A Comparative Study and Prediction of the Ex Vivo Permeation of Six Vaginally Administered Drugs across Five Artificial Membranes and Vaginal Tissue
Abstract
1. Introduction
2. Results and Discussion
2.1. HPLC Methods
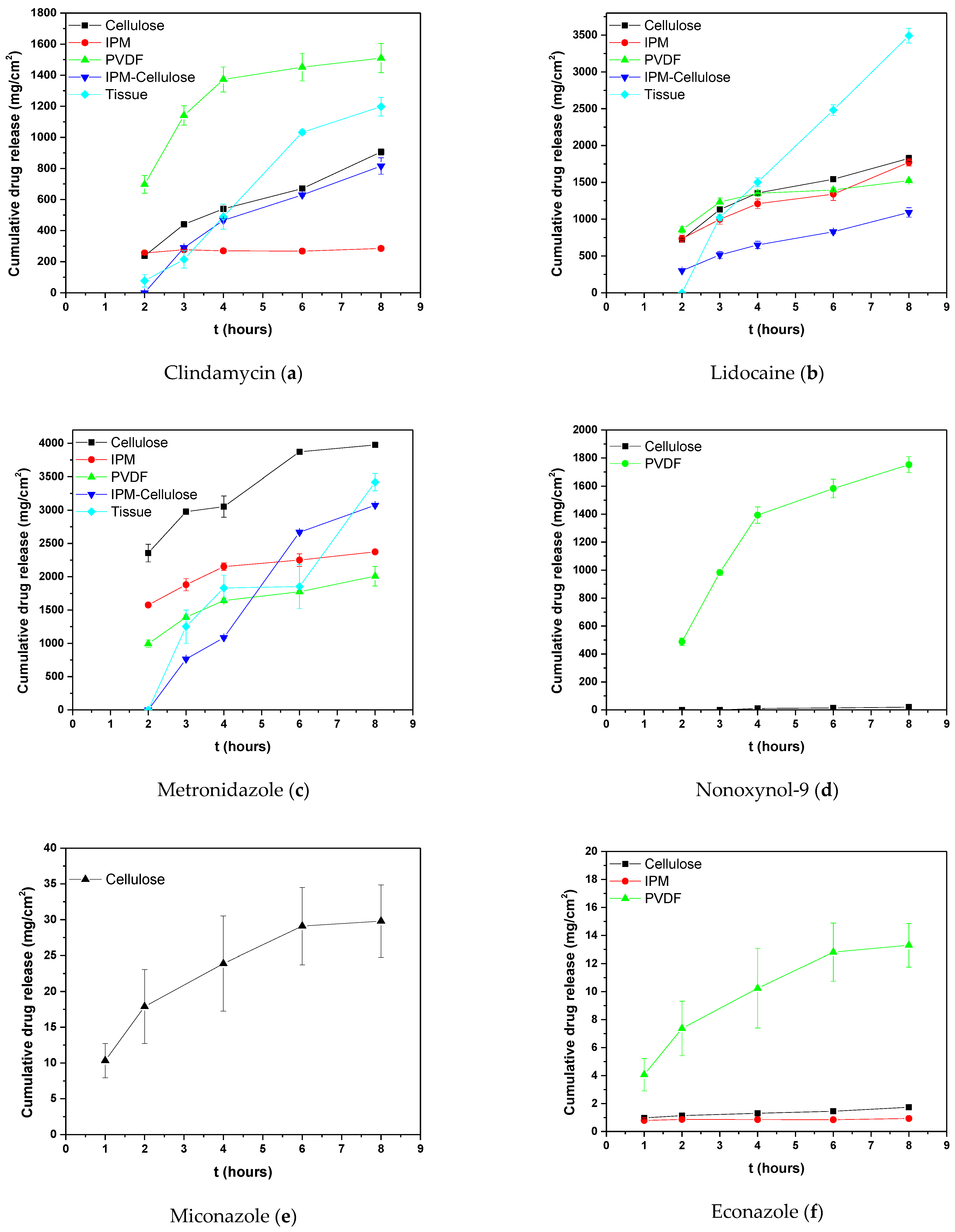
2.2. Permeability Studies
2.3. Penetration Prediction with In Silico Models
3. Materials
4. Methods
4.1. HPLC Analysis/Chromatographic Conditions
4.2. Validation of Quantification Method
4.3. Drug Solubility in Donor Medium
4.4. Synthetic Membranes Preparation
4.5. In Vitro Permeability Studies
4.6. Vagina Tissue—Ex Vivo Permeability Studies
4.7. PLS Methodology
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, N.J.; Baker, E.; Kaptein, M.; Karck, U.; Miller, L.; Zampaglione, E. Why consider vaginal drug administration? Fertil. Steril. 2004, 82, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Brannon-Peppas, L. Novel vaginal drug release applications. Adv. Drug Deliv. Rev. 1993, 11, 169–177. [Google Scholar] [CrossRef]
- Bijl, P.; Thompson, I.O.C.; Squier, C.A. Comparative permeability of human vaginal and buccal mucosa to water. Eur. J. Oral Sci. 1997, 105, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Van Der Bijl, P.; Van Eyk, A.D. Comparative in vitro permeability of human vaginal, small intestinal and colonic mucosa. Int. J. Pharm. 2003, 261, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.C.; Desai, K.J. Method for Augmentation of Intraepthellal and Systemic Exposure of Therapeutic Agents Having Substrate Activity for Cytochrome P450 Enzymes and Membrane Efflux Systems Following Vaginal and Oral Cavity Administration. United States Pat. US8178123B2, 15 May 2012. [Google Scholar]
