Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives
Abstract
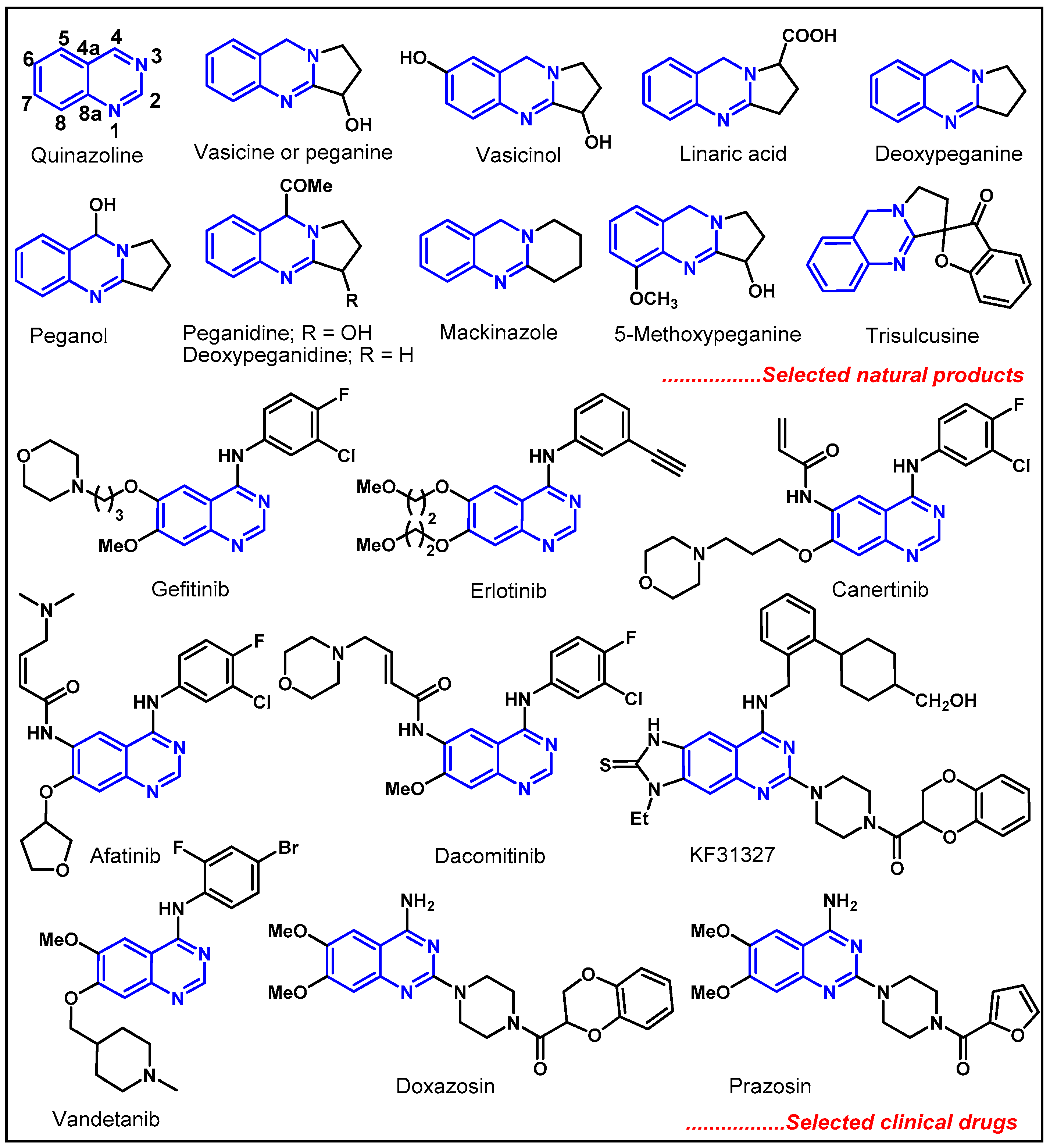
1. Introduction
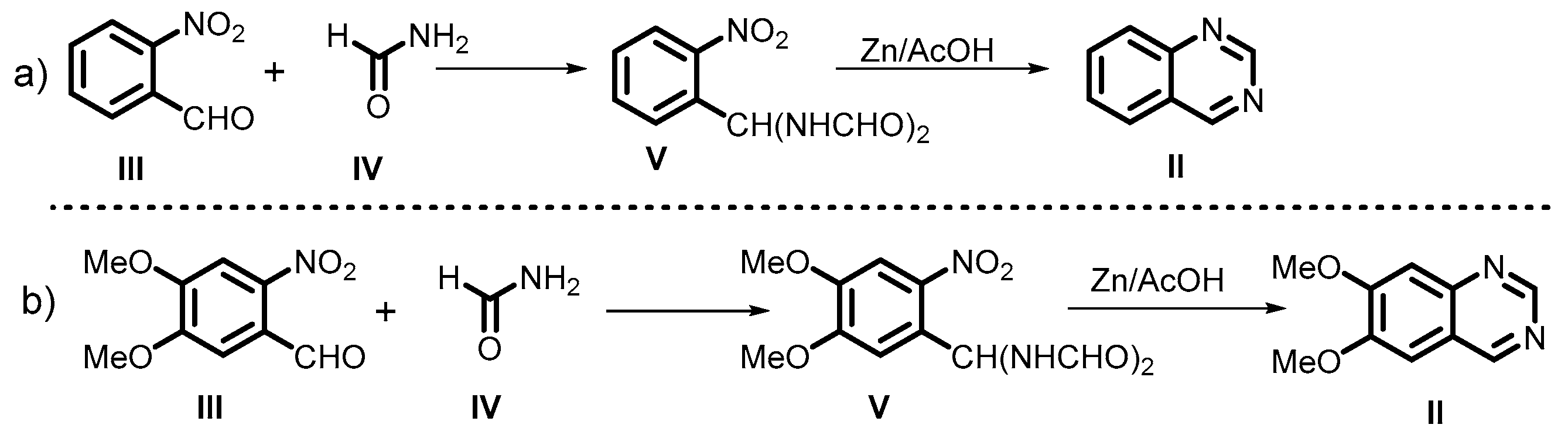
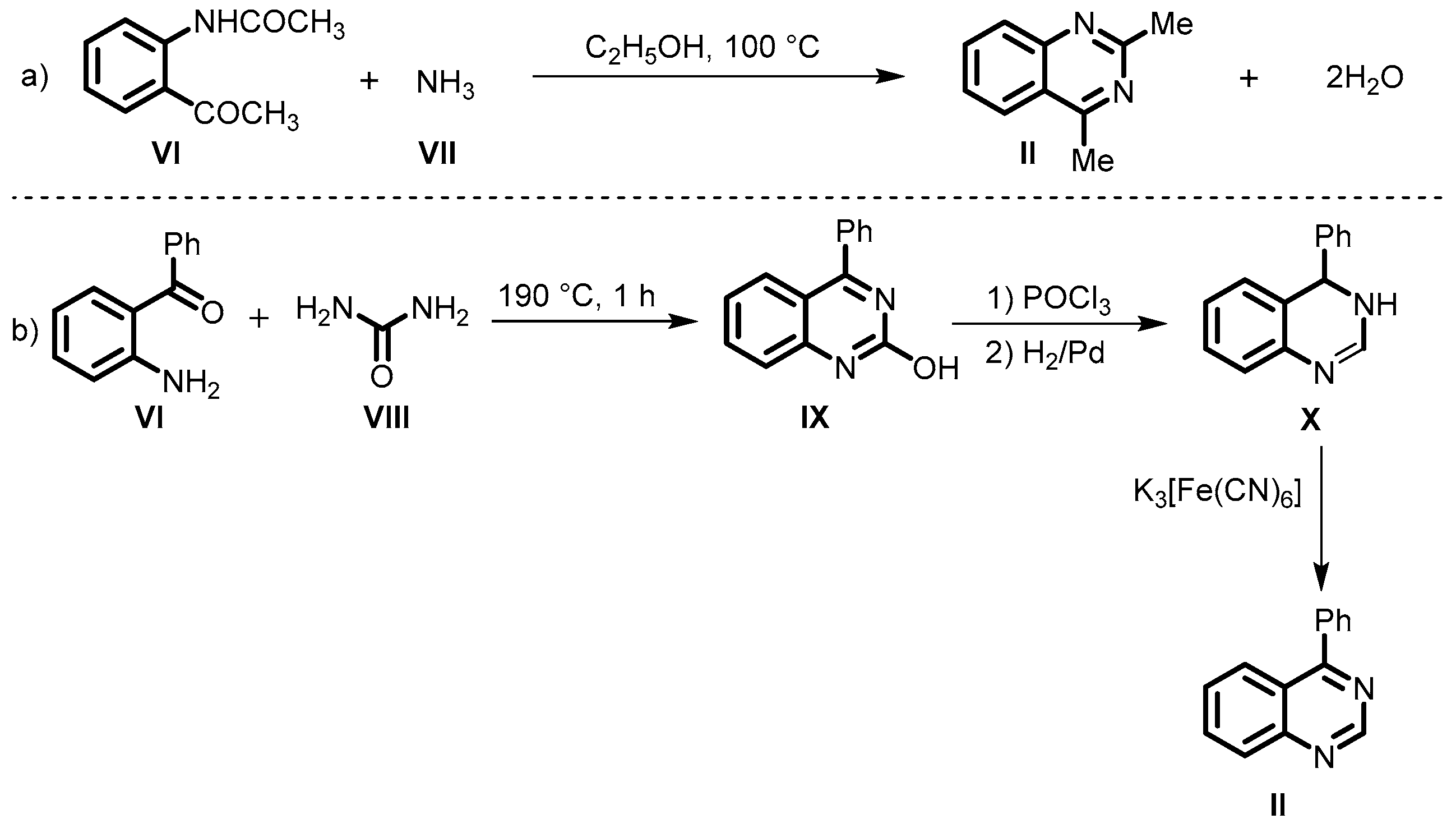
2. Classical Method
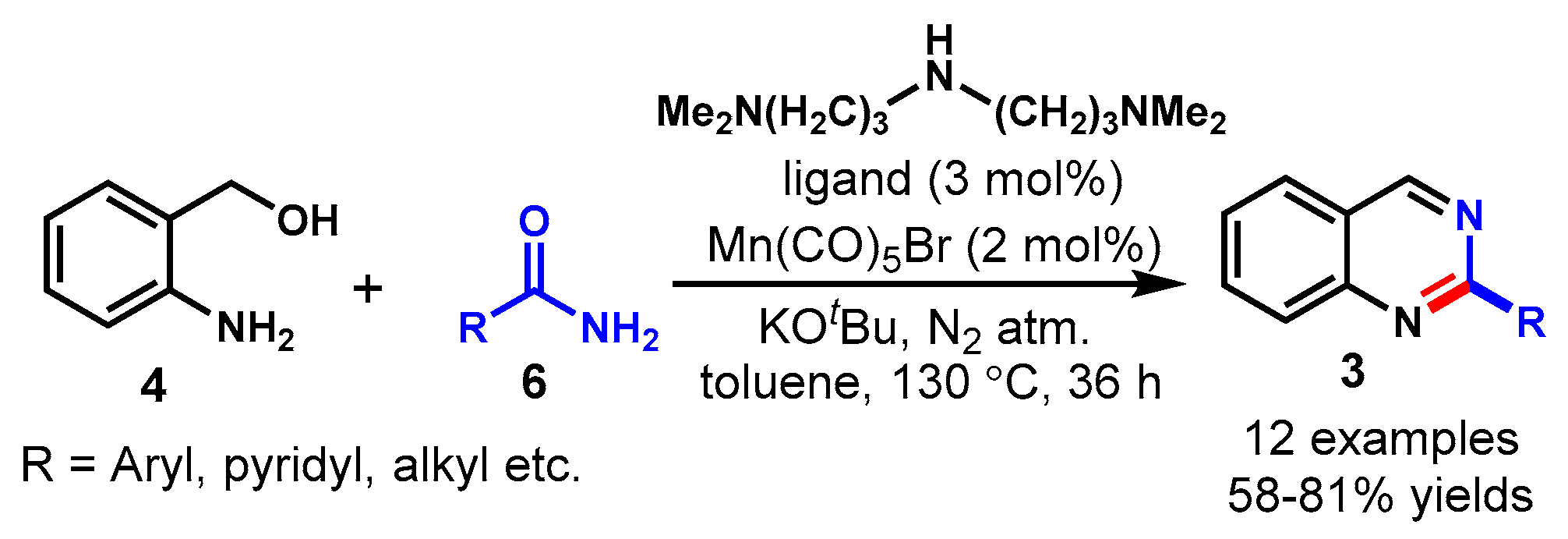
3. Manganese-Catalyzed Protocols
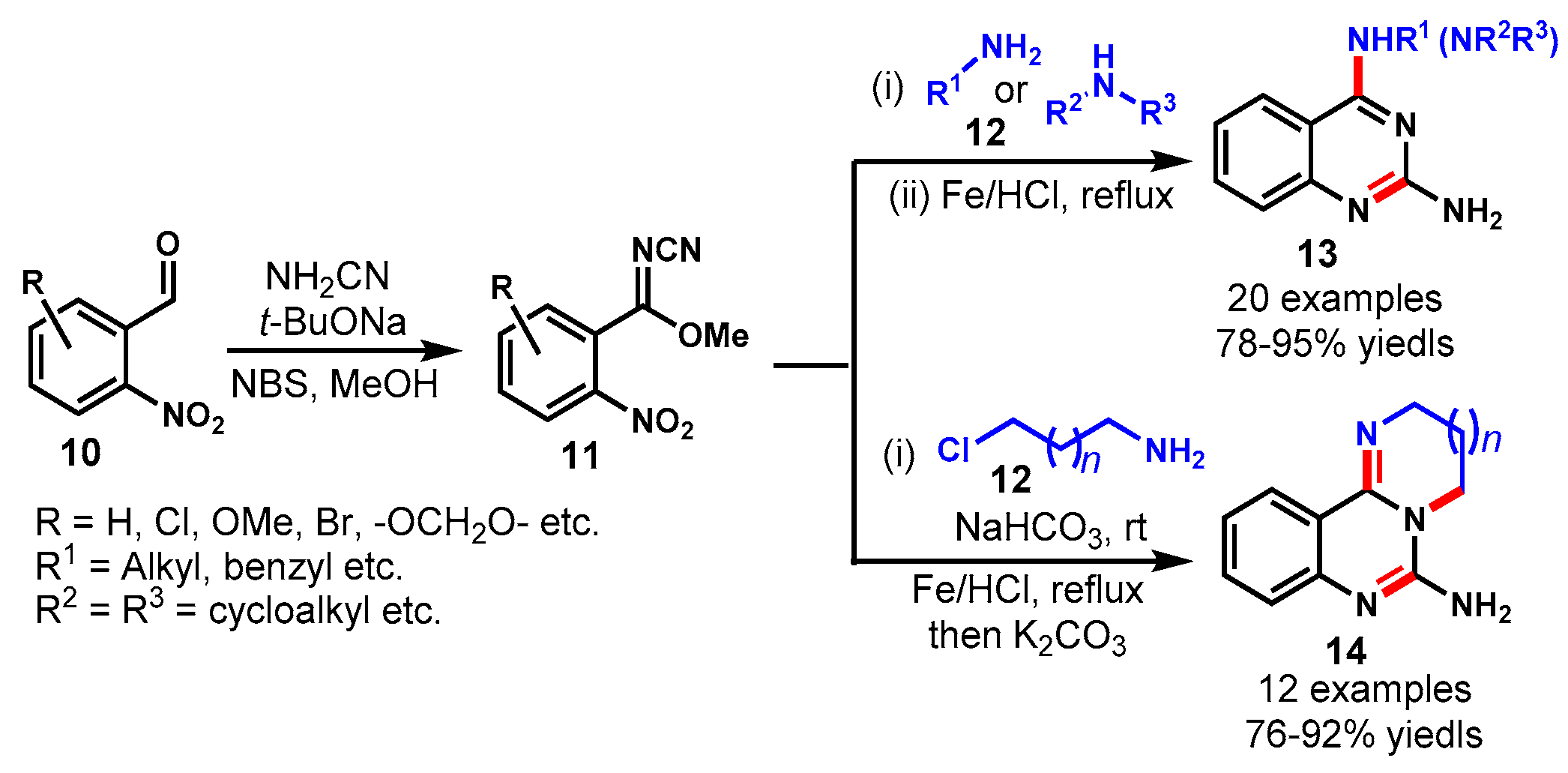
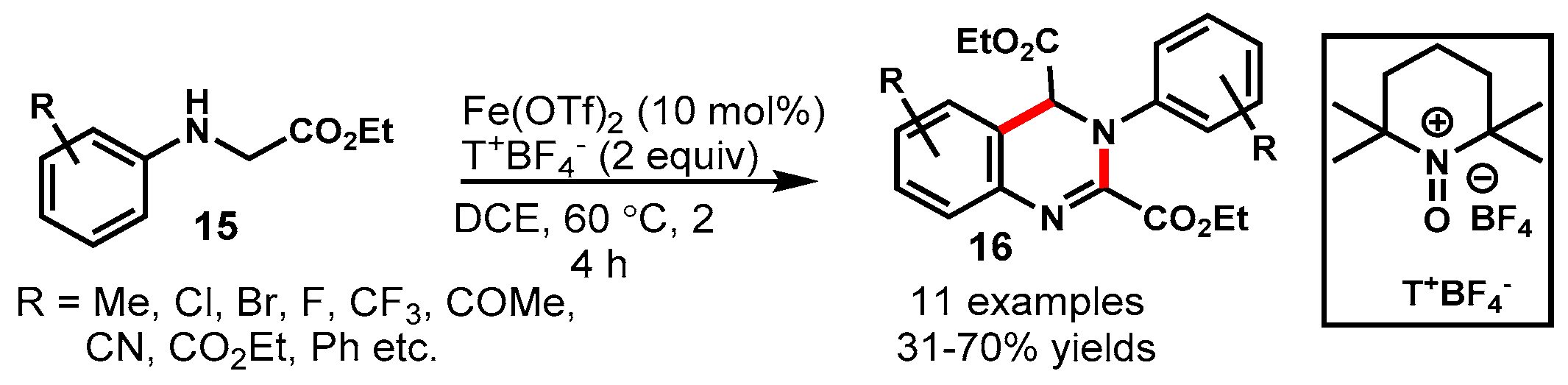
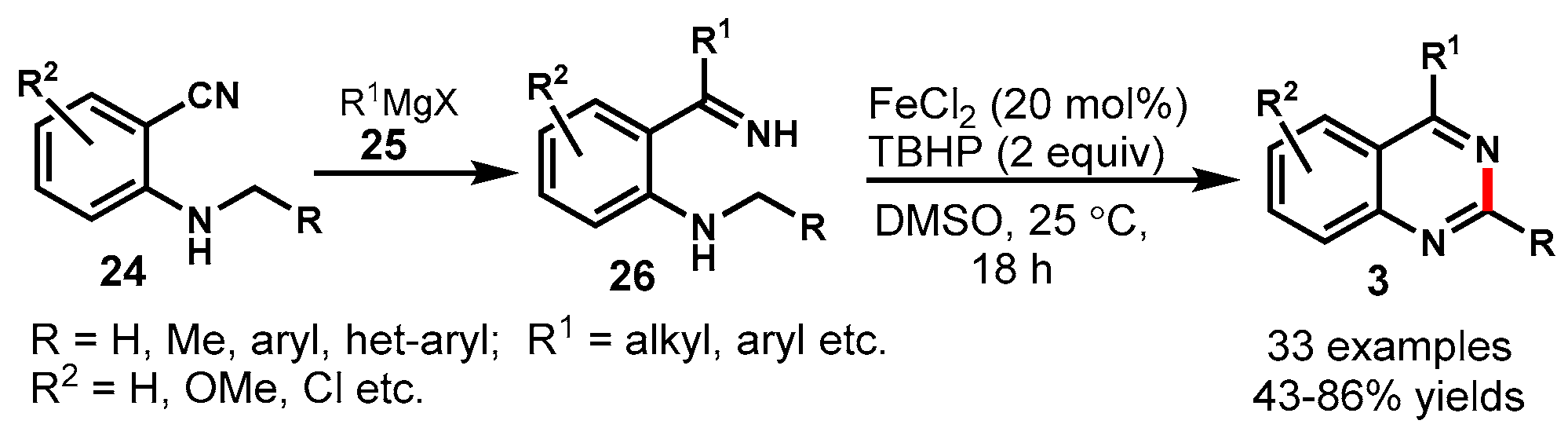
4. Iron-Catalyzed Protocols
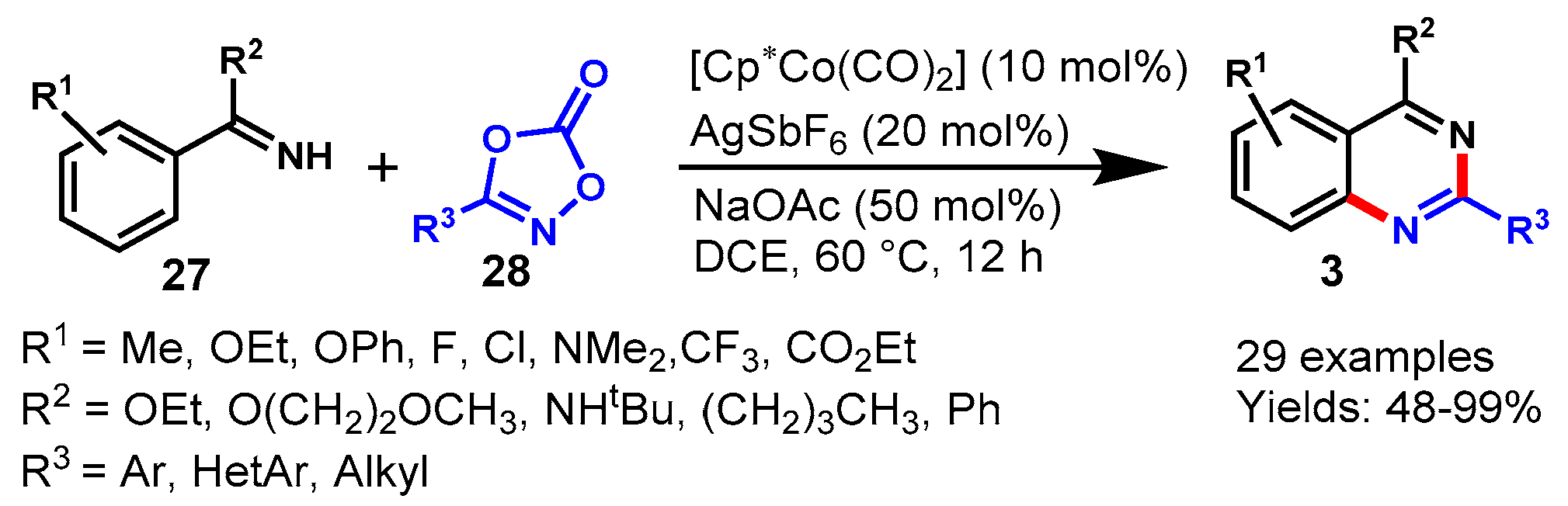
5. Cobalt-Catalyzed Protocols
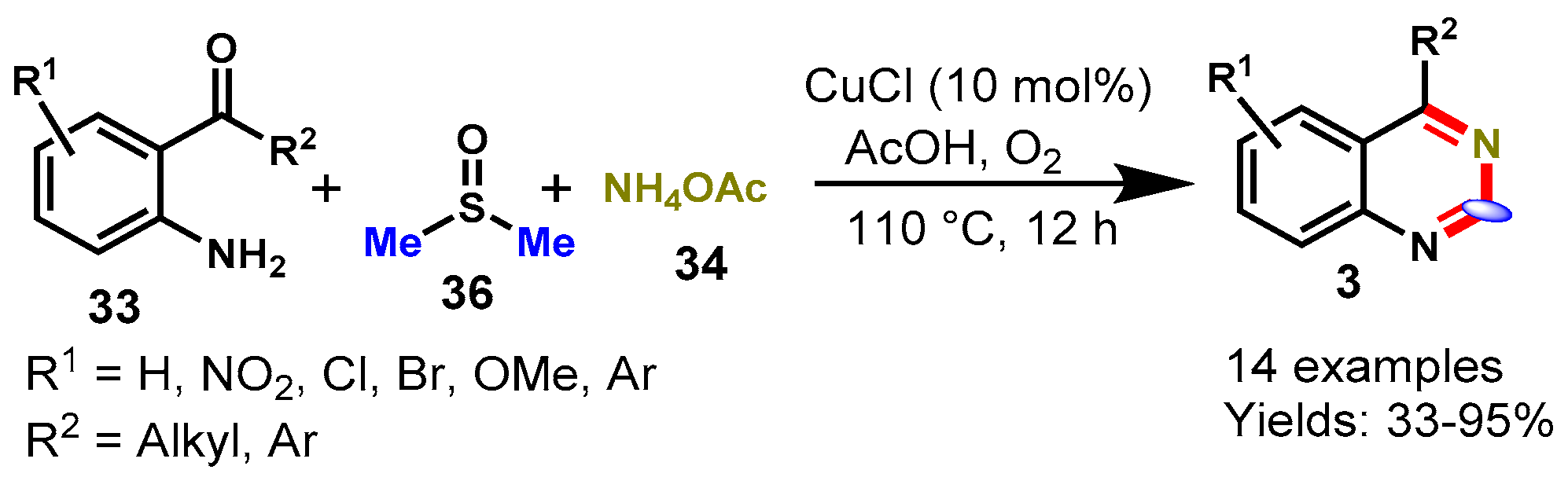
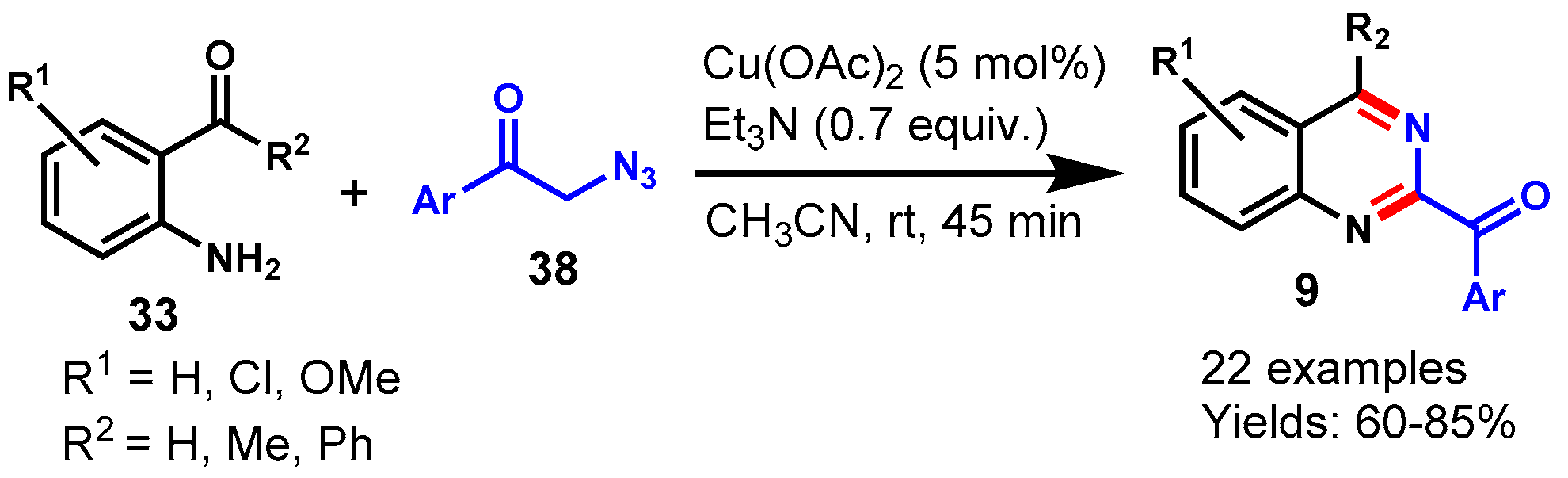
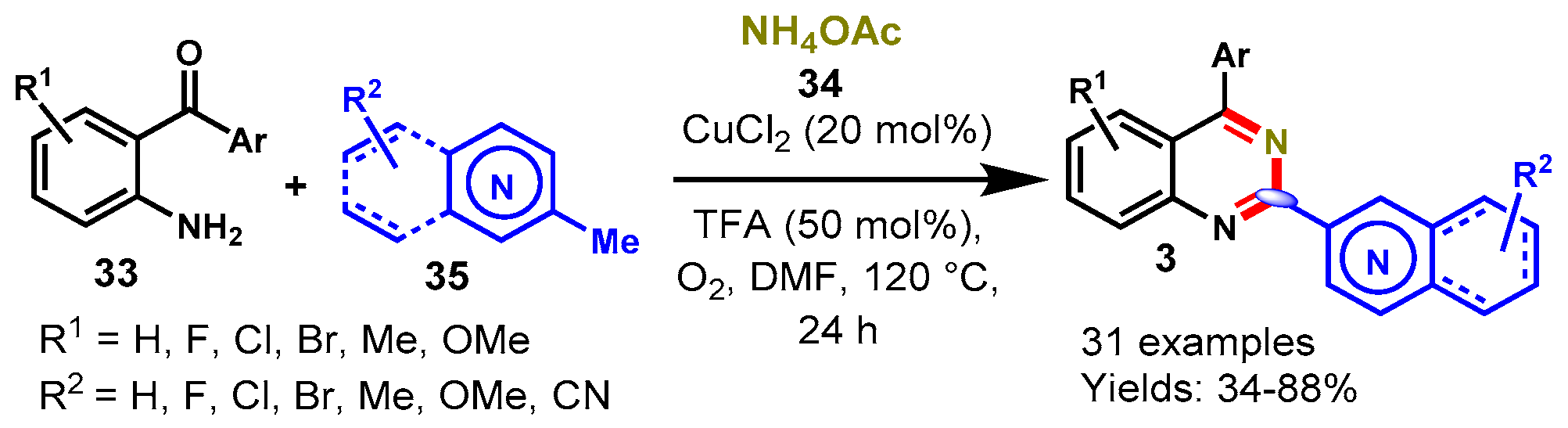
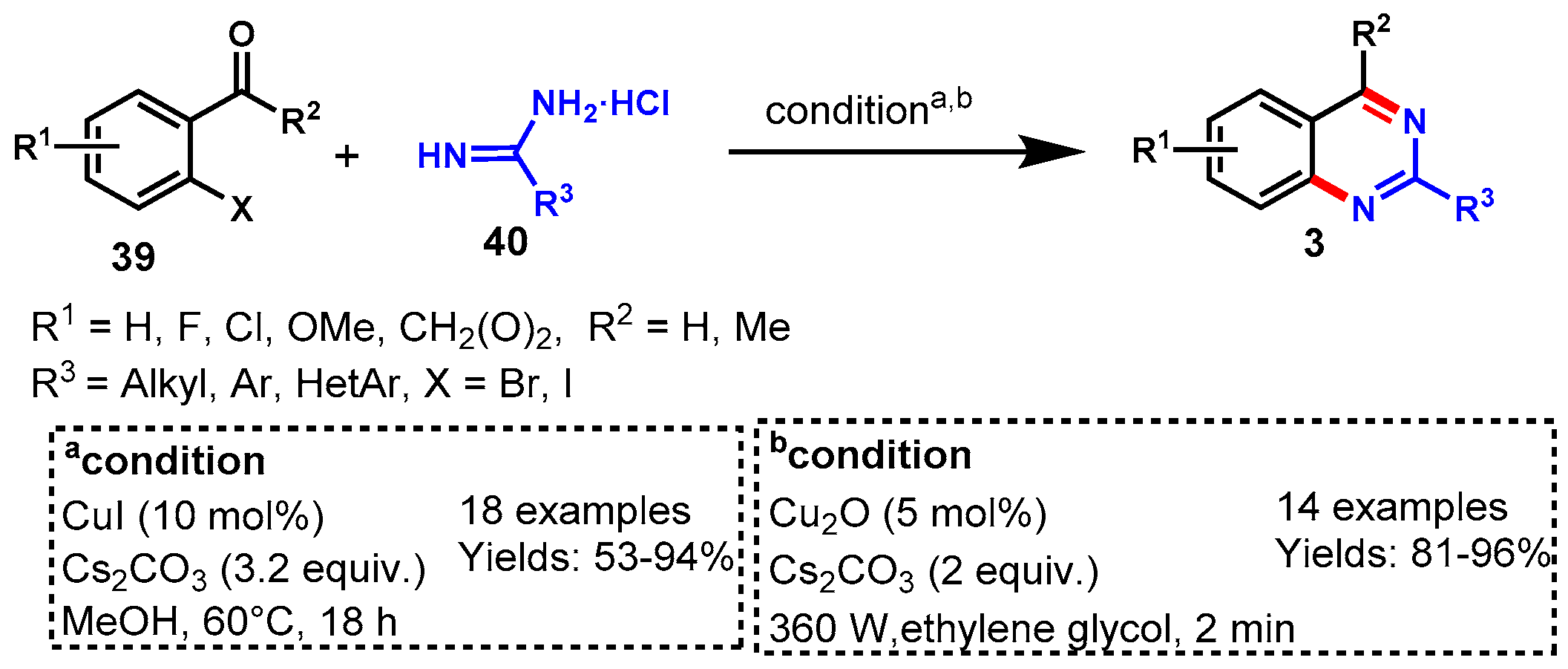
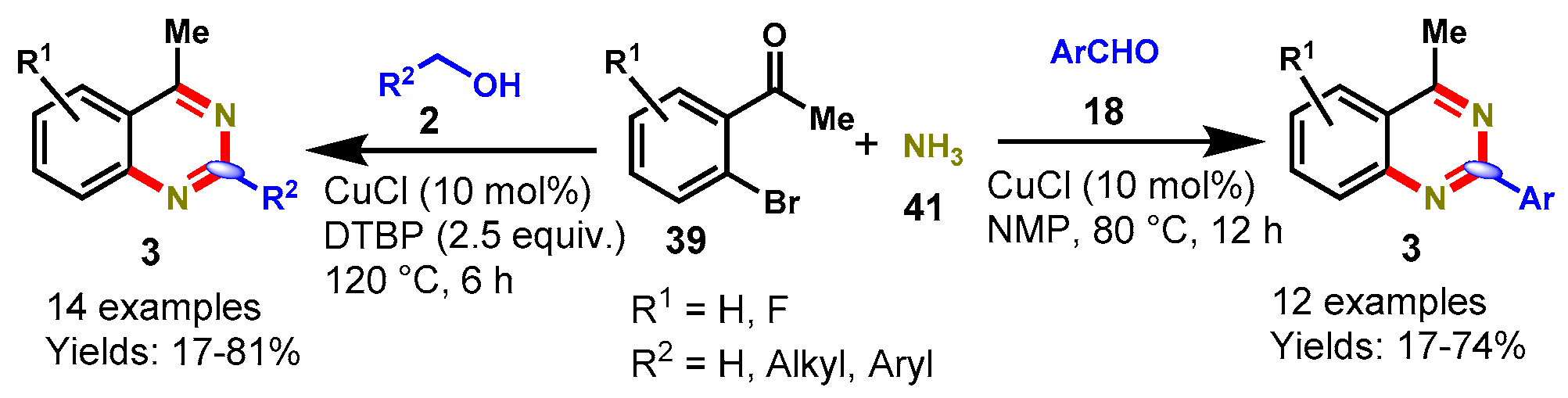
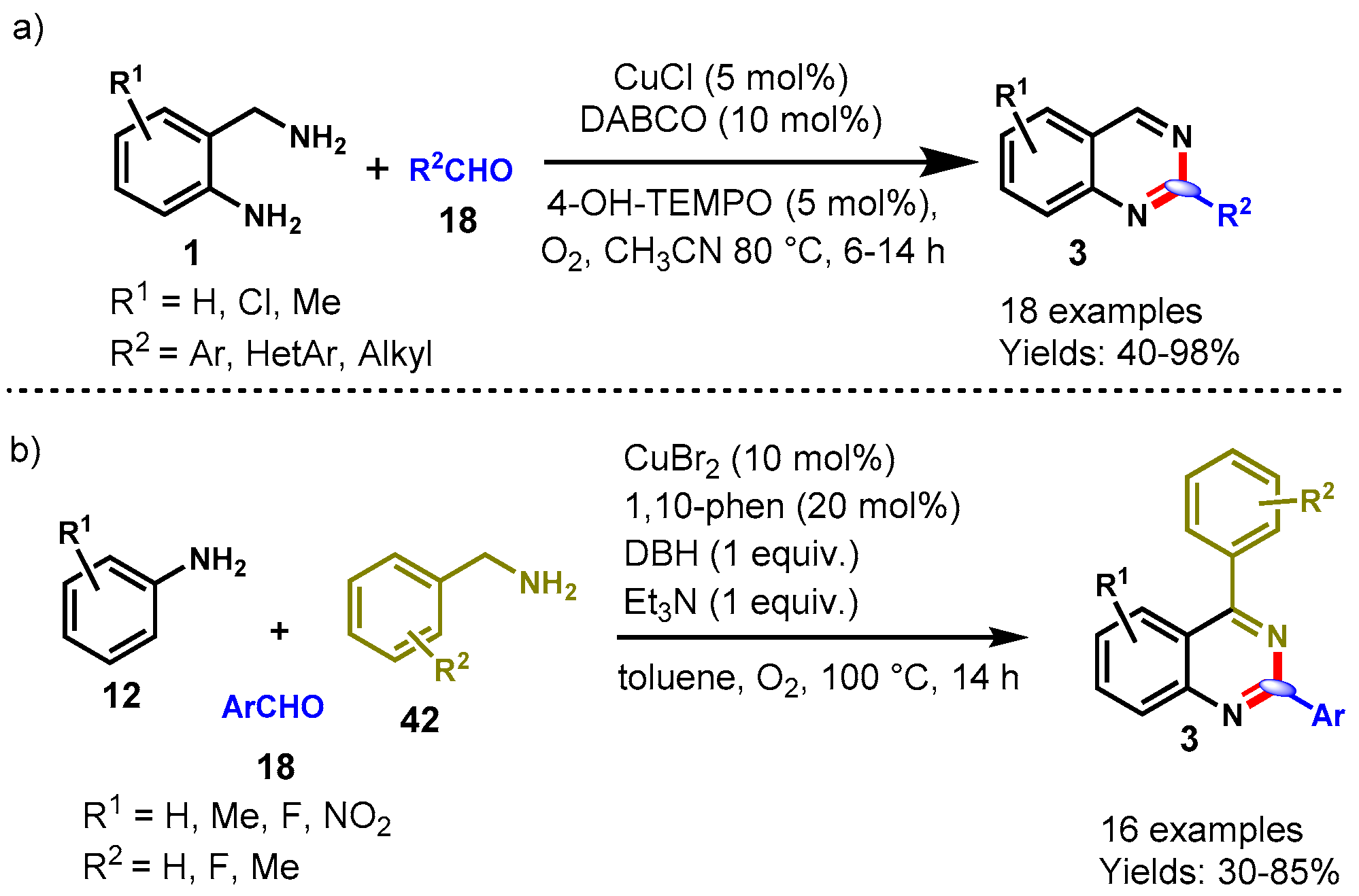
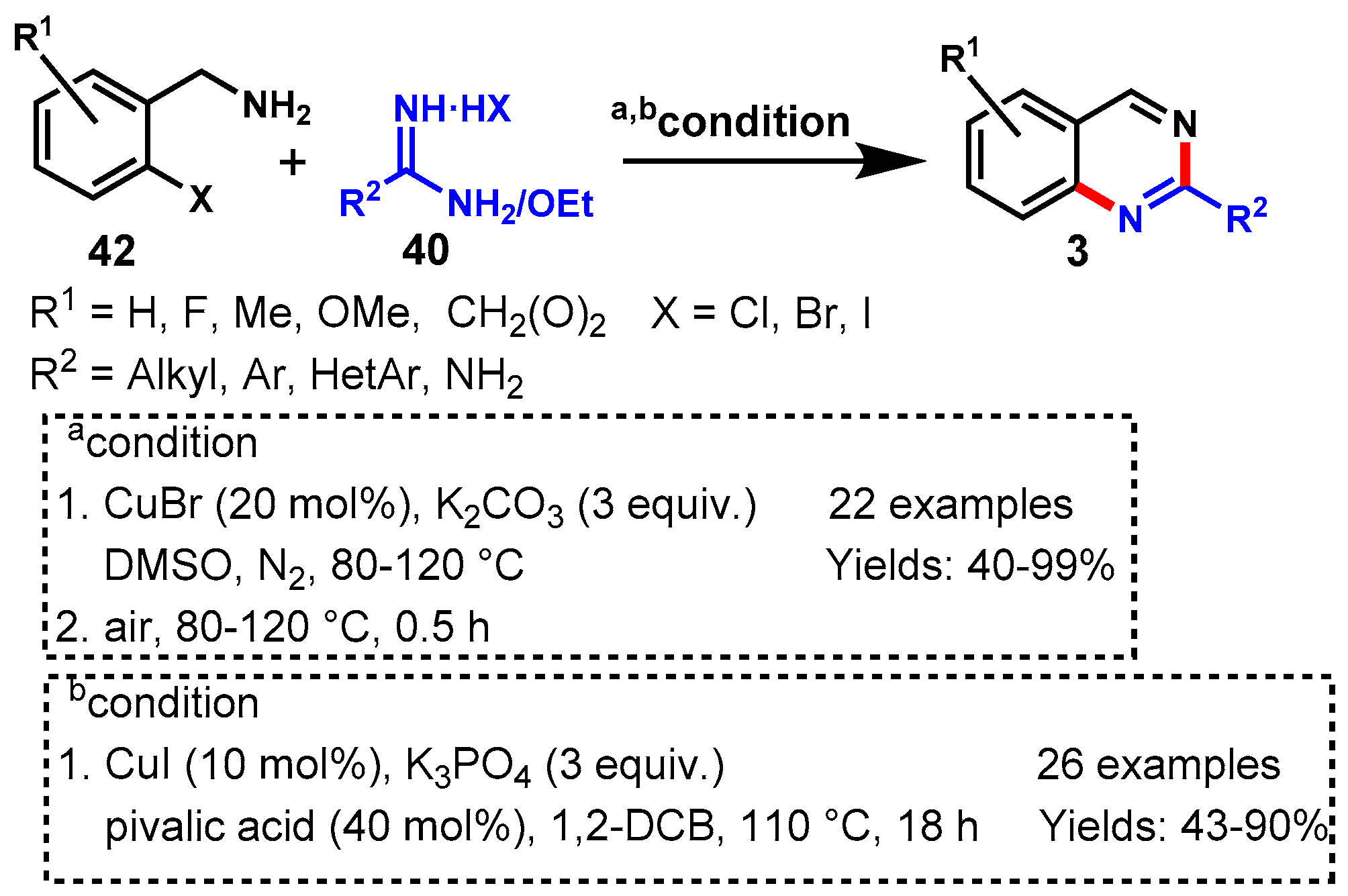
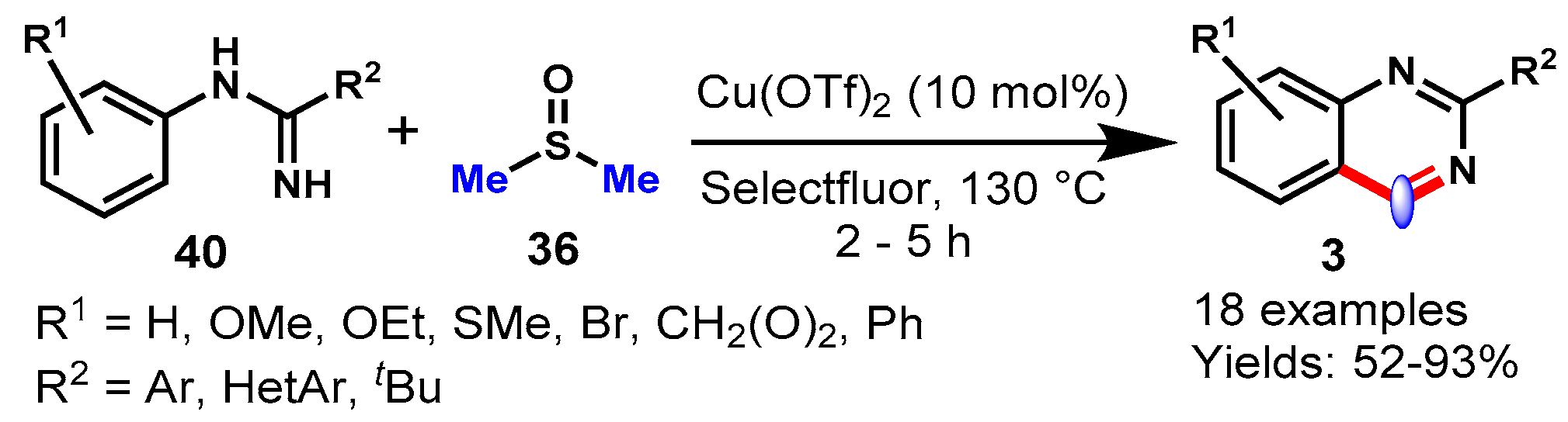
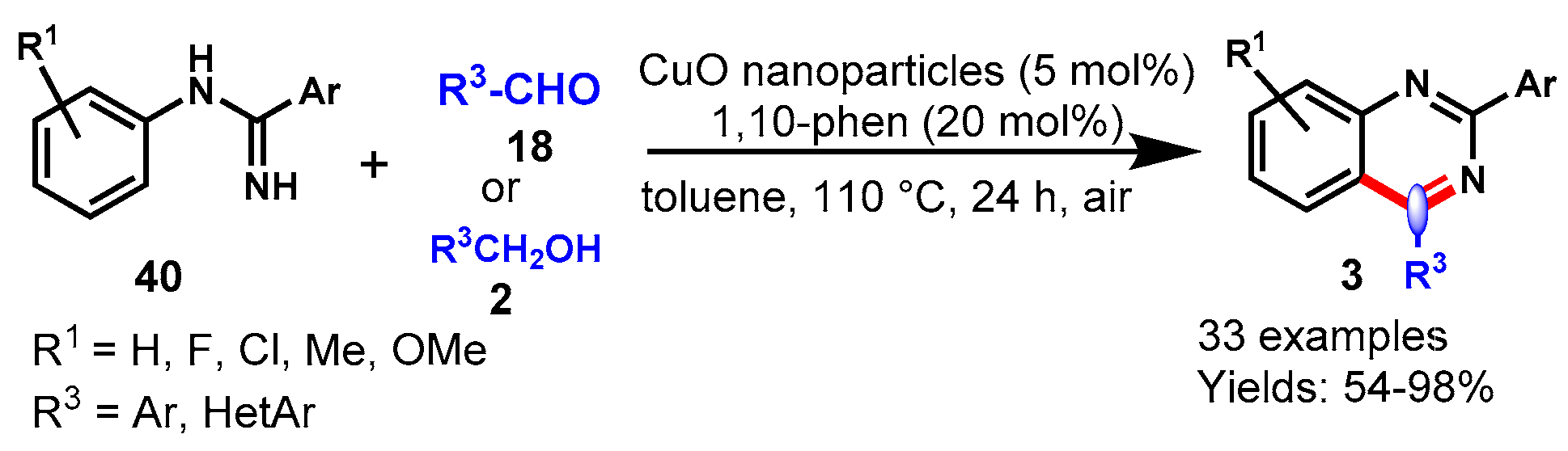
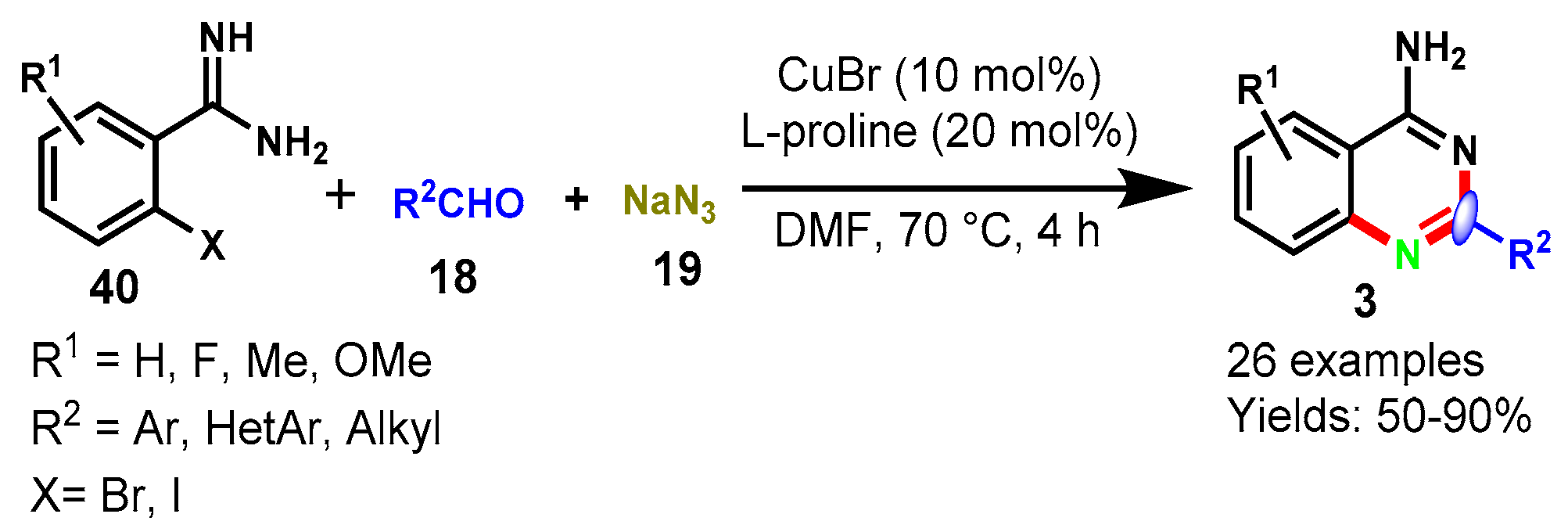
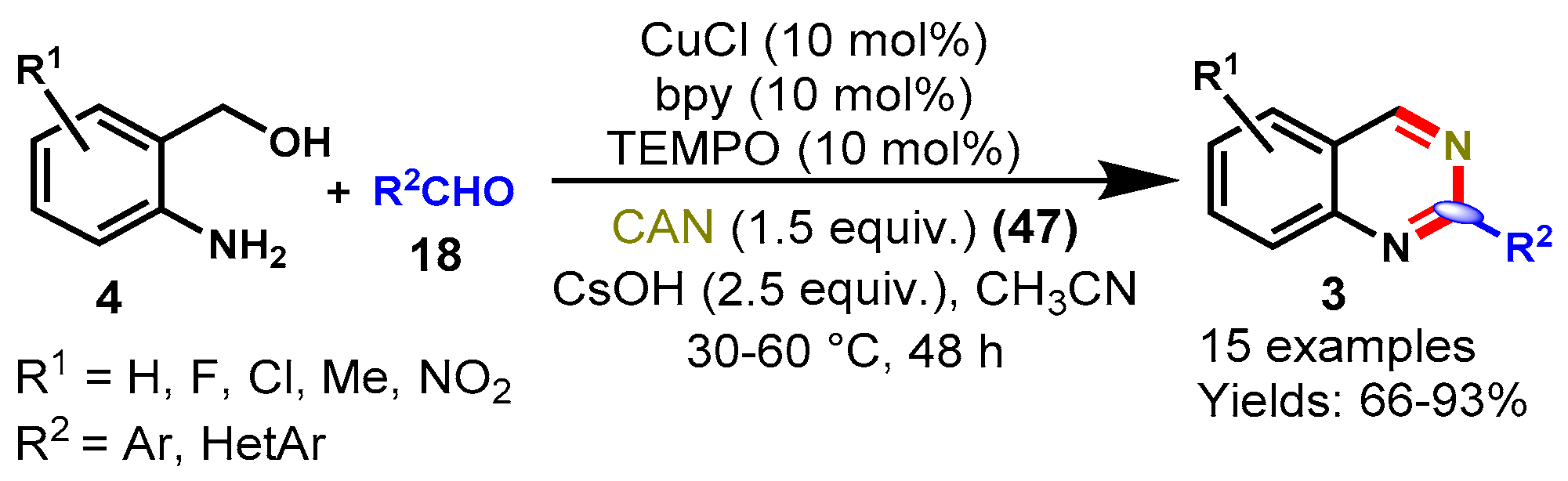
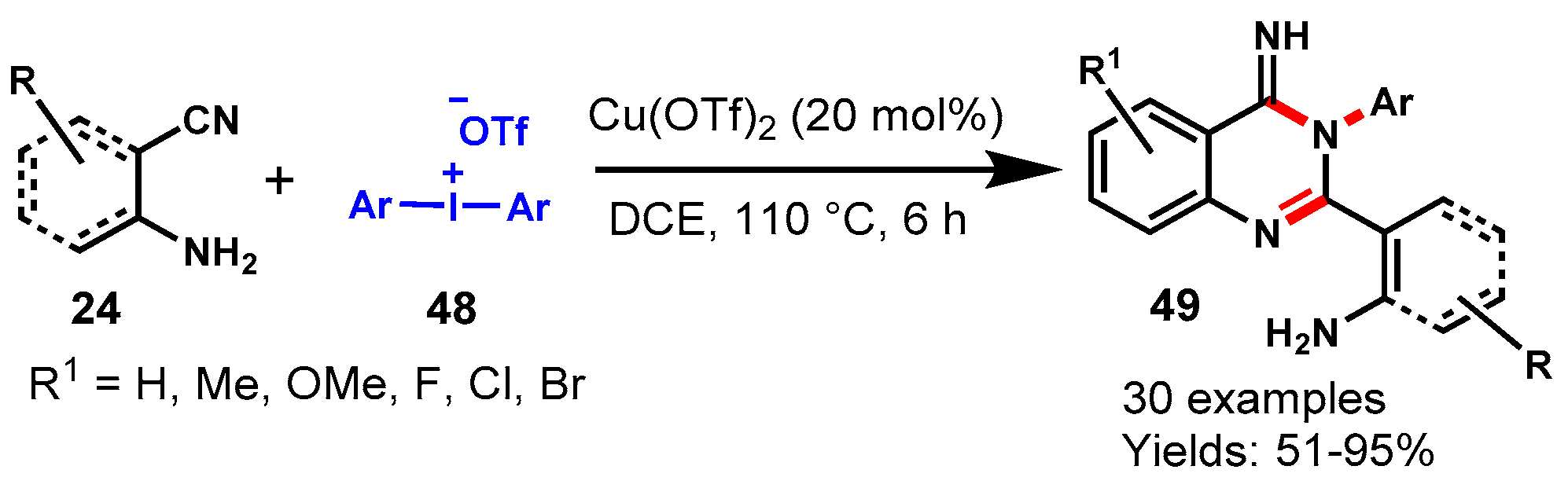
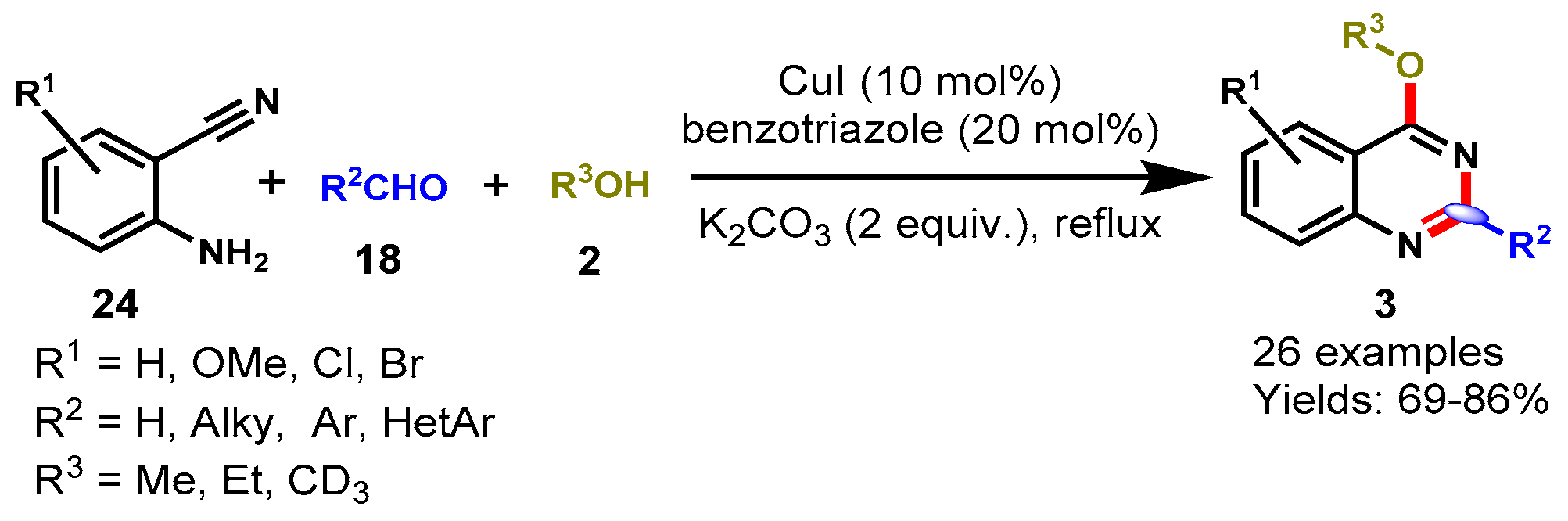
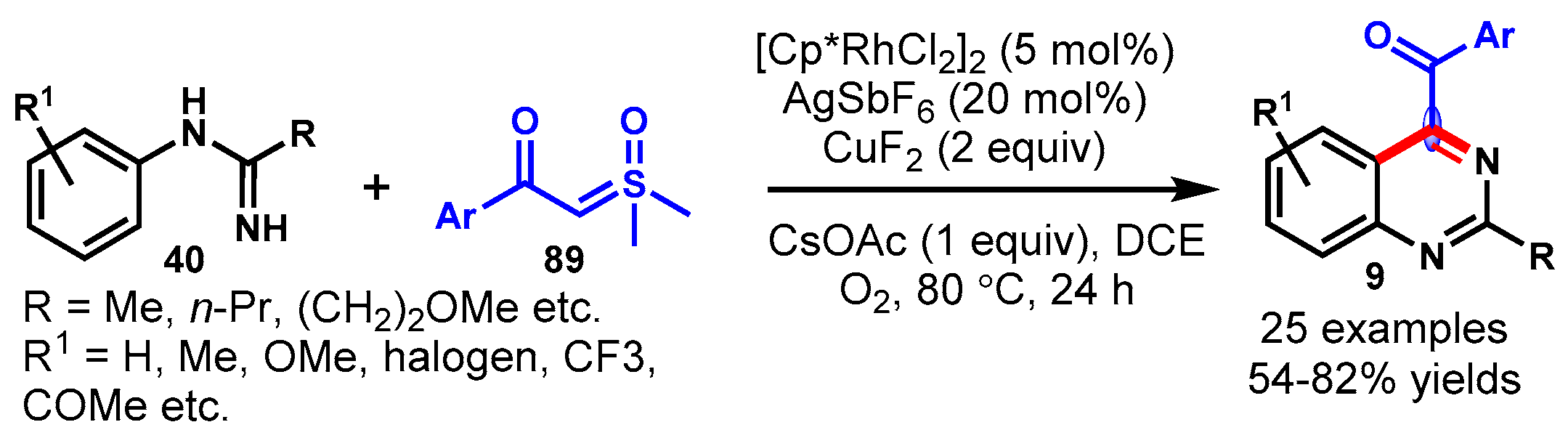
6. Copper-Catalyzed Protocols
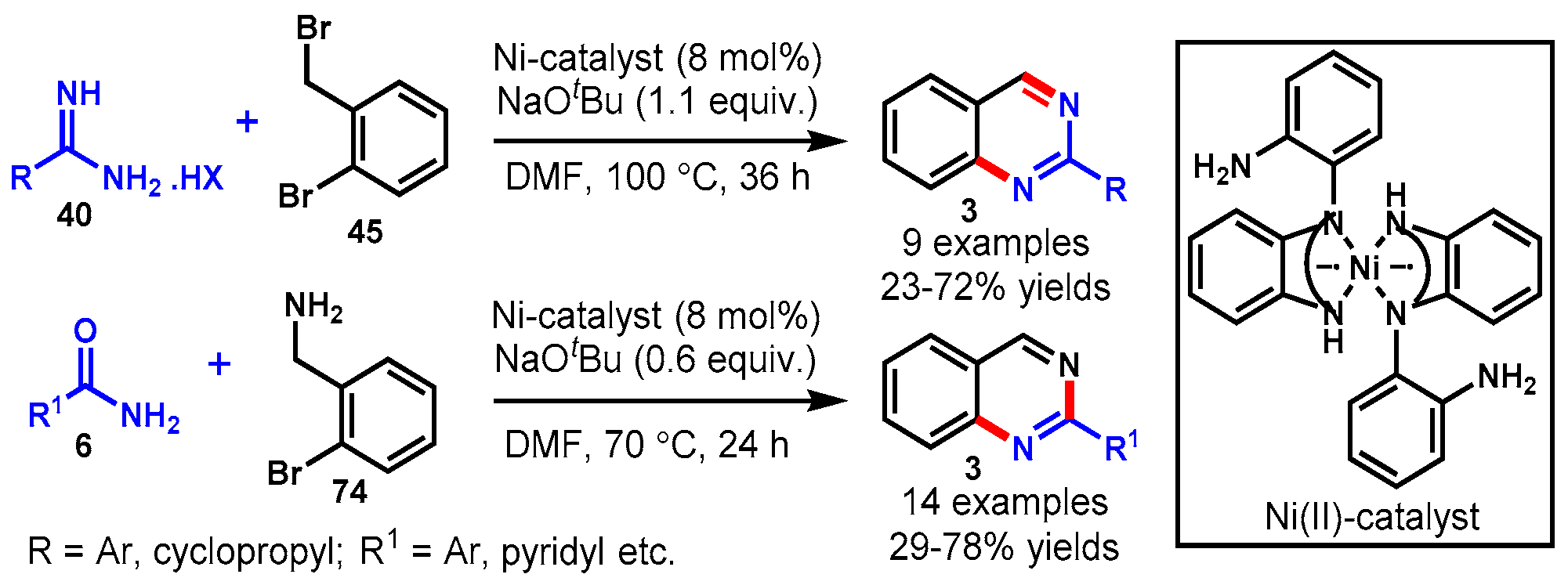
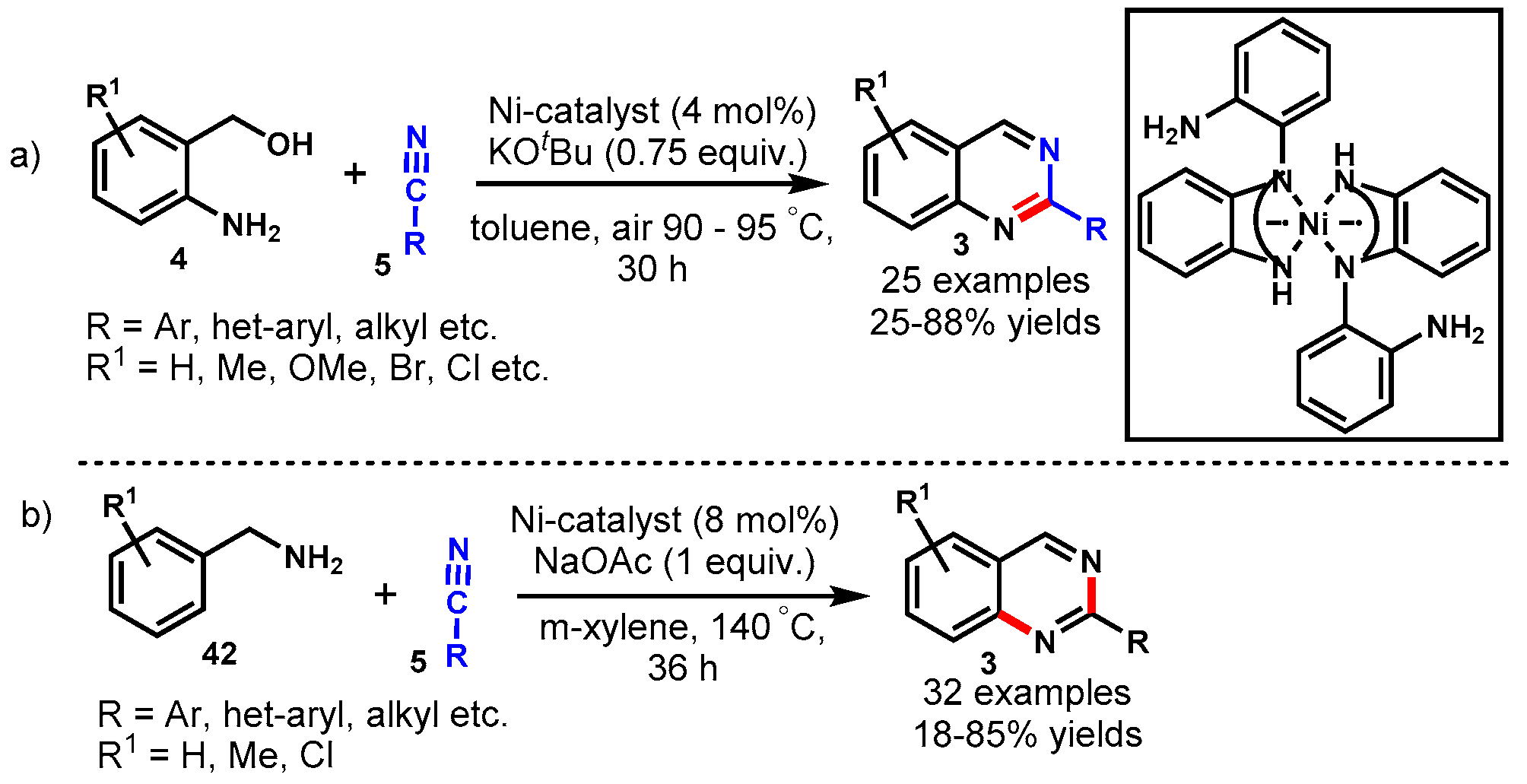
7. Nickel-Catalyzed Protocols
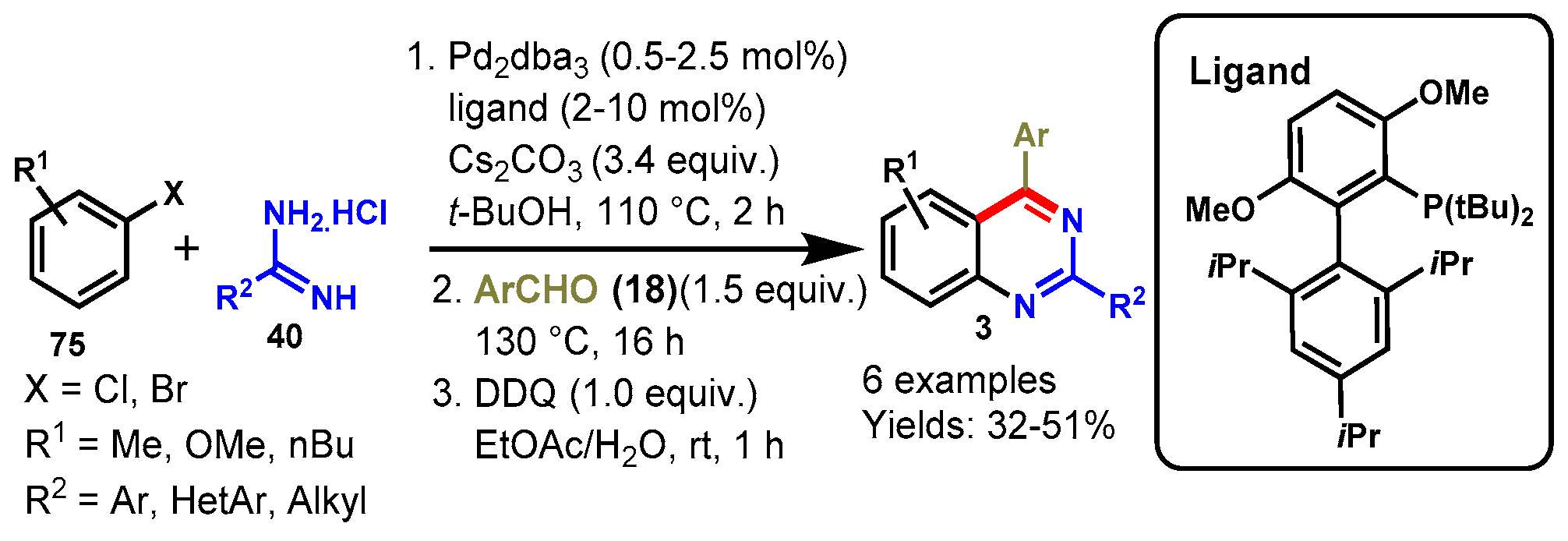
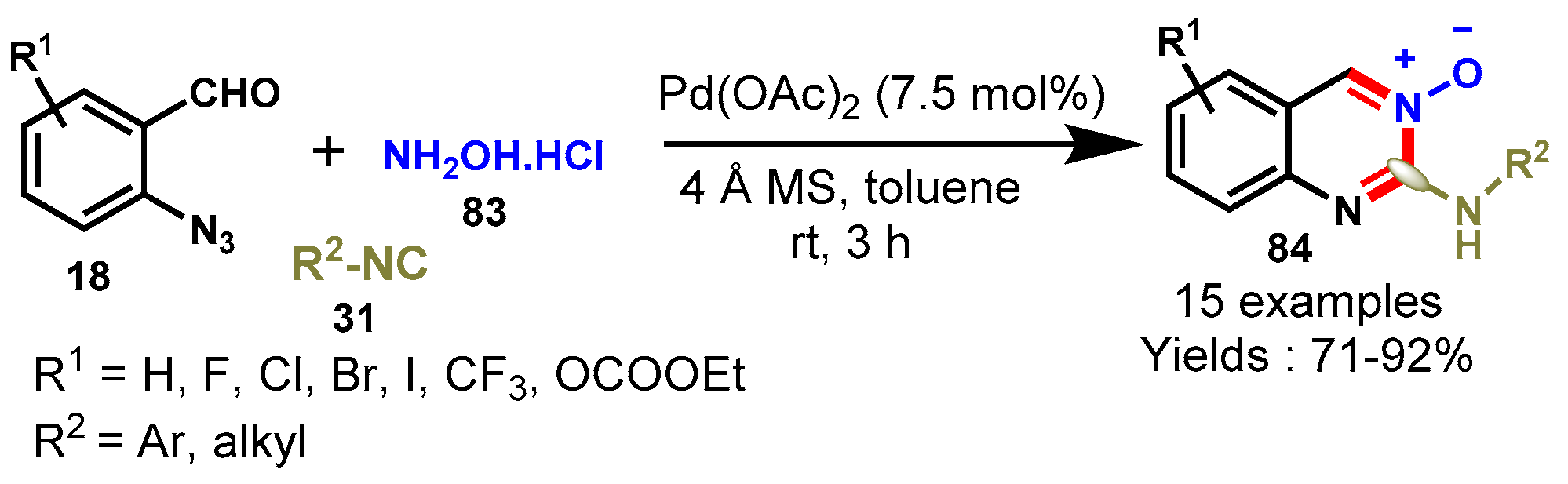
8. Palladium-Catalyzed Protocols
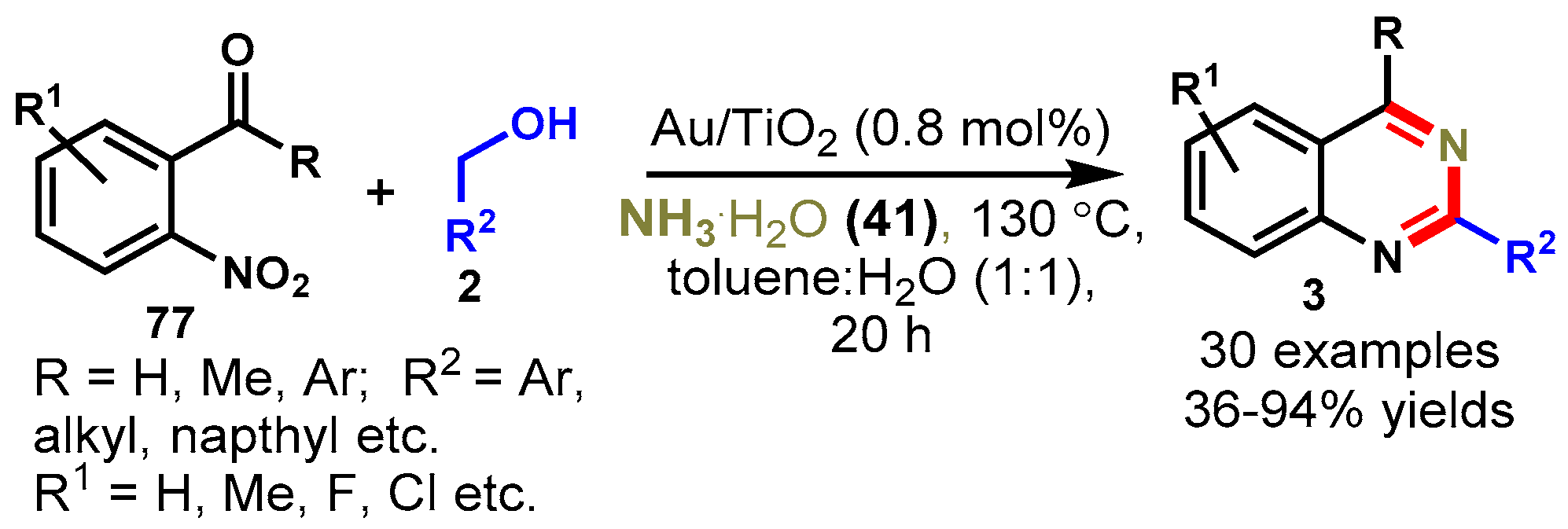
9. Gold-Catalyzed Protocols
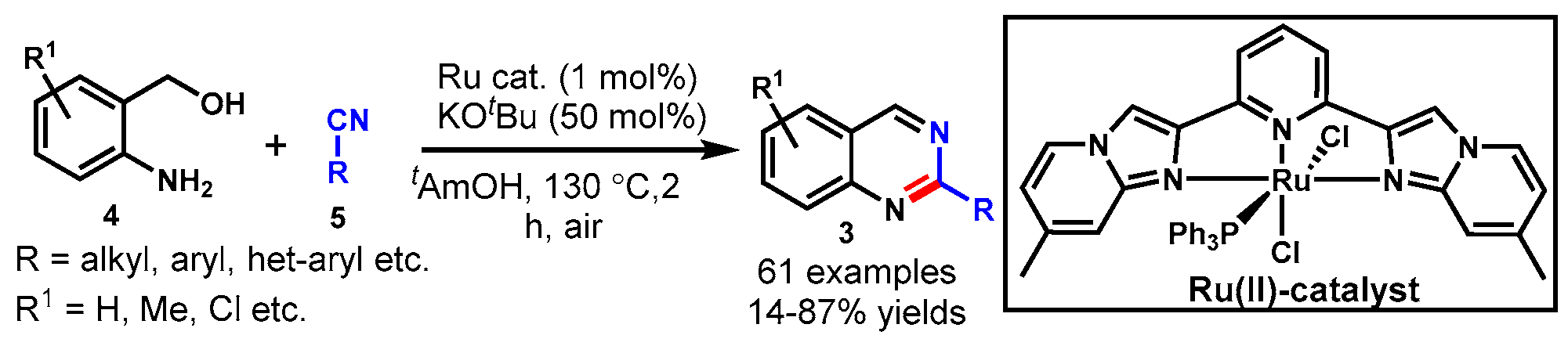
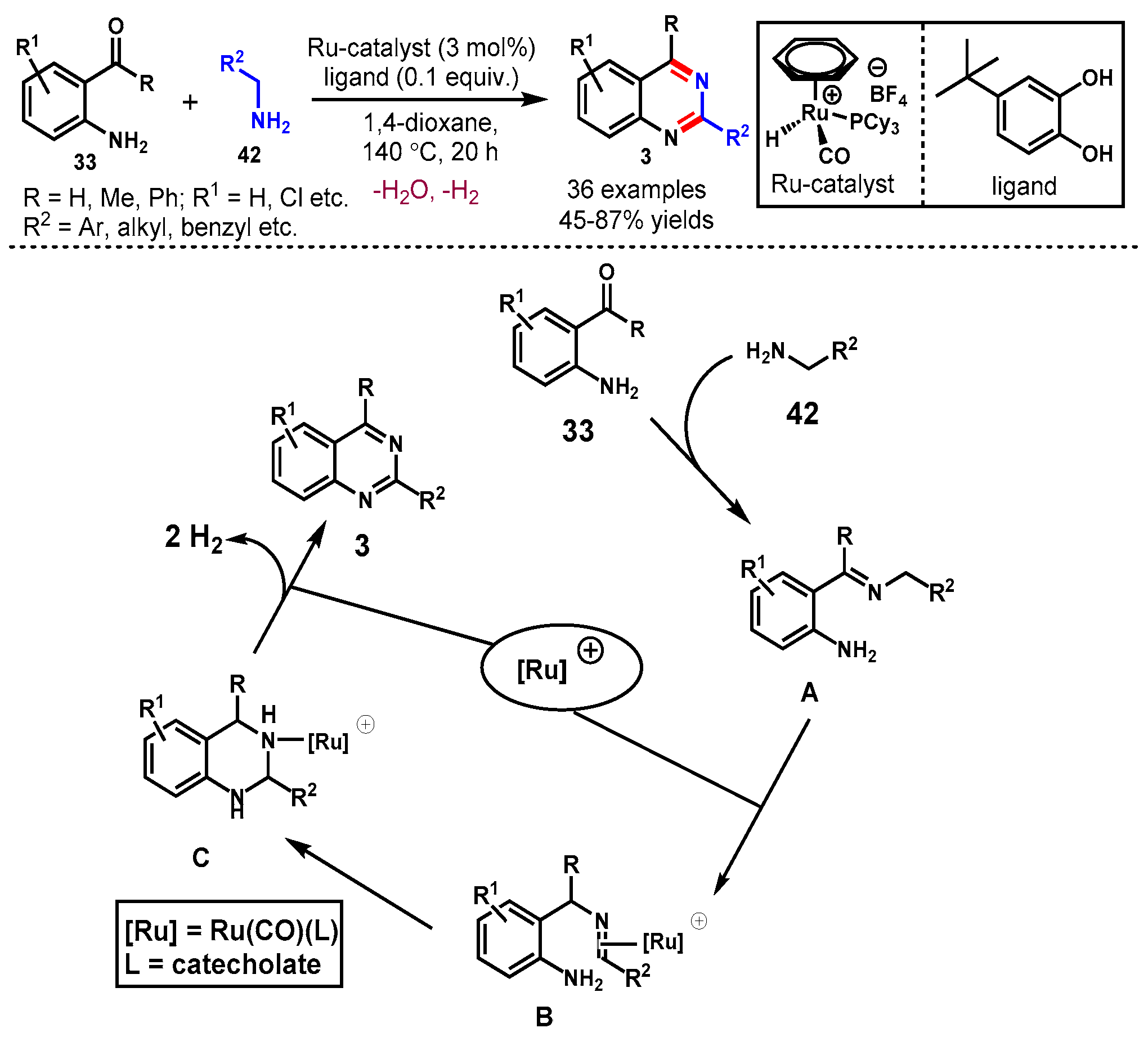
10. Ruthenium-Catalyzed Protocols
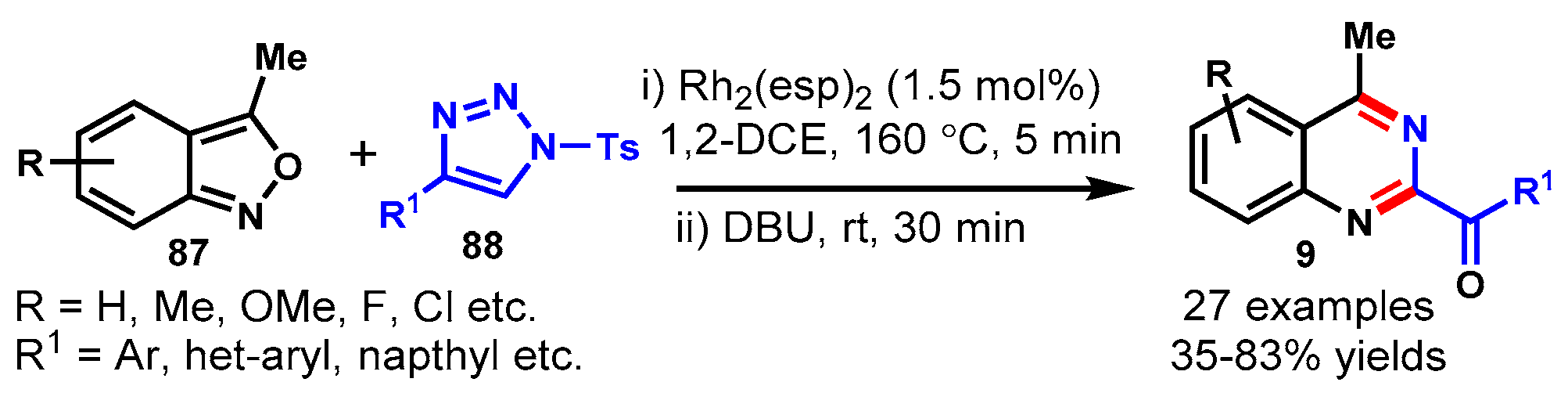
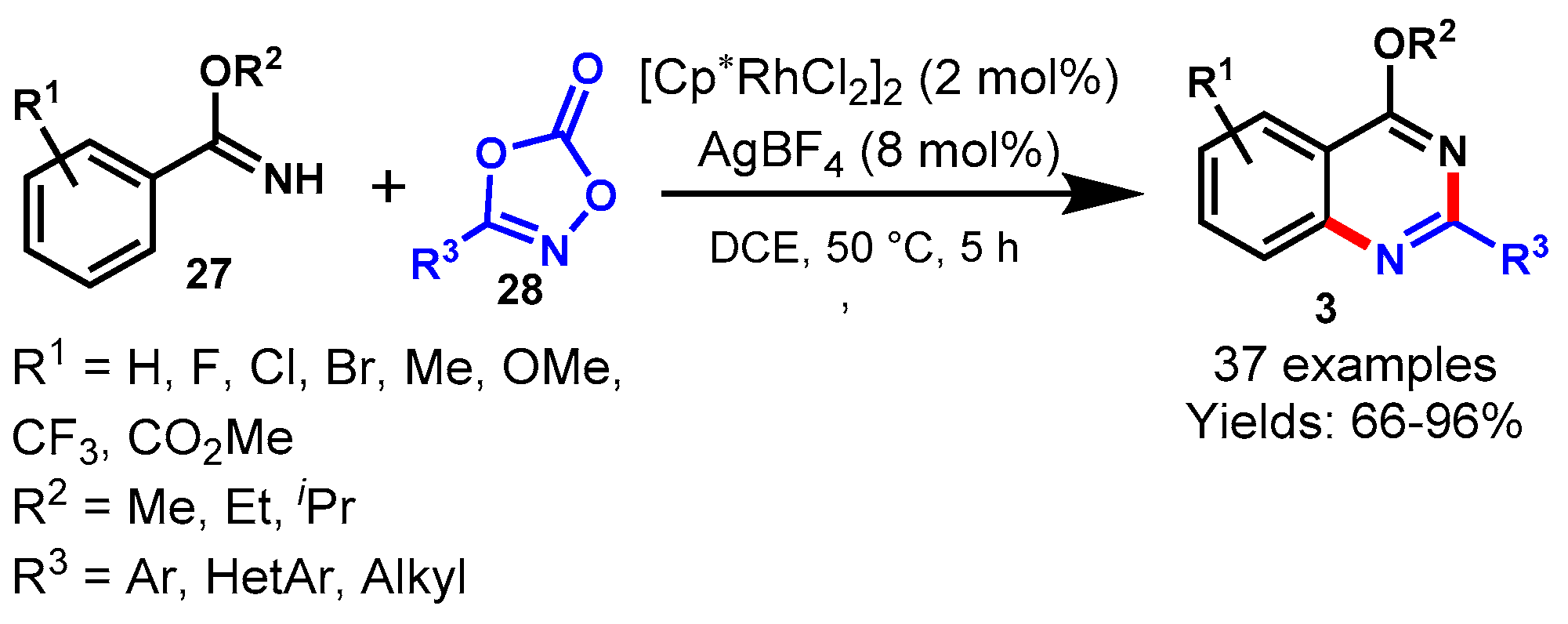
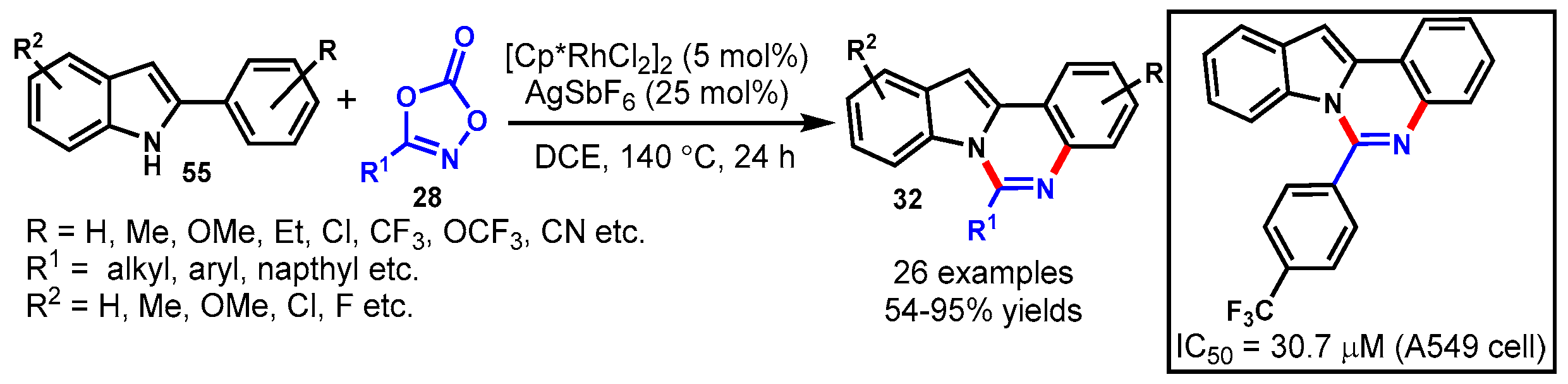
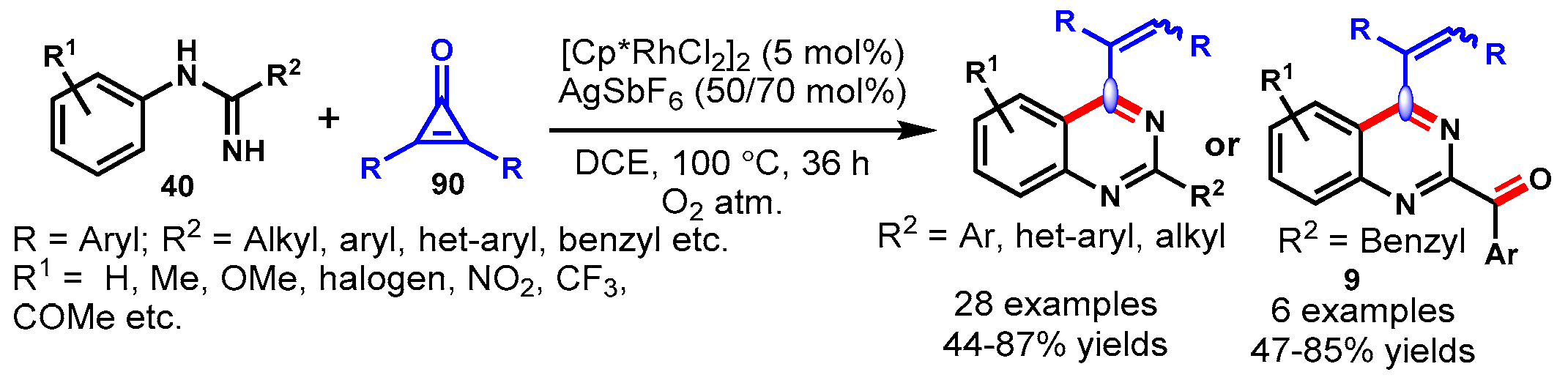
11. Rhodium-Catalyzed Protocols
12. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Deiters, A.; Martin, S.F. Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis. Chem. Rev. 2004, 104, 2199–2238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, G.R.; Kuethe, J.T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef] [PubMed]
- Maiden, T.; Harrity, J. Recent developments in transition metal catalysis for quinazolinone synthesis. Org. Biomol. Chem. 2016, 14, 8014–8025. [Google Scholar] [CrossRef] [PubMed]
- Patel, O.P.S.; Nandwana, N.K.; Legoabe, L.J.; Das, B.C.; Kumar, A. Recent Advances in Radical C−H Bond Functionalization of Imidazoheterocycles. Adv. Synth. Catal. 2020, 362, 4226–4255. [Google Scholar] [CrossRef]
- Pericherla, K.; Kaswan, P.; Pandey, K.; Kumar, A. Recent Developments in the Synthesis of Imidazo[1,2-a]pyridines. Synthesis 2015, 47, 887–912. [Google Scholar]
- Taylor, A.P.; Robinson, R.P.; Fobian, Y.M.; Blakemore, D.C.; Jones, L.H.; Fadeyi, O. Modern advances in heterocyclic chemistry in drug discovery. Org. Biomol. Chem. 2016, 14, 6611–6637. [Google Scholar] [CrossRef]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Hameed, A.; Al-Rashida, M.; Uroos, M.; Ali, S.A.; Arshia; Ishtiaq, M.; Khan, K.M. Quinazoline and quinazolinone as important medicinal scaffolds: A comparative patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 281–297. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Ahmed, W.; Saeed, A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. [Google Scholar] [CrossRef]
- Brewster, W.K.; Klittich, C.J.; Yao, C.; Zhu, Y.; Rieder, B.J. 5,8-Difluoro-4-(2-(4-(heteroaryloxy)-phenyl) ethylamino) Quinazolines and Their Use as Agrochemicals. U.S. Patent 8,268,843, 18 September 2012. [Google Scholar]
- Lipunova, G.N.; Nosova, E.V.; Charushin, V.N.; Chupakhin, O.N. Functionalized quinazolines and pyrimidines for optoelectronic materials. Curr. Org. Synth. 2018, 15, 793–814. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, J.; Wang, H.; Shen, B.; Zhang, J.; Hao, J.; Cao, J.; Wang, Z. Synthesis, photophysical and optoelectronic properties of quinazoline-centered dyes and their applications in organic light-emitting diodes. Dye. Pigment. 2016, 125, 299–308. [Google Scholar] [CrossRef]
- Gupta, T.; Rohilla, A.; Pathak, A.; Akhtar, M.J.; Haider, M.R.; Yar, M.S. Current perspectives on quinazolines with potent biological activities: A review. Synth. Commun. 2018, 48, 1099–1127. [Google Scholar] [CrossRef]
- Kshirsagar, U. Recent developments in the chemistry of quinazolinone alkaloids. Org. Biomol. Chem. 2015, 13, 9336–9352. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.F.; Morris-Natschke, S.L.; Liu, Y.Q.; Guo, X.; Xu, X.S.; Goto, M.; Li, J.C.; Yang, G.Z.; Lee, K.H. Biologically active quinoline and quinazoline alkaloids part I. Med. Res. Rev. 2017, 38, 775–828. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Begum, S.; Ahmed, S.; Choudhary, M.; Khan, I. Quantitative determination of vasicine and vasicinone in Adhatoda vasica by high performance capillary electrophoresis. Die Pharm.-Int. J. Pharm. Sci. 2008, 63, 20–22. [Google Scholar]
- Khan, I.; Ibrar, A.; Abbas, N.; Saeed, A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorganic Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar] [CrossRef]
- Grover, G.; Kini, S.G. Synthesis and evaluation of new quinazolone derivatives of nalidixic acid as potential antibacterial and antifungal agents. Eur. J. Med. Chem. 2006, 41, 256–262. [Google Scholar] [CrossRef]
