, 1927. [Google Scholar] [CrossRef] [PubMed]
- Rončević, A.; Koruga, N.; Soldo Koruga, A.; Rončević, R.; Rotim, T.; Šimundić, T.; Kretić, D.; Perić, M.; Turk, T.; Štimac, D. Personalized Treatment of Glioblastoma: Current State and Future Perspective. Biomedicines 2023, 11, 1579. [Google Scholar] [CrossRef] [PubMed]
- Rong, L.; Li, N.; Zhang, Z. Emerging therapies for glioblastoma: Current state and future directions. J. Exp. Clin. Cancer Res. 2022, 41, 142. [Google Scholar] [CrossRef]
- Khan, I.; Mahfooz, S.; Hatiboglu, M.A. Herbal Medicine for Glioblastoma: Current and Future Prospects. Med. Chem. 2020, 16, 1022–1043. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qi, F.; Wang, Z.; Zhang, Z.; Pan, N.; Huai, L.; Qu, S.; Zhao, L. A review of traditional Chinese medicine for treatment of glioblastoma. Biosci. Trends 2020, 13, 476–487. [Google Scholar] [CrossRef] [PubMed]
- Arabzadeh, A.; Mortezazadeh, T.; Aryafar, T.; Gharepapagh, E.; Majdaeen, M.; Farhood, B. Therapeutic potentials of resveratrol in combination with radiotherapy and chemotherapy during glioblastoma treatment: A mechanistic review. Cancer Cell Int. 2021, 21, 391. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Cao, S.; Qiu, F.; Zhang, B. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacol. Ther. 2020, 216, 107650. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef] [PubMed]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the History of Medicine and Its Possible Contemporary Applications Substantiated by Phytochemical and Pharmacological Studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef] [PubMed]
- Siwan, D.; Nandave, D.; Nandave, M. Artemisia vulgaris Linn: An updated review on its multiple biological activities. Future J. Pharm. Sci. 2022, 8, 47. [Google Scholar] [CrossRef]
- Umam, K.; Feng, C.S.; Yang, G.; Tu, P.C.; Lin, C.Y.; Yang, M.T.; Kuo, T.F.; Yang, W.C.; Tran Nguyen Minh, H. Phytochemistry, Pharmacology and Mode of Action of the Anti-Bacterial Artemisia Plants. Bioengineering 2023, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Anibogwu, R.; Jesus, K.; Pradhan, S.; Pashikanti, S.; Mateen, S.; Sharma, K. Extraction, Isolation and Characterization of Bioactive Compounds from Artemisia and Their Biological Significance: A Review. Molecules 2021, 26, 6995. [Google Scholar] [CrossRef] [PubMed]
- Abiri, R.; Silva, A.L.M.; de Mesquita, L.S.S.; de Mesquita, J.W.C.; Atabaki, N.; de Almeida, E.B., Jr.; Shaharuddin, N.A.; Malik, S. Towards a better understanding of Artemisia vulgaris: Botany, phytochemistry, pharmacological and biotechnological potential. Food Res. Int. 2018, 109, 403–415. [Google Scholar] [CrossRef]
