Discovery of a New Class of Lipophilic Pyrimidine-Biphenyl Herbicides Using an Integrated Experimental-Computational Approach
Abstract
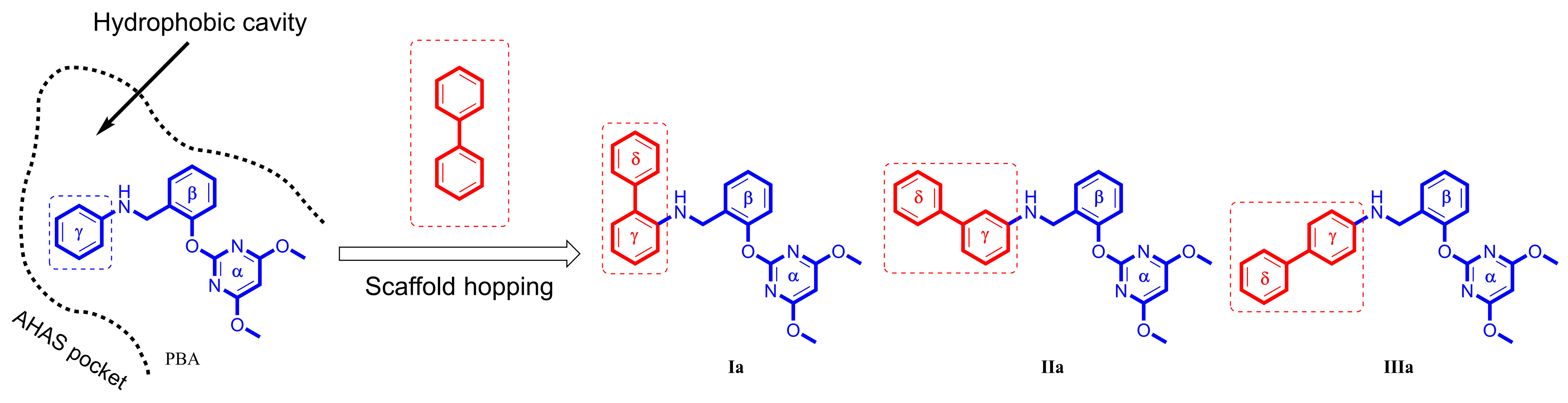
1. Introduction
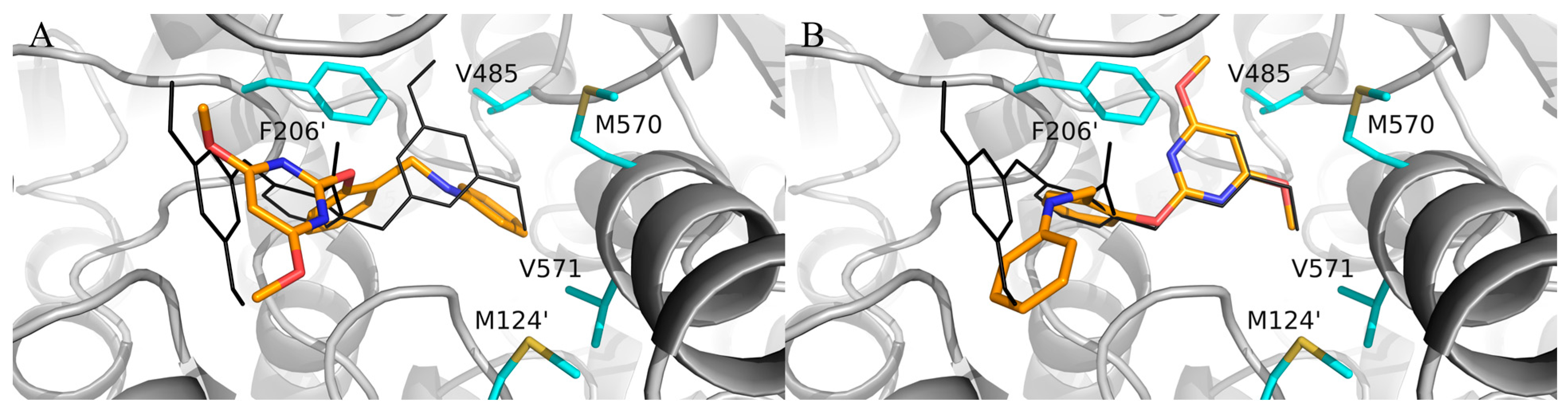
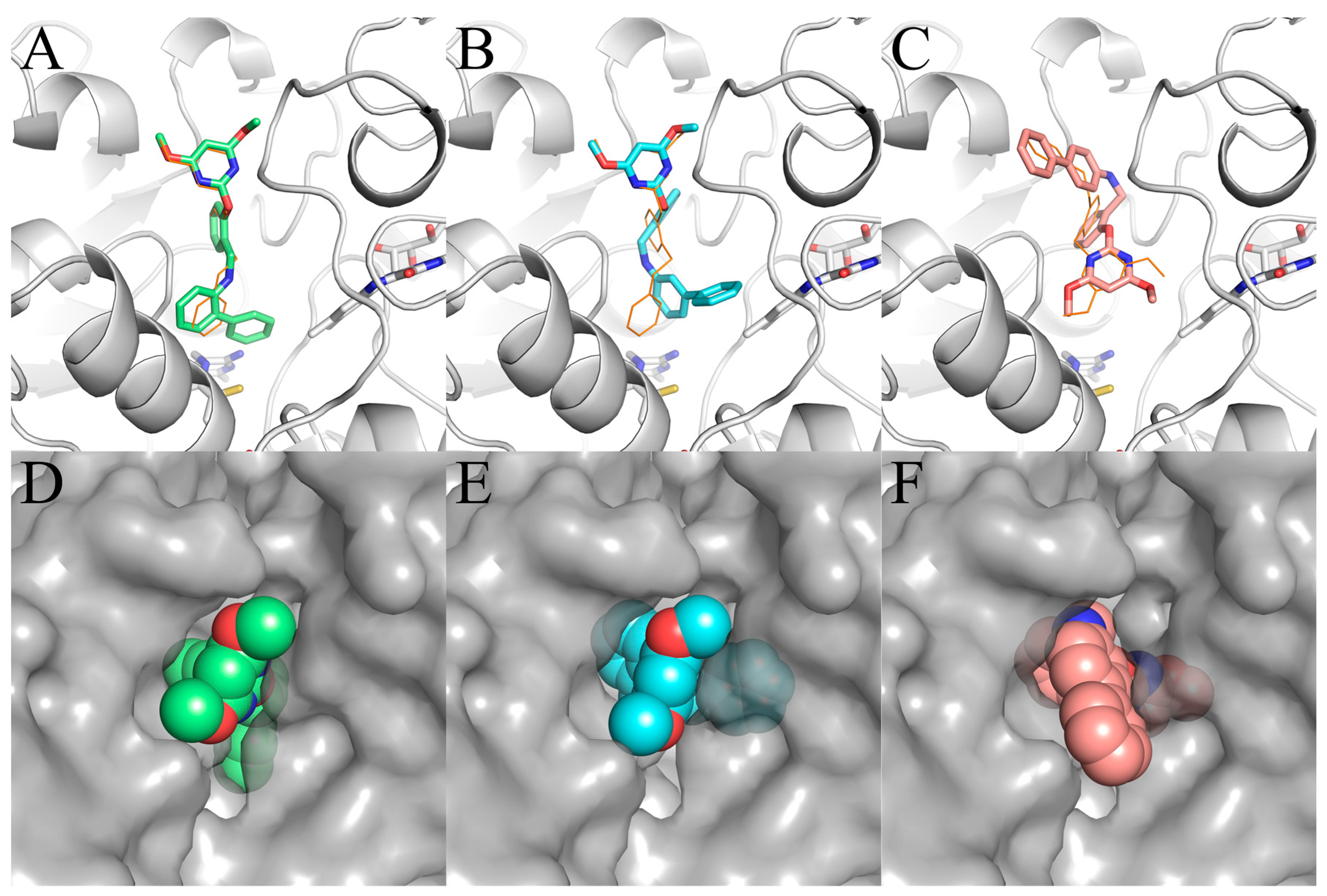
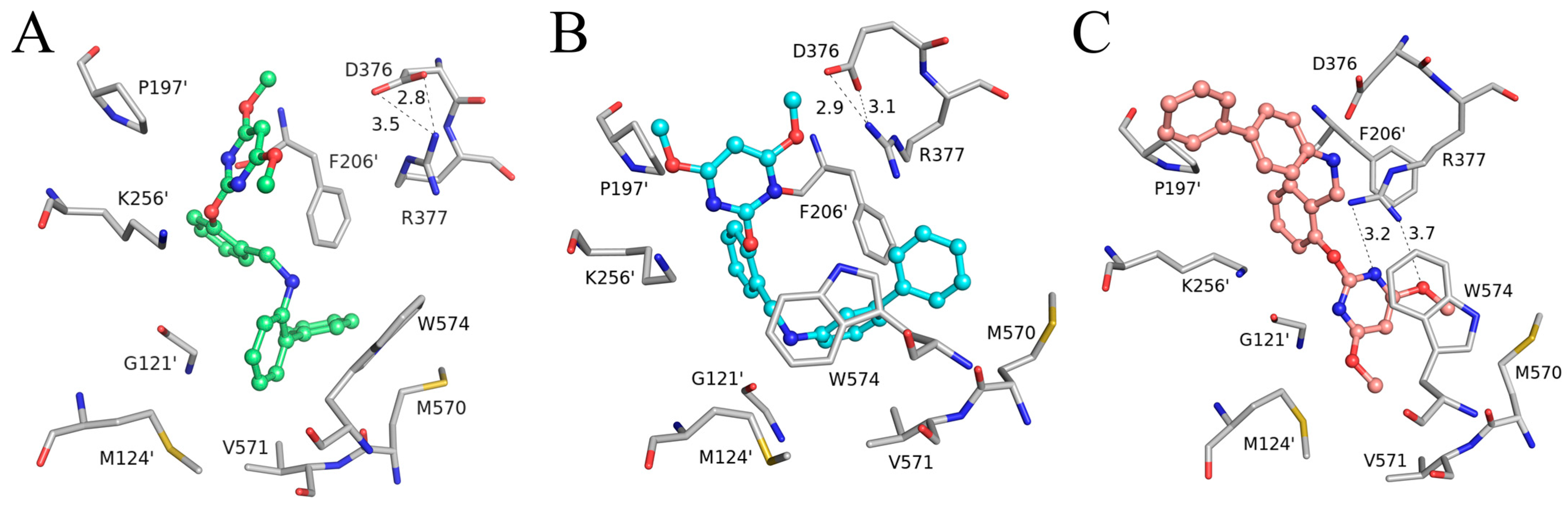
2. Results
3. Discussion
4. Materials and Methods
4.1. Enzyme Assays
4.2. Herbicidal Activities under Greenhouse Conditions
4.3. Molecular Docking Study
4.4. MD Simulation Study
4.5. Binding Free Energy Calculation
4.6. Solvent Accessible Surface Area (SASA) Calculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, S.; Shao, Y.; Fu, X.; Chen, Q.; Du, X.; Tan, C. Design, Synthesis and Insecticidal Activities of Pyridyl Thiazole Diamide Compounds. Chin. J. Org. Chem. 2022, 42, 3870. [Google Scholar]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Li, F. Research Progress on Benzimidazole Fungicides: A Review. Molecules 2024, 29, 1218. [Google Scholar] [CrossRef]
- Lonhienne, T.; Low, Y.S.; Garcia, M.D.; Croll, T.; Gao, Y.; Wang, Q.; Brillault, L.; Williams, C.M.; Fraser, J.A.; McGeary, R.P.; et al. Structures of Fungal and Plant Acetohydroxyacid Synthases. Nature 2020, 586, 317–321. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Wang, X. Acetohydroxyacid Synthases: Evolution, Structure, and Function. Appl. Microbiol. Biotechnol. 2016, 100, 8633–8649. [Google Scholar] [CrossRef]
- Lonhienne, T.; Garcia, M.D.; Low, Y.S.; Guddat, L.W. Herbicides that Inhibit Acetolactate Synthase. Front. Agric. Sci. Eng. 2022, 9, 155–160. [Google Scholar] [CrossRef]
- Souza, T.C.S.; Josa, D.; Ramalho, T.C.; Caetano, M.S.; da Cunha, E.F.F. Molecular Modelling of Mycobacterium Tuberculosis Acetolactate Synthase Catalytic Subunit and Its Molecular Docking Study with Inhibitors. Mol. Simul. 2008, 34, 707–713. [Google Scholar] [CrossRef]
- Garcia, M.D.; Nouwens, A.; Lonhienne, T.G.; Guddat, L.W. Comprehensive Understanding of Acetohydroxyacid Synthase Inhibition by Different Herbicide Families. Proc. Natl. Acad. Sci. USA 2017, 114, E1091–E1100. [Google Scholar] [CrossRef]
