In Vitro and Molecular Docking Evaluation of the Anticholinesterase and Antidiabetic Effects of Compounds from Terminalia macroptera Guill. & Perr. (Combretaceae)
Abstract
1. Introduction
2. Results
2.1. Isolated Compounds
2.2. Anticholinesterase and Antidiabetic Effects of the Isolated Compounds
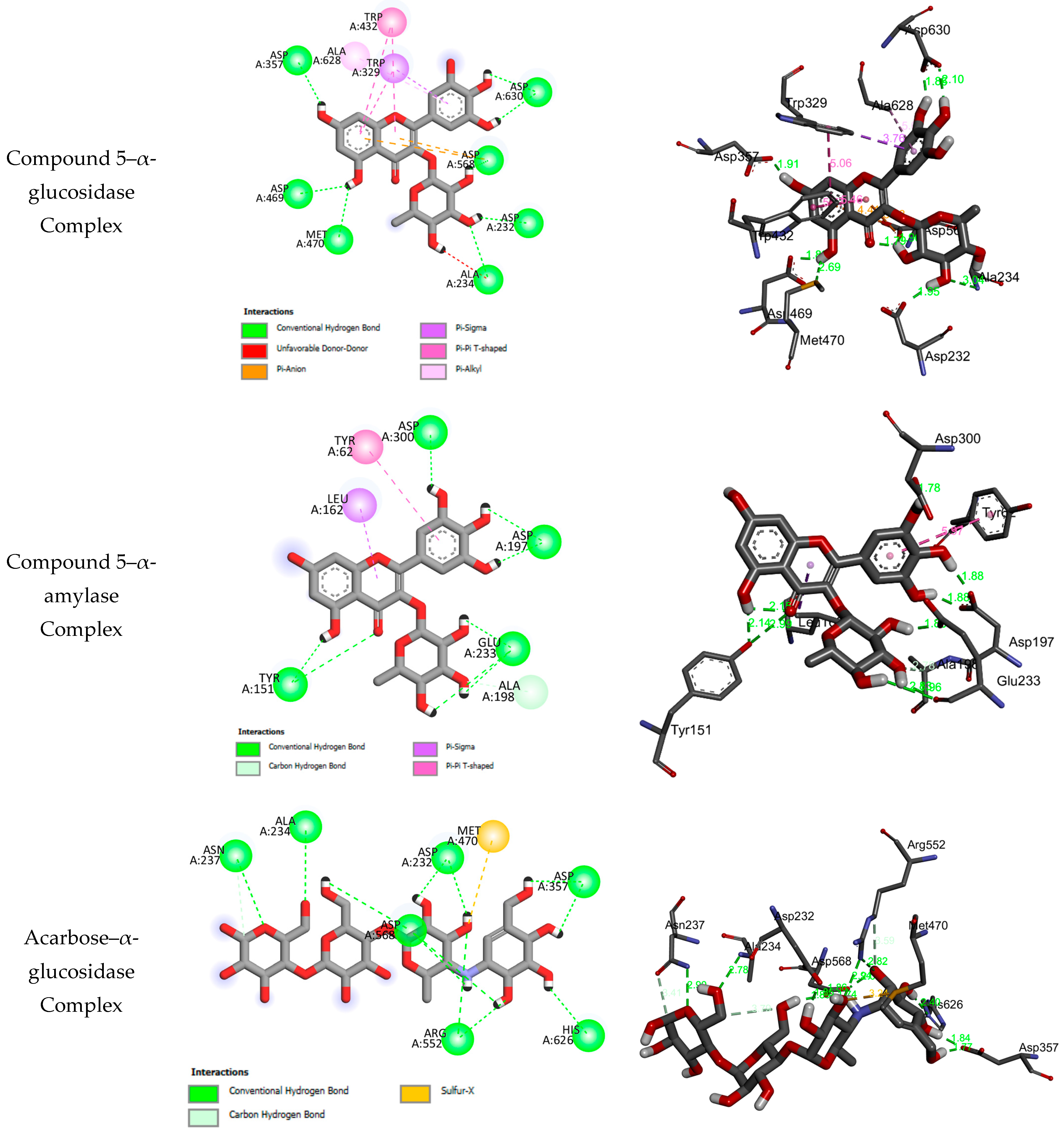
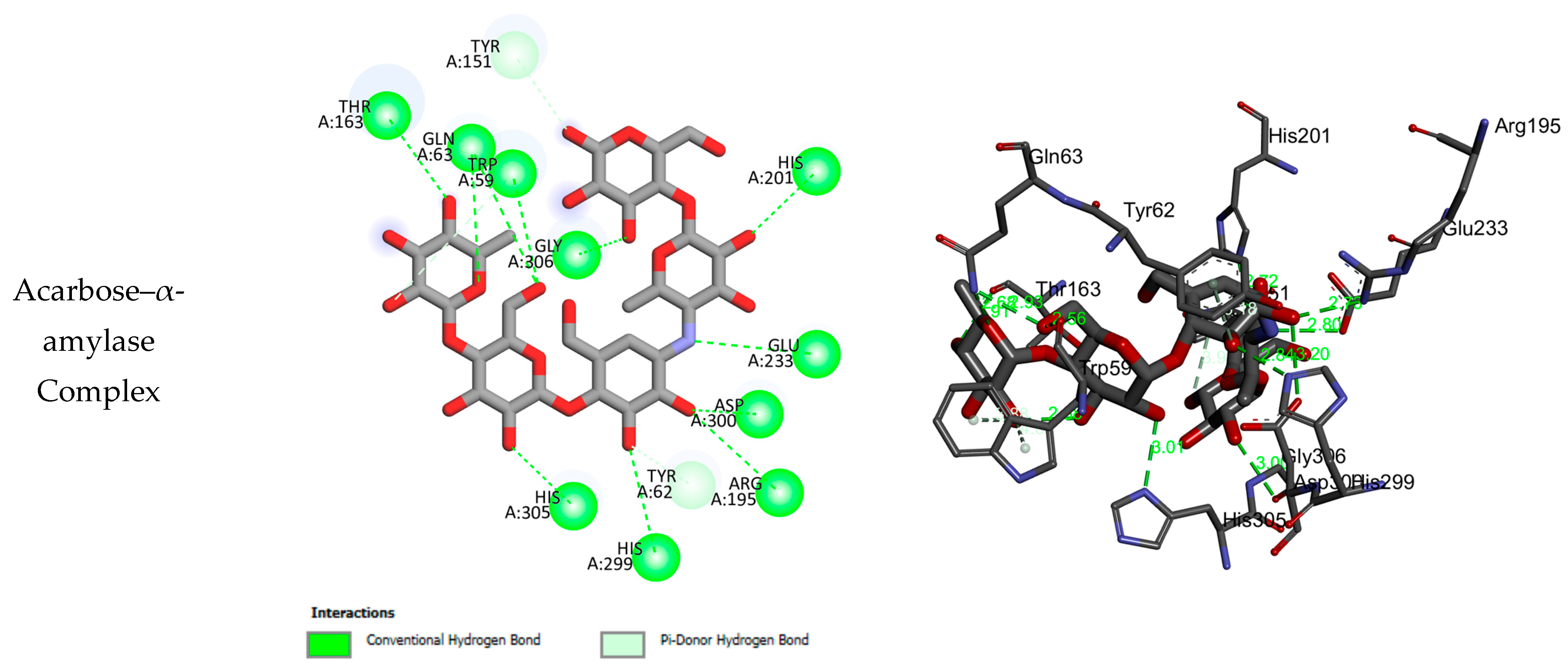
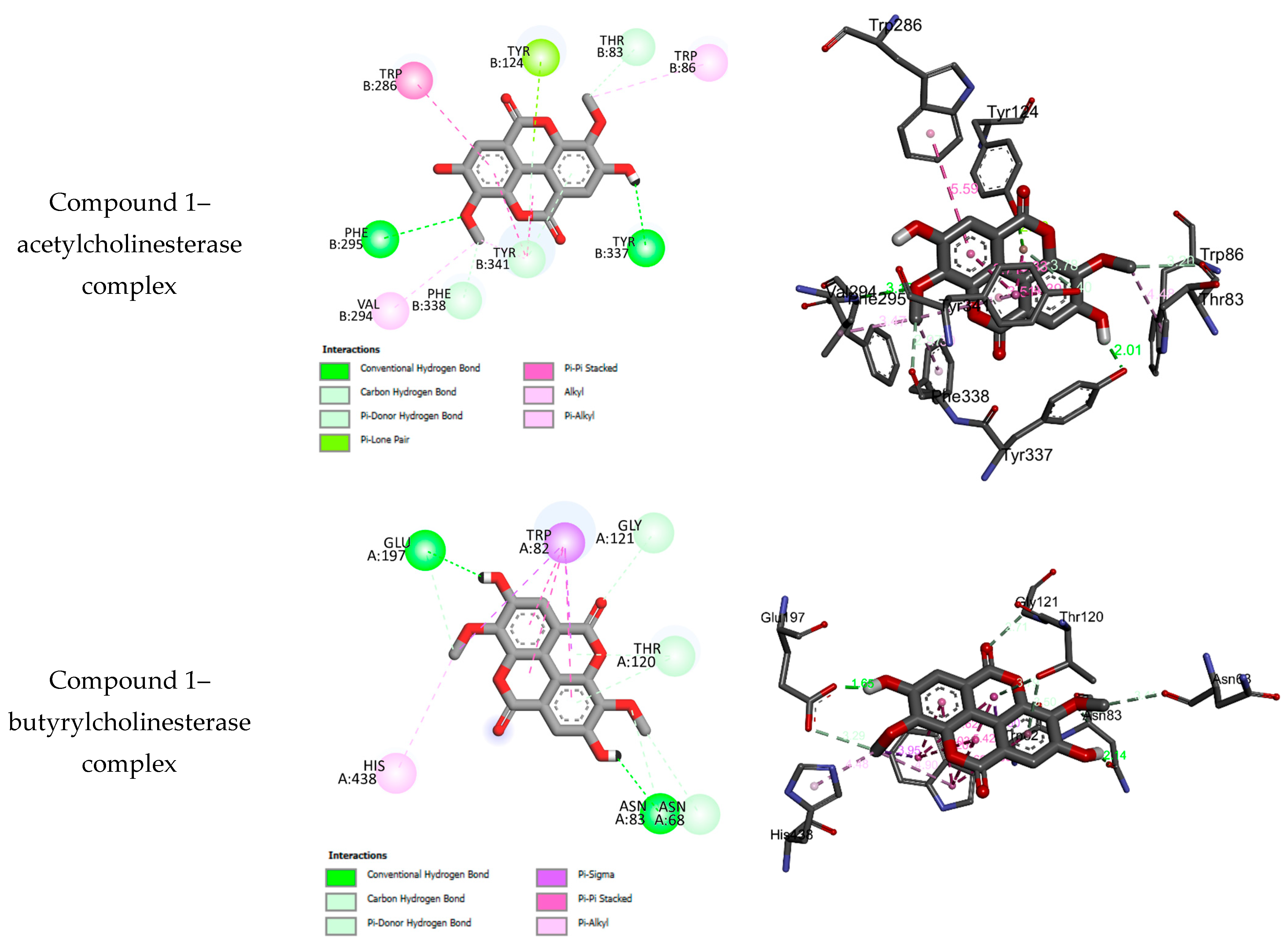
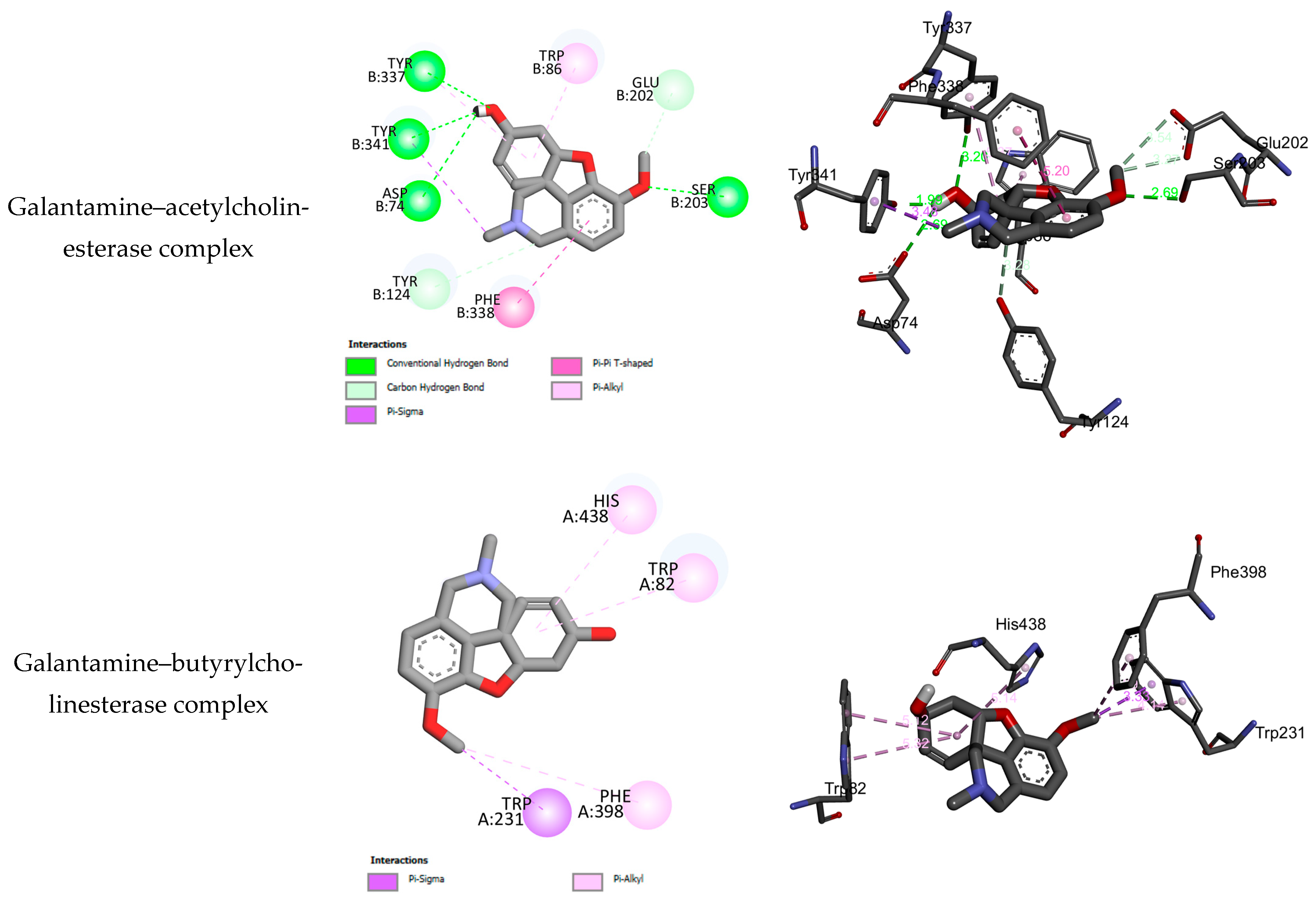
2.3. Molecular Docking Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. Isolation and Purification of the Compounds
4.3. Anticholinesterase Activity
4.4. α-Glucosidase and α-Amylase Inhibitory Assay
4.5. Molecular Docking Details
4.6. Statistical Analysis
4.7. NMR Data of the Isolated Compounds
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mishra, P.; Beura, S.; Modak, R. Significance of Enzymes in Modern Healthcare: From Diagnosis to Therapy. In Bioprospecting of Enzymes in Industry, Healthcare and Sustainable Environment; Thatoi, H., Mohapatra, S., Das, S.K., Eds.; Springer: Singapore, 2011. [Google Scholar] [CrossRef]
- Murakawa, T.; Matsushita, Y.; Suzuki, T.; Khan, M.T.; Kurita, N. Ab initio molecular simulations for proposing potent inhibitors to butyrylcholinesterase. J. Mol. Graph. Model. 2014, 54, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Boudiba, S.; Kucukaydin, S.; Tamfu, A.N.; Blaise, K.; Munvera, A.M.; Arab, Y.; Ceylan, O.; Dinica, R.M. HPLC-DAD Phenolic Composition, Antioxidant, Anticholinesterase, Antidiabetic and Anti-quorum Sensing Properties of Bitter Kola (Garcinia kola) and Kolanut (Cola acuminata). Pharmacog. Res. 2023, 15, 373–383. [Google Scholar] [CrossRef]
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, 105–125. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Kucukaydin, S.; Yeskaliyeva, B.; Ozturk, M.; Dinica, R.M. Non-alkaloid cholinesterase inhibitory compounds from natural sources. Molecules 2021, 26, 5582. [Google Scholar] [CrossRef] [PubMed]
- Zuin, M.; Cherubini, A.; Volpato, S.; Ferrucci, L.; Zuliani, G. Acetyl-cholinesterase-inhibitors slow cognitive decline and decrease overall mortality in older patients with dementia. Sci. Rep. 2022, 12, 12214. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, M.B.; Rezaei, Z.; Asadi, M.; Behnammanesh, H.; Nadri, H.; Afsharirad, F.; Moradi, A.; Larijani, B.; Mohammadi-Khanaposhtan, M.; Mahdavi, M. Design, Synthesis, and Cholinesterase Inhibition Assay of Coumarin-3-carboxamide-N-morpholine Hybrids as New Anti-Alzheimer Agents. Chem. Biodivers. 2019, 16, 900144. [Google Scholar] [CrossRef] [PubMed]