- Beylerli, O.; Beilerli, A.; Shumadalova, A.; Wang, X.; Yang, M.; Sun, H.; Teng, L. Therapeutic effect of natural polyphenols against glioblastoma. Front. Cell Dev. Biol. 2022, 10, 1036809. [Google Scholar] [CrossRef]
- Luís, Â.; Amaral, L.; Domingues, F.; Pereira, L.; Cascalheira, J.F. Action of Curcumin on Glioblastoma Growth: A Systematic Review with Meta-Analysis of Animal Model Studies. Biomedicines 2024, 12, 268. [Google Scholar] [CrossRef]
- Persano, F.; Gigli, G.; Leporatti, S. Natural Compounds as Promising Adjuvant Agents in The Treatment of Gliomas. Int. J. Mol. Sci. 2022, 23, 3360. [Google Scholar] [CrossRef]
- Udroiu, I.; Marinaccio, J.; Sgura, A. Epigallocatechin-3-gallate induces telomere shortening and clastogenic damage in glioblastoma cells. Environ. Mol. Mutagen. 2019, 60, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Giordo, R.; Pintus, G.; Mohammed, S.A.; Orhan, I.E.; Fokou, P.V.T.; Sharopov, F.; Adetunji, C.O.; Gulsunoglu-Konuskan, Z.; Ydyrys, A.; et al. Medicinal and mechanistic overview of artemisinin in the treatment of human diseases. Biomed. Pharmacother. 2023, 163, 114866. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.W.; Chen, D.; Chen, L.; He, B.; Li, Y. A comprehensive overview of Artemisinin and its derivatives as anticancer agents. Eur. J. Med. Chem. 2023, 247, 115000. [Google Scholar] [CrossRef] [PubMed]
- Strik, H.; Efferth, T.; Kaina, B. Artesunate in glioblastoma therapy: Case reports and review of clinical studies. Phytomedicine 2024, 123, 155274. [Google Scholar] [CrossRef] [PubMed]
- Park, K.R.; Jeong, Y.; Lee, J.; Kwon, I.K.; Yun, H.M. Anti-tumor effects of jaceosidin on apoptosis, autophagy, and necroptosis in human glioblastoma multiforme. Am. J. Cancer Res. 2021, 11, 4919–4930. [Google Scholar] [PubMed]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.A.; Martínez-Ávila, G.C.G.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-drying encapsulation of microwave-assisted extracted polyphenols from Moringa oleifera: Influence of tragacanth, locust bean, and carboxymethyl-cellulose formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red Clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef] [PubMed]
- Blundell, R.; Butterworth, P.; Charlier, A.; Daurio, D.; Degenhardt, M.; Harris, D.; Hancock, B.; Johnston, M.; Kasina, R.; Kaye, J.; et al. The Role of Titanium Dioxide (E171) and the Requirements for Replacement Materials in Oral Solid Dosage Forms: An IQ Consortium Working Group Review. J. Pharm. Sci. 2022, 111, 2943–2954. [Google Scholar] [CrossRef] [PubMed]