- Sassi, A.B.; McCullough, K.D.; Cost, M.R.; Hillier, S.L.; Rohan, L.C. Permeability of tritiated water through human cervical and vaginal tissue. J. Pharm. Sci. 2004, 93, 2009–2016. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-de-Oliveira, R.; Palmeira-de-Oliveira, A.; Martinez-de-Oliveira, J. New strategies for local treatment of vaginal infections. Adv. Drug Deliv. Rev. 2015, 92, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Robinson, J.R. Vaginal epithelial models. Pharm. Biotechnol. 1996, 8, 409–424. [Google Scholar]
- Machado, R.M.; Palmeira-de-Oliveira, A.; Gaspar, C.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Studies and methodologies on vaginal drug permeation. Adv. Drug Deliv. Rev. 2015, 92, 14–26. [Google Scholar] [CrossRef]
- Neves, J.D.; Amaral, M.H.; Bahia, M.F. Performance of an in vitro mucoadhesion testing method for vaginal semisolids: Influence of different testing conditions and instrumental parameters. Eur. J. Pharm. Biopharm. 2008, 69, 622–632. [Google Scholar] [CrossRef]
- Franz, T.J. Percutaneous absorption on the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- FDA-SUPAC-SS Guidance for Industry. SUPAC-SS Non-sterile Semisolid Dosage Forms. Scale-up and Postapproval Changes: Chemistry, Manufacturing and Controls. In vitro Release Testing and In Vivo Bioequivalence Documentation. 1997; pp. 19–24. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ss-nonsterile-semisolid-dosage-forms-scale-and-post-approval-changes-chemistry-manufacturing (accessed on 15 April 2024).
