Research Progress of Natural Active Substances with Immunosuppressive Activity
Abstract
1. Introduction
2. The Immunosupperssive Activity of Animal and Cell Models
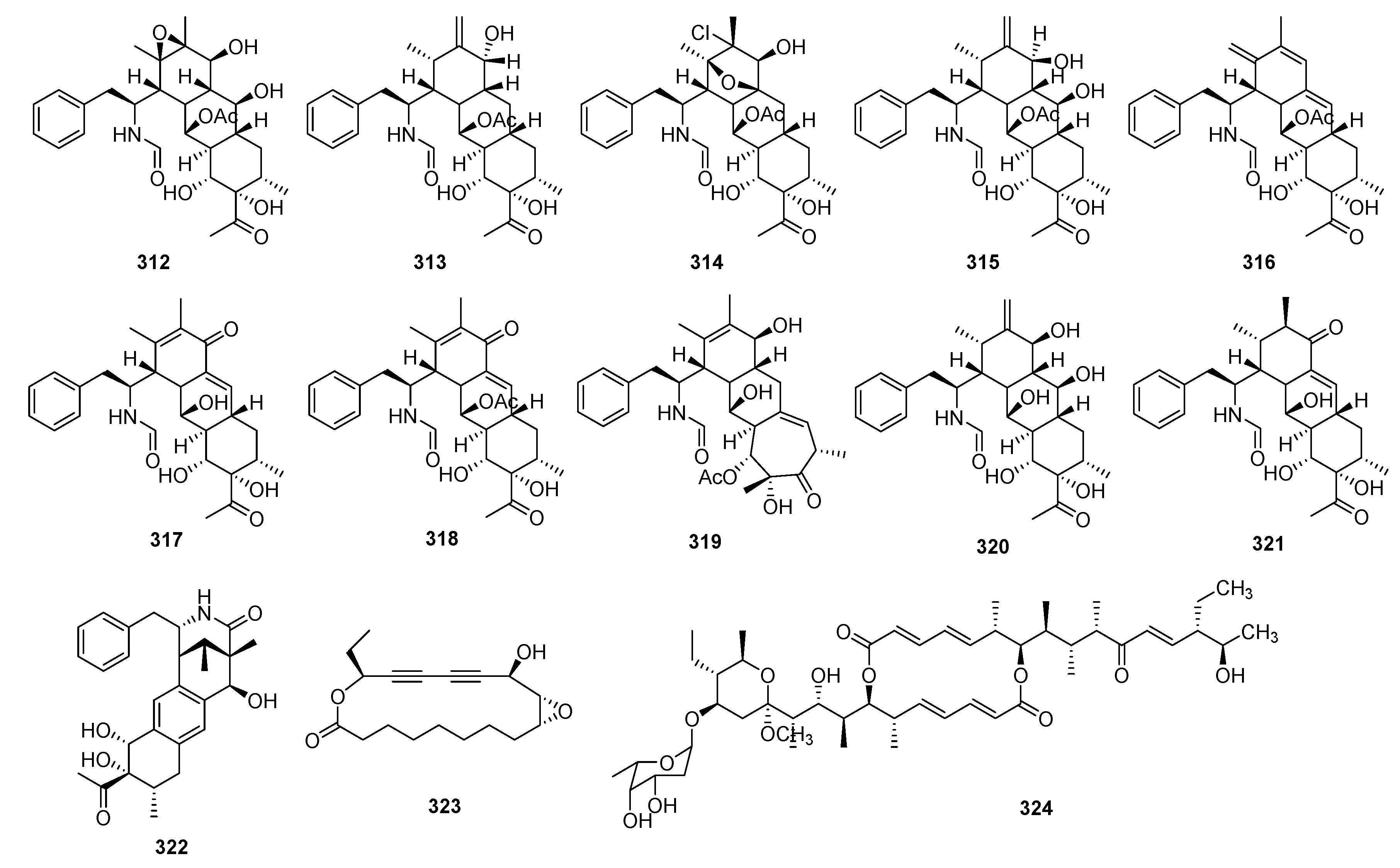
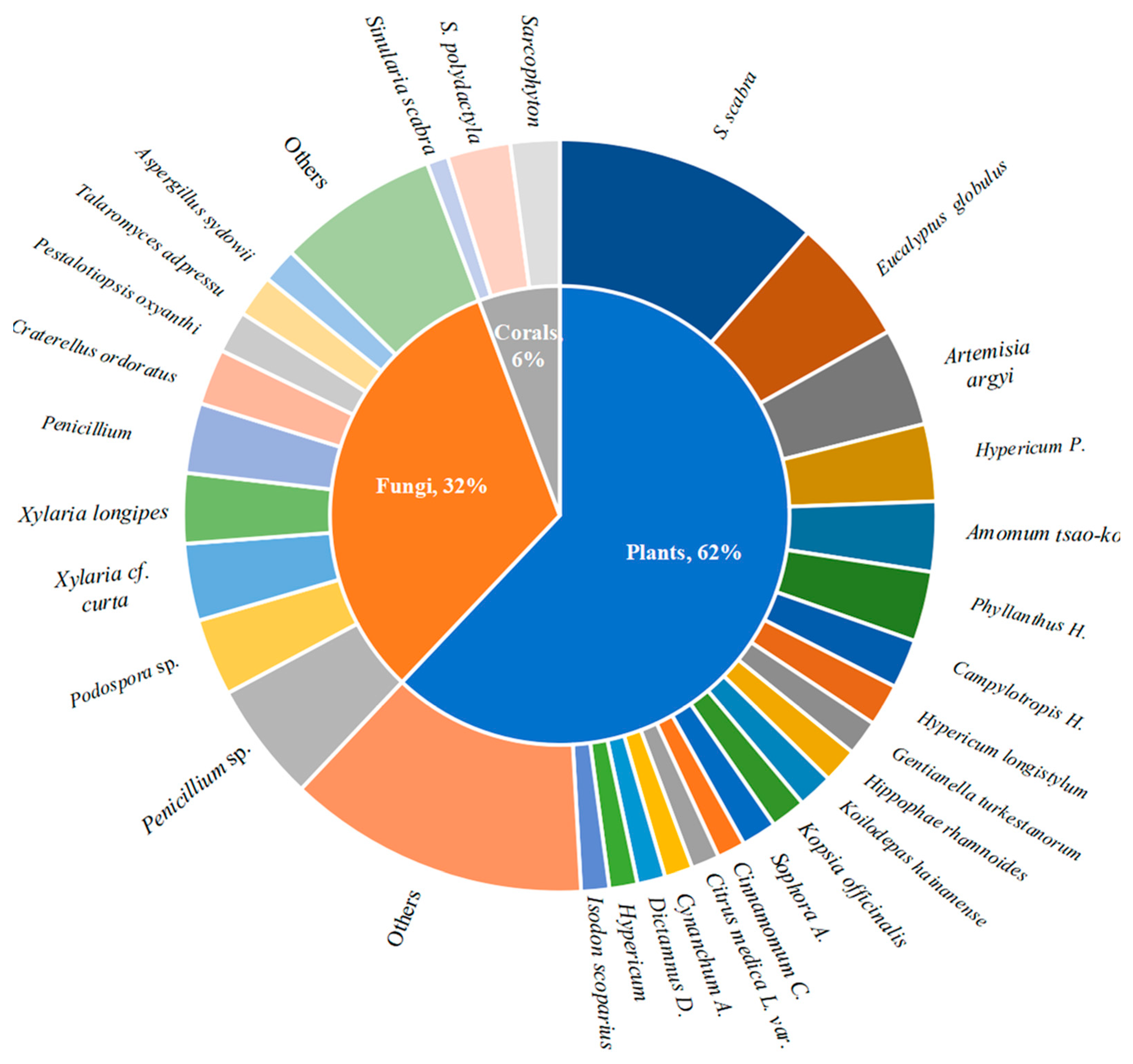
3. Natural Products with Immunosuppressive Activity
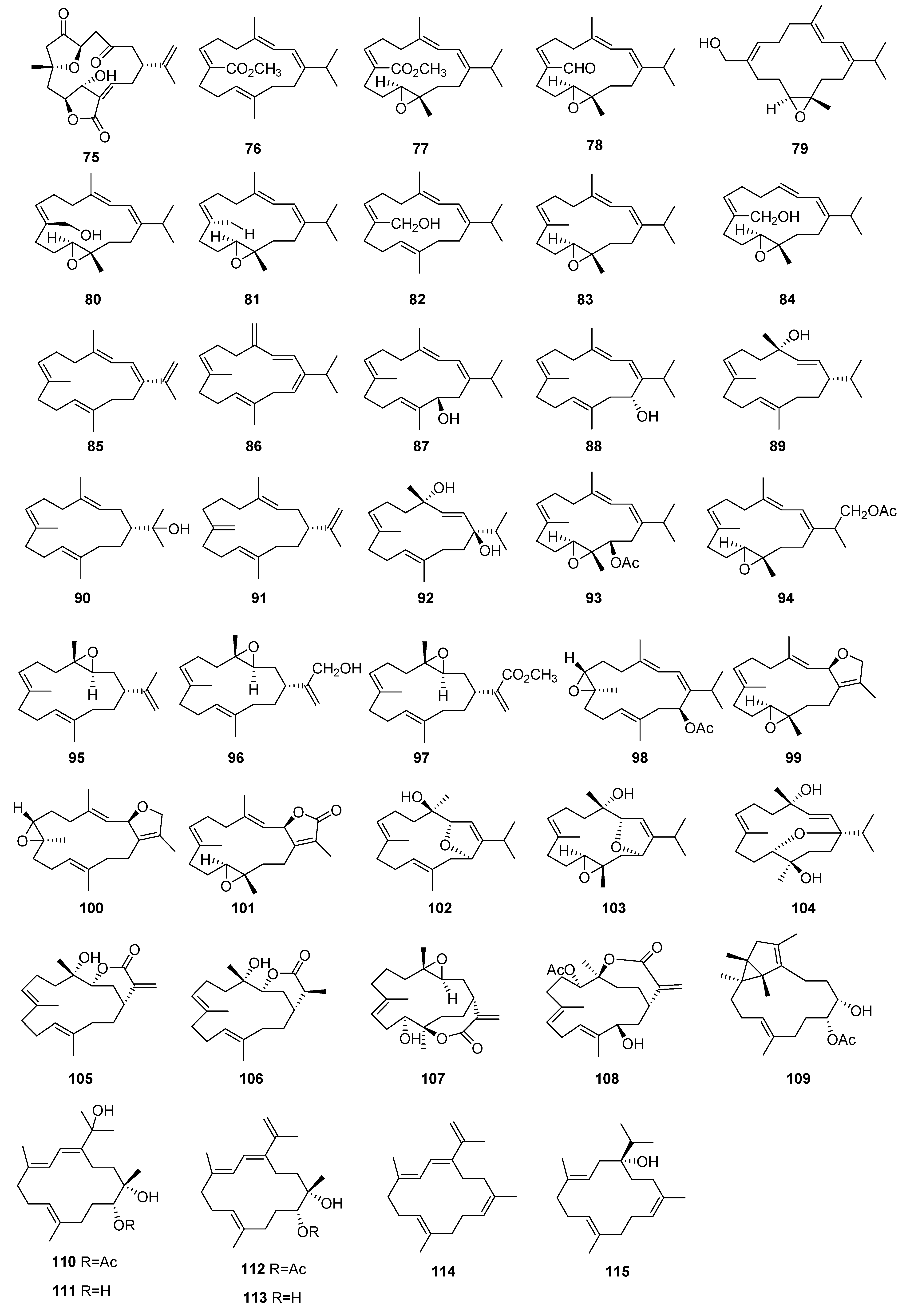
3.1. Terpenoids
3.1.1. Sesquiterpenoids
3.1.2. Diterpenoids
3.1.3. Triterpenoids
3.1.4. Other Terpenoids
3.2. Alkaloids
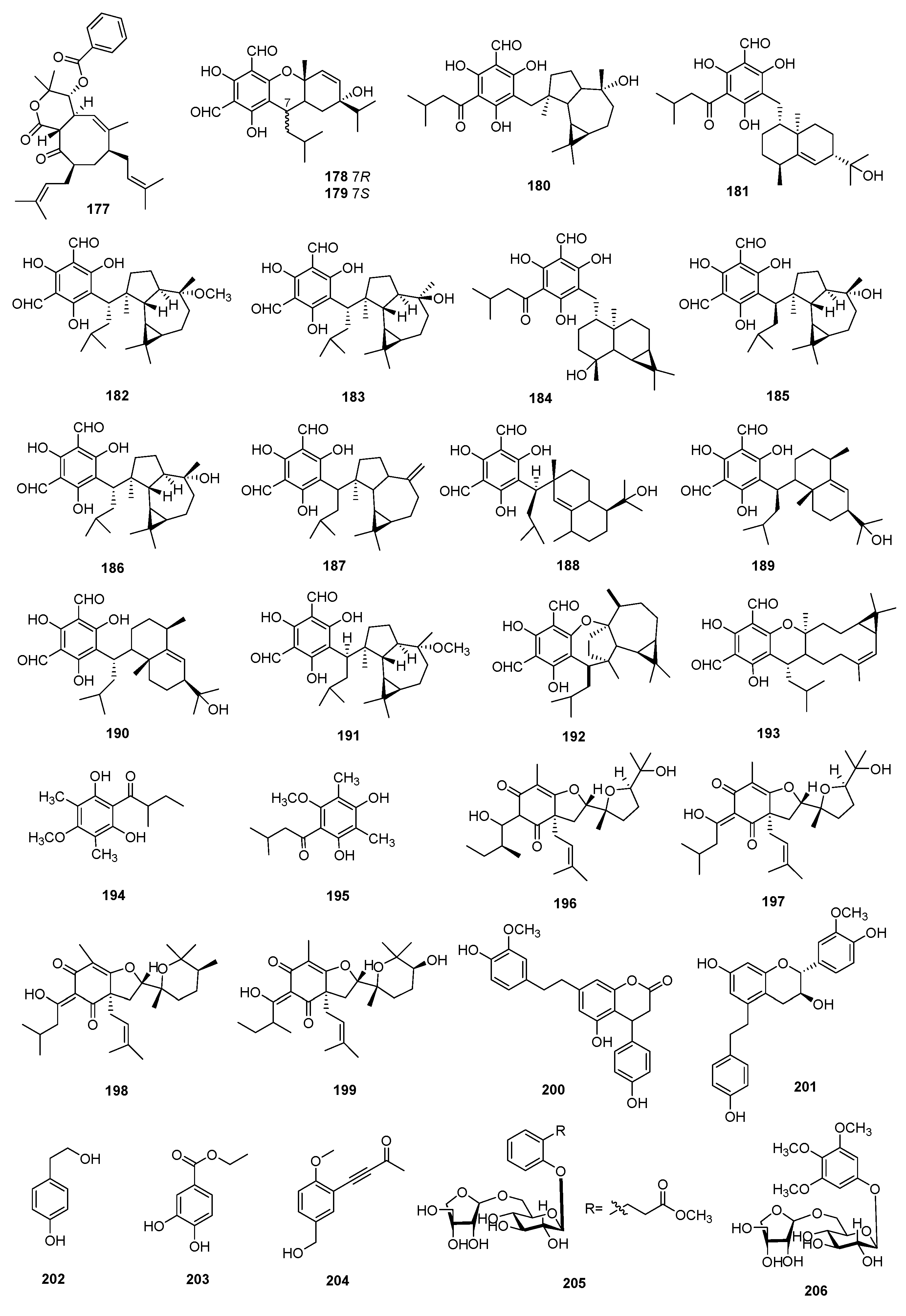
3.3. Phenolic Compounds
3.4. Flavonoids
3.5. Steroids
3.6. Others
4. Structure-Activity Relationship
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alexander, H.; Eirik, B.; Eystein, S.H. Autoimmune Addison’s disease—An update on pathogenesis. Ann. D’Endocrinol. 2018, 79, 157–163. [Google Scholar]
- Conrad, N.; Misra, S.; Verbakel, J.Y.; Verbeke, G.; Molenberghs, G.; Taylor, P.N.; Mason, J.; Sattar, N.; McMurray, J.J.V.; McInnes, I.B.; et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: A population-based cohort study of 22 million individuals in the UK. Lancet 2023, 401, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.; Satveer, K.M.; Jonathan, N.B. Psoriasis: A brief overview. Clin. Med. 2021, 21, 170–173. [Google Scholar]
- Lin, S.; Yan, S.; Liu, Y.; Zhang, X.; Cao, F.; He, Y.; Li, F.; Liu, J.; Wang, J.; Hu, Z.; et al. New secondary metabolites with immunosuppressive and BChE inhibitory activities from an endophytic fungus Daldinia sp. TJ403-LS1. Bioorganic Chem. 2021, 114, 105091. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Xie, S.; Bu, P.; Guo, Y.; Shi, Z.; Guo, Y.; Cao, Y.; Sun, W.; Qi, C.; Zhang, Y. Hypaluton A, an Immunosuppressive 3,4-nor-Polycyclic Polyprenylated Acylphloroglucinol from Hypericum patulum. J. Org. Chem. 2021, 86, 6478–6485. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Nagata, S. Efferocytosis and autoimmune disease. Int. Immunol. 2018, 30, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Roberto, P.; Alessandra, F.; Alberto, F. Autoimmune Addison’s Disease as Part of the Autoimmune Polyglandular Syndrome Type 1: Historical Overview and Current Evidence. Front. Immunol. 2021, 12, 606860. [Google Scholar]
- Pascual, J.; Berger, S.P.; Witzke, O. Everolimus with Reduced Calcineurin Inhibitor Exposure in Renal Transplantation. J. Am. Soc. Nephrol. 2018, 29, 1979–1991. [Google Scholar] [CrossRef] [PubMed]
- Leroy, C.; Rigot, J.M.; Leroy, M. Immunosuppressive drugs and fertility. Orphanet J. Rare Dis. 2015, 10, 136. [Google Scholar] [CrossRef]
- Shin, J.S.; Chung, S.H.; Lee, W.S.; Lee, J.Y.; Kim, J.L.; Lee, K.T. Immunostimulatory effects of cordycepin-enriched WIB-801CE from Cordyceps militaris in splenocytes and cyclophosphamide induced immunosuppressed mice. Phytother. Res. 2018, 32, 132–139. [Google Scholar] [CrossRef]
- Ali, H.; Musharraf, S.G.; Iqbal, N.; Adhikari, A.; Abdalla, O.M.; Ahmed Mesaik, M.; Kabir, N. Immunosuppressive and hepatoprotective potential of Sarcococca saligna and its biomarker components. Int. Immunopharmacol. 2015, 28, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Jiyan, S.; Jingjin, H.; Ziren, S.; Lian, Z.; Yao, Z.; Xiao, L.; Yu, L. T cell inhibition by pogostone from Pogostemon cablin (Blanco) Benth: In vitro and in vivo immunosuppressive analysis. Mol. Med. Rep. 2017, 16, 4511–4520. [Google Scholar]
- Zeynep, T.; Jing, X.; Jian, H.; Yu, L.; Hua, H.; Tao, Y. Cytotoxic lanostane triterpenoids from the fruiting bodies of Piptoporus betulinus. Phytochemistry 2017, 143, 98–103. [Google Scholar]
