Environmental Dyeing and Functionalization of Silk Fabrics with Natural Dye Extracted from Lac
Abstract
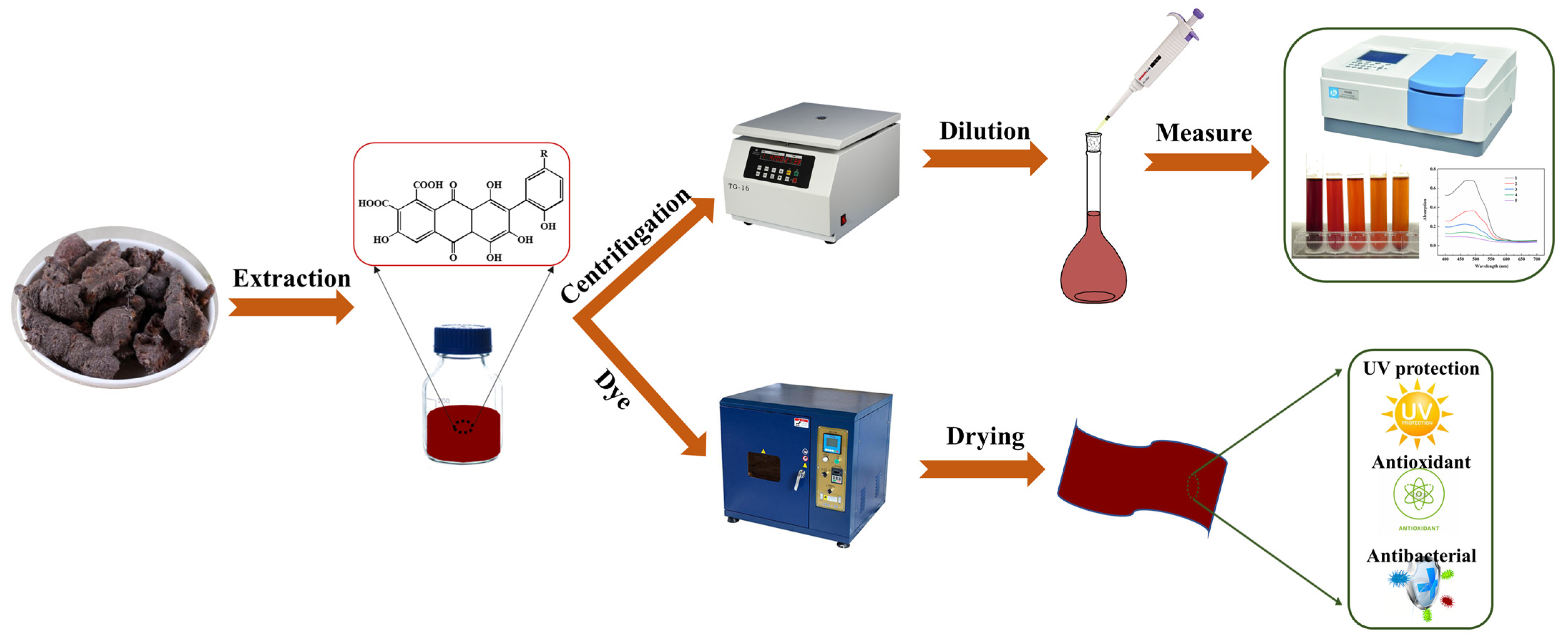
1. Introduction
2. Results and Discussion
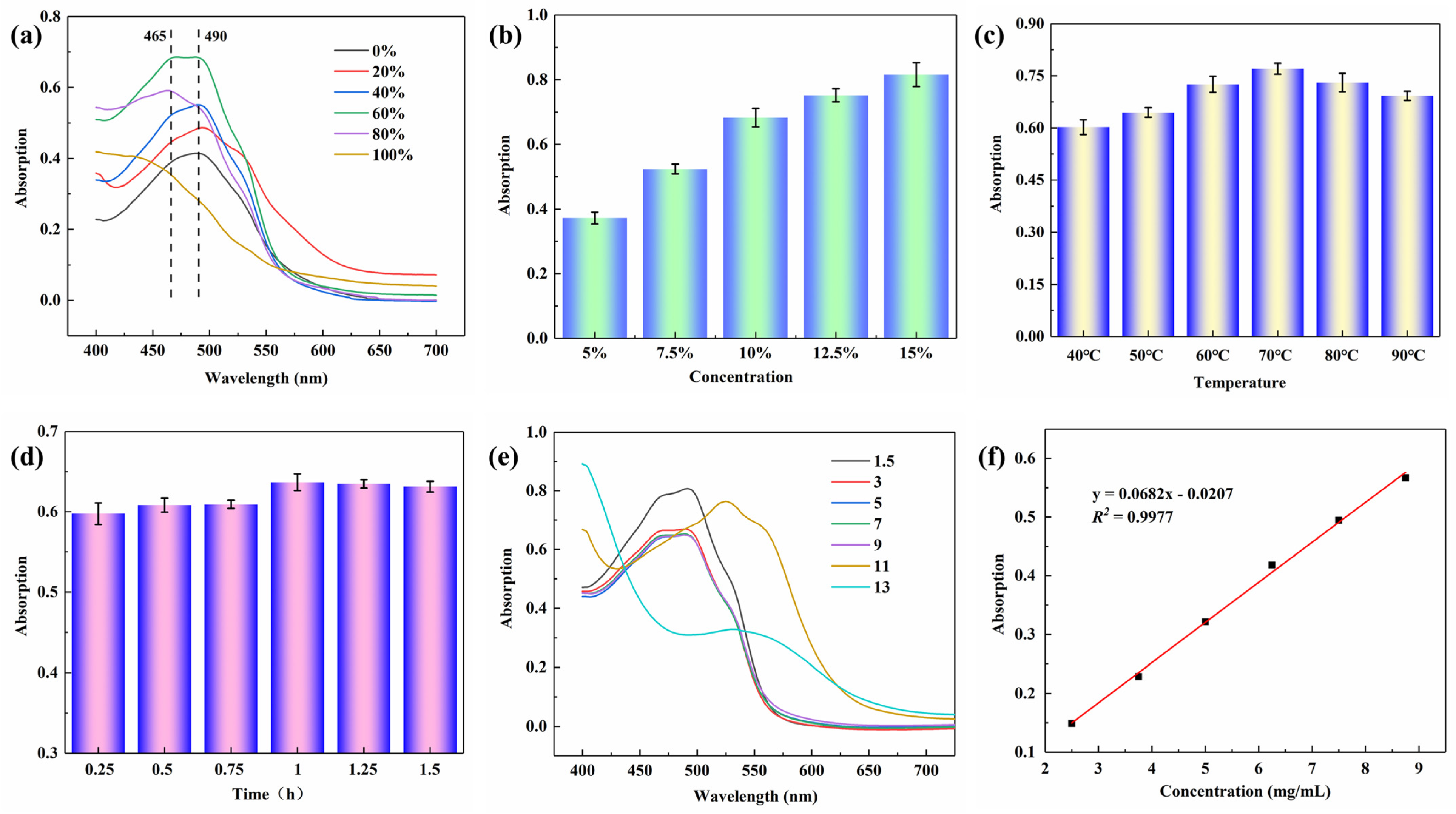
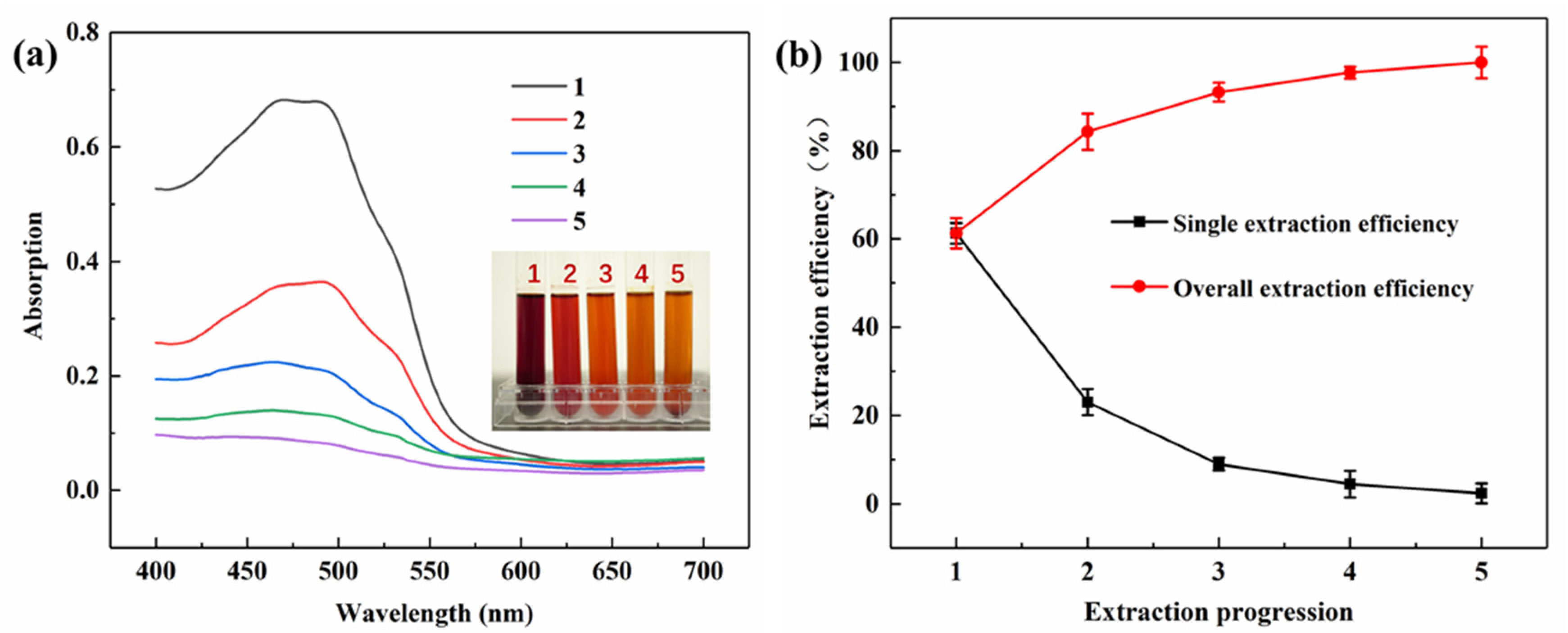
2.1. Optimization of Extracting Process of Lac Pigment
2.2. Dyeing Fabrics by Lac Extracts
2.3. Color Fastness of Dyed Fabrics
2.4. Functionality of Dyed Fabrics
2.4.1. UV Protection
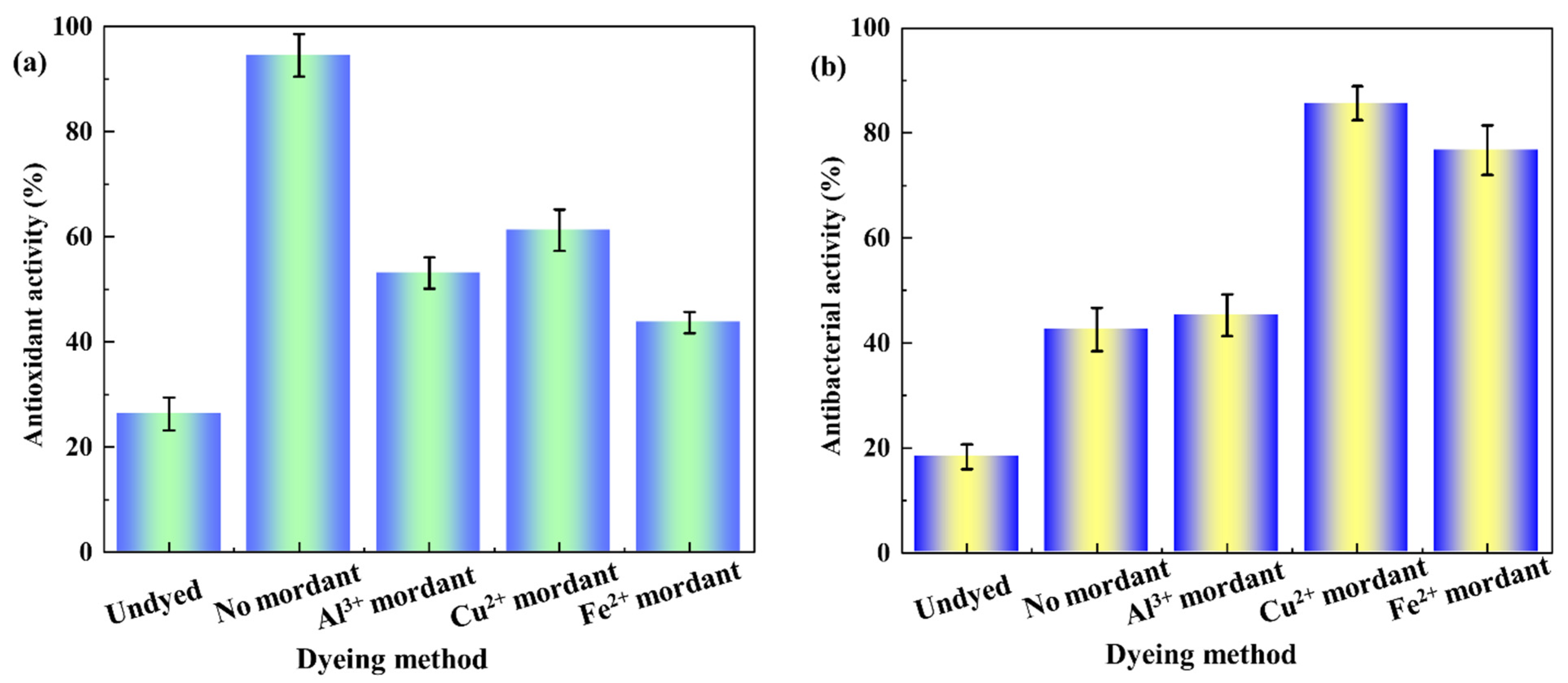
2.4.2. Antioxidant Activity
2.4.3. Antibacterial Performances
3. Materials and Methods
3.1. Materials
3.2. Extraction of Lac Pigment
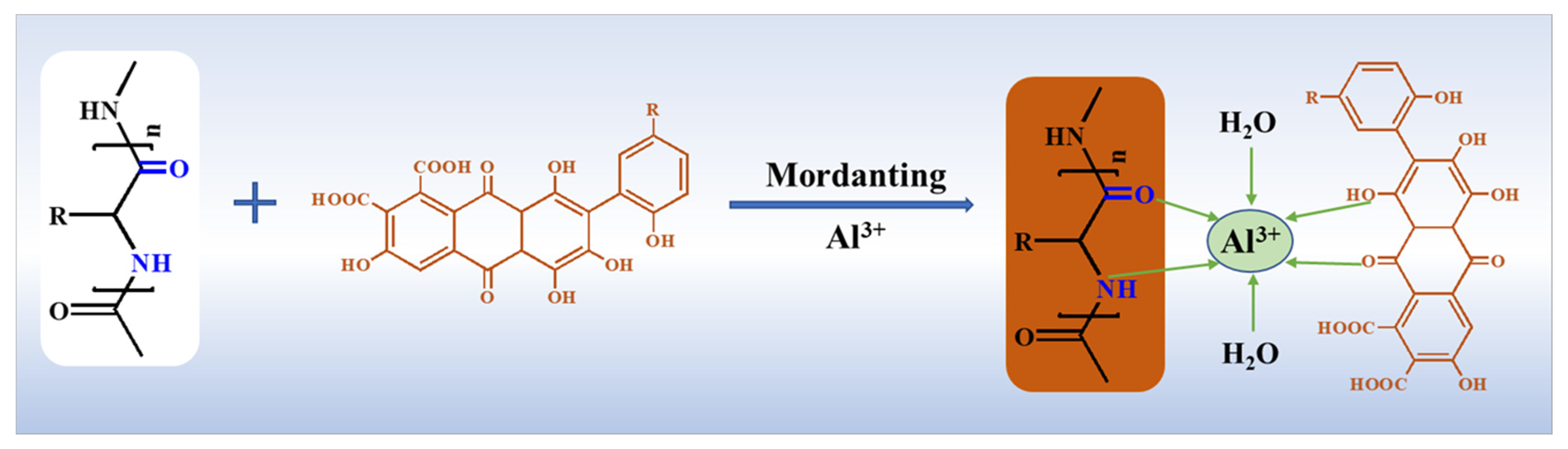
3.3. Dyeing and Mordanting of Fabrics
3.4. Measurements and Characterizations
3.4.1. Color Parameter Measurement
3.4.2. Durability Analysis of Dyed Fabrics
3.4.3. Ultraviolet Protection Performance (UPF) Analysis
3.4.4. Antioxidant Efficacy Assessment
3.4.5. Antibacterial Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Wang, L.; Hu, H.; Du, Y.; Mi, X.; Zhu, Q.; Chen, Q.; Gui, Z.; Zhang, B.; Yu, Z. Eco-Friendly and Sustainable Application of Gardenia Yellow Extraction as Natural Dye Source for Dyeing and Bio-Functional Finishing of Cotton Fabric. Cellulose 2024, 31, 2583–2601. [Google Scholar] [CrossRef]
