Evaluation of Alternative Sources of Proteins and Other Nutrients with Potential Applications in Fish Nutrition
Abstract
1. Introduction
2. Results
2.1. Animal Base Protein Sources
2.2. Plant-Based Protein Sources
3. Discussion
4. Materials and Methods
4.1. Animal-Based Protein Sources
- Drying: Initially, the muscle tissue underwent a dehydration process in a drying oven (UFE 400 oven from Memmert, Büchenbach, Germany). This process was conducted at a controlled temperature of 60 °C. The aim of this stage was to reduce the moisture content in the tissue until a constant mass was achieved, thus ensuring complete and uniform drying of the biological material.
- Mass Monitoring: The mass of the tissue was periodically monitored (WSP4000/C/2, Partner Radwag, Radom, Poland) to determine the point of constant mass achievement, indicating the completion of the dehydration process. This stage is crucial for ensuring the quality and uniformity of the final product.
- Grinding: After dehydration completion, the dried tissue was transferred for grinding. The grinding process was performed using specialized equipment to achieve a fine and uniform granulation of the material (PM 100, Retsch GmbH, Haan, Germany). The purpose of this stage was to transform the dehydrated tissue into a powder form, thereby facilitating its subsequent use in various applications, including protein extraction.
4.2. Plant-Based Protein Sources
4.3. Reagents and Materials
4.4. Nutritional Value Analysis
4.5. Humidity Content Analysis
4.6. Elemental Analysis
4.7. PAH Analysis
4.8. Statistical Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EIT Food. Protein Diversification—An EIT Food White Paper. Available online: https://www.eitfood.eu/files/EIT-FOOD-WHITE-PAPER-PROTEIN-DIVERSITICATION-2022_FINAL15-12-22.pdf (accessed on 13 November 2023).
- Agriculture. Available online: https://agriculture.ec.europa.eu/common-agricultural-policy/cap-overview/cap-glance_ro (accessed on 10 November 2023).
- Europarl. Available online: https://www.europarl.europa.eu/news/ro/headlines/society/20180301STO98928/emisii-de-gaze-cu-efect-de-sera-pe-tari-si-sectoare-infografic (accessed on 10 November 2023).
- Agridata. Oilseeds and Protein Crops Production (europa.eu). Available online: https://agridata.ec.europa.eu/extensions/DashboardCereals/OilseedProduction.html (accessed on 10 November 2023).
- Agridata. Protein Crops Short-Term Outlook, Directorate-General for Agriculture and Rural Development. Available online: https://agridata.ec.europa.eu/extensions/DashboardSTO/STO_ProteinCrops.html (accessed on 10 November 2023).