- Smits, R.A.; Adami, M.; Istyastono, E.P.; Zuiderveld, O.P.; van Dam, C.M.; de Kanter, F.J.; Jongejan, A.; Coruzzi, G.; Leurs, R.; de Esch, I.J. Synthesis and QSAR of quinazoline sulfonamides as highly potent human histamine H4 receptor inverse agonists. J. Med. Chem. 2010, 53, 2390–2400. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.K.; Singh, A.; Singh, A. Antimicrobial studies of some novel quinazolinones fused with [1,2,4]-triazole,[1,2,4]-triazine and [1,2,4,5]-tetrazine rings. Eur. J. Med. Chem. 2009, 44, 1188–1197. [Google Scholar] [CrossRef] [PubMed]
- Kashaw, S.K.; Kashaw, V.; Mishra, P.; Jain, N.; Stables, J. Synthesis, anticonvulsant and CNS depressant activity of some new bioactive 1-(4-substituted-phenyl)-3-(4-oxo-2-phenyl/ethyl-4H-quinazolin-3-yl)-urea. Eur. J. Med. Chem. 2009, 44, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bian, M.; Deng, X.Q.; Wang, S.B.; Quan, Z.S. Synthesis and Anticonvulsant Activity Evaluation of 5-Phenyl-[1,2,4] triazolo [4, 3-c] quinazolin-3-amines. Arch. Der Pharm. 2013, 346, 119–126. [Google Scholar] [CrossRef]
- Malamas, M.S.; Millen, J. Quinazolineacetic acids and related analogs as aldose reductase inhibitors. J. Med. Chem. 1991, 34, 1492–1503. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Barker, S.; Abou El Ella, D.A.; Abouzid, K.A.; Toubar, R.A.; Todd, M.H. Design and synthesis of new tetrazolyl-and carboxy-biphenylylmethyl-quinazolin-4-one derivatives as angiotensin II AT1 receptor antagonists. J. Med. Chem. 2006, 49, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, P.M.; Yakaiah, T.; Rao, A.R.R.; Narsaiah, B.; Reddy, N.C.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Chilin, A.; Conconi, M.T.; Marzaro, G.; Guiotto, A.; Urbani, L.; Tonus, F.; Parnigotto, P. Exploring epidermal growth factor receptor (EGFR) inhibitor features: The role of fused dioxygenated rings on the quinazoline scaffold. J. Med. Chem. 2010, 53, 1862–1866. [Google Scholar] [CrossRef]
- Ravez, S.; Castillo-Aguilera, O.; Depreux, P.; Goossens, L. Quinazoline derivatives as anticancer drugs: A patent review (2011–present). Expert Opin. Ther. Pat. 2015, 25, 789–804. [Google Scholar] [CrossRef]
- Marvania, B.; Lee, P.-C.; Chaniyara, R.; Dong, H.; Suman, S.; Kakadiya, R.; Chou, T.-C.; Lee, T.-C.; Shah, A.; Su, T.-L. Design, synthesis and antitumor evaluation of phenyl N-mustard-quinazoline conjugates. Bioorganic Med. Chem. 2011, 19, 1987–1998. [Google Scholar] [CrossRef]
- Rosowsky, A.; Wright, J.E.; Vaidya, C.M.; Forsch, R.A. The effect of side-chain, para-aminobenzoyl region, and B-ring modifications on dihydrofolate reductase binding, influx via the reduced folate carrier, and cytotoxicity of the potent nonpolyglutamatable antifolate Nα-(4-amino-4-deoxypteroyl)-Nδ-hemiphthaloyl-l-ornithine. Pharmacol. Ther. 2000, 85, 191–205. [Google Scholar] [PubMed]
- Gangjee, A.; Kothare, M.; Kisliuk, R.L. The synthesis of novel nonclassical reversed bridge quinazoline antifolates as inhibitors of thymidylate synthase. J. Heterocycl. Chem. 2000, 37, 1097–1102. [Google Scholar] [CrossRef]
- Garofalo, A.; Goossens, L.; Lemoine, A.; Ravez, S.; Six, P.; Howsam, M.; Farce, A.; Depreux, P. [4-(6,7-Disubstituted quinazolin-4-ylamino) phenyl] carbamic acid esters: A novel series of dual EGFR/VEGFR-2 tyrosine kinase inhibitors. MedChemComm 2011, 2, 65–72. [Google Scholar] [CrossRef]
- Cruz-Lopez, O.; Conejo-Garcia, A.; Nunez, M.C.; Kimatrai, M.; Garcia-Rubino, M.E.; Morales, F.; Gomez-Perez, V.; M Campos, J. Novel substituted quinazolines for potent EGFR tyrosine kinase inhibitors. Curr. Med. Chem. 2011, 18, 943–963. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, H.; Chen, J.; Wu, X. Recent advances in 4 (3 H)-quinazolinone syntheses. RSC Adv. 2014, 4, 12065. [Google Scholar]
- Khan, I.; Zaib, S.; Batool, S.; Abbas, N.; Ashraf, Z.; Iqbal, J.; Saeed, A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. [Google Scholar] [CrossRef] [PubMed]
- Theivendren, P.; Palanirajan, V. Quinazoline Marketed drugs–A review. Res. Pharm. 2011, 1, 1–21. [Google Scholar]
- Crenshaw, R.R.; Luke, G.M.; Partyka, R.A. Process for Preparing Quinazolines. U.S. Patent 4098788, 4 July 1978. [Google Scholar]
- Wang, D.; Gao, F. Quinazoline derivatives: Synthesis and bioactivities. Chem. Cent. J. 2013, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F.; Stang, P.J. Metal-Catalyzed Cross-Coupling Reactions; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Bräse, S.; Meijere, A.D. Cross-coupling of organyl halides with alkenes: The Heck reaction. Met.-Catalyzed Cross-Coupling React. 2004, 217–315. [Google Scholar]
- Gabriel, S. Ueber das Chinazolin. Eur. J. Inorg. Chem. 1903, 36, 800–813. [Google Scholar] [CrossRef]
- Elderfield, R.C.; Williamson, T.A.; Gensler, W.J.; Kremer, C.B. Synthesis of bz-substituted quinazolines and antimalarials from them; a contribution to the chemistry of quinazoline. J. Org. Chem. 1947, 12, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Chen, C.; Wang, Y.; Chen, J.; Lou, Z.; Li, M. One-pot synthesis of quinazoline derivatives via [2 + 2 + 2] cascade annulation of diaryliodonium salts and two nitriles. Chem. Commun. 2013, 49, 6752–6754. [Google Scholar] [CrossRef] [PubMed]
- Armarego, W. Quinazolines. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 1963; Volume 1, pp. 253–309. [Google Scholar]
