Green Extraction of Polyphenols from Elaeagnus angustifolia L. Using Natural Deep Eutectic Solvents and Evaluation of Bioactivity
Abstract
1. Introduction
2. Results and Discussion
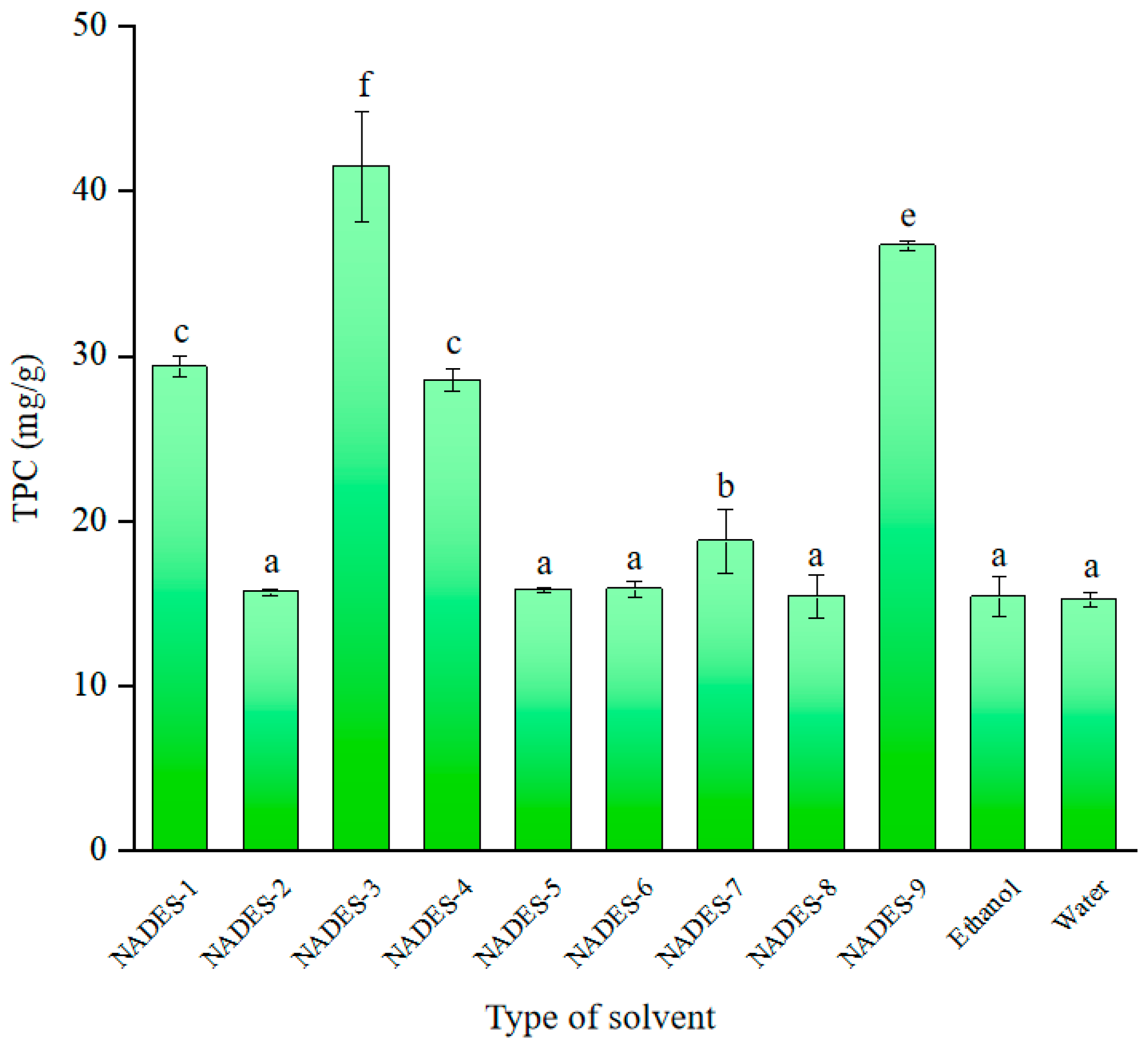
2.1. Selection of Extraction Solvent
2.2. Single-Factor Experiment
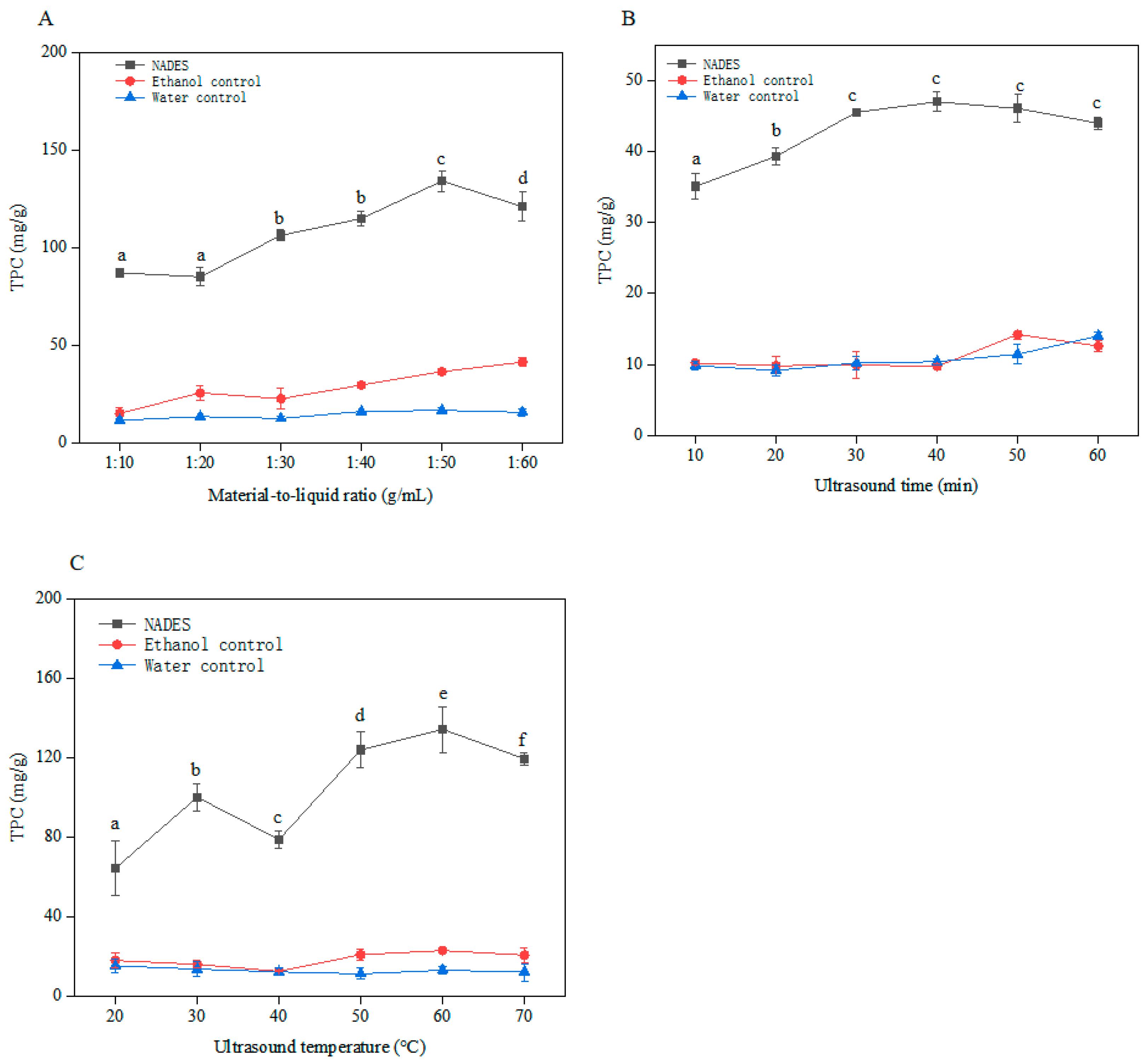
2.2.1. Effect of Material-to-Liquid Ratio
2.2.2. Effect of Ultrasound Time
2.2.3. Effect of Ultrasound Temperature
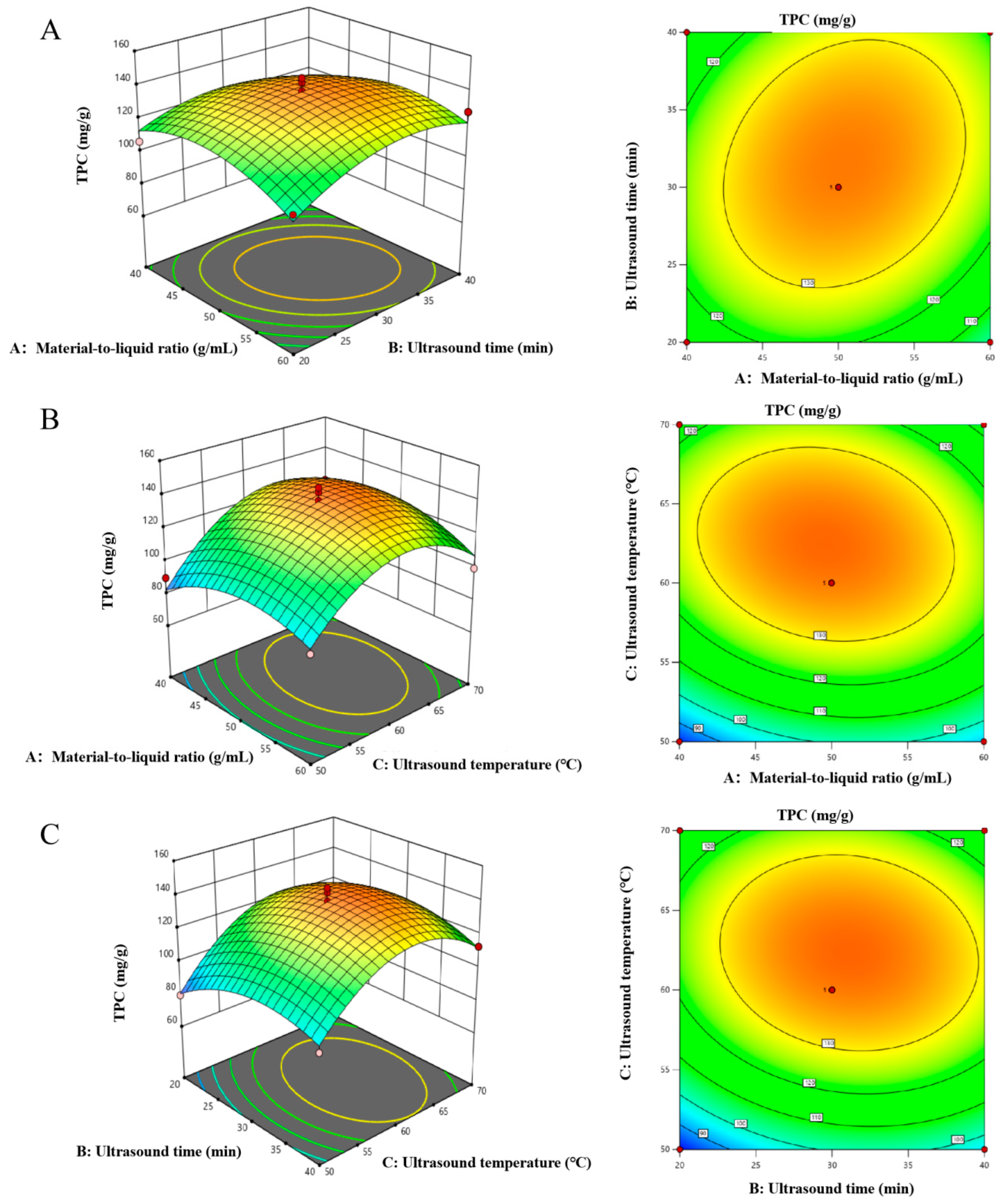
2.3. RMS-BBD Model Fitting and Response Surface Analysis
2.4. Comparison of Antioxidant Capacity
2.5. Qualitative Analysis of E. angustifolia L. Polyphenols
3. Materials and Methods
3.1. Material and Chemicals
3.2. Preparation of NADES
3.3. Extraction Procedure
3.4. Determination of Total Phenolic Content in the Extracts
3.5. Experimental Design
3.5.1. Single Variable Experiment
3.5.2. RSM Using BBD
3.6. Antioxidant Activity
3.6.1. DPPH Radical Scavenging Activity
3.6.2. ABTS Radical Scavenging Activity Assay
3.7. Identification of Phenolic Compounds Using UPLC–IMS–QTOF–MS Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, J.X.; Chen, X.Y.; Liu, W.; Cui, H.; Yuan, T. Triterpenoid saponin and lignan glycosides from the traditional medicine Elaeagnus angustifolia flowers and their cytotoxic activities. Molecules 2020, 25, 462. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Chen, X.; Li, M.; Aisa, H.A.; Yuan, T. Angustifolinoid B, a flavonoid glycoside dimer with cyclobutane from Elaeagnus angustifolia flowers. Tetrahedron Lett. 2020, 61, 151946. [Google Scholar] [CrossRef]
- Jabeen, A.; Sharma, A.; Gupta, I.; Kheraldine, H.; Vranic, S.; Al Moustafa, A.-E.; Al Farsi, H.F. Elaeagnus angustifolia plant extract inhibits epithelialmesenchymal transition and induces apoptosis via HER2 inactivation and JNK pathway in HER2-positive breast cancer cells. Molecules 2020, 25, 4240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.N.; Ma, M.H.; Ma, X.L.; Ma, F.L.; Du, Q.Y.; Liu, J.N.; Wang, X.C.; Zhao, Q.P.; Yu, Y.J.; She, Y. A comprehensive study of the effect of drying methods on compounds in Elaeagnus angustifolia L. flower by GC-MS and UHPLC-HRMS based untargeted metabolomics combined with chemometrics. Ind. Crop. Prod. 2023, 195, 116452. [Google Scholar] [CrossRef]
