Abstract
Polyhydroxyalkanoates (PHAs) are intracellular biopolymers that microorganisms use for energy and carbon storage. They are mechanically similar to petrochemical plastics when chemically extracted, but are completely biodegradable. While they have potential as a replacement for petrochemical plastics, their high production cost using traditional carbon sources remains a significant challenge. One potential solution is to modify heterotrophic PHA-producing strains to utilize alternative carbon sources. An alternative approach is to utilize methylotrophic or autotrophic strains. This article provides an overview of bacterial strains employed for PHA production, with a particular focus on those exhibiting the highest PHA content in dry cell mass. The strains are organized according to their carbon source utilization, encompassing autotrophy (utilizing CO2, CO) and methylotrophy (utilizing reduced single-carbon substrates) to heterotrophy (utilizing more traditional and alternative substrates).
1. Introduction
The term ‘bio-based polymers’, recommended by IUPAC and colloquially referred to as ‘bioplastics’, refers to polymeric substances produced from renewable resources and at least partially of biological origin. This includes compounds whose starting material was produced naturally, such as starch, cellulose, and lactic acid, but which was later re-molded or polymerized abiotically. Such material is typically hydrophilic and brittle. There are also more robust materials, such as polylactic acid, but it has been found that these materials accumulate in the environment similarly to petrochemical plastics [1]. Bio-based polymers can also refer to compounds that are produced entirely through biological means. These substances are known as polyhydroxyalkanoates (PHAs), which serve as carbon and energy reserves for cells. PHAs are biodegradable in the environment due to their biological origin, while still retaining some characteristics of petrochemical plastics. These polymers are predominantly employed in the field of packaging [2,3], with ongoing research exploring their potential applications in the medical field [4] and in agriculture [5]. Figure 1 illustrates the general structure of PHAs.
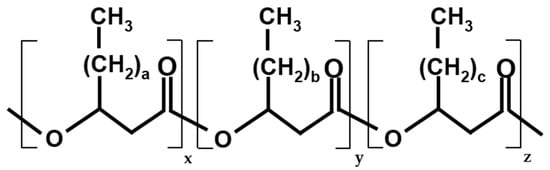
Figure 1.
General structure of PHA (co-)polymers. a,b,c—number of additional methylene bridges in the chain (0–14), x,y,z—number of monomer units of each hydroxyalkanoate in the polymer chain (0 → ∞).
PHAs are divided into two categories based on the length of the monomer used for polymerization: short-chain-length PHAs (scl-PHAs), which use C3–C5 substrates, and medium-chain-length PHAs (mcl-PHAs), which use C6+ substrates (usually up to C14). The most widely produced storage polymer is scl-PHA, specifically the C4-derived poly(3-hydroxybutyrate) (PHB). It can form copolymers with C5-derived polyhydroxyvalerate (PHV), which improves the mechanical properties of the resulting material. C3- and C4-derived polyhydroxypropionate and poly(4-hydroxybutyrate) are not naturally occurring storage polymers. However, their desirable mechanical properties have led to efforts to produce these copolymers in bulk through genetic modifications to organisms or using carbon precursors. While scl-PHAs have been studied more extensively, mcl-PHAs such as polyhydroxyhexanoate (PHH) have higher elasticity and a lower melting point. Therefore, copolymers of scl- and mcl-PHAs are desirable due to their decreased brittleness. Table 1 compares the characteristics of various PHA copolymers.

Table 1.
Comparison of mechanistic properties of various PHA copolymers.
PHA production is highly dependent on the organism, cultivation, and extraction method used. Cupriavidus necator (previously called Ralstonia eutropha) is one of the most studied organisms for PHA production due to its ability to naturally produce PHAs in high amounts and due its high metabolic versatility. PHA accumulation can also be increased by a high C/N ratio, depletion of N, S, and P, or a low rate of respiration (resulting in more reducing conditions in the cell). The primary disadvantage of PHAs is their high production cost, which is mainly due to the expensive carbon source that can account for up to 40% of total costs. A study conducted in 1997 estimated the cost of investments and maintenance, using sugars as a carbon source, and the extraction of PHAs using hypochlorite digestion and a detergent [13]. In 1995, the Copersugar company constructed a pilot plant in a sugar mill in Brazil to produce PHB. The plant processed biomass with a PHA content of 65–70%, which was extracted using non-halogenated solvents (medium-length alcohols) [14]. A 2016 study used a computer simulation to evaluate the potential for PHB production from methane, with extraction being carried out using acetone. The use of thermophilic organisms would eliminate the need for cooling, which is the most expensive operation [15]. For an overview of the cost distribution of PHA production, refer to Figure A1 in Appendix A. The current commercial producers of PHA include, among others, Danimer Scientific (Bainbridge, GA, USA), Shenzhen Ecomann Biotechnology Co., Ltd. (Shenzhen, China), Kaneka Corporation (Osaka, Japan), RWDC Industries (Singapore), TianAn Biologic Materials Co., Ltd. (Ningbo, China), Newlight Technologies LLC (Huntington Beach, CA, USA), and Biomer (Schwalbach, Germany). These producers primarily utilize sugars and vegetable oils as raw materials. Figure 2 provides an overview of the production of PHAs using microorganisms.
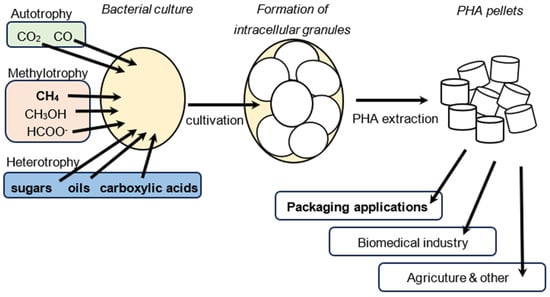
Figure 2.
Diagram of PHA production by microorganisms. The carbon substrate is assimilated by the bacterial strain, resulting in the formation of intracellular PHA granules. These serve as carbon and energy storage for the cell. The PHA granules can be extracted and processed into pellets or powder, which can then be further processed to be used, for instance, as a packaging material.
In the environment, polyhydroxyalkanoates (PHAs) are degraded by microbial PHA-degraders that excrete PHA depolymerases, which themselves are not necessarily PHA producers. These include bacteria and fungi. Some bacterial lipases are also capable of degrading PHAs [16]. Abiotic factors, such as elevated temperature, low pH, UV radiation, high relative humidity, and the surface area of the polymer (plates vs. granules), facilitate PHA degradation. A review of various studies on the environmental degradation of PHA was conducted [17]. The rate of degradation is also influenced by the diversity of the microbial community. The time frame for degradation varies between weeks and months. The majority of studies indicate that copolymers, such as PHBV, degrade at a faster rate than PHB due to their lower crystallinity and more porous surface [17]. A more recent study has highlighted the significance of relative humidity in soil. Samples were observed to degrade within two weeks at 100% relative humidity, whereas degradation was negligible within the same time frame at 40% humidity [18].
2. Metabolism of PHAs
2.1. Biosynthesis
Figure 3 shows general pathways related to the synthesis of storage PHA. These PHAs can be produced by bacteria and archaea that inhabit soil and water environments.
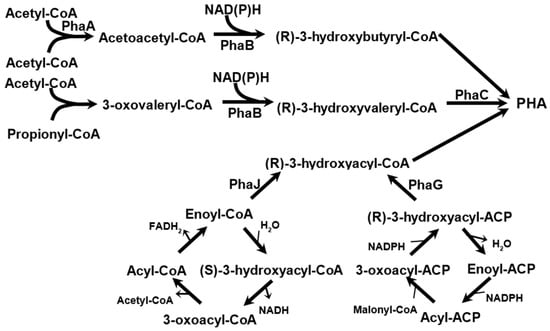
Figure 3.
Pathways to form scl- and mcl-PHAs.
The main pathway for PHB synthesis begins with the condensation of two molecules of acetyl-CoA into acetoacetyl-CoA, which is then reduced to 3-hydroxybutyryl-CoA, the active form of 3-hydroxybutyrate. These activated monomers are then polymerized into PHB. The enzymes that catalyze these reactions are commonly referred to as PhaA, PhaB, and PhaC. Other acyl-CoA compounds, such as valeryl-CoA, can be utilized for polymerization. Valeryl-CoA can be synthesized from acetyl-CoA and propionyl-CoA. The synthesis of mcl-PHAs involves the use of (R)-3-hydroxyacyl-CoAs, which can be obtained through the beta-oxidation of fatty acids (using enzyme PhaJ [19]) or during the de novo synthesis of fatty acids (using enzyme PhaG [20]).
2.2. PHA Granules
Synthetized PHA chains are known to form intracellular inclusion bodies, which are commonly referred to as PHA granules. These granules contain amorphous PHA. Subsequent industrial processing results in the denaturation and transformation of these PHAs into a more crystalline structure. The majority of the surface area of a native PHA granule is covered with an amphiphilic protein called phasin (PhaP), which forms a boundary between the hydrophobic PHA chains and hydrophilic cytosol. The expression of the phasin gene is controlled by the PhaR regulator protein, which competes with phasin on the granule surface. Phasin has a higher affinity for the granule and eventually replaces all PhaR, which then binds upstream of phaP and represses its expression [21]. The remaining surface of the granule is covered with PHA polymerases and depolymerases. In C. necator, an additional protein PhaM anchors the granule to the nucleoid and affects the total number of granules in the cell [22].
There are three main models for granule formation. The first is the micelle model, where PHA polymerases surround a micelle that accumulates synthetized PHA chains. The second is the budding model, in which PHA synthases are located on the cytosolic side of the cell membrane, PHA accumulates in the phospholipid bilayer, and eventually buds into cytosolic side. Finally, the scaffolding model is similar to the micelle model, but nucleation centers are referred to as ‘mediation elements’, which are dark-stained structures that appear during electron microscopy but have not been identified further. A modified micelle model emerged later, where nascent PHA-chains first create a complex with phasins to maintain solubility and later accumulate into larger granules (as reviewed in [23]).
2.3. Mobilization of PHAs
The utilization of these storage compounds requires the enzymatic degradation of PHA by PHA depolymerases (PhaZ). This enzyme cleaves the long chain into both monomers of 3-hydroxybutyrate and into oligomers of 3-hydroxybutyrate. The oligomers of 3-hydroxybutyrate require a dedicated enzyme to be further split into monomers [24]. Subsequently, monomeric 3-hydroxybutyrate is oxidized to acetoacetate. After its activation to acetoacetyl-CoA, it can re-enter metabolism. The degradation of mcl-PHA yields fatty acids that can enter beta-oxidation.
2.4. Role of PHAs in Cell Physiology
Long-chained PHAs are well-known for their role as storage compounds in bacteria and archaea. Nevertheless, short methyl-esterified dimers and trimers of 3-hydroxybutyrate are highly effective antioxidants against hydroxyl radicals (3-fold more effective than glutathione and 11-fold more effective than ascorbic acid and monomeric 3-hydroxybutyrate) in cells that possess PhaC and PhaZ enzymes. These findings provide new insights into the utilization of PHA reserves in microorganisms that inhabit stressful environments and in endophytic microorganisms that infect plant cells [25].
Small amounts of PHB seem to be present in all organisms. This has been evidenced by the detection of PHB in samples from organisms that do not form storage PHA granules, including E. coli, yeast, spinach leaves, and animal and human tissues. The elevated concentration of PHB in competent E. coli cells suggests its importance for exogenous DNA uptake during bacterial transformation [26]. Furthermore, it has been identified in human plasma [27]. Oligomers of PHB (100–200 units) have been observed to create a complex with polyphosphates and Ca2+ ions to form channels that facilitate ion transport, as reported in the membranes of E. coli [28]. Furthermore, PHB has been implicated in the formation of the mitochondrial permeability transition pore during apoptosis, as reported for tissue samples from rat livers [29]. Additionally, short PHB chains were found to be covalently linked to the outer membrane protein OmpW of E. coli. This may be involved in its targeting to the outer membrane and assembly [30]. Furthermore, data indicate that histones and analogous DNA-binding proteins in prokaryotes are covalently modified by oligomeric PHB [31].
