Abstract
An iodophor-catalyzed direct disulfenylation of amino naphthalenes with aryl sulfonyl hydrazines in water was developed. A series of aryl sulfides were obtained in moderate to excellent yields. The advantages of this green protocol were the simple reaction conditions (metal-free, water as the solvent, under air), the odorless and easily available sulfur reagent, the broad substrate scope, and gram-scale synthesis. Moreover, the potential application of aryl sulfides was exemplified by further transformations.
1. Introduction
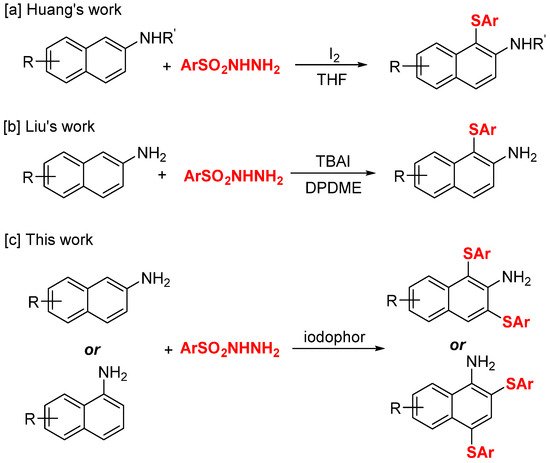
As important organosulfides, diaryl sulfides have a wide range of applications in the fields of organic synthesis, medicinal chemistry, and materials science due to their unique biological activities and physical properties [1,2,3,4]. Therefore, the development of green and efficient strategies for the synthesis of diaryl sulfides is one of the hot spots in current research [5]. In recent years, the synthesis of diaryl sulfides by direct C-H sulfenylation of arenes has received much attention because of the shortening of the reaction steps and the reduction in waste generation [6,7]. In particular, the use of water as a safe, cheap, and environmentally friendly solvent makes sulfenylation greener and more economical. Examples of relevant processes include the following: (i) copper-catalyzed three-component reaction of arenes, iodoaromatics (or iodoalkanes), and sulfur [8]; (ii) iodine-mediated C-H sulfenylation of arenes [9,10,11,12]; (iii) cobalt-catalyzed aerobic couplings of C-H and thiols [13]; (iv) potassium persulfate–glucose-mediated sulfenylation of indole and thiophenols [14]. In 2015, Kang et al. reported an iodine-mediated thiolation of substituted naphthylamines and aryl sulfonyl hydrazides via direct C-H bond functionalization (Scheme 1a) [15]. In 2020, Liu et al. developed the TBAI-promoted sulfenylation of 2-aminonaphthalene and aryl sulfonyl hydrazides in the presence of DPDME (dipropylene glycol dimethyl ether) as a green solvent (Scheme 1b) [16]. However, all of the above reactions result in mono-substituted aryl thioethers. Double-substituted aryl sulfides have not been realized as yet.
Scheme 1.
Sulfenylation of naphthalene amines. [a] Huang’s work; [b] Liu’s work; [c] This work.
It is well known that iodophors are now widely used as bactericidal products for surface disinfection, wound treatment, pre- and post-operative care, mouthwashes, gargles, and nasal sprays [17]. Studies have shown that iodophor as a highly effective topical disinfectant is not significantly cytotoxic or irritating [18]. However, to the best of our knowledge, the use of iodophor as a catalyst for organic synthesis reactions has not been reported. Considering that povidone iodine as a commercially readily available reagent has the advantage of possessing a two-component reagent (iodine and polyvinylpyrrolidone surfactant), this provides a rare opportunity for iodine-catalyzed organic reactions in the aqueous phase. On the basis of our previous research work [19,20,21,22], we herein report the iodophor-catalyzed disulfenylation of naphthylamine with aryl sulfonyl hydrazines; a series of 1,3-bis(arylthio)naphthalene-2-amines and 2,4-bis(arylthio)naphthalene-1-amines were obtained in moderate to excellent yields (Scheme 1c).
2. Results and Discussion
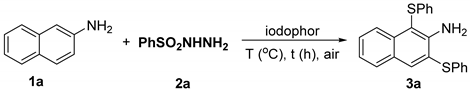
Initially, 0.3 mmol of 2-aminonaphthalene 1a and 0.6 mmol of phenylsulfonyl hydrazine 2a were chosen for a model reaction to investigate the feasibility of the Sulfite reaction (Table 1). The results showed that the product 1,3-bis(phenylthio)naphthalene-2-amine 3a was successfully obtained in the presence of 7 mL of iodophor at 100 °C for 24 h (Table 1, entry 1). The dosage study of iodophor showed that 2 mL was the most efficient in producing the corresponding product 3a in a 60% yield (entries 2–5). When the ratio of 1a to 2a was 1:3, 2 mL of iodophor was still the most catalytically effective and the desired product was obtained in a 90% yield (entries 6–8). Changes in reaction temperature are detrimental to the reaction (entries 9–11). Shortening the reaction time resulted in a significant decrease in the reaction yield (entries 12). The yield of product 3a declined to 71% when the ratio of 1a to 2a was adjusted to 1:4 (entries 13). Finally, the optimum reaction conditions were determined: 1a (0.3 mmol), 2a (0.9 mmol), and iodophor (2 mL) under air at 100 °C for 24 h.

Table 1.
Optimization of the reaction conditions a.