- Caliskan, E.; Elmastas, M.; Gokce, I. Evaluation of antioxidant properties of Elaeagnus angustifolia flowers. Asian J. Chem. 2010, 22, 2840–2848. [Google Scholar]
- Chen, Q.Q.; Chen, J.C.; Du, H.T.; Li, Q.; Chen, J.; Zhang, G.C. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int. J. Mol. Sci. 2014, 15, 11446–11455. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Z.; Ma, R. Study on the anti fatigue effect and mechanism of jujube polysaccharides. Food Sci. 2010, 31, 255–257. [Google Scholar]
- Chen, Q.; Yang, J.; Liu, H. Purification and Antioxidant Activity of Polysaccharide from Elaeagnus angustifolia in Xinjiang. Food Rev. Dev. 2016, 37, 37–40. [Google Scholar]
- Sharifian-Nejad, M.S.; Shekarchizadeh, H. Physicochemical and functional properties of oleaster (Elaeagnus angustifolia L.) polysaccharides extracted under optimal conditions. Int. J. Biol. Macromol. 2019, 124, 946–954. [Google Scholar] [CrossRef]
- Du, H.; Chen, J.; Tian, S.; Gu, H.; Li, N.; Sun, Y.; Ru, J.; Wang, J. Extraction optimization, preliminary characterization and immunological activities in vitro of polysaccharides from Elaeagnus angustifolia L. pulp. Carbohyd. Polym. 2016, 151, 348–357. [Google Scholar] [CrossRef]
- Ardestani, N.S.; Amani, M.; Sajadian, S.A. Phenomenological modeling of supercritical fluid extraction of oil from Elaeagnus angustifolia seeds. J. Appl. Res. Med. Aroma. 2023, 35, 100468. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Alirezalu, K.; Hesari, J.; Peighambardoust, S.H.; Marcinkowska-Lesiak, M.; Barzegar, Y.; Hoseinian-Khosrowshahi, S.R.; Marszałek, K.; Khaneghah, A.M. Application of oleaster leaves (Elaeagnus angustifolia L.) essential oil and natural nanoparticle preservatives in frankfurter-type sausages: An assessment of quality attributes and stability during refrigerated storage. Meat Sci. 2023, 198, 109097. [Google Scholar] [CrossRef]
- Faramarz, S.; Dehghan, G.; Jahanban-Esfahlan, A. Antioxidants in different parts of oleaster as a function of genotype. Bioimpacts 2015, 5, 79–85. [Google Scholar] [CrossRef]
- Saboonchian, F.; Jamei, R.; Hosseini Sarghein, S. Phenolic and flavonoid content of Elaeagnus angustifolia L. (leaf and flower). Avicenna J. Phytomed. 2013, 4, 231–238. [Google Scholar]