- Pang, S.S.; Guddat, L.W.; Duggleby, R.G. Crystallization of Arabidopsis thaliana Acetohydroxyacid Synthase in Complex with the Sulfonylurea Herbicide Chlorimuron Ethyl. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 153–155. [Google Scholar] [CrossRef]
- McCourt, J.A.; Pang, S.S.; King-Scott, J.; Guddat, L.W.; Duggleby, R.G. Herbicide-Binding Sites Revealed in the Structure of Plant Acetohydroxyacid Synthase. Proc. Natl. Acad. Sci. USA 2006, 103, 569–573. [Google Scholar] [CrossRef]
- Lonhienne, T.; Garcia, M.D.; Pierens, G.; Mobli, M.; Nouwens, A.; Guddat, L.W. Structural Insights into the Mechanism of Inhibition of AHAS by Herbicides. Proc. Natl. Acad. Sci. USA 2018, 115, E1945–E1954. [Google Scholar] [CrossRef]
- Lonhienne, T.; Cheng, Y.; Garcia, M.D.; Hu, S.H.; Low, Y.S.; Schenk, G.; Williams, C.M.; Guddat, L.W. Structural Basis of Resistance to Herbicides that Target Acetohydroxyacid Synthase. Nat. Commun. 2022, 13, 3368. [Google Scholar] [CrossRef]
- Cheng, Y.; Lonhienne, T.; Garcia, M.D.; Williams, C.M.; Schenk, G.; Guddat, L.W. Crystal Structure of the Commercial Herbicide, Amidosulfuron, in Complex with Arabidopsis thaliana Acetohydroxyacid Synthase. J. Agric. Food Chem. 2023, 71, 5117–5126. [Google Scholar] [CrossRef]
- Chen, C.-N.; Lv, L.-L.; Ji, F.-Q.; Chen, Q.; Xu, H.; Niu, C.-W.; Xi, Z.; Yang, G.-F. Design and Synthesis of N-2,6-difluorophenyl-5-methoxyl-1,2,4-triazolo[1,5-a]-pyrimidine-2-sulfonamide as Acetohydroxyacid Synthase Inhibitor. Bioorg. Med. Chem. 2009, 17, 3011–3017. [Google Scholar] [CrossRef]
- Li, K.J.; Qu, R.Y.; Liu, Y.C.; Yang, J.F.; Devendar, P.; Chen, Q.; Niu, C.W.; Xi, Z.; Yang, G.F. Design, Synthesis, and Herbicidal Activity of Pyrimidine-Biphenyl Hybrids as Novel Acetohydroxyacid Synthase Inhibitors. J. Agric. Food Chem. 2018, 66, 3773–3782. [Google Scholar] [CrossRef]
- Qu, R.Y.; Yang, J.F.; Devendar, P.; Kang, W.M.; Liu, Y.C.; Chen, Q.; Niu, C.W.; Xi, Z.; Yang, G.F. Discovery of New 2-[(4,6-Dimethoxy-1,3,5-triazin-2-yl)oxy]-6-(substituted phenoxy)benzoic Acids as Flexible Inhibitors of Arabidopsis thaliana Acetohydroxyacid Synthase and Its P197L Mutant. J. Agric. Food Chem. 2017, 65, 11170–11178. [Google Scholar] [CrossRef]