- d’Angremont, E.; Begemann, M.J.H.; Van, L.T.; Sommer, I.E.C. Cholinesterase Inhibitors for Treatment of Psychotic Symptoms in Alzheimer Disease and Parkinson Disease: A Meta-analysis. JAMA Neurol. 2023, 80, 813–823. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.A.S.; Camara, A.L.; Paes, A.M.A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Ceylan, O.; Kucukaydin, S.; Duru, M.E. HPLC-DAD phenolic profiles, antibiofilm, anti-quorum sensing and enzyme inhibitory potentials of Camellia sinensis (L.) O. Kuntze and Curcuma longa L. LWT Food Sci. Technol. 2020, 133, 110150. [Google Scholar] [CrossRef]
- Ahmed, F.; Ghalib, R.M.; Sasikala, P.; Ahmed, K.K. Cholinesterase inhibitors from botanicals. Pharmacogn Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef]
- Munvera, A.M.; Alfred, N.T.; Ouahouo, B.M.W.; Kucukaydin, S.; Nyemb, J.N.; Fokam, M.M.A.; Djappa, T.E.C.; Mkounga, P.; Nkengfack, A.E. Cholinesterase, α-glucosidase, tyrosinase and urease inhibitory activities of compounds from fruits of Rinorea oblongifolia C.H. Wright (Violaceae). Nat. Prod. Res. 2023, 9, 4169–4180. [Google Scholar] [CrossRef]
- GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: An update. Mini Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Riyaphan, J.; Jhong, C.H.; Lin, S.R.; Chang, C.H.; Tsai, M.J.; Lee, D.N.; Sung, P.J.; Leong, M.K.; Weng, C.F. Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase. Molecules 2018, 23, 2260. [Google Scholar] [CrossRef] [PubMed]
- Feunaing, R.T.; Tamfu, A.N.; Gbaweng, A.J.Y.; Magnibou, M.L.; Ntchapda, F.; Henoumont, C.; Laurent, S.; Talla, E.; Dinica, R.M. In Vitro Evaluation of α-amylase and α-glucosidase Inhibition of 2,3-Epoxyprocyanidin C1 and Other Constituents from Pterocarpus erinaceus Poir. Molecules 2023, 28, 126. [Google Scholar] [CrossRef]
- Owokotomo, I.A.; Ekundayo, O.; Abayomi, T.G.; Chukwuka, A.V. In-vitro anti-cholinesterase activity of essential oil from four tropical medicinal plants. Toxicol. Rep. 2015, 2, 850–857. [Google Scholar] [CrossRef]
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of hyperglycaemia in type 2 diabetes: A Patient-Centered Approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia 2012, 55, 1577–1596. [Google Scholar] [CrossRef] [PubMed]
- Holman, R.R.; Coleman, R.L.; Chan, J.C.N.; Chiasson, J.-L.; Feng, H.; Ge, J.; Gerstein, H.C.; Gray, R.; Huo, Y.; Lang, Z.; et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017, 5, 877–886. [Google Scholar] [CrossRef]
- Stumpfe, D.; Geppert, H.; Bajorath, J. In SilicoScreening. In Lead Generation Approaches in Drug Discovery; Wiley & Sons Press: Hoboken, NJ, USA, 2010; pp. 73–103. [Google Scholar]
- Metiefeng, N.T.; Tamfu, A.N.; Fotsing Tagatsing, M.; Tabopda, T.K.; Kucukaydin, S.; Noah Mbane, M.; de Theodore Atchade, A.; Talla, E.; Henoumont, C.; Laurent, S.; et al. In Vitro and In Silico Evaluation of Anticholinesterase and Antidiabetic Effects of Furanolabdanes and Other Constituents from Graptophyllum pictum (Linn.) Griffith. Molecules 2023, 28, 4802. [Google Scholar] [CrossRef]