The existence of oligomeric PHB in organisms that lack PhaC has remained a mystery, as it has been unclear how these polymers can be synthesized. In E. coli, it has been demonstrated that YdcS, a component of a putative periplasmic ABC transporter, exhibits PHB synthase activity [32]. A simple database search reveals that the sequence of this protein is widely distributed among bacteria. To date, no eukaryotic protein with a similar function has been reported. The significance of non-storage PHB for cellular physiology remained obscure for a long time, due to the lability of its connection and the polymer’s absence in protein crystallographic studies, which is attributed to sample preparation methods that do not preserve it [33].
3. Determination of PHA Content in Cells
There are a number of different methods that can be employed to determine the content of polyhydroxyalkanoates (PHA) in biomass. The intracellular PHA granules can be observed in high definition using transmission electron microscopy (TEM), although this method requires laborious sample preparation and expensive equipment. A rapid and relatively simple approach is fluorescence microscopy using Nile Red dye, which binds to intracellular granules (emission maximum around 610 nm) [34]. However, due to its lipophilic nature, this dye is not suitable for quantitative analysis.
The content of PHA can be determined gravimetrically by its extraction into organic solvents, which are subsequently evaporated or dissolved PHAs are selectively precipitated. Nevertheless, such extractions do not ensure sufficient PHA purity. Indeed, the purity of the extracted PHA can vary considerably, ranging from 66 to 96%, depending on the pretreatment of the cells [35]. PHB can also be dehydrated to crotonic acid, which can be determined via high-performance liquid chromatography (HPLC) with a UV detector unit. A traditional method for determining the composition of PHA is transesterification, which converts the polymer into volatile methylesters. These can be identified by gas chromatography. In recent years, Fourier transform infrared (FTIR) spectroscopy has emerged as a valuable tool for the qualitative and quantitative determination of PHAs [36,37]. This method can be applied to intact cells, eliminating the need for laborious pretreatment. For further details on the methodologies employed for the determination of PHA, please refer to [38].
4. Extraction Methods
It may be useful to provide a brief summary of the methods for subsequent PHA extraction from biomass. To date, the most effective method for disruptive extraction in terms of purity, recovery rate, and high levels of polymerization of extracted PHAs (high molecular weight) is the use of halogenated organic solvents, such as chloroform. Their high ecotoxicity raises concerns about the environmental impact and limits their use in bulk. In an effort to reduce their use, researchers have explored the possibility of pretreating the cells with supercritical CO2 [39], or replacing these solvents entirely with non-halogenated solvents, such as alcohols [14], butyl acetate [40], or methyl isobutyl ketone [41]. A combination of sonication and detergents [42,43], sonication and extraction by NH3 [44,45], or the use of subcritical water [46] have also been attempted, with varying levels of success. Additionally, cells were genetically modified to excrete PHAs, as observed in the case of E. coli, which excreted 36% PHB [47]. A mutant strain of Alcanivorax borkumensis was observed to excrete PHA in large but undetermined amounts [48].
5. Overview of Artificial Modifications to Enhance PHA Production
Recently, modifications to enhance PHA production have been reviewed [49]. Figure 4 provides an overview of several modifications that have been employed to enhance PHA accumulation, which will be discussed in greater detail in this section.
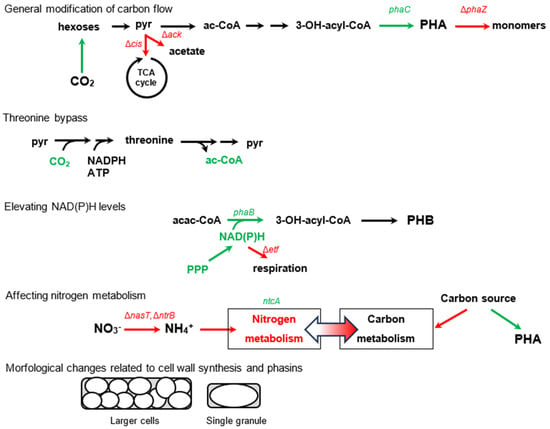
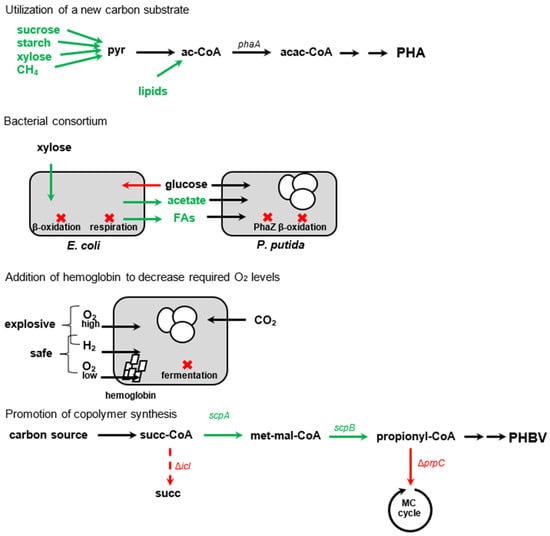
Figure 4.
Overview of some modifications of metabolic pathways to promote PHA accumulation. Newly introduced or stimulated paths are in green; suppressed paths are in red. Red crosses indicate paths that have been suppressed in cells (grey rounded rectangles). Abbreviations: pyr—pyruvate, ac−CoA—acetyl coenzyme A, cis—citrate synthase, ack—acetate kinase, TCA—tricarboxylic, acac−CoA—acetoacetyl coenzyme A, PPP—penthose phosphate pathway, etf—electron transfer flavoprotein, FAs—fatty acids, succ−CoA—succinyl coenzyme A, succ—succinate, met−malo−CoA—methylmalonyl coenzyme A, MC—methylcitrate, scpA—methylmalonyl coenzyme A mutase, scpB—mehylmalonyl coenzyme A decarboxylase, icl—isocitrate lyase, prpC—2−methylcitrate synthase.
One strategy involves modifying the carbon flow towards PHA accumulation by overexpressing genes related to PHA synthesis (phaC) [50] (from 6% PHA content to 8% PHA content), carbon assimilation (Calvin–Benson cycle) (from 49% to 58%) [51], or decreasing catabolism by downregulating citrate synthase within the citric acid cycle (from 49% to 55%) [52]. To utilize the excess reducing power, a “threonine bypass” was developed to assimilate CO2 during growth on glucose, which was further enhanced by the addition of stress-tolerant genes (from 34% to 57%) [53,54].
To reduce acetoacetyl-CoA and promote the synthesis of PHA precursors, one can increase the concentration of NAD(P)H by overexpressing pentose phosphate pathway enzymes (co-expression with phaBRC for precursors, 2.6-fold increase in g/L titre, 24% PHA max) [55] or by deleting electron transfer flavoproteins (from 84% to 90%) [56]. As carbon and nitrogen metabolisms are interconnected, modifying nitrogen regulators can redirect carbon flow towards PHAs. For example, deleting sensors of NH4+ (NtrB) (from 17% to 93 93%) [57] or nitrate (NasT) (from below 1% to 15%) [58], or by overexpressing the sensor related to 2-oxoglutarate concentration (NtcA) (from 2% to 5%) [59], can be effective.
The amount of PHA content in cells is limited by the size of the cell wall. When specific acyltransferases are deleted, the compactness of the outer cell membrane decreases, resulting in an increase in PHA content (from 70% to 81%) [60]. Knocking out certain phasin genes and overexpressing genes for cell-fission-ring-blocking proteins can cause PHA to form a single large granule, which increases in size along with the cells [61]. Although larger granules could be beneficial for subsequent PHA extractions, the percentage of PHA was somewhat lower in comparison to single-granule non-producing strains (drop from 80% to 73%).
There have been many modifications made to strains in order to increase their metabolic versatility. For instance, transport systems and kinases have been added for glycerol (cultivation time dropped to 72 h from 268 h, and PHA content increased from 60% to 64%) [62], sucrose (resulting in 74%) [63], xylose (up to in 73%, together with NDH-II knockout) [64], and raw starch (up to 63%) [65]. The addition of lipases has also been reported (up to 66%) [66]. Lignocellulose can serve as a carbon source through the introduction of PHA synthesis into natural lignocellulose utilizers, which are resistant to various toxins in the substrate (39%, mixture of xylose and glucose originating from wheat straw) [67]. It can also be utilized by modifying strains capable of growing on both lignocellulose and methane (1%) [68]. A bacterial consortium was created in which xylose is first converted to acetate by E. coli before being utilized for PHA production by Pseudomonas putida. This was achieved through the deletion of glucose uptake, suppression of respiration, promotion of fatty acid export, deletion of PHA depolymerase, and beta-oxidation pathway (1.30 g/L, %PHA not determined) [69]. To ensure safety, aerobic cultivation on H2 requires a low concentration of O2. In this instance, PHA accumulation was increased by deleting enzymes related to fermentation, such as lactate dehydrogenase, acetate kinase, and isocitrate lyase, and by introducing low-O2-tolerant hemoglobin to improve O2 diffusion in the cell (from 39% to 50%) [70]. Modifications can also be used to produce copolymers by increasing the accumulation of propionyl-CoA, which can be achieved by deleting isocitrate lyase, succinate dehydrogenase, and methylcitrate synthase (65% with 25% PHV) [71].
6. Application of PHA-Producing Strains
In this context, PHA-producing organisms are classified into groups based on their carbon source, source of electrons, and source of energy to power metabolism. Organisms can obtain carbon in two primary forms: as oxidized inorganic compounds, such as CO2 and CO, which are utilized in autotrophic processes, or in its reduced organic form, which is employed in heterotrophic processes. By convention, an autotroph must obtain at least 50% of its carbon from oxidized carbon forms and vice versa [72]. On occasion, the capability to metabolize one-carbon (1C) organic compounds, such as methanol and formate, is designated as “methylotrophy” to differentiate it from growth on two-carbon (2C+) substrates [73]. This distinction is maintained here. In order to reduce oxidized carbon and/or use it for ATP production via oxidative phosphorylation, a source of electrons is necessary. This donor can be either an organic (organotrophy) or inorganic (lithotrophy) compound. The energy for the system can come from conversion of chemical bonds (chemotrophy) towards a state with minimum energy, or from electromagnetic radiation (phototrophy). To illustrate this with a more specific example, there exist cyanobacteria that produce PHA and use carboxylic acids as a growth source. However, they acquire energy from sunlight, making them photo-organoheterotrophs. In contrast, more traditional PHA producers, which grow on sources such as glucose, obtain energy by oxidizing the carbon substrate, thus classifying them as chemo-organoheterotrophs. PHA-producing cyanobacteria that fix CO2 via oxygenic (O2 evolving from inorganic H2O) photosynthesis are photolithoautotrophs. Table 2 provides a summary of possible combinations.

Table 2.
Overview of types of “-trophs”.
The main parameters related to cell cultivation for PHA accumulation are the weight of PHAs per dry mass of cells (w/w) and the titer of PHA in the volume of cell suspension (g/L). While a high PHA yield in grams per liter is desirable, the concentration of cells in the suspension can be increased by filtration or centrifugation. The w/w ratio determines the amount of the carbon source that is converted into PHAs rather than PHA-unrelated biomass. Although a high conversion ratio may not be necessary for inexpensive carbon sources, the ballast biomass must still be separated, which can require expensive and/or ecotoxic chemicals and can negatively impact PHA purity (as discussed in Extraction of PHAs). Therefore, we were primarily interested in the strains that produce the highest PHA (w/w), although we are aware that scaling up such cultivation may pose challenges. This section mainly concerns the production of PHB, which is the most widely studied polymer, with several copolymers and mcl-PHA mentioned. The production of copolymers usually requires the utilization of more direct or indirect precursors, such as propanol, propionic acid, or valeric acid. Table 3 shows an overview of categories subsequently discussed here.

Table 3.
Overview of metabolism of PHA producers discussed in this article.