- Fang, J.; Meng, C.; Zhang, G. Agricultural Waste of Ipomoea Batatas Leaves as a Source of Natural Dye for Green Coloration and Bio-Functional Finishing for Textile Fabrics. Ind. Crops Prod. 2022, 177, 114440. [Google Scholar] [CrossRef]
- Haque, M.A.; Mia, R.; Mahmud, S.T.; Bakar, M.A.; Ahmed, T.; Farsee, M.S.; Hossain, M.I. Sustainable Dyeing and Functionalization of Wool Fabrics with Black Rice Extract. Resour. Environ. Sustain. 2022, 7, 100045. [Google Scholar] [CrossRef]
- Yadav, S.; Tiwari, K.S.; Gupta, C.; Tiwari, M.K.; Khan, A.; Sonkar, S.P. A Brief Review on Natural Dyes, Pigments: Recent Advances and Future Perspectives. Results Chem. 2023, 5, 100733. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, S.; Fang, K.; Zhao, Z.; Shu, D.; Xie, R.; Chen, W. Green Fabrication of Inkjet Printed Antibacterial Wool Fabric with Natural Gardenia Yellow Dye. Ind. Crops Prod. 2023, 206, 117700. [Google Scholar] [CrossRef]
- Pizzicato, B.; Pacifico, S.; Cayuela, D.; Mijas, G.; Riba-Moliner, M. Advancements in Sustainable Natural Dyes for Textile Applications: A Review. Molecules 2023, 28, 5954. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Cotton Textile Dyeing by Plant-Derived Colorants in the Presence of Natural Additives. Fibers Polym. 2023, 24, 3641–3655. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, M.; Youm, K.; Kumar, S.; Koh, J.; Hong, K.H. Sustainable One-Bath Natural Dyeing of Cotton Fabric Using Turmeric Root Extract and Chitosan Biomordant. J. Clean. Prod. 2023, 382, 135303. [Google Scholar] [CrossRef]