- Twist, J.N.; Zatz, J.L. Membrane-solvent-solute interaction in a model permeation system. J. Pharm. Sci. 1988, 77, 536–540. [Google Scholar] [CrossRef]
- Pharmacopeia, U.S. Topical and Transdermal Drug Products—Product Quality Tests. United States Pharmacop. 2013, 35, 3–6. [Google Scholar]
- Ng, S.; Rouse, J.J.; Sanderson, F.D.; Eccleston, G.M. The Relevance of Polymeric Synthetic Membranes in Topical Formulation Assessment and Drug Diffusion Study. Arch. Pharm. Res. 2012, 35, 579–593. [Google Scholar] [CrossRef]
- Thakker, K.D.; Chern, W.H. Development and validation of in vitro release tests for semisolid dosage forms—Case study. Dissolution Technol. 2003, 10, 10–15. [Google Scholar] [CrossRef]
- Ng, S.F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative study of transmembrane diffusion and permeation of ibuprofen across synthetic membranes using franz diffusion cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Barry, B.W.; Eini, D.I.D.E. Influence of non-ionic surfactants on permeation of hydrocortisone, dexamethasone, testosterone and progesterone across cellulose acetate membrane. J. Pharm. Pharmacol. 1976, 28, 219–227. [Google Scholar] [CrossRef]
- Simon, A.; Amaro, M.I.; Healy, A.M.; Cabral, L.M.; de Sousa, V.P. Comparative evaluation of rivastigmine permeation from a transdermal system in the Franz cell using synthetic membranes and pig ear skin with in vivo-in vitro correlation. Int. J. Pharm. 2016, 512, 234–241. [Google Scholar] [CrossRef]
- Clément, P.; Laugel, C.; Marty, J.P. Influence of three synthetic membranes on the release of caffeine from concentrated W/O emulsions. J. Control. Release 2000, 66, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.; Church, M.K.; Duchnik, W.; Różewicka-Czabańska, M.; Bielecka-Grzela, S.; Prowans, P.; Pietriczko, J.; Czapla, N.; Bargiel, P.; Klimowicz, A. Comparison of artificial hydrophilic and lipophilic membranes and human skin to evaluate niacinamide penetration in vitro. Acta Pol. Pharm. Drug Res. 2020, 77, 271–279. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A vaginal fluid simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Zhu, C.; Jiang, L.; Chen, T.M.; Hwang, K.K. A comparative study of artificial membrane permeability assay for high throughput profiling of drug absorption potential. Eur. J. Med. Chem. 2002, 37, 399–407. [Google Scholar] [CrossRef]
- Wu, S.T.; Shiu, G.K.; Simmons, J.E.; Bronaugh, R.L.; Skelly, J.P. In vitro release of nitroglycerin from topical products by use of artificial membranes. J. Pharm. Sci. 1992, 81, 1153–1156. [Google Scholar] [CrossRef]
- Franc, I.; Lipinski, A.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar]
- Tsanaktsidou, E.; Krestenitis, M.; Karavasili, C.; Zacharis, C.K.; Fatouros, D.G.; Markopoulou, C.K. In vitro and in silico computational methods for assessing vaginal permeability. Drug Dev. Ind. Pharm. 2023, 49, 249–259. [Google Scholar] [CrossRef]
- Dahan, A.; Miller, J.M. The Solubility–Permeability Interplay and Its Implications in Formulation Design and Development for Poorly Soluble Drugs. AAPS J. 2012, 14, 244–251. [Google Scholar] [CrossRef]
- Jung, Y.J.; Yoon, J.H.; Kang, N.G.; Park, S.G.; Jeong, S.H. Diffusion properties of different compounds across various synthetic membranes using Franz-type diffusion cells. J. Pharm. Investig. 2012, 42, 271–277. [Google Scholar] [CrossRef]
- Kouskoura, M.G.; Kachrimanis, K.G.; Markopoulou, C.K. Modeling the drugs’ passive transfer in the body based on their chromatographic behavior. J. Pharm. Biomed. Anal. 2014, 100, 94–102. [Google Scholar] [CrossRef] [PubMed]
- ICH. ICH Topic Q2 (R1) Validation of Analytical Procedures: Text and Methodology. ICH Harmon. Tripart. Guidel. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2r1-validation-analytical-procedures-text-and-methodology-guidance-industry (accessed on 15 April 2024).
