Abstract
Background: Staphylococcus aureus is a common pathogenic microorganism in humans and animals. Type II NADH oxidoreductase (NDH-2) is the only NADH:quinone oxidoreductase present in this organism and represents a promising target for the development of anti-staphylococcal drugs. Recently, myricetin, a natural flavonoid from vegetables and fruits, was found to be a potential inhibitor of NDH-2 of S. aureus. The objective of this study was to evaluate the inhibitory properties of myricetin against NDH-2 and its impact on the growth and expression of virulence factors in S. aureus. Results: A screening method was established to identify effective inhibitors of NDH-2, based on heterologously expressed S. aureus NDH-2. Myricetin was found to be an effective inhibitor of NDH-2 with a half maximal inhibitory concentration (IC50) of 2 μM. In silico predictions and enzyme inhibition kinetics further characterized myricetin as a competitive inhibitor of NDH-2 with respect to the substrate menadione (MK). The minimum inhibitory concentrations (MICs) of myricetin against S. aureus strains ranged from 64 to 128 μg/mL. Time–kill assays showed that myricetin was a bactericidal agent against S. aureus. In line with being a competitive inhibitor of the NDH-2 substrate MK, the anti-staphylococcal activity of myricetin was antagonized by MK-4. In addition, myricetin was found to inhibit the gene expression of enterotoxin SeA and reduce the hemolytic activity induced by S. aureus culture on rabbit erythrocytes in a dose-dependent manner. Conclusions: Myricetin was newly discovered to be a competitive inhibitor of S. aureus NDH-2 in relation to the substrate MK. This discovery offers a fresh perspective on the anti-staphylococcal activity of myricetin.
1. Introduction
Staphylococcus aureus is a Gram-positive opportunistic pathogen that colonizes in about one-third of the general population [1]. While this pathogen typically does not infect healthy individuals, its entry into internal tissues or the bloodstream can lead to various complications, ranging from wound infections to more severe and life-threatening conditions such as septic arthritis, pneumonia, gastroenteritis, bacteremia, osteomyelitis, and meningitis [2,3,4]. Of particular concern is the escalating prevalence of antibiotic-resistant strains, notably methicillin-resistant S. aureus (MRSA) [5]. Infections caused by MRSA are widespread and associated with high mortality rates. Additionally, S. aureus ranks among the most frequent causes of bacterial foodborne diseases globally [4,6]. Consumption of food contaminated with S. aureus can induce symptoms of acute gastroenteritis, including diarrhea, vomiting, and fever. The pathogenicity of S. aureus primarily arises from its capacity to generate a variety of toxins. These toxins act synergistically, allowing S. aureus to evade host immune responses during infections and subsequently induce a spectrum of diseases [2,3,4,5,6]. Therefore, there is a growing interest in identifying small molecules and compounds that can disrupt the virulence mechanisms of S. aureus, including toxin production [4].
Type II NADH dehydrogenase (NDH-2) is a monotopic membrane flavoprotein that catalyzes the transfer of electrons from NADH to quinone via FAD [7]. This respiratory enzyme contributes indirectly to the generation of proton motive force and helps to maintain the NADH/NAD+ redox balance. Given its essential role in ATP synthesis or growth in certain pathogens and its absence in mammals, NDH-2 has emerged as a promising antibiotic target [7]. Different from mammals and many pathogens, S. aureus lacks type I NADH dehydrogenase but possesses two NDH-2 isoforms, namely, NdhC and NdhF [8]. These NDH-2s represent the sole NADH: quinone oxidoreductases in this organism. The impact on the growth of S. aureus in Mueller–Hinton medium was notably pronounced when NdhC was absent, while the absence of NdhF exhibited minimal effects [9]. This observation underscores the predominant role of NdhC as the primary dehydrogenase under such conditions. Importantly, inactivation of NDH-2, particularly NdhC, resulted in a significant compromise in the pathogenicity and virulence of S. aureus [9]. Consequently, targeting NDH-2 has been proposed as a potential approach to combat S. aureus infections.
Many compounds with diverse structures, such as phenothiazines [10,11,12], quinolones [13,14], quinolinyl pyrimidines [15,16], 2-mercapto-quinazolinones [17], and tricyclic spiroLactams [18], have been identified as potent antibacterial agents targeting NDH-2 in Mycobacterium tuberculosis. However, there is limited information available on inhibitors that target S. aureus NDH-2. Currently, only 2-heptyl-4-hydroxyquinoline-N-oxide (HQNO) and three phenothiazine compounds (thioridazine, trifluoperazine, and chlorpromazine) have been characterized as inhibitors of S. aureus NDH-2 in vitro [8,19]. Recent screening efforts of natural compounds derived from plants have unveiled myricetin, a 3, 3′, 4′, 5, 5′, 7-hexahydroxy flavone (Figure 1), as a more potent in vitro inhibitor of S. aureus NDH-2 compared to HQNO. This discovery prompted our investigation into the inhibitory properties of myricetin against NDH-2. Therefore, this study aims to explore the inhibitory effect and mechanism of myricetin on NDH-2, as well as its impact on the growth and virulence factor expression in S. aureus.
Figure 1.
Structure of myricetin.