- Mia, R.; Bakar, M.A.; Islam, M.R.; Ahmed, T. Eco-Friendly Coloration from Mahogany Wood Waste for Sustainable Dyeing of Organic Nonwoven Cotton Fabric. Results Eng. 2023, 17, 101032. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Zhang, Y.; Wu, S.; Liu, R. Flavonoid Dyes from Vine Tea (Ampelopsis Grossedentata) Have Excellent Bioactive Properties for Dyeing and Finishing of Silk Fabrics. Sustain. Chem. Pharm. 2022, 28, 100708. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, R.C. Natural Flavonoid-Functionalized Silk Fiber Presenting Antibacterial, Antioxidant, and Uv Protection Performance. ACS Sustain. Chem. Eng. 2017, 5, 10518–10526. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.Y.; Tang, R.C. Bioactive and UV Protective Silk Materials Containing Baicalin—The Multifunctional Plant Extract from Scutellaria Baicalensis Georgi. Mater. Sci. Eng. C 2016, 67, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhou, Y.; Tang, R.C. Bridging Phycocyanin onto Silk by Genipin Towards Durable Colouristic, Antioxidant and Uv Protective Properties: A Sustainable Strategy for Fully Bio-Based Textile. Chem. Eng. J. 2023, 477, 146808. [Google Scholar] [CrossRef]
- Fazal-ur, R.; Adeel, S.; Liaqat, S.; Hussaan, M.; Mia, R.; Ahmed, B.; Wafa, H. Environmental Friendly Bio-Dyeing of Silk Using Alkanna Tinctoria Based Alkannin Natural Dye. Ind. Crops Prod. 2022, 186, 115301. [Google Scholar]