- Aneeja, T.; Neetha, M.; Afsina, C.M.A.; Anilkumar, G. Recent advances and perspectives in manganese-catalyzed C–H activation. Catal. Sci. Technol. 2021, 11, 444–458. [Google Scholar] [CrossRef]
- Das, K.; Waiba, S.; Jana, A.; Maji, B. Manganese-catalyzed hydrogenation, dehydrogenation, and hydroelementation reactions. Chem. Soc. Rev. 2022, 51, 4386–4464. [Google Scholar] [CrossRef] [PubMed]
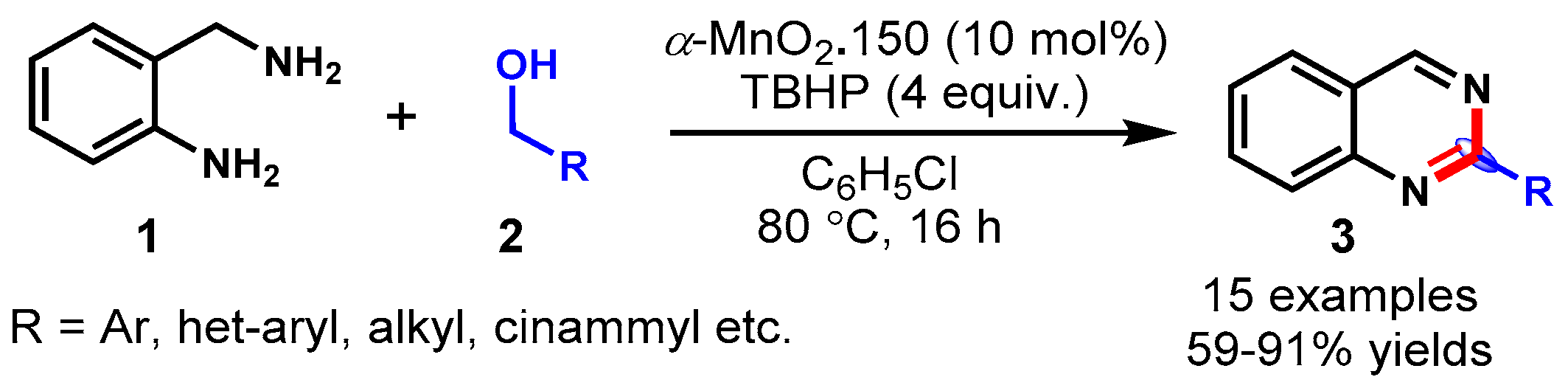
- Zhang, Z.; Wang, M.; Zhang, C.; Zhang, Z.; Lu, J.; Wang, F. The cascade synthesis of quinazolinones and quinazolines using an α-MnO2 catalyst and tert-butyl hydroperoxide (TBHP) as an oxidant. Chem. Commun. 2015, 51, 9205–9207. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Mondal, A.; Pal, D.; Srimani, D. Sustainable Synthesis of Quinazoline and 2-Aminoquinoline via Dehydrogenative Coupling of 2-Aminobenzyl Alcohol and Nitrile Catalyzed by Phosphine-Free Manganese Pincer Complex. Org. Lett. 2019, 21, 3223–3227. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Sahoo, M.K.; Subaramanian, M.; Balaraman, E. Manganese (I)-Catalyzed Sustainable Synthesis of Quinoxaline and Quinazoline Derivatives with the Liberation of Dihydrogen. J. Org. Chem. 2020, 85, 7181–7191. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.R.Y.; Begum, N.S.; Imran, K.M. Mn-mediated oxidative radical cyclization of 2-(azidomethyl) phenyl isocyanides with carbazate: Access to quinazoline-2-carboxylates. New J. Chem. 2020, 44, 7001–7006. [Google Scholar] [CrossRef]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-Catalyzed C–H Bond Activation. Chem. Rev. 2017, 117, 9086–9139. [Google Scholar] [CrossRef]
- Hoyt, J.M.; Schmidt, V.A.; Tondreau, A.M.; Chirik, P.J. Iron-catalyzed intermolecular [2 + 2] cycloadditions of unactivated alkenes. Science 2015, 349, 960–963. [Google Scholar] [CrossRef]
- Watile, R.A.; Bunrit, A.; Margalef, J.; Akkarasamiyo, S.; Ayub, R.; Lagerspets, E.; Biswas, S.; Repo, T.; Samec, J.S. Intramolecular substitutions of secondary and tertiary alcohols with chirality transfer by an iron (III) catalyst. Nat. Commun. 2019, 10, 3826. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Liu, N.; Deng, Y.-X.; Chen, Y.; Deng, Y.; He, L. Synthesis of 2,4-diaminoquinazolines and tricyclic quinazolines by cascade reductive cyclization of methyl N-cyano-2-nitrobenzimidates. J. Org. Chem. 2012, 77, 2649–2658. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, R.; Stopka, T.; Richter, H.; García Mancheño, O. Iron-catalyzed oxidative tandem reactions with TEMPO oxoammonium salts: Synthesis of dihydroquinazolines and quinolines. J. Org. Chem. 2013, 78, 6050–6064. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.-C.; Zhou, Z.-W.; Xu, C.; Cai, Q.; Li, D.-K.; Wu, A.-X. Expeditious synthesis of 2-phenylquinazolin-4-amines via a Fe/Cu relay-catalyzed domino strategy. Org. Lett. 2015, 17, 4236–4239. [Google Scholar] [CrossRef] [PubMed]
- Shinde, V.V.; Jeong, Y.T. Sonochemical FeF3 catalyzed three-component synthesis of densely functionalized tetrahydroindazolo [3,2-b] quinazoline under solvent-free conditions. Tetrahedron Lett. 2016, 57, 3795–3799. [Google Scholar] [CrossRef]
- Gopalaiah, K.; Saini, A.; Devi, A. Iron-catalyzed cascade reaction of 2-aminobenzyl alcohols with benzylamines: Synthesis of quinazolines by trapping of ammonia. Org. Biomol. Chem. 2017, 15, 5781–5789. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-y.; He, F.; Tang, G.; Yuan, H.; Li, N.; Wang, J.; Faessler, R. Synthesis of quinazolines via an iron-catalyzed oxidative amination of N–H ketimines. J. Org. Chem. 2018, 83, 2395–2401. [Google Scholar] [CrossRef] [PubMed]
- Gopalaiah, K.; Tiwari, A.; Choudhary, R.; Mahiya, K. Straightforward Access to 3, 4-Dihydro-2H-1, 2, 4-benzothiadiazine 1, 1-dioxides and Quinazolines via Iron-Catalyzed Aerobic Oxidative Condensation of Amines. ChemistrySelect 2019, 4, 5200–5205. [Google Scholar] [CrossRef]
- Ujwaldev, S.M.; Harry, N.A.; Divakar, M.A.; Anilkumar, G. Cobalt-catalyzed C–H activation: Recent progress in heterocyclic chemistry. Catal. Sci. Technol. 2018, 8, 5983–6018. [Google Scholar] [CrossRef]
- Moselage, M.; Li, J.; Ackermann, L. Cobalt-Catalyzed C–H Activation. ACS Catal. 2016, 6, 498–525. [Google Scholar] [CrossRef]
- Wang, X.; Lerchen, A.; Glorius, F. A Comparative Investigation: Group 9 Cp* M (III)-Catalyzed Formal [4 + 2] Cycloaddition as an Atom-Economic Approach to Quinazolines. Org. Lett. 2016, 18, 2090–2093. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Wang, Q.; Yu, S.; Li, X. Co (III)-catalyzed synthesis of quinazolines via C–H activation of N-sulfinylimines and benzimidates. Org. Lett. 2016, 18, 1306–1309. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, F.; Bazgir, A. Synthesis of benzoimidazoquinazolines by cobalt-catalyzed isocyanide insertion–cyclization. RSC Adv. 2016, 6, 61955–61958. [Google Scholar] [CrossRef]
- Hao, Z.; Zhou, X.; Ma, Z.; Zhang, C.; Han, Z.; Lin, J.; Lu, G.-L. Dehydrogenative Synthesis of Quinolines and Quinazolines via Ligand-Free Cobalt-Catalyzed Cyclization of 2-Aminoaryl Alcohols with Ketones or Nitriles. J. Org. Chem. 2022, 87, 12596–12607. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-X.; Gu, D.-W.; Wu, Z.; Zhang, W. Copper-Catalyzed C–H Functionalization Reactions: Efficient Synthesis of Heterocycles. Chem. Rev. 2015, 115, 1622–1651. [Google Scholar] [CrossRef] [PubMed]