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Sampath, W.W.H.A.; Rathnayake, R.M.D.S.; Yang, M.; Zhang, W.; Mai, K. Roles of dietary taurine in fish nutrition. Mar. Life Sci. Technol. 2020, 2, 360–375. [Google Scholar] [CrossRef]
- Roques, S.; Deborde, C.; Richard, N.; Skiba-Cassy, S.; Moing, A.; Fauconneau, B. Metabolomics and fish nutrition: A review in the context of sustainable feed development. Rev. Aquac. 2020, 12, 261–282. [Google Scholar] [CrossRef]
- Lall, S.P.; Kaushik, S.J. Nutrition and Metabolism of Minerals in Fish. Animals 2021, 11, 2711. [Google Scholar] [CrossRef] [PubMed]
- Fokina, N.N.; Sukhovskaya, I.V.; Kantserova, N.P.; Lysenko, L.A. Tissue Lipid Profiles of Rainbow Trout, Oncorhynchus mykiss, Cultivated under Environmental Variables on a Diet Supplemented with Dihydroquercetin and Arabinogalactan. Animals 2024, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Islam, S.; Hasanuzzaman, A.F.M. Water Quality, Nutritional, Hematological, and Growth Profiles of Ompok pabda Fish Fry Reared in Biofloc Technology and Traditional Culture System with Different Stocking Densities. Animals 2024, 14, 90. [Google Scholar] [CrossRef] [PubMed]
- Rennemo, J.; Berge, K.; Yousaf, M.N.; Eriksen, T.B.; Welde, E.; Robertsen, C.; Johansen, B.; McGurk, C.; Rimstad, E.; Koppang, E.O.; et al. An Atypical Course of Cardiomyopathy Syndrome (CMS) in Farmed Atlantic Salmon (Salmo salar) Fed a Clinical Nutrition Diet. Microorganisms 2024, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Roasto, M.; Mäesaar, M.; Püssa, T.; Anton, D.; Rätsep, R.; Elias, T.; Jortikka, S.; Pärna, M.; Kapp, K.; Tepper, M.; et al. The Effect of Fruit and Berry Pomaces on the Growth Dynamics of Microorganisms and Sensory Properties of Marinated Rainbow Trout. Microorganisms 2023, 11, 2960. [Google Scholar] [CrossRef]
- Tefal, E.; Jauralde, I.; Martínez-Llorens, S.; Tomás-Vidal, A.; Milián-Sorribes, M.C.; Moyano, F.J.; Peñaranda, D.S.; Jover-Cerdá, M. Organic Ingredients as Alternative Protein Sources in the Diet of Juvenile Organic Seabass (Dicentrarchus labrax). Animals 2023, 13, 3816. [Google Scholar] [CrossRef]
- Hasan, I.; Gai, F.; Cirrincione, S.; Rimoldi, S.; Saroglia, G.; Terova, G. Chitinase and Insect Meal in Aquaculture Nutrition: A Comprehensive Overview of the Latest Achievements. Fishes 2023, 8, 607. [Google Scholar] [CrossRef]
- Nunes, A.J.P.; Yamamoto, H.; Simões, J.P.; Pisa, J.L.; Miyamoto, N.; Leite, J.S. The Black Soldier Fly (Hermetia illucens) Larvae Meal Can Cost-Effectively Replace Fish Meal in Practical Nursery Diets for Post-Larval Penaeus vannamei under High-Density Culture. Fishes 2023, 8, 605. [Google Scholar] [CrossRef]