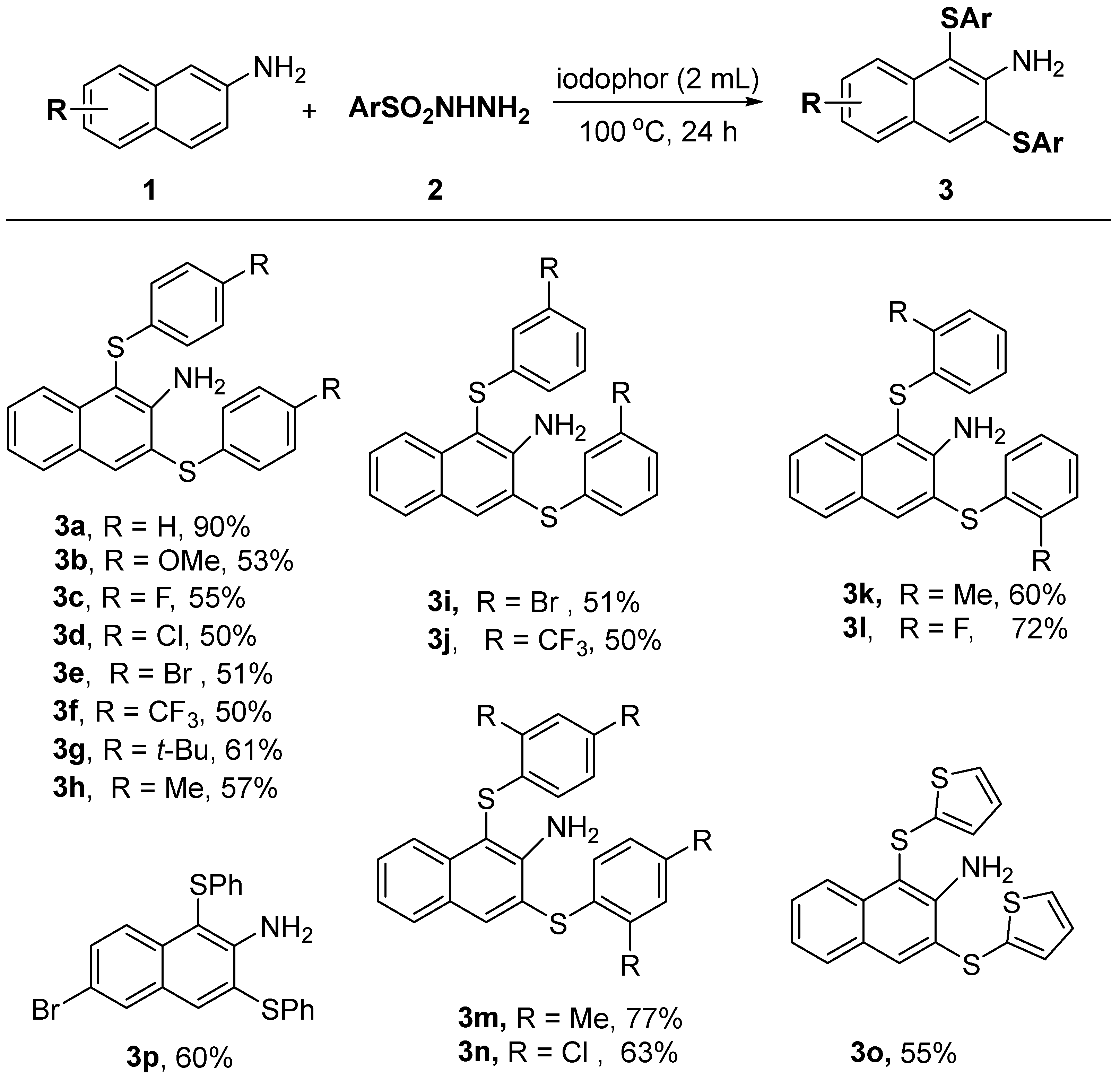
Under optimal reaction conditions, we investigated the range of 1,3-bisulfenylation reactions of β-naphthylamine with aryl sulfonyl hydrazides, and the results are summarized in Table 2. Benzenesulfonyl hydrazide substrates with various substituents attached to the 4-position such as methyl, methoxy, tert-butyl, fluoro, chloro, bromo, trifluoromethyl, and trifluoromethoxy were able to react smoothly with 2-naphthylamine, and the 1,3-bisulfenylated products 3a–3h were obtained in moderate yields. The electronic effect of the substituent group seems to have no influence on the reaction. Likewise, the meso-substituted aryl sulfonyl hydrazides showed similar reactivity to afford the products 3i and 3j. It is worth mentioning that substrates with some spatial site resistance such as 2-methyl-, 2-fluoro-, 2,4-dimethyl-, and 2,4-dichloro-substituted benzenesulfonyl hydrazides are also suitable reactants to provide the desired products 3k–3n in good yields. Heterocyclic substrates such as 2-thiophenesulfonyl hydrazide were also successfully converted to 1,3-bis(thiophen-2-ylthio)-2-naphthyl amine product 3o in a 55% yield. 6-Bromon-2-naphthyl-amine was used as a substrate to react successfully with phenylsulfonyl hydrazide to give the desired product 3p in a 60% yield. Predictably, the presence of bromine substituent offered the possibility of further conversions of the product 3p.

Table 2.
Iodophor-catalyzed disulfenylation of β-naphthylamine with ArSO2NHNH2 a.
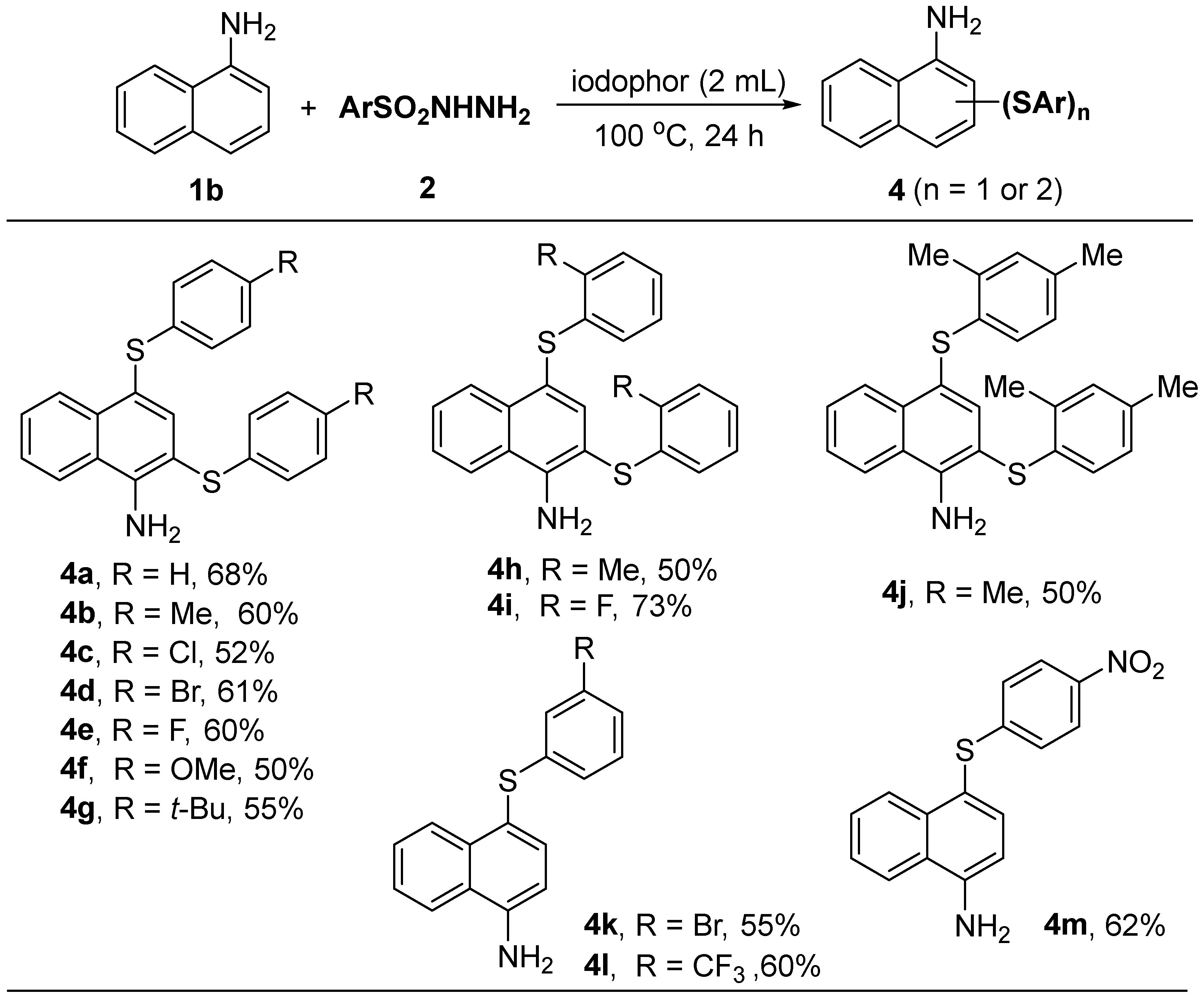
Subsequently, we investigated the 1,3-disulfenylation of 1-aminonaphthalene with aryl sulfonyl hydrazides (Table 3). The results showed that aryl sulfonyl hydrazides with all types of substituents reacted equally well, and a series of 2,4-diaryl sulfide products 4a–4j [12] were obtained in good yields. However, for benzenesulfonyl hydrazides with strong electron-withdrawing groups (4-nitro, 3-bromo, and 3-trifluoromethyl) in the para- or meso-positions as reactants, only mono-substituted 4-sulfenylated products 4k–4m [12] could be obtained, and we speculate that this may be mainly due to the electronic effect of the substituent groups.