- Zha, P.; Liu, H. Study on the Extraction Conditions of Polyphenols from Elaeagnus angustifolia L. J. Anhui Agri. Sci. 2012, 40, 8967–8968+8976. [Google Scholar]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromat. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, M.; Alirezalu, K.; Hesari, J.; Peighambardoust, S.H.; Marcinkowska-Lesiak, M.; Barzegar, Y.; Hoseinian-Khosrowshahi, S.R.; Marszałek, K.; Khaneghah, A.M. Greener is better: First approach for the use of natural deep eutectic solvents (NADES) to extract antioxidants from the medicinal halophyte Polygonum maritimum L. Molecules 2021, 26, 6136. [Google Scholar] [CrossRef]
- Dong, J.N.; Dong, Z.Q.; Zhao, L.S.; Yang, D.; Bo, Y.K.; Zhang, X.Q.; Wu, G.D.; An, M. The evaluation of five bioactive compounds content and in vitro antioxidant of Caryophylli Flos extracts obtained by natural deep eutectic solvents. Sustain. Chem. Pharm. 2022, 30, 100838. [Google Scholar] [CrossRef]
- Socas-Rodríguez, B.; Torres-Cornejo, M.V.; Alvarez-Rivera, G.; Mendiola, J.A. Deep eutectic solvents for the extraction of bioactive compounds from natural sources and agricultural by-products. Appl. Sci. 2021, 11, 4897. [Google Scholar] [CrossRef]
- Dong, J.N.; Wu, G.D.; Dong, Z.Q.; Yang, D.; Bo, Y.K.; An, M.; Zhao, L.S. Natural deep eutectic solvents as tailored and sustainable media for the extraction of five compounds from compound liquorice tablets and their comparison with conventional organic Solvents. RSC Adv. 2021, 11, 37649–37660. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; An, M.; Zhao, G.; Wang, Y.; Yang, D.; Zhang, D.; Zhao, L.; Han, J.; Wu, G.; Bo, Y. Ultrasonic-assisted customized natural deep eutectic solvents extraction of polyphenols from Chaenomeles speciosa. Microchem. J. 2023, 193, 108952. [Google Scholar] [CrossRef]
- Duru, K.C.; Slesarev, G.P.; Aboushanab, S.A.; Kovalev, I.S.; Zeidler, D.M.; Kovaleva, E.G.; Bhat, R. An eco-friendly approach to enhance the extraction and recovery efficiency of isoflavones from kudzu roots and soy molasses wastes using ultrasound-assisted extraction with natural deep eutectic solvents (NADES). Ind. Crop. Prod. 2022, 182, 114886. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Yu, J.; Chen, X.; Chen, B.; Mao, Y.; Shao, P. Lycopene in hydrophobic deep eutectic solvent with natural catalysts: A promising strategy to simultaneously promote lycopene Z-isomerization and extraction. Food Chem. 2023, 426, 136627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural deep eutectic solvents: Properties, applications, and perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pan, Y.; Zhao, J. Development and optimization of green extraction of polyphenols in Michelia alba using natural deep eutectic solvents (NADES) and evaluation of bioactivity. Sustain. Chem. Pharm. 2024, 37, 101425. [Google Scholar] [CrossRef]
- Mohd Fuad, F.; Mohd Nadzir, M. Ultrasound-assisted extraction of asiaticoside from Centella asiatica using betaine-based natural deep eutectic solvent. Ind. Crop. Prod. 2023, 192, 116069. [Google Scholar] [CrossRef]
- Zhen, S.; Chen, S.; Geng, S.; Zhang, H.; Chen, Y.; Liu, B. Ultrasound-assisted natural deep eutectic solvent extraction and bioactivities of flavonoids in Ampelopsis grossedentata leaves. Foods 2022, 11, 668. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Anderson, J.L. Deep eutectic solvents in separations: Methods of preparation, polarity, and applications in extractions and capillary electrochromatography. J. Chromat. A 2020, 1633, 461613. [Google Scholar] [CrossRef]
- Jiao, P.; He, X.; Ma, S.; Wang, S.; Niu, Q. Ultrasonic-Assisted Extraction of Antioxidants from Perilla frutescens Leaves Based on Tailor-Made Deep Eutectic Solvents: Optimization and Antioxidant Activity. Molecules 2023, 28, 7554. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, P.; Li, Y.B.; Liu, T.C.; Zhang, L.; Zhou, Y.H. Natural deep eutectic solvents as new green solvents to extract anthraquinones from Rheum palmatum L. RSC Adv. 2018, 8, 15069–15077. [Google Scholar] [CrossRef] [PubMed]
- Bener, M.; Sen, F.B.; Onem, A.N.; Bekdeser, B.; Celik, S.E.; Lalikoglu, M.; Asci, Y.S.; Capanoglu, E.; Apak, R. Microwave-assisted extraction of antioxidant compounds from by-products of Turkish hazelnut (Corylus avellana L.) using natural deep eutectic solvents: Modeling, optimization and phenolic characterization. Food Chem. 2022, 385, 132633. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of ultrasound-assisted extraction parameters on total polyphenols and its antioxidant activity from Mango residues (Mangifera indica L. Var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- Feng, S.; Deng, G.; Liu, H.; Shi, H.; Li, P.; Li, X.; Chen, T.; Zhou, L.; Yuan, M.; Ding, C. Extraction and identification of polyphenol from Camellia oleifera leaves using tailor-made deep eutectic solvents based on COSMO-RS design. Food Chem. 2024, 444, 138473. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Yuan, Y.; Xiang, J.; Jin, W.; Johnson, J.B.; Li, Z.; Wang, C.; Luo, D. Green extraction of phenolic compounds from foxtail millet bran by ultrasonic-assisted deep eutectic solvent extraction: Optimization, comparison and bioactivities. LWT 2022, 154, 112740. [Google Scholar] [CrossRef]
- Tan, M.-J.; Li, Y.; Zhao, S.-Q. Synergistic ultrasound pulsed electric field extraction of litchi peel polyphenols and determination of their properties. Int. J. Biol. Macromol. 2024, 260, 129613. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Gogoi, M.; Goswami, R. Ultrasound assisted hydrotropic extraction of polyphenols from green tea leaves in aqueous media. Ind. Crop. Prod. 2024, 209, 117986. [Google Scholar] [CrossRef]
- Bakirtzi, C.; Triantafyllidou, K.; Makris, D.P. Novel lactic acid-based natural deep eutectic solvents: Efficiency in the ultrasound-assisted extraction of antioxidant polyphenols from common native greek medicinal plants. J. Appl. Res. Med. Aromat. Plants 2016, 3, 120–127. [Google Scholar] [CrossRef]
- Luo, J.; Lv, X.; Wang, X.; Kong, L. Sesquiterpenoids from thefruits of Alpinia oxyphyla and inhibition of nitric oxide pro-duction in lipopolysaccaride-activated macrophages. Phyto-Chem. Lett. 2012, 5, 134–138. [Google Scholar] [CrossRef]
- Chang, S.Y.; S, Q.H.; Tong, Z.; Xing, W.; X, Y.D.; Ping, B.O. Study on coumarins of Trigonostemon lutescens. Chin. Herbal Med. 2018, 49, 5751–5755. [Google Scholar]
- Hnit, S.S.T.; Ding, R.; Bi, L.; Xie, C.; Yao, M.; De Souza, P.; Xu, L.; Li, Z.; Dong, Q. Agrimol B present in Agrimonia pilosa Ledeb impedes cell cycle progression of cancer cells through G0 state arrest. Biomed Pharmacother. 2021, 141, 111795. [Google Scholar] [CrossRef]