- Magnani, M.; Claret, A.; Gisbert, E.; Guerrero, L. Consumer Expectation and Perception of Farmed Rainbow Trout (Oncorhynchus mykiss) Fed with Insect Meal (Tenebrio molitor). Foods 2023, 12, 4356. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Francis, P.; Rohani, F.; Azam, M.S.; Mock, T.S.; Francis, D.S. Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants 2023, 12, 2066. [Google Scholar] [CrossRef]
- Ghosh, K.; Harikrishnan, R.; Mukhopadhyay, A.; Ringø, E. Fungi and Actinobacteria: Alternative Probiotics for Sustainable Aquaculture. Fishes 2023, 8, 575. [Google Scholar] [CrossRef]
- Zuluaga-Hernández, C.D.; Hincapié, C.A.; Osorio, M. Non-Conventional Ingredients for Tilapia (Oreochromis spp.) Feed: A Systematic Review. Fishes 2023, 8, 556. [Google Scholar] [CrossRef]
- Saleh, H.H. Review on use of macro algae (seaweed) in fish nutrition. J. Zoöl. Res. 2020, 2, 12–26. [Google Scholar] [CrossRef]
- Gasco, L.; Acuti, G.; Bani, P.; Dalle Zotte, A.; Danieli, P.P.; De Angelis, A.; Fortina, R.; Marino, R.; Parisi, G.; Piccolo, G.; et al. Insect and fish by-products as sustainable alternatives to conventional animal proteins in animal nutrition. Ital. J. Anim. Sci. 2020, 19, 360–372. [Google Scholar] [CrossRef]
- Hong, Y.; Chu, J.; Kirby, R.; Sheen, S.; Chien, A. The effects of replacing fish meal protein with a mixture of poultry by-product meal and fermented soybean meal on the growth performance and tissue nutritional composition of Asian seabass (Lates calcarifer). Aquac. Res. 2021, 52, 4105–4115. [Google Scholar] [CrossRef]
- Randazzo, B.; Zarantoniello, M.; Cardinaletti, G.; Cerri, R.; Giorgini, E.; Belloni, A.; Contò, M.; Tibaldi, E.; Olivotto, I. Hermetia illucens and Poultry by-Product Meals as Alternatives to Plant Protein Sources in Gilthead Seabream (Sparus aurata) Diet: A Multidisciplinary Study on Fish Gut Status. Animals 2021, 11, 677. [Google Scholar] [CrossRef]
- Irm, M.; Taj, S.; Jin, M.; Luo, J.; Andriamialinirina, H.J.T.; Zhou, Q. Effects of replacement of fish meal by poultry by-product meal on growth performance and gene expression involved in protein metabolism for juvenile black sea bream (Acanthoparus schlegelii). Aquaculture 2020, 528, 735544. [Google Scholar] [CrossRef]
- Glencross, B.D.; Booth, M.; Allan, G.L. A feed is only as good as its ingredients? A review of ingredient evaluation strategies for aquaculture feeds. Aquac. Nutr. 2007, 13, 17–34. [Google Scholar] [CrossRef]