Table 3.
Iodophor-catalyzed disulfenylation of α-naphthylamine with ArSO2NHNH2 a.
To demonstrate the utility of this reaction, we performed a scale-up reaction based on optimal conditions (Scheme 2). The reaction of 5 mmol of 2-aminonaphthalene with phenylsulfonyl hydrazine (10 mmol) in the presence of 20 mL of iodophor afforded 1.06 g of 3a product in a 60% yield (Scheme 2a). Subsequently, further derivatization of 3a was explored. Under the condition of acetic acid as a solvent, ferric nitrate hydrate was able to oxidize 3a to the corresponding sulfoxide product 1,3-bis(phenylsulfinyl)-2-naphthyl amine 3aa in 55% yield (Scheme 2b).
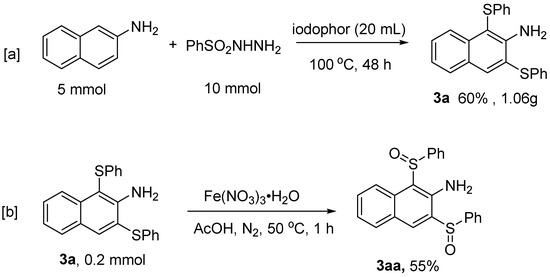
Scheme 2.
[a] Gram-scale synthesis and transformations of 3a. [b] The oxidation reaction of 3a.
In order to determine the innovation of the iodophor catalytic system and the selectivity of sulfinylation, related control experiments were designed and carried out. The controls used the same substrate concentration ratio, iodine dosage, temperature, and reaction time. According to the reaction (Scheme 3), a substituted 1-(phenylthio)-2-naphthyl-amine is mainly formed in the reaction system of iodine, while a disubstituted 1,3-bis(phenylthio)-2-naphthyl-amine is mainly formed in the reaction system of iodophor according to the reaction (Scheme 3). Therefore, it is speculated that iodophor provides a method to generate disulfide products.
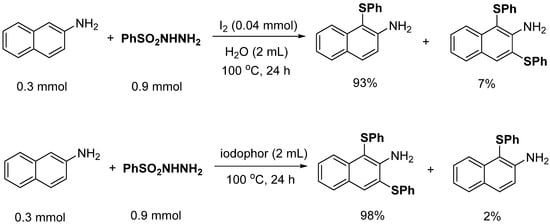
Scheme 3.
Control experiment of catalytic system.
In order to probe the mechanism of this reaction, a series of control experiments were performed (Scheme 4). The reaction of 2-aminonaphthalene with phenylsulfonyl hydrazine occurred equally well without any significant decrease in the yield of the product 3a when the radical trapping agents TEMPO (2,2,6,6- tetramethylpiperidine oxide) or BHT (butylated hydroxytoluene) were added to the system of this reaction (Scheme 4a), which suggests that the disulfenylation reaction should not be a free radical-involved process. When benzenesulfonyl hydrazide was reacted under standard conditions alone, diphenyl disulfide 2aa and S-phenyl benzenesulfonyl thioate 2ab were obtained in 80% and 16% yields, respectively (Scheme 4b), which suggests that 2aa and 2ab may be important intermediates produced during the reaction. In order to prove this conjecture, the feasibility of 2-aminonaphthalene disulfenylation reaction was explored using 2aa and 2ab as sulfur sources, respectively. It was shown that the reaction of 2-aminonaphthalene with 2aa had difficulty proceeding in the absence of iodophor (Scheme 4c), whereas the reaction of 2-aminonaphthalene with 2ab was possible under the same conditions, and the presence of iodophor significantly increased the yield of the disulfenylation reaction (Scheme 4d). All these facts demonstrate the important role played by povidone iodine in catalyzing the disulfenylation reaction.
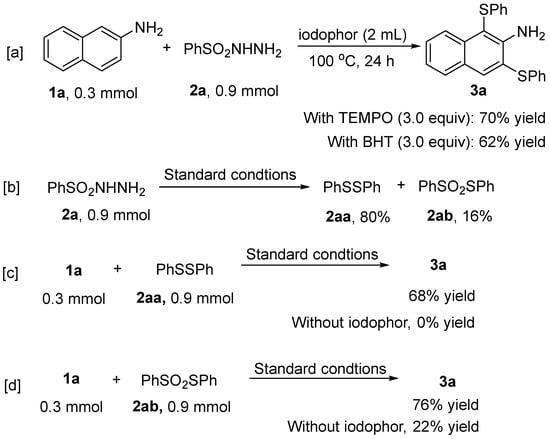
Scheme 4.
The control experiments. [a] Reactions in the presence of radical trapping reagents. [b] The reactivity of 2a alone. [c] The reactivity of 2aa without iodophor. [d] The reactivity of 2ab without iodophor.
Based on the above investigation and analysis, a plausible mechanism is outlined in Scheme 5. The reaction is initiated by benzenesulfonyl hydrazine, which can be transformed by I2 to form the intermediate 2aa and 2ab. Next, the sulfenylation of naphthylamine is mainly achieved through the following two routes: (1) Phenyl hypoiodothioite formed by the reaction of 2aa with I2 undergoes an electrophilic addition reaction with β-naphthylamine to give intermediate I. This intermediate loses HI to provide intermediate II. Subsequently, intermediate II repeats the above reaction process to obtain product 3a (Path 1). (2) The intermediate 2ab as an electrophile reacts with 1a to form III. The generated benzenesulfinic acid can be converted into 2ab in the presence of I2. Finally, the intermediate II reacts with the generated 2ab to obtain the product 3a (Path 2).
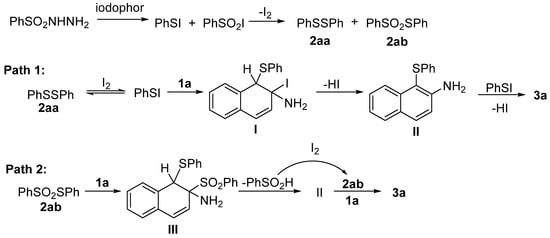
Scheme 5.
Proposed reaction mechanism.
3. Materials and Methods
Unless otherwise stated, all reactions were carried out experimentally in Schlenk tubes. Melting points were determined with a fusion meter and no other corrections were made. Materials obtained from commercial suppliers were used directly without further purification. Chromatography was performed using Qingdao Ocean Chemical 200–300 mesh silica gel. Spectra of 1H NMR, 13C NMR (in the Supplementary Materials) were recorded on a Bruker Avance III HD 400 MHz spectrometer in CDCl3 or DMSO-d6 solution, and chemical shifts (δ) relative to the internal standard tetramethylsilane (TMS) (0 ppm) were reported. High-resolution mass spectrometry (HRMS) was performed on a Thermo Scientific LTQ or a bitrap XL mass spectrometer, Thermo-fisher Q Exactive.