2. Results
2.1. Protein Purification and Enzymatic Characterization
The gene encoding NADH dehydrogenase II protein (NdhC) was cloned into plasmid pET28a (+) and transformed into E. coli BL21(DE3). The protein was induced with isopropyl β-D-1-thiogalactopyranoside (IPTG) at 25 °C for 8 h. Consistent with the previous report [20], cells expressing NDH-2 were light greenish in color compared to the cream color of the wild-type pellet. The bacterial cells were disrupted using a pre-cooled French Press and sonicated on ice for 20 min. It is worth mentioning that the soluble protein content of NDH-2 showed a significant increase after ultrasound treatment. One explanation for this phenomenon is that the process might enhance the product release from the cell membrane, as NDH-2 is a monotopic membrane protein anchored to the lipid bilayer. Furthermore, we observed that the stability of NDH-2 was greatly improved by adding 500 mM NaCl and 5% (v/v) glycerol to the lysis and elution buffers.
The His-tagged NDH-2 was then purified using an affinity chromatography column and analyzed by SDS-PAGE (Figure 2A, Supplementary Figure S1A). The purified enzyme showed a molecular mass of approximately 42 kDa, in line with the predicted molecular weights of NDH-2, which was confirmed by Western blotting using an anti-His antibody (Figure 2B, Supplementary Figure S1B). The full-length gel and blot images are shown in Figure S1. The UV–visible spectra of the purified NDH-2 proteins showed no appearance peak near 450 nm, indicating that the enzyme carried little or no FAD, which has an absorption maxima at 450 nm in its oxidized form. Flavoproteins that are heterologously expressed commonly experience FAD loss [21]. Fortunately, the addition of exogenous FAD to the reaction mix restored the NADH dehydrogenase activity of the recombinant protein. As the purified NDH-2 contained sub-stoichiometric amounts of FAD, subsequent assays were performed with the addition of 20 μM FAD to the reaction system.
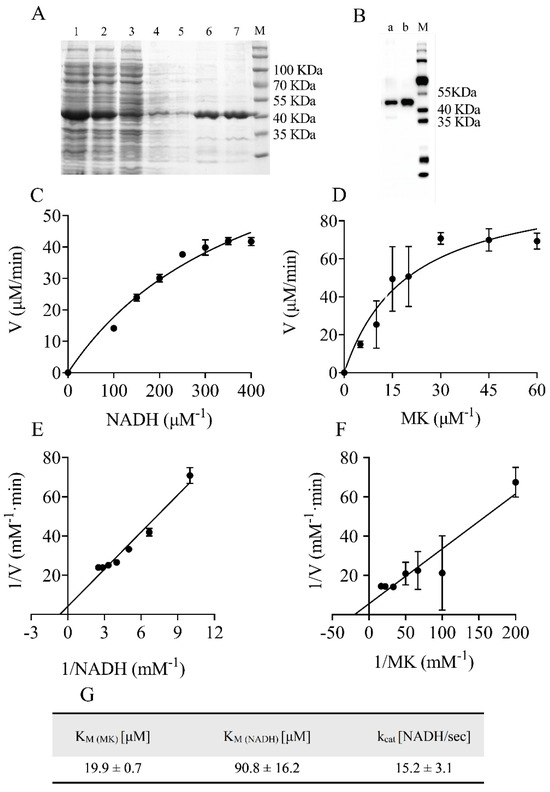
Figure 2.
Preparation and enzymatic properties of heterologously expressed NDH−2. (A) SDS−PAGE of purified protein. 1: Lysate, 2: Supernatant, 3: Pellet, 4: Wash, 5: 30 mM imidazole, 6: 80 mM imidazole, 7: 150 mM imidazole. (B) Western blotting of NDH−2 using anti−6His−tag antibody. a: 80 mM imidazole, b: 150 mM imidazole, M: Protein Marker. (C,D) The plot of the reaction rate (V) vs. substrate concentration. (E,F) The kinetic parameters Km were calculated by Lineweaver−Burk plot of 1/V vs. 1/S. (G) The turnover number (kcat) and affinity (Km) for the substrates of the enzyme were determined. Data are expressed as mean ± SD of three independent experiments.
Optimal enzyme activity also required 500 mM NaCl and 5% glycerol. NADH:quinone oxidoreductase activity was tested in the presence of NADH and water-soluble menadione (MK) as an oxidant. The results showed that the substrate NADH or MK had a dose-dependent effect on the enzyme activity of NDH-2 (Figure 2C,D). The turnover number under Vmax conditions estimated for the reaction was 15.2 ± 3.1 NADH/s, and the Km values for MK and NADH were 19.9 ± 0.7 µM and 90.8 ± 16.2 µM, respectively (Figure 2E–G). These values were close to those reported in the literature [19]. Thus, the purified NDH-2 was used to screen inhibitors of the NDH-2 enzyme.
2.2. Myricetin Acted as a Competitive Inhibitor of NDH-2 Substrate Menadione
We used 96-well plates to conduct the screens with a reaction mixture consisting of 2.5 μg/mL purified NDH-2 and various drugs at a concentration of 32 μg/mL. To validate the screenings, enzyme inhibition assays were performed with 3.2% DMSO (negative control) and HQNO, a well-known NDH-2 inhibitor (positive control) [22]. As shown in Figure 3A, 32 μg/mL HQNO almost completely inhibited the NADH oxidation catalyzed by NDH-2. Similar results were observed for myricetin. To determine the half maximal inhibitory concentration (IC50) of myricetin, different concentrations of drugs were added into the assay mixture, and the reaction was monitored via absorbance at 340 nm (Figure 3B,C). Probit analysis was performed on the data to obtain probability of response curves (Figure 3D,E). The calculated IC50 of myricetin was found to be 0.7 μg/mL (2 μM), which was about 4.5 times lower than that of the positive agent HQNO (2.3 μg/mL or 9 μM).