6.1. Autotrophy
The reduction in CO2 is accomplished through either the oxidation of inorganic compounds such as H2 (chemolithoautotrophy) or the utilization of sunlight to split inorganic compounds and obtain electrons (photolithoautotrophy). The most well-known example of the latter is oxygenic photosynthesis, which utilizes H2O as the electron source and fixes CO2 using the Calvin–Benson cycle (reductive pentose phosphate pathway), which utilizes the RuBisCO enzyme. In this pathway, CO2 reacts with ribulose-1,5-bisphosphate to form glyceraldehyde-3-phosphate. RuBisCO itself is estimated to be the most abundant protein on Earth (0.7 Gt total) [74]. H2 can serve as an electron source when oxidized by O2 in aerobic hydrogenotrophs utilizing Calvin–Benson cycle, such as C. necator. Anaerobic chemolithoautotrophs are non-native PHA producers that utilize the Wood–Ljungdahl pathway (WLP, reductive acetyl-CoA pathway) for both CO2 assimilation and energy production. This pathway involves the subsequent reduction of CO2 to formate and acetyl-CoA, which is either assimilated or used for ATP production and dissimilated by acetogens. Other autotrophic pathways not associated with PHA production are not discussed here. Figure 5 shows autotrophic pathways related to reported PHA-producing organisms. For more information on autotrophic and methylotrophic strains for PHA production, refer to [75].
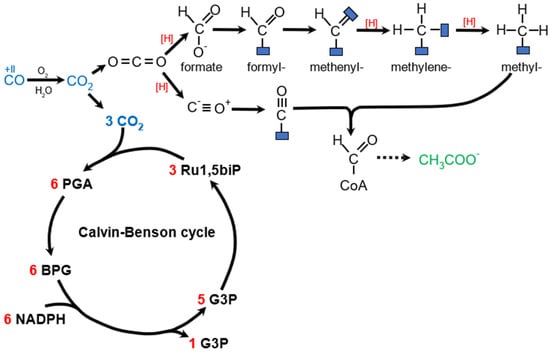
Figure 5.
Autotrophic pathways related to PHA production. CO is oxidized to CO2 in aerobic or anaerobic conditions. Upper part: schematic representation of Wood–Ljungdahl pathway. For simplicity, all reducing equivalents are represented by [H]. Bottom part: schematic representation of Calvin–Benson cycle. Ru1,5biP—ribulose−1,5−bisphosphate, PGA—3−phosphoglycerate, BPG—1,3−bisphosphoglycerate, G3P—glyceraldehyde−3−phosphate.
6.1.1. Photoautotrophy (Sunlight)
Cyanobacteria are a promising source for PHA production due to their oxygenic photosynthesis, which eliminates the need for dangerous chemicals during cultivation. Currently, the main challenge is to suppress the accumulation of glycogen, the preferred storage compound, and increase PHA content. According to a study, a random mutant of Synechocystis sp. PCC 6714 was able to increase PHB accumulation by 37% [76]. Similarly, Synechocystis sp. PCC 6803, overexpressing RuBisCO genes, produced 39% PHB when they were limited by nitrogen and phosphorus and were supplied with NaHCO3 [77]. Oscillatoria okeni, on the other hand, is capable of producing 14% PHA with 6% PHV using only CO2 during nitrogen limitation. When supplied with 0.4% acetate, PHA increased to 42% with 6.5% PHV [78]. Anabaena sp. can produce 40% PHA with 5% PHV through pure autotrophy. The introduction of different concentrations of acetate resulted in an increase in the proportion of PHV, up to 24%, but a decrease in total PHA content, down to 14% [79]. Mixotrophic cultivation with heterotrophic carbon sources can enhance PHA production, as discussed in the Photoheterotrophy section.
6.1.2. Hydrogenotrophy (H2)
Hydrogenotrophs can utilize H2 to power carbon assimilation, either aerobically (using the Calvin–Benson cycle) or possibly anaerobically (using the Wood–Ljungdahl pathway) to produce PHAs. Currently, most H2 is produced from fossil fuels, but more sustainable approaches are being considered for the future. All of these methods use H2O as a starting material. They include electrolysis (using renewable or nuclear energy), thermochemical production, photocatalytic production, and production by living cells (using cyanobacteria or algae).
The highest reported PHA content was achieved through aerobic cultivation of hydrogenotrophic C. necator, which produced 85% PHB a decade ago [80]. H2 and O2 can form an explosive mixture, so it is necessary to exercise enhanced care. Coupling bioreactors to water electrolysis outputs could improve the economics of such production. For copolymer production, C. necator was genetically modified with transgenic PHA synthases and an acyl-carrying protein. This resulted in the synthesis of various scl/mcl-PHA copolymers using only CO2 as a carbon source, with a total PHA content of 23–55%. The study found that the highest yields were achieved with 55% PHA and 20% polyhydroxydodecanoate, 46% PHA with 39% polyhydroxydodecanoate, and 42% PHA with 55% polyhydroxyoctanoate (the latter two were obtained by adding acrylic acid to limit beta-oxidation of fatty acids) [81].
Anaerobic hydrogenotrophs, including methanogens and acetogens, are not natural producers of PHAs. However, they could be genetically modified to produce PHAs. The use of anaerobic strains eliminates the need for O2 and prevents the formation of explosive mixtures, but there are inherent bioenergetic limitations that must be considered. In order to obtain enough ATP, most of the carbon source must be dissimilated into waste CH4 or acetate, rather than being assimilated into PHAs. The net yield of ATP per substrate molecule is only 0.5–2 for methanogens and 0.25 for acetogens [82]. The ratio of carbon assimilation to dissimilation has been experimentally determined for acetogens grown on H2: for every 24 carbon atoms, 1 is converted into biomass, 20 into acetate, and 3 are unrecovered during measurements [83]. Therefore, a high intracellular PHA concentration would result in the production of large volumes of side products. However, these side products could be further utilized by employing a mixed culture of PHA-producing hydrogenotrophs together with methanotrophs and/or acetotrophs. It is important to note that chemoheterotrophs require an electron acceptor to obtain energy. Aerated co-culture is not possible because the Wood–Ljungdahl pathway is extremely sensitive to oxygen. However, both methanotrophs and acetotrophs could be denitrifiers or sulfate reducers, requiring either NO3− or SO42− in the medium. Alternatively, photoheterotrophs that do not require such oxidant, such as Rhodobacter capsulatus, could be used. This bacterium is able to grow anaerobically on acetate as a sole carbon source [84]. The acetogenic bacterium Clostridium autoethanogenum has been genetically modified to produce PHAs. However, as its main carbon source is CO, it is discussed in the following section.
6.1.3. Carboxydotrophy (CO)
Carbon monoxide (CO) is naturally produced by volcanic activity, but it is also present in industrial gases, such as syngas, which is made abiotically from various fossil fuels. Syngas can contain up to 60% CO, with the remaining gases mainly consisting of H2 and low amounts of CH4 and CO2. CO is more reduced than CO2 and can be used as an electron donor. However, the ability to grow on CO, known as carboxydotrophy, is usually considered a form of autotrophy (e.g., in [85]) because it utilizes oxidized inorganic carbon. To utilize CO, it must first be oxidized to CO2 using either O2 or H2O and can later be assimilated using either the Calvin–Benson cycle or WLP. Although carbon monoxide (CO) is an intermediate of the WLP, it is unlikely that external CO would be utilized directly. This is because carbon monoxide dehydrogenase and acetyl-CoA synthase are connected via a channel that CO travels through [86]. When grown aerobically on 30% CO and limited via sulfur, Seliberia carboxydohydrogena Z-1062 was able to accumulate polyhydroxybutyrate (PHB) up to 63% [87]. Some components of crude syngas can inhibit cell growth, but these can be resolved by using charcoal filters [88]. Acetogenic Clostridrium autoethanogenum has been genetically modified to produce PHAs using substrates that mimic syngas (50% CO, 20% CO2, 20% H2, Ar) or steel mill-off gas (50% CO, 20% CO2, 2% H2, N2) [89]. The ratio between carbon in biomass and acetate ranged between 1:8 and 1:13, based on the available data. This is consistent with [83], as it is unclear how much carbon substrate was used to achieve these ratios. A significant amount of CO2 was also present, but it is unclear how much of it originated from the oxidation of CO. To enhance PHA synthesis, the authors aimed to minimize the ATP used for cell maintenance. They discovered that a low pH has a negative impact on PHA accumulation. This is because the excreted waste acetate is protonated and flows back into the cell, and its intracellular deprotonation depletes the proton gradient. Increasing the pH [89,90] resulted in a 6% accumulation of PHB [89]. Even with optimized ATP consumption, the ratio of waste to biomass will remain constant, as acetate dissimilation is required for ATP production. Any excess acetate waste must be properly processed or separated.
One potential concern for future PHA production using syngas is its origin from fossil fuels. While biomass production is feasible, the biological conversion of biomass to biogas appears to be a more viable option. It is important to note that, in addition to the danger of forming explosive mixtures with air in case of an accident, CO is highly toxic, unlike H2 or CH4. Therefore, it requires more a strictly controlled operation.
6.2. Methylotrophy
Methylotrophic organisms are able to grow on 1C organic substrates (e.g., CH4, methanol, formate). Such reduced 1C substrates require specific assimilation pathways. These include the ribulose monophosphate (RuMP) cycle and the serine cycle (other pathways such as the xylulose monophosphate pathway exclusive to Saccharomyces or the methylotrophic use of WLP have not been reported in the context of PHA synthesis). Alternatively, reduced 1C substrates can be oxidized to CO2 and utilized by autotrophic pathways. Figure 6 provides an overview of the methylotrophic pathways used for PHA production.
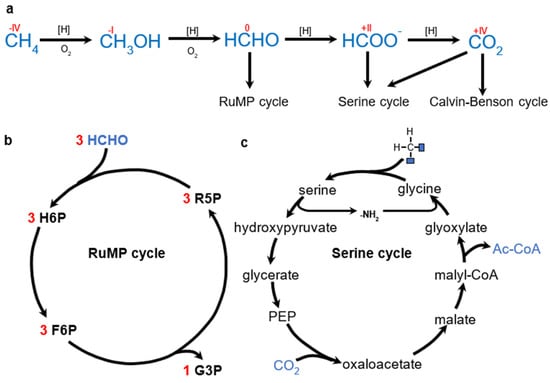
Figure 6.
(a) Overview of methylotrophic pathways considered for PHA production so far. For simplicity, all reducing equivalents are represented by [H]. (b) Schematic representation of RuMP. R5P—ribulose−5−phosphate, H6P—hexulose−6−phosphate, F6P—fructose−6−phosphate, G3P—glyceraldehyde−3−phosphate. (c) Schematic representation of serine cycle. Formate is converted to methylene before entering the cycle.
6.2.1. Methane (CH4)
CH4 is a major component of natural gas, is present in coal beds, and forms methane clathrates in frozen soils under high pressure. Besides geological processes, CH4 is produced by methanogenic archaea, which is widely used for biogas production (containing 50–70% CH4). Anaerobic digestion is performed by microbial consortium, where complex biopolymers are mainly degraded to H2, CO2, and acetate, which are utilized by hydrogenotrophic and acetoclastic methanogens. Biogas can also be further purified to biomethane.
Reported PHA-producing methanotrophs are aerobic. CH4 is first oxidized to methanol and then to formaldehyde (HCHO). Based on the subsequent fate of HCHO, methanogens are divided into three groups: Type I utilizes the RuMP cycle, Type II utilizes the serine cycle, and Type X utilizes the RuMP cycle and the Calvin–Benson cycle (reviewed by [91]).
Methylocystis sp. GB 25 accumulates 51% PHB in the stationary phase when grown on CH4 and when it is limited by nitrogen availability [92]. A mixed culture dominated mainly by Methylophilus and Methylocystis was capable of achieving 59% PHB accumulation when limited by nitrogen in the stationary phase [93]. Methylocystis hirsuta grown using mixotrophic cultivation with natural gas supplied with 0.5% methanol and ethanol in the medium was capable of achieving 73% PHB accumulation [94]. Methylobacterium organophilum is able to accumulate 60% PHA with 5% PHV on CH4 as its sole carbon source [95]. Other methods for copolymer production require mixotrophic cultivation. The introduction of citrate (250 mg/L) yielded 88% PHA with 55:35:10 PHB:PHV:PHO, and the introduction of propionate (250 mg/L) yielded 60% PHA with 75:25:0 ratio with the same strain [95]. Using an artificial biogas mixture, Methylocystis hirsuta accumulated 54% PHA with 25% PHV when supplied with valeric acid (136 mg/L) [96]. An enriched anaerobic sludge accumulated 52% PHA with 33% PHV when valeric acid (50 mg/L) was added [97]. Methylocystis parvus accumulated 50% PHA with 10% poly-4-hydroxybutyrate when grown on 1.2 mM 4-hydroxybutyrate [98]. Methylocystis sp. WRRC1 accumulated 60% PHA with 50% PHV on 0.34% valerate [99]. It should be noted that CH4 can form explosive mixtures with air in the event of an accident, so careful handling is required. CH4 is also a greenhouse gas.