- Baghbanian, S.M.; Farhang, M. CuFe2O4 nanoparticles: A magnetically recoverable and reusable catalyst for the synthesis of quinoline and quinazoline derivatives in aqueous media. RSC Adv. 2014, 4, 11624–11633. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Yan, Y.-Z.; Bao, Y.-J.; Wang, Z.-Y. Efficient synthesis of 2-arylquinazolines via copper-catalyzed dual oxidative benzylic CH aminations of methylarenes. Chin. Chem. Lett. 2015, 26, 1216–1220. [Google Scholar] [CrossRef]
- Duan, T.; Zhai, T.; Liu, H.; Yan, Z.; Zhao, Y.; Feng, L.; Ma, C. One-pot three-component synthesis of quinazolines via a copper-catalysed oxidative amination reaction. Org. Biomol. Chem. 2016, 14, 6561–6567. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shi, M.; Niu, B.; Meng, X.; Zhu, C.; Liu, G.; Chen, T.; Liu, Y. Copper-catalyzed aerobic oxidative decarboxylative amination of arylacetic acids: A facile access to 2-arylquinazolines. RSC Adv. 2016, 6, 36192–36197. [Google Scholar] [CrossRef]
- Visweswara Sastry, K.N.; Prasad, B.; Nagaraju, B.; Ganga Reddy, V.; Alarifi, A.; Babu, B.N.; Kamal, A. Copper-Catalysed Tandem Synthesis of Substituted Quinazolines from Phenacyl Azides and O-Carbonyl Anilines. ChemistrySelect 2017, 2, 5378–5383. [Google Scholar] [CrossRef]
- Liang, E.; Wu, Y.; Chen, J.; Xiong, W.; Zhao, J.; Yao, X.; Tang, X. Copper-catalyzed aerobic oxidative cyclization protocol for the synthesis of quinazolines via amination of C (sp3)-H bonds of methylazaarenes. Tetrahedron 2019, 75, 130783. [Google Scholar] [CrossRef]
- Truong, V.L.; Morrow, M. Mild and efficient ligand-free copper-catalyzed condensation for the synthesis of quinazolines. Tetrahedron Lett. 2010, 51, 758–760. [Google Scholar] [CrossRef]
- Raut, A.B.; Tiwari, A.R.; Bhanage, B.M. Ultrasound-Assisted Preparation of Copper (I) Oxide Nanocubes: High Catalytic Activity in the Synthesis of Quinazolines. ChemCatChem 2017, 9, 1292–1297. [Google Scholar] [CrossRef]
- Ju, J.; Hua, R.; Su, J. Copper-catalyzed three-component one-pot synthesis of quinazolines. Tetrahedron 2012, 68, 9364–9370. [Google Scholar] [CrossRef]
- Han, B.; Yang, X.-L.; Wang, C.; Bai, Y.-W.; Pan, T.-C.; Chen, X.; Yu, W. CuCl/DABCO/4-HO-TEMPO-Catalyzed Aerobic Oxidative Synthesis of 2-Substituted Quinazolines and 4 H-3,1-Benzoxazines. J. Org. Chem. 2011, 77, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Rui, X.; Si, D.; Dai, R.; Zhu, Y.; Wen, H.; Li, W.; Liu, J. Copper-Catalyzed Three-Component Cascade Reaction of Benzaldehyde with Benzylamine and Hydroxylamine or Aniline: Synthesis of 1,2,4-Oxadiazoles and Quinazolines. Adv. Synth. Catal. 2021, 363, 2825–2833. [Google Scholar]
- Wang, C.; Li, S.; Liu, H.; Jiang, Y.; Fu, H. Copper-catalyzed synthesis of quinazoline derivatives via ullmann-type coupling and aerobic oxidation. J. Org. Chem. 2010, 75, 7936–7938. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhao, Y.; Fu, H.; Cheng, C. Copper-catalyzed sequential n-arylation and aerobic oxidation: Synthesis of quinazoline derivatives. Synlett 2013, 24, 2089–2094. [Google Scholar] [CrossRef]
- Omar, M.A.; Conrad, J.; Beifuss, U. Copper-catalyzed domino reaction between 1-(2-halophenyl) methanamines and amidines or imidates for the synthesis of 2-substituted quinazolines. Tetrahedron 2014, 70, 3061–3072. [Google Scholar] [CrossRef]
- Lv, Y.; Li, Y.; Xiong, T.; Pu, W.; Zhang, H.; Sun, K.; Liu, Q.; Zhang, Q. Copper-catalyzed annulation of amidines for quinazoline synthesis. Chem. Commun. 2013, 49, 6439–6441. [Google Scholar] [CrossRef]
- Ohta, Y.; Tokimizu, Y.; Oishi, S.; Fujii, N.; Ohno, H. Direct synthesis of quinazolines through copper-catalyzed reaction of aniline-derived benzamidines. Org. Lett. 2010, 12, 3963–3965. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Guo, F.; Wang, F.; Zhao, N.; Liu, L.; Li, J.; Wang, Z. Synthesis of quinazolines via CuO nanoparticles catalyzed aerobic oxidative coupling of aromatic alcohols and amidines. Org. Biomol. Chem. 2014, 12, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, H.; Sun, Y.; Shi, Z.; Ni, K.; Li, F.; Chen, D. A Highly Efficient Copper-Catalyzed Three-Component Synthesis of 4-Aminoquinazolines. Synthesis 2017, 49, 2535–2543. [Google Scholar] [CrossRef]
- Malakar, C.C.; Baskakova, A.; Conrad, J.; Beifuss, U. Copper-Catalyzed Synthesis of Quinazolines in Water Starting from o-Bromobenzylbromides and Benzamidines. Chem. A Eur. J. 2012, 18, 8882–8885. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Li, B.; Guo, S.; Wang, Y.; Zhang, X. Synthesis of Quinazolines and Tetrahydroquinazolines: Copper-Catalyzed Tandem Reactions of 2-Bromobenzyl Bromides with Aldehydes and Aqueous Ammonia or Amines. Chem. Asian J. 2014, 9, 739–743. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, J.; Liu, M.; Ding, J.; Gao, W.; Huang, X.; Wu, H. Unexpected copper-catalyzed cascade synthesis of quinazoline derivatives. J. Org. Chem. 2013, 78, 11342–11348. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yu, L.; Zhu, L.; Xia, X. One-Pot Tandem Synthesis of 2-Arylquinazolines by a Multicomponent Cyclization Reaction. Molecules 2013, 18, 13860–13869. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Chen, C.; Su, X.; Li, M.; Wen, L. Diverse tandem cyclization reactions of o-cyanoanilines and diaryliodonium salts with copper catalyst for the construction of quinazolinimine and acridine scaffolds. Org. Lett. 2014, 16, 6228–6231. [Google Scholar] [CrossRef]