- Qun, W.; Wen, Y.L.; Yu, W. A immunosuppressive triterpenoid saponin from the stems of Epigynum griffithianum. Nat. Prod. Res. 2020, 34, 1389–1393. [Google Scholar]
- Renda, G.; Gökkaya, O.; Şöhretoğlu, D. Immunomodulatory properties of triterpenes. Phytochem. Rev. 2022, 21, 537–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, W. Anti-inflammatory and immunoregulatory effects of paeoniflorin and total glucosides of paeony. Pharmacol. Ther. 2020, 207, 107452. [Google Scholar] [CrossRef] [PubMed]
- Chamani, S.; Moossavi, M.; Naghizadeh, A.; Abbasifard, M.; Majeed, M.; Johnston, T.P.; Sahebkar, A. Immunomodulatory effects of curcumin in systemic autoimmune diseases. Phytother. Res. 2022, 36, 1616–1632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Li, J.; Wang, L.; Wang, S.; Nie, X.; Chen, Y.; He, Y. Total glucosides of paeony: A review of its phytochemistry, role in autoimmune diseases, and mechanisms of action. J. Ethnopharmacol. 2020, 258, 112913. [Google Scholar] [CrossRef] [PubMed]
- Yeung, C.H.C.; Yeung, S.L.A.; Schooling, C.M. Association of autoimmune diseases with Alzheimer’s disease: A mendelian randomization study. J. Psychiatr. Res. 2022, 155, 550–558. [Google Scholar] [CrossRef]
- Kahaly, G.J.; Frommer, L.; Schuppan, D. Celiac disease and endocrine autoimmunity-the genetic link. Autoimmun. Rev. 2018, 17, 1169–1175. [Google Scholar] [CrossRef]
- Craft, J.E. Follicular helper T cells in immunity and systemic autoimmunity. Nat. Rev. Rheumatol. 2012, 8, 337–347. [Google Scholar] [CrossRef]
- Wang, L.; Wang, F.S.; Gershwin, M.E. Human autoimmune diseases: A comprehensive update. J. Intern. Med. 2015, 278, 369–395. [Google Scholar] [CrossRef]
- Huang, M.; Xu, H. Genetic susceptibility to autoimmunity-Current status and challenges. Adv. Immunol. 2022, 156, 25–54. [Google Scholar]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients with Cancer and Preexisting Autoimmune Disease: A Systematic Review. Ann. Intern. Med. 2018, 168, 121–130. [Google Scholar] [CrossRef]
- Grigore, A. Plant Phenolic Compounds as Immunomodulatory Agents; IntechOpen: London, UK, 2017. [Google Scholar]
- Yu, C.; Gershwin, M.E.; Chang, C. Diagnostic criteria for systemic lupus erythematosus: A critical review. J. Autoimmun. 2014, 48, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, J.K.; Klemd, A.M.; Danton, O.; De Mieri, M.; Smieško, M.; Huber, R.; Bürgi, T.; Gründemann, C.; Hamburger, M. Sesquiterpene Lactones from Artemisia argyi: Absolute Configuration and Immunosuppressant Activity. J. Nat. Prod. 2019, 82, 1424–1433. [Google Scholar] [CrossRef]
- Wang, M.; Du, J.X.; Huang, Y.; Dai, Q.; Liu, Y.P.; He, J.; Liu, J.K. Irpex lacteusSesquiterpenoids from Cultures of the Basidiomycetes. J. Nat. Prod. 2020, 83, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Tan, X.; Gu, L.; Liu, J.; Hao, X.; Tao, L.; Zhang, Y. New secondary metabolites with immunosuppressive activity from the phytopathogenic fungus Bipolaris maydis. Bioorganic Chem. 2020, 99, 103816. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, C.; Chen, C.; Wang, N.; Liao, H.; Li, Q.; Zhang, Y. Two Pairs of New Bisabolane-Type Sesquiterpenoids from Aspergillus sydowii. Chem. Biodivers. 2023, 20, e202301047. [Google Scholar] [CrossRef]