- Shabir, G.A. Validation of high-performance liquid chromatography methods for pharmaceutical analysis. J. Chromatogr. A 2003, 987, 57–66. [Google Scholar] [CrossRef]
- Smoleński, M.; Karolewicz, B.; Gołkowska, A.M.; Nartowski, K.P.; Małolepsza-Jarmołowska, K. Emulsion-based multicompartment vaginal drug carriers: From nanoemulsions to nanoemulgels. Int. J. Mol. Sci. 2021, 22, 6455. [Google Scholar] [CrossRef]
- Köllner, S.; Nardin, I.; Markt, R.; Griesser, J.; Prüfert, F.; Bernkop-Schnürch, A. Self-emulsifying drug delivery systems: Design of a novel vaginal delivery system for curcumin. Eur. J. Pharm. Biopharm. 2017, 115, 268–275. [Google Scholar] [CrossRef]
- D’cruz, O.J.; Uckun, F.M. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception 2001, 64, 113–123. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, O.J.; Yiv, S.H.; Waurzyniak, B.; Uckun, F.M. Contraceptive efficacy and safety studies of a novel microemulsion-based lipophilic vaginal spermicide. Fertil. Steril. 2001, 75, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Garg, S.; Tambwekar, K.R.; Vermani, K.; Garg, A.; Kaul, C.L.; Zaneveld, L.J.D. Compendium of pharmaceutical excipients for vaginal formulations. Pharm. Technol. 2001, 25, 14–24. [Google Scholar]
- Chen, Y.A.; Ou, S.M.; Lin, C.C. Influence of Dialysis Membranes on Clinical Outcomes: From History to Innovation. Membranes 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Jurca, T.; Józsa, L.; Suciu, R.; Pallag, A.; Marian, E.; Bácskay, I.; Mureșan, M.; Stan, R.L.; Cevei, M.; Cioară, F.; et al. Formulation of topical dosage forms containing synthetic and natural anti-inflammatory agents for the treatment of rheumatoid arthritis. Molecules 2021, 26, 24. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, D.; Meng, J.; Wang, H. Introduction to SIMCA-P and Its Application. In Handbook of Partial Least Squares: Concepts, Methods and Applications; Vinzi, V.E., Chin, W.W., Henseler, J., Wang, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 757–774. [Google Scholar]
- Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. DataWarrior: An Open-Source Program for Chemistry Aware Data Visualization and Analysis. J. Chem. Inf. Model. 2015, 55, 460–473. [Google Scholar] [CrossRef]