- Silva, O.; Duarte, A.; Pimentel, M.; Viegas, S.; Barroso, H.; Machado, J.; Pires, I.; Cabrita, J.; Gomes, E. Antimicrobial activity of Terminalia macroptera root. J. Ethnopharmacol. 1997, 5, 203–207. [Google Scholar] [CrossRef]
- Anh, T.P.; Karl, E.M.; Berit, S.P.; Drissa, D.; Helle, W. α-Glucosidase inhibition, 15-lipoxygenase inhibition, and brine shrimptoxicity of extracts and isolated compounds from Terminalia macroptera leaves. Pharm. Biol. 2014, 52, 1166–1169. [Google Scholar]
- Anh, T.P.; Christina, D.; Adiaratou, T.; Helle, W.; Drissa, D.; Berit, S.P.; Karl, E.M. Terminalia macroptera, its current medicinal use and future perspectives. J. Ethnopharmacol. 2011, 137, 1486–1491. [Google Scholar]
- Mahamane, H.; Adama, D.; Mohamed, H.; Aïssata, C.; Korotoumou, T.; Agnès, A.; Geneviève, B.; Rokia, S. Evaluation of Anti-inflammatory, Anti-pyretic, Analgesic, and Hepatoprotective Properties of Terminalia macroptera. Planta Med. Int. Open 2020, 7, 58–67. [Google Scholar]
- Mahamane, H.; Mohamed, H.; Adama, D.; Guillaume, M.; Sandra, B.-D.; Rokia, S.; Geneviève, B.; Agnès, A. In vivo validation of anti-malarial activity of crude extracts of Terminalia macroptera, a Malian medicinal plant. Malar. J. 2018, 17, 68. [Google Scholar]
- Silva, O.; Viegas, S.; De Mello-Sampayo, C.; Costa, M.J.P.; Serrano, R.; Cabrita, J.; Gomes, E.T. Anti-Helicobacter pylori activity of Terminalia macroptera root. Fitoterapia 2012, 83, 872–876. [Google Scholar] [CrossRef]
- Anh, T.P.; Karl, E.M.; Berit, S.P.; Drissa, D.; Helle, W. DPPH radical scavenging and xanthine oxidase inhibitory activity of Terminalia macroptera leaves. Nat. Prod. Commun. 2011, 6, 1125–1128. [Google Scholar]
- Conrad, J.; Vogler, B.; Klaiber, I.; Roos, G.; Walter, U.; Kraus, W. Two triterpene esters from Terminalia macroptera bark. Phytochemistry 1998, 48, 647–650. [Google Scholar] [CrossRef]
- Nongonierma, R.; Proliac, A.; Raynaud, J. O-glycosyl flavonoids from the flowers of Terminalia macroptera Guill and Perr (Combretaceae). Pharm. Acta Helv. 1990, 65, 233–235. [Google Scholar]
- Conrad, J.; Vogler, B.; Klaiber, I.; Reeb, S.; Guse, J.H.; Roos, G. Vanillic acid 4-O-d-(6(-O-galloyl) glucopyranoside and other constituents from the bark of Terminalia macroptera Guill. et Perr. Nat. Prod. Lett. 2001, 15, 35–42. [Google Scholar] [CrossRef]
- Silva, O.; Gomes, E.T.; Wolfender, J.L.; Marston, A.; Hostettmann, K. Application of high performance chromatography coupled with ultraviolet spectroscopy and electrospray mass spectrometry to the characterisation of ellagitannins from Terminalia macroptera roots. Pharm. Res. 2000, 17, 1396–1401. [Google Scholar] [CrossRef] [PubMed]