- Zhou, M.; Duan, F.; Gao, Y.; Peng, X.; Meng, X.; Ruan, H. Eremophilane sesquiterpenoids from the whole plant of Parasenecio albus with immunosuppressive activity. Bioorganic Chem. 2021, 115, 105247. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Yang, H.X.; He, J.; Yan, B.C.; Feng, T.; Liu, J.K. Steccherins A-D, chamigrane-type sesquiterpenes from the fungus Steccherinum ochraceum with selective inhibition on B lymphocyte proliferation. Phytochemistry 2023, 214, 113830. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Wang, X.; Jin, B.; Yang, Y.; Chen, K.; Jia, Q.; Li, Y. Sesquiterpenes with immunosuppressive effect from the stems of Solanum torvum. Phytochem. Lett. 2016, 17, 126–130. [Google Scholar] [CrossRef]
- Dai, Q.; Zhang, F.L.; Li, Z.H.; He, J.; Feng, T. Immunosuppressive Sesquiterpenoids from the Edible Mushroom Craterellus odoratus. J. Fungi 2021, 7, 1052. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.X.; Yang, M.; Li, H.; Li, S.W.; Yao, L.G.; Li, G.; Tang, W.; Wang, C.H.; Liang, L.F.; Guo, Y.W. Polycyclic furanobutenolide-derived norditerpenoids from the South China Sea soft corals Sinularia scabra and Sinularia polydactyla with immunosuppressive activity. Bioorganic Chem. 2020, 94, 103350. [Google Scholar] [CrossRef]
- Junfen, Z.; Yongbo, X.; Penghua, S.; Huiqin, Q.; Rongjian, S.; Ming, X.; Yonghui, Z. Diterpenoids with Immunosuppressive Activities from Cinnamomum cassia. J. Nat. Prod. 2014, 77, 1948–1954. [Google Scholar]
- Ghanadian, S.M.; Ayatollahi, A.M.; Mesaik, M.A.; Abdalla, O.M. New immunosuppressive cyclomyrsinol diterpenes from Euphorbia kopetdaghi Prokh. Nat. Prod. Res. 2013, 27, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Li, J.; Zhao, Z.Z.; Li, X.; Liu, S.L.; Wang, Q.Y.; Liu, J.K. Diterpenes with bicyclo[2.2.2]octane moieties from the fungicolous fungus Xylaria longipes HFG1018. Org. Biomol. Chem. 2020, 18, 2410–2415. [Google Scholar] [CrossRef]
- Gobu, F.R.; Chen, J.J.; Zeng, J.; Wei, W.J.; Wang, W.F.; Lin, C.J.; Gao, K. Isolation, Structure Elucidition, and Immunosuppressive Activity of Diterpenoids from Ligularia fischeri. J. Nat. Prod. 2017, 80, 2263–2268. [Google Scholar] [CrossRef]
- Ren, Y.; Tong, X.; Zhao, Y.; He, S.J.; Fan, Y.Y.; Yue, J.M. Dolabrane-Type Diterpenoids with Immunosuppressive Activity from Koilodepas hainanense. J. Nat. Prod. 2022, 85, 1581–1590. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhaso, Z.Z.; Cheng, G.G.; Zhao, K.; Han, K.Y.; Zhou, L.; Liu, J.K. Immunosuppressive Nor-isopimarane Diterpenes from Cultures of the Fungicolous Fungus HFG1018. J. Nat. Prod. 2020, 83, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Lv, X.; Zhang, X.; Wei, P.P.; Li, Z.H.; Ai, H.L.; Liu, J.K. Immunosuppressive Isopimarane Diterpenes from Cultures of the Endophytic Fungus. Front. Pharmacol. 2021, 12, 766441. [Google Scholar] [CrossRef] [PubMed]
- Li, X.R.; Hu, K.; Yan, B.C.; Li, X.N.; Sun, H.D.; Liu, Y.; Puno, P.T. Scopariusicides D-M, ent-clerodane-based isomeric meroditerpenoids with a cyclobutane-fused γ/δ-lactone core from Isodon scoparius. Bioorganic Chem. 2022, 127, 105973. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, H.B.; Wang, W.G.; Gong, N.B.; Zhan, R.; Li, X.N.; Sun, H.D. Scopariusic acid, a new meroditerpenoid with a unique cyclobutane ring isolated from Isodon scoparius. Org. Lett. 2013, 15, 4446–4449. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.P.; Xu, J.B.; Zhao, P.; Zhou, Y.; Zuo, J.P.; Zhao, J.X.; Yue, J.M. Highly Oxygenated Cephalotane-Type Diterpenoids from Cephalotaxus fortunei var. alpina and C. sinensis. ChemRxiv 2023. [Google Scholar]