6.2.2. Methanol (CH3OH)
Hydrogen is often mentioned in the context of a sustainable future, but it suffers from low energy density, problematic storage, and the need to build costly infrastructure. Even with the elimination of fossil fuels, many materials and chemicals still require carbon precursors. Nobel laureate George Andrew Olah proposed the so-called “methanol economy” [100], in which methanol serves as such precursor. Methanol can be synthesized from fossil fuels (syngas), but also from CO2, either by chemical hydrogenation or electrocatalytically with H2O (current methods and production challenges are reviewed in [101]). Today, methanol is used for the industrial production of many compounds and can be used as a combustible fuel or in fuel cells. It is a liquid at room temperature, has a 3.4 times higher volumetric energy density (MJ/L) than H2 compressed at 70 MPa, and a 1.8 times higher volumetric energy density than liquid H2 (−253 °C) (see Appendix A for calculations). Therefore, methanol may be a suitable medium for PHA production in the future.
To date, the highest PHA content reported for methanol is for Methylobacterium extorquens, which was able to be used to accumulate 53% PHB almost three decades ago [102]. A genetically engineered strain of Methylobacterium extorquens AM1 accumulates 44% PHA with 3% PHV content when limited by Co2+ concentration, due to the accumulation of propionyl-CoA [103]. However, due to the strong inhibitory effect on cells, the methanol concentration must be kept low (about 0.1%).
6.2.3. Formate (HCOO−)
Formic acid is currently produced from fossil fuels; however, similar to methanol, it can also be produced electrochemically or via the direct hydrogenation of CO2. Although the volumetric energy density of formic acid is 2.7 times lower than that of MeOH, it is still 1.3 times higher than that of pressurized H2 (70 MPa) (see Appendix A for calculations), making it another potential medium.
The aforementioned Methylobacterium extorquens is able to accumulate 43% PHB when grown on formate [104]. Formate can also be produced in situ for subsequent microbial consumption (from a more industrial point of view, such system facilitates autotrophy, as CO2 is used as the carbon source). Formate can be electrochemically synthesized from CO2 and H2O separately and then dropped into a bioreactor with C. necator, resulting in 34% PHB accumulation [105]. Formate can also be synthesized directly in the cell medium using immobilized formate dehydrogenase on a carbon cloth electrode, NADH, and neutral red dye. C. necator with transgenic RuBisCO was able to be used to obtain 485 mg PHB/L (w/w not determined) in such a system [106].
6.3. Non-Methylotrophic Heterotrophy
Most PHA-producing heterotrophs are chemoorganotrophs that oxidize carbon sources for energy, which naturally results in the loss of some carbon source as CO2. PHA production has also been reported for photoheterotrophs, which (in theory) could more efficiently utilize the carbon source to produce PHAs, given sufficient illumination.
6.3.1. Food Products
Traditional PHA production uses food products such as sugars and oils as carbon substrates. The use of these substrates can achieve very high yields of PHAs, but the disadvantage is the overlap/competition with food production. The use of extremophilic bacteria can help prevent contamination via PHA non-producing strains and can also result in high PHA content. The halophilic bacterium Halomonas halophila is capable of accumulating up to 85% PHB when grown on glucose [107]. A mutant strain of Halomonas bluephagenesis can accumulate PHB up to 94% when limited by respiration (knockout of electron transport flavoproteins) on a mixture of glucose (30 g/L) and acetate (3 g/L) [56]. Halomonas sp. YLGW01 was able to accumulate 95% PHB during non-sterile cultivation on fructose [108]. However, most publications are related to non-extremophilic bacteria. To reduce costs, refined sugars can be replaced by more crude ones. A bacterial consortium of Bacillus subtilis and C. necator 5119 grown on cane sugar (90% sucrose) can accumulate 75% PHA [109]. C. necator can accumulate up to 92% PHB when grown on maltose-rich brewery wastewater as long as a high C/N ratio is maintained [110]. Using hydrolyzed sugarcane molasse, C. necator can accumulate PHB up to 58% [111]. A modified strain of C. necator is able to utilize crude corn (maize) starch to produce 63% PHB [65]. The use of oils for PHA production has recently been reviewed by [112]. Palm oil, despite its controversial production, can also be used as a source, and C. necator B-10646 can accumulate up to 72% PHA [113]. A modified strain of C. necator has also been used to utilize date seed oil with 81% PHA accumulation [114]. As for copolymer production, C. necator using fructose and rapeseed oil produced 86% PHA with 17% PHH [115]. Methylobacterium organophilum can produce 48% PHA with a ratio of 35:53:12 (PHB:PHV:PHO) on sodium citrate [95].
It should be emphasized that the use of such food industry products can be controversial in the eyes of the public, as it can be seen as a “waste” of food or of arable land (related to the food vs. fuel dilemma). It is also highly dependent on feasible agricultural conditions, intensive use of fertilizers, and it is very water intensive.
6.3.2. Food and Other Industry Waste
An alternative approach is to use food industry wastes such as desugarized sugar beet molasse (the remaining fraction after sugar and betaine isolation from sugar beet). Bacillus megaterium yielded 68% PHB content when used as a carbon source [116]. More crude sources require a hydrolysis step, such as inedible rice (C. necator, 69% PHA) [117]) or sugarcane bagasse (Lysinibacillus sp., 62% PHA) [118]). Another source can be xylose extracted from lignocellulosic biomass. Bacillus sp. SM01 yielded 62% PHB on this substrate [119]. It is also worth mentioning a bacterial consortium that utilizes xylose and glucose to produce 1.30 g of PHA per liter. However, the %PHA was not determined [69]. Glycerol is produced in large quantities as a by-product of the transesterification of natural triacylglycerides. Paracoccus denitrificans was able to accumulate PHB up to 72% in the stationary growth phase when nitrogen was depleted [120]. Activated sludge mainly containing Alphaproteobacteria and Betaproteobacteria contained 80% PHA when grown on crude glycerol [121]. Using a mixture of glycerol (20 g/L) and levulinic acid (2 g/L), C. necator was able to accumulate 80% PHA when limited by nitrogen [122]. Residues from a specific food industry can also be used, as Bacillus subtilis achieved 89% PHA accumulation when grown on onion peels, and Bacillus siamensis yielded 82% PHA when grown on orange peels [123]. Whey is a by-product of cheese production and is often discarded as waste. After cheese whey was ultrafiltered and used as a carbon source, Bacillus megaterium was able to accumulate 76% PHB [124]. Fats extracted from slaughterhouse waste can also be used for PHA production; a modified C. necator strain with high lipase activity was able to accumulate 66% PHA [66]). C. necator also accumulated 73% PHA when grown on waste fish oil [125]. Waste cooking oil is also being investigated for PHA synthesis. Bacillus thermoamylovorans was able to accumulate 88% PHA [126]. As for copolymer production, sludge palm oil, a solid by-product of palm oil processing, was emulsified with Tween 80 and used as a carbon substrate for C. necator, yielding 74% PHA with 22% PHH [127]. Waste rapeseed oil with 1% propanol was also used for C. necator to yield 80% PHA with 9% HV [128]. Halomonas hydrothermalis was grown on waste frying oil, yielding 69% PHA with 50% PHV when valeric acid (2 g/L) was added and yielding 70% PHA with 8% PHV when propanol (2 g/L) was used [129].
6.3.3. Hydrocarbons
Hydrocarbons are not a typical carbon source for PHA production because petrochemical plastics are cheaper to produce and because their use as a cellular carbon source does not eliminate our dependence on fossil fuels. However, PHAs have been reported to be produced from both aliphatic and aromatic compounds. A mutant strain of Alcanivorax borkumensis SK2, a petroleum-degrading marine bacterium, was able to accumulate PHA when fed octadecane at 2.56 g/L (OD600 = 1, dry cell mass not determined) [48]. Pseudomonas putida CA-3 was able to accumulate up to 42% PHA when grown on styrene with a nitrogen limitation [130]. However, no recent research has been reported on the use of crude hydrocarbons for PHA production.
6.3.4. Short-Chain Carboxylic Acids
Short-chain carboxylic acids, such as acetate and butyrate, are being investigated for PHA production because their metabolic pathways to PHA are relatively short. Most acetic acid today is produced via the carbonylation of methanol with CO, and a small amount is produced via fermentation. Although methanol could be produced via the capture of CO2 in the future, the need for CO complicates the process. Recently, the use of acetogens (bacteria and archaea that use WLP) for acetic acid production from CO2 and H2 has gained great interest. Their metabolism, which produces a large amount of waste compared to biomass (as mentioned in the Section 6.1), would be advantageous here (reviewed in [131]). As for the current PHA production on acetate, Cobetia sp. MC34 was able to accumulate 72% PHB in the stationary growth phase [132]. C. necator grown on acetate accumulates 72% PHB when limited by nitrogen [133]. Using a mixed carbon source of acetate (16 g/L) and butyrate (8 g/L), the halophilic bacterium Salinivibrio sp. TGB19 was able to accumulate PHA up to 89% [134].
Similar to acetic acid, butyric acid is mostly produced using syngas and propene to form butyraldehyde, which is subsequently oxidized to butyric acid, but it can also be produced via fermentation. An enriched culture dominated by Plasticicumulans acidivorans was capable of obtaining 88% PHB production, both on butyrate alone and on a mixture of acetate with butyrate. Butyrate was the preferred carbon source for the bacteria [135]. C. necator is able to accumulate 66% PHA with up to 13% PHV when using butyrate as its sole carbon source [136]. The cultivation of C. necator on butyrate supplied by γ-valerolactone resulted in 78% PHA accumulation with 31% PHV [137].
Propionate is often used to promote the synthesis of PHA copolymers. Most of it is produced industrially from fossil fuels via the carbonylation of ethylene, but it can also be produced via fermentation. Methylobacterium organophilum can accumulate 37% PHA with a PHB:PHV:PHO ratio of 37:56:7 when grown on propionate as its sole carbon source [95].
6.3.5. Photo-Organoheterotrophy
Some photo-organoheterotrophs are capable of PHA accumulation. Dinoroseobacter sp. JL1447 accumulated 72% PHA when grown on sodium acetate at a high C/N ratio. The use of a light/dark cycle resulted in a quarter increase in PHA content compared to the unilluminated strain [138]. Oscillatoria okeni was able to produce 14% PHA at 6% PHV using only CO2 during nitrogen-limited conditions (as mentioned in the Section 6.1); however, when supplied with 0.4% acetate, PHA increased to 42% at 7% PHV [78]. The introduction of acetate resulted in 81% PHB content when the autotrophically grown mutant Synechocystis sp. PCC 6803 was starved for 20 days in a phosphorus- and nitrogen-free medium [139]. A consortium of phototrophic bacteria grown on butyrate (3 g/L) and acetate (1.75 g/L) was able to accumulate up to 67% PHA when limited by nitrogen, with an undetermined amount of PHV [140]. Rhodobacter sphaeroides can accumulate up to 72% PHA with 2% PHV when grown on acetate supplemented with malate under nitrogen-limited conditions [141].
7. Discussion and Outlook
PHAs have the potential to replace petrochemical plastics due to their biological production and biodegradability. For sustainable PHA production, in addition to using safe solvents for extraction, the carbon source should be independent of fossil fuels. There are several possibilities for large-scale PHA production in the future based on the carbon sources available.
Food products can be used for PHA production with high yields. However, such production requires feasible agricultural conditions that are not easily met worldwide and could be perceived negatively by the public. Worldwide PHA production using more ubiquitous carbon sources could be an alternative. Lignocellulose is an abundant and globally available carbon source that can be used either directly or via the conversion to CH4-rich biogas for use by methylotrophs. Sustainably produced methanol (or formate) can also become a ubiquitous carbon source for methylotrophs, but such use is highly dependent on its future price (the production of carbon-neutral fuels has recently been reviewed by [142]). Direct use of CO2 by autotrophs is also feasible. Sufficient sunlight is available on most of the Earth (and could be augmented by low-energy electric lights if needed). Another source of reducing energy for autotrophs is H2, which is often mentioned in connection with a sustainable future. If the price of sustainably produced H2 decreases sufficiently, PHA production with hydrogenotrophs would not be limited to any location. The aerobic hydrogenotrophic production of copolymers with mcl-PHAs is also very feasible. Anaerobic hydrogenotrophs can also be used for acetate production (not requiring food), which can be used as a reduced carbon source for (photo-)heterotrophic PHA-producing strains. Figure 7 provides an overview of the pathways leading to PHA production discussed in this article. Table 4 lists all the strains discussed in this article.