- Chahad, A.M.; Adey, S.A.; Chatté, A.; Louis, Z.; Dijoux-Franca, M.-G. Chemical analysis and Biological properties of Terminalia macroptera Guill. & Perr. from Eastern Chad. Mediterr. J. Chem. 2021, 11, 104–114. [Google Scholar] [CrossRef]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Javaid, N.; Shah, M.A.; Rasul, A.; Chauhdary, Z.; Saleem, U.; Khan, H.; Ahmed, N.; Uddin, M.S.; Mathew, B.; Behl, T.; et al. Neuroprotective Effects of Ellagic Acid in Alzheimer’s Disease: Focus on Underlying Molecular Mechanisms of Therapeutic Potential. Curr Pharm Des. 2021, 27, 3591–3601. [Google Scholar] [CrossRef]
- Kumar, M.; Bansal, N. Ellagic acid prevents dementia through modulation of PI3-kinase-endothelial nitric oxide synthase signalling in streptozotocin-treated rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 987–1001. [Google Scholar] [CrossRef]
- Oh, J.M.; Jang, H.J.; Kang, M.G.; Song, S.; Kim, D.Y.; Kim, J.H.; Noh, J.I.; Park, J.E.; Park, D.; Yee, S.T.; et al. Acetylcholinesterase and monoamine oxidase-B inhibitory activities by ellagic acid derivatives isolated from Castanopsis cuspidata var. sieboldii. Sci. Rep. 2021, 11, 13953. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Menard, C.; Hennion, M.C.; Lawali, Y.D.; Mehmet, A.; Tuba, A.; Ahmet, C. Antidiabetic and Anticholinesterase Properties of Extracts and Pure Metabolites of Fruit Stems of Pistachio (Pistacia vera L.). Curr. Org. Chem. 2020, 24, 785–797. [Google Scholar]
- Haouam, C.; Boudiba, S.; Tamfu, A.N.; Kucukaydin, S.; Hanini, K.; Zohra, H.F.; Hioun, S.; Botezatu, A.D.; Ceylan, Ö.; Boudiba, L. Assessment of Chemical Composition and In Vitro Antioxidant, Antidiabetic, Anticholinesterase and Microbial Virulence-Quenching Effects of Salad Burnet (Sanguisorba minor L.) Harvested from Algeria. Plants 2023, 12, 4134. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, R.L.; Ganapathy, A.; Jaisankar, P. A Review of A-Glucosidase and A-Amylase Inhibitors for Type 2 Diabetes Isolated From Some Important Indian Medicinal Plants. Ann. Clin. Pharmacol. Ther. 2018, 1, 1003. [Google Scholar]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef]
- Quradha, M.M.; Duru, M.E.; Kucukaydin, S.; Tamfu, A.N.; Iqbal, M.; Bibi, H.; Khan, R.; Ceylan, O. Comparative assessment of phenolic composition profile and biological activities of green extract and conventional extracts of Salvia sclarea. Sci. Rep. 2024, 14, 1885. [Google Scholar] [CrossRef]
- Tran, T.D.; Tu, V.L.; Hoang, T.M.; Dat, T.V.; Tam, D.N.H.; Phat, N.T.; Hung, D.T.; Huynh, H.H.; Do, T.C.; Le, H.H.; et al. A Review of the In Vitro Inhibition of α-Amylase and α-Glucosidase by Chalcone Derivatives. Cureus 2023, 15, e37267. [Google Scholar] [CrossRef]
- Amor, A.J.; Gómez-Guerrero, C.; Orteg, A.E.; Sala-Vila, A.; Lázaro, I. Ellagic Acid as a Tool to Limit the Diabetes Burden: Updated Evidence. Antioxidants 2020, 9, 1226. [Google Scholar] [CrossRef]