- Yang, M.; Li, H.; Zhang, Q.; Wu, Q.H.; Li, G.; Chen, K.X.; Li, X.W. Highly diverse cembranoids from the South China Sea soft coral Sinularia scabra as a new class of potential immunosuppressive agents. Bioorg. Med. Chem. 2019, 27, 3469–3476. [Google Scholar] [CrossRef]
- Yang, M.; Li, X.L.; Wang, J.R.; Lei, X.; Tang, W.; Li, X.W.; Guo, Y.W. Sarcomililate A, an Unusual Diterpenoid with Tricyclo [11.3.0.0] hexadecane Carbon Skeleton, and Its Potential Biogenetic Precursors from the Hainan Soft Coral Sarcophyton mililatensis. J. Org. Chem. 2019, 84, 2568–2576. [Google Scholar] [CrossRef]
- Gu, W.; Huang, M.; Zhang, Y. A new limonoid with immunosuppressive activity from Munronia pinnata. Phytochem. Lett. 2023, 57, 106–109. [Google Scholar] [CrossRef]
- Yan, S.; Feng, H.; Sun, L.; Shi, Z.; Hu, H.; Duan, Y.; Zhang, Y. Discovery of immunosuppressive Lupane-type Triterpenoids from Hypericum longistylum. Nat. Prod. Res. 2022, 36, 4394–4400. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, L.; Tang, Z.; Chen, M.; Jia, J.; Wang, A. Research progress on the triterpenoids from the genus Schisandra and their biological activities. Asian J. Tradit. Med. 2022, 17, 116–136. [Google Scholar]
- Zhao, J.Y.; Mu, L.H.; Dong, X.Z.; Hu, Y.; Liu, P. One new cycloartane triterpene glycoside from Beesia calthaefolia. Nat. Prod. Res. 2016, 30, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Cho, K.W.; Hong, S.S.; Hwang, B.Y.; Chun, T.; Lee, D. Antiproliferative glabretal-type triterpenoids from the root bark of Dictamnus dasycarpus. Bioorg. Med. Chem. Lett. 2015, 25, 621–625. [Google Scholar] [CrossRef]
- Song, J.; Zhou, M.; Zhou, J.; Liang, J.J.; Peng, X.G.; Liu, J.; Ruan, H.L. Schincalactones A and B, Two 5/5/6/11/3 Fused Schinortriterpenoids with a 13-Membered Carbon Ring System from Schisandra incarnata. Org. Lett. 2018, 20, 2499–2502. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Zhang, H.; Zhou, Y.; Liu, H.B.; Tang, W.; Zhou, B.; Yue, J.M. Phainanoids A–F, a new class of potent immunosuppressive triterpenoids with an unprecedented carbon skeleton from Phyllanthus hainanensis. J. Am. Chem. Soc. 2015, 137, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.L.; Gan, Y.T.; Peng, X.G.; Ouyang, Q.X.; Pei, J.; Ruan, H.L. Peniandranoids A–E: Meroterpenoids with Antiviral and Immunosuppressive Activity from a Penicillium sp. J. Nat. Prod. 2023, 86, 66–75. [Google Scholar] [CrossRef]
- Guo, K.; Liu, Y.C.; Liu, Y.; Zhang, H.; Li, W.Y.; Shi, Q.M.; Li, S.H. Immunosuppressive gentianellane-type sesterterpenoids from the traditional Uighur medicine Gentianella turkestanorum. Phytochemistry 2021, 187, 112780. [Google Scholar] [CrossRef]
- Jing, S.; Fu, R.; Li, C.; Zhou, T.; Liu, Y.; Liu, Y.; Luo, S.; Li, X.; Zeng, F.; Li, S. Immunosuppresive Sesterterpenoids and Norsesterterpenoids from Colquhounia coccinea var. mollis. J. Org. Chem. 2021, 86, 11169–11176. [Google Scholar] [CrossRef]
- Szabó, T.; Volk, B.; Milen, M. Recent Advances in the Synthesis of β-Carboline Alkaloids. Molecules 2021, 26, 663. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Zhang, Z.; Chen, M.; Wu, X.; Ma, S. Immunosuppressive Sesquiterpene Pyridine Alkaloids from Tripterygium wilfordii Hook. f. Molecules 2022, 27, 7274. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhu, Z.; Hao, T.T.; Fang, Q.Q.; Jiang, K.; Qu, S.J.; Tan, C.H. Alopecines A–E, five chloro-containing matrine-type alkaloids with immunosuppressive activities from the seeds of Sophora alopecuroides. Bioorganic Chem. 2020, 99, 103812. [Google Scholar] [CrossRef]