- Nageeb, A.; Al-Tawashi, A.; Mohammad Emwas, A.H.; Abdel-Halim Al-Talla, Z.; Al-Rifai, N. Comparison of Artemisia annua Bioactivities between Traditional Medicine and Chemical Extracts. Curr. Bioact. Compd. 2013, 9, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.; Mandrioli, R.; Mandrone, M.; Cappadone, C.; Farruggia, G.; Chiocchio, I.; Malucelli, E.; Isani, G.; Poli, F.; Mercolini, L. Analysis of Artemisia annua extracts and related products by high performance liquid chromatography-tandem mass spectrometry coupled to sample treatment miniaturisation. J. Pharm. Biomed. Anal. 2019, 174, 81–88. [Google Scholar] [CrossRef]
- Saunoriūtė, S.; Ragažinskienė, O.; Ivanauskas, L.; Marksa, M.; Laužikė, K.; Raudonė, L. Phenolic Diversity and Antioxidant Activity of Artemisia abrotanum L. and Artemisia absinthium L. during Vegetation Stages. Separations 2023, 10, 545. [Google Scholar] [CrossRef]
- Slimestad, R.; Johny, A.; Thomsen, M.G.; Karlsen, C.R.; Rosnes, J.T. Chemical Profiling and Biological Activity of Extracts from Nine Norwegian Medicinal and Aromatic Plants. Molecules 2022, 27, 7335. [Google Scholar] [CrossRef] [PubMed]
- Sungpud, C.; Panpipat, W.; Sae Yoon, A.; Chaijan, M. Tuning of virgin coconut oil and propylene glycol ratios for maximizing the polyphenol recovery and in vitro bioactivities of mangosteen (Garcinia mangostana L.) pericarp. Process Biochem. 2019, 87, 179–186. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Costa, R.D.; Domínguez-Perles, R.; Abraão, A.; Gomes, V.; Gouvinhas, I.; Barros, A.N. Exploring the Antioxidant Potential of Phenolic Compounds from Winery By-Products by Hydroethanolic Extraction. Molecules 2023, 28, 6660. [Google Scholar] [CrossRef]
- Gil-Martín, E.; Forbes-Hernández, T.; Romero, A.; Cianciosi, D.; Giampieri, F.; Battino, M. Influence of the extraction method on the recovery of bioactive phenolic compounds from food industry by-products. Food Chem. 2022, 378, 131918. [Google Scholar] [CrossRef]
- Alanazi, A.M.; Mostafa, G.A.E.; Al-Badr, A.A. Chapter Two—Glutathione. In Profiles of Drug Substances, Excipients and Related Methodology; Brittain, H.G., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 40, pp. 43–158. [Google Scholar]
- Maheshwari, N.; Sharma, M.C. Anticancer Properties of Some Selected Plant Phenolic Compounds: Future Leads for Therapeutic Development. J. Herbal. Med. 2023, 42, 100801. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Sharma, E.; Attri, D.C.; Sati, P.; Dhyani, P.; Szopa, A.; Sharifi-Rad, J.; Hano, C.; Calina, D.; Cho, W.C. Recent updates on anticancer mechanisms of polyphenols. Front. Cell Dev. Biol. 2022, 10, 1005910. [Google Scholar] [CrossRef]
- Nwafor, E.O.; Lu, P.; Zhang, Y.; Liu, R.; Peng, H.; Xing, B.; Liu, Y.; Li, Z.; Zhang, K.; Zhang, Y.; et al. Chlorogenic acid: Potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl. Oncol. 2022, 15, 101294. [Google Scholar] [CrossRef]