- Agboola, J.O.; Øverland, M.; Skrede, A.; Hansen, J. Yeast as major protein-rich ingredient in aquafeeds: A review of the implications for aquaculture production. Rev. Aquac. 2021, 13, 949–970. [Google Scholar] [CrossRef]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.Q.; Nguyen, T.T.; Prokešová, M.; Gebauer, T.; Doan, H.V.; Stejskal, V. Systematic review and meta-analysis of production performance of aquaculture species fed dietary insect meals. Rev. Aquac. 2022, 14, 1637–1655. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.; Zhu, Z.; Barba, F.J.; Toldrá, F.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Challenges and opportunities regarding the use of alternative protein sources: Aquaculture and insects. Adv. Food Nutr. Res. 2019, 89, 259–295. [Google Scholar] [PubMed]
- Li, X.; Zheng, S.; Ma, X.; Cheng, K.; Wu, G. Use of alternative protein sources for fishmeal replacement in the diet of largemouth bass (Micropterus salmoides). Part I: Effects of poultry by-product meal and soybean meal on growth, feed utilization, and health. Amino Acids 2021, 53, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Bhattarai, N.; Pahlow, M.; Xu, Z. Environmental sustainability and footprints of global aquaculture. Resour. Conserv. Recycl. 2022, 180, 106183. [Google Scholar] [CrossRef]
- Aragão, C.; Gonçalves, A.T.; Costas, B.; Azeredo, R.; Xavier, M.J.; Engrola, S. Alternative Proteins for Fish Diets: Implications beyond Growth. Animals 2022, 12, 1211. [Google Scholar] [CrossRef] [PubMed]
- Allam, B.W.; Khalil, H.S.; Mansour, A.T.; Srour, T.M.; Omar, E.A.; Nour, A.A.M. Impact of substitution of fish meal by high protein distillers dried grains on growth performance, plasma protein and economic benefit of striped catfish (Pangasianodon hypophthalmus). Aquaculture 2020, 517, 734792. [Google Scholar] [CrossRef]
- Campanati, C.; Willer, D.; Schubert, J.; Aldridge, D.C. Sustainable intensification of aquaculture through nutrient recycling and circular economies: More fish, less waste, blue growth. Rev. Fish. Sci. Aquac. 2022, 30, 143–169. [Google Scholar] [CrossRef]
- Benes, E.; Gere, A.; Fodor, M. Predicting macronutrients and energy content of snack products using FT-NIR analysis and chemometric techniques. J. Food Eng. 2020, 280, 109954. [Google Scholar] [CrossRef]
- Leion, H.; Folestad, S.; Josefson, M.; Sparén, A. Evaluation of basic algorithms for transferring quantitative multivariate calibrations between scanning grating and FT NIR spectrometers. J. Pharm. Biomed. Anal. 2005, 37, 37–55. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; Shao, X. Correcting multivariate calibration model for near infrared spectral analysis without using standard samples. J. Near Infrared Spectrosc. 2015, 23, 285–291. [Google Scholar] [CrossRef]
- Agelet, L.E.; Hurburgh Jr, C.R. A tutorial on near infrared spectroscopy and its calibration. Crit. Rev. Anal. Chem. 2010, 40, 246–260. [Google Scholar] [CrossRef]