- Wei, P.; Ji, J.; Ma, X.; Li, Z.; Ai, H.; Lei, X.; Liu, J. Three new pyrrole alkaloids from the endophytic fungus Albifimbria viridis. Nat. Prod. Bioprospecting 2022, 12, 4–5. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, X.; Ma, J.; Yang, Y.; Zhou, J.; Xu, J. Secondary metabolites produced by mangrove endophytic fungus Aspergillus fumigatus HQD24 with immunosuppressive activity. Biochem. Syst. Ecol. 2020, 93, 104166. [Google Scholar] [CrossRef]
- Zeng, T.; Wu, X.Y.; Yang, S.X.; Lai, W.C.; Shi, S.D.; Zou, Q.; Li, L.M. Monoterpenoid Indole Alkaloids from Kopsia officinalis and the Immunosuppressive Activity of Rhazinilam. J. Nat. Prod. 2017, 80, 864–871. [Google Scholar] [CrossRef]
- Guo, K.; Liu, X.; Zhou, T.; Liu, Y.; Liu, Y.; Shi, Q.; Li, X.; Li, S. Gentianelloids A and B: Immunosuppressive 10,11-seco-gentianellane sesterterpenoids from the traditional Uighur medicine gentianella turkestanorum. J. Org. Chem. 2020, 85, 5511–5515. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Mu, R.; Liu, Y.; Xiao, C.; Li, D.; Gao, J.; Guo, K.; Li, X.; Liu, Y.; Zeng, F.; et al. Immunosuppressive and adipogenesis inhibitory sesterterpenoids with a macrocyclic ether system from Eurysolen gracilis. Org. Lett. 2021, 23, 2232–2237. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, H.Y.; Xu, H.Z.; Liu, J.J.; Meng, X.G.; Zhou, M.; Ruan, H.L. Alkaloids with Immunosuppressive Activity from the Bark of Pausinystalia yohimbe. J. Nat. Prod. 2018, 81, 1841–1849. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, H.X.; Li, Z.H.; Feng, T.; Liu, J.K. Indole alkaloids from Ophiorrhiza cantoniensis with immunosuppressive activity. Fitoterapia 2021, 148, 104777. [Google Scholar]
- Shi, B.B.; Ai, H.L.; Duan, K.T.; Feng, T.; Liu, J.K. Ophiorrhines F and G, key biogenetic intermediates of ophiorrhine alkaloids from Ophiorrhiza japonica and their immunosuppressant activities. J. Nat. Prod. 2022, 85, 453–457. [Google Scholar] [CrossRef]
- Im, S.H.; Wang, Z.; Lim, S.S.; Lee, O.H.; Kang, I.J. Bioactivity-Guided Isolation and Identification of New and Immunosuppressive Monoterpenoid Indole Alkaloids from Tsiang. Molecules 2019, 24, 4574. [Google Scholar]
- Simsek, M.; Quezada-Calvillo, R.; Nichols, B.L.; Hamaker, B.R. Phenolic compounds increase the transcription of mouse intestinal maltase-glucoamylase and sucrase-isomaltase. Food Funct. 2017, 8, 1915–1924. [Google Scholar] [CrossRef]
- Zeng, J.; Xue, Y.; Lai, Y.; Yao, G.; Luo, Z.; Zhang, Y.; Zhang, J. A new phenolic glycoside from the barks of Cinnamomum cassia. Molecules 2014, 19, 17727–17734. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.; Hu, X.L.; Huang, X.J.; Ma, M.X.; Feng, J.H.; Li, J.Y.; Wang, H. Phloroglucinols with Immunosuppressive Activities from the Fruits of Eucalyptus globulus. J. Nat. Prod. 2019, 82, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhao, X.; Song, F.; Hou, Z.; Hao, X.; Guo, J.; Sun, L.; Feng, H.; Wu, M.; Xie, P.; et al. Prenyllongnols A–D, New Prenylated Acylphloroglucinols that Fight Concanavalin A-Induced Autoimmune Hepatitis. J. Agric. Food Chem. 2023, 71, 17801–17809. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Tan, X.; Qiao, Y.; Sun, W.; Xie, S.; Shi, Z.; Lu, Y.; Chen, G.; Qi, C.; Zhang, Y. New secondary metabolites from the endophytic fungus Aspergillus sp. from Tripterygium wilfordii. Nat. Prod. Res. 2022, 36, 3544–3552. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Li, Y.; Liu, B.; Zhan, R. Bibenzyls and lignans from Dendrobium devonianum and their immunosuppressive activities. Phytochem. Lett. 2023, 54, 137–140. [Google Scholar] [CrossRef]