- ACD/Labs. Advanced Chemistry Development, Inc. 2015. Available online: https://www.acdlabs.com/index.php (accessed on 9 September 2019).
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2019 update: Improved access to chemical data. Nucleic Acids Res. 2018, 47, D1102–D1109. [Google Scholar] [CrossRef]
- Simca-P 9.0. In User Guide and Tutorial; Umetrics: Malmo, Sweden, 2001.
- Haaland, D.M.; Thomas, E.V.; Haaland, D.M.; Thomas, E.V. Partial least-squares methods for spectral analyses. 1. Relation to other quantitative calibration methods and the extraction of qualitative information. Anal. Chem. 1988, 60, 1193–1202. [Google Scholar] [CrossRef]
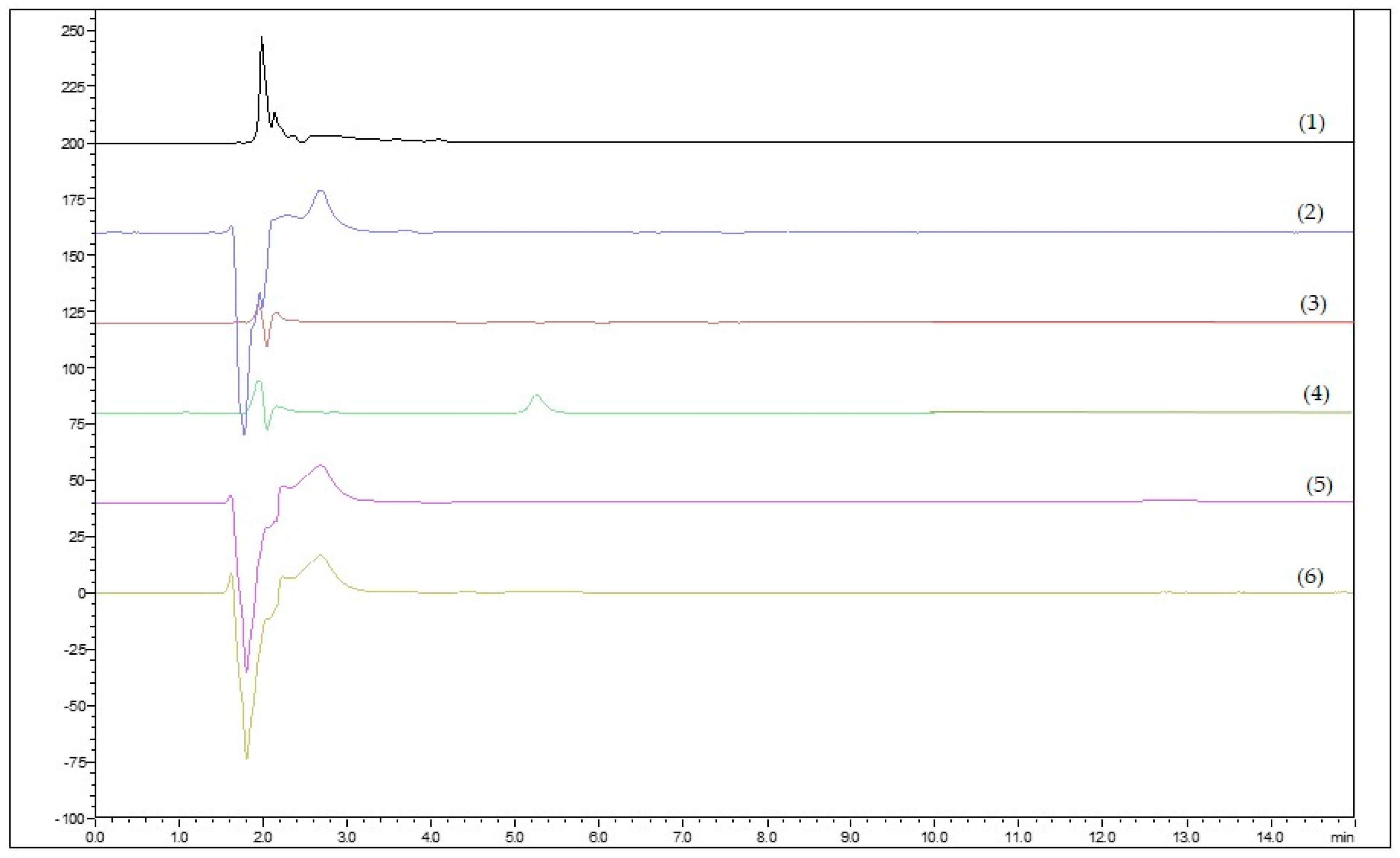
| Comp. | Calibration Curve | LOD (μg/mL) | LOQ (μg/mL) | R² | Mobile Phase | Retention Factor (k’) |
|---|---|---|---|---|---|---|
| NONO | y = 28002x − 56015 | 1.04 | 3.14 | 0.9993 | 20 mM KH2PO4 buffer, pH 3:MeOH, (20:80 v/v) | 8.285 |
| CLIND | y = 4887.3x − 743.42 | 0.08 | 0.25 | 1 | 20 mM KH2PO4 buffer, pH 3:ACN, (85:15 v/v) | 1.061 |
| LIDO | y = 11490x − 333.93 | 0.15 | 0.45 | 1 | 20 mM KH2PO4 buffer, pH 3:MeOH, (35:65 v/v) | 0.728 |
| METRO | y = 14236x − 1443.2 | 0.23 | 0.68 | 0.9999 | 20 mM KH2PO4 buffer, pH 3:MeOH, (80:20 v/v) | 2.041 |
| ECO | y = 90527x − 19250 | 0.34 | 1.02 | 0.9991 | 20 mM KH2PO4 buffer, pH 3:MeOH, (50:50 v/v) | 2.105 |
| MICO | y = 42121x − 9117.2 | 0.25 | 0.77 | 0.9996 | 20 mM KH2PO4 buffer, pH 3:MeOH, (30:70 v/v) | 2.231 |
| Comp. | Precision | Intermediate Precision | Accuracy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Theoretical (μg/mL) | av. Experimental Conc | RSD% of 6 Preparations | av. EXPERIMENTAL Conc | RSD% of 6 Preparations | ||||||
| 2nd Day | 3rd Day | 2nd Day | 3rd Day | |||||||
| NONO | 20 | 20.02 ± 0.33 | 1.67 | 20.1 (±0.2) | 19.8 (±0.1) | 1.5 | 1.8 | 40 μg/mL | 15.5 μg/mL | 5 μg/mL |
| 39.54 ± 0.14 (RSD: 0.36)/R%: 98.8 | 15.4 ± 0.2 (RSD: 1.4)/R%: 102.8 | 5.09 ± 0.02 (RSD: 0.38)/R%: 101.9 | ||||||||
| CLIND | 5 | 5.01 ± 0.04 | 0.76 | 5.1 (±0.1) | 4.8 (±0.3) | 1 | 0.8 | 10 μg/mL | 2.5 μg/mL | 0.5 μg/mL |
| 9.91 ± 0.07 (RSD: 0.71)/R%: 99.1 | 2.48 ± 0.06 (RSD: 2.3)/R%: 99.5 | 0.49 ± 0.01 (RSD: 2.7)/R%: 99.7 | ||||||||
| LIDO | 8 | 7.85 ± 0.02 | 0.21 | 7.92 (±0.1) | 8.1 (±0.3) | 0.8 | 0.6 | 12.5 μg/mL | 5 μg/mL | 1 μg/mL |