- Deb, A.K.; Shaikh, M.A.A.; Sultan, M.Z.; Rafi, M.I.H. Application of Lac Dye in Shoe Upper Leather Dyeing. Leath Footwear J. 2017, 17, 97–106. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, S.H.; Kim, Y.S.; Kwon, E.; Lim, H.J.; Han, K.M.; Choi, Y.K.; Jung, C.W.; Kang, B.C. Nonclinical Safety Evaluation of Food Colorant Lac Dye Via Systematic Toxicity Profiling with Assessment of in Vivo Antigenic Potential. Front. Pharmacol. 2022, 13, 1020379. [Google Scholar] [CrossRef]
- Berbers, S.V.J.; Tamburini, D.; van-Bommel, M.R.; Dyer, J. Historical Formulations of Lake Pigments and Dyes Derived from Lac: A Study of Compositional Variability. Dyes Pigments 2019, 170, 107579. [Google Scholar] [CrossRef]
- Liu, L.; Yi, G.; Li, K.; Ma, J.; Xu, J.; Zhang, W.; Sun, Y.; Zhang, H. Synthesis of Carbon Quantum Dots from Lac Dye for Silicon Dioxide Imaging and Highly Sensitive Ethanol Detecting. Dyes Pigments 2019, 171, 107681. [Google Scholar] [CrossRef]
- Liu, L.; Xu, J.; Zheng, H.; Li, K.; Zhang, W.; Li, K.; Zhang, H. Inclusion Complexes of Laccaic Acid a with β-Cyclodextrin or Its Derivatives: Phase Solubility, Solubilization, Inclusion Mode, and Characterization. Dyes Pigments 2017, 139, 737–746. [Google Scholar] [CrossRef]