- Fási, L.; Latif, A.D.; Zupkó, I.; Lévai, S.; Dékány, M.; Béni, Z.; Könczöl, Á.; Balogh, G.T.; Hunyadi, A. AAPH or Peroxynitrite-Induced Biorelevant Oxidation of Methyl Caffeate Yields a Potent Antitumor Metabolite. Biomolecules 2020, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, L.; Chen, S.; Wang, L.; Lin, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from Moringa oleifera L. leaves: Optimization, comparison and antioxidant activity. Sep. Purif. Technol. 2020, 247, 117014–117024. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Marques, C.; Igarashi-Mafra, L.; Coutinho, J.A.P.; Mafra, M.R. Extraction of phenolic compounds from rosemary using choline chloride—Based Deep Eutectic Solvents. Sep. Purif. Technol. 2021, 258, 117975–117982. [Google Scholar] [CrossRef]
- Georgantzi, C.; Lioliou, A.-E.; Paterakis, N.; Makris, D. Combination of lactic acid-based deep eutectic solvents (DES) with β-cyclodextrin: Performance screening using ultrasound-assisted extraction of polyphenols from selected native Greek medicinal plants. Agronomy 2017, 7, 54. [Google Scholar] [CrossRef]
- Shopska, V.; Kostova, R.D.; Zarcheva, M.D.; Teneva, D.; Denev, P.; Kostov, G. Comparative study on phenolic content and antioxidant activity of different malt types. Antioxidants 2021, 10, 1124. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Y.; Gao, B.; Geng, P.; Huang, H.; Yu, L.; Choe, U.; Liu, J.; Sun, J.; Chen, P.; et al. Chemical profile and in vitro gut microbiota modulatory, anti-inflammatory and free radical scavenging properties of chrysanthemum morifolium cv. Fubaiju. J. Funct. Foods 2019, 58, 114–122. [Google Scholar] [CrossRef]

| No. | A: Material-to-Liquid Ratio, g/mL | B: Ultrasound Time, min | C: Ultrasound Temperature, °C | Yield (mg/mL) |
|---|---|---|---|---|
| 1 | −1 | −1 | 0 | 106.32 |
| 2 | 0 | 0 | 0 | 145.76 |
| 3 | −1 | 0 | −1 | 89.92 |
| 4 | 0 | −1 | 1 | 115.29 |
| 5 | 1 | 0 | 1 | 99.49 |
| 6 | 0 | 0 | 0 | 129.57 |
| 7 | 0 | 0 | 0 | 138.14 |
| 8 | 1 | 0 | −1 | 90.03 |
| 9 | 0 | 1 | 1 | 112.9 |
| 10 | −1 | 0 | 1 | 119.28 |
| 11 | 0 | −1 | −1 | 79.55 |
| 12 | 0 | 1 | −1 | 91.21 |
| 13 | 1 | 1 | 0 | 127.49 |
| 14 | 0 | 0 | 0 | 143.14 |
| 15 | −1 | 1 | 0 | 105.94 |
| 16 | 0 | 0 | 0 | 131.95 |
| 17 | 1 | −1 | 0 | 106.34 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 6003.37 | 9 | 667.04 | 10.01 | 0.0031 |