3.1. General Procedure for Iodophor-Catalyzed Disulfenylation of Amino Naphthalenes with Aryl Sulfonyl Hydrazines
A mixture of amino naphthalene (0.3 mmol), aryl sulfonyl hydrazine (0.9 mmol), and iodophor (2 mL) was added to a Schlenk tube. The solution was stirred continuously at 100 °C for 24 h. After completion of the reaction, the mixture was saturated with NaCl (10 mL) and extracted with CH2Cl2 (10 mL × 3). The combined CH2Cl2 extracts were dried with anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude residue was purified by fast column chromatography on silica gel by using PE (petroleum ether)/EA (ethyl acetate) as eluents.
3.2. Gram Synthesis Procedure for 3a
A mixture of β-amino naphthalene (5 mmol), phenylsulfonyl hydrazine (10 mmol), and iodophor (20 mL) was added to a Schlenk tube. The solution was stirred continuously at 100 °C for 48 h. After completion of the reaction, the mixture was saturated with NaCl (20 mL) and extracted with CH2Cl2 (20 mL × 3). The combined CH2Cl2 extracts were dried with anhydrous Na2SO4, filtered, and concentrated under reduced pressure. The crude residue was purified by fast column chromatography on silica gel by using PE/EA as eluents (3a, 60% yield, 1.06 g).
3.3. Synthesis Procedure of 3aa
To a 10 mL reaction tube, we added 3a (0.2 mmol), acetic acid (1 mL), ferric nitrate nonahydrate (0.5 equiv.), and iodophor (2 mL). The solution was stirred at 50 °C for 1 h. When the reaction was complete, it was neutralized with saturated sodium bicarbonate solution and extracted with ethyl acetate (20 mL). The solvent was removed under reduced pressure and the crude product was purified by fast chromatography on silica gel (PE/EA) to afford the final product 1,3-bis(phenylsulfinyl)-2-naphthyl-amine 3aa in a 55% yield.
1,3-bis(phenylthio)-2-naphthyl-amine (3a): red oily liquid; 1H NMR (400 MHz, CDCl3) δ 8.19 (d, J = 8.5 Hz, 1H), 7.69–7.60 (m, 2H), 7.48 (d, J = 8.3 Hz, 3H), 7.32 (d, J = 7.4 Hz, 2H), 7.19–7.15 (m, 1H), 7.06 (t, J = 7.5 Hz, 2H), 7.00–6.88 (m, 4H), 4.61 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 148.22, 142.63, 136.31, 133.41, 131.54, 131.18, 129.19, 128.70, 128.56, 128.12, 127.49, 127.27, 125.58, 124.76, 123.93, 122.29, 117.45, 104.25. HRMS (ESI): m/z calcd for C22H18NS2 (M+H)+: 360.0883, found: 360.0881.
1,3-bis((4-methoxyphenyl)thio)-2-naphthyl-amine (3b): red solid; mp = 68–71 °C; 1H NMR (400 MHz, CDCl3) δ 8.31 (s, 1H), 7.71 (d, J = 9.4 Hz, 3H), 7.47–7.42 (m, 2H), 7.03–7.00 (m, 4H), 6.73 (d, J = 9.1 Hz, 3H), 4.74 (s, 2H), 3.71 (d, J = 1.2 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 148.91, 136.48, 134.59, 132.64, 131.91, 130.97, 130.97, 129.43, 129.43, 128.66, 128.61, 128.29, 127.60, 126.88, 123.89, 123.02, 117.79, 102.51, 32.08, 29.86. HRMS (ESI): m/z calcd for C24H21NO2S2 (M+H)+: 420.1086, found: 420.1092.
1,3-bis((4-fluorophenyl)thio)-2-naphthyl-amine (3c): red oily liquid; 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 8.4 Hz, 1H), 7.80 (d, J = 8.8 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.45 (t, J = 8.4 Hz, 3H), 7.39 (d, J = 3.3 Hz, 2H), 7.21 (d, J = 8.6 Hz, 1H), 7.07 (d, J = 8.8 Hz, 1H), 6.88 (d, J = 8.5 Hz, 1H), 6.33 (d, J = 8.6 Hz, 1H), 5.30 (s, 1H), 4.72 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 134.17 (d, J = 87.4 Hz), 132.81 (d, J = 33.4 Hz). δ 134.60, 133.73, 132.97, 132.64, 129.78, 129.78, 129.43, 128.76, 128.66, 128.29, 128.12, 128.12, 127.60, 126.88, 123.89, 123.89, 123.01, 117.79. HRMS (APCI): m/z calcd for C22H15F2NS2 (M+H)+: 396.0690, found: 396.0686.
1,3-bis((4-chlorophenyl)thio)-2-naphthyl-amine (3d): red solid; mp = 80–83 °C; 1H NMR (400 MHz, CDCl3) δ 8.21 (d, J = 8.5 Hz, 1H), 7.77 (dd, J = 22.6, 8.4 Hz, 3H), 7.47–7.39 (m, 3H), 7.30 (d, J = 7.4 Hz, 2H), 7.23 (d, J = 7.9 Hz, 2H), 7.09 (d, J = 8.8 Hz, 1H), 7.02 (d, J = 7.8 Hz, 1H), 5.30 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 138.57, 136.50, 135.81, 132.53, 132.53, 129.50, 129.50, 128.74, 128.66, 128.19, 124.00, 124.00, 123.00, 122.78, 121.97, 121.06, 117.81, 117.81. HRMS (APCI): m/z calcd for C22H15Cl2NS2 (M+H)+: 428.0100, found: 428.0095.
1,3-bis((4-bromophenyl)thio)-2-naphthyl-amine (3e): red oily liquid; 1H NMR (400 MHz, CDCl3) δ 8.35–8.23 (m, 1H), 7.78–7.73 (m, 1H), 7.61 (d, J = 7.7 Hz, 1H), 7.49–7.46 (m, 1H), 7.40 (d, J = 9.8 Hz, 2H), 7.33 (d, J = 7.6 Hz, 1H), 7.17 (s, 1H), 7.05 (t, J = 7.4 Hz, 2H), 6.93 (t, J = 6.6 Hz, 2H), 6.69 (d, J = 7.8 Hz, 1H), 4.28 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 137.16, 131.93, 129.19, 129.19, 129.09, 129.09, 129.04, 128.48, 127.90, 127.64, 127.64, 127.28, 127.28, 125.96, 125.15, 124.36, 122.71, 117.76. HRMS (APCI): m/z calcd for C22H15Br2NS2 (M+H)+: 515.9090, found: 515.9085.
1,3-bis((4-(trifluoromethyl)phenyl)thio)-2-naphthyl-amin (3f): red solid; mp = 85–87 °C; 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 7.86–7.65 (m, 3H), 7.49–7.36 (m, 3H), 7.30 (d, J = 7.7 Hz, 3H), 7.07 (d, J = 8.6 Hz, 3H), 4.73 (s, 2H). 13C NMR (101 MHz, CDCl3), δ 128.62 (d, J = 8.4 Hz), δ 148.78, 142.18, 136.51, 132.54, 132.54, 128.67, 128.58, 128.23, 125.94, 125.94, 125.66, 125.66, 125.66, 123.92, 122.97, 122.97, 117.77, 117.77, 102.97, 98.23. HRMS (APCI): m/z calcd for C24H15F6NS2 (M+H)+: 494.0477, found: 494.0550.