- Koko, W.S.; Mesaik, M.A.; Ranjitt, R.; Galal, M.; Choudhary, M.I. Immunosuppressive phenolic compounds from Hydnora abyssinica A. Braun. BMC Complement. Altern. Med. 2015, 15, 400. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, X.; Shen, L.; Mo, S.; Liu, J.; Wang, J.; Zhang, Y. A new abietane-type diterpenoid and a new long-chain alkenone from fungus sp. TJ403-LS1. Nat. Prod. Res. 2022, 36, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Bu, P.; Xie, S.; Guo, Y.; Shi, Z.; Qi, C.; Zhang, Y. (±)-hyperzewalsins A–D, four pairs of nor-monocyclic polyprenylated acylphloroglucinols with immunosuppressive activity from hypericum przewalskii maxim. Phytochemistry 2021, 187, 112779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, B.; Li, F.; Liu, M.; Lin, S.; Wang, J.; Xue, Y.; Zhu, H.; Sun, W.; Hu, Z.; et al. Mycophenolic Acid Derivatives with Immunosuppressive Activity from the Coral-Derived Fungus Penicillium bialowiezense. Mar. Drugs 2018, 16, 230. [Google Scholar] [CrossRef]
- Guo, Y.; Cao, Y.; Qi, C.; Tong, Q.; Chen, C.; Yang, J.; Zhu, H.; Zhang, Y. Polycyclic polyprenylated acylphloroglucinols with immunosuppressive activity from Hypericum perforatum and absolute configurations assignment of previously reported analogues. Bioorganic Chem. 2021, 114, 105144. [Google Scholar] [CrossRef]
- Duan, Y.; Xie, S.; Guo, Y.; Qiao, Y.; Shi, Z.; Tao, L.; Deng, M.; Cao, Y.; Xue, Y.; Qi, C.; et al. Przewalcyrones A–F, epoxychromene-containing polycyclic polyprenylated acylphloroglucinols with immunosuppressive activity from Hypericum przewalskii Maxim. Org. Biomol. Chem. 2019, 17, 8234–8242. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Li, Y.; Shi, Z.; Duan, Y.; Guo, Y.; Huang, X.; Li, J.; Zhang, Y.; Chen, M.; Song, F.; et al. Discovery of bioactive polycyclic polyprenylated acylphloroglucinols with adamantine/homoadamantane skeletons from Hypericum wilsonii. Phytochemistry 2024, 218, 113953. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Jang, H.; Le, T.P.L.; Hong, H.R.; Lee, M.K.; Hong, J.T.; Hwang, B.Y. Pyranoflavanones and Pyranochalcones from the Fruits of Amomum tsaoko. J. Nat. Prod. 2019, 82, 1886–1892. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xuan, B.; Shou, Q.; Shen, Z. New flavonoids from Campylotropis hirtella with immunosuppressive activity. Fitoterapia 2014, 95, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Tang, Y.; Sang, Z.; Dong, J.; Wei, R. Structurally diverse biflavonoids from the fruits of Citrus medica L. var. sarcodactylis Swingle and their hypolipidemic and immunosuppressive activities. Bioorganic Chem. 2021, 117, 105450. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Wei, R.; Shang, D.; Sang, Z.; Dong, J. Structurally diverse flavonolignans with immunosuppressive and neuroprotective activities from the fruits of Hippophae rhamnoides L. J. Agric. Food Chem. 2020, 68, 6564–6575. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, X.; Liao, H.; Liu, L.; Li, Q.; Sun, W.; Chen, C.; Zhu, H.; Zhang, Y. Quadristerols A–G: Seven undescribed ergosterols from Aspergillus quadrilineata. Phytochemistry 2023, 213, 113785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ding, M.; Tao, L.; Zhang, M.; Xu, X.; Zhang, C. Immunosuppressive C21 steroidal glycosides from the root of Cynanchum atratum. Fitoterapia 2015, 105, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Zhou, C.; Liao, H.; Li, Q.; Bao, A.; Chen, C.; He, F.; Wu, P.; Sun, W.; Zhu, H.; et al. Enantiomeric α-pyrone derivatives with immunosuppressive activity from Talaromyces adpressus. Phytochemistry 2024, 218, 113931. [Google Scholar] [CrossRef]