- Battula, S.; Vishwakarma, R.A.; Ahmed, Q.N. Cu–benzotriazole-catalyzed electrophilic cyclization of N-arylimines: A methodical tandem approach to O-protected-4hydroxyquinazolines. RSC Adv. 2014, 4, 38375–38378. [Google Scholar] [CrossRef]
- Gawande, S.D.; Zanwar, M.R.; Kavala, V.; Kuo, C.W.; Rajawinslin, R.; Yao, C.F. One-Pot Synthesis of 2-Arylquinazolines and Tetracyclic Isoindolo [1, 2-a] quinazolines via Cyanation Followed by Rearrangement of ortho-Substituted 2-Halo-N-arylbenzamides. Adv. Synth. Catal. 2015, 357, 168–176. [Google Scholar] [CrossRef]
- Tran, L.Q.; Li, J.; Neuville, L. Copper-catalyzed domino three-component approach for the assembly of 2-Aminated benzimidazoles and quinazolines. J. Org. Chem. 2015, 80, 6102–6108. [Google Scholar] [CrossRef]
- Rodrigues, R.; Tran, L.Q.; Darses, B.; Dauban, P.; Neuville, L. Copper-Promoted Tandem Three-Component Access to Quinazolin-4 (H)-imines and Benzimidazo [1, 2-c] quinazolines. Adv. Synth. Catal. 2019, 361, 4454–4460. [Google Scholar] [CrossRef]
- Xu, C.; Jia, F.-C.; Zhou, Z.-W.; Zheng, S.-J.; Li, H.; Wu, A.-X. Copper-catalyzed multicomponent domino reaction of 2-bromoaldehydes, benzylamines, and sodium azide for the assembly of quinazoline derivatives. J. Org. Chem. 2016, 81, 3000–3006. [Google Scholar] [CrossRef]
- Wang, X.; He, D.; Huang, Y.; Fan, Q.; Wu, W.; Jiang, H. Copper-Catalyzed Synthesis of Substituted Quinazolines from Benzonitriles and 2-Ethynylanilines via Carbon–Carbon Bond Cleavage Using Molecular Oxygen. J. Org. Chem. 2018, 83, 5458–5466. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lu, J.; Fu, H. Copper-catalyzed cascade synthesis of benzimidazoquinazoline derivatives under mild condition. Chem. Commun. 2011, 47, 5596–5598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jin, Y.; Liu, H.; Jiang, Y.; Fu, H. Copper-Catalyzed Cascade Synthesis of 1H-Indolo [1, 2-c] quinazoline Derivatives. Eur. J. Org. Chem. 2012, 2012, 6798–6803. [Google Scholar] [CrossRef]
- Sang, P.; Xie, Y.; Zou, J.; Zhang, Y. Copper-Catalyzed Sequential Ullmann N-Arylation and Aerobic Oxidative C–H Amination: A Convenient Route to Indolo [1,2-c] quinazoline Derivatives. Org. Lett. 2012, 14, 3894–3897. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yang, H.; Jiang, Y.; Zhao, Y.; Fu, H. General and efficient copper-catalyzed aerobic oxidative synthesis of N-fused heterocycles using amino acids as the nitrogen source. RSC Adv. 2013, 3, 15636–15644. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Pericherla, K.; Kaswan, P.; Kumar, A. Synthesis of novel azole-fused quinazolines via one-pot, sequential Ullmann-type coupling and intramolecular dehydrogenative C–N bonding. Org. Biomol. Chem. 2015, 13, 2947–2950. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Singh, R.P.; Patel, O.P.; Dhiman, S.; Saini, H.K.; Jha, P.N.; Kumar, A. Design and synthesis of imidazo/benzimidazo [1,2-c] quinazoline derivatives and evaluation of their antimicrobial activity. ACS Omega 2018, 3, 16338–16346. [Google Scholar] [CrossRef]
- Rai, B.; Kumar, P.; Kumar, A. Copper-mediated aerobic oxidative synthesis of benzimidazo fused quinazolines via a multicomponent approach. RSC Adv. 2015, 5, 85915–85918. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Shinde, V.N.; Kumar, A. Copper-Catalyzed One-Pot, Three-Component Synthesis of Imidazo [1, 2-c] quinazolines and Benzimidazo [1, 2-c] quinazolines. ChemistrySelect 2021, 6, 12205–12208. [Google Scholar] [CrossRef]
- Guo, S.; Tao, L.; Zhang, W.; Zhang, X.; Fan, X. Regioselective Synthesis of Indolo [1,2-c] quinazolines and 11 H-Indolo [3,2-c] quinolines via Copper-Catalyzed Cascade Reactions of 2-(2-Bromoaryl)-1 H-indoles with Aldehydes and Aqueous Ammonia. J. Org. Chem. 2015, 80, 10955–10964. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, N.K.; Dhiman, S.; Shelke, G.M.; Kumar, A. Copper-catalyzed tandem Ullmann type C–N coupling and dehydrative cyclization: Synthesis of imidazo[1,2-c]quinazolines. Org. Biomol. Chem. 2016, 14, 1736–1741. [Google Scholar] [CrossRef] [PubMed]
- Nandwana, N.K.; Dhiman, S.; Saini, H.K.; Kumar, I.; Kumar, A. Synthesis of Quinazolinones, Imidazo [1, 2-c] quinazolines and Imidazo [4, 5-c] quinolines through Tandem Reductive Amination of Aryl Halides and Oxidative Amination of C (sp3)–H Bonds. Eur. J. Org. Chem. 2017, 2017, 514–522. [Google Scholar] [CrossRef]
- Nandwana, N.K.; Shinde, V.N.; Saini, H.K.; Kumar, A. Copper-Catalyzed One-Pot Tandem Reaction for the Synthesis of Imidazo [1, 2-c][1, 2, 3] triazolo [1, 5-a] quinazolines. Eur. J. Org. Chem. 2017, 2017, 6445–6449. [Google Scholar] [CrossRef]
- Dao, P.D.Q.; Lee, H.K.; Sohn, H.-S.; Yoon, N.S.; Cho, C.S. Synthesis of Benzo [4, 5] imidazo [1,2-c] pyrimidin-1-amines and Their Analogs via Copper-Catalyzed C–N Coupling and Cyclization. ACS Omega 2017, 2, 2953–2958. [Google Scholar] [CrossRef]