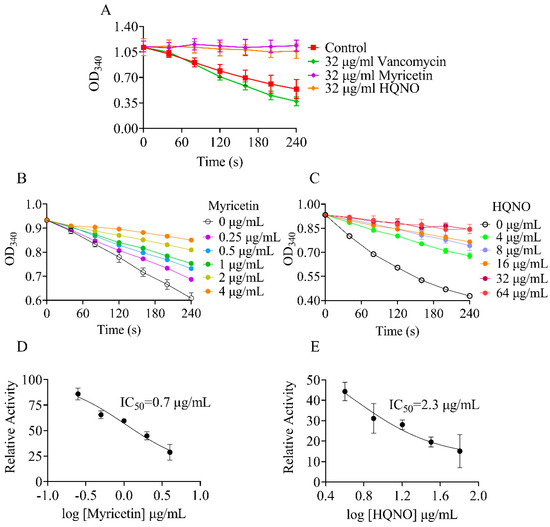
Figure 3.
The effect of myricetin on enzyme activity of NDH−2. Concentration of NADH was set at 300 μM for all experiments. A variable slope model was fitted to determine the IC50 values. In each panel, NADH: quinone oxidoreduction activities in the absence of drugs were used for 100%. (A) The NADH UV absorbance at 340 nm was monitored after the addtion of various agents; (B) The NADH UV absorbance at 340 nm was monitored after the addtion of myricetin; (C) The NADH UV absorbance at 340 nm was monitored after the addtion of HQNO; (D) Myricetin inhibition curves for NDH−2 in the presence of 30 μM MK; (E) HQNO inhibition curves for NDH−2 in the presence of 30 μM MK.
In order to understand the molecular interactions between the inhibitor and enzyme, a molecular docking study was performed. The binding poses were evaluated and ranked using a scoring function. The top-ranked binding pose of myricetin was found around the binding pocket of substrate MK (Figure 4A). The docking simulation revealed that myricetin interacted hydrophobically with several amino acid residues, including T48, F168, M209, A319, Q320, T352, K379, I382, and D383. These residues partially overlapped with the binding sites of the MK substrate [19], suggesting that myricetin acted as a competitive inhibitor of NDH-2 in relation to the substrate MK. The inhibition mechanism of the NDH-2 inhibitor was subsequently investigated by checking the residual enzymatic activity of NDH-2 at different concentrations of MK under a set of fixed myricetin concentrations (Figure 4B). As shown in Figure 4C, all double reciprocal plots had intersecting lines on the Y-axis, confirming that myricetin competitively inhibited NDH-2 for substrate MK.
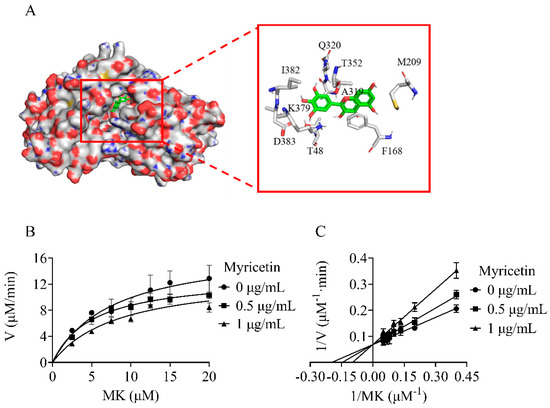
Figure 4.
Myricetin acting as a competitive inhibitor of NDH−2 substrate MK. (A) Electronic cloud plot of NDH−2 and the interaction of myricetin with NDH−2 (PDB code 5AN1). (B) Michaelis−Menten plot of myricetin against NDH−2. (C) Lineweaver−Burk plot of myricetin against NDH−2.
2.3. The Anti-Staphylococcal Activity of Myricetin Was Antagonized by MK
The sole respiratory NADH dehydrogenase found in S. aureus is NDH-2 [8], which facilitates electron transfer by reducing menaquinone to menaquinol. Deletion of the ndh gene significantly impaired staphylococcal growth when compared to the parent strain in MH medium [9]. To assess the antibacterial activity of the biochemically active NDH-2 inhibitor myricetin, we determined its ability to inhibit the growth of S. aureus. As shown in Table 1, myricetin showed antibacterial activity against S. aureus with MICs ranging from 64 to 128 μg/mL. To evaluate the bactericidal/bacteriostatic behavior of the flavonoid, time–kill assays against S. aureus ATCC 25923 and 43300 were performed. As shown in Figure 5A,B, no living cell was detected at 24 h for the two tested strains treated with 4 × MIC of myricetin, indicating that this flavonoid was a bactericidal agent against S. aureus. Subsequent research revealed that adding menaquinone-4 (MK-4) to the culture medium rescued the growth defects caused by myricetin (Figure 5C,D), which was in agreement with the above finding that myricetin acted as a competitive inhibitor of the NDH-2 substrate MK.

Table 1.
MICs of myricetin against Staphylococcus aureus strains.
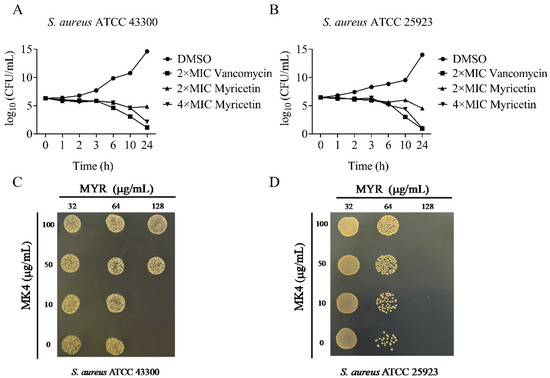
Figure 5.