- Sharma, K.K.; Chowdhury, A.R.; Srivastava, S. Chemistry and Applications of Lac and Its by-Product. In Natural Materials and Products from Insects: Chemistry and Applications; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Rather, L.J.; Ali, A.; Zhou, Q.; Ganie, S.A.; Gong, K.; Rizwanul Haque, Q.M.; Li, Q. Instrumental Characterization of Merino Wool Fibers Dyed with Cinnamomum Camphora Waste/Fallen Leaves Extract: An Efficient Waste Management Alternative. J. Clean. Prod. 2020, 273, 123021. [Google Scholar] [CrossRef]
- Gong, X.; Li, Y.; Qu, H. Removing Tannins from Medicinal Plant Extracts Using an Alkaline Ethanol Precipitation Process: A Case Study of Danshen Injection. Molecules 2014, 19, 18705–18720. [Google Scholar] [CrossRef] [PubMed]
- Jabar, J.M.; Ogunsade, A.F.; Odusote, Y.A.; Yılmaz, M. Utilization of Nigerian Mango (Mangifera indica L) Leaves Dye Extract for Silk Fabric Coloration: Influence of Extraction Technique, Mordant and Mordanting Type on the Fabric Color Attributes. Ind. Crops Prod. 2023, 193, 116235. [Google Scholar] [CrossRef]
- Ke, G.; Mulla, M.S.; Peng, F.; Chen, S. Dyeing Properties of Natural Gardenia on the Lyocell Fabric Pretreated with Tannic Acid. Cellulose 2022, 30, 611–624. [Google Scholar] [CrossRef]
- Amutha, K.; Sudhapriya, N. Dyeing of Textiles with Natural Dyes Extracted from Terminalia Arjuna and Thespesia Populnea Fruits. Ind. Crops Prod. 2020, 148, 112303. [Google Scholar]