- Elbandrawy, M.M.; Sweef, O.; Elgamal, D.; Mohamed, T.M.; Ehab, T.; Elgharabawy, R.M. Ellagic acid regulates hyperglycemic state through modulation of pancreatic IL-6 and TNF-α immunoexpression. Saudi J. Biol. Sci. 2022, 29, 3871–3880. [Google Scholar] [CrossRef]
- Naraki, K.; Ghasemzadeh, R.M.; Ajiboye, B.O.; Hosseinzadeh, H. The effect of ellagic acid on the metabolic syndrome: A review article. Heliyon 2023, 9, 21844. [Google Scholar] [CrossRef]
- Guo, S.; Ren, X.; He, K.; Chen, X.; Zhang, S.; Roller, M.; Zheng, B.; Zheng, Q.; Ho, C.T.; Bai, N. The anti-diabetic effect of eight Lagerstroemia speciosa leaf extracts based on the contents of ellagitannins and ellagic acid derivatives. Food Funct. 2020, 11, 1560–1571. [Google Scholar] [CrossRef]
- Qian, J.; Zhang, J.; Chen, Y.; Dai, C.; Fan, J.; Guo, H. Hypoglycemic activity and mechanisms of myricetin. Nat. Prod. Res. 2022, 36, 6177–6180. [Google Scholar] [CrossRef]
- Liu, X.; Fu, Y.; Ma, Q.; Yi, J.; Cai, S. Anti-Diabetic Effects of Different Phenolic-Rich Fractions from Rhus Chinensis Mill. Fruits in vitro. eFood 2021, 2, 37–46. [Google Scholar] [CrossRef]
- Mohammed, A.; Kumar, D.; Rizvi, S.I. Antidiabetic potential of some less commonly used plants in traditional medicinal systems of India and Nigeria. J. Intercult. Ethnopharmacol. 2015, 4, 78–85. [Google Scholar] [CrossRef]
- Mohanty, I.R.; Borde, M.; Kumar, C.S.; Maheshwari, U. Dipeptidyl peptidase IV Inhibitory activity of Terminalia arjuna attributes to its cardioprotective effects in experimental diabetes: In silico, in vitro and in vivo analyses. Phytomedicine 2019, 57, 158–165. [Google Scholar] [CrossRef]
- Feunaing, R.T.; Tamfu, A.N.; Ntchapda, F.; Gade, I.S.; Mbane, M.N.; Tagatsing, M.F.; Talla, E.; Henoumont, C.; Laurent, S.; Dinica, R.M. A new abietane-type diterpenoid from roots of Burkea africana Hook (Fabaceae) with α-amylase inhibitory potential. Nat. Prod. Res. 2022, 36, 4132–4139. [Google Scholar] [CrossRef]
- Peitzika, S.-C.; Pontiki, E. A Review on Recent Approaches on Molecular Docking Studies of Novel Compounds Targeting Acetylcholinesterase in Alzheimer Disease. Molecules 2023, 28, 1084. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In Vitro and In Vivo Antidiabetic Activity, Isolation of Flavonoids, and in Silico Molecular Docking of Stem Extract of Merremia Tridentata (L.). Biomed. Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef]
- Olaokun, O.O.; Zubair, M.S. Antidiabetic Activity, Molecular Docking, and ADMET Properties of Compounds Isolated from Bioactive Ethyl Acetate Fraction of Ficus lutea Leaf Extract. Molecules 2023, 28, 7717. [Google Scholar] [CrossRef]