- Liu, Y.C.; Qu, R.Y.; Chen, Q.; Yang, J.F.; Cong-Wei, N.; Zhen, X.; Yang, G.F. Triazolopyrimidines as a New Herbicidal Lead for Combating Weed Resistance Associated with Acetohydroxyacid Synthase Mutation. J. Agric. Food Chem. 2016, 64, 4845–4857. [Google Scholar] [CrossRef]
- Qu, R.Y.; Yang, J.F.; Chen, Q.; Niu, C.W.; Xi, Z.; Yang, W.C.; Yang, G.F. Fragment-Based Discovery of Flexible Inhibitor Targeting Wild-Type Acetohydroxyacid Synthase and P197L Mutant. Pest Manag. Sci. 2020, 76, 3403–3412. [Google Scholar] [CrossRef]
- He, Y.-Z.; Li, Y.-X.; Zhu, X.-L.; Xi, Z.; Niu, C.; Wan, J.; Zhang, L.; Yang, G.-F. Rational Design Based on Bioactive Conformation Analysis of Pyrimidinylbenzoates as Acetohydroxyacid Synthase Inhibitors by Integrating Molecular Docking, CoMFA, CoMSIA, and DFT Calculations. J. Chem. Inf. Model. 2007, 47, 2335–2344. [Google Scholar] [CrossRef]
- Chen, C.-N.; Chen, Q.; Liu, Y.-C.; Zhu, X.-L.; Niu, C.-W.; Xi, Z.; Yang, G.-F. Syntheses and Herbicidal Activity of New Triazolopyrimidine-2-Sulfonamides as Acetohydroxyacid Synthase Inhibitor. Bioorg. Med. Chem. 2010, 18, 4897–4904. [Google Scholar] [CrossRef]
- Chandler, D. Interfaces and the Driving Force of Hydrophobic Assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Jain, Z.J.; Gide, P.S.; Kankate, R.S. Biphenyls and Their Derivatives as Synthetically and Pharmacologically Important Aromatic Structural Moieties. Arabian J. Chem. 2017, 10, S2051–S2066. [Google Scholar] [CrossRef]
- Wang, D.-m.; Feng, B.; Fu, H.; Liu, A.-l.; Wang, L.; Du, G.-h.; Wu, S. Design, Synthesis, and Biological Evaluation of a New Series of Biphenyl/Bibenzyl Derivatives Functioning as Dual Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Molecules 2017, 22, 172. [Google Scholar] [CrossRef]
- Devendar, P.; Qu, R.-Y.; Kang, W.-M.; He, B.; Yang, G.-F. Palladium-Catalyzed Cross-Coupling Reactions: A Powerful Tool for the Synthesis of Agrochemicals. J. Agric. Food Chem. 2018, 66, 8914–8934. [Google Scholar] [CrossRef]
- Han, F.-S. Transition-Metal-Catalyzed Suzuki–Miyaura Cross-Coupling Reactions: A Remarkable Advance from Palladium to Nickel Catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef]
- Yang, Z.-M.; Lu, L. Synthesis of Deuterated Herbicidal ZJ0273, ZJ0702, ZJ0777, and SIOC0163. J. Labelled Compd. Radiopharm. 2010, 53, 192–197. [Google Scholar] [CrossRef]