- Agu, P.C.; Afiukwa, C.A.; Orji, O.U.; Ezeh, E.M.; Ofoke, I.H.; Ogbu, C.O.; Ugwuja, E.I.; Aja, P.M. Molecular docking as a tool for the discovery of molecular targets of nutraceuticals in diseases management. Sci. Rep. 2023, 13, 13398. [Google Scholar] [CrossRef] [PubMed]
- Grinter, S.Z.; Zou, X. Challenges, applications, and recent advances of protein-ligand docking in structure-based drug design. Molecules 2014, 19, 10150–10176. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Dang, T.; Chai, J. From Apoptosis to Necroptosis: The Death Wishes to Cancer. Cancer Control 2021, 28, 10732748211066311. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Lee, H.J.; Jung, Y.M.; Jung, Y.J. Regulated Necrotic Cell Death in Alternative Tumor Therapeutic Strategies. Cells 2020, 9, 2709. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Tian, Z.; Cui, Y.; Liu, Z.; Ma, X. Chlorogenic acid: A comprehensive review of the dietary sources, processing effects, bioavailability, beneficial properties, mechanisms of action, and future directions. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3130–3158. [Google Scholar] [CrossRef] [PubMed]
- Roda, D.; Veiga, P.; Melo, J.B.; Carreira, I.M.; Ribeiro, I.P. Principles in the Management of Glioblastoma. Genes 2024, 15, 501. [Google Scholar] [CrossRef] [PubMed]
- Giakoumettis, D.; Kritis, A.; Foroglou, N. C6 cell line: The gold standard in glioma research. Hippokratia 2018, 22, 105–112. [Google Scholar]
- Belkaid, A.; Currie, J.C.; Desgagnés, J.; Annabi, B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006, 6, 7. [Google Scholar] [CrossRef]
- Huang, S.; Wang, L.L.; Xue, N.N.; Li, C.; Guo, H.H.; Ren, T.K.; Zhan, Y.; Li, W.B.; Zhang, J.; Chen, X.G.; et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics 2019, 9, 6745–6763. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Paliwal, P.; Mukherjee, S.; Patnaik, N.; Krishnamurthy, S.; Patnaik, R. Pharmacokinetics and brain penetration study of chlorogenic acid in rats. Xenobiotica 2019, 49, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, J.S.; Jang, H.J.; Kim, S.M.; Chang, M.S.; Park, S.H.; Kim, K.S.; Bae, J.; Park, J.W.; Lee, B.; et al. Chlorogenic acid ameliorates brain damage and edema by inhibiting matrix metalloproteinase-2 and 9 in a rat model of focal cerebral ischemia. Eur. J. Pharmacol. 2012, 689, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Keskin, C.; Ölçekçi, A.; Baran, A.; Baran, M.F.; Eftekhari, A.; Omarova, S.; Khalilov, R.; Aliyev, E.; Sufianov, A.; Beilerli, A.; et al. Green synthesis of silver nanoparticles mediated Diospyros kaki L. (Persimmon): Determination of chemical composition and evaluation of their antimicrobials and anticancer activities. Front. Chem. 2023, 11, 1187808. [Google Scholar] [CrossRef] [PubMed]