1,3-bis((4-(tert-butyl)phenyl)thio)-2-naphthyl-amine (3g): red solid; mp = 103 -106 °C; 1H NMR (400 MHz, CDCl3) δ 8.31 (d, J = 8.5 Hz, 1H), 7.76–7.71 (m, 2H), 7.55–7.33 (m, 3H), 7.19 (d, J = 8.4 Hz, 3H), 7.01 (dd, J = 38.8, 8.6 Hz, 4H), 4.71 (s, 2H), 1.43–1.10 (m, 18H). 13C NMR (101 MHz, CDCl3) δ 148.39, 148.10, 136.75, 136.33, 133.23, 131.67, 131.67, 128.40, 128.31, 127.70, 127.46, 126.05, 126.05, 125.61, 124.38, 122.53, 117.63, 105.11, 34.31, 31.28, 31.28, 31.16. HRMS (APCI): m/z calcd for C30H33NS2 (M+H)+: 472.2132, found: 471.2127.
1,3-bis(p-tolylthio)-2-naphthyl-amine (3h): red oily liquid; 1H NMR (400 MHz, CDCl3) δ 8.28 (s, 1H), 7.73 (d, J = 3.8 Hz, 1H), 7.47 (d, J = 8.0 Hz, 2H), 7.25–7.20 (m, 4H), 7.15 (d, J = 7.8 Hz, 2H), 6.99–6.94 (m, 3H), 4.59 (s, 2H), 2.40 (d, J = 16.4 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 148.46, 144.70, 144.70, 142.16, 142.16, 140.57, 136.61, 133.30, 131.74, 130.32, 129.86, 127.81, 127.71, 126.17, 124.40, 122.62, 117.77, 105.31, 21.78, 20.99. HRMS (APCI): m/z calcd for C24H21NS2 (M+H)+: 388.1122, found: 388.1188.
1,3-bis((3-bromophenyl)thio)-2-naphthyl-amine (3i): brown liquid; 1H NMR (400 MHz, CDCl3) δ 8.18 (s, 1H), 7.65 (s, 1H), 7.48 (d, J = 8.6 Hz, 2H), 7.33 (d, J = 11.7 Hz, 2H), 7.23 (s, 1H), 7.17 (s, 1H), 7.05 (d, J = 7.6 Hz, 2H), 6.95 (t, J = 8.1 Hz, 3H), 4.38 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 148.51, 136.68, 136.68, 133.73, 131.90, 131.90, 129.53, 129.06, 129.06, 128.90, 128.46, 127.86, 127.64, 125.93, 125.93, 125.12, 124.31, 122.68, 122.68, 117.78, 117.78, 104.71. HRMS (APCI): m/z calcd for C22H15Br2NS2 (M+Na)+: 537.8915, found: 537.8910.
1,3-bis((3-(trifluoromethyl)phenyl)thio)-2-naphthyl-amine (3j): red solid; mp = 88–91 °C; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.5 Hz, 1H), 7.77 (dd, J = 22.3, 8.4 Hz, 3H), 7.47–7.40 (m, 2H), 7.32–7.28 (m, 2H), 7.22 (t, J = 7.8 Hz, 2H), 7.04 (dd, J = 22.4, 8.4 Hz, 3H), 4.73 (s, 2H).13C NMR (101 MHz, CDCl3), δ 128.55 (d, J = 103.5 Hz), δ 137.42 (d, J = 208.3 Hz), δ 148.64, 138.46, 136.39, 132.37, 132.37, 131.47, 130.49, 129.35, 129.35, 128.57, 128.51, 128.51, 128.03, 125.96, 123.82, 123.82, 122.77, 122.61, 121.76, 117.60, 103.07, 103.07. HRMS (APCI): m/z calcd for C24H15F6NS2 (M-H)+: 494.0464, found: 494.0477.
1,3-bis(o-tolylthio)-2-naphthyl-amine (3k): red solid; mp = 101–103 °C; 1H NMR (400 MHz, CDCl3) δ 8.21 (d, J = 8.5 Hz, 1H), 7.76 (dd, J = 17.6, 8.3 Hz, 3H), 7.43 (t, J = 7.6 Hz, 2H), 7.18 (d, J = 7.4 Hz, 1H), 7.09 (d, J = 8.8 Hz, 2H), 6.99 (t, J = 7.0 Hz, 2H), 6.87 (d, J = 7.5 Hz, 1H), 6.44 (d, J = 7.9 Hz, 1H), 4.41 (s, 2H), 2.41 (d, J = 128.3 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 148.50, 136.82, 135.67, 135.01, 131.89, 130.23, 129.90, 128.68, 128.50, 128.50, 127.90, 127.90, 126.65, 126.22, 124.78, 124.78, 124.40, 124.30, 122.79, 122.79, 117.83, 104.34, 20.18, 20.18. HRMS (ESI): m/z calcd for C24H21NS2 (M+H)+: 388.1187, found: 388.1194.
1,3-bis((2-fluorophenyl)thio)-2-naphthyl-amine (3l): red liquid; 1H NMR (400 MHz, CDCl3) δ 8.16 (s, 1H), 7.82–7.68 (m, 3H), 7.55–7.42 (m, 2H), 7.39 (d, J = 7.9 Hz, 3H), 7.29 (s, 1H), 7.11–7.02 (m, 3H), 4.72 (s, 2H). 13C NMR (101 MHz, CDCl3), 134.94 (d, J = 103.9 Hz), 126.91 (d, J = 61.8 Hz), 124.96 (d, J = 57.1 Hz) 118.64 (d, J = 35.2 Hz), δ 143.86, 141.85, 138.04, 135.46, 134.43, 132.09, 131.66, 130.70, 128.40, 128.37, 127.93, 127.21, 126.60, 126.14, 125.66, 125.25, 124.68, 121.25, 120.59, 118.82, 118.47, 109.51. HRMS (ESI): m/z calcd for C22H15F2NS2 (M-H)+: 395.0614, found: 395.0614.
1,3-bis((2,4-dimethylphenyl)thio)-2-naphthyl-amine (3m): brown liquid; 1H NMR (400 MHz, CDCl3) δ 8.22 (d, J = 8.5 Hz, 1H), 7.76 (dd, J = 17.1, 8.4 Hz, 3H), 7.43 (t, J = 7.6 Hz, 2H), 7.07 (dd, J = 11.9, 8.2 Hz, 3H), 6.80 (d, J = 7.4 Hz, 2H), 6.27 (s, 2H), 2.52 (s, 6H), 2.01 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 148.16, 138.54, 136.58, 135.94, 134.92, 134.11, 132.48, 132.28, 131.71, 131.48, 130.51, 130.39, 129.83, 128.36, 128.13, 127.50, 125.43, 124.47, 124.17, 122.40, 117.50, 104.23, 20.93, 20.26, 19.92, 19.41. HRMS (ESI): m/z calcd for C22H13Cl4NS2 (M+Na)+: 438.1317, found: 438.1326.