- Daura, X.; Gademann, K.; Jaun, B.; Seebach, D.; van Gunsteren, W.F.; Mark, A.E. Peptide Folding: When Simulation Meets Experiment. Angew. Chem. Int. Ed. 1999, 38, 236–240. [Google Scholar] [CrossRef]
- Wang, J.; Yao, L. Dissecting C–H∙∙∙π and N−H∙∙∙π Interactions in Two Proteins Using a Combined Experimental and Computational Approach. Sci. Rep. 2019, 9, 20149. [Google Scholar] [CrossRef]
- Sitkoff, D.; Sharp, K.A.; Honig, B. Accurate Calculation of Hydration Free Energies Using Macroscopic Solvent Models. J. Phys. Chem. 1994, 98, 1978–1988. [Google Scholar] [CrossRef]
- Fitch, C.A.; Platzer, G.; Okon, M.; Garcia-Moreno, E.B.; McIntosh, L.P. Arginine: Its pKa Value Revisited. Protein Sci. 2015, 24, 752–761. [Google Scholar] [CrossRef]
- Loladze, V.V.; Ermolenko, D.N.; Makhatadze, G.I. Thermodynamic Consequences of Burial of Polar and Non-polar Amino Acid Residues in the Protein Interior. J. Mol. Biol. 2002, 320, 343–357. [Google Scholar] [CrossRef]
- Meng, J.-P.; Wang, W.-W.; Chen, Y.-L.; Bera, S.; Wu, J. Switchable Solvent-Controlled Divergent Synthesis: An Efficient and Regioselective Approach to Pyrimidine and Dibenzo[b,f][1,4]oxazepine Derivatives. Org. Chem. Front. 2020, 7, 267–272. [Google Scholar] [CrossRef]
- Nezu, Y.; Miyazaki, M.; Sugiyama, K.; Wada, N.; Kajiwara, I.; Miyazawa, T. Dimethoxypyrimidines as Novel Herbicides. Part 2. Synthesis and Herbicidal Activity of O-pyrimidinylsalicylates and Analogues. Pestic. Sci. 1996, 47, 115–124. [Google Scholar] [CrossRef]
- Schwertz, G.; Frei, M.S.; Witschel, M.C.; Rottmann, M.; Leartsakulpanich, U.; Chitnumsub, P.; Jaruwat, A.; Ittarat, W.; Schäfer, A.; Aponte, R.A.; et al. Conformational Aspects in the Design of Inhibitors for Serine Hydroxymethyltransferase (SHMT): Biphenyl, Aryl Sulfonamide, and Aryl Sulfone Motifs. Chem. Eur. J. 2017, 23, 14345–14357. [Google Scholar] [CrossRef]
- Grein, F. Twist Angles and Rotational Energy Barriers of Biphenyl and Substituted Biphenyls. J. Phys. Chem. A 2002, 106, 3823–3827. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Guzik, K.; Zieba, B.J.; Musielak, B.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Basis for Small Molecule Targeting of the Programmed Death Ligand 1 (PD-L1). Oncotarget 2016, 7, 30323–30335. [Google Scholar] [CrossRef]