- Guo, S.; Wang, F.; Tao, L.; Zhang, X.; Fan, X. Solvent-Dependent Copper-Catalyzed Indolyl C3-Oxygenation and N1-Cyclization Reactions: Selective Synthesis of 3 H-Indol-3-ones and Indolo [1,2-c] quinazolines. J. Org. Chem. 2018, 83, 3889–3896. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, L.; Bao, P.; Shao, Y.; Yue, H.; Yang, D.; Yang, X.; Zhao, X.; Wang, H. Metal-Free C(sp2)–H/N–H Cross-Dehydrogenative Coupling of Quinoxalinones with Aliphatic Amines under Visible-Light Photoredox Catalysis. Org. Lett. 2018, 20, 7125–7130. [Google Scholar] [CrossRef]
- Chen, D.; Huang, L.; Yang, J.; Ma, J.; Zheng, Y.; Luo, Y.; Shen, Y.; Wu, J.; Feng, C.; Lv, X. Copper-catalyzed C–N coupling/C–H functionalization: A tandem approach to azole-fused quinazoline derivatives. Tetrahedron Lett. 2018, 59, 2005–2009. [Google Scholar] [CrossRef]
- Diep, T.D.; Dao, P.D.Q.; Ho, S.L.; Cho, C.S. Copper-Catalyzed Synthesis of Trinuclear N-Fused Hybrid Scaffolds by Double C (sp2)-N Bond Formation between 2-(2-Bromoaryl) indoles and 2-Aminoazoles. Eur. J. Org. Chem. 2020, 2020, 2807–2812. [Google Scholar] [CrossRef]
- Guo, S.; Wang, J.; Fan, X.; Zhang, X.; Guo, D. Synthesis of pyrazolo [1,5-c] quinazoline derivatives through copper-catalyzed tandem reaction of 5-(2-bromoaryl)-1 H-pyrazoles with carbonyl compounds and aqueous ammonia. J. Org. Chem. 2013, 78, 3262–3270. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jin, Y.; Liu, H.; Jiang, Y.; Fu, H. Easy and efficient one-pot synthesis of pyrazolo [1,5-c] quinazolines under mild copper-catalyzed conditions. RSC Adv. 2012, 2, 11061–11066. [Google Scholar] [CrossRef]
- Kiruthika, S.E.; Perumal, P.T. CuI-Catalyzed Coupling of gem-Dibromovinylanilides and Sulfonamides: An Efficient Method for the Synthesis of 2-Amidoindoles and Indolo [1,2-a] quinazolines. Org. Lett. 2013, 16, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Song, Y.; Zhang, X.; Guo, S.; Fan, X. Copper-catalyzed tandem reactions of 2-bromobenzaldehydes/ketones with aminopyrazoles toward the synthesis of pyrazolo [1,5-a] quinazolines. Tetrahedron Lett. 2014, 55, 4997–5002. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, H.; Gao, J.; Yang, K.; Niu, Q.; Mao, H.; Wang, X.; Lv, X. Copper-Catalyzed Domino Addition/Double Cyclization: An Approach to Polycyclic Benzimidazole Derivatives. J. Org. Chem. 2014, 79, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, W.-T.; Wang, X.-S. CuI-Catalyzed C–N Bond Formation and Cleavage for the Synthesis of Benzimidazo [1,2-a] quinazoline Derivatives. J. Org. Chem. 2014, 79, 5847–5851. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.-C.; Xu, C.; Zhou, Z.-W.; Cai, Q.; Li, D.-K.; Wu, A.-X. Consecutive cycloaddition/SNAr/reduction/cyclization/oxidation sequences: A copper-catalyzed multicomponent synthesis of fused N-heterocycles. Org. Lett. 2015, 17, 2820–2823. [Google Scholar] [CrossRef]
- Pang, X.; Chen, C.; Li, M.; Xi, C. A concise and efficient synthesis of benzimidazo [1,2-c] quinazolines through CuI-catalyzed intramolecular N-arylations. Beilstein J. Org. Chem. 2015, 11, 2365–2369. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Mao, S.; Wang, X.; Zhou, M.; Zhao, Y.-l.; Wang, H. Synthesis of Pyrazolo [1,5-c] quinazoline Derivatives through Copper-Catalyzed Domino Reaction of o-Alkenyl Aromatic Isocyanides with Diazo Compounds. Chem. Commun. 2020, 56, 7665–7668. [Google Scholar] [CrossRef]
- Muto, K.; Yamaguchi, J.; Musaev, D.G.; Itami, K. Decarbonylative organoboron cross-coupling of esters by nickel catalysis. Nat. Commun. 2015, 6, 7508. [Google Scholar] [CrossRef]
- Dhiman, S.; Nandwana, N.K.; Saini, H.K.; Kumar, D.; Rangan, K.; Robertson, K.N.; Jha, M.; Kumar, A. Nickel-Catalyzed Tandem Knoevenagel Condensation and Intramolecular Direct Arylation: Synthesis of Pyrazolo [5, 1-a]-isoquinoline Derivatives. Adv. Synth. Catal. 2018, 360, 1973–1983. [Google Scholar] [CrossRef]
- Subaramanian, M.; Sivakumar, G.; Balaraman, E. Recent advances in nickel-catalyzed C–C and C–N bond formation via HA and ADC reactions. Org. Biomol. Chem. 2021, 19, 4213–4227. [Google Scholar] [CrossRef]
- Shinde, A.H.; Arepally, S.; Baravkar, M.D.; Sharada, D.S. Nickel-Catalyzed Aerobic Oxidative Isocyanide Insertion: Access to Benzimidazoquinazoline Derivatives via a Sequential Double Annulation Cascade (SDAC) Strategy. J. Org. Chem. 2016, 82, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Parua, S.; Sikari, R.; Sinha, S.; Chakraborty, G.; Mondal, R.; Paul, N.D. Accessing Polysubstituted Quinazolines via Nickel Catalyzed Acceptorless Dehydrogenative Coupling. J. Org. Chem. 2018, 83, 11154–11166. [Google Scholar] [CrossRef]
- Sikari, R.; Sinha, S.; Chakraborty, G.; Das, S.; van Leest, N.P.; Paul, N.D. C−N Cross-Coupling Reactions Under Mild Conditions Using Singlet Di-Radical Nickel (II)-Complexes as Catalyst: N-Arylation and Quinazoline Synthesis. Adv. Synth. Catal. 2019, 361, 4342–4353. [Google Scholar] [CrossRef]
- Chakraborty, G.; Sikari, R.; Das, S.; Mondal, R.; Sinha, S.; Banerjee, S.; Paul, N.D. Dehydrogenative synthesis of quinolines, 2-aminoquinolines, and quinazolines using singlet diradical Ni (II)-catalysts. J. Org. Chem. 2019, 84, 2626–2641. [Google Scholar] [CrossRef] [PubMed]
- Sikari, R.; Chakraborty, G.; Guin, A.K.; Paul, N.D. Nickel-catalyzed [4 + 2] annulation of nitriles and benzylamines by C–H/N–H activation. J. Org. Chem. 2020, 86, 279–290. [Google Scholar] [CrossRef]