1,3-bis((2,4-dichlorophenyl)thio)-2-naphthyl-amine (3n): red oily liquid; 1H NMR (400 MHz, CDCl3) δ 8.21 (d, J = 8.6 Hz, 1H), 7.75 (dd, J = 20.4, 8.4 Hz, 3H), 7.45 (s, 1H), 7.18 (s, 2H), 7.08–6.98 (m, 3H), 6.88 (d, J = 8.0 Hz, 1H), 5.35 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 143.51, 143.09, 136.73, 133.75, 133.75, 131.54, 129.57, 129.21, 129.19, 129.11, 129.11, 128.93, 127.70, 127.70, 127.64, 127.10, 126.47, 125.52, 125.45, 121.66, 118.71, 108.21. HRMS (ESI): m/z calcd for C22H13Cl4NS2 (M+H)+: 495.9310, found: 495.9322.
1,3-bis(thiophen-2-ylthio)-2-naphthyl-amine (3o): red oily liquid; 1H NMR (400 MHz, CDCl3) δ 7.71 (dd, J = 15.2, 8.4 Hz, 3H), 7.62 (d, J = 8.2 Hz, 1H), 7.40 (t, J = 7.5 Hz, 2H), 7.26 (t, J = 7.4 Hz, 2H), 7.01–6.95 (m, 3H), 3.77 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 144.21, 135.02, 129.31, 129.31, 128.08, 128.08, 127.83, 127.83, 126.45, 126.45, 125.91, 125.91, 122.58, 122.58, 118.34, 118.34, 108.70, 108.70. HRMS (ESI): m/z calcd for C18H13NS2 (M+H)+: 371.9998, found: 372.0009.
6-bromo-1,3-bis(phenylthio)-2-naphthyl-amine (3p): red liquid; 1H NMR (400 MHz, CDCl3) δ 8.14 (d, J = 9.0 Hz, 1H), 7.85 (s, 1H), 7.66 (d, J = 8.8 Hz, 1H), 7.57 (d, J = 7.0 Hz, 1H), 7.49–7.46 (m, 1H), 7.44–7.39 (m, 1H), 7.34 (d, J = 6.9 Hz, 2H), 7.17 (t, J = 7.6 Hz, 2H), 7.08 (t, J = 7.9 Hz, 2H), 6.99 (d, J = 7.7 Hz, 2H), 4.79 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 148.82, 136.74, 136.49, 135.46, 133.75, 131.54, 130.99, 130.90, 130.34, 129.57, 129.19, 129.19, 128.93, 127.71, 126.38, 125.98, 125.98, 125.39, 125.39, 118.79, 116.29, 104.86. HRMS (APCI): m/z calcd for C22H16BrNS2 (M+H)+ 437.9980, found: 437.9981.
2,4-bis(phenylthio)naphthalen-1-amine (4a) [16]: white solid; mp 100–102 °C; 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 9.3 Hz, 1H), 7.97 (s, 1H), 7.79 (d, J = 7.8 Hz, 1H), 7.54–7.42 (m, 2H), 7.18 (t, J = 7.5 Hz, 2H), 7.1–7.06 (m, 5H), 7.03 (t, J = 7.5 Hz, 3H), 5.20 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 147.85, 143.47, 139.24, 136.60, 136.07, 129.19, 128.91, 128.31, 127.20, 126.62, 126.49, 125.99, 125.73, 125.11, 123.89, 122.03, 117.26, 108.14, 77.16
2,4-bis(p-tolylthio)naphthalen-1-amine (4b) [16]: white solid; mp 101–103 °C; 1H NMR (400 MHz, CDCl3) δ 8.38 (d, J = 9.0 Hz, 1H), 7.93 (s, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.49 (t, J = 6.8 Hz, 1H), 7.43 (t, J = 7.5 Hz, 1H), 7.05–6.97 (m, 4H), 6.95 (t, J = 6.5 Hz, 4H), 5.14 (s, 2H), 2.24 (s, 3H), 2.21 (s, 3H); 13C NMR (101 MHz, CDCl3) δ 147.29, 142.79, 135.80, 135.72, 135.41, 135.03, 132.91, 129.95, 129.70, 128.06, 127.16, 127.04, 125.86, 123.92, 121.97, 118.03, 109.02, 77.16, 21.03, 20.99;
2,4-bis((4-chlorophenyl)thio)naphthalen-1-amine (4c) [16]: white solid; mp 97–99 °C; 1H NMR (400 MHz, CDCl3) δ 8.32 (d, J = 8.7 Hz, 1H), 7.92 (s, 1H), 7.81 (d, J = 8.9 Hz, 1H), 7.58–7.47 (m, 2H), 7.15 (d, J = 8.6 Hz, 2H), 7.09 (d, J = 8.6 Hz, 2H), 7.00 (d, J = 8.7 Hz, 2H), 6.93 (d, J = 8.6 Hz, 2H), 5.24 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 148.03, 143.23, 137.74, 135.91, 135.08, 131.69, 130.99, 129.31, 129.02, 128.62, 127.88, 127.75, 127.03, 126.23, 123.86, 122.11, 116.99, 107.63, 77.16;
2,4-bis((4-bromophenyl)thio)naphthalen-1-amine (4d): white solid; mp 115–118 °C; 1H NMR (400 MHz, CDCl3) δ 8.32 (s, 1H), 7.93 (s, 1H), 7.84 (d, J = 10.8 Hz, 2H), 7.59–7.49 (m, 3H), 7.33 (d, J = 8.5 Hz, 2H), 6.92 (dd, J = 29.1, 8.6 Hz, 4H), 5.26 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 143.65, 133.85, 132.57, 132.41, 132.27, 129.13, 129.03, 128.48, 128.36, 128.28, 127.63, 127.63, 126.62, 125.99, 122.47, 121.97, 119.21, 107.82. HRMS (APCI): m/z calcd for C22H15Br2NS2 (M-H)+ 513.8941, found: 513.8939.
2,4-bis((4-fluorophenyl)thio)naphthalen-1-amine (4e): white solid; mp 103–105 °C; 1H NMR (400 MHz, CDCl3) δ 8.37 (d, J = 7.3 Hz, 1H), 7.91 (s, 1H), 7.85 (d, J = 7.2 Hz, 1H), 7.58–7.51 (m, 2H), 7.10 (dd, J = 8.6, 5.3 Hz, 2H), 7.04 (dd, J = 8.6, 5.3 Hz, 2H), 6.90 (dt, J = 25.3, 8.6 Hz, 4H), 5.25 (s, 2H). 13C NMR (101 MHz, CDCl3), 126.31 (d, J = 90.0 Hz), 122.72 (d, J = 189.1 Hz), δ 147.21, 142.32, 135.43, 128.66, 128.58, 128.50, 128.10, 126.76, 125.86, 123.66, 121.78, 117.90, 116.16, 116.16, 115.94, 115.87, 115.66, 108.55. HRMS (APCI): m/z calcd for C22H15F2NS2 (M-H)+ 394.0545, found: 394.0541.