- Wang, J.; Tan, H.; Li, Y.; Ma, Y.; Li, Z.; Guddat, L.W. Chemical Synthesis, In Vitro Acetohydroxyacid Synthase (AHAS) Inhibition, Herbicidal Activity, and Computational Studies of Isatin Derivatives. J. Agric. Food Chem. 2011, 59, 9892–9900. [Google Scholar] [CrossRef]
- Shang, J.; Wang, W.-M.; Li, Y.-H.; Song, H.-B.; Li, Z.-M.; Wang, J.-G. Synthesis, Crystal Structure, In Vitro Acetohydroxyacid Synthase Inhibition, In Vivo Herbicidal Activity, and 3D-QSAR of New Asymmetric Aryl Disulfides. J. Agric. Food Chem. 2012, 60, 8286–8293. [Google Scholar] [CrossRef]
- Lv, X.-H.; Ren, Z.-L.; Liu, H.; Li, H.-d.; Li, Q.-S.; Wang, L.; Zhang, L.-S.; Yao, X.-K.; Cao, H.-Q. Design, Synthesis and Biological Evaluation of Novel Pyrazole Sulfonamide Derivatives as Potential AHAS Inhibitors. Chem. Pharm. Bull. 2018, 66, 358–362. [Google Scholar] [CrossRef]
- Kalnmals, C.A.; Benko, Z.L.; Hamza, A.; Bravo-Altamirano, K.; Siddall, T.L.; Zielinski, M.; Takano, H.K.; Riar, D.S.; Satchivi, N.M.; Roth, J.J.; et al. A New Class of Diaryl Ether Herbicides: Structure–Activity Relationship Studies Enabled by a Rapid Scaffold Hopping Approach. J. Agric. Food Chem. 2023, 71, 18171–18187. [Google Scholar] [CrossRef]
- Davis, A.M.; Teague, S.J. Hydrogen Bonding, Hydrophobic Interactions, and Failure of the Rigid Receptor Hypothesis. Angew. Chem. Int. Ed. 1999, 38, 736–749. [Google Scholar] [CrossRef]
- Paarakh, M.P.; Shruthi, S.D.; Sharath, B.S. Evaluation of Plant-Derived Compounds to Inhibit COVID-19 through in Silico Studies. Indian J. Nat. Prod. Resour. 2021, 12, 26–33. [Google Scholar]
- Shaji, D.; Das, A.; Suzuki, R.; Nagura, Y.; Sabishiro, H.; Kurita, N. Proposal of Novel ApoE4 Inhibitors from the Natural Spice Cinnamon for the Treatment of Alzheimer’s Disease: Ab initio Molecular Simulations. Biophys. Chem. 2023, 296, 106990. [Google Scholar] [CrossRef]
- Zabkiewicz, J.A. Adjuvants and Herbicidal Efficacy–Present Status and Future Prospects. Weed Res. 2000, 40, 139–149. [Google Scholar] [CrossRef]
- Adams, E.; Gerstle, V.; Schmitt, T.; Brühl, C.A. Co-Formulants and Adjuvants Affect the Acute Aquatic and Terrestrial Toxicity of a Cycloxydim Herbicide Formulation to European Common Frogs (Rana temporaria). Sci. Total Environ. 2021, 789, 147865. [Google Scholar] [CrossRef]