- Wan, Z.; Yao, Y.C.; Gao, F.; Cai, S.B.; Khan, A.; Zhao, T.R.; Cheng, G.G. A new immunosuppressive pregnane glycoside from aqueous fraction of Epigynum cochinchinensis. Nat. Prod. Res. 2017, 31, 2893–2899. [Google Scholar] [CrossRef]
- Song, X.; Zhang, X.; Han, Q.; Li, X.; Li, G.; Li, R.; Jiao, Y.; Zhou, J.; Lou, H. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliata S. Lac and their immunosuppressive activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Wei, C.; Sun, C.; Feng, Z.; Zhang, X.; Xu, J. Four New Chromones from the Endophytic Fungus Phomopsis asparagi DHS-48 Isolated from the Chinese Mangrove Plant Rhizophora mangle. Mar. Drugs 2021, 19, 348. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, S.; Liu, W.; Liu, Y.; Huang, X.; She, Z. Polyketides with Immunosuppressive Activities from Mangrove Endophytic Fungus Penicillium sp. ZJ-SY2. Mar. Drugs 2016, 14, 217. [Google Scholar] [CrossRef]
- Deng, M.; Chen, X.; Qiao, Y.; Shi, Z.; Wang, J.; Zhu, H.; Gu, L.; Qi, C.; Zhang, Y. Isolation, absolute configurations and bioactivities of pestaphilones A–I: Undescribed methylated side chain containing-azaphilones from Pestalotiopsis oxyanthi. Phytochemistry 2022, 194, 113045. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Duan, F.; Liu, L.; Peng, X.; Meng, X.; Ruan, H. Hypothemycin-Type Resorcylic Acid Lactones with Immunosuppressive Activities from a Podospora sp. J. Nat. Prod. 2021, 84, 483–494. [Google Scholar] [CrossRef]
- Wang, W.; Cheng, G.; Li, Z.; Ai, H.; He, J.; Li, J.; Feng, T.; Liu, J. Curtachalasins, immunosuppressive agents from the endophytic fungus Xylaria cf. curta. Org. Biomol. Chem. 2019, 17, 7985–7994. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Q.; Xue, J.; Zhou, Y.; Yu, H.; Yang, S.; Zhang, B.; Zuo, J.; Li, Y.; Yue, J. Ivorenolide B, an immunosuppressive 17-membered macrolide from khaya ivorensis: Structural Determination and Total Synthesis. Org. Lett. 2014, 16, 2062–2065. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, X.; Huang, F.; Li, G.; Leadlay, P.F. Efophylins A and B, Two C2-Asymmetric Macrodiolide Immunosuppressants from Streptomyces malaysiensis. J. Nat. Prod. 2021, 84, 1579–1586. [Google Scholar] [CrossRef]
- Kanako, S.; Junnosuke, M.; Manabu, K.; Yasushi, K.; Hisashi, Y.; Yasuko, U. Genetic differences between type 1 diabetes with and without other autoimmune diseases. Diabetes/Metab. Res. Rev. 2018, 34, e3023. [Google Scholar]
- Bossini-Castillo, L.; López-Isac, E.; Martín, J. Immunogenetics of systemic sclerosis: Defining heritability, functional variants and shared-autoimmunity pathways. J. Autoimmun. 2015, 64, 53–65. [Google Scholar] [CrossRef]