| 12.37 ± 0.02 (RSD: 0.13)/R%: 99.0 | 4.93 ± 0.01 (RSD: 0.18)/R%: 98.7 | 1.0 ± 0.01 (RSD: 0.81)/R%: 100.3 | ||||||||
| METRO | 8 | 8.08 ± 0.02 | 0.23 | 7.95 (±0.1) | 7.85 (±0.2) | 0.5 | 0.8 | 12 μg/mL | 5 μg/mL | 1 μg/mL |
| 12.04 ± 0.04 (RSD: 0.4)/R%: 100.3 | 4.99 ± 0.01 (RSD: 0.11)/R%: 99.9 | 1.02 ± 0.01 (RSD: 0.18)/R%: 101.9 | ||||||||
| ECO | 13 | 13.4 ± 0.06 | 0.48 | 13.1 (±0.03) | 12.8 (±0.1) | 0.7 | 0.9 | 15 μg/mL | 5 μg/mL | 1 μg/mL |
| 14.94 ± 0.04 (RSD: 0.3)/R%: 100.3 | 4.97 ± 0.03 (RSD: 0.2)/R%: 99.7 | 1.05 ± 0.03 (RSD: 0.25)/R%: 101.7 | ||||||||
| MICO | 5.5 | 5.46 ± 0.06 | 1.14 | 5.3 (±0.04) | 5.1 (±0.1) | 0.9 | 1.0 | 15 μg/mL | 5 μg/mL | 1 μg/mL |
| 15.04 ± 0.07 (RSD: 0.27)/R%: 99.3 | 4.99 ± 0.01 (RSD: 0.7)/R%: 101.1 | 1.02 ± 0.01 (RSD: 1.1)/R%: 98.9 | ||||||||
| Compound | Initial Donor Concentration (mg/mL) | Initial Mass Added (mg) | Final Mass Donor (mg) 1 | Acceptor’s Mass (mg) | Tissue Extraction (mg) |
|---|---|---|---|---|---|
| CLIND | 9.94 | 1.988 | 1.4 (±0.1) | 0.23 (±0.07) | 0.3 (±0.01) |
| ECO | 1.24 | 0.248 | 0.2 (±0.01) | 0 | 0.01 (±0.001) |
| LIDO | 9.94 | 1.988 | 0.9 (±0.1) | 0.53 (±0.09) | 0.5 (±0.09) |
| METRO | 9.98 | 1.996 | 0.01 (±0.001) | 0.65 (±0.08) | 1.4 (±0.2) |
| MICO | 1.3 | 0.26 | 0.26 (±0.02) | 0 | 0 |
| NONO | 10.04 | 2.008 | 1.85 (±0.2) | 0 | 0.15 (±0.05) |
| (a) | |||||||
| Papp (h/cm²) 1 | |||||||
| Membranes | Cellulose (Hydrophilic) | Impregnated IPM | PVDF (Hydrophobic) | IPM- Cellulose | PVDF-Cellulose | Tissue | |
| Compounds | |||||||
| LIDO | 0.011 (±0.003) | 0.011 (±0.003) | 0.005 (±0.002) | 0.012 (±0.001) | 0.012 (±0.0007) | 0.042 (±0.01) | |
| METRO | 0.023 (±0.0017) | 0.014 (±0.0007) | 0.009 (±0.003) | 0.002 (±0.0001) | 0.004 (±0.0009) | 0.046 (±0.016) | |
| CLIND | 0.009 (±0.001) | 0.0003 (±0.0001) | 0.005 (±0.0007) | 0.012 (±0.001) | 0.015 (±0.0002) | 0.02 (±0.0016) | |
| NONO | 2 × 10−4 (±3 × 10−5) | 0 | 0.009 (±4.5 × 10−5) | 0 | 0 | 0 | |
| MICO | 0 | 0 | 0.0029 (±2 × 10−5) | 0 | 0 | 0 | |
| ECO | 8.6 × 10−4 (±2 × 10−5) | 1.4 × 10−4 (±3 × 10−6) | 0.00015 (±9 × 10−6) | 0 | 0 | 0 | |
| (b) | |||||||
| Cumulative Amount Permeated (μg/cm2) | |||||||
| Membranes | Cellulose (Hydrophilic) | Impregnated IPM | PVDF (Hydrophobic) | IPM- Cellulose | PVDF-Cellulose | Tissue | |
| Compounds | |||||||