- Blanco, M.; Zamora, D.; Planells, J.; Mulero, R. Analytical Control of Adjuvants in Herbicide Formulations by NIR Spectroscopy. Anal. Bioanal. Chem. 2009, 395, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Palma-Bautista, C.; Tataridas, A.; Kanatas, P.; Travlos, I.S.; Bastida, F.; Domínguez-Valenzuela, J.A.; De Prado, R. Can Control of Glyphosate Susceptible and Resistant Conyza sumatrensis Populations be Dependent on the Herbicide Formulation or Adjuvants? Agronomy 2020, 10, 1599. [Google Scholar] [CrossRef]
- Stillway, M.E.; Teh, S.J. The Effect of Herbicide Formulations and Herbicide–Adjuvant Mixtures on Aquatic Food Web Species of the Sacramento–San Joaquin Delta, California, USA. Environ. Toxicol. Chem. 2020, 39, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Jibrin, M.O.; Liu, Q.; Jones, J.B.; Zhang, S. Surfactants in Plant Disease Management: A Brief Review and Case Studies. Plant Pathol. 2021, 70, 495–510. [Google Scholar] [CrossRef]
- Chang, A.K.; Duggleby, R.G. Expression, Purification and Characterization of Arabidopsis thaliana Acetohydroxyacid Synthase. Biochem. J. 1997, 327, 161–169. [Google Scholar] [CrossRef]
- Fan, Z.-J.; Chen, J.-P.; Hu, J.-Y.; Qian, C.-F.; Li, Z.-M. Activity of Acetolactate Synthase from Maize (Zea mays L.) as Influenced by Chlorsulfuron and Tribenuron-Methyl. Agric. Sci. China 2003, 2, 176–182. [Google Scholar]
- Min, J.; Zhang, X.; Wang, L.; Zou, X.; Zhang, Q.; He, J. Mutational Analysis of the Interaction Between a Potential Inhibitor luteolin and Enoyl-ACP Reductase (FabI) from Salmonella Enterica. J. Mol. Catal. B Enzym. 2011, 68, 174–180. [Google Scholar] [CrossRef]
- Ma, D.; Yin, Y.; Chen, Y.-L.; Yan, Y.-T.; Wu, J. Design, Step-Economical Diversity-Oriented Synthesis of an N-Heterocyclic Library Containing a Pyrimidine Moiety: Discovery of Novel Potential Herbicidal Agents. RSC Adv. 2021, 11, 15380–15386. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field: A Force Field for Drug-Like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Vanommeslaeghe, K.