The antimicrobial activity of myricetin against Staphylococcus aureus. (A,B) Time–kill curves for Staphylococcus aureus treated with myricetin. The concentrations are as follows: ●, 3.2% DMSO; ▴, 2 × MIC of myricetin; ▾, 4 × MIC of myricetin; ■, 2 × MIC of vancomycin. (C,D) MK4 antagonizes the antibacterial activity of myricetin.
2.4. Myricetin Exhibited a Complex Impact on the Virulence Factors of S. aureus
S. aureus infections heavily rely on the production of a series of toxins. A recent study has demonstrated that the inactivation of NDH-2 significantly impacts the production of factors contributing to staphylococcal virulence, such as staphyloxanthin and α-hemolysin [9]. To assess the effect of myricetin on staphylococcal virulence, we first determined its impact on the gene expression of the two above-mentioned toxins using quantitative real-time PCR (qRT-PCR). Myricetin did not significantly affect the expression of crtM in S. aureus (Figure 6A). The crtM gene encodes dehydrosqualene synthase, which catalyzes the initial step of the biosynthetic pathway of staphyloxanthin. Conversely, myricetin marginally but significantly upregulated the expression of the gene hla, responsible for encoding α-hemolysin (Figure 6B). The production of α-hemolysin was further quantitatively analyzed by assessing the lysis of rabbit red blood cells, which are highly susceptible to hemolysis induced by α-hemolysin. Inconsistent with the findings from the qRT-PCR assay, myricetin inhibited the hemolytic activity of S. aureus at concentrations ranging from 2 to 16 μg/mL, without interfering with growth (Figure 6C,D). Such results were understandable given that myricetin has been reported to directly interfere with the hemolytic activity of α-hemolysin [23].
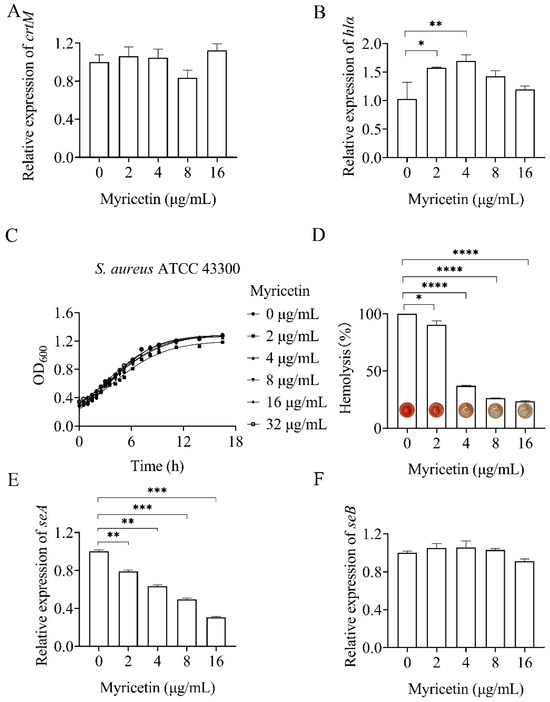
Figure 6.
Effect of myricetin on Staphylococcus aureus toxins. Changes in the transcript levels of crtM (A), hlα (B), seA (E), and seB (F) in myricetin-treated S. aureus were analyzed relative to those in untreated control cultures. Relative normalized expression values were determined by RT-qPCR. (C) Bacterial growth was measured by optical density at 600 nm. (D) Hemolytic activity of myricetin-treated Staphylococcus aureus. Data represent means ± SD (n = 3). Statistically significant differences between differences in drug concentrations are shown as * p < 0.1, ** p < 0.01, *** p < 0.001 and **** p < 0.0001.
In addition, the impact of myricetin on the gene expression of staphylococcal enterotoxins (SEA and SEB) was investigated using qRT-PCR. As depicted in Figure 6E, myricetin inhibited the expression of seA in a dose-dependent manner within the concentration range of 2–16 μg/mL. Conversely, only minor or non-significant effects of the compounds on seB expression were observed (Figure 6F).
3. Discussion
Myricetin is a plant-derived flavonoid compound famous for its versatile activity, and widely found in vegetables, fruits, nuts, tea, and wine [24,25]. It has been shown to have antimicrobial activity against Mycobacterium tuberculosis H37Rv, Streptococcus mutans, Bacillus subtilis, E. coli, Pseudomonas aeruginosa, and Helicobacter pylori, with a MIC of 50, 512, 250, 500, 500, and 160 μg/mL [26,27,28,29,30], respectively. Myricetin has also been reported to be capable of inhibiting biofilm formation and virulence factors produced by S. mutans, S. aureus, and Streptococcus suis, without interfering with growth [23,26,31,32]. Several molecular targets have been reported to be associated with the anti-infective effects of the flavonoid compound. Myricetin has been characterized as an inhibitor of various DNA polymerases, RNA polymerases, and reverse transcriptases [33,34]. It has also demonstrated significant inhibitory activity against E. coli DnaB helicase with an IC50 of 11.3 ± 1.6 μM [35]. Recently, myricetin has been identified as an inhibitor of sortase A [23,27,36], effectively attenuating the adhesion and biofilm formation of S. mutans and S. aureus. The IC50 of this flavonoid against SrtA from S. mutans and S. aureus has been determined to be 4.63 and 48.66 μM, respectively. Here, myricetin was identified for the first time as an effective inhibitor of NDH-2 from S. aureus with an IC50 of 2 μM. Through in silico prediction and enzyme inhibition kinetics, myricetin was further characterized as a competitive inhibitor of NDH-2 in relation to the substrate MK.