- Zhao, C.N.; Xu, Z.; Wu, G.C.; Mao, Y.M.; Liu, L.N.; Dan, Y.L.; Pan, H.F. Emerging role of air pollution in autoimmune diseases. Autoimmun. Rev. 2019, 18, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Lavery, A.M.; Collins, B.N.; Waldman, A.T.; Hart, C.N.; Bar-Or, A.; Marrie, R.A.; Banwell, B. The contribution of secondhand tobacco smoke exposure to pediatric multiple sclerosis risk. Mult. Scler. 2019, 25, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Kahaly, G.J.; Frommer, L. Autoimmune polyglandular diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101344. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.R.; Liberal, R.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Regulatory T-cells in autoimmune diseases: Challenges, controversies and--yet--unanswered questions. Autoimmun. Rev. 2015, 14, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ventura, S.P.; e Silva, F.A.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Cools, K.; Terry, L.A. Comparative study between extraction techniques and column separation for the quantification of sinigrin and total isothiocyanates in mustard seed. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 901, 115–118. [Google Scholar] [CrossRef]
- Ohno, H.; Miyoshi, S.; Araho, D.; Kanamoto, T.; Terakubo, S.; Nakashima, H.; Tsuda, T.; Sunaga, K.; Amano, S.; Ohkoshi, E.; et al. Efficient utilization of licorice root by alkaline extraction. In Vivo 2014, 28, 785–794. [Google Scholar]
- Fibigr, J.; Šatínský, D.; Solich, P. A UHPLC method for the rapid separation and quantification of anthocyanins in acai berry and dry blueberry extracts. J. Pharm. Biomed. Anal. 2017, 143, 204–213. [Google Scholar] [CrossRef]
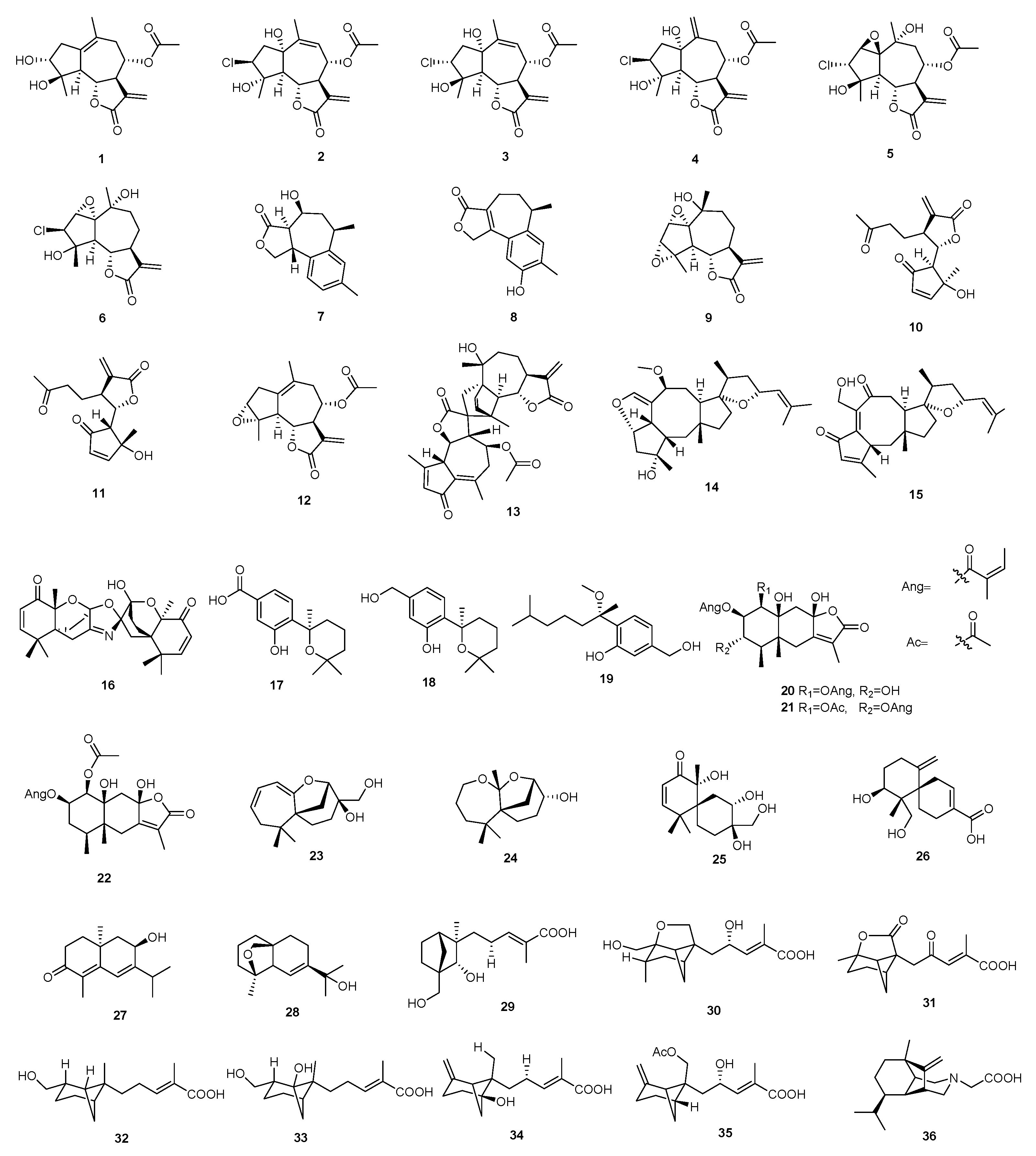




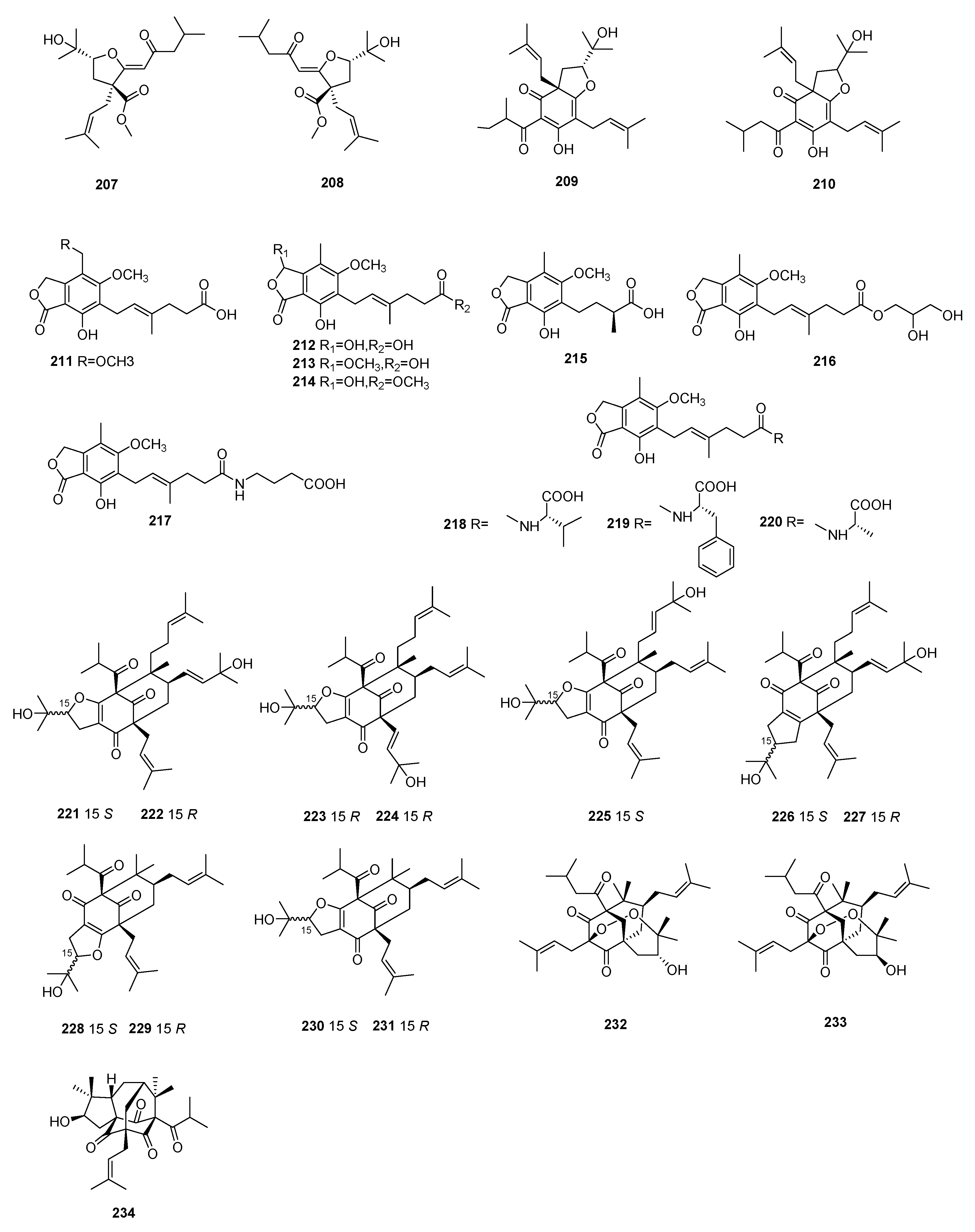


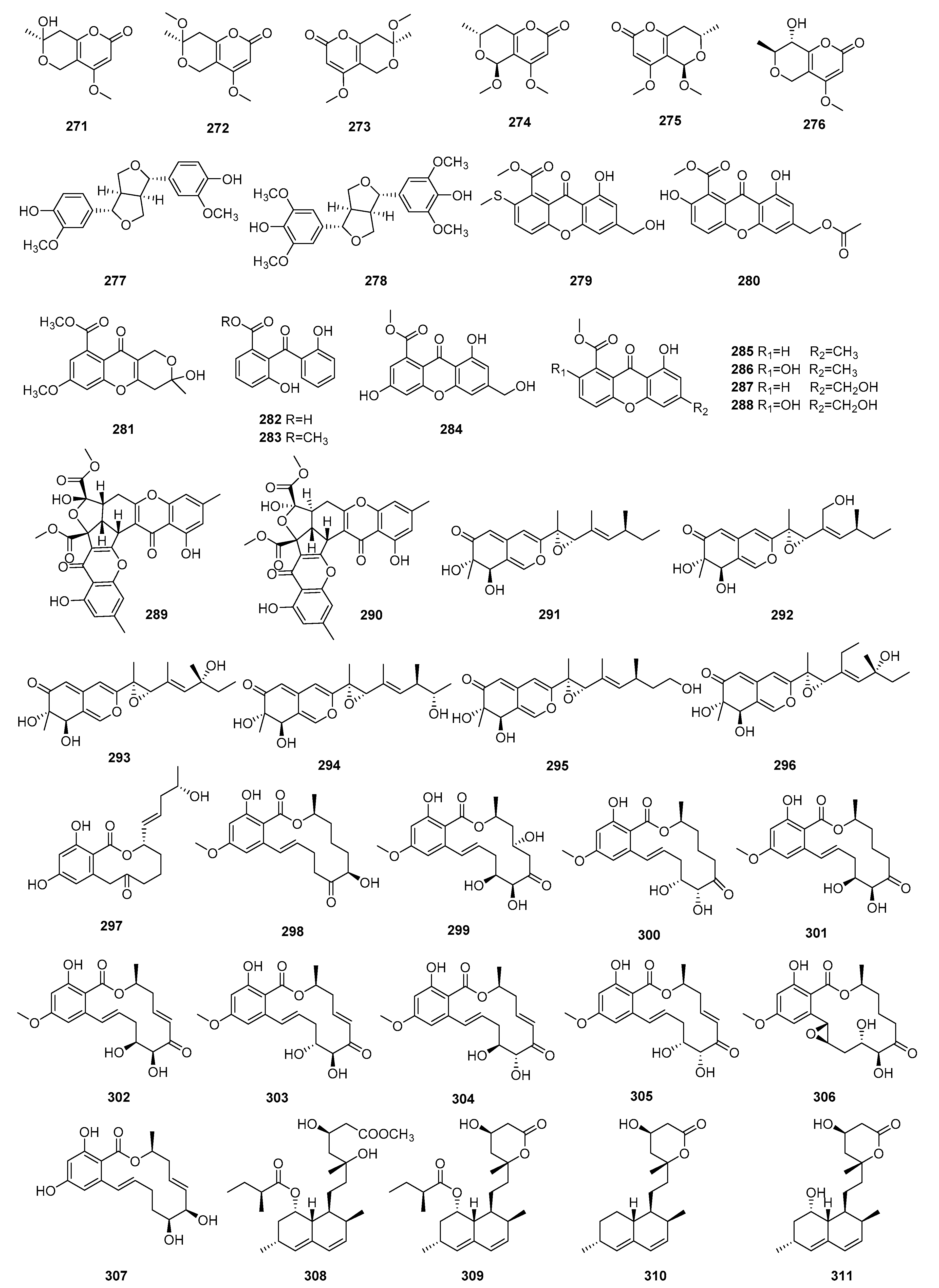
| Number | Model | Inducing Drugs |
|---|---|---|
| 1 | human peripheral blood T-lymphocytes | ConA |
| 2 | human mononuc-lear cell + stimulated RAW264.7 macrophage cell | ConA |
| 3 | isolated peritoneal macrophages | LPS |
| 4 | splenocytes | ConA/LPS |
| 5 | RAW264.7 macrophage cell | ConA |
| 6 | stimulated spleen cells | anti-CD3/anti-CD28 |
| 7 | T-cell proliferation | phytohaemagglutinin |
| 8 | BV-2 microglia | LPS |
| Number | Model Making Drug | Animal | Mode of Administration |
|---|---|---|---|
| 1 | Mitogen and OVA | ICR mice | immunized subcutaneously in one hind limb of OVA and in saline soln |
| 2 | Cyclophosphamide | C57BL/6 mice | intraperitoneal injection |
| 3 | CCl4 | Wistar rats | intraperitoneal injection |
| 4 | Xylene | Balb/c mice | injected xylene into the left ear of each mouse |
| 5 | DTH | Balb/c mice | smearing DNCB on the shaved abdominal skin of the mice |
| 6 | SRBC | Swiss albino mice | the mice were primed with SRBC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, F.; Shen, Q.; Yang, Z.; Yang, W.; Lu, Z.; Zheng, J.; Zhang, L.; Li, H. Research Progress of Natural Active Substances with Immunosuppressive Activity. Molecules 2024, 29, 2359. https://doi.org/10.3390/molecules29102359
Shao F, Shen Q, Yang Z, Yang W, Lu Z, Zheng J, Zhang L, Li H. Research Progress of Natural Active Substances with Immunosuppressive Activity. Molecules. 2024; 29(10):2359. https://doi.org/10.3390/molecules29102359
Chicago/Turabian StyleShao, Fei, Qiying Shen, Zhengfei Yang, Wenqian Yang, Zixiang Lu, Jie Zheng, Liming Zhang, and Hangying Li. 2024. "Research Progress of Natural Active Substances with Immunosuppressive Activity" Molecules 29, no. 10: 2359. https://doi.org/10.3390/molecules29102359
APA StyleShao, F., Shen, Q., Yang, Z., Yang, W., Lu, Z., Zheng, J., Zhang, L., & Li, H. (2024). Research Progress of Natural Active Substances with Immunosuppressive Activity. Molecules, 29(10), 2359. https://doi.org/10.3390/molecules29102359