- Chennai, H.Y.; Belaidi, S.; Bourougaa, L.; Ouassaf, M.; Sinha, L.; Samadi, A.; Chtita, S. Identification of Potent Acetylcholinesterase Inhibitors as New Candidates for Alzheimer Disease via Virtual Screening, Molecular Docking, Dynamic Simulation, and Molecular Mechanics–Poisson–Boltzmann Surface Area Calculations. Molecules 2024, 29, 1232. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Alain, K.Y.; Tamfu, A.N.; Kucukaydin, S.; Ceylan, O.; Pascal, A.D.; Félicien, A.; Dominique, S.C.; Duru, M.E.; Dinica, R.M. Phenolic profiles, antioxidant, antiquorum sensing, antibiofilm and enzyme inhibitory activities of selected Acacia species collected from Benin. LWT 2022, 171, 114162. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Yang, X.-W.; Huang, M.-Z.; Jin, Y.-S.; Sun, L.-N.; Song, Y.; Chen, H.-S. Phenolics from Bidens bipinnata and their amylase inhibitory properties. Fitoterapia 2012, 83, 1169–1175. [Google Scholar] [CrossRef]
- Tamfu, A.N.; Munvera, A.M.; Botezatu, A.V.D.; Talla, E.; Ceylan, O.; Fotsing, M.T.; Mbafor, J.T.; Shaheen, F.; Dinica, R.M. Synthesis of benzoyl esters of β-amyrin and lupeol and evaluation of their antibiofilm and antidiabetic activities. Results Chem. 2022, 4, 100322. [Google Scholar] [CrossRef]

| Anticholinesterase Activity | Antidiabetic Activity | |||
|---|---|---|---|---|
| AChE | BChE | α-Amylase | α-Glucosidase | |
| Compound/Standard | IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) | IC50 (µg/mL) |
| Extract | 56.91 ± 0.47 | 80.50 ± 1.25 | 164.71 ± 1.12 | 148.20 ± 1.00 |
| 1 | 46.77 ± 0.90 | 50.48 ± 1.10 | 67.08 ± 0.42 | 74.18 ± 0.29 |
| 2 | 61.25 ± 0.11 | >100 | 102.97 ± 0.73 | 121.51 ± 0.65 |
| 3 | 59.75 ± 0.33 | 75.21 ± 0.31 | 110.13 ± 0.80 | 137.51 ± 0.81 |
| 4 | 63.55 ± 0.72 | 89.81 ± 0.43 | 91.26 ± 0.55 | 97.75 ± 0.49 |
| 5 | >100 | >100 | 65.17 ± 0.43 | 69.02 ± 0.65 |
| 6 | 55.25 ± 0.25 | 58.70 ± 0.46 | >200 | >200 |
| 7 | 53.61 ± 0.81 | >100 | 186.32 ± 0.21 | 192.17 ± 0.78 |
| 8 | 65.72 ± 0.33 | >100 | 145.91 ± 0.56 | 155.84 ± 0.31 |
| 9 | >100 | >100 | >200 | >200 |
| 10 | 98.71 ± 1.02 | >100 | >200 | >200 |
| 11 | >100 | >100 | >200 | >200 |
| Galantamine | 5.50 ± 0.25 | 42.10 ± 0.15 | NT | NT |
| Acarbose | NT | NT | 32.25 ± 0.36 | 87.70 ± 0.68 |
| No. | BEs | HBs | Number of Closest Residues to the Docked Ligand in the Active Site | IC50 ± SEM |
|---|---|---|---|---|
| α-glucosidase | ||||
| 1 | −5.97 | 2 | HIS A626, PHE A601, ASP A357, TRP A329, ASP A469, TRP A432, MET A470, ARG A552, ASP A568, ASPA232, ALA A234 | 74.18 ± 0.29 |
| 2 | −7.86 | 6 | ASP A469, ARG A552, ASP A568, ALA A234, ILE A233, ASPA232, PHE A476, TRP A432 | 121.51 ± 0.65 |
| 3 | −7.54 | 7 | ASP A469, ASP A357, TRP A432, PHE A476, ASP A232, ASN A237, PHE A236, ALA A234, ASP A568, ARG A552 | 137.51 ± 0.81 |
| 4 | −7.78 | 5 | ASP A469, ASP A357, ASP A232, ALA A234, TRP A329, PHE A601, ALA A628, ASP A568 | 97.75 ± 0.49 |
| 5 | −7.30 | 8 | ASP A357, ASP A469, MET A470, ALA A234, ASP A232, ASP A568, ASP A630, TRP A329, TRP A432, ALA A628 | 69.02 ± 0.65 |