- Ruiz-Castillo, P.; Buchwald, S.L. Applications of Palladium-Catalyzed C–N Cross-Coupling Reactions. Chem. Rev. 2016, 116, 12564–12649. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; Peng, J.; Zhu, Q. Palladium-catalyzed intramolecular C (sp2)–H amidination by isonitrile insertion provides direct access to 4-aminoquinazolines from N-arylamidines. Org. Lett. 2011, 13, 4604–4607. [Google Scholar] [CrossRef]
- Van Baelen, G.; Kuijer, S.; Rýček, L.; Sergeyev, S.; Janssen, E.; de Kanter, F.J.; Maes, B.U.; Ruijter, E.; Orru, R.V. Synthesis of 4-Aminoquinazolines by Palladium-Catalyzed Intramolecular Imidoylation of N-(2-Bromoaryl) amidines. Chem.-A Eur. J. 2011, 17, 15039–15044. [Google Scholar] [CrossRef] [PubMed]
- McGowan, M.A.; McAvoy, C.Z.; Buchwald, S.L. Palladium-catalyzed N-monoarylation of amidines and a one-pot synthesis of quinazoline derivatives. Org. Lett. 2012, 14, 3800–3803. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, H.; Chen, Y.; Deng, G.-J. Palladium-catalyzed one pot 2-arylquinazoline formation via hydrogen-transfer strategy. Org. Biomol. Chem. 2014, 12, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Natte, K.; Neumann, H.; Wu, X.-F. A convenient palladium-catalyzed carbonylative synthesis of quinazolines from 2-aminobenzylamine and aryl bromides. RSC Adv. 2014, 4, 56502–56505. [Google Scholar] [CrossRef]
- Vlaar, T.; Bensch, L.; Kraakman, J.; Vande Velde, C.M.; Maes, B.U.; Orru, R.V.; Ruijter, E. Synthesis of Diverse Azolo [c] quinazolines by Palladium (II)-Catalyzed Aerobic Oxidative Insertion of Isocyanides. Adv. Synth. Catal. 2014, 356, 1205–1209. [Google Scholar] [CrossRef]
- Xu, L.; Li, H.; Liao, Z.; Lou, K.; Xie, H.; Li, H.; Wang, W. Divergent synthesis of imidazoles and quinazolines via Pd (OAc) 2-catalyzed annulation of N-allylamidines. Org. Lett. 2015, 17, 3434–3437. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Zhen, Q.; Gong, J.; Cheng, T.; Qi, L.; Shao, Y.; Chen, J. Palladium-catalyzed three-component tandem process: One-pot assembly of quinazolines. Org. Lett. 2018, 20, 3083–3087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shao, Y.; Gong, J.; Hu, K.; Cheng, T.; Chen, J. Palladium-Catalyzed Tandem Reaction of Quinazolinone-Based Nitriles with Arylboronic Acids: Synthesis of 2-(4-Arylquinazolin-2-yl) anilines. Adv. Synth. Catal. 2018, 360, 3260–3265. [Google Scholar] [CrossRef]
- Zhu, J.; Shao, Y.; Hu, K.; Qi, L.; Cheng, T.; Chen, J. Pd-Catalyzed tandem reaction of N-(2-cyanoaryl) benzamides with arylboronic acids: Synthesis of quinazolines. Org. Biomol. Chem. 2018, 16, 8596–8603. [Google Scholar] [CrossRef]
- Gong, J.; Hu, K.; Zhang, Y.; Shao, Y.; Cheng, T.; Hu, M.; Chen, J. Efficient Tandem Addition/Cyclization for Access to 2,4-Diarylquinazolines via Catalytic Carbopalladation of Nitriles. Molecules 2019, 24, 463. [Google Scholar] [CrossRef]
- Guo, X.; Hu, J.; Zhang, M.; Wang, L. Palladium-Catalyzed C (sp2)−H Activation for the Formation of C−N Bonds: Rapid Access to Benzimidazoquinazolines. Asian J. Org. Chem. 2019, 8, 417–421. [Google Scholar] [CrossRef]
- Pathare, R.S.; Maurya, A.K.; Kumari, A.; Agnihotri, V.K.; Verma, V.P.; Sawant, D.M. Synthesis of quinazoline-3-oxides via a Pd (ii) catalyzed azide–isocyanide coupling/cyclocondensation reaction. Org. Biomol. Chem. 2019, 17, 363–368. [Google Scholar] [CrossRef]
- Demakova, M.Y.; Islamova, R.; Suslonov, V. Palladium-Catalyzed Synthesis of 4-Aminoquinazolines from Amide Oxime Ethers and 2-Iodobenzonitrile. Russ. J. Gen. Chem. 2019, 89, 668–672. [Google Scholar] [CrossRef]
- Meera, G.; Rohit, K.; Treesa, G.S.; Anilkumar, G. Advances and Prospects in Gold-Catalyzed C−H Activation. Asian J. Org. Chem. 2020, 9, 144–161. [Google Scholar] [CrossRef]
- Tang, L.; Yang, Y.; Wen, L.; Zhang, S.; Zha, Z.; Wang, Z. Supported gold-catalyzed and ammonia-promoted selective synthesis of quinazolines in aqueous media. Org. Chem. Front. 2015, 2, 114–118. [Google Scholar] [CrossRef]
- Tang, H.-T.; Xiong, K.; Li, R.-H.; Ding, Z.-C.; Zhan, Z.-P. Synthesis of 5,6-Dihydropyrazolo [1,5-c] quinazolines through Gold-Catalyzed Chemoselective Bicyclization of N-Propargylic Sulfonylhydrazones. Org. Lett. 2015, 17, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Sonam; Nandwana, N.K.; Shinde, V.N.; Rangan, K.; Kumar, A. Ruthenium(II)-Catalyzed Annulation of 2-Arylimidazoles with Arylglyoxals: Synthesis of 5-Hydroxyimidazo[2,1-a]isoquinolin-6(5H)-ones and Their Photophysical Studies. Eur. J. Org. Chem. 2023, 26, e202201065. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, M.; Xiong, B.; Tan, Z.; Lv, W.; Jiang, H. A novel ruthenium-catalyzed dehydrogenative synthesis of 2-arylquinazolines from 2-aminoaryl methanols and benzonitriles. Org. Lett. 2014, 16, 6028–6031. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.-M.; Liu, Z.-L.; Liu, W.-Q.; Cao, X.-N.; Zhu, X.; Zhao, X.-M.; Song, B.; Hao, X.-Q.; Liu, G. NNN pincer Ru (II)-catalyzed dehydrogenative coupling of 2-aminoarylmethanols with nitriles for the construction of quinazolines. Tetrahedron 2019, 75, 2697–2705. [Google Scholar] [CrossRef]
- Prakash, R.; Bora, B.R.; Boruah, R.C.; Gogoi, S. Ru (II)-catalyzed C–H activation and annulation reaction via carbon–carbon triple bond cleavage. Org. Lett. 2018, 20, 2297–2300. [Google Scholar] [CrossRef]