- Song, J.; Luo, C.; Lim, L.; Cheong, K.-L.; Farhadi, A.; Tan, K. Protein quality of commercially important edible bivalves. Crit. Rev. Food Sci. Nutr. 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Monge-Ortiz, R.; Martínez-Llorens, S.; Lemos-Neto, M.; Falcó-Giaccaglia, S.; Pagán, M.; Godoy-Olmos, S.; Jover-Cerdá, M.; Tomás-Vidal, A. Growth, sensory and chemical characterization of Mediterranean yellowtail (Seriola dumerili) fed diets with partial replacement of fish meal by other protein sources. Aquac. Rep. 2020, 18, 100466. [Google Scholar] [CrossRef]
- Glencross, B.D.; Baily, J.; Berntssen, M.H.; Hardy, R.; MacKenzie, S.; Tocher, D.R. Risk assessment of the use of alternative animal and plant raw material resources in aquaculture feeds. Rev. Aquac. 2019, 12, 703–758. [Google Scholar] [CrossRef]
- Cheng, Z.; Mo, W.-Y.; Lam, C.-L.; Choi, W.-M.; Wong, M.-H. Replacing fish meal by food waste to produce lower trophic level fish containing acceptable levels of polycyclic aromatic hydrocarbons: Health risk assessments. Sci. Total. Environ. 2015, 523, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Gomot, A. Biochemical Composition of Helix Snails: Influence of Genetic and Physiological Factors. J. Molluscan Stud. 1998, 64, 173–181. [Google Scholar] [CrossRef]
- Çağiıltay, F.; Erkan, N.; Tosun, D.; Selçuk, A. Amino Acid, Fatty Acid, Vitamin and Mineral Contents of the Edible Garden Snail (Helix aspersa). J. Fish. Sci. 2011, 5, 354–363. [Google Scholar] [CrossRef]
- Zhong, Y.-F.; Shi, C.-M.; Zhou, Y.-L.; Chen, Y.-J.; Lin, S.-M.; Tang, R.-J. Optimum dietary fiber level could improve growth, plasma biochemical indexes and liver function of largemouth bass, Micropterus salmoides. Aquaculture 2020, 518, 734661. [Google Scholar] [CrossRef]
- Nkansah, M.A.; Agyei, E.A.; Opoku, F. Mineral and proximate composition of the meat and shell of three snail species. Heliyon 2021, 7, e08149. [Google Scholar] [CrossRef]
- Corda, A.; Mara, L.; Virgilio, S.; Pisanu, M.; Chessa, G.; Parisi, A.; Cogoni, M.P. Microbiological and Chemical Evaluation of Helix Spp. Snails from Local and Non-EU Markets, Utilised as Food in Sardinia. Ital. J. Food Saf. 2014, 3, 1732. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Q.; Li, Y.; Bi, L.; Jin, L.; Peng, R. Toxic Effects of Cadmium on Fish. Toxics 2022, 10, 622. [Google Scholar] [CrossRef]
- Recabarren-Villalón, T.; Ronda, A.C.; Oliva, A.L.; Cazorla, A.L.; Marcovecchio, J.E.; Arias, A.H. Seasonal distribution pattern and bioaccumulation of Polycyclic aromatic hydrocarbons (PAHs) in four bioindicator coastal fishes of Argentina. Environ. Pollut. 2021, 291, 118125. [Google Scholar] [CrossRef]