- Kour, R.; Sharma, N.; Showkat, S.; Sharma, S.; Nagaiah, K.; Kumar, S.; Kaur, S. Methanolic fraction of Cassia fistula L. bark exhibits potential to combat oxidative stress and possess antiproliferative activity. J. Toxicol. Environ. Health A 2023, 86, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Zvikas, V.; Urbanaviciute, I.; Bernotiene, R.; Kulakauskiene, D.; Morkunaite, U.; Balion, Z.; Majiene, D.; Liaudanskas, M.; Viskelis, P.; Jekabsone, A.; et al. Investigation of Phenolic Composition and Anticancer Properties of Ethanolic Extracts of Japanese Quince Leaves. Foods 2020, 10, 18. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Nieborowska-Skorska, M.; Kolasa, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl-2-p53 axis by transformed and non-transformed root extracts of Leonurus sibiricus L. Tumour Biol. 2016, 37, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef]
- Lewandowska, U.; Gorlach, S.; Owczarek, K.; Hrabec, E.; Szewczyk, K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postep. Hig. Med. Dosw. (Online) 2014, 68, 528–540. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef] [PubMed]
- Laurutis, A.; Liobikas, J.; Stanciauskaite, M.; Marksa, M.; Ramanauskiene, K.; Majiene, D. Comparison of the Formulation, Stability and Biological Effects of Hydrophilic Extracts from Black Elder Flowers (Sambucus nigra L.). Pharmaceutics 2022, 14, 2831. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Balion, Z.; Cėpla, V.; Svirskiene, N.; Svirskis, G.; Druceikaitė, K.; Inokaitis, H.; Rusteikaitė, J.; Masilionis, I.; Stankevičienė, G.; Jelinskas, T.; et al. Cerebellar Cells Self-Assemble into Functional Organoids on Synthetic, Chemically Crosslinked ECM-Mimicking Peptide Hydrogels. Biomolecules 2020, 10, 754. [Google Scholar] [CrossRef]
- Balion, Z.; Ramanauskienė, K.; Jekabsone, A.; Majienė, D. The Role of Mitochondria in Brain Cell Protection from Ischaemia by Differently Prepared Propolis Extracts. Antioxidants 2020, 9, 1262. [Google Scholar] [CrossRef]

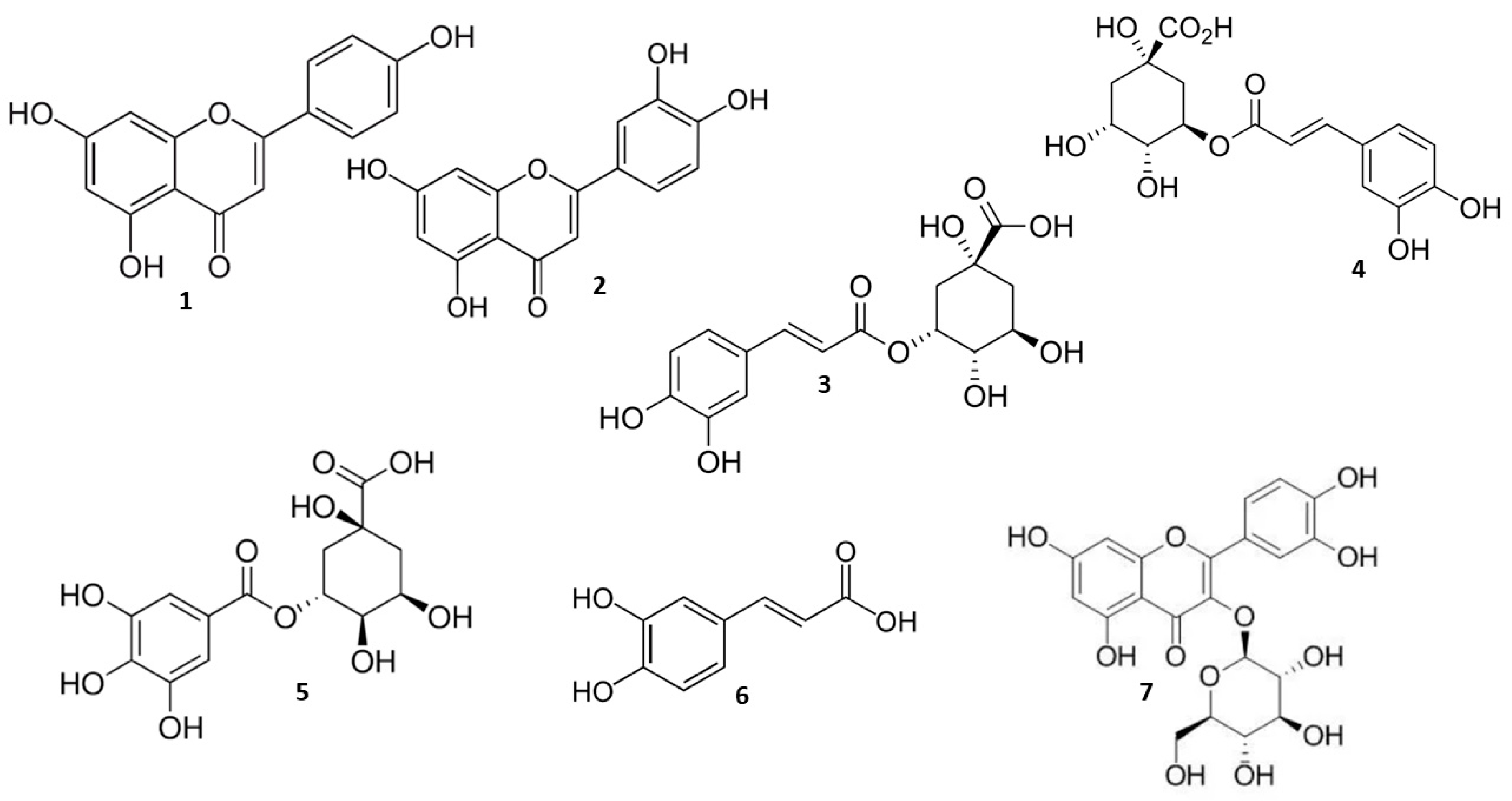

| Concentration, µg/mL | Control | Titanium Dioxide 2% | L-Glutathione 1% | Propylene Glycol 10% | β-Cyclodextrin 5% |
|---|---|---|---|---|---|
| Artemisia annua | |||||
| Chlorogenic acid | 582.0 ± 30.2 | 664.5 ± 28.0 * | 643.9 ± 34.7 * | 596.3 ± 29.1 | 498.5 ± 25.6 |
| Luteolin | 387.8 ± 18.3 | 443.9 ± 24.1 * | 623.1 ± 35.4 * | 556.7 ± 24.2 * | 500.1 ± 100.4 |
| Isoquercitrin | 48.64 ± 4.20 | 59.49 ± 5.53 * | 61.10 ± 3.20 * | 55.84 ± 5.65 | 47.87 ± 4.54 |