NDH-2 enzymes in S. aureus are crucial for the regeneration of NAD+, redox balance maintenance, and energy production [9]. NDH-2 inactivation impairs staphylococcal growth and reduces biofilm formation and hemolytic activity, but enhances staphyloxanthin production [9]. To investigate the potential targeting of NDH-2 in S. aureus by myricetin, we evaluated its effect on bacterial growth and the expression of virulence factors. As anticipated, myricetin exhibited good activity against S. aureus. Importantly, the anti-staphylococcal activity of myricetin was antagonized by MK-4, which is an NDH-2 substrate as an electron acceptor. The impact of myricetin on the virulence of S. aureus is multifaceted. At sub-inhibitory concentrations, myricetin was observed to have no effect on the expression of crtM and seB, but caused a small but statistically significant inhibition on the gene expression of enterotoxin seA. Although myricetin slightly stimulated the gene expression of α-hemolysin, it significantly attenuated the hemolytic activity induced by S. aureus culture on rabbit erythrocytes in a dose-dependent manner. This may be due to the fact that myricetin can bind directly to certain amino acids of α-hemolysin, even at concentrations well below the threshold for growth inhibition, thereby disrupting the toxin’s hemolytic activity [23]. The regulation of virulence factors in S. aureus is subject to a complex network that integrates cues from the host and environment into a coordinated response [37]. It is noteworthy that numerous antibiotics have been documented to exert an impact on the production of toxins by S. aureus when present at sub-inhibitory concentrations [38,39]. At lower concentrations, myricetin altered the virulence of S. aureus, despite the requirement of higher concentrations to inhibit bacterial growth. Therefore, myricetin appears to possess greater potential as a candidate for the development of an anti-virulence compound.
In response to the growing threat of antibiotic resistance, the compounds that neutralize bacterial toxins or block the pathways regulating toxin production have received increasing attention [4,40,41,42]. Compared to traditional antibiotics, anti-toxin compounds have less selective pressure to evolve resistance because they affect bacterial virulence rather than cell viability. Although no anti-virulence approaches have yet been approved for clinical use, a growing body of evidence points to their potential benefits and efficacy, particularly in the context of infections caused by resistant bacterial strains. Future studies should prioritize in-depth mechanistic analysis of carefully selected, highly potent virulence candidates to advance their potential therapeutic applications against S. aureus infections.
4. Methods
4.1. Strains and Chemicals
S. aureus ATCC 25923, S. aureus ATCC 29213, S. aureus ATCC 43300, and S. aureus Newman were used in this study. S. aureus strains were routinely grown in Luria–Bertani (LB) media at 37 °C. Mueller–Hinton (MH) medium (Oxoid, Hants, UK) was used for drug susceptibility testing. A concentration of 50 μg/mL kanamycin was employed for plasmid selection and maintenance, as required. Myricetin (>98%, HPLC) was purchased from aladdin (Shanghai, China), while Menadione (>98%, HPLC) and NADH (>98%, HPLC) were purchased from Macklin (Shanghai, China). Myricetin was dissolved in Dimethyl sulfoxide (DMSO) and then diluted to ensure a final DMSO concentration of <3.2% (v/v).
4.2. Expression Plasmid Construction
For heterologous expression of NDH-2, the ndhC gene [WP 002463246.1] was amplified from chromosomal DNA of S. aureus 25923 using primers NdhC-F/NdhC-R (Table 2), and inserted into the plasmid pET28a(+) using a One-Step Seamless Cloning kit (Accurate Biology, Changsha, Hunan, China). To facilitate purification, a 6His-tag was introduced at the N-terminus of the NDH-2 gene. The recombinant plasmid was transformed into Escherichia coli DH5α. The transformants were spread on LB plates containing kanamycin at 37 °C, and the resulting strains were then selected and confirmed by PCR using two pairs of primers: YZ-1-F/YZ-1-R and YZ-2-F/YZ-2-R (Table 2). After confirmation by DNA sequencing, the recombinant plasmid pET28a(+)-NdhC was transformed into E. coli BL21(DE3).

Table 2.
Primers used in the study.
4.3. Protein Preparation
The overnight cultures of E. coli BL21(DE3) were diluted into fresh LB media at a dilution of 1:100 and grown with 250 rpm shaking till an OD600 of 1 was reached. Gene expression was induced by the addition of isopropyl β-D-1-thiogalactopyranoside (IPTG) at a concentration of 1 mM to the media. The induced culture was further incubated at 25 °C for 8 h and then harvested in a centrifuge at 4 °C. All the purification procedures were carried out at 4–8 °C. Cell pellets were resuspended in lysis buffer (50 mM Tris-HCl, 500 mM NaCl, 5% glycerol, pH 8.0) and disrupted by passing three through a high-pressure homogenizer (EmulsiFlex-C5, Avestin, Ottawa, ON, Canada) at a pressure of 15,000 psi. The lysate underwent an additional 20-min ultrasound treatment (SK2510HP, Shanghai, China) in an ice bath, followed by centrifugation at 8000 rpm for 30 min to eliminate cell debris. The supernatant was added to Ni-NTA resin (Ni Bestarose FF, Jiaxing, Zhejiang, China) pre-equilibrated with lysis buffer. The resin was washed with lysis buffer plus 30–80 mM imidazole, and then the bound proteins were eluted with lysis buffer containing 150 mM imidazole. The purified protein was loaded on SDS-PAGE for the purity and confirmed by Western blotting using an anti-His antibody (HA1006, Hangzhou, China).