- Ademolu, K.O.; Onadeko, D.E.; Mselbwala, F.M.; Oropo, A. Nutritional value of the visceral mass of three giant African land snail species (and). Niger. J. Anim. Prod. 2017, 44, 133–138. [Google Scholar] [CrossRef]
- Ng, H.S.; Kee, P.E.; Yim, H.S.; Chen, P.-T.; Wei, Y.-H.; Lan, J.C.-W. Recent advances on the sustainable approaches for conversion and reutilization of food wastes to valuable bioproducts. Bioresour. Technol. 2020, 302, 122889. [Google Scholar] [CrossRef] [PubMed]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oilcakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef] [PubMed]
- Sinkovič, L.; Kolmanič, A. Elemental composition and nutritional characteristics of Cucurbita pepo subsp. Pepo seeds, oil cake and pumpkin oil. J. Elementology 2021, 26, 97–107. [Google Scholar] [CrossRef]
- Moura, M.A.F.E.; Martins, B.d.A.; de Oliveira, G.P.; Takahashi, J.A. Alternative protein sources of plant, algal, fungal and insect origins for dietary diversification in search of nutrition and health. Crit. Rev. Food Sci. Nutr. 2023, 63, 10691–10708. [Google Scholar] [CrossRef]
- Lee, Y.-N.; Lee, S.; Kim, J.-S.; Patra, J.K.; Shin,, H.-S. Chemical analysis techniques and investigation of polycyclic aromatic hydrocarbons in fruit, vegetables and meats and their products. Food Chem. 2019, 277, 156–161. [Google Scholar]
- Escobar, M.I.R.; Cadena, E.; Nhu, T.T.; Cooreman-Algoed, M.; De Smet, S.; Dewulf, J. Analysis of the Cultured Meat Production System in Function of Its Environmental Footprint: Current Status, Gaps and Recommendations. Foods 2021, 10, 2941. [Google Scholar] [CrossRef] [PubMed]
- Abozeid, A.M.; Abdel-Rahim, M.M.; Abouelenien, F.; Elkaradawy, A.; Mohamed, R.A. Quillaja saponaria and/or Yucca schidigera ameliorate water quality, growth performance, blood health, intestine and gills histomorphology of Nile tilapia, Oreochromis niloticus. Aquac. Res. 2021, 52, 6117–6131. [Google Scholar] [CrossRef]
- Masithoh, R.E.; Amanah, H.Z.; Cho, B.K. Application of Fourier Transform Near-Infrared (FT-NIR) and Fourier Transform Infrared (FT-IR) Spectroscopy Coupled with Wavelength Selection for Fast Discrimination of Similar Color of Tuber Flours. Indones. J. Chem. 2020, 20, 680–687. [Google Scholar] [CrossRef]
- Mæhre, H.K.; Dalheim, L.; Edvinsen, G.K.; Elvevoll, E.O.; Jensen, I.-J. Protein Determination—Method Matters. Foods 2018, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Manirakiza, P.; Covaci, A.; Schepens, P. Comparative study on total lipid determination using Soxhlet, Roese-Gottlieb, Bligh & Dyer, and Modified Bligh & Dyer Extraction Methods. J. Food Compos. Anal. 2001, 14, 93–100. [Google Scholar] [CrossRef]