- Yan, X.; Hong, L.; Pei, S.; Hamilton, A.; Sun, H.; Yang, R.; Liu, A.; Yang, L. A Natural Yellow Colorant from Buddleja Officinalis for Dyeing Hemp Fabric. Ind. Crops Prod. 2021, 171, 113968. [Google Scholar] [CrossRef]
- Benli, H.; Aydınlıoğlu, Ö.; Yılmaz, F.; Bahtiyari, M.İ. Topping of Naturally Dyed Wool Fabrics with Different Natural Dye Sources. Color. Technol. 2022, 139, 171–181. [Google Scholar] [CrossRef]
- Amin, N.; Rehman, F.-u.; Adeel, S.; Ahamd, T.; Muneer, M.; Haji, A. Sustainable Application of Cochineal-Based Anthraquinone Dye for the Coloration of Bio-Mordanted Silk Fabric. Environ. Sci. Pollut. Res. 2019, 27, 6851–6860. [Google Scholar] [CrossRef] [PubMed]
- Adeel, S.; Habib, N.; Arif, S.; Rehman, F.u.; Azeem, M.; Batool, F.; Amin, N. Microwave-Assisted Eco-Dyeing of Bio Mordanted Silk Fabric Using Cinnamon Bark (Cinnamomum Verum) Based Yellow Natural Dye. Sustain. Chem. Pharm. 2020, 17, 100306. [Google Scholar] [CrossRef]
- Rahman Liman, M.L.; Islam, M.T.; Repon, M.R.; Hossain, M.M.; Sarker, P. Comparative Dyeing Behavior and Uv Protective Characteristics of Cotton Fabric Treated with Polyphenols Enriched Banana and Watermelon Biowaste. Sustain. Chem. Pharm. 2021, 21, 100417. [Google Scholar] [CrossRef]
- GB 18401–2010; National General Safety Technical Code for Textile Products. National Technical Committee for Textile Standardization: Jiaxin, China, 2011.