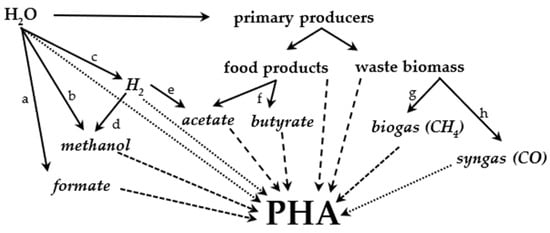
Figure 7.
Overview of PHA production pathways. Terms in italics refer to sources that are or can be currently mass-produced using fossil fuels. Dotted lines: autotrophs. Densely dashed lines: methylotrophs. Loosely dashed lines: other heterotrophs. Solid lines: processes related to PHA production: a—electrochemical synthesis of formate, b—electrochemical synthesis of methanol, c—production of hydrogen from water, d—production of methanol by hydrogenation, e—production of acetate by acetogens, f—fermentation of sugars, g—anaerobic digestion, h—biomass gasification.

Table 4.
Overview of strains discussed in this article.
Author Contributions
Conceptualization, I.F. and I.K.; writing—original draft praparation, I.F.; visualization, I.F.; writing—review and editing, I.F. and I.K. All authors have read and agreed to the published version of the manuscript.
Funding
This paper is part of the Ph.D. thesis of the first author and was funded by the internal resources of Masaryk University (MUNI/A/1582/2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
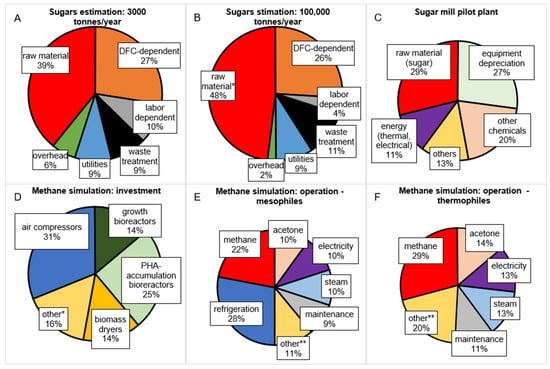
Figure A1.
Visualization of distribution of PHA production costs using three studies. (A,B) Estimation. Carbon source accounts for 70–80% of total raw material costs. DFC—direct fixed capital (initial investment costs) [13], (C) pilot plant [14], (D–F) simulation [15]. *—other represents centrifuges, coolers, filtration systems, etc. **—other represents supervision, labor, administration, solids handling, etc.
Table A1 shows volumetric energy densities (EVs) of certain fuels mentioned in this paper in relation to the growth sources for bacterial strains. These values were calculated as the enthalpy of combustion (ΔH°comb, “higher heating value”) related to a volume of 1 L of fuel. Standard formation entropy (ΔfH°comb) values were taken from the NIST database of the National Institute of Standards and Technology [143]; densities and molecular weights were taken from the PubChem database of the National Library of Medicine [144]. The value for 70 MPa H2 was taken from [145]. Only values relevant for calculations are shown.

Table A1.
Overview of tabulated values used for calculations.
Table A1.
Overview of tabulated values used for calculations.
| Compound | ΔfH°comb (kJ/mol) | ρ (g/cm3) | M (g/mol) |
|---|---|---|---|
| MeOH (l) | −238.4 | 0.792 | 32.042 |
| HCOOH (l) | −425.09 | 1.22 | 46.025 |
| CO2 (g) | −393.51 | ||
| H2O (l) | −285.83 | ||
| H2 (l, −253 °C) | 0.071 | 2.016 | |
| H2 (g, 70 MPa) | 37 | 2.016 |
References: ΔfH°comb: [143], ρ: [144], ρ (H2, g, 70 MPa): [145], M: [144].
Hydrogen: H2 + 1/2 O2 → H2O
ΔH°comb = −285.83 kJ/mol
Liquid H2:
c = 35.2 mol/L
EV = −10.06 MJ/L
Gaseous H2:
c = 18.35 mol/L
EV = −5.25 MJ/L
Formic acid: HCOOH + 1/2 O2 → CO2 + H2O
ΔH°comb = −393.51 + (−285.83) − (−425.09) = −254.25 kJ/mol
c = 26.51 mol/L
EV = −6.74 MJ/L
Methanol: CH3OH + O2 → CO2 + 2 H2O
ΔH°comb = −393.51 + (−285.83 × 2) − (−238.4) = −726.77 kJ/mol
c = 24.7 mol/L
EV = −17.95 MJ/L
References
- Ainali, N.M.; Kalaronis, D.; Evgenidou, E.; Kyzas, G.Z.; Bobori, D.C.; Kaloyianni, M.; Yang, X.; Bikiaris, D.N.; Lambropoulou, D.A. Do poly(lactic acid) microplastics instigate a threat? A perception for their dynamic towards environmental pollution and toxicity. Sci. Total. Environ. 2022, 832, 155014. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A.; González-Martínez, C.; Vargas, M. Production of Polyhydroxyalkanoates for Biodegradable Food Packaging Applications Using Haloferax mediterranei and Agrifood Wastes. Foods 2024, 13, 950. [Google Scholar] [CrossRef]
- Bulantekin, Ö.; Alp, D. Perspective Chapter: Development of Food Packaging Films from Microorganism-Generated Polyhydroxyalkanoates. In Food Processing and Packaging Technologies—Recent Advances; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Bonartsev, A.P.; Bonartseva, G.A.; Reshetov, I.V.; Kirpichnikov, M.P.; Shaitan, K.V. Application of Polyhydroxyalkanoates in Medicine and the Biological Activity of Natural Poly(3-Hydroxybutyrate). Acta Nat. 2019, 11, 4–16. [Google Scholar] [CrossRef]
- Levett, I.; Pratt, S.; Donose, B.C.; Brackin, R.; Pratt, C.; Redding, M.; Laycock, B. Understanding the Mobilization of a Nitrification Inhibitor from Novel Slow Release Pellets, Fabricated through Extrusion Processing with PHBV Biopolymer. J. Agric. Food Chem. 2019, 67, 2449–2458. [Google Scholar] [CrossRef]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial Synthesis and Characterization of Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Bhubalan, K.; Rathi, D.-N.; Abe, H.; Iwata, T.; Sudesh, K. Improved synthesis of P(3HB-co-3HV-co-3HHx) terpolymers by mutant Cupriavidus necator using the PHA synthase gene of Chromobacterium sp. USM2 with high affinity towards 3HV. Polym. Degrad. Stabil. 2010, 95, 1436–1442. [Google Scholar] [CrossRef]
- Bhubalan, K.; Lee, W.-H.; Loo, C.-Y.; Yamamoto, T.; Tsuge, T.; Doi, Y.; Sudesh, K. Controlled biosynthesis and characterization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) from mixtures of palm kernel oil and 3HV-precursors. Polym. Degrad. Stabil. 2008, 93, 17–23. [Google Scholar] [CrossRef]
- Yamane, T.; Chen, X.F.; Ueda, S. Growth-associated production of poly(3-hydroxyvalerate) from n-pentanol by a methylotrophic bacterium, Paracoccus denitrificans. Appl. Environ. Microbiol. 1996, 62, 380–384. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial Synthesis and Properties of Poly(3-Hydroxybutyrate-Co-4-Hydroxybutyrate) in Comamonas acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shi, Z.-Y.; Meng, D.-C.; Wu, Q.; Chen, J.-C.; Chen, G.-Q. Production of 3-hydroxypropionate homopolymer and poly(3-hydroxypropionate-co-4-hydroxybutyrate) copolymer by recombinant Escherichia coli. Metab. Eng. 2011, 13, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.M.; Wang, Z.H.; Luo, H.N.; Xu, M.; Ren, X.Y.; Zheng, G.X.; Wu, B.J.; Zhang, X.H.; Lu, X.Y.; Chen, F.; et al. Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)-based scaffolds for tissue engineering. Braz. J. Med. Biol. Res. 2014, 47, 533–539. [Google Scholar] [CrossRef]
- Choi, J.I.; Lee, S.Y. Process analysis and economic evaluation for poly(3-hydroxybutyrate) production by fermentation. Bioprocess. Eng. 1997, 17, 335–342. [Google Scholar] [CrossRef]
- Nonato, R.V.; Mantelatto, P.E.; Rossell, C.E.V. Integrated production of biodegradable plastic, sugar and ethanol. Appl. Microbiol. Biotechnol. 2001, 57, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Levett, I.; Birkett, G.; Davies, N.; Bell, A.; Langford, A.; Laycock, B.; Lant, P.; Pratt, S. Techno-economic assessment of poly-3-hydroxybutyrate (PHB) production from methane—The case for thermophilic bioprocessing. J. Environ. Chem. Eng. 2016, 4, 3724–3733. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Steinbüchel, A.; Jendrossek, D. Substrate Specificities of Bacterial Polyhydroxyalkanoate Depolymerases and Lipases: Bacterial Lipases Hydrolyze Poly(ω-Hydroxyalkanoates). Appl. Environ. Microbiol. 1995, 8, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Salvador, A.; Alves, M.M.; Vicente, A.A. Factors affecting polyhydroxyalkanoates biodegradation in soil. Polym. Degrad. Stab. 2020, 182, 109408. [Google Scholar] [CrossRef]
- Kim, J.; Gupta, N.S.; Bezek, L.B.; Linn, J.; Bejagam, K.K.; Banerjee, S.; Dumont, J.H.; Nam, S.Y.; Kang, H.W.; Park, C.H.; et al. Biodegradation Studies of Polyhydroxybutyrate and Polyhydroxybutyrate-co-Polyhydroxyvalerate Films in Soil. Int. J. Mol. Sci. 2023, 24, 7638. [Google Scholar] [CrossRef]
- Davis, R.; Chandrashekar, A.; Shamala, T.R. Role of (R)-specific enoyl coenzyme A hydratases of Pseudomonas sp. in the production of polyhydroxyalkanoates. Antonie Van. Leeuwenhoek 2008, 93, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Rehm, B.H.A.; Krüger, N.; Steinbüchel, A. A new metabolic link between fatty acid de novo synthesis and polyhydroxyalkanoic acid synthesis: The phaG gene from Pseudomonas putida KT2440 encodes a 3-hydroxyacyl-acyl carrier protein coenzyme A transferase. J. Biol. Chem. 1998, 273, 24044–24051. [Google Scholar] [CrossRef]
- Maehara, A.; Ueda, S.; Nakano, H.; Yamane, T. Analyses of a polyhydroxyalkanoic acid granule-associated 16-kilodalton protein and its putative regulator in the pha locus of Paracoccus denitrificans. J. Bacteriol. 1999, 181, 2914–2921. [Google Scholar] [CrossRef]
- Pfeiffer, D.; Wahl, A.; Jendrossek, D. Identification of a multifunctional protein, PhaM, that determines number, surface to volume ratio, subcellular localization and distribution to daughter cells of poly(3-hydroxybutyrate), PHB, granules in Ralstonia eutropha H16. Mol. Microbiol. 2011, 82, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Brigham, C.J.; Sinskey, A.J. Polyhydroxybutyrate Production Enzymes: A Survey and Biological perspective. J. Sib. Fed. Univ. Biol. 2012, 3, 220–242. [Google Scholar]
- Lu, J.; Takahashi, A.; Ueda, S. 3-Hydroxybutyrate Oligomer Hydrolase and 3-Hydroxybutyrate Dehydrogenase Participate in Intracellular Polyhydroxybutyrate and Polyhydroxyvalerate Degradation in Paracoccus denitrificans. Appl. Environ. Microbiol. 2014, 80, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Koskimäki, J.J.; Kajula, M.; Hokkanen, J.; Ihantola, E.-L.; Kim, J.H.; Hautajärvi, H.; Hankala, E.; Suokas, M.; Pohjanen, J.; Podolich, O.; et al. Methyl-esterified 3-hydroxybutyrate oligomers protect bacteria from hydroxyl radicals. Nat. Chem. Biol. 2016, 12, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Seebach, D.; Brunner, A.; Bürger, H.M.; Schneider, J.; Reusch, R.N. Isolation and 1H-NMR spectroscopic identification of poly(3-hydroxybutanoate) from prokaryotic and eukaryotic organisms—Determination of the absolute-configuration (R) of the monomeric unit 3-hydroxybutanoic acid from Escherichia coli and spinach. Eur. J. Biochem. 1994, 224, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Reusch, R.N.; Sparrow, A.W.; Gardiner, J. Transport of poly-β-hydroxybutyrate in human plasma. Biochim. Biophys. Acta 1992, 1123, 33–40. [Google Scholar] [CrossRef]