| LIDO | 1825.7 2 2nd (±44.9) | 1772.0 2nd (±72.8) | 1524.8 3rd (±18) | 1091.9 1st (±91.9) | 975.8 2nd (±61.8) | 2789.3 2nd (±99.4) | |
| METRO | 3976.4 1st (±8.9) | 2373.1 1st (±298) | 2008.4 1st (±147) | 307.3 3rd (±40.7) | 370.2 3rd (±67.1) | 3419.5 1st (±29.6) | |
| CLIND | 905.9 3rd (±38.8) | 285.0 3rd (±48) | 1510.1 4th (±188.6) | 815.5 2nd (±85.2) | 1047.1 1st (±22) | 1197.5 3rd (±159.5) | |
| NONO | 20.1 4th (±1.1) | - | 1752.2 2nd (±56) | - | - | - | |
| MICO | - | - | 33.4 5th (±3.07) | - | - | - | |
| ECO | 1.7 5th (±0.1) | 0.92 4th (±0.02) | 15.3 6th (±1.6) | - | - | - | |
| Compound | tr (min) |
|---|---|
| CLIND | 6.10 |
| ECO | 9.94 |
| LIDO | 5.94 |
| METRO | 3.93 |
| MICO | 10.2 |
| NONO | 39.48 |
| Compound | Papp (h/cm²) | YPredPS[3] (Papp (h/cm²)) |
|---|---|---|
| ECO | 8.6 × 10−4 | −5.6 × 10−4 ≡ 0 |
| METRO | 0.023 | 0.027 |
| MICO | 0 | −0.005 ≡ 0 |
| LIDO | 0.011 | 0.016 |
| CLIND | 0.009 | 0.007 |
| NONO | 2 × 10−4 | 8 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsanaktsidou, E.; Chatzitaki, A.-T.; Chatzichristou, A.; Fatouros, D.G.; Markopoulou, C.K. A Comparative Study and Prediction of the Ex Vivo Permeation of Six Vaginally Administered Drugs across Five Artificial Membranes and Vaginal Tissue. Molecules 2024, 29, 2334. https://doi.org/10.3390/molecules29102334
Tsanaktsidou E, Chatzitaki A-T, Chatzichristou A, Fatouros DG, Markopoulou CK. A Comparative Study and Prediction of the Ex Vivo Permeation of Six Vaginally Administered Drugs across Five Artificial Membranes and Vaginal Tissue. Molecules. 2024; 29(10):2334. https://doi.org/10.3390/molecules29102334
Chicago/Turabian StyleTsanaktsidou, Eleni, Aikaterini-Theodora Chatzitaki, Anatoli Chatzichristou, Dimitrios G. Fatouros, and Catherine K. Markopoulou. 2024. "A Comparative Study and Prediction of the Ex Vivo Permeation of Six Vaginally Administered Drugs across Five Artificial Membranes and Vaginal Tissue" Molecules 29, no. 10: 2334. https://doi.org/10.3390/molecules29102334
APA StyleTsanaktsidou, E., Chatzitaki, A.-T., Chatzichristou, A., Fatouros, D. G., & Markopoulou, C. K. (2024). A Comparative Study and Prediction of the Ex Vivo Permeation of Six Vaginally Administered Drugs across Five Artificial Membranes and Vaginal Tissue. Molecules, 29(10), 2334. https://doi.org/10.3390/molecules29102334