4.4. Enzyme Activity Assay
Enzyme activity assays were determined using a UV-Vis spectrophotometer (Technologies model 8453, Santa Clara, CA, USA) by monitoring absorbance at 340 nm (NADH ε = 6.22 mM−1 cm−1). The reaction mixture (200 μL) contained 0.5 μg of the purified NDH-2, 20 μM FAD, 300 μM NADH, 500 mM NaCl, 5% glycerol, and 50 mM Tris-HCl buffer, pH 7.5. The reaction was initiated by adding 30 μM menadione (MK). For determination of kinetic constants, 0 to 400 μM of NADH and 0 to 60 μM of MK were added to the reaction mixture. Kinetic parameters were calculated by nonlinear regression analysis using GraphPad Prism Software 8.2 (GraphPad Software, La Jolla, CA, USA). All experiments were performed with three replicates.
4.5. Molecular Docking Simulation
Molecular docking using AutoDock Vina [43] was employed to explore the molecular basis of myricetin interaction with NDH-2. The 3D structure of the small molecule was obtained from the PubChem database (https://pubchem.ncbi.nlm.nih.gov (accessed on 25 August 2022)), and the crystal structure of NDH-2 (PDB ID: 5NA1) from S. aureus RF144 was obtained from the PDB database (http://www.rcsb.org (accessed on 25 August 2022)).
4.6. Antimicrobial Activity Assays
Antimicrobial activity assays were performed by the minimum inhibitory concentration (MIC) method and time–kill experiments, as described previously [44]. The MIC was defined as the lowest concentration of drug with invisible bacterial growth after incubation at 37 °C for 18–20 h. DMSO (3.2%) was used as a negative control, and vancomycin was used as a positive control of bactericidal antibiotic.
4.7. Quantitative Polymerase Chain Reaction
The levels of expression for gyrb, hlα, crtM, seA, and seB were analyzed using reverse transcription (RT) followed by real-time quantitative PCR (qPCR). Briefly, a total of 2 mL of S. aureus grown with different concentrations of myricetin (0 to 32 μg/mL) was incubated at 37 °C with shaking at 200 rpm for 16 h. S. aureus grown without myricetin was treated in the same manner and used as a negative control. Subsequently, each sample was centrifuged at 10,000× g for 10 min to collect the bacteria. RNA was extracted from each culture using the SteadyPure RNA Extraction Kit (Accurate Biology, Changsha, Hunan, China), and its purity and concentration were determined using a SpectraMax ABS Puls (Molecular Devices, Shanghai, China). The RNA was then reverse-transcribed using the Evo M-MLV RT Mix Kit (Accurate Biology, Changsha, Hunan, China). The resulting cDNA was quantified using 2 × SYBR Green Abstart qPCR Mix from Sangon Biotech in a qTOWER3 G real-time PCR system (Analytic Jena, Jena, Germany). The primers used are listed in Table 2. The RNA transcript levels were determined using the 2−ΔΔCT method. The experiment was conducted in triplicate for each reaction.
5. Conclusions
Myricetin was characterized as a competitive inhibitor of NDH-2 in relation to the substrate MK. In accordance with this, the antimicrobial activity of myricetin against S. aureus was attenuated by MK-4. The impact of myricetin on the production of S. aureus virulence factors is multifaceted. Most observations seem to be consistent with the finding that myricetin acts as a competitive inhibitor of NDH-2. However, further experiments are needed to clarify whether NDH-2 is the antibacterial target for myricetin.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29102354/s1. Figure S1. (A) SDS-PAGE of purified protein. 1: Lysate, 2: Supernatant, 3: Pellet, 4: Wash, 5: 30 mM imidazole, 6: 80 mM imidazole, 7: 150 mM imidazole, M: Protein Marker. (B) Western blotting of the NDH-2 using anti 6His tag antibody. a: 30 mM imidazole, b: 80 mM imidazole, c: 150 mM imidazole. M: Protein Marker.
Author Contributions
C.-D.Q. designed the study. C.-D.Q. and J.X. wrote the manuscript. J.-L.Z. and H.-H.C. performed the experiments. M.-Y.H., J.-F.W., H.-J.S., S.-X.S. and C.-X.G. contributed to manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by Key Projects of Zhejiang Science Technology Plan of Traditional Chinese Medicine (2022ZZ009) and Special Research Funds of Zhejiang Chinese Medical University (2022GJYY021).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Acknowledgments
We appreciate the great help/technical support from Key Laboratory for Green Pharmaceutical Technologies and Related Equipment of Ministry of Education, and the Public Platform of Medical Research Center, Academy of Chinese Medical Science, Zhejiang Chinese Medical University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.Y.C.; Bae, J.S.; Otto, M. Pathogenicity and virulence of Staphylococcus aureus. Virulence 2021, 12, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.A.; Sharma-Kuinkel, B.K.; Maskarinec, S.A.; Eichenberger, E.M.; Shah, P.P.; Carugati, M.; Holland, T.L.; Fowler, V.G., Jr. Methicillin-resistant Staphylococcus aureus: An overview of basic and clinical research. Nat. Rev. Microbiol. 2019, 17, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Hennekinne, J.A.; De Buyser, M.L.; Dragacci, S. Staphylococcus aureus and its food poisoning toxins: Characterization and outbreak investigation. FEMS Microbiol. Rev. 2012, 36, 815–836. [Google Scholar] [CrossRef] [PubMed]