2,4-bis((4-methoxyphenyl)thio)naphthalen-1-amine (4f): red solid; mp 75–78 °C; 1H NMR (400 MHz, CDCl3) δ 8.41 (s, 1H), 7.82 (d, J = 6.8 Hz, 2H), 7.52 (d, J = 8.5 Hz, 2H), 7.11 (dd, J = 13.9, 8.8 Hz, 4H), 6.76 (dd, J = 18.7, 8.8 Hz, 4H), 5.35 (s, 2H), 3.75 (d, J = 9.3 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 158.58, 158.23, 141.17, 129.90, 129.90, 129.83, 129.01, 127.85, 127.84, 127.04, 125.91, 124.05, 121.95, 121.95, 121.95, 114.98, 114.98, 114.77, 55.50, 55.46. HRMS (APCI): m/z calcd for C24H21NO2S2 (M+H)+ 420.1088, found: 420.1086.
2,4-bis((4-(tert-butyl)phenyl)thio)naphthalen-1-amine (4g): white solid; mp 103–106 °C 1H NMR (400 MHz, CDCl3) δ 8.45 (d, J = 8.9 Hz, 1H), 7.97 (s, 1H), 7.86 (d, J = 7.4 Hz, 1H), 7.56–7.50 (m, 2H), 7.25 (d, J = 9.9 Hz, 2H), 7.18 (d, J = 8.4 Hz, 2H), 7.06 (d, J = 8.4 Hz, 2H), 6.99 (d, J = 8.4 Hz, 2H), 5.24 (s, 2H), 1.27 (s, 9H), 1.24 (s, 9H). 13C NMR (101 MHz, CDCl3) δ 148.85, 147.47, 143.19, 143.19, 135.55, 135.55, 132.99, 132.99, 128.04, 127.22, 126.52, 126.38, 126.15, 125.86, 125.80, 125.80, 123.81, 121.88, 117.74, 108.71, 34.40, 31.28. HRMS (APCI): m/z calcd for C30H33NS2 (M-H)+ 470.1983, found: 470.1981.
2,4-bis(o-tolylthio)naphthalen-1-amine (4h): red liquid; 1H NMR (400 MHz, CDCl3) δ 8.33 (s, 1H), 7.88–7.81 (m, 3H), 7.50 (dd, J = 26.8, 8.5 Hz, 4H), 7.18 (d, J = 7.6 Hz, 2H), 7.05–6.97 (m, 3H), 5.20 (s, 2H), 2.47 (d, J = 10.9 Hz, 6H). 13C NMR (101 MHz, CDCl3) δ 147.29, 142.62, 137.81, 133.34, 130.12, 129.76, 127.90, 126.83, 126.40, 126.14, 125.65, 125.25, 124.93, 124.64, 121.70, 118.81, 118.56, 117.09, 109.52, 107.78, 19.90, 19.90. HRMS (APCI): m/z calcd for C24H21NS2 (M-H)+ 388.1046, found: 386.1042.
2,4-bis((2-fluorophenyl)thio)naphthalen-1-amine] (4i): red solid; mp 75–78 °C; 1H NMR (400 MHz, CDCl3) δ 8.38 (s, 1H), 7.97 (s, 1H), 7.55 (d, J = 9.0 Hz, 2H), 7.10–7.01 (m, 4H), 6.94 (d, J = 8.0 Hz, 2H), 6.84 (d, J = 8.3 Hz, 2H), 6.65 (d, J = 7.9 Hz, 2H), 5.35 (s, 2H). 13C NMR (101 MHz, CDCl3), 132.64 (d, J = 222.7 Hz), 127.84 (d, J = 28.6 Hz), δ 136.73, 136.73, 133.75, 131.54, 129.57, 129.57, 129.22, 129.11, 128.93, 127.98, 127.70, 127.10, 126.66, 126.66, 126.52, 126.47, 126.47, 125.52, 125.45, 121.66, 118.71, 118.71.HRMS (APCI): m/z calcd for C22H15F2NS2 (M-H)+ 394.0544, found:395.0541.
2,4-bis(thiophen-2-ylthio)naphthalen-1-amine (4j): red liquid; 1H NMR (400 MHz, CDCl3) δ 7.67 (d, J = 8.6 Hz, 1H), 7.36 (d, J = 7.6 Hz, 1H), 7.27 (d, J = 8.5 Hz, 1H), 7.12–7.09 (m, 2H), 7.03 (d, J = 5.0 Hz, 2H), 6.93 (dq, J = 15.2, 7.8 Hz, 4H), 6.77–6.67 (m, 2H), 2.04–1.98 (m, 12H). 13C NMR (101 MHz, CDCl3) δ 141.18, 140.71, 138.85, 136.52, 135.85, 134.76, 134.42, 133.49, 132.80, 132.60, 132.57, 130.82, 130.70, 130.25, 128.74, 126.94, 126.90, 126.47, 126.34, 125.48, 121.66, 118.94, 20.65, 20.57, 20.22, 20.11. HRMS (APCI): m/z calcd for C26H25NS2 (M-H)+ 415.1430, found:415.1428.
4-((3-bromophenyl)thio)naphthalen-1-amine (4k): red liquid; 1H NMR (400 MHz, CDCl3) δ 8.32 (s, 1H), 7.84 (s, 1H), 7.71 (s, 1H), 7.51 (d, J = 4.1 Hz, 2H), 7.15 (d, J = 7.7 Hz, 2H), 6.98 (s, 1H), 6.89 (s, 1H), 6.80 (s, 1H), 4.43 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 145.03, 142.53, 137.24, 135.52, 130.15, 128.56, 127.90, 127.57, 126.83, 125.59, 124.65, 124.42, 123.00, 121.42, 116.10, 109.53. HRMS (APCI): m/z calcd for C16H16BrNS (M+H)+ 329.9948, found:329.9946.
4-((3-(trifluoromethyl)phenyl)thio)naphthalen-1-amin (4l): red liquid; 1H NMR (400 MHz, CDCl3) δ 8.25 (s, 1H), 7.79 (s, 1H), 7.64 (s, 1H), 7.44 (d, J = 9.6 Hz, 2H), 7.21 (s, 2H), 7.13 (s, 1H), 6.97 (s, 1H), 6.75 (s, 1H), 4.38 (s, 2H). 13C NMR (101 MHz, CDCl3), δ 143.07 (d, J = 352.6 Hz), 136.07 (d, J = 180.5 Hz), δ 144.83, 141.32, 136.97, 135.18, 132.11, 128.88, 128.73, 127.31, 126.40, 125.31, 124.11, 122.32, 121.16, 119.11, 115.44, 109.22, 109.22. HRMS (APCI): m/z calcd for C16H16BrNS (M+H)+ 320.0711,found:320.0715.
4-((4-nitrophenyl)thio)naphthalen-1-amin (4m) [16]: yellow liquid; 1H NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.9 Hz, 2H), 7.90–7.78 (m, 2H), 7.63–7.49 (m, 2H), 7.45 (d, J = 8.5 Hz, 1H), 7.31 (d, J = 8.5 Hz, 1H), 7.11 (d, J = 8.9 Hz, 2H), 5.01 (s, 2H); 13C NMR (101 MHz, CDCl3) δ 147.40, 146.44, 145.38, 135.56, 133.18, 128.88, 127.76, 125.91, 125.61, 124.21, 123.16, 121.67, 119.22, 105.00, 77.16.