| A | 0.4465 | 1 | 0.4465 | 0.0067 | 0.9370 |
| B | 112.80 | 1 | 112.80 | 1.69 | 0.2344 |
| C | 1158.01 | 1 | 1158.01 | 17.38 | 0.0042 |
| AB | 115.89 | 1 | 115.89 | 1.74 | 0.2287 |
| AC | 99.00 | 1 | 99.00 | 1.49 | 0.2623 |
| BC | 49.35 | 1 | 49.35 | 0.7406 | 0.4180 |
| A² | 725.16 | 1 | 725.16 | 10.88 | 0.0131 |
| B² | 718.82 | 1 | 718.82 | 10.79 | 0.0134 |
| C² | 2612.35 | 1 | 2612.35 | 39.21 | 0.0004 |
| Residual | 466.43 | 7 | 66.63 | ||
| Lack of Fit | 272.52 | 3 | 90.84 | 1.87 | 0.2749 |
| Pure Error | 193.91 | 4 | 48.48 | ||
| Cor Total | 6469.79 | 16 | |||
| R2 | 0.9279 |
| ID | RT (min) | Observed [M−H]− m/z | Response | Chemical Formula | Component Name | Type |
|---|---|---|---|---|---|---|
| 1 | 0.81 | 379.0834 | 192 | C20H14O5 | Sophoracoumestan A | Coumarin |
| 2 | 8.63 | 853.461 | 129 | - | Pomodic acid 3-β-O-α-L-2′-Acetoxypyranoarabinyl-28-O-β-D-glucopyranose ester | Flavonoids |
| 3 | 9.21 | 935.5035 | 239 | C14H24O8 | Marsdekoiside B,2 | Flavonoids |
| 4 | 10.93 | 421.1868 | 178 | C21H28O6 | Octahydrocurcumin | Metabolites of curcumin |
| 5 | 11.38 | 401.0871 | 13935 | C19H16O7 | 6-Aldehydoisoophiopogonanone A | Flavonoids |
| 6 | 11.78 | 207.1029 | 207 | C12H16O3 | β-Asarone | Phenols |
| 7 | 12.56 | 239.1289 | 283 | C12H10O3 | Cnidiumlac | Coumarin |
| 8 | 12.9 | 587.3597 | 601 | C34H52O8 | Quinatoside A | Flavonoid glycoside |
| 9 | 15.99 | 347.1713 | 12607 | - | Schizonepetoside E | Phenolic glycoside |
| 10 | 16.92 | 681.2966 | 21984 | C37H46O12 | Agrimol B | Phenols |
| 11 | 17.04 | 297.1529 | 2846 | C19H22O3 | Ostruthins | Coumarin |
| 12 | 17.05 | 359.1534 | 11376 | C15H16O4 | Citroenol | Coumarin |
| 13 | 17.13 | 239.0591 | 3553 | C10H10O4 | Methyl caffeate | Phenols |
| 14 | 17.18 | 464.0986 | 179 | C21H21O11 | Delphinidin-3-glucoside | Flavonoids |
| 15 | 17.86 | 387.0961 | 3726 | - | Caffeic acid-β-D-glucopyranoside | Phenolic acid |
| 16 | 17.91 | 391.2084 | 179 | C22H32O6 | Picrasinol B | Phenols |
| 17 | 17.94 | 359.1829 | 949 | C20H26O3 | Oxyphyllacinol | Flavonoids |
| 18 | 18 | 671.1424 | 128 | - | Quercetin 7-O-[β-D-glucopyranose group (1 → 6)-β-D-glucopyranoside | Flavonoids |
| 19 | 18.06 | 337.236 | 1115 | C20H34O4 | Kirenol | Phenols |