- Reusch, R.M.; Sadoff, H.L. Putative structure and functions of a poly-β-hydroxybutyrate/calcium polyphosphate channel in bacterial plasma membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 4176–4180. [Google Scholar] [CrossRef]
- Elustondo, P.A.; Nichols, M.; Negoda, A.; Thirumaran, A.; Zakharian, E.; Robertson, G.S.; Pavlov, E.V. Mitochondrial permeability transition pore induction is linked to formation of the complex of ATPase C-Subunit, polyhydroxybutyrate and inorganic polyphosphate. Cell Death Discov. 2016, 2, 16070. [Google Scholar] [CrossRef] [PubMed]
- Xian, M.; Fuerst, M.M.; Shabalin, Y.; Rosetta, R.N. Sorting Signal of Escherichia coli OmpA is Modified by Oligo-(R)-3-Hydroxybutyrate. Biochim. Biophys. Acta Biomemb. 2007, 1768, 2660–2666. [Google Scholar] [CrossRef] [PubMed]
- Reusch, R.N.; Shabalin, O.; Crumbaugh, A.; Wagner, R.; Schröder, O.; Wurm, R. Posttranslational modification of E. coli histone-like protein H-NS and bovine histones by short-chain poly-(R)-3-hydroxybutyrate (cPHB). FEBS Lett. 2002, 527, 319–322. [Google Scholar] [CrossRef]
- Dai, D.; Reusch, R.N. Poly-3-hydroxybutyrate Synthase from the Periplasm of Escherichia coli. Biochem. Biophys. Res. Commun. 2008, 374, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Reusch, R.N. Physiological Importance of Poly-(R)-3-Hydroxybutyrates. Chem. Biodivers. 2009, 9, 2343–2366. [Google Scholar] [CrossRef] [PubMed]
- Zuriani, R.; Vigneswari, S.; Azizan, M.N.M.; Majid, M.I.A.; Amirul, A.A. A high throughput Nile red fluorescence method for rapid quantification of intracellular bacterial polyhydroxyalkanoates. Biotechnol. Bioprocess Eng. 2013, 18, 472–478. [Google Scholar] [CrossRef]
- Lakshman, K.; Shamala, T.R. Extraction of polyhydroxyalkanoate from Sinorhizobium meliloti cells using Microbispora sp. culture and its enzymes. Enzyme Microb. Technol. 2006, 39, 1471–1475. [Google Scholar] [CrossRef]
- Randriamahefa, S.; Renard, E.; Guerin, P.; Langlois, V. Fourier transform infrared spectroscopy for screening and quantifying production of PHAs by Pseudomonas grown on sodium octanoate. Biomacromolecules 2003, 4, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Isak, I.; Patel, M.; Riddell, M.; West, M.; Bowers, T.; Wijeyekoon, S.; Lloyd, J. Quantification of polyhydroxyalkanoates in mixed and pure cultures biomass by Fourier transform infrared spectroscopy: Comparison of different approaches. Lett. Appl. Microbiol. 2016, 63, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Censi, V.; Saiano, F.; Bongiorno, D.; Indelicato, S.; Napoli, A.; Piazzese, D. Bioplastics: A new analytical challenge. Front. Chem. 2022, 10, 987669. [Google Scholar] [CrossRef] [PubMed]
- Hejazi, P.; Vasheghani-Farahani, E.; Yamini, Y. Supercritical fluid disruption of Ralstonia eutropha for poly(beta-hydroxybutyrate) recovery. Biotechnol. Prog. 2003, 19, 1519–1523. [Google Scholar] [CrossRef] [PubMed]
- Aramvash, A.; Gholami-Banadkuki, N.; Moazzeni-Zavareh, F.; Hajizadeh-Turchi, S. An Environmentally Friendly and Efficient Method for Extraction of PHB Biopolymer with Non-Halogenated Solvents. J. Microbiol. Biotechnol. 2015, 25, 1936–1943. [Google Scholar] [CrossRef]
- Riedel, S.L.; Brigham, C.J.; Budde, C.F.; Bader, J.; Rha, C.; Stah, U.; Sinskey, A.J. Recovery of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Ralstonia eutropha cultures with non-halogenated solvents. Biotechnol. Bioeng. 2013, 110, 461–470. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Brigham, C.; Willis, L.; Rha, C.; Sinskey, A. Improved detergent-based recovery of polyhydroxyalkanoates (PHAs). Biotechnol. Lett. 2011, 33, 937–942. [Google Scholar] [CrossRef]
- Xiong, B.; Fang, Q.; Wei, T.; Wang, Z.; Shen, R.; Cheng, M.; Zhou, W. Chemical digestion method to promote activated sludge cell wall breaking and optimize the polyhydroxyalkanoate (PHA) extraction process. Int. J. Biol. Macromol. 2023, 240, 124369. [Google Scholar] [CrossRef]
- Burniol-Figols, A.; Skiadas, I.; Daugaard, A.E.; Gavala, H.N. Polyhydroxyalkanoate (PHA) purification through dilute aqueous ammonia digestion at elevated temperatures. J. Chem. Technol. Biotechnol. 2020, 95, 1519–1532. [Google Scholar] [CrossRef]
- Page, W.; Cornish, A. Growth of Azotobacter vinelandii Uwd in Fish Peptone Medium and Simplified Extraction of Poly-Beta-Hydroxybutyrate. Appl. Environ. Microbiol. 1993, 59, 4236–4244. [Google Scholar] [CrossRef]
- Meneses, L.; Esmail, A.; Matos, M.; Sevrin, C.; Grandfils, C.; Barreiros, S.; Reis, M.A.M.; Freitas, F.; Paiva, A. Subcritical Water as a Pre-Treatment of Mixed Microbial Biomass for the Extraction of Polyhydroxyalkanoates. Bioengineering 2022, 9, 302. [Google Scholar] [CrossRef]
- Rahman, A.; Linton, E.; Hatch, A.D.; Sims, R.C.; Miller, C.D. Secretion of polyhydroxybutyrate in Escherichia coli using a synthetic biological engineering approach. J. Biol. Eng. 2013, 7, 24. [Google Scholar] [CrossRef]
- Sabirova, J.S.; Ferrer, M.; Luensdorf, H.; Wray, V.; Kalscheuer, R.; Steinbuechel, A.; Timmis, K.N.; Golyshin, P.N. Mutation in a “tesB-like” hydroxyacyl-coenzyme A-specific thioesterase gene causes hyperproduction of extracellular polyhydroxyalkanoates by Alcanivorax borkumensis SK2. J. Bacteriol. 2006, 188, 8452–8459. [Google Scholar] [CrossRef]
- Wang, J.; Liu, S.; Huang, J.; Cui, R.; Xu, Y.; Song, Z. Genetic engineering strategies for sustainable polyhydroxyalkanoate (PHA) production from carbon-rich wastes. Environ. Technol. Innov. 2023, 30, 103069. [Google Scholar] [CrossRef]
- Barati, F.; Asgarani, E.; Gharavi, S.; Soudi, M.R. Considerable increase in Poly(3-hydroxybutyrate) production via phbC gene overexpression in Ralstonia eutropha PTCC 1615. BioImpacts 2021, 11, 53–57. [Google Scholar] [CrossRef]
- Kim, S.; Jang, Y.J.; Gong, G.; Lee, S.-M.; Um, Y.; Kim, K.H.; Ko, J.K. Engineering Cupriavidus necator H16 for enhanced lithoautotrophic poly(3-hydroxybutyrate) production from CO2. Microb. Cell. Fact. 2022, 21, 231. [Google Scholar] [CrossRef]
- Lin, L.; Chen, J.; Mitra, R.; Gao, Q.; Cheng, F.; Xu, T.; Zuo, Z.; Xiang, H.; Han, J. Optimising PHBV biopolymer production in haloarchaea via CRISPRi-mediated redirection of carbon flux. Commun. Biol. 2021, 4, 1007. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, G.B.; Kim, H.U.; Park, S.J.; Choi, J. Enhanced production of poly-3-hydroxybutyrate (PHB) by expression of response regulator DR1558 in recombinant Escherichia coli. Int. J. Biol. Macromol. 2019, 131, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Y.; Yuan, Q.; Liu, Q.; Li, Y.; Wang, Z.; Ma, H.; Chen, T.; Zhao, X. Metabolic engineering of Escherichia coli for poly(3-hydroxybutyrate) production via threonine bypass. Microb. Cell Factories 2015, 14, 185. [Google Scholar] [CrossRef]
- Tadi, S.R.R.; Ravindran, S.D.; Balakrishnan, R.; Sivaprakasam, S. Recombinant production of poly-(3-hydroxybutyrate) by Bacillus megaterium utilizing millet bran and rapeseed meal hydrolysates. Bioresour. Technol. 2021, 326, 124800. [Google Scholar] [CrossRef] [PubMed]
- Ling, C.; Qiao, G.-Q.; Shuai, B.-W.; Olavarria, K.; Yin, J.; Xiang, R.-J.; Song, K.-N.; Shen, Y.-H.; Guo, Y.; Chen, G.-Q. Engineering NADH/NAD(+) ratio in Halomonas bluephagenesis for enhanced production of polyhydroxyalkanoates (PHA). Metab. Eng. 2018, 49, 275–286. [Google Scholar] [CrossRef]
- Olaya-Abril, A.; Luque-Almagro, V.M.; Manso, I.; Gates, A.J.; Moreno-Vivian, C.; Richardson, D.J.; Roldán, M.D. Poly(3-hydroxybutyrate) hyperproduction by a global nitrogen regulator NtrB mutant strain of Paracoccus denitrificans PD1222. FEMS Microbiol. Lett. 2018, 365, fnx251. [Google Scholar] [CrossRef]
- Hobmeier, K.; Loewe, H.; Liefeldt, S.; Kremling, A.; Pflueger-Grau, K. A Nitrate-Blind, P. putida Strain Boosts PHA Production in a Synthetic Mixed Culture. Front. Bioeng. Biotechnol. 2020, 8, 486. [Google Scholar] [CrossRef]
- Arisaka, S.; Terahara, N.; Oikawa, A.; Osanai, T. Increased polyhydroxybutyrate levels by ntcA overexpression in Synechocystis sp. PCC 6803. Algal. Res. 2019, 41, 101565. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, Y.; Ji, M.; Zhang, X.; Wang, H.; Chen, Y.; Wu, Q.; Chen, G.-Q. Hyperproduction of PHA copolymers containing high fractions of 4-hydroxybutyrate (4HB) by outer membrane-defected Halomonas bluephagenesis grown in bioreactors. Microb. Biotechnol. 2022, 15, 1586–1597. [Google Scholar] [CrossRef]
- Shen, R.; Ning, Z.-Y.; Lan, Y.-X.; Chen, J.-C.; Chen, G.-Q. Manipulation of polyhydroxyalkanoate granular sizes in Halomonas bluephagenesis. Metab. Eng. 2019, 54, 117–126. [Google Scholar] [CrossRef]
- Fukui, T.; Mukoyama, M.; Orita, I.; Nakamura, S. Enhancement of glycerol utilization ability of Ralstonia eutropha H16 for production of polyhydroxyalkanoates. Appl. Microbiol. Biotechnol. 2014, 98, 7559–7568. [Google Scholar] [CrossRef] [PubMed]
- Arikawa, H.; Matsumoto, K.; Fujiki, T. Polyhydroxyalkanoate production from sucrose by Cupriavidus necator strains harboring csc genes from Escherichia coli. W. Appl. Microbiol. Biotechnol. 2017, 101, 7497–7507. [Google Scholar] [CrossRef] [PubMed]
- Huo, G.; Zhu, Y.; Liu, Q.; Tao, R.; Diao, N.; Wang, Z.; Chen, T. Metabolic engineering of an E. coli ndh knockout strain for PHB production from mixed glucose-xylose feedstock. J. Chem. Technol. Biotechnol. 2017, 92, 2739–2745. [Google Scholar] [CrossRef]