- Sellamuthu, S.; Singh, M.; Kumar, A.; Singh, S.K. Type-II NADH Dehydrogenase (NDH-2): A promising therapeutic target for antitubercular and antibacterial drug discovery. Expert. Opin. Ther. Targets 2017, 21, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Schurig-Briccio, L.A.; Yano, T.; Rubin, H.; Gennis, R.B. Characterization of the type 2 NADH: Menaquinone oxidoreductases from Staphylococcus aureus and the bactericidal action of phenothiazines. Biochim. Biophys. Acta 2014, 1837, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Schurig-Briccio, L.A.; Parraga Solorzano, P.K.; Lencina, A.M.; Radin, J.N.; Chen, G.Y.; Sauer, J.D.; Kehl-Fie, T.E.; Gennis, R.B. Role of respiratory NADH oxidation in the regulation of Staphylococcus aureus virulence. EMBO Rep. 2020, 21, e45832. [Google Scholar] [CrossRef]
- Weinstein, E.A.; Yano, T.; Li, L.S.; Avarbock, D.; Avarbock, A.; Helm, D.; McColm, A.A.; Duncan, K.; Lonsdale, J.T.; Rubin, H. Inhibitors of type II NADH: Menaquinone oxidoreductase represent a class of antitubercular drugs. Proc. Natl. Acad. Sci. USA 2005, 102, 4548–4553. [Google Scholar] [CrossRef]
- Dunn, E.A.; Roxburgh, M.; Larsen, L.; Smith, R.A.; McLellan, A.D.; Heikal, A.; Murphy, M.P.; Cook, G.M. Incorporation of triphenylphosphonium functionality improves the inhibitory properties of phenothiazine derivatives in Mycobacterium tuberculosis. Bioorg Med. Chem. 2014, 22, 5320–5328. [Google Scholar] [CrossRef] [PubMed]
- Nizi, M.G.; Desantis, J.; Nakatani, Y.; Massari, S.; Mazzarella, M.A.; Shetye, G.; Sabatini, S.; Barreca, M.L.; Manfroni, G.; Felicetti, T.; et al. Antitubercular polyhalogenated phenothiazines and phenoselenazine with reduced binding to CNS receptors. Eur. J. Med. Chem. 2020, 201, 112420. [Google Scholar] [CrossRef]
- Heikal, A.; Hards, K.; Cheung, C.Y.; Menorca, A.; Timmer, M.S.; Stocker, B.L.; Cook, G.M. Activation of type II NADH dehydrogenase by quinolinequinones mediates antitubercular cell death. J. Antimicrob. Chemother. 2016, 71, 2840–2847. [Google Scholar] [CrossRef]
- Hong, W.D.; Gibbons, P.D.; Leung, S.C.; Amewu, R.; Stocks, P.A.; Stachulski, A.; Horta, P.; Cristiano, M.L.S.; Shone, A.E.; Moss, D.; et al. Rational Design, Synthesis, and Biological Evaluation of Heterocyclic Quinolones Targeting the Respiratory Chain of Mycobacterium tuberculosis. J. Med. Chem. 2017, 60, 3703–3726. [Google Scholar] [CrossRef]
- Shirude, P.S.; Paul, B.; Roy Choudhury, N.; Kedari, C.; Bandodkar, B.; Ugarkar, B.G. Quinolinyl Pyrimidines: Potent Inhibitors of NDH-2 as a Novel Class of Anti-TB Agents. ACS Med. Chem. Lett. 2012, 3, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Åkerbladh, L.; Ahmad, S.; Konda, V.; Cao, S.; Vocat, A.; Maes, L.; Cole, S.T.; Hughes, D.; Larhed, M.; et al. Synthesis and In Vitro Biological Evaluation of Quinolinyl Pyrimidines Targeting Type II NADH-Dehydrogenase (NDH-2). ACS Infect. Dis. 2022, 8, 482–498. [Google Scholar] [CrossRef]
- Nguyen, N.; Wilson, D.W.; Nagalingam, G.; Triccas, J.A.; Schneider, E.K.; Li, J.; Velkov, T.; Baell, J. Broad activity of diphenyleneiodonium analogues against Mycobacterium tuberculosis, malaria parasites and bacterial pathogens. Eur. J. Med. Chem. 2018, 148, 507–518. [Google Scholar] [CrossRef]
- Dam, S.; Tangara, S.; Hamela, C.; Hattabi, T.; Faïon, L.; Carre, P.; Antoine, R.; Herledan, A.; Leroux, F.; Piveteau, C.; et al. Tricyclic SpiroLactams Kill Mycobacteria in vitro and in vivo by Inhibiting Type II NADH Dehydrogenases. J. Med. Chem. 2022, 65, 16651–16664. [Google Scholar] [CrossRef] [PubMed]
- Sena, F.V.; Batista, A.P.; Catarino, T.; Brito, J.A.; Archer, M.; Viertler, M.; Madl, T.; Cabrita, E.J.; Pereira, M.M. Type-II NADH:quinone oxidoreductase from Staphylococcus aureus has two distinct binding sites and is rate limited by quinone reduction. Mol. Microbiol. 2015, 98, 272–288. [Google Scholar] [CrossRef]
- Vamshi Krishna, K.; Venkata Mohan, S. Purification and Characterization of NDH-2 Protein and Elucidating Its Role in Extracellular Electron Transport and Bioelectrogenic Activity. Front. Microbiol. 2019, 10, 880. [Google Scholar] [CrossRef]
- Lencina, A.M.; Franza, T.; Sullivan, M.J.; Ulett, G.C.; Ipe, D.S.; Gaudu, P.; Gennis, R.B.; Schurig-Briccio, L.A. Type 2 NADH Dehydrogenase Is the Only Point of Entry for Electrons into the Streptococcus agalactiae Respiratory Chain and Is a Potential Drug Target. mBio 2018, 9, e01034-18. [Google Scholar] [CrossRef] [PubMed]