- Leamkaew, V.; Thongsamai, P.; Bremner, J.B.; Chairat, M. Adsorption Kinetics of Lac Dye on Eri Silk Yarn. J. Text. Inst. 2021, 113, 2480–2490. [Google Scholar] [CrossRef]
- Do, K.L.; Su, M.; Mushtaq, A.; Zhao, F. Dyeing of Silk with Natural Lac Dye from Laccifer lacca Kerr. and Evaluation of Antibacterial and Uv-Protective Properties. Fibers Polym. 2023, 24, 2773–2783. [Google Scholar]
- Yin, M.; Lin, X.; Ren, T.; Li, Z.; Ren, X.; Huang, T.S. Cytocompatible Quaternized Carboxymethyl Chitosan/Poly(Vinyl Alcohol) Blend Film Loaded Copper for Antibacterial Application. Int. J. Biol. Macromol. 2018, 120, 992–998. [Google Scholar] [CrossRef] [PubMed]
- ISO 105–C06:2010; Textiles—Tests for Colour Fastness—Part C06: Colour Fastness to Domestic and Commercial Laundering. ISO: Geneva, Switzerland, 2010.
- ISO 105–X12:2016; Textiles—Tests for Colour Fastness—Part X12: Colour Fastness to Rubbing. ISO: Geneva, Switzerland, 2016.
- ISO 105–B02:2014; Textiles—Tests for Colour Fastness—Part B02: Colour Fastness to Artificial Light: Xenon Arc Fading Lamp Test. ISO: Geneva, Switzerland, 2014.
- Yin, M.; Wang, Y.; Zhang, Y.; Ren, X.; Qiu, Y.; Huang, T.S. Novel Quaternarized N-Halamine Chitosan and Polyvinyl Alcohol Nanofibrous Membranes as Hemostatic Materials with Excellent Antibacterial Properties. Carbohydr. Polym. 2020, 232, 115823. [Google Scholar] [CrossRef] [PubMed]
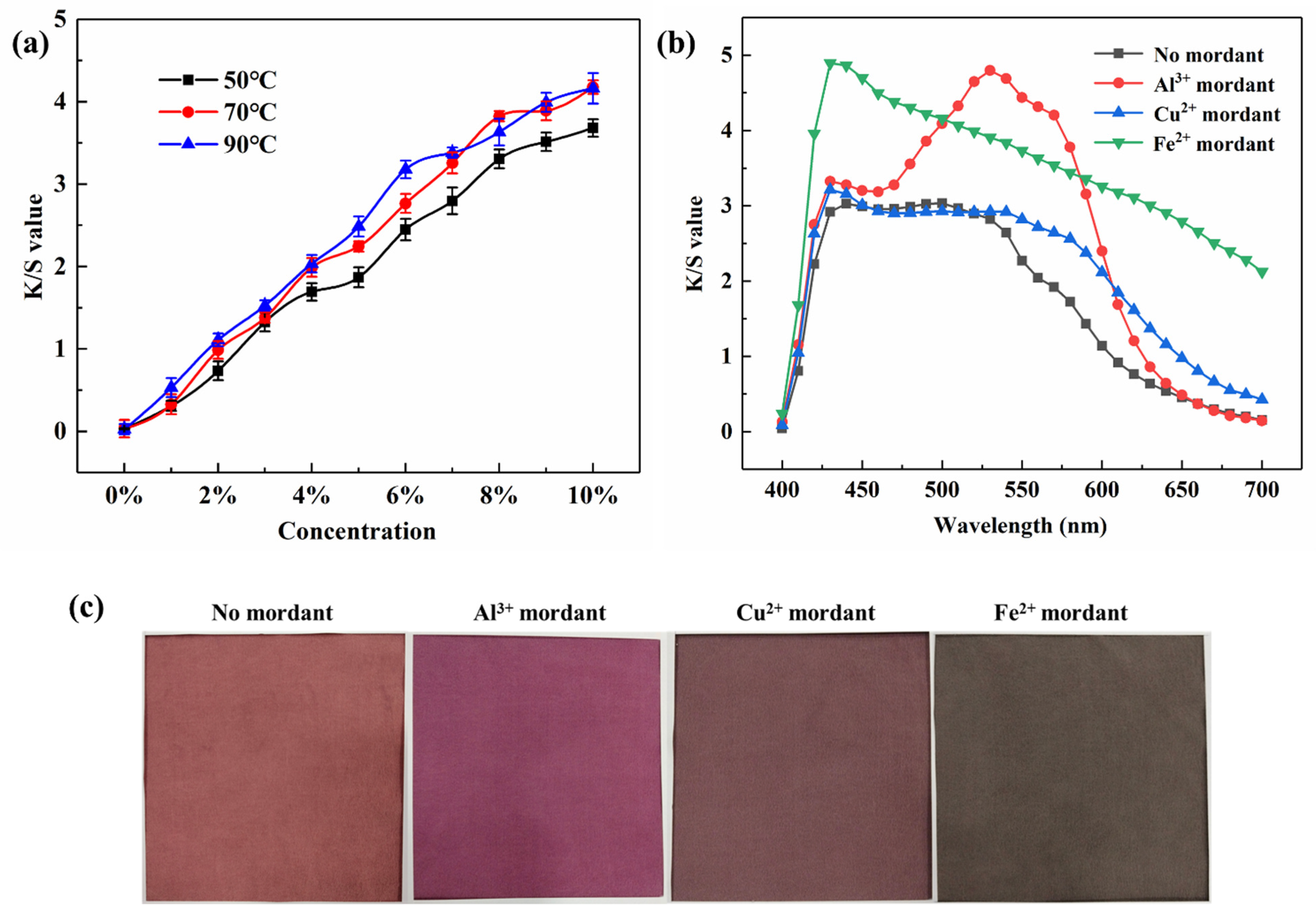

| Dyeing Method | λmax | K/S Value | L | a | b |
|---|---|---|---|---|---|
| No mordant | 440 | 3.03 | 49.44 | 19.65 | 9.55 |
| Al3+ mordant | 530 | 4.80 | 42.82 | 23.11 | −0.8 |
| Cu2+ mordant | 430 | 3.22 | 46.50 | 9.89 | 3.06 |
| Fe2+ mordant | 430 | 4.90 | 40.76 | 3.45 | 4.01 |
| Dyeing Method | Rubbing Fastness | Washing Fastness | Light Fastness | ||
|---|---|---|---|---|---|
| Dry | Wet | Fade | Staining | ||
| No mordant | 3 | 2–3 | 2 | 3 | 4 |
| Al3+ mordant | 4–5 | 4 | 4 | 4–5 | 4–5 |
| Cu2+ mordant | 4–5 | 4 | 3–4 | 4 | 4–5 |
| Fe2+ mordant | 4 | 4 | 3–4 | 4–5 | 4 |