| Apigenin | 2.25 ± 0.10 | 2.75 ± 0.16 * | 3.63 ± 0.18 * | 3.25 ± 0.17 * | 2.67 ± 0.46 |
| Neochlorogenic acid | 20.81 ± 1.65 | 25.14 ± 1.32 * | 24.73 ± 1.32 * | 22.31 ± 1.31 | 18.59 ± 1.74 |
| 4-o-Caffeoyl-quinic acid | 9.23 ± 0.24 | 10.11 ± 0.35 * | 9.68 ± 0.15 * | 9.00 ± 0.96 | 7.62 ± 0.26 |
| Caffeic acid | 15.50 ± 2.42 | 14.49 ± 2.25 | 15.15 ± 2.65 | 17.34 ± 3.20 | 15.50 ± 2.43 |
| Artemisia vulgaris | |||||
| Chlorogenic acid | 1233.7 ± 20.1 | 1432.6 ± 27.5 * | 1293.9 ± 28.9 * | 1351.9 ± 52.7 * | 1320.2 ± 83.6 |
| Luteolin | 265.1 ± 13.2 | 691.8 ± 23.4 * | 387.0 ± 19.2 * | 581.3 ± 20.8 * | 439.3 ± 26.5 * |
| Isoquercitrin | 1.46 ± 0.12 | 1.76 ± 0.14 * | 1.85 ± 0.17 * | 3.26 ± 0.35 * | 2.50 ± 0.25 * |
| Apigenin | 1.59 ± 0.17 | 3.61 ± 0.42 * | 2.53 ± 0.19 * | 2.54 ± 0.21 * | 2.18 ± 0.58 |
| Neochlorogenic acid | 24.00 ± 1.60 | 35.26 ± 5.83 * | 29.39 ± 1.71 * | 29.82 ± 4.95 | 26.32 ± 3.54 |
| 4-o-Caffeoyl-quinic acid | 22.67 ± 2.25 | 33.16 ± 4.87 * | 27.90 ± 2.27 * | 27.71 ± 4.78 | 27.20 ± 5.1 |
| Caffeic acid | 12.06 ± 0.35 | 15.42 ± 1.72 * | 13.03 ± 0.23 * | 14.14 ± 1.21 * | 12.32 ± 1.87 |
| Bax | Bcl-2 | MLKL/RIPK3 | |
|---|---|---|---|
| Chlorogenic acid | −7.4 kcal/mol N6 | −7.4 kcal/mol N9 | −6.8 kcal/mol N18 |
| Luteolin | −6.6 kcal/mol N6 | −8.1 kcal/mol N9 | −8.5 kcal/mol N5 |
| Isoquercitrin | −7.6 kcal/mol N10 | −8.3 kcal/mol N7 | −9.2 kcal/mol N11 |
| Apigenin | −6.3 kcal/mol N5 | −7.8 kcal/mol N6 | −7.2 kcal/mol N4 |
| Neochlorogenic acid | −6.9 kcal/mol N7 | −7.6 kcal/mol N8 | −6.9 kcal/mol N10 |
| 4-o-Caffeoylquinic acid | −7.0 kcal/mol N10 | −7.7 kcal/mol N5 | −7.2 kcal/mol N5 |
| Caffeic acid | −5.2 kcal/mol N4 | −6.1 kcal/mol N6 | −6.3 kcal/mol N10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernatoniene, J.; Nemickaite, E.; Majiene, D.; Marksa, M.; Kopustinskiene, D.M. In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L. Molecules 2024, 29, 2460. https://doi.org/10.3390/molecules29112460
Bernatoniene J, Nemickaite E, Majiene D, Marksa M, Kopustinskiene DM. In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L. Molecules. 2024; 29(11):2460. https://doi.org/10.3390/molecules29112460
Chicago/Turabian StyleBernatoniene, Jurga, Emilija Nemickaite, Daiva Majiene, Mindaugas Marksa, and Dalia M. Kopustinskiene. 2024. "In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L." Molecules 29, no. 11: 2460. https://doi.org/10.3390/molecules29112460
APA StyleBernatoniene, J., Nemickaite, E., Majiene, D., Marksa, M., & Kopustinskiene, D. M. (2024). In Vitro and In Silico Anti-Glioblastoma Activity of Hydroalcoholic Extracts of Artemisia annua L. and Artemisia vulgaris L. Molecules, 29(11), 2460. https://doi.org/10.3390/molecules29112460