| 6 | −4.03 | 4 | ASP A232, LYS A506, ASN A475, SER A474 | >200 |
| 7 | −7.27 | 4 | ASP A630, ASP A469, ASP A232, GLU A603 | 192.17 ± 0.78 |
| 8 | −6.71 | 6 | ASP A232, ASP A568, MET A470, ARG A552, ASP A630, GLU A603 | 155.84 ± 0.31 |
| 9 | −10.16 | 6 | ARG A552, ASP A568, ASP A357, ASP A469, TRP A432 | >200 |
| 10 | −9.60 | 5 | ASP A630, GLU A603, ARG A552, ASP A568, ASP A469, ASP A357 | >200 |
| 11 | −10.20 | 6 | ASP A630, ASP A469, ASP A357, ASP A568, ARG A552 | >200 |
| Acarbose | −10.56 | 12 | ASN A237, ALA A234, ASP A232, ASP A568, MET A470, ASP A357, HIS A626, ARG A552 | 87.70 ± 0.68 |
| α-amylase | ||||
| 1 | −6.22 | 2 | TRP A58, HIS A305, TRP A59, LEU A165, HIS A101, ASP A197 | 67.08 ± 0.42 |
| 2 | −9.41 | 7 | TRP A59, GLN A63, ASP A197, ARG A195, GLU A133, HIS A305 | 102.97 ± 0.73 |
| 3 | −8.84 | 7 | TRP A59, GLN A63, GLU A233, ASP A197, ARG A195, HIS A305 | 110.13 ± 0.80 |
| 4 | −8.72 | 7 | TRP A59, GLN A63, GLU A233, ASP A197, ARG A195, HIS A305 | 91.26 ± 0.55 |
| 5 | −7.83 | 7 | ASP A197, GLU A233, ALA A198, TYR A151, LEU A162, TYR A62, ASP A300 | 65.17 ± 0.43 |
| 6 | −4.72 | 4 | GLU A233, ILE A235, VAL A234 | >200 |
| 7 | −8.78 | 3 | ASP A300, ASP A197 | 186.32 ± 0.21 |
| 8 | −10.18 | 4 | TRP A59, HIS A305, LYS A200 | 145.91 ± 0.56 |
| 9 | −8.84 | 7 | ASP A197, TYR A151, THR A163 | >200 |
| 10 | −8.95 | 8 | ASP A197, ASP A300, TYR A151, THR A163 | >200 |
| 11 | −9.60 | 7 | ASP A197, ASP A300, TYR A151, THR A163, ILE A148 | >200 |
| Acarbose | −14.46 | 11 | THR A163, GLN A63, TRP A59, GLY A306, TYR A151, HIS A201, GLU A233, ASP A300, ARG A195, TYR A62, HIS A299, HIS A305 | 32.25 ± 0.36 |
| No. | BEs (kcal/mol) | HBs | Number of Closest Residues to the Docked Ligand in the Active Site | IC50 ± SEM |
|---|---|---|---|---|
| Acetylcholinesterase | ||||
| 1 | −8.21 | 2 | PHE B295, VAL B295, PHE B338, TYR B341, TYR B337, TRP B86, THR B83, TYR B124, TRP B286 | 46.77 ± 0.90 |
| 2 | −10.08 | 3 | HIS B447, TYR B337, PHE B338, TYR B341, TRP B286, TYR B124, THR B83, TRP B86 | 61.25 ± 0.11 |
| 3 | −10.21 | 3 | SER B203, TYR B124, TYR B72, TRP B286, PHE B295, TYR B341, TYR B337, PHE B338 | 59.75 ± 0.33 |
| 4 | −9.86 | 3 | SER B203, TYR B124, TYR B72, TRP B286, PHE B295, TYR B341, PHE B338 | 63.55 ± 0.72 |
| 5 | −8.45 | 5 | TRP B286, TYR B341, TYR B124, ASP B74, THR B83, ASN B87, GLY B122 | >100 |
| 6 | −4.13 | 5 | TYR B341, TYR B124, PHE B295, ARG B296 | 55.25 ± 0.25 |
| 7 | −7.10 | 1 | TRP B286, TYR B341, ARG B296, PHE B338 | 53.61 ± 0.81 |
| 8 | −7.56 | 2 | SER B293, TYR B341 | 65.72 ± 0.33 |
| 9 | −6.13 | 3 | ARG B296, TYR B341, ASP B74, TRP B286, TRP B86, PHE B338 | >100 |
| 10 | −5.72 | 2 | ARG B296, PHE B338, TRP B86, TYR B341, TRP B286, ASP B74 | 98.71 ± 1.02 |
| 11 | −6.57 | 5 | TRP B86, ASP B74, TYR B341, TYR B72, TRP B86 | >100 |