1,3-bis(phenylsulfinyl)-2-naphthyl-amine (3aa): red liquid; 1H NMR (400 MHz, CDCl3) δ 8.20 (d, J = 8.5 Hz, 1H), 7.67–7.62 (m, 2H), 7.50–7.44 (m, 1H), 7.37–7.30 (m, 2H), 7.17 (t, J = 7.4 Hz, 2H), 7.05 (d, J = 7.3 Hz, 2H), 6.96 (dd, J = 14.3, 7.5 Hz, 5H), 4.57 (s, 2H). 13C NMR (101 MHz, CDCl3) δ 148.27, 136.59, 136.37, 136.37, 131.58, 129.21, 128.75, 128.58, 128.16, 128.14, 127.55, 127.33, 125.61, 124.80, 123.99, 122.35, 117.43, 104.30. HRMS (APCI): m/z calcd for C22H17NO2S2 (M-H)+ 390.0631, found: 390.0627.
4. Conclusions
In summary, we developed the iodophor-catalyzed direct disulfenylation of amino naphthalenes with aryl sulfonyl hydrazines. This reaction was able to proceed smoothly under air in the aqueous phase without any additional phase-transfer reagents, and a series of novel aryl sulfides were obtained in moderate to excellent yields. The advantages of this green approach are the use of odorless and readily available sulfur reagents, a wide range of substrates, and gram-scale synthesis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29112411/s1: the charts of 1H-, 13C-NMR, and HRMS of products.
Author Contributions
X.M. and Y.L. designed the experiments and wrote the paper; Y.Y. performed the experiments; J.H. conducted the data analysis; S.H. contributed reagents/materials. All authors have read and agreed to the published version of the manuscript.
Funding
We gratefully acknowledge the financial support of this work by the Science and Technology Innovation Talents Program of Shihezi University (No. ZG010603) and the Innovation Development Program of Shihezi University (CXFZ202204).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mansy, S.S.; Cowan, J.A. Iron-sulfur cluster biosynthesis: Toward an understanding of cellular machinery and molecular mechanism. Acc. Chem. Res. 2004, 37, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Block, E. Fifty years of smelling sulfur. J. Sulfur Chem. 2013, 34, 158–207. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Gupta, L.; Talwar, A.; Chauhan, P.M. Bis and tris indole alkaloids from marine organisms: New leads for drug discovery. Curr. Med. Chem. 2007, 14, 1789–1803. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Mitsudo, T.-A. Metal-catalyzed carbon-sulfur bond formation. Chem. Rev. 2000, 100, 3205–3220. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, P.; Sun, Q.; Bai, S.; Hor, T.S.A.; Liu, X. Recent advances in C-S bond formation via C-H bond functionalization and decarboxylation. Chem. Soc. Rev. 2015, 44, 291–314. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.-Q.; Hao, S.-H.; Yang, D.-S.; Li, L.-X.; Wang, Z.-L. Sulfenylation of C-H bonds for C-S bond formation under metal-free conditions. Eur. J. Org. Chem. 2017, 2017, 6576–6592. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, S.; Li, C.; Huang, H.; Deng, G.-J. Coppercatalyzed three-component one-pot synthesis of aryl sulfides with sulfur powder under aqueous conditions. Adv. Synth. Catal. 2016, 358, 3881–3886. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Lu, G.-P.; Wang, G.-X.; Yi, W.-B. Odorless, regioselective synthesis of diaryl sulfides and α-thioaryl carbonyls from sodium arylsulfinates via a metal-free radical strategy in water. Adv. Synth. Catal. 2016, 358, 4100–4105. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, D.; Hong, M.; Wang, F.; Huang, S. Phenyliodine (III) bis (trifluoroacetate)(PIFA)-mediated synthesis of aryl sulfides in water. J. Org. Chem. 2018, 83, 7553–7558. [Google Scholar] [CrossRef]
- Saima, S.; Equbal, D.; Lavekar, A.G.; Sinha, A.K. Cooperative catalysis by bovine serum albumin-iodine towards cascade oxidative coupling-C(sp2)-H sulfenylation of indoles/hydroxyaryls with thiophenols on water. Org. Biomol. Chem. 2016, 14, 6111–6118. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, Y.; Li, A.; Liao, J.; Zhang, L.; Yang, T.; Zhou, C. Iodine-mediated sulfenylation of 4-hydroxycoumarins with sulfonyl hydrazides under aqueous conditions. New J. Chem. 2018, 42, 14738–14741. [Google Scholar] [CrossRef]
- Huang, X.; Chen, Y.; Zhen, S.; Song, L.; Gao, M.; Zhang, P.; Li, H.; Yuan, B.; Yang, G. Cobalt-catalyzed aerobic cross-dehydrogenative coupling of C-H and thiols in water for C-S formation. J. Org. Chem. 2018, 83, 7331–7340. [Google Scholar] [CrossRef]
- Kumar, N.; Venkatesh, R.; Singh, S.; Kandasamy, J. Potassium Persulfate-Glucose Mediated Synthesis of (3)-S-Arylthioindoles from Indole and Thiophenols in Water. Eur. J. Org. Chem. 2023, 26, e202300679. [Google Scholar] [CrossRef]
- Kang, X.; Yan, R.; Yu, G.; Pang, X.; Liu, X.; Li, X.; Xiang, L.; Huang, G. Iodine-mediated thiolation of substituted naphthols/naphthylamines and aryl sulfonyl hydrazides via C(sp2)-H bond functionalization. J. Org. Chem. 2015, 46, 10605–10610. [Google Scholar]
- Yueting, W.; Yali, L.; Jing, H.; Xuezhen, L.; Ping, L.; Jie, Z. TBAI-mediated sulfenylation of arenes with arylsulfonyl hydrazides in DPDME. Tetrahedron 2020, 76, 131646. [Google Scholar] [CrossRef]
- Makhayeva, D.N.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Advances in antimicrobial polymeric iodophors. Eur. Polym. J. 2023, 201, 112573. [Google Scholar] [CrossRef]
- Freeman, C.; Duan, E.; Kessler, J. Molecular iodine is not responsible for cytotoxicity in iodophors. J. Hosp. Infect. 2022, 122, 194–202. [Google Scholar] [CrossRef]
- Yang, F.L.; Tian, S.K. Iodine-catalyzed regioselective sulfenylation of indoles with sulfonyl hydrazides. Angew. Chem. Int. Ed. 2013, 52, 4929–4932. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Tang, L.; Hu, Y.; Zha, Z.; Wang, Z. Catalyst-free thiolation of indoles with sulfonyl hydrazides for the synthesis of 3-sulfenylindoles in water. Green. Chem. 2018, 18, 2609–2613. [Google Scholar] [CrossRef]
- Zhan, Z.; Ma, H.; Wei, D.; Pu, J.; Zhang, Y.; Huang, G. Metal-free catalyzed synthesis of the (E)-vinyl sulfones via aromatic olefins with arylsulfonyl hydrazides. Tetrahedron Lett. 2018, 59, 1446–1450. [Google Scholar] [CrossRef]
- Jia, F.; He, J.; Wei, Y.; Liu, Y.; Gu, Y.; Vaccaro, L.; Liu, P. C4-sulfenylation of 4-iodine-1H-pyrazole-5-amine with arylsulfonyl hydrazide in water. Mol. Catal. 2022, 528, 112485. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).