- Petri, J.; Shimaki, Y.; Jiao, W.; Bridges, H.R.; Russell, E.R.; Parker, E.J.; Aragão, D.; Cook, G.M.; Nakatani, Y. Structure of the NDH-2—HQNO inhibited complex provides molecular insight into quinone-binding site inhibitors. Biochim. Biophys. Acta Bioenerg. 2018, 1859, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A comprehensive review on its biological potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef] [PubMed]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement. Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Yadav, A.K.; Thakur, J.; Prakash, O.; Khan, F.; Saikia, D.; Gupta, M.M. Screening of flavonoids for antitubercular activity and their structure–activity relationships. Med. Chem. Res. 2013, 22, 2706–2716. [Google Scholar] [CrossRef]
- Hu, P.; Lv, B.; Yang, K.; Lu, Z.; Ma, J. Discovery of myricetin as an inhibitor against Streptococcus mutans and an anti-adhesion approach to biofilm formation. Int. J. Med. Microbiol. 2021, 311, 151512. [Google Scholar] [CrossRef]
- Xu, H.; Ziegelin, G.; Schröder, W.; Frank, J.; Ayora, S.; Alonso, J.C.; Lanka, E.; Saenger, W. Flavones inhibit the hexameric replicative helicase RepA. Nucleic Acids Res. 2001, 29, 5058–5066. [Google Scholar] [CrossRef]
- Jayaraman, P.; Sakharkar, M.K.; Lim, C.S.; Tang, T.H.; Sakharkar, K.R. Activity and interactions of antibiotic and phytochemical combinations against Pseudomonas aeruginosa in vitro. Int. J. Biol. Sci. 2010, 6, 556–568. [Google Scholar] [CrossRef]
- Krzyżek, P.; Migdał, P.; Paluch, E.; Karwańska, M.; Wieliczko, A.; Gościniak, G. Myricetin as an Antivirulence Compound Interfering with a Morphological Transformation into Coccoid Forms and Potentiating Activity of Antibiotics against Helicobacter pylori. Int. J. Mol. Sci. 2021, 22, 2695. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, P.; Lv, H.; Deng, X.; Wang, J. A Natural Dietary Flavone Myricetin as an α-Hemolysin Inhibitor for Controlling Staphylococcus aureus Infection. Front. Cell Infect. Microbiol. 2020, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, G.; Si, X.; Zhang, X.; Liu, W.; Li, L.; Wang, J. Inhibition of suilysin activity and inflammation by myricetin attenuates Streptococcus suis virulence. Life Sci. 2019, 223, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Nakane, H.; Fukushima, M.; Chermann, J.C.; Barré-Sinoussi, F. Differential inhibitory effects of various flavonoids on the activities of reverse transcriptase and cellular DNA and RNA polymerases. Eur. J. Biochem. 1990, 190, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.C.; Hsieh, Y.S.; Lin, J.Y. Inhibitory effects of flavonoids on Moloney murine leukemia virus reverse transcriptase activity. J. Nat. Prod. 1992, 55, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Griep, M.A.; Blood, S.; Larson, M.A.; Koepsell, S.A.; Hinrichs, S.H. Myricetin inhibits Escherichia coli DnaB helicase but not primase. Bioorg. Med. Chem. 2007, 15, 7203–7208. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.; Nicorescu, I.M.; Olaru, O.T.; Ungurianu, A.; Mihai, D.P.; Zanfirescu, A.; Nitulescu, G.M.; Margina, D. Molecular docking and screening studies of new natural Sortase a inhibitors. Int. J. Mol. Sci. 2017, 18, 2217. [Google Scholar] [CrossRef]
- Jenul, C.; Horswill, A.R. Regulation of Staphylococcus aureus Virulence. Microbiol. Spectr. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.; Huang, J.; Zhang, R.; Rao, X. Virulence alterations in Staphylococcus aureus upon treatment with the sub-inhibitory concentrations of antibiotics. J. Adv. Res. 2021, 31, 165–175. [Google Scholar] [CrossRef]
- Hodille, E.; Rose, W.; Diep, B.A.; Goutelle, S.; Lina, G.; Dumitrescu, O. The Role of Antibiotics in Modulating Virulence in Staphylococcus aureus. Clin. Microbiol. Rev. 2017, 30, 887–917. [Google Scholar] [CrossRef]
- Kong, C.; Neoh, H.M.; Nathan, S. Targeting Staphylococcus aureus Toxins: A Potential form of Anti-Virulence Therapy. Toxins 2016, 8, 72. [Google Scholar] [CrossRef]
- Sabino, Y.N.V.; Cotter, P.D.; Mantovani, H.C. Anti-virulence compounds against Staphylococcus aureus associated with bovine mastitis: A new therapeutic option? Microbiol. Res. 2023, 271, 127345. [Google Scholar] [CrossRef] [PubMed]
- Pecoraro, C.; Carbone, D.; Parrino, B.; Cascioferro, S.; Diana, P. Recent Developments in the Inhibition of Bacterial Adhesion as Promising Anti-Virulence Strategy. Int. J. Mol. Sci. 2023, 24, 4872. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Le, C.Y.; Ye, Y.J.; Xu, J.; Li, L.; Feng, X.Q.; Chen, N.P.; Zhu, B.Q.; Ding, Z.S.; Qian, C.D. Hinokitiol Selectively Enhances the Antibacterial Activity of Tetracyclines against Staphylococcus aureus. Microbiol. Spectr. 2023, 11, e0320522. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).