- Brojanigo, S.; Gronchi, N.; Cazzorla, T.; Wong, T.S.; Basaglia, M.; Favaro, L.; Casella, S. Engineering Cupriavidus necator DSM 545 for the one-step conversion of starchy waste into polyhydroxyalkanoates. Bioresour. Technol. 2022, 347, 126383. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, J.E.; Brojanigo, S.; Basaglia, M.; Favaro, L.; Casella, S. Efficient production of polyhydroxybutyrate from slaughterhouse waste using a recombinant strain of Cupriavidus necator DSM 545. Sci. Total Environ. 2021, 794, 148754. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Li, J.; Huang, Z.; Han, X.; Bao, J. Engineering Corynebacterium glutamicum for synthesis of poly(3-hydroxybutyrate) from lignocellulose biomass. Biotechnol. Bioeng. 2022, 119, 1598–1613. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.H.T.; Nguyen, A.D.; Lee, E.Y. Engineering type I methanotrophic bacteria as novel platform for sustainable production of 3-hydroxybutyrate and biodegradable polyhydroxybutyrate from methane and xylose. Bioresour. Technol. 2022, 363, 127898. [Google Scholar] [CrossRef]
- Qin, R.; Zhu, Y.; Ai, M.; Jia, X. Reconstruction and optimization of a Pseudomonas putida-Escherichia coli microbial consortium for mcl-PHA production from lignocellulosic biomass. Front. Bioeng. Biotechnol. 2022, 10, 1023325. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Weng, C.; Peng, X.; Han, Y. Metabolic engineering of Cupriavidus necator H16 for improved chemoautotrophic growth and PHB production under oxygen-limiting conditions. Metab. Eng. 2020, 61, 11–23. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.-Y.; Du, H.-T.; Zhang, X.; Ma, Y.-M.; Chen, J.-C.; Ye, J.-W.; Jiang, X.-R.; Chen, G.-Q. Chromosome engineering of the TCA cycle in Halomonas bluephagenesis for production of copolymers of 3-hydroxybutyrate and 3-hydroxyvalerate (PHBV). Metab. Eng. 2019, 54, 69–82. [Google Scholar] [CrossRef]
- Schönheit, P.; Buckel, W.; Martin, W.F. On the Origin of Heterotrophy. Trends. Microbiol. 2016, 24, 12–25. [Google Scholar] [CrossRef] [PubMed]
- De Marco, P. Methylotrophy versus heterotrophy: A misconception. Microbiology 2004, 150, 1606–1607. [Google Scholar] [CrossRef] [PubMed]
- Bar-On, Y.M.; Milo, R. The global mass and average rate of rubisco. Proc. Natl. Acad. Sci. USA 2019, 116, 4738–4743. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Oh, M.-K. Strategies for Biosynthesis of C1 Gas-derived Polyhydroxyalkanoates: A review. Bioresour. Technol. 2022, 344, 126307. [Google Scholar] [CrossRef] [PubMed]
- Kamravamanesh, D.; Kovacs, T.; Pflugl, S.; Druzhinina, I.; Kroll, P.; Lackner, M.; Herwig, C. Increased poly-beta-hydroxybutyrate production from carbon dioxide in randomly mutated cells of cyanobacterial strain Synechocystis sp. PCC 6714: Mutant generation and characterization. Bioresour. Technol. 2018, 266, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Tharasirivat, V.; Jantaro, S. Increased Biomass and Polyhydroxybutyrate Production by Synechocystis sp. PCC 6803 Overexpressing RuBisCO Genes. Int. J. Mol. Sci. 2023, 24, 6415. [Google Scholar] [CrossRef] [PubMed]
- Taepucharoen, K.; Tarawat, S.; Puangcharoen, M.; Incharoensakdi, A.; Monshupanee, T. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) under photoautotrophy and heterotrophy by non-heterocystous N2-fixing cyanobacterium. Bioresour. Technol. 2017, 239, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Tarawat, S.; Incharoensakdi, A.; Monshupanee, T. Cyanobacterial production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) from carbon dioxide or a single organic substrate: Improved polymer elongation with an extremely high 3-hydroxyvalerate mole proportion. J. Appl. Phycol. 2020, 32, 1095–1102. [Google Scholar] [CrossRef]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef]
- Nangle, S.N.; Ziesack, M.; Buckley, S.; Trivedi, D.; Loh, D.M.; Nocera, D.G.; Silver, P.A. Valorization of CO2 through lithoautotrophic production of sustainable chemicals in Cupriavidus necator. Metab. Eng. 2020, 62, 207–220. [Google Scholar] [CrossRef]
- Buan, N.R. Methanogens: Pushing the boundaries of biology. Emerg. Top. Life Sci. 2018, 2, 629–646. [Google Scholar] [CrossRef]
- Daniel, S.; Hsu, T.; Dean, S.; Drake, H. Characterization of the H2-Dependent and CO-Dependent Chemolithotrophic Potentials of the Acetogens Clostridium thermoaceticum and Acetogenium kivui. J. Bacteriol. 1990, 172, 4464–4471. [Google Scholar] [CrossRef]
- Petushkova, E.P.; Tsygankov, A.A. Acetate metabolism in the purple non-sulfur bacterium Rhodobacter capsulatus. Biochemistry 2017, 82, 587–605. [Google Scholar] [CrossRef] [PubMed]
- Brady, A.L.; Sharp, C.E.; Grasby, S.E.; Dunfield, P.F. Anaerobic carboxydotrophic bacteria in geothermal springs identified using stable isotope probing. Front. Microbiol. 2015, 6, 897. [Google Scholar] [CrossRef]
- Volbeda, A.; Fontecilla-Camps, J.C. Crystallographic evidence for a CO/CO2 tunnel gating mechanism in the bifunctional carbon monoxide dehydrogenase/acetyl coenzyme A synthase from Moorella thermoacetica. J. Biol. Inorg. Chem. 2004, 9, 525–532. [Google Scholar] [CrossRef]
- Volova, T.; Zhila, N.; Shishatskaya, E. Synthesis of poly(3-hydroxybutyrate) by the autotrophic CO-oxidizing bacterium Seliberia carboxydohydrogena Z-1062. J. Ind Microbiol. Biotechnol. 2015, 42, 1377–1387. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.S.; Smeenk, J.; Broer, K.M.; Kisting, C.J.; Brown, R.; Heindel, T.J.; Bobik, T.A.; DiSpirito, A.A. Growth of Rhodospirillum rubrum on synthesis gas: Conversion of CO to H2 and poly-beta-hydroxyalkanoate. Biotechnol. Bioeng. 2007, 97, 279–286. [Google Scholar] [CrossRef]
- Lemgruber, R.S.P.; Valgepea, K.; Tappel, R.; Behrendorff, J.B.; Palfreyman, R.W.; Plan, M.; Hodson, M.P.; Simpson, S.D.; Nielsen, L.K.; Kopke, M.; et al. Systems-level engineering and characterisation of Clostridium autoethanogenum through heterologous production of poly-3-hydroxybutyrate (PHB). Metab. Eng. 2019, 53, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Valgepea, K.; Lemgruber, R.S.P.; Meaghan, K.; Palfreyman, R.W.; Abdalla, T.; Heijstra, B.D.; Behrendorff, J.B.; Tappel, R.; Kopke, M.; Simpson, S.D.; et al. Maintenance of ATP Homeostasis Triggers Metabolic Shifts in Gas-Fermenting Acetogens. Cell Syst. 2017, 4, 505–515. [Google Scholar] [CrossRef]
- Khider, M.L.K.; Brautaset, T.; Irla, M. Methane monooxygenases: Central enzymes in methanotrophy with promising biotechnological applications. World J. Microbiol. Biotechnol. 2021, 37, 72. [Google Scholar] [CrossRef]
- Wendlandt, K.D.; Jechorek, M.; Helm, J.; Stottmeister, U. Producing poly-3-hydroxybutyrate with a high molecular mass from methane. J. Biotechnol. 2001, 86, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Salem, R.; Soliman, M.; Fergala, A.; Audette, G.E.; ElDyasti, A. Screening for Methane Utilizing Mixed Communities with High Polyhydroxybutyrate (PHB) Production Capacity Using Different Design Approaches. Polymers 2021, 13, 1579. [Google Scholar] [CrossRef] [PubMed]
- Ghoddosi, F.; Golzar, H.; Yazdian, F.; Khosravi-Darani, K.; Vasheghani-Farahani, E. Effect of carbon sources for PHB production in bubble column bioreactor: Emphasis on improvement of methane uptake. J. Environ. Chem. Eng. 2019, 7, 102978. [Google Scholar] [CrossRef]
- Zuñiga, C.; Morales, M.; Revah, S. Polyhydroxyalkanoates accumulation by Methylobacterium organophilum CZ-2 during methane degradation using citrate or propionate as cosubstrates. Bioresour. Technol. 2013, 129, 686–689. [Google Scholar] [CrossRef] [PubMed]
- López, J.C.; Arnaiz, E.; Merchan, L.; Lebrero, R.; Munoz, R. Biogas-based polyhydroxyalkanoates production by Methylocystis hirsuta: A step further in anaerobic digestion biorefineries. Chem. Eng. J. 2018, 333, 529–536. [Google Scholar] [CrossRef]
- Fergala, A.; AlSayed, A.; Khattab, S.; Ramirez, M.; Eldyasti, A. Development of Methane-Utilizing Mixed Cultures for the Production of Polyhydroxyalkanoates (PHAs) from Anaerobic Digester Sludge. Environ. Sci. Technol. 2018, 52, 12376–12387. [Google Scholar] [CrossRef] [PubMed]
- Myung, J.; Flanagan, J.C.A.; Waymouth, R.M.; Criddle, C.S. Expanding the range of polyhydroxyalkanoates synthesized by methanotrophic bacteria through the utilization of omega-hydroxyalkanoate co-substrates. AMB Express 2017, 7, 118. [Google Scholar] [CrossRef] [PubMed]
- Cal, A.J.; Sikkema, W.D.; Ponce, M.I.; Franqui-Villanueva, D.; Riiff, T.J.; Orts, W.J.; Pieja, A.J.; Lee, C.C. Methanotrophic production of polyhydroxybutyrate-co-hydroxyvalerate with high hydroxyvalerate content. Int. J. Biol. Macromol. 2016, 87, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Olah, G.A. Beyond oil and gas: The methanol economy. Angew. Chem.-Int. Edit. 2005, 44, 2636–2639. [Google Scholar] [CrossRef]
- Mondal, U.; Yadav, G.D. Methanol economy and net zero emissions: Critical analysis of catalytic processes, reactors and technologies. Green Chem. 2021, 23, 8361–8405. [Google Scholar] [CrossRef]
- Bourque, D.; Pomerleau, Y.; Groleau, D. High cell density production of poly-beta-hydroxybutyrate (PHB) from methanol by Methylobacterium extorquens: Production of high-molecular-mass PHB. Appl. Microbiol. Biotechnol. 1995, 44, 367–376. [Google Scholar] [CrossRef]
- Orita, I.; Nishikawa, K.; Nakamura, S.; Fukui, T. Biosynthesis of polyhydroxyalkanoate copolymers from methanol by Methylobacterium extorquens AM1 and the engineered strains under cobalt-deficient conditions. Appl. Microbiol. Biotechnol. 2014, 98, 3715–3725. [Google Scholar] [CrossRef]
- Chang, W.; Yoon, J.; Oh, M.-K. Production of Polyhydroxyalkanoates with the Fermentation of Methylorubrum extorquens Using Formate as a Carbon Substrate. Biotechnol. Bioprocess Eng. 2022, 27, 268–275. [Google Scholar] [CrossRef]
- Stöckl, M.; Harms, S.; Dinges, I.; Dimitrova, S.; Holtmann, D. From CO2 to Bioplastic—Coupling the Electrochemical CO2 Reduction with a Microbial Product Generation by Drop-in Electrolysis. ChemSusChem 2020, 13, 4086–4093. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cao, Y.; Li, F.; Tian, Y.; Song, H. Enzyme-Assisted Microbial Electrosynthesis of Poly(3-hydroxybutyrate) via CO2 Bioreduction by Engineered Ralstonia eutropha. ACS Catal. 2018, 8, 4429–4437. [Google Scholar] [CrossRef]