| Galantamine | −7.59 | 4 | ASP B74, TYR B341, TYR B337, TRP B86, GLU B202, SER B203, PHE B338, TYR B124 | 5.50 ± 0.25 |
| Butyrylcholinesterase | ||||
| 1 | −7.83 | 2 | HIS A438, ASN A83, ASN A68, THR A120, GLY A121, TRP A82, GLU A197 | 50.48 ± 1.10 |
| 2 | −8.10 | 5 | GLU A197, ALA A328, PHE A329, GLY A117, VAL A288, TRP A231, LEU A286, SER A198, TRP A82 | >100 |
| 3 | −8.04 | 5 | LEU A286, SER A287, PRO A285, ASP A70, THR A120, GLY A115, TYR A128, LEU A125, GLU A197, GLY A116, SER A198, TRP A82 | 75.21 ± 0.31 |
| 4 | −8.14 | 6 | TYR A128, GLU A197, TRP A82, SER A198, LEU A286, VAL A288, SER A287, PHE A329 | 89.81 ± 0.43 |
| 5 | −9.66 | 11 | SER A198, GLY A116, TYR A128, GLU A197, ASP A70, TYR A332, ALA A328, HIS A438, TRP A231, LEU A286 | >100 |
| 6 | −5.13 | 4 | LEU A286, PRO A285, SER A198, GLY A117 | 58.70 ± 0.46 |
| 7 | −5.00 | 3 | HIS A438, TRP A82, SER A287 | >100 |
| 8 | −6.77 | 3 | HIS A438, TRP A82, SER A287 | >100 |
| 9 | NB | NA | NA | >100 |
| 10 | NB | NA | NA | >100 |
| 11 | NB | NA | NA | >100 |
| Galantamine | −6.69 | 0 | HIS A438, TRP A82, PHE A398, TRP A231 | 42.10 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feunaing, R.T.; Tamfu, A.N.; Gbaweng, A.J.Y.; Kucukaydin, S.; Tchamgoue, J.; Lannang, A.M.; Lenta, B.N.; Kouam, S.F.; Duru, M.E.; Anouar, E.H.; et al. In Vitro and Molecular Docking Evaluation of the Anticholinesterase and Antidiabetic Effects of Compounds from Terminalia macroptera Guill. & Perr. (Combretaceae). Molecules 2024, 29, 2456. https://doi.org/10.3390/molecules29112456
Feunaing RT, Tamfu AN, Gbaweng AJY, Kucukaydin S, Tchamgoue J, Lannang AM, Lenta BN, Kouam SF, Duru ME, Anouar EH, et al. In Vitro and Molecular Docking Evaluation of the Anticholinesterase and Antidiabetic Effects of Compounds from Terminalia macroptera Guill. & Perr. (Combretaceae). Molecules. 2024; 29(11):2456. https://doi.org/10.3390/molecules29112456
Chicago/Turabian StyleFeunaing, Romeo Toko, Alfred Ngenge Tamfu, Abel Joel Yaya Gbaweng, Selcuk Kucukaydin, Joseph Tchamgoue, Alain Meli Lannang, Bruno Ndjakou Lenta, Simeon Fogue Kouam, Mehmet Emin Duru, El Hassane Anouar, and et al. 2024. "In Vitro and Molecular Docking Evaluation of the Anticholinesterase and Antidiabetic Effects of Compounds from Terminalia macroptera Guill. & Perr. (Combretaceae)" Molecules 29, no. 11: 2456. https://doi.org/10.3390/molecules29112456
APA StyleFeunaing, R. T., Tamfu, A. N., Gbaweng, A. J. Y., Kucukaydin, S., Tchamgoue, J., Lannang, A. M., Lenta, B. N., Kouam, S. F., Duru, M. E., Anouar, E. H., Talla, E., & Dinica, R. M. (2024). In Vitro and Molecular Docking Evaluation of the Anticholinesterase and Antidiabetic Effects of Compounds from Terminalia macroptera Guill. & Perr. (Combretaceae). Molecules, 29(11), 2456. https://doi.org/10.3390/molecules29112456