| Dyeing Method | UPF | T (UVA, %) | T (UVB, %) |
|---|---|---|---|
| Undyed | 8.52 | 11.14 | 5.21 |
| No mordant | 28.56 | 4.03 | 4.57 |
| Al3+ mordant | 42.68 | 1.97 | 2.01 |
| Cu2+ mordant | 35.61 | 2.94 | 2.85 |
| Fe2+ mordant | 38.91 | 2.03 | 2.08 |
| Dye | Antibacterial | Antioxidant | References | |
|---|---|---|---|---|
| S. aureus | E. coli | |||
| Gardenia yellow | 100% | 100% | 38% | (Wang et al., 2024) [2] |
| Blue bio-colorant phycocyanin | / | / | 70% | (Wu et al., 2023) [14] |
| Gardenia yellow | >95% | >95% | / | (Wang et al., 2023) [6] |
| Nigerian mango leaves | / | / | 80% | (Jabar et al., 2023) [24] |
| Lac | 40.8% | 69.11% | (Do et al., 2023) [34] | |
| Flavonoid dyes from vine tea | 64.7% | 73% | 45.13% | (Zhang et al., 2022) [11] |
| Black rice extract | >80% | >80% | (Haque et al., 2022) [4] | |
| Lac | / | 42.58% | 98.57% | This work |
| Lac + Cu2+ | / | 85.68% | 61.27% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Wang, Z.; Zhao, L.; Li, X.; Cai, H.; Yang, S.; Yin, M.; Xing, J. Environmental Dyeing and Functionalization of Silk Fabrics with Natural Dye Extracted from Lac. Molecules 2024, 29, 2358. https://doi.org/10.3390/molecules29102358
Huang Q, Wang Z, Zhao L, Li X, Cai H, Yang S, Yin M, Xing J. Environmental Dyeing and Functionalization of Silk Fabrics with Natural Dye Extracted from Lac. Molecules. 2024; 29(10):2358. https://doi.org/10.3390/molecules29102358
Chicago/Turabian StyleHuang, Qinru, Zhao Wang, Liwei Zhao, Xiaojuan Li, Haohao Cai, Shuang Yang, Maoli Yin, and Jian Xing. 2024. "Environmental Dyeing and Functionalization of Silk Fabrics with Natural Dye Extracted from Lac" Molecules 29, no. 10: 2358. https://doi.org/10.3390/molecules29102358
APA StyleHuang, Q., Wang, Z., Zhao, L., Li, X., Cai, H., Yang, S., Yin, M., & Xing, J. (2024). Environmental Dyeing and Functionalization of Silk Fabrics with Natural Dye Extracted from Lac. Molecules, 29(10), 2358. https://doi.org/10.3390/molecules29102358