- ISO 1442:2023; Determination of Moisture Content, Reference Method. ISO Organization: Geneva, Switzerland, 2023. Available online: https://www.iso.org/standard/82664.html (accessed on 5 January 2024).
- Karasakal, A. Determination of Trace and Major Elements in Vegan Milk and Oils by ICP-OES After Microwave Digestion. Biol. Trace Element Res. 2020, 197, 683–693. [Google Scholar] [CrossRef]
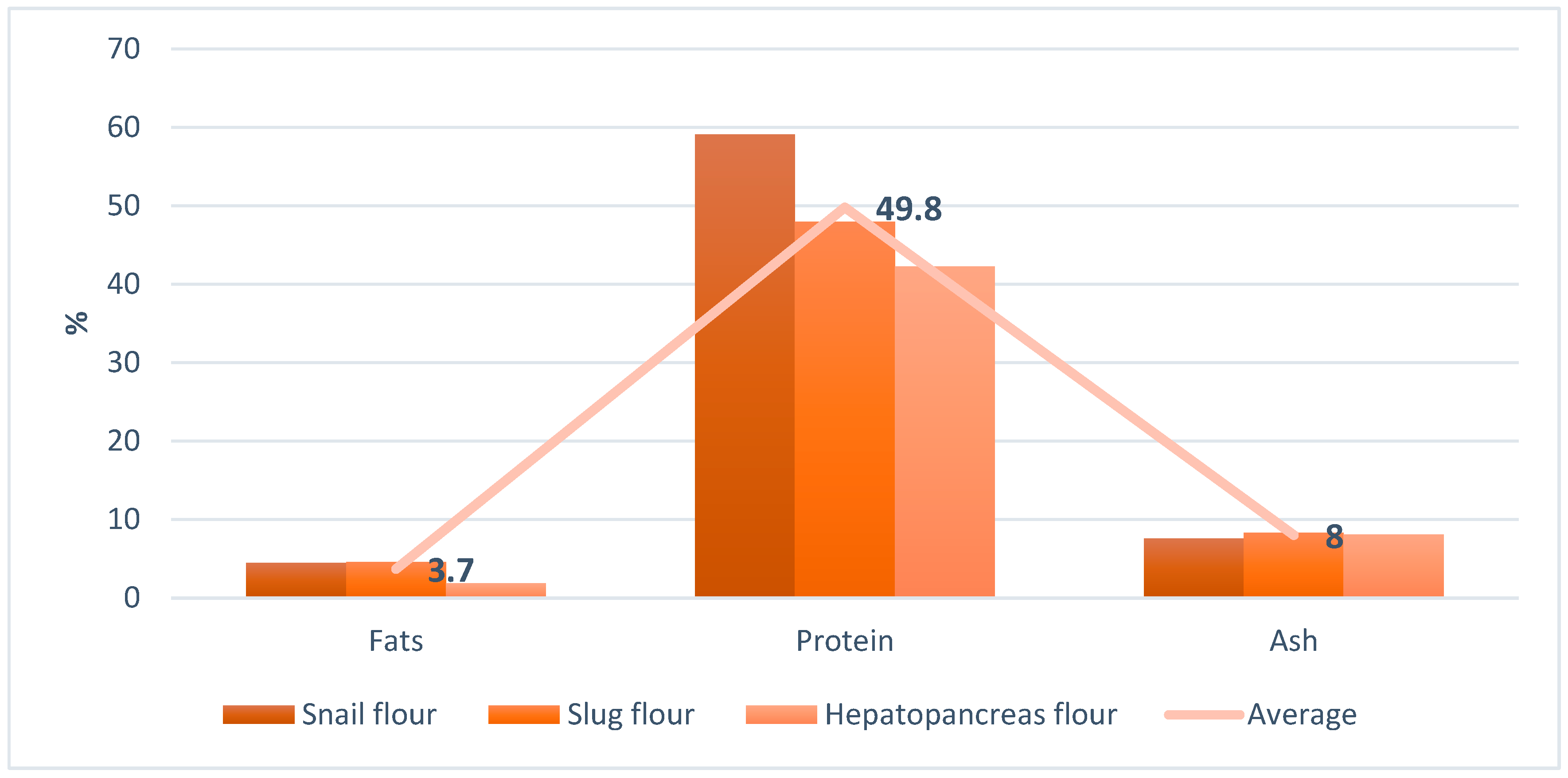
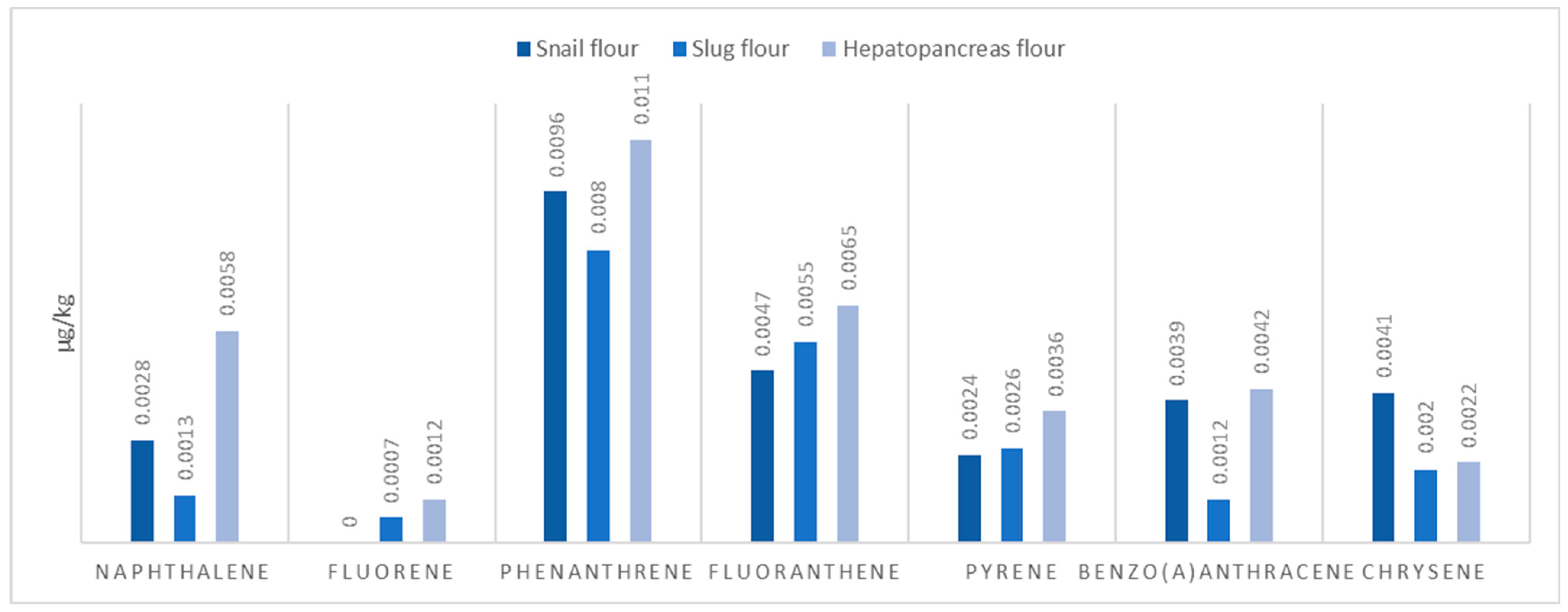
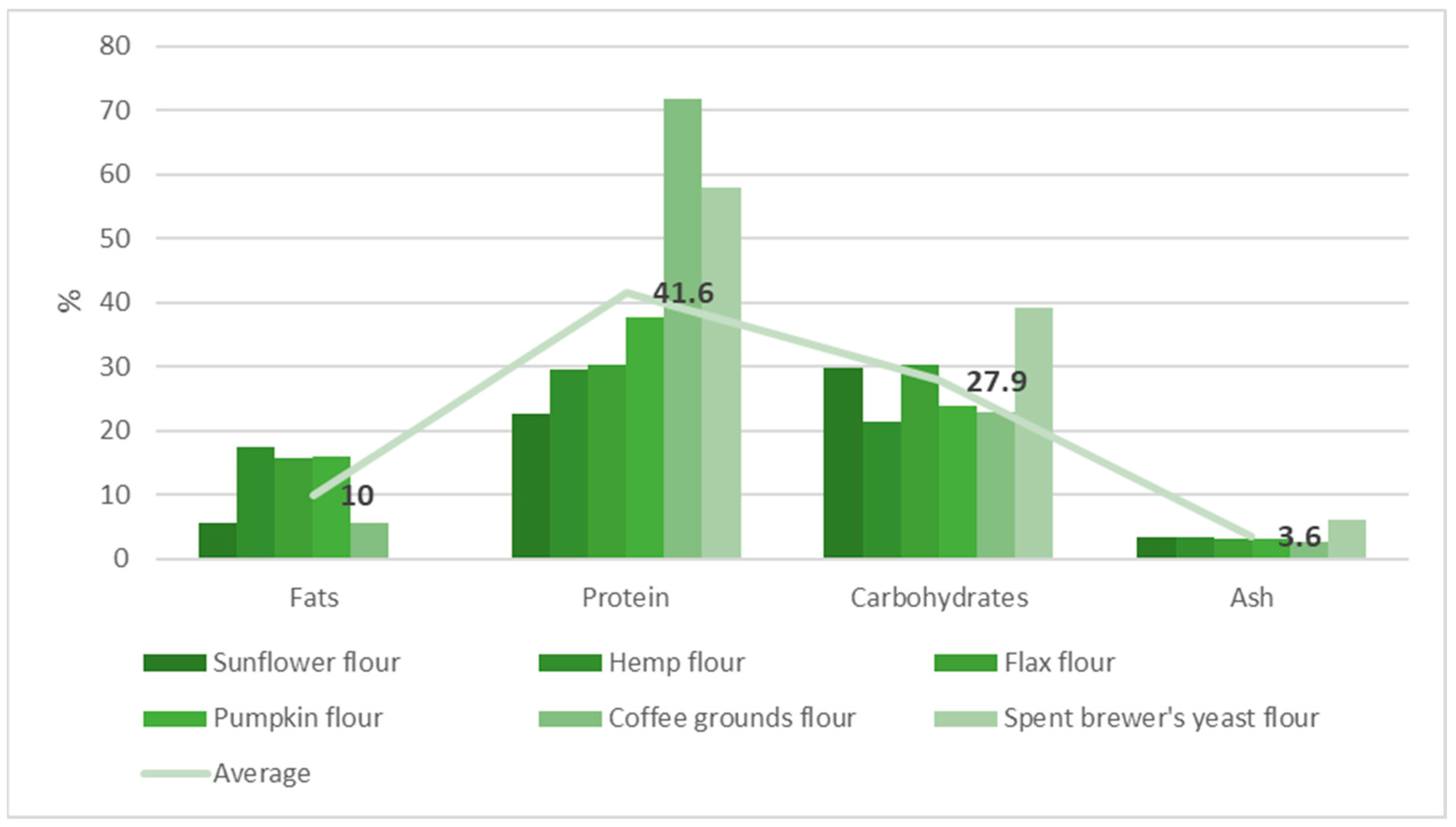
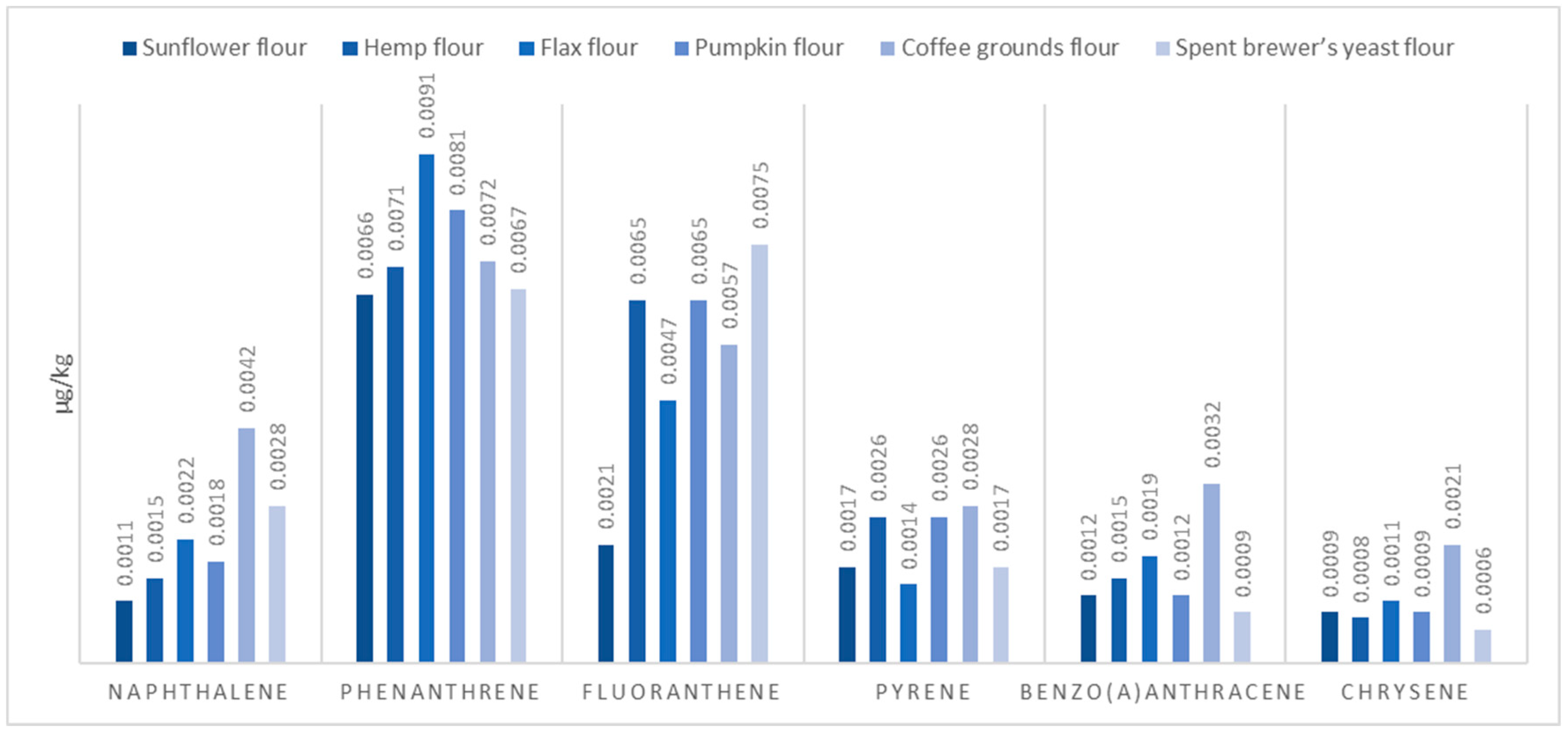


| Sample Matrix | Protein | Fats |
|---|---|---|
| Snail Flour | 1.024 | 1.037 |
| Slug Flour | 1.055 | 1.021 |
| Hepatopancreas Flour | 1.077 | 1.023 |
| Sunflower Flour | 1.034 | 1.014 |
| Hemp Flour | 1.051 | 1.025 |
| Flax Flour | 1.027 | 1.020 |
| Pumpkin Flour | 1.020 | 1.011 |
| Coffee Grounds Flour | 1.029 | 1.009 |
| Spent Brewer’s Yeast Flour | 1.014 | ND |
| Compound Name | UM | Snail Flour | Slug Flour | Hepatopancreas Flour | |||
|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | ||
| Na | mg/kg | 177.12 | 11.57 | 5549.02 | 95.71 | 2470.85 | 85.06 |
| Mg | mg/kg | 3821.48 | 82.57 | 2631.10 | 61.54 | 5719.10 | 101.24 |
| Al | mg/kg | 21.78 | 2.71 | 211.90 | 15.33 | 62.50 | 5.91 |
| Si | mg/kg | 285.01 | 5.24 | 271.10 | 11.09 | 115.65 | 9.54 |
| K | mg/kg | 15,831.76 | 345.12 | 16,688.02 | 651.02 | 18,623.77 | 751.30 |
| Ca | mg/kg | 8409.02 | 235.13 | 48,640.11 | 851.24 | 14,770.03 | 245.13 |
| Mn | mg/kg | 15.58 | 0.99 | 283.25 | 19.04 | 96.80 | 4.98 |
| Fe | mg/kg | 96.18 | 8.24 | 263.05 | 18.73 | 212.10 | 18.37 |
| Ni | mg/kg | 4.18 | 0.42 | 1.55 | 0.09 | 1.21 | 0.59 |
| Cu | mg/kg | 28.77 | 1.84 | 18.75 | 2.03 | 129.70 | 11.43 |
| Zn | mg/kg | 43.12 | 3.51 | 112.95 | 8.42 | 116.65 | 8.82 |
| As | mg/kg | <LQ | ND | 1.56 | 0.74 | 0.11 | 0.02 |
| Se | mg/kg | 0.22 | 0.01 | <LQ | ND | 0.34 | 0.05 |
| Sr | mg/kg | 8.30 | 0.76 | 109.20 | 7.13 | 88.10 | 2.46 |
| Cd | mg/kg | 0.06 | 0.001 | 1.17 | 0.85 | 11.45 | 0.93 |
| Ba | mg/kg | 1.60 | 0.09 | 32.35 | 2.67 | 98.80 | 6.44 |
| Pb | mg/kg | 0.24 | 0.02 | 0.53 | 0.0002 | 1.72 | 0.0003 |
| Analysis | UM | Sunflower Flour | Hemp Flour | Flax Flour | Pumpkin Flour | Coffee Grounds Flour | Spent Brewer’s Yeast Flour | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | Average | SD | ||
| Na | mg/kg | 96.25 | 6.41 | 110.6 | 9.41 | 354.55 | 26.55 | 127.71 | 10.02 | 33.35 | 3.45 | 5.5 | 0.41 |
| Mg | mg/kg | 1245.8 | 95.03 | 1858.45 | 102.78 | 1407.25 | 76.95 | 1940.51 | 143.67 | 936.71 | 40.12 | 90.9 | 7.84 |