- Kourilova, X.; Novackova, I.; Koller, M.; Obruca, S. Evaluation of mesophilic Burkholderia sacchari, thermophilic Schlegelella thermodepolymerans and halophilic Halomonas halophila for polyhydroxyalkanoates production on model media mimicking lignocellulose hydrolysates. Bioresour. Technol. 2021, 325, 124704. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-L.; Bhatia, S.K.; Gurav, R.; Choi, T.-R.; Kim, H.J.; Song, H.-S.; Park, J.-Y.; Han, Y.-H.; Lee, S.M.; Park, S.L.; et al. Fructose based hyper production of poly-3-hydroxybutyrate from Halomonas sp. YLGW01 and impact of carbon sources on bacteria morphologies. Int. J. Biol. Macromol. 2020, 154, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yoon, J.-J.; Kim, H.-J.; Hong, J.W.; Hong, Y.G.; Song, H.-S.; Moon, Y.-M.; Jeon, J.-M.; Kim, Y.-G.; Yang, Y.-H. Engineering of artificial microbial consortia of Ralstonia eutropha and Bacillus subtilis for poly(3-hydroxybutyrate-co-3-hydroxyvalerate) copolymer production from sugarcane sugar without precursor feeding. Bioresour. Technol. 2018, 257, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Yousefi-Massumabad, H.; Younesi, H.; Abyar, H.; Bahramifar, N. Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J. Environ. Chem. Eng. 2020, 8, 103588. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; Falcao de Aragdo, G.M.; Poletto, P. Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process. Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Lim, S.W.; Kansedo, J.; Tan, I.S.; Tan, Y.H.; Nandong, J.; Lam, M.K.; Ongkudon, C.M. Microbial valorization of oil-based substrates for polyhydroxyalkanoates (PHA) production—Current strategies, status, and perspectives. Process. Biochem. 2023, 130, 715–733. [Google Scholar] [CrossRef]
- Volova, T.; Sapozhnikova, K.; Zhila, N. Cupriavidus necator B-10646 growth and polyhydroxyalkanoates production on different plant oils. Int. J. Biol. Macromol. 2020, 164, 121–130. [Google Scholar] [CrossRef]
- Purama, R.K.; Al-Sabahi, J.N.; Sudesh, K. Evaluation of date seed oil and date molasses as novel carbon sources for the production of poly(3Hydroxybutyrate-co-3Hydroxyhexanoate) by Cupriavidus necator H16 Re 2058/pCB113. Ind. Crop. Prod. 2008, 119, 83–92. [Google Scholar] [CrossRef]
- Santolin, L.; Waldburger, S.; Neubauer, P.; Riedel, S.L. Substrate-Flexible Two-Stage Fed-Batch Cultivations for the Production of the PHA Copolymer P(HB-co-HHx) With Cupriavidus necator Re2058/pCB113. Front. Bioeng. Biotechnol. 2021, 9, 623890. [Google Scholar] [CrossRef]
- Schmid, M.T.; Sykacek, E.; O’Connor, K.; Omann, M.; Mundigler, N.; Neureiter, M. Pilot scale production and evaluation of mechanical and thermal properties of P(3HB) from Bacillus megaterium cultivated on desugarized sugar beet molasses. J. Appl. Polym. Sci. 2022, 139, e51503. [Google Scholar] [CrossRef]
- Tu, W.-L.; Chu, H.-K.; Huang, C.-M.; Chen, C.-H.; Ou, C.-M.; Guo, G.-L. Polyhydroxyalkanoate Production by Cupriavidus necator with Inedible Rice. BioResources 2022, 17, 2202–2213. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Ghodake, G.S.; Bharagava, R.N.; Kim, D.S.; Nair, S.; Shin, H.S. Efficient bioconversion of sugarcane bagasse into polyhydroxybutyrate (PHB) by Lysinibacillus sp. and its characterization. Bioresour. Technol. 2021, 324, 124673. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, H.-J.; Kim, S.H.; Suh, M.J.; Cho, J.Y.; Ham, S.; Jeon, J.-M.; Yoon, J.-J.; Bhatia, S.K.; Gurav, R.; et al. Screening of the strictly xylose-utilizing Bacillus sp. SM01 for polyhydroxybutyrate and its co-culture with Cupriavidus necator NCIMB 11599 for enhanced production of PHB. Int. J. Biol. Macromol. 2021, 181, 410–417. [Google Scholar] [CrossRef]
- Kalaiyezhini, D.; Ramachandran, K.B. Biosynthesis of Poly-3-Hydroxybutyrate (PHB) from Glycerol by Paracoccus denitrificans in a Batch Bioreactor: Effect of Process Variables. Prep. Biochem. Biotechnol. 2015, 45, 69–83. [Google Scholar] [CrossRef]
- Fauzi, A.H.M.; Chua, A.S.M.; Yoon, L.W.; Nittami, T.; Yeoh, H.K. Enrichment of PHA-accumulators for sustainable PHA production from crude glycerol. Process. Saf. Environ. Protect. 2019, 122, 200–208. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Yu, J. Miscibility of natural polyhydroxyalkanoate blend with controllable material properties. J. Appl. Polym. Sci. 2013, 129, 2004–2016. [Google Scholar] [CrossRef]
- Vijay, R.; Tarika, K. Microbial Production of Polyhydroxyalkanoates (Phas) Using Kitchen Waste as an Inexpensive Carbon Source. Biosci. Biotechnol. Res. Asia 2019, 16, 155–166. [Google Scholar] [CrossRef]
- Das, S.; Majumder, A.; Shukla, V.; Suhazsini, P.; Radha, P. Biosynthesis of Poly(3-hydroxybutyrate) from Cheese Whey by Bacillus megaterium NCIM 5472. J. Polym. Environ. 2018, 26, 4176–4187. [Google Scholar] [CrossRef]
- Loan, T.T.; Trang, D.T.Q.; Huy, P.Q.; Ninh, P.X.; Van Thuoc, D. A fermentation process for the production of poly(3-hydroxybutyrate) using waste cooking oil or waste fish oil as inexpensive carbon substrate. Biotechnol. Rep. 2022, 33, e00700. [Google Scholar] [CrossRef] [PubMed]
- Sangkharak, K.; Khaithongkaeo, P.; Chuaikhunupakarn, T.; Choonut, A.; Prasertsan, P. The production of polyhydroxyalkanoate from waste cooking oil and its application in biofuel production. Biomass Convers Biorefin. 2021, 11, 1651–1664. [Google Scholar] [CrossRef]
- Thinagaran, L.; Sudesh, K. Evaluation of Sludge Palm Oil as Feedstock and Development of Efficient Method for its Utilization to Produce Polyhydroxyalkanoate. Waste Biomass Valorization 2019, 10, 709–720. [Google Scholar] [CrossRef]
- Obruca, S.; Marova, I.; Snajdar, O.; Mravcova, L.; Svoboda, Z. Production of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by Cupriavidus necator from waste rapeseed oil using propanol as a precursor of 3-hydroxyvalerate. Biotechnol. Lett. 2010, 32, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Pernicova, I.; Kucera, D.; Nebesarova, J.; Kalina, M.; Novackova, I.; Koller, M.; Obruca, S. Production of polyhydroxyalkanoates on waste frying oil employing selected Halomonas strains. Bioresour. Technol. 2019, 292, 122028. [Google Scholar] [CrossRef] [PubMed]
- Goff, M.; Ward, P.G.; O’Connor, K.E. Improvement of the conversion of polystyrene to polyhydroxyalkanoate through the manipulation of the microbial aspect of the process: A nitrogen feeding strategy for bacterial cells in a stirred tank reactor. J. Biotechnol. 2007, 132, 283–286. [Google Scholar] [CrossRef]
- Merli, G.; Becci, A.; Amato, A.; Beolchini, F. Acetic acid bioproduction: The technological innovation change. Sci. Total. Environ. 2021, 798, 149292. [Google Scholar] [CrossRef]
- Christensen, M.; Jablonski, P.; Altermark, B.; Irgum, K.; Hansen, H. High natural PHA production from acetate in Cobetia sp. MC34 and Cobetia marina DSM 4741(T) and in silico analyses of the genus specific PhaC(2) polymerase variant. Microb. Cell. Fact. 2021, 20, 225. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gonzalez, L.; De Wever, H. Acetic Acid as an Indirect Sink of CO2 for the Synthesis of Polyhydroxyalkanoates (PHA): Comparison with PHA Production Processes Directly Using CO2 as Feedstock. Appl. Sci. 2019, 8, 1416. [Google Scholar] [CrossRef]
- Tao, G.-B.; Tian, L.; Pu, N.; Li, Z.-J. Efficient production of poly-3-hydroxybutyrate from acetate and butyrate by halophilic bacteria Salinivibrio spp. TGB4 and TGB19. Int. J. Biol. Macromol. 2022, 221, 1365–1372. [Google Scholar] [CrossRef] [PubMed]
- Marang, L.; Jiang, Y.; van Loosdrecht, M.C.M.; Kleerebezem, R. Butyrate as preferred substrate for polyhydroxybutyrate production. Bioresour. Technol. 2013, 142, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-M.; Brigham, C.J.; Kim, Y.-H.; Kim, H.-J.; Yi, D.-H.; Kim, H.; Rha, C.; Sinskey, A.J.; Yang, Y.-H. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (P(HB-co-HHx)) from butyrate using engineered Ralstonia eutropha. Appl. Microbiol. Biotechnol. 2014, 98, 5461–5469. [Google Scholar] [CrossRef] [PubMed]
- Zhila, N.O.; Sapozhnikova, K.Y.; Kiselev, E.G.; Nemtsev, I.; Lukyanenko, A.; Shishatskaya, E.; Volova, T.G. Biosynthesis and Properties of a P(3HB-co-3HV-co-4HV) Produced by Cupriavidus necator B-10646. Polymers 2022, 14, 4226. [Google Scholar] [CrossRef] [PubMed]
- Xiao, N.; Jiao, N. Formation of Polyhydroxyalkanoate in Aerobic Anoxygenic Phototrophic Bacteria and Its Relationship to Carbon Source and Light Availability. Appl. Environ. Microbiol. 2011, 77, 7445–7450. [Google Scholar] [CrossRef]
- Koch, M.; Bruckmoser, J.; Scholl, J.; Hauf, W.; Rieger, B.; Forchhammer, K. Maximizing PHB content in Synechocystis sp. PCC 6803: A new metabolic engineering strategy based on the regulator PirC. Microb. Cell Fact. 2020, 19, 231. [Google Scholar] [CrossRef]
- Cortes, O.; Guerra-Blanco, P.; Chairez, I.; Poznyak, T.; Garcia-Pena, E.I. Polymers, the Light at the End of Dark Fermentation: Production of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) by a Photoheterotrophic Consortium. J. Polym. Environ. 2022, 30, 2392–2404. [Google Scholar] [CrossRef]
- Brandl, H.; Gross, R.; Lenz, R.; Lloyd, R.; Fuller, R. The Accumulation of Poly(3-Hydroxyalkanoates) in Rhodobacter sphaeroides. Arch. Microbiol. 1991, 155, 337–340. [Google Scholar] [CrossRef]
- Nemmour, A.; Inayat, A.; Janajreh, I.; Ghenai, C. Green hydrogen-based E-fuels (E-methane, E-methanol, E-ammonia) to support clean energy transition: A literature review. Int. J. Hydrog. 2023, 48, 29011–29033. [Google Scholar] [CrossRef]
- NIST database of National Institute of Standards and Technology, U.S. Department of Commerce Webbook. Available online: https://webbook.nist.gov/ (accessed on 29 March 2024).
- PubChem Database of National Library of Medicine, Nacional Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 March 2024).
- Eberle, U.; Felderhoff, M.; Schueth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem.-Int. Edit. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).