- Kirinde Arachchige, P.T.; Yi, C.S. Synthesis of Quinazoline and Quinazolinone Derivatives via Ligand-Promoted Ruthenium-Catalyzed Dehydrogenative and Deaminative Coupling Reaction of 2-Aminophenyl Ketones and 2-Aminobenzamides with Amines. Org. Lett. 2019, 21, 3337–3341. [Google Scholar] [CrossRef]
- Shinde, V.N.; Rangan, K.; Kumar, D.; Kumar, A. Rhodium(III)-Catalyzed Dehydrogenative Annulation and Spirocyclization of 2-Arylindoles and 2-(1H-Pyrazol-1-yl)-1H-indoles with Maleimides: A Facile Access to Isogranulatimide Alkaloid Analogues. J. Org. Chem. 2021, 86, 2328–2338. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.K.; Guin, S.; Maiti, S.; Biswas, J.P.; Porey, S.; Maiti, D. Toolbox for Distal C–H Bond Functionalizations in Organic Molecules. Chem. Rev. 2022, 122, 5682–5841. [Google Scholar] [CrossRef]
- Lei, X.; Gao, M.; Tang, Y. Rh (II)-catalyzed transannulation of N-sulfonyl-1,2,3-triazoles with 2,1-benzisoxazoles or 1,2-benzisoxazoles. Org. Lett. 2016, 18, 4990–4993. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zha, S.; Chen, K.; Zhang, F.; Song, C.; Zhu, J. Quinazoline Synthesis via Rh(III)-Catalyzed Intermolecular C–H Functionalization of Benzimidates with Dioxazolones. Org. Lett. 2016, 18, 2062–2065. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Yang, X.; Zhou, X.; Li, X. Rh (III)-and Zn (II)-Catalyzed Synthesis of Quinazoline N-Oxides via C–H Amidation–Cyclization of Oximes. Org. Lett. 2016, 18, 6144–6147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiao, N. Rh-and Cu-Cocatalyzed Aerobic Oxidative Approach to Quinazolines via [4 + 2] C–H Annulation with Alkyl Azides. Org. Lett. 2016, 18, 2150–2153. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, S.; Xu, S.; Cheng, J. Rh-Catalyzed Annulation of ortho-C−H Bonds of 2-Arylimidazoles with 1,4,2-Dioxazol-5-ones toward 5-Arylimidazo [1,2-c] quinazolines. Adv. Synth. Catal. 2018, 360, 1111–1115. [Google Scholar] [CrossRef]
- Xu, L.; Li, T.; Wang, L.; Cui, X. Rh (III)-Catalyzed One-Pot Synthesis of Benzimidazoquinazolines via C–H Amidation–Cyclization of N-LG-2-phenylbenzoimidazoles. J. Org. Chem. 2018, 84, 560–567. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Chen, D.; Wang, B.; Yang, X.; Ma, Y.; Szostak, M. Rh (III)-Catalyzed C–H Amidation of 2-Arylindoles with Dioxazolones: A Route to Indolo [1,2-c] quinazolines. Org. Lett. 2019, 21, 7038–7043. [Google Scholar] [CrossRef]
- Lai, R.; Wu, X.; Lv, S.; Zhang, C.; He, M.; Chen, Y.; Wang, Q.; Hai, L.; Wu, Y. Synthesis of indoles and quinazolines via additive-controlled selective C–H activation/annulation of N-arylamidines and sulfoxonium ylides. Chem. Commun. 2019, 55, 4039–4042. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Chen, J.; Shi, Y.; Huang, T.; Hai, L.; Wu, Y. Synthesis of 4-ethenyl quinazolines via rhodium (iii)-catalyzed [5 + 1] annulation reaction of N-arylamidines with cyclopropenones. Org. Chem. Front. 2020, 7, 672–677. [Google Scholar] [CrossRef]
- Xu, H.-B.; Zhu, Y.-Y.; Yang, J.-H.; Chai, X.-Y.; Dong, L. Rh III-Catalyzed one-pot cascade synthesis of quinazolines with N-alkoxyamide as an amidating reagent. Org. Chem. Front. 2020, 7, 1230–1234. [Google Scholar] [CrossRef]


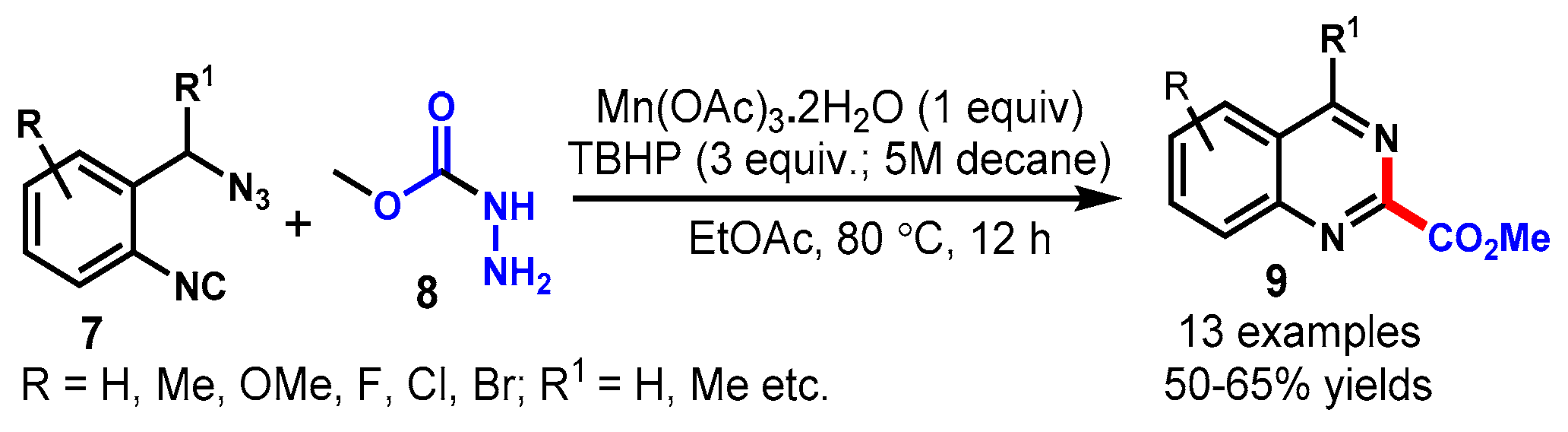


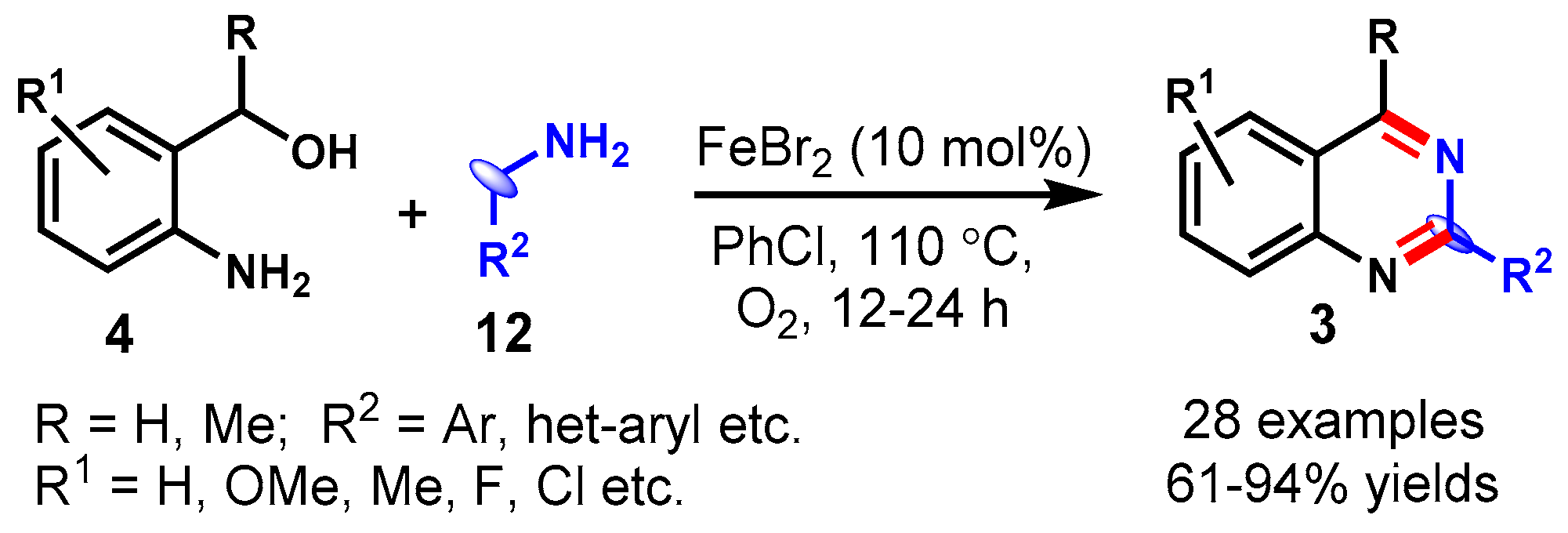
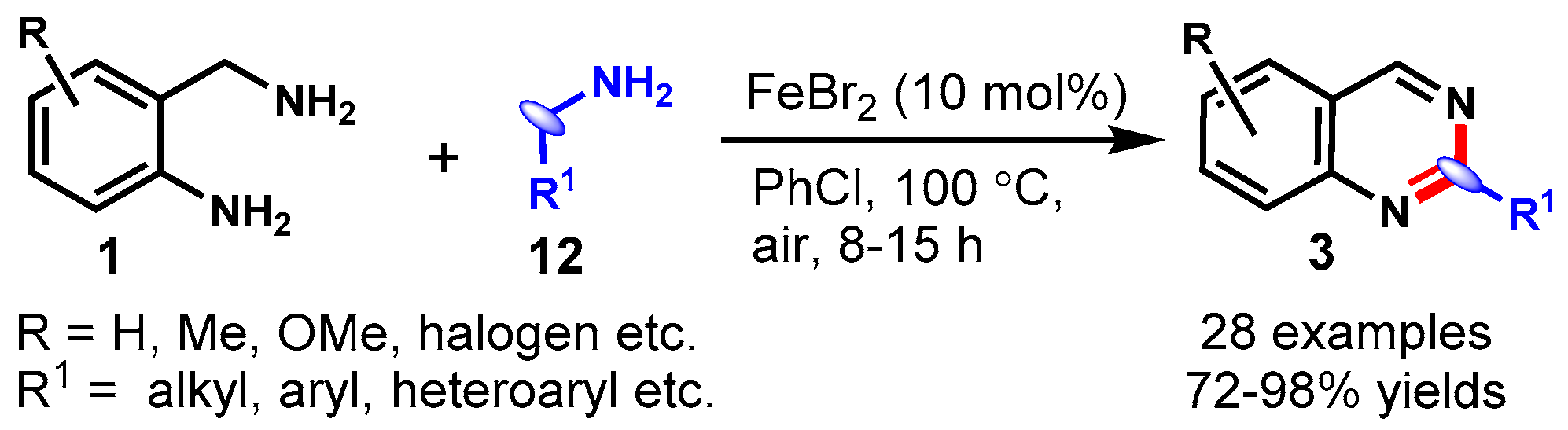

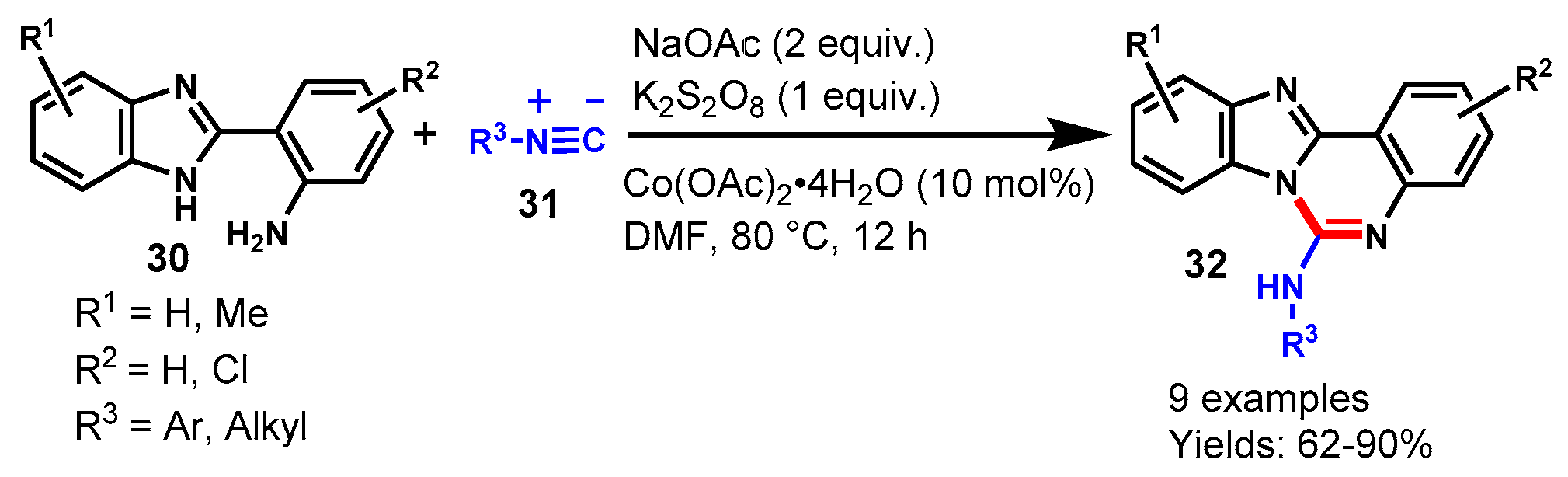
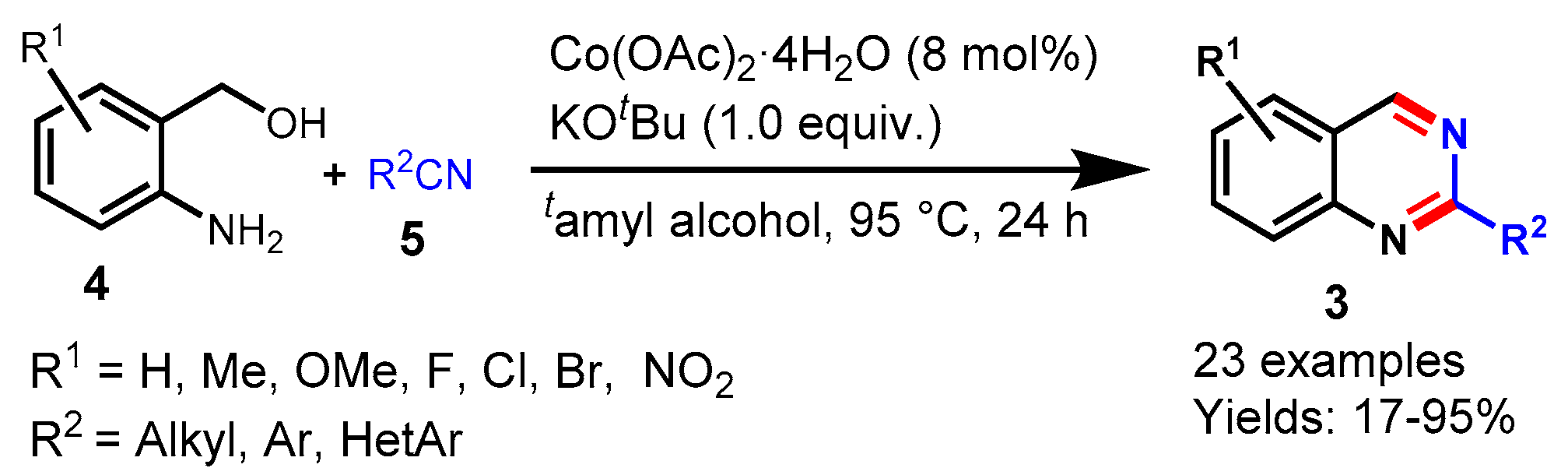








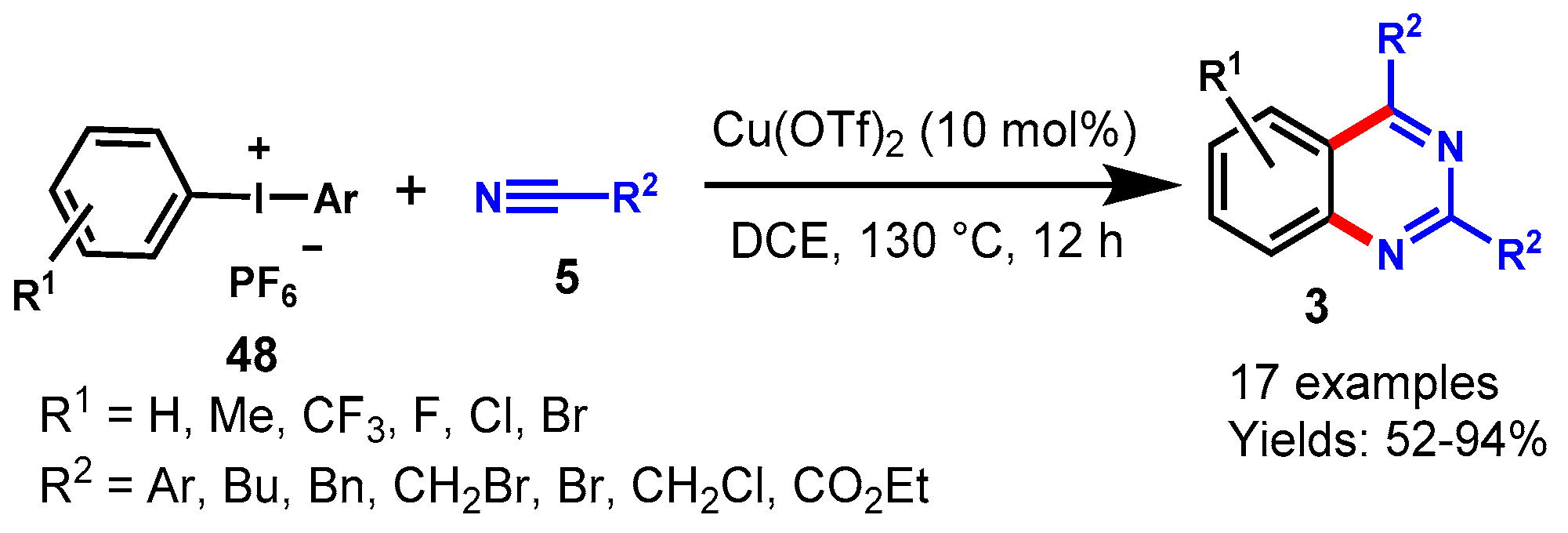
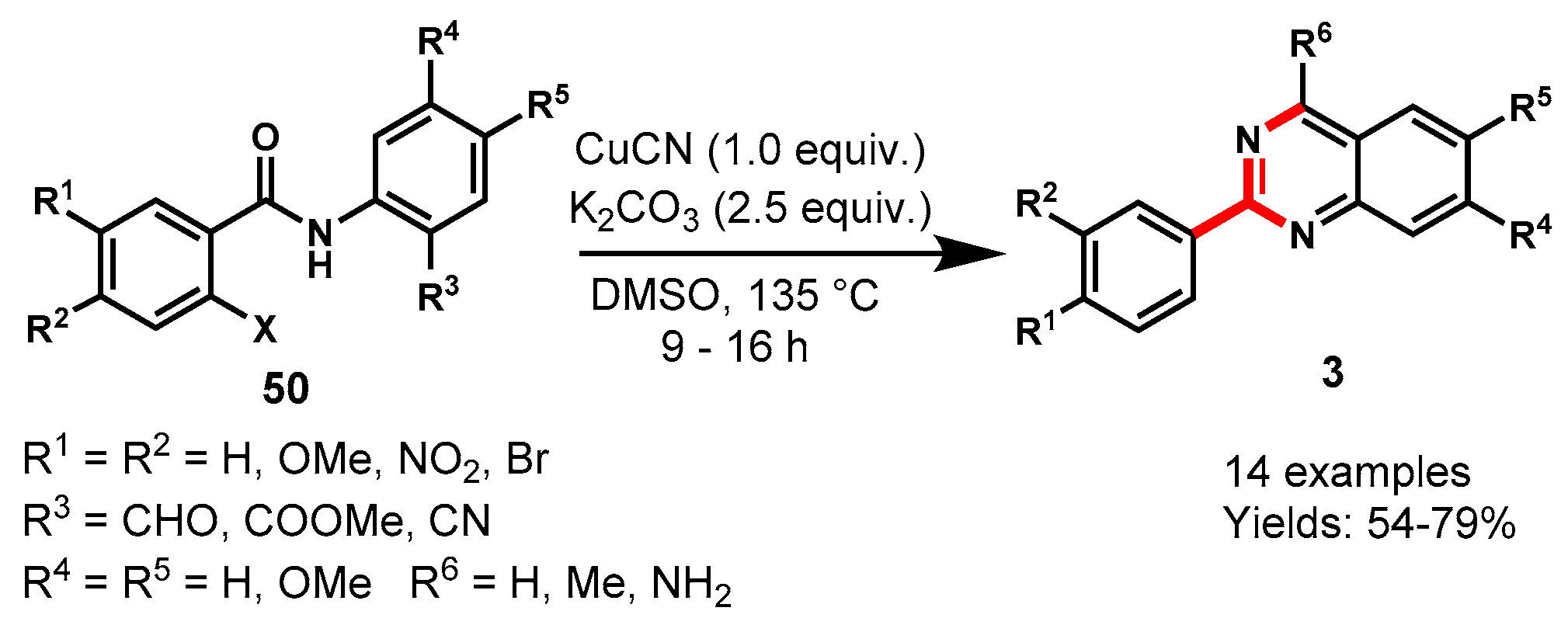
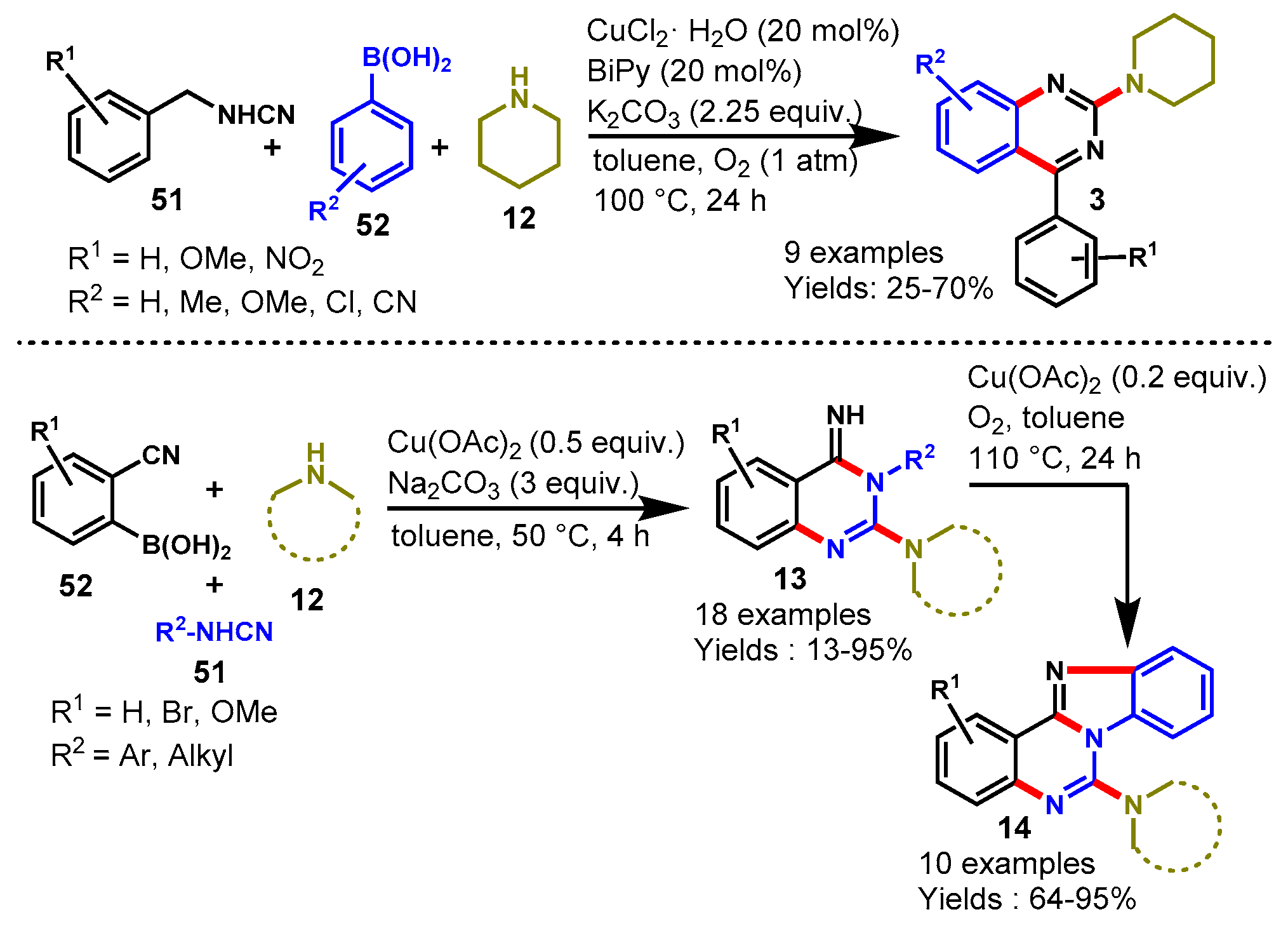
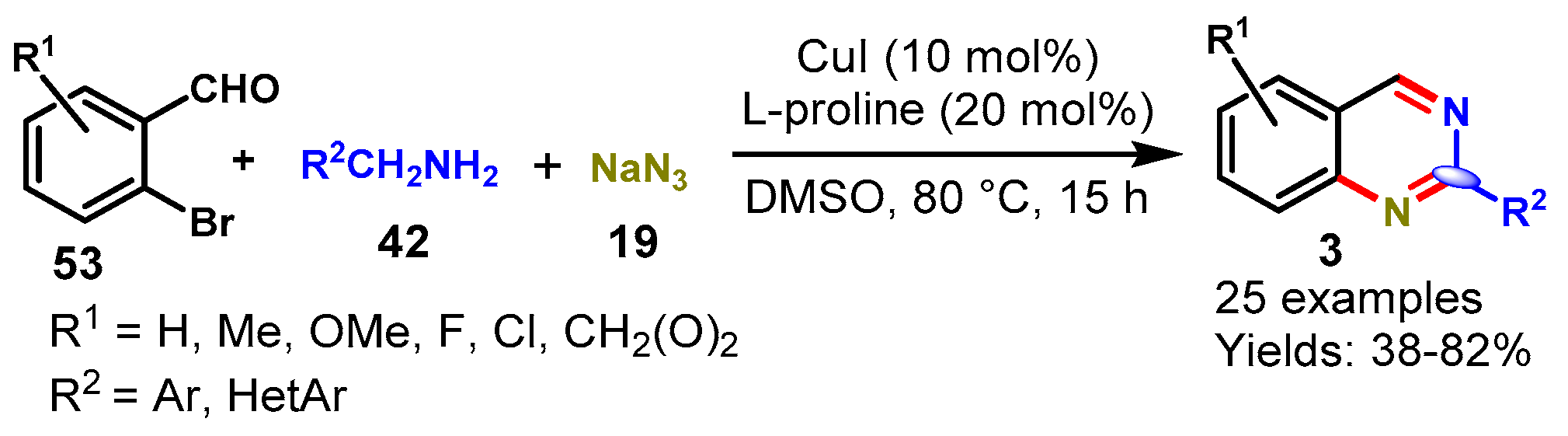
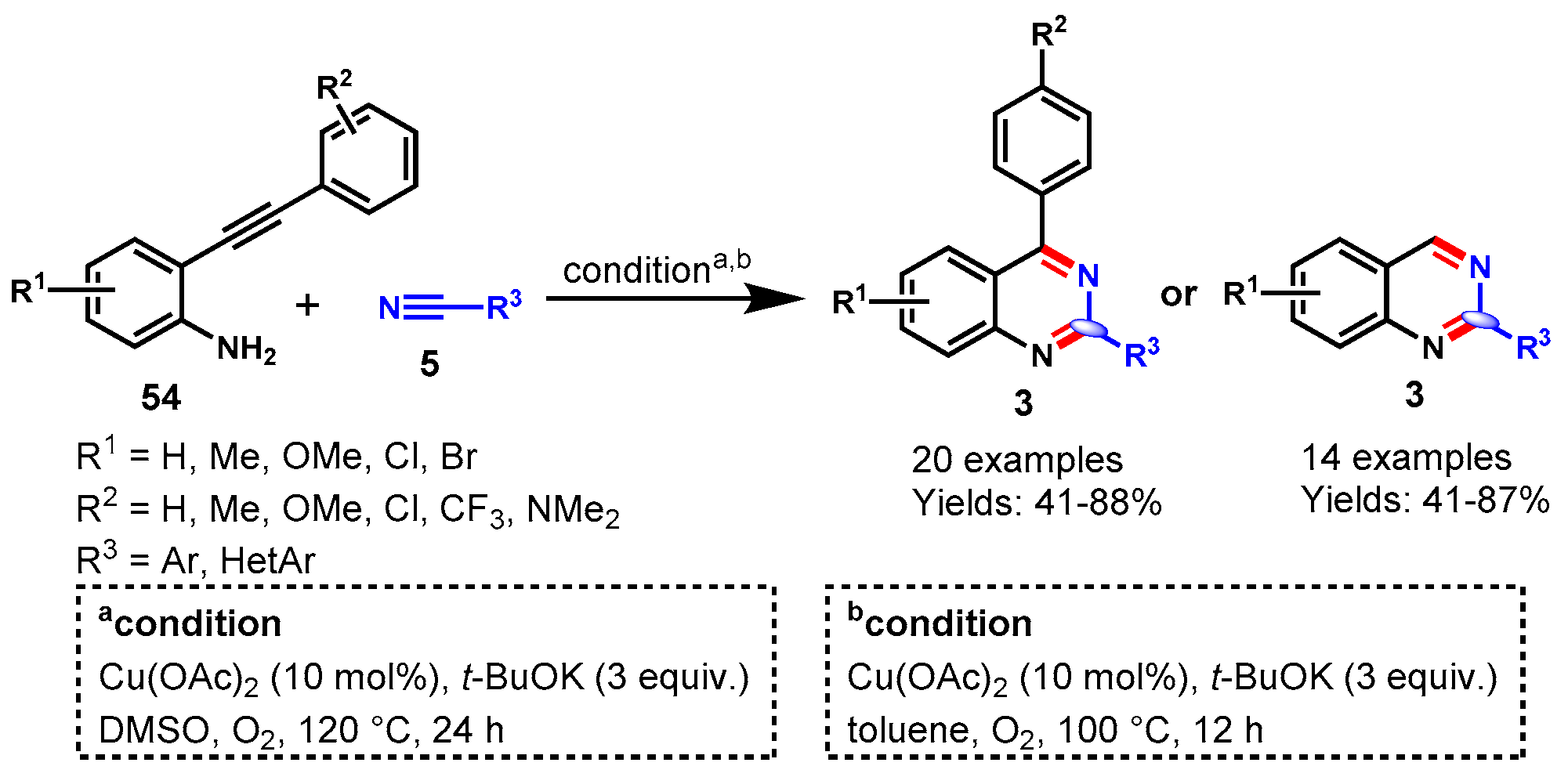

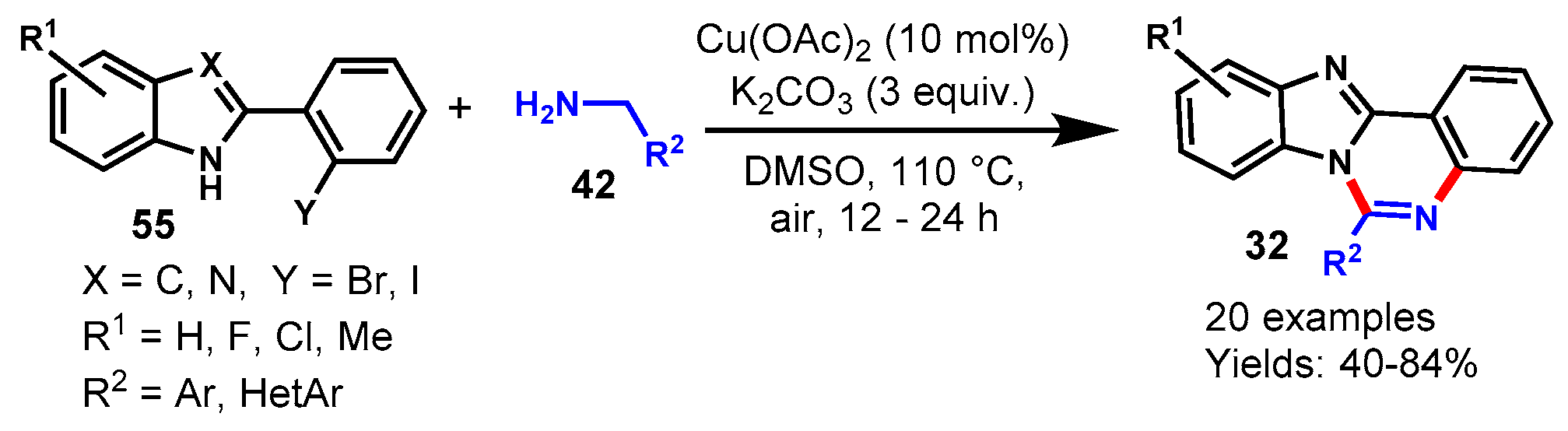

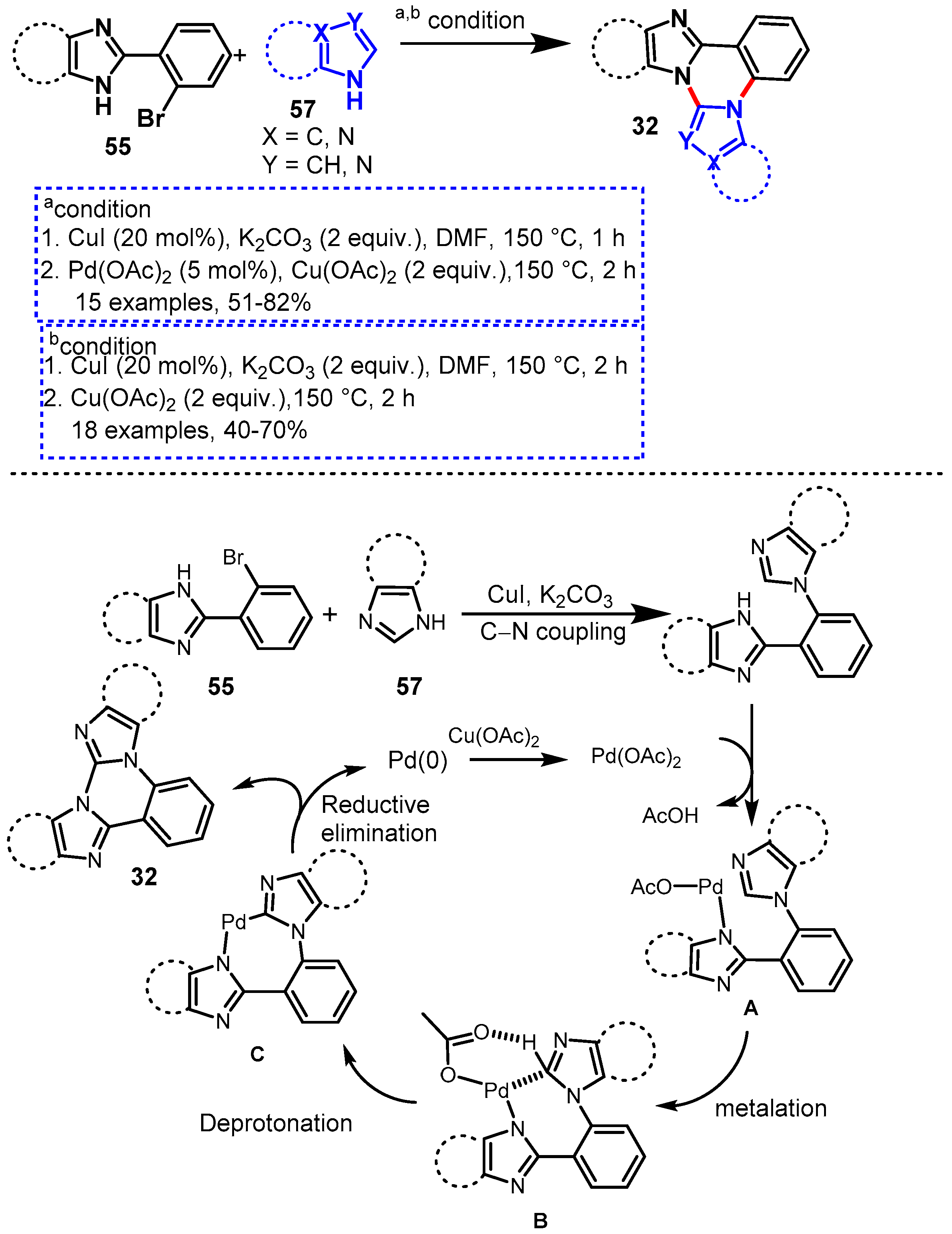
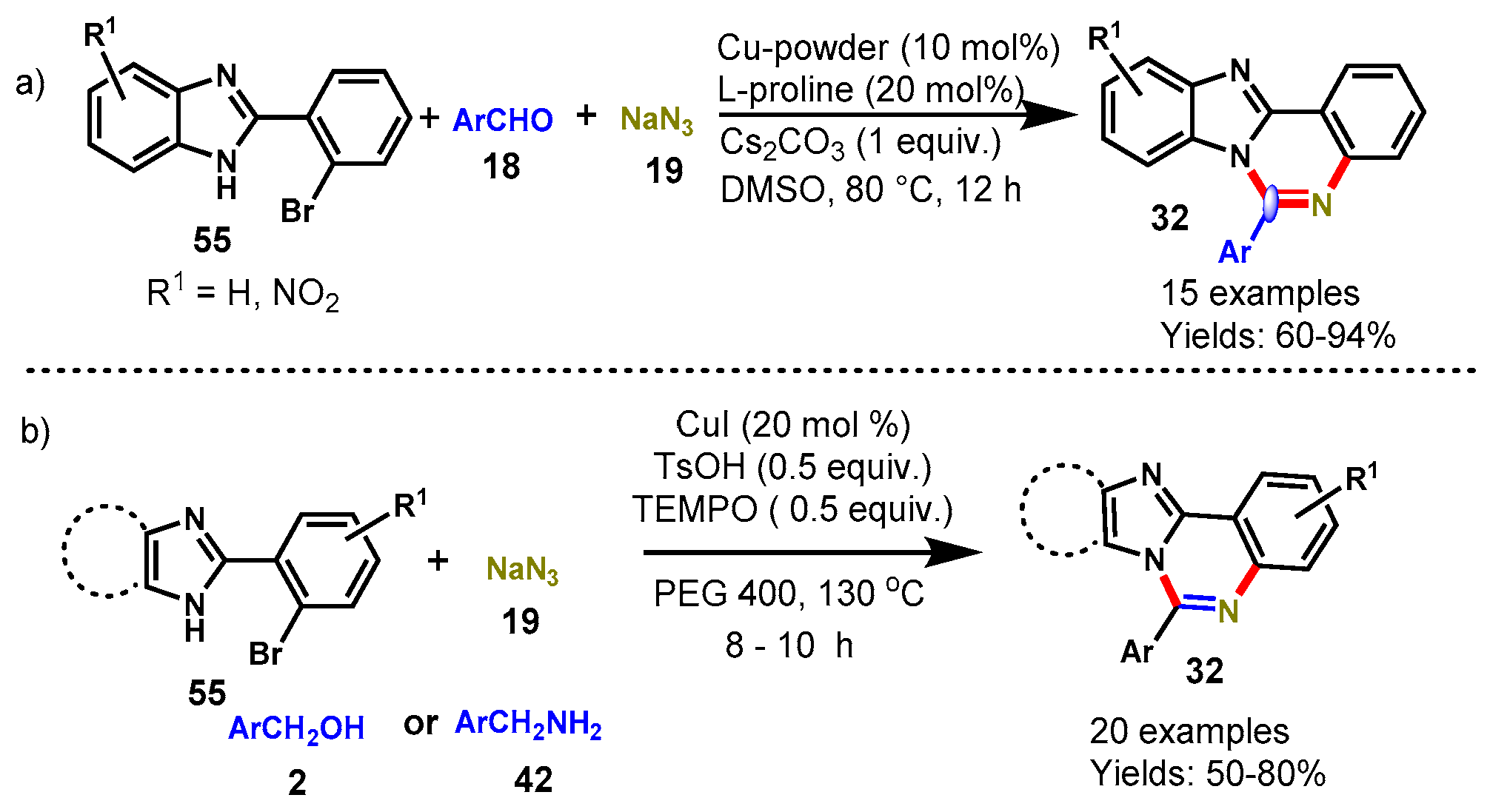
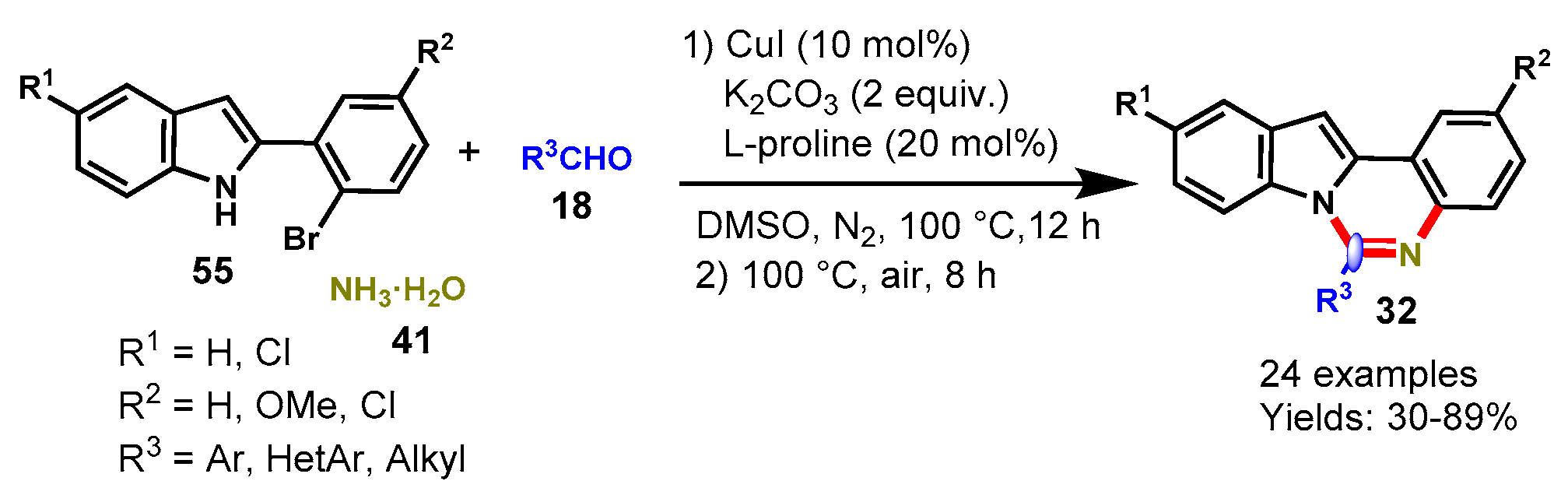
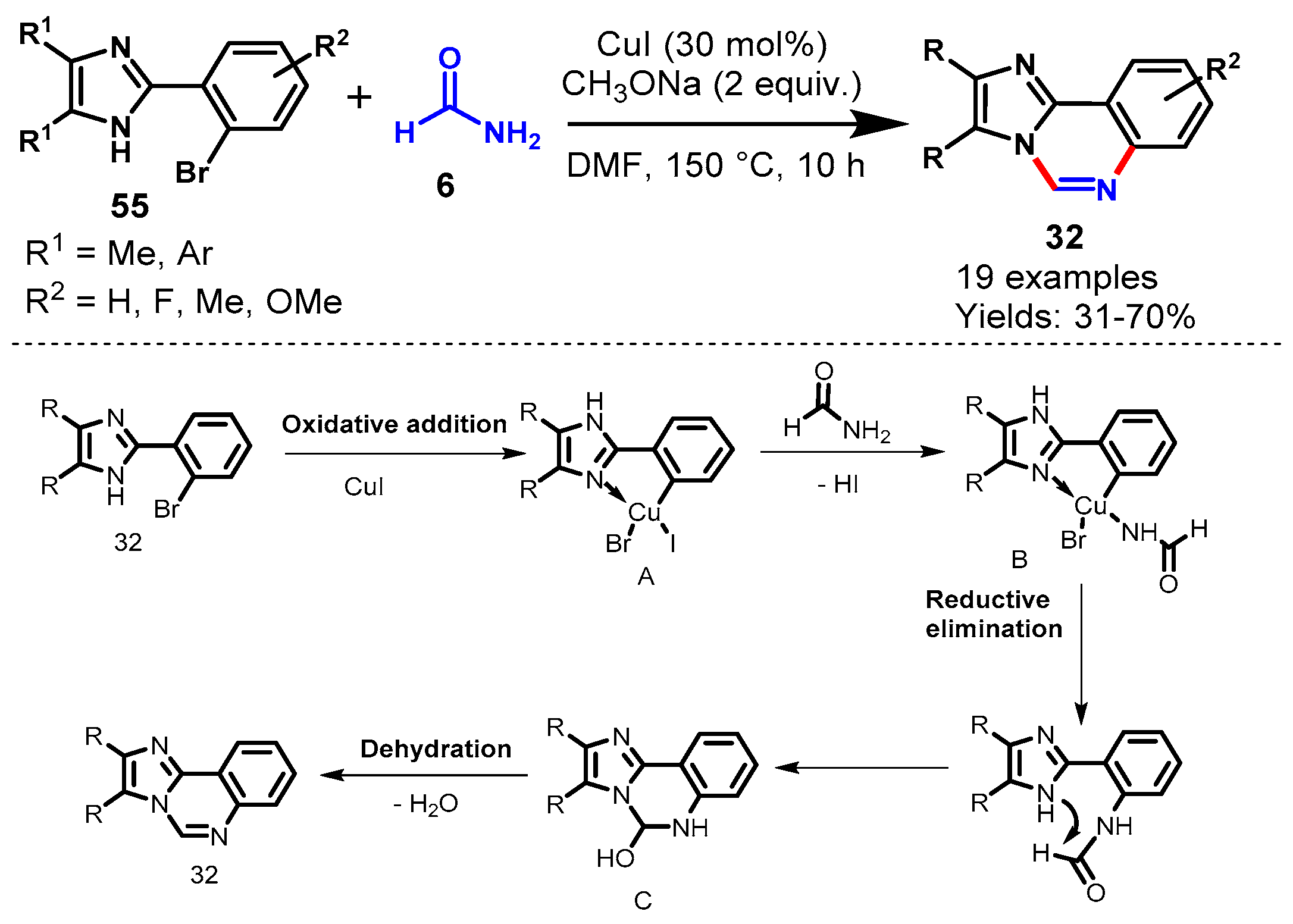
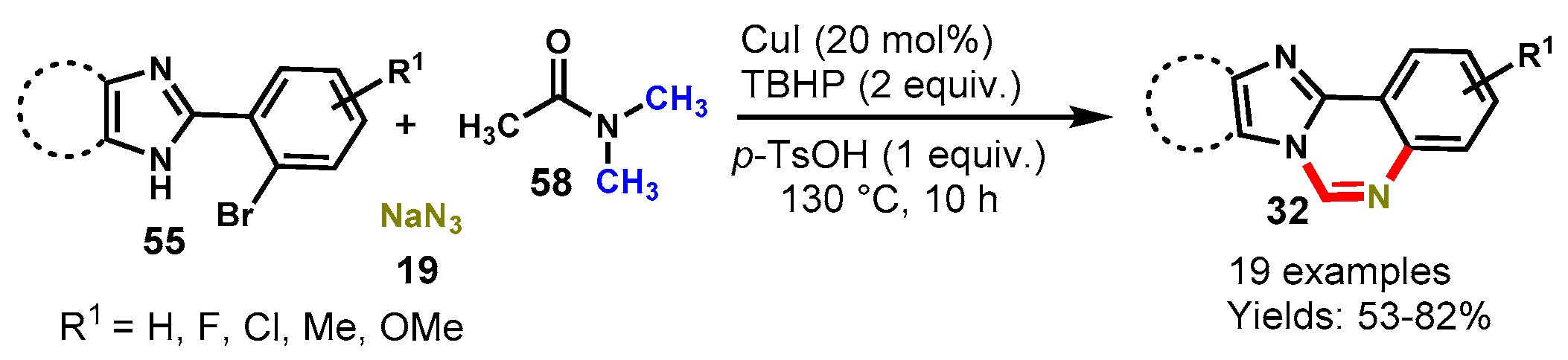
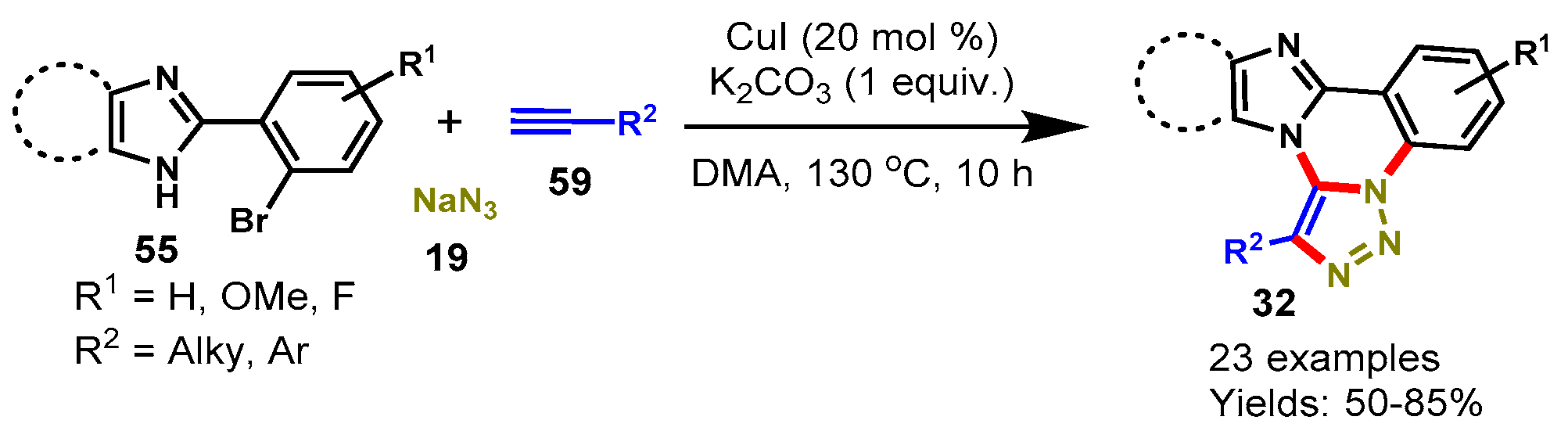
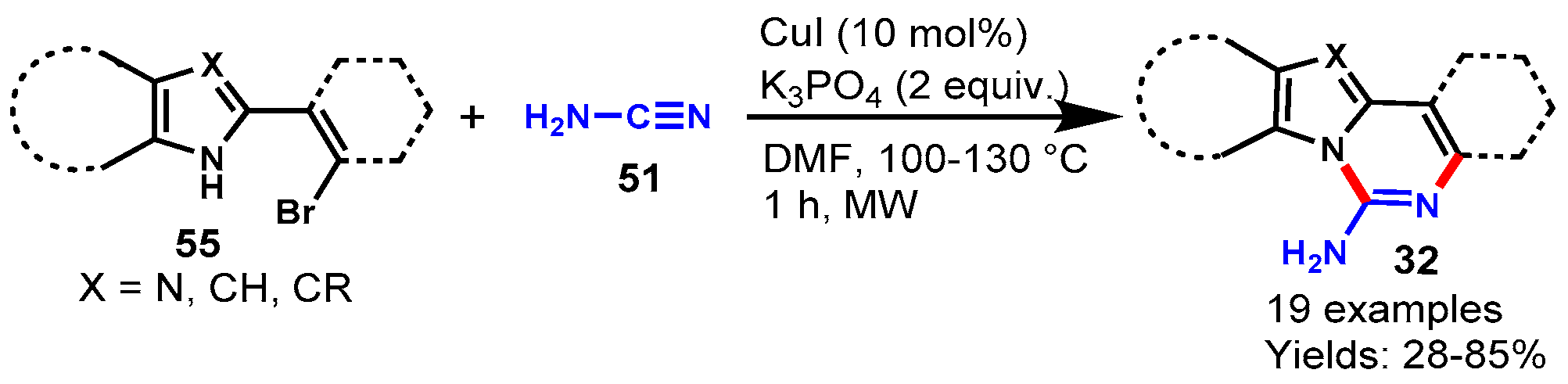
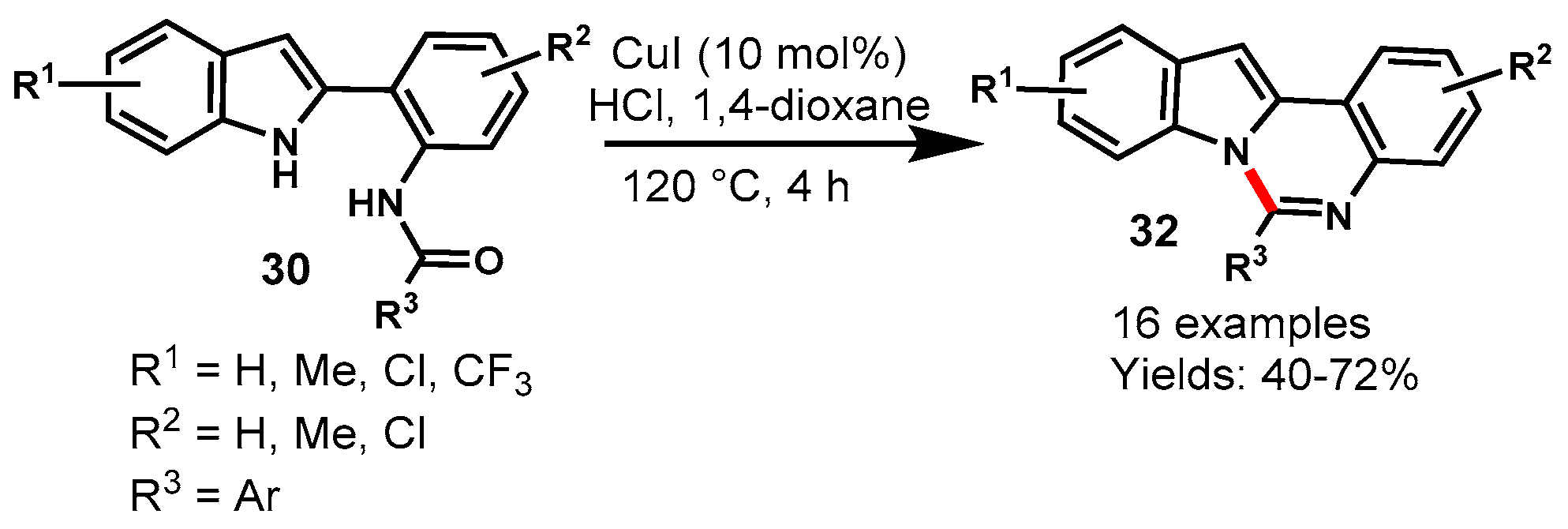
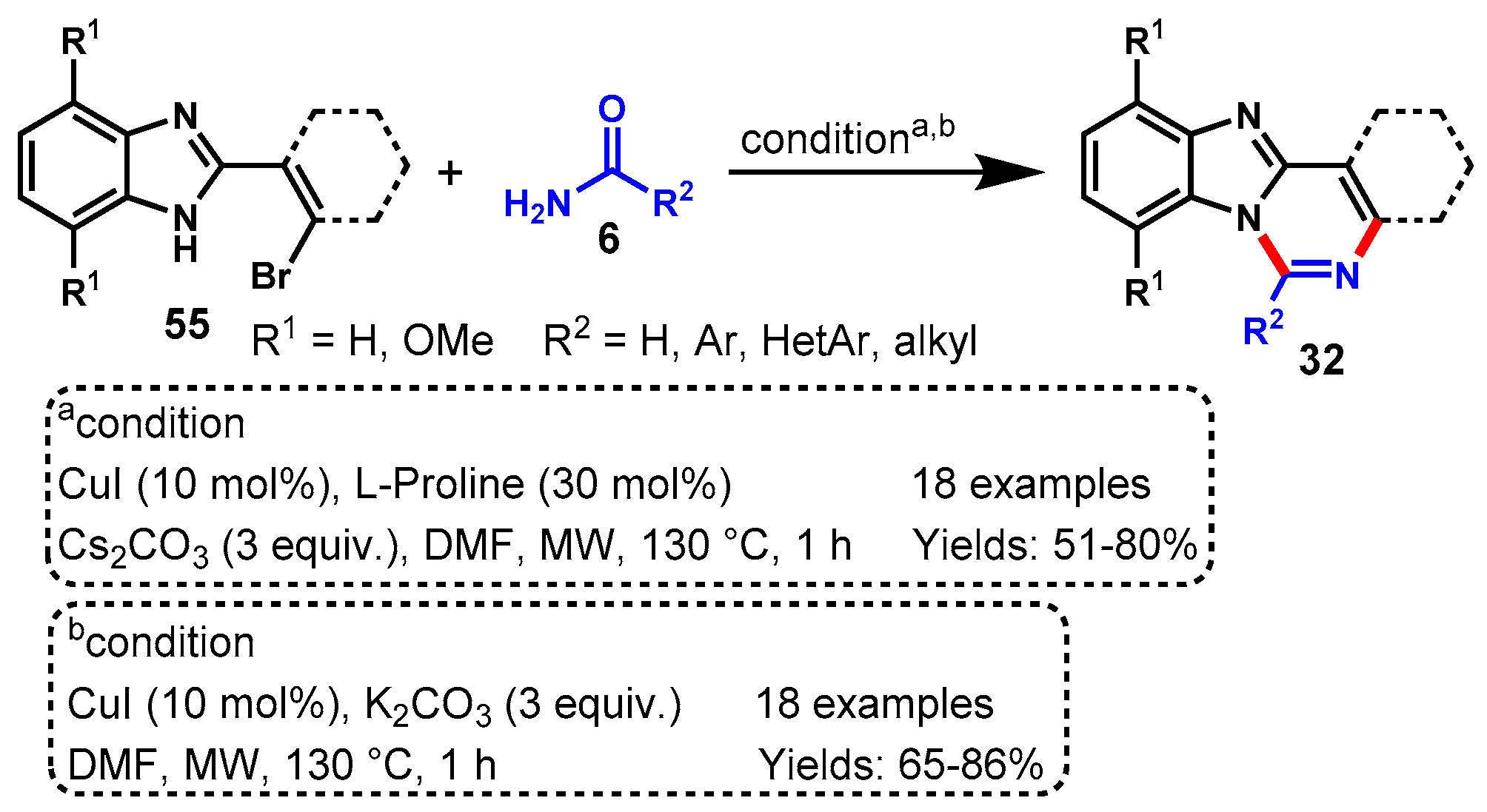

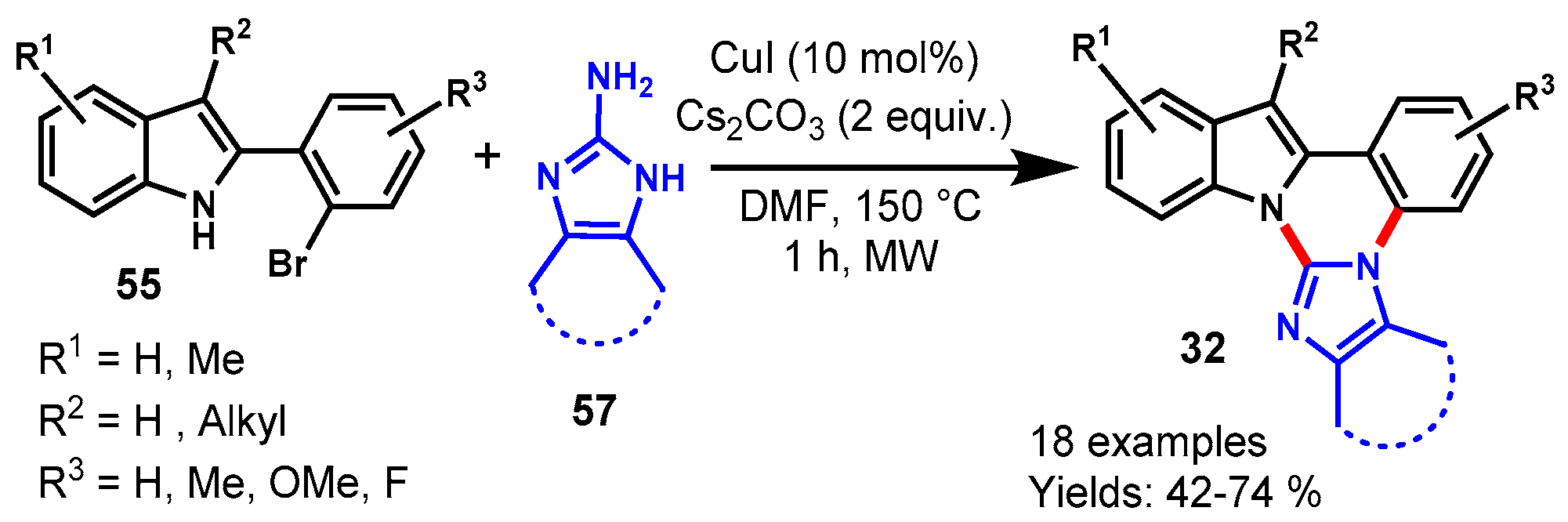
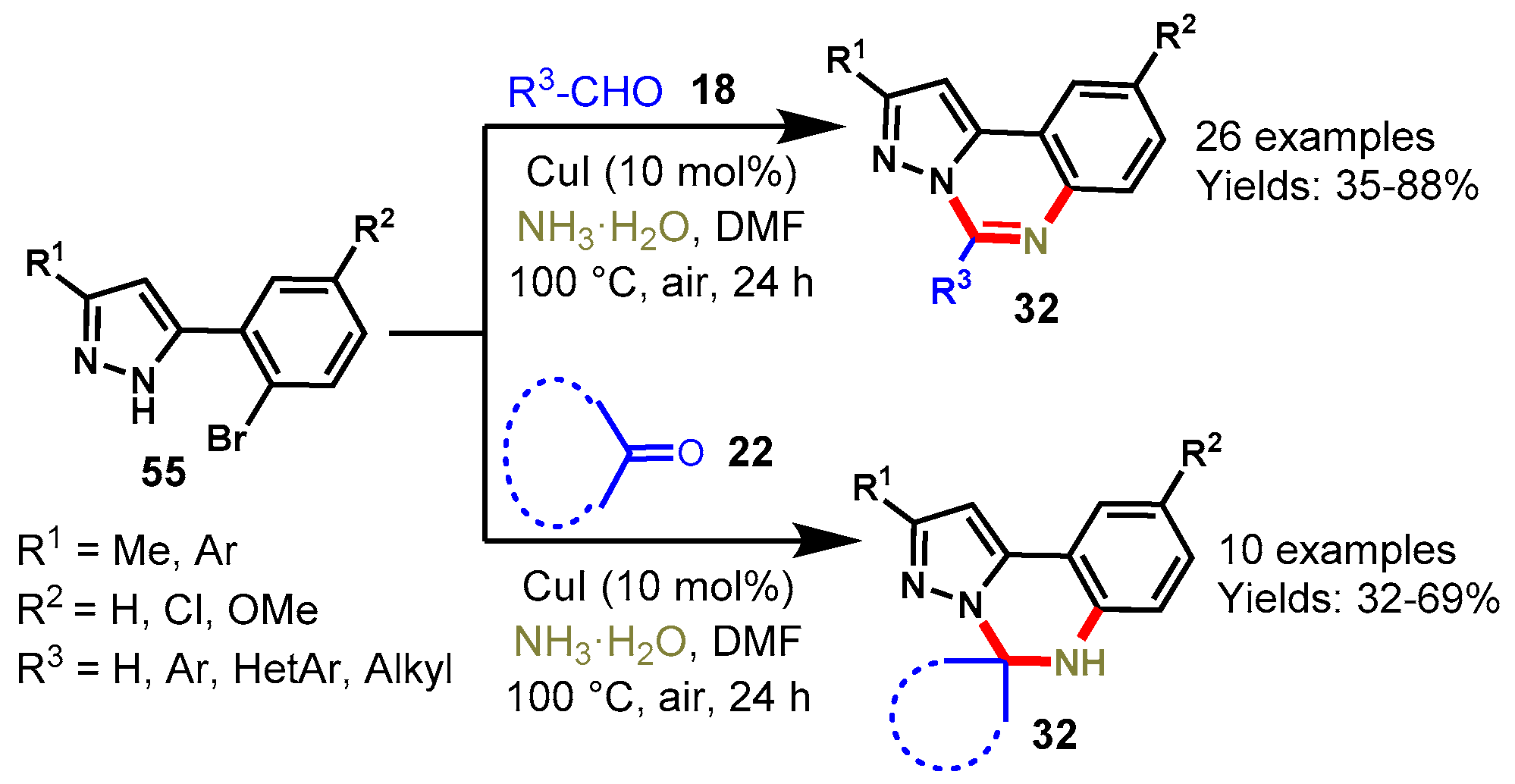




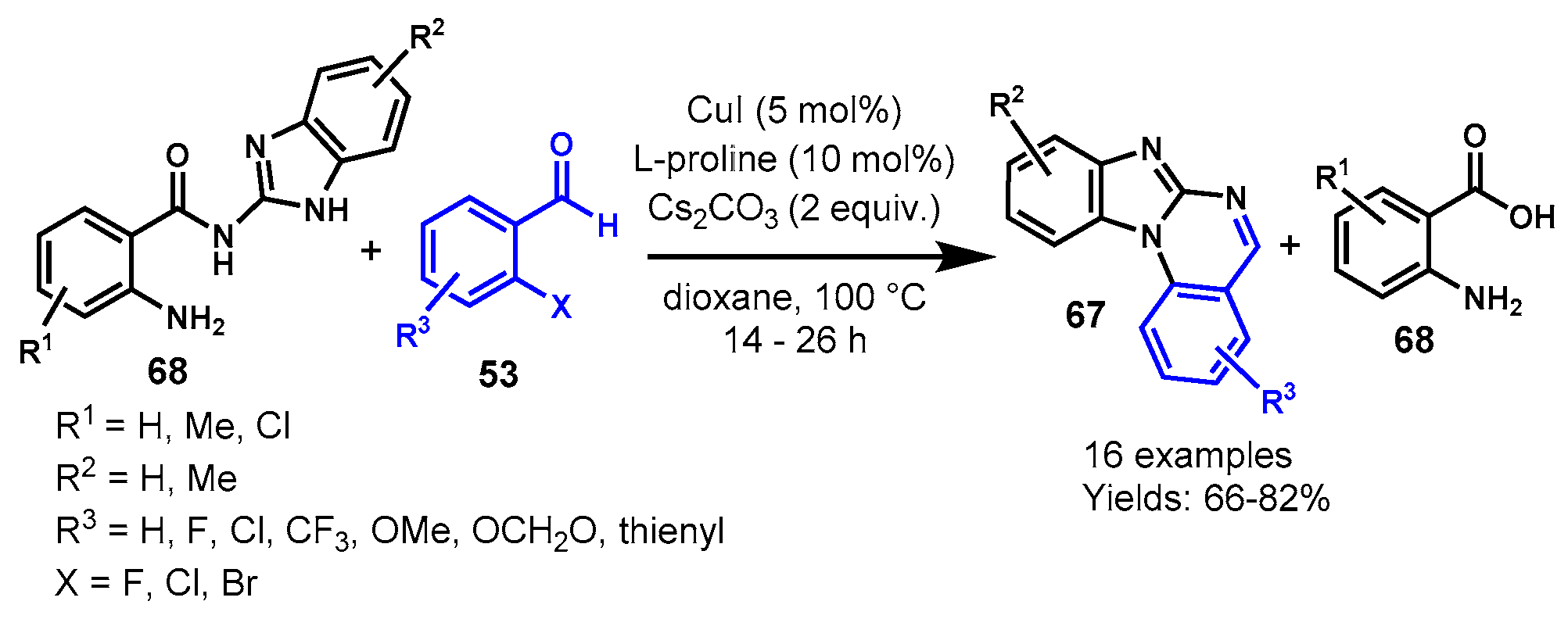
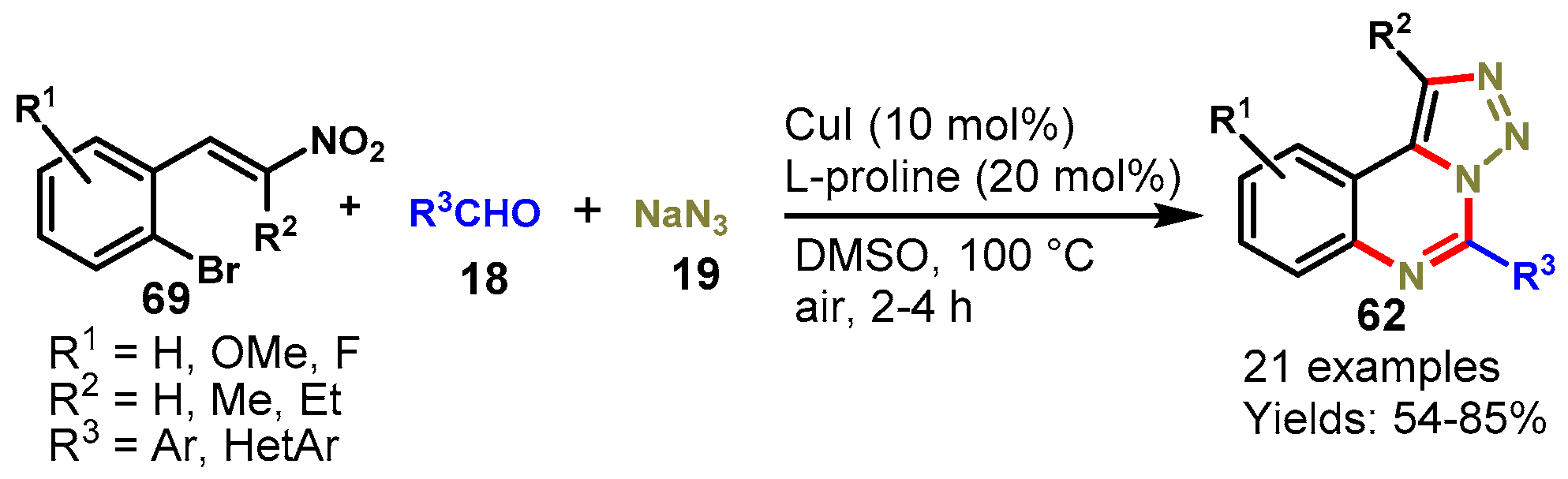
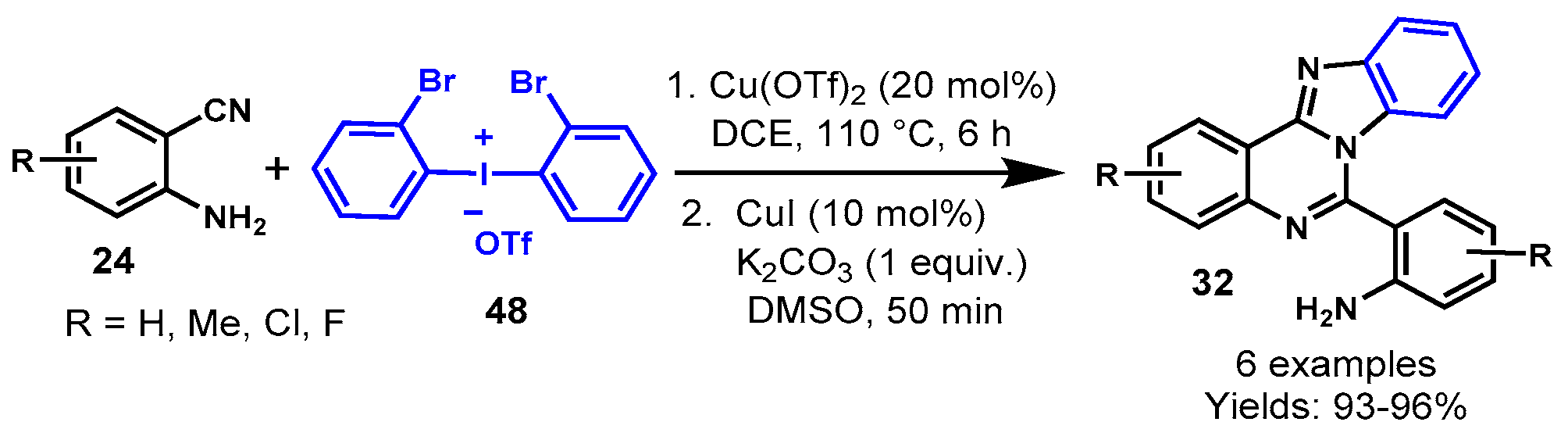
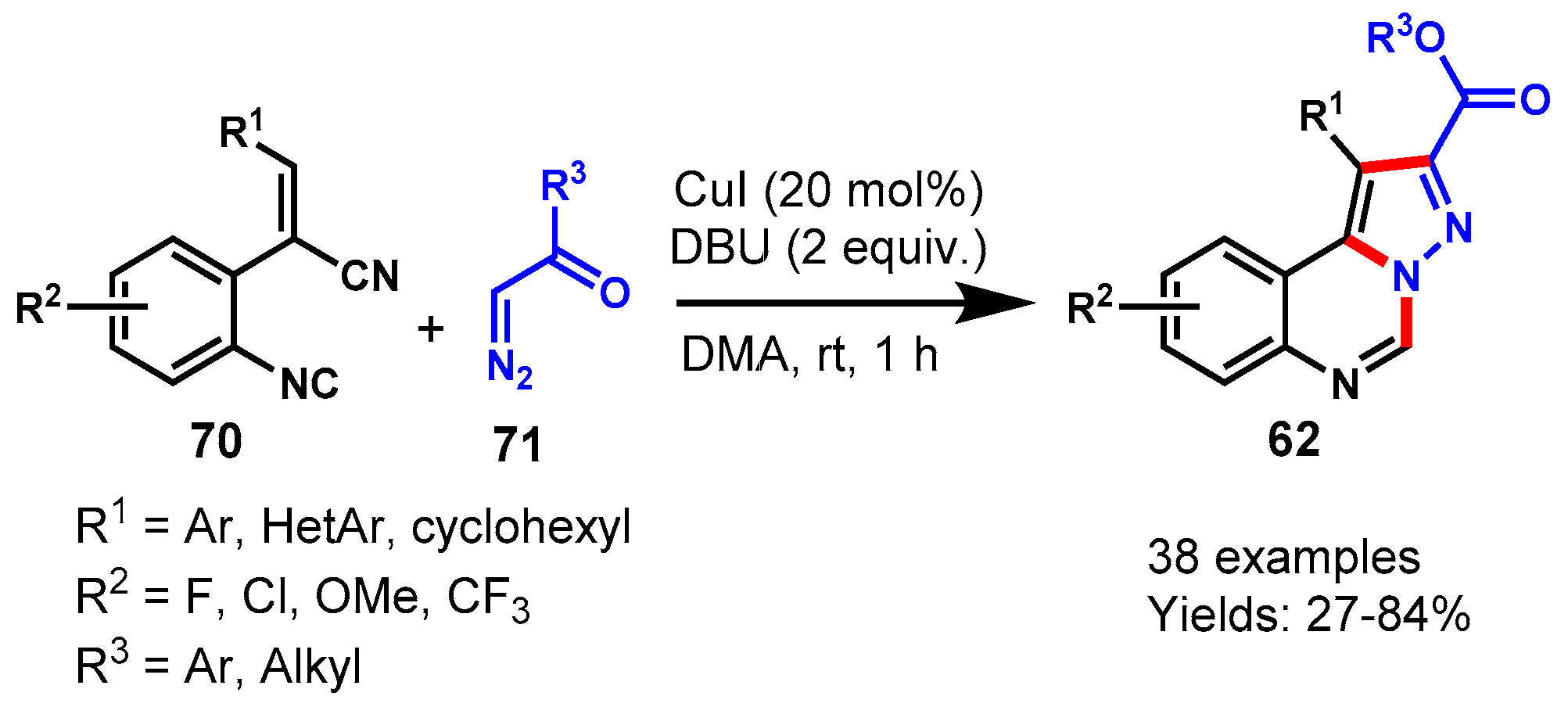


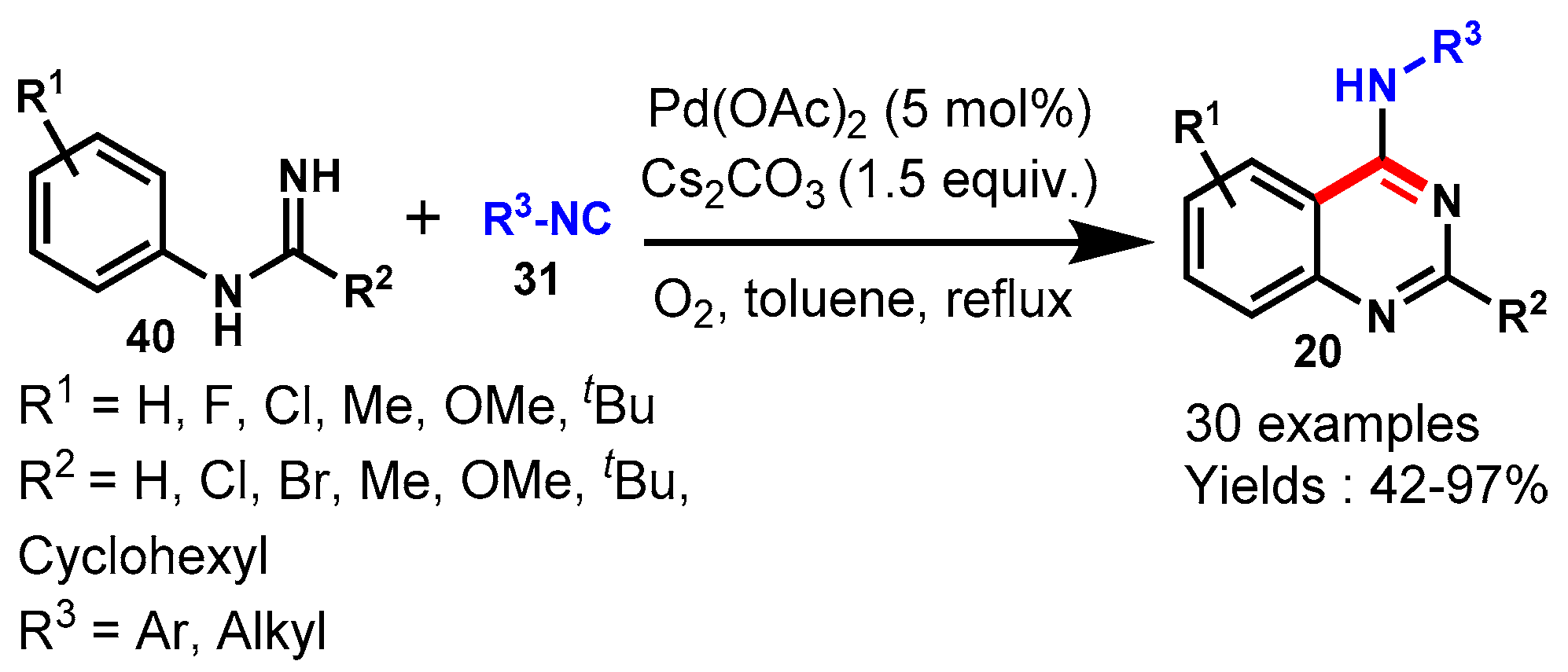
















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nandwana, N.K.; Patel, O.P.S.; Mehra, M.K.; Kumar, A.; Salvino, J.M. Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives. Molecules 2024, 29, 2353. https://doi.org/10.3390/molecules29102353
Nandwana NK, Patel OPS, Mehra MK, Kumar A, Salvino JM. Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives. Molecules. 2024; 29(10):2353. https://doi.org/10.3390/molecules29102353
Chicago/Turabian StyleNandwana, Nitesh K., Om P. S. Patel, Manish K. Mehra, Anil Kumar, and Joseph M. Salvino. 2024. "Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives" Molecules 29, no. 10: 2353. https://doi.org/10.3390/molecules29102353
APA StyleNandwana, N. K., Patel, O. P. S., Mehra, M. K., Kumar, A., & Salvino, J. M. (2024). Recent Advances in Metal-Catalyzed Approaches for the Synthesis of Quinazoline Derivatives. Molecules, 29(10), 2353. https://doi.org/10.3390/molecules29102353