| 20 | 18.13 | 553.2432 | 709 | C30H36O7 | Kushenol M | Flavonoids |
| 21 | 18.16 | 533.1563 | 159 | C17H14O3 | Draconin | Anthraquinone |
| 22 | 18.26 | 223.0278 | 146563 | C9H6O4 | 5,7-dihydroxychromogen ketone | Phenols |
| 23 | 18.44 | 297.0725 | 243 | C17H14O5 | 5-hydroxy-7,4′-dimethoxyflavonoid | Flavonoids |
| 24 | 18.66 | 675.2257 | 322 | C25H28O4 | AMulberrofuran A | Phenols |
| No. | Solvent Abbreviation | HBA | HBD | Molar Ratio | Moisture Content |
|---|---|---|---|---|---|
| 1 | NADES-1 | Choline chloride | Malic acid | 1:1 | 20% |
| 2 | NADES-2 | Propylene Glycol | 1:2 | ||
| 3 | NADES-3 | Malonic acid | 1:2 | ||
| 4 | NADES-4 | Ethylene glycol | 1:2 | ||
| 5 | NADES-5 | Ammonium acetate | 1:2 | ||
| 6 | NADES-6 | Glycerol | 1:2 | ||
| 7 | NADES-7 | Butanediol | 1:2 | ||
| 8 | NADES-8 | Urea | 1:2 | ||
| 9 | NADES-9 | Lactic acid | 1:2 |
| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A(material-to-liquid ratio, g/mL) | 1:40 | 1:50 | 1:60 |
| B(ultrasound time, min) | 20 | 30 | 40 |
| C(ultrasound temperature, °C) | 50 | 60 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Lv, J.; Wang, X.; Li, X.; Guo, D.; Wang, L.; Zhang, N.; Jia, Q. Green Extraction of Polyphenols from Elaeagnus angustifolia L. Using Natural Deep Eutectic Solvents and Evaluation of Bioactivity. Molecules 2024, 29, 2412. https://doi.org/10.3390/molecules29112412
Li L, Lv J, Wang X, Li X, Guo D, Wang L, Zhang N, Jia Q. Green Extraction of Polyphenols from Elaeagnus angustifolia L. Using Natural Deep Eutectic Solvents and Evaluation of Bioactivity. Molecules. 2024; 29(11):2412. https://doi.org/10.3390/molecules29112412
Chicago/Turabian StyleLi, Lu, Jingjing Lv, Xiaoqin Wang, Xiujun Li, Dongqi Guo, Liling Wang, Na Zhang, and Qinghua Jia. 2024. "Green Extraction of Polyphenols from Elaeagnus angustifolia L. Using Natural Deep Eutectic Solvents and Evaluation of Bioactivity" Molecules 29, no. 11: 2412. https://doi.org/10.3390/molecules29112412
APA StyleLi, L., Lv, J., Wang, X., Li, X., Guo, D., Wang, L., Zhang, N., & Jia, Q. (2024). Green Extraction of Polyphenols from Elaeagnus angustifolia L. Using Natural Deep Eutectic Solvents and Evaluation of Bioactivity. Molecules, 29(11), 2412. https://doi.org/10.3390/molecules29112412