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) I: Bond Perception and Atom Typing. J. Chem. Inf. Model. 2012, 52, 3144–3154. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Raman, E.P.; MacKerell, A.D., Jr. Automation of the CHARMM General Force Field (CGenFF) II: Assignment of Bonded Parameters and Partial Atomic Charges. J. Chem. Inf. Model. 2012, 52, 3155–3168. [Google Scholar] [CrossRef]
- Izadi, S.; Onufriev, A.V. Accuracy Limit of Rigid 3-Point Water Models. J. Phys. Chem. 2016, 145, 074501. [Google Scholar] [CrossRef]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of Nanosystems: Application to Microtubules and the Ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef]
- Eisenhaber, F.; Lijnzaad, P.; Argos, P.; Sander, C.; Scharf, M. The Double Cubic Lattice Method: Efficient Approaches to Numerical Integration of Surface Area and Volume and to Dot Surface Contouring of Molecular Assemblies. J. Comput. Chem. 1995, 16, 273–284. [Google Scholar] [CrossRef]
- Tekin, H.O.; ALMisned, G.; Issa, S.A.; Kasikci, E.S.; Arooj, M.; Ene, A.; Al-Buriahi, M.; Konuk, M.; Zakaly, H.M. Molecular Polar Surface Area, Total Solvent Accessible Surface Area (SASA), Heat of Formation, and Gamma-Ray Attenuation Properties of Some Flavonoids. Front. Phys. 2022, 10, 838725. [Google Scholar] [CrossRef]

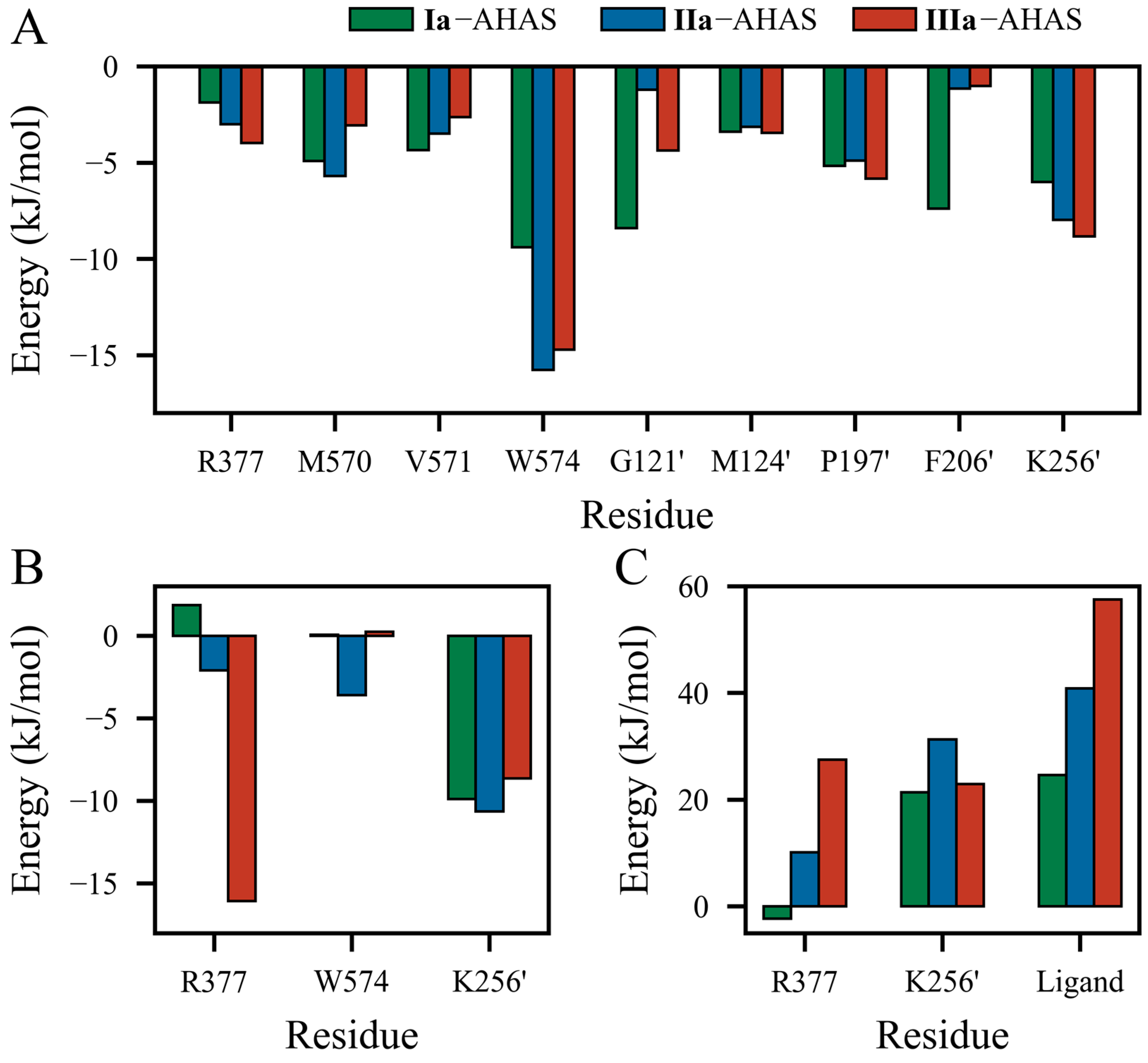
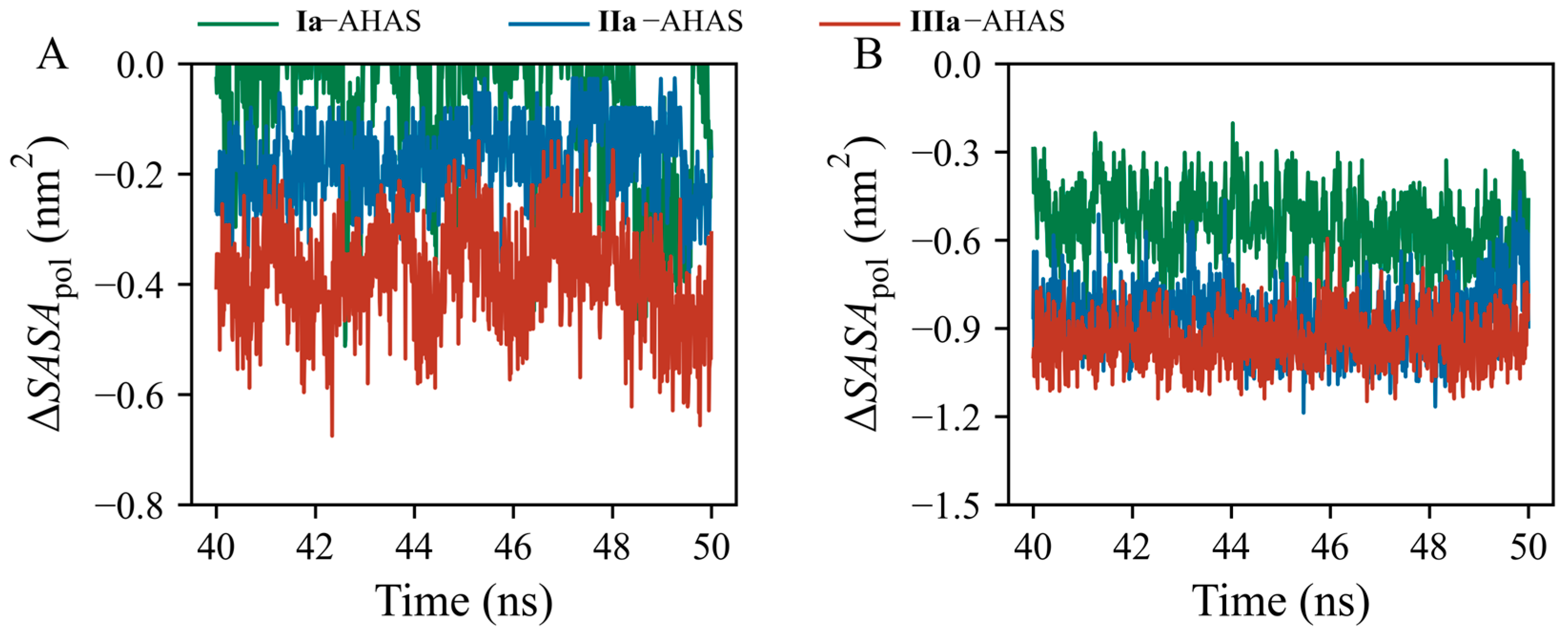
| System | Calculated Energy (kJ/mol) | ||||
|---|---|---|---|---|---|
| ΔEVdW | ΔEELE | ΔEMM | ΔGSOL | ΔGbind | |
| Ia–AHAS | −190.013 | −24.397 | −214.410 | 89.455 | −124.955 |
| IIa–AHAS | −190.307 | −42.914 | −233.222 | 122.967 | −110.255 |
| IIIa–AHAS | −191.220 | −68.200 | −259.420 | 170.556 | −88.864 |
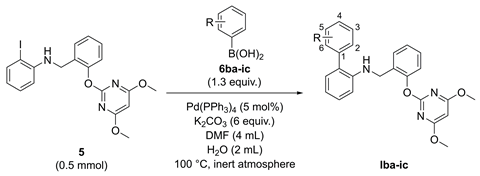
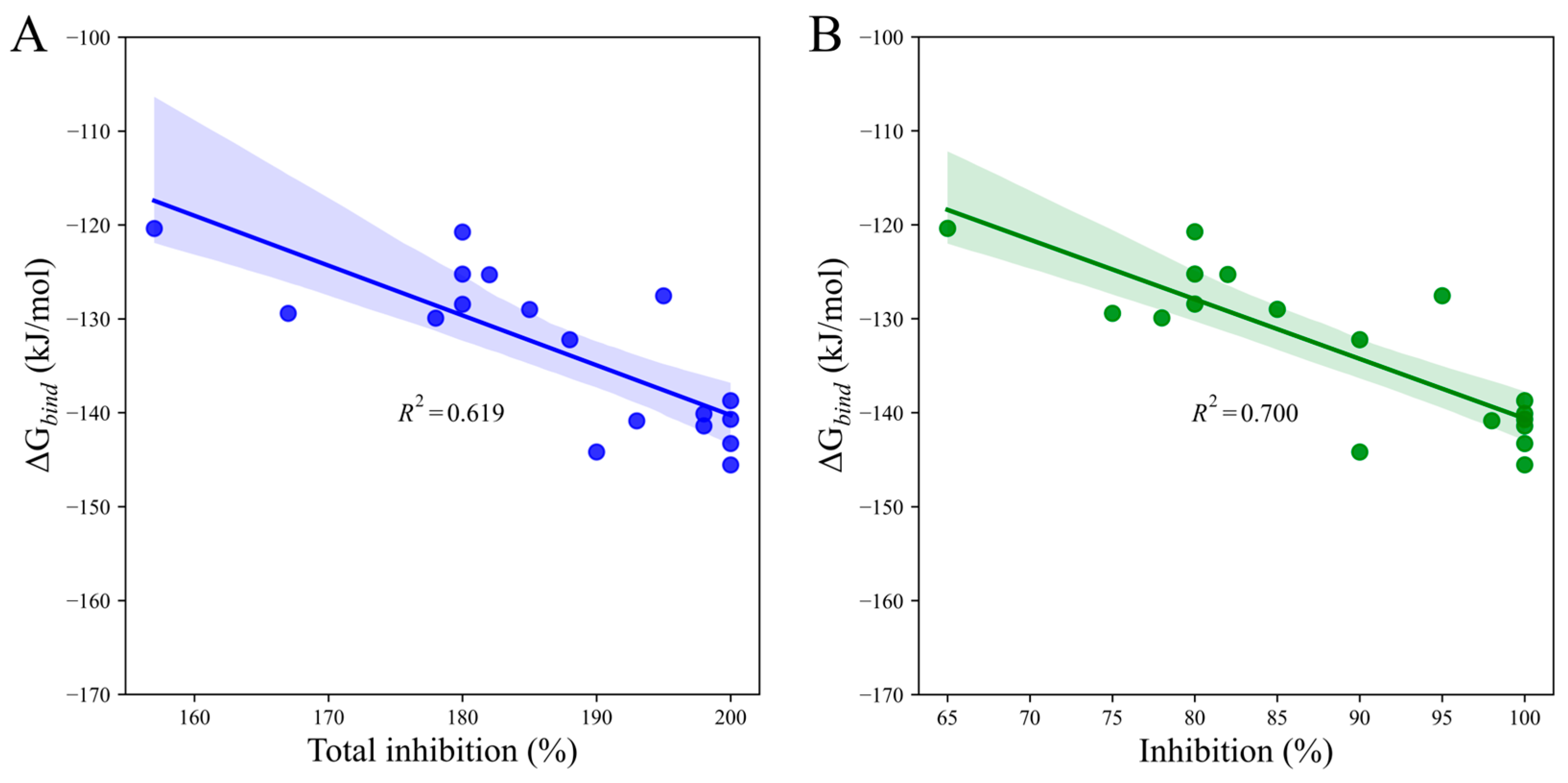
| Compd. | R | ΔGbind (kJ/mol) | Inhibition a (Post-Emergence, %) | ||||
|---|---|---|---|---|---|---|---|
| EC b | DS | AT | CT | Total | |||
| Ia | -H | −124.955 | 60 | 85 | 85 | 85 | 315 |
| Iba | 2-Me | −141.408 c | 98 | 100 | 45 | 98 | 341 |
| Ibb | 3-Me | −128.998 | 100 | 85 | 45 | 80 | 310 |
| Ibc | 4-Me | −144.177 | 100 | 90 | 60 | 80 | 330 |
| Ica | 2-OMe | −145.547 | 100 | 100 | 50 | 88 | 338 |
| Icb | 3-OMe | −138.720 | 100 | 100 | 40 | 85 | 325 |