| Al | mg/kg | 3.03 | 0.05 | 5.05 | 0.39 | 3.54 | 0.22 | 15.2 | 1.06 | 0.16 | 0.01 | 6.1 | 0.51 |
| Si | mg/kg | <LQ | ND | 29.3 | 1.99 | <LQ | ND | <LQ | ND | 58.4 | 4.61 | 5.9 | 0.42 |
| K | mg/kg | 3431.75 | 261.09 | 3843.35 | 70.03 | 3484.25 | 251.08 | 3951.55 | 297.78 | 7338.9 | 120.34 | 417.2 | 38.47 |
| Ca | mg/kg | 1045.35 | 52.61 | 761.12 | 39.13 | 1449.65 | 307.21 | 153.02 | 84.31 | 221.15 | 25.04 | 179.9 | 12.57 |
| Mn | mg/kg | 16.71 | 1.05 | 94.45 | 7.43 | 26.22 | 2.09 | 29.51 | 2.38 | <LQ | ND | 0.39 | 0.01 |
| Fe | mg/kg | 28.75 | 2.01 | 91.51 | 7.68 | 49.45 | 3.84 | 87.45 | 6.26 | 4.10 | 0.37 | 2.54 | 0.14 |
| Ni | mg/kg | 6.11 | 0.22 | 5.95 | 0.14 | 1.50 | 0.08 | 1.67 | 0.09 | 0.08 | 0.01 | 0.12 | 0.01 |
| Cu | mg/kg | 14.65 | 1.21 | 12.61 | 0.91 | 12.51 | 1.05 | 8.45 | 0.69 | 3.46 | 0.31 | 0.81 | 0.01 |
| Zn | mg/kg | 31.45 | 3.04 | 41.15 | 3.82 | 43.85 | 3.61 | 43.25 | 3.23 | 1.74 | 0.09 | 1.16 | 0.09 |
| As | mg/kg | <LQ | ND | <LQ | ND | <LQ | ND | <LQ | ND | <LQ | ND | <LQ | ND |
| Se | mg/kg | <LQ | ND | <LQ | ND | <LQ | ND | <LQ | ND | <LQ | ND | <LQ | ND |
| Sr | mg/kg | 3.74 | 0.18 | 5.72 | 0.24 | 8.82 | 0.94 | 2.85 | 0.11 | 0.92 | 0.05 | 0.19 | 0.01 |
| Cd | mg/kg | 0.49 | 0.01 | <LQ | ND | 0.28 | 0.02 | <LQ | ND | <LQ | ND | 0.01 | 0.001 |
| Ba | mg/kg | 1.64 | 0.43 | 2.92 | 0.13 | 1.31 | 0.04 | 1.14 | 0.09 | <LQ | ND | 0.04 | 0.002 |
| Pb | mg/kg | 0.15 | 0.01 | 0.14 | 0.01 | 0.11 | 0.01 | 0.16 | 0.01 | <LQ | ND | 0.01 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muntean, G.-C.; Simedru, D.; Uiuiu, P.; Tanaselia, C.; Cadar, O.; Becze, A.; Coroian, A. Evaluation of Alternative Sources of Proteins and Other Nutrients with Potential Applications in Fish Nutrition. Molecules 2024, 29, 2332. https://doi.org/10.3390/molecules29102332
Muntean G-C, Simedru D, Uiuiu P, Tanaselia C, Cadar O, Becze A, Coroian A. Evaluation of Alternative Sources of Proteins and Other Nutrients with Potential Applications in Fish Nutrition. Molecules. 2024; 29(10):2332. https://doi.org/10.3390/molecules29102332
Chicago/Turabian StyleMuntean, George-Cătălin, Dorina Simedru, Paul Uiuiu, Claudiu Tanaselia, Oana Cadar, Anca Becze, and Aurelia Coroian. 2024. "Evaluation of Alternative Sources of Proteins and Other Nutrients with Potential Applications in Fish Nutrition" Molecules 29, no. 10: 2332. https://doi.org/10.3390/molecules29102332
APA StyleMuntean, G.-C., Simedru, D., Uiuiu, P., Tanaselia, C., Cadar, O., Becze, A., & Coroian, A. (2024). Evaluation of Alternative Sources of Proteins and Other Nutrients with Potential Applications in Fish Nutrition. Molecules, 29(10), 2332. https://doi.org/10.3390/molecules29102332