| Icc | 4-OMe | −143.275 | 100 | 100 | 70 | 88 | 358 |
| Ida | 2-NH2 | −120.744 | 100 | 80 | 65 | 20 | 265 |
| Idb | 3-NH2 | −125.291 | 100 | 82 | 65 | 90 | 337 |
| Idc | 4-NH2 | −127.550 | 100 | 95 | 25 | 88 | 308 |
| Iea | 2-OH | −125.229 | 100 | 80 | 50 | 85 | 315 |
| Iec | 4-OH | −120.380 | 92 | 65 | 35 | 75 | 267 |
| Ifa | 2-F | −135.177 | 92 | 75 | 40 | 82 | 289 |
| Ifc | 4-F | −132.218 | 98 | 90 | 45 | 70 | 303 |
| Iga | 2-Cl | −128.437 | 100 | 80 | 40 | 80 | 300 |
| Igc | 4-Cl | −140.856 | 95 | 98 | 55 | 70 | 318 |
| Iha | 2-Br | −140.702 | 100 | 100 | 50 | 45 | 295 |
| Iia | 2-CF3 | −140.103 | 98 | 100 | 60 | 80 | 338 |
| Iic | 4-CF3 | −129.919 | 100 | 78 | 45 | 90 | 313 |
| Compd. | Dosage (g ai/ha) | Inhibition a (%) | |
|---|---|---|---|
| EC b | DS | ||
| Iba | 375 | 90 | 80 |
| 187.5 | 85 | 70 | |
| Ica | 375 | 90 | 85 |
| 187.5 | 85 | 80 | |
| Icb | 375 | 80 | 70 |
| 187.5 | 75 | 60 | |
| Icc | 375 | 80 | 75 |
| 187.5 | 75 | 70 | |
| Iha | 375 | 85 | 70 |
| 187.5 | 75 | 60 | |
| Iia | 375 | 80 | 85 |
| 187.5 | 70 | 65 | |
| PYB1 c | 375 | 90 | 90 |
| 187.5 | 85 | 85 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Chen, Y.; Hu, H.; Jiang, Y.; Kang, Z.; Wu, J. Discovery of a New Class of Lipophilic Pyrimidine-Biphenyl Herbicides Using an Integrated Experimental-Computational Approach. Molecules 2024, 29, 2409. https://doi.org/10.3390/molecules29112409
Yan Y, Chen Y, Hu H, Jiang Y, Kang Z, Wu J. Discovery of a New Class of Lipophilic Pyrimidine-Biphenyl Herbicides Using an Integrated Experimental-Computational Approach. Molecules. 2024; 29(11):2409. https://doi.org/10.3390/molecules29112409
Chicago/Turabian StyleYan, Yitao, Yinglu Chen, Hanxian Hu, Youwei Jiang, Zhengzhong Kang, and Jun Wu. 2024. "Discovery of a New Class of Lipophilic Pyrimidine-Biphenyl Herbicides Using an Integrated Experimental-Computational Approach" Molecules 29, no. 11: 2409. https://doi.org/10.3390/molecules29112409
APA StyleYan, Y., Chen, Y., Hu, H., Jiang, Y., Kang, Z., & Wu, J. (2024). Discovery of a New Class of Lipophilic Pyrimidine-Biphenyl Herbicides Using an Integrated Experimental-Computational Approach. Molecules, 29(11), 2409. https://doi.org/10.3390/molecules29112409






