1. Introduction
Alzheimer’s disease (AD) is a type of dementia that affects around 50 million people worldwide, and this number is expected to increase to 152 million people by 2050. The World Alzheimer’s Report 2019 stated that annual care costs for AD treatment among American people amounted to USD 340 billion [
1], which could rise to USD 1.1 trillion by 2050. AD is an irreversible neurodegenerative disorder that causes a decline in cognitive abilities and impairs general behaviors [
2]. The expected increase in AD patients in the near future will lead to significantly increased expenses for governments, communities, families, and people, as well as a decline in economic productivity [
3]. The speedy development of AD medicines is urgently required and has attracted intensive research.
Several hypotheses have been posited regarding AD pathogenesis, including oxidative stress, impairment of the cholinergic nervous system, and the aggregation of amyloid (Aβ) peptides [
4]. Naturally occurring amyloid peptides in the brain are generated through the proteolytic cleavage of amyloid precursor proteins (APPs) via two pathways. Non-toxic amyloid peptides are produced through non-amyloidogenic pathways, whereas toxic amyloid peptides, specifically Aβ
1–40 and Aβ
1–42, are formed via amyloidogenic pathways involving the cleavage of APPs by β-secretase-1 (BACE-1) and γ-secretase [
5,
6]. The accumulation of cytotoxic amyloid peptides leads to several adverse events in neurons, including amyloid plaques, oxidative stress, mitochondrial dysfunctions, neuroinflammation, endoplasmic reticulum (ER) stress, and eventually neuronal death [
7,
8,
9]. Therefore, the reduction of oxidative stress and BACE-1 could be one approach to ameliorate the impact of AD. The US FDA has approved both lecanemab and aducanumab as novel immunotherapies to reduce amyloid plaques [
10,
11], highlighting the importance of the amyloid hypothesis in AD.
Diplazium esculentum (Retz.) Sw. (Pak-Kood in Thai), an edible fern, is a member of the Athyriaceae family, and is widespread across moist climatic areas, including the Philippines, India, China, and Thailand [
12].
D. esculentum (DE) also contains non-nutritive compounds, including phytochemicals, and has been reported to promote health benefits such as treating skin diseases, asthma, and cancer as well as aiding in scar drying [
13,
14,
15]. Previous studies demonstrated that the ethanolic extract of DE functioned as an anti-AD agent by quenching oxidative stress, inhibiting acetylcholine (AChE)-degrading enzymes (acetylcholinesterase (AChE) and butyrylcholinesterase (BChE)) in vitro, and inhibiting BACE-1 activities, resulting in the reduction of Aβ peptides in the brain and improved locomotor functions in
Drosophila models of AD [
16]. Interestingly, the ethanolic extract of DE also exhibited additive and synergistic effects with donepezil, which is an AD drug inhibiting both AChE and BACE-1 [
17], to suppress BACE-1 [
18].
These results implied that the ethanolic extract of DE obtained from a non-optimized extraction condition suppressed the development of AD by inhibiting BACE-1. Therefore, this study developed extraction conditions to achieve optimal BACE-1 inhibitory activities from
D. esculentum using a Box–Behnken design (BBD) and response surface methodology (RSM). A BBD was utilized to examine the number of trials using a full factorial design that was both time-efficient and cost-effective, hence decreasing the probability of complications and simplifying the analysis while preserving a high level of precision, while RSM was employed to analyze the correlation between the studied factors under ideal circumstances using statistical and mathematical concepts [
19]. Both BBD and RSM are widely used in the pharmaceutical and food industries to optimally recover bioactive phenolic and flavonoid compounds from various sources including plants for human health benefits while consuming less energy and raw materials [
20]. This study investigated the effect of four ethanol extraction factors, including solvent concentration, extraction temperature, extraction time, and solid–liquid ratio (SLR), on BACE-1 inhibitory activities using BBD and RSM. The optimized DE extract showed significantly different antioxidant and BACE-1 inhibitory activities and required less extraction time compared to the non-optimized DE extract obtained from previous studies [
16,
18], affirming the advantages of BBD and RSM implementation in the optimization process. The optimized DE extract also impacted a number of genes linked to AD pathogenesis in a
Drosophila model of the amyloid hypothesis, indicating a multi-target anti-AD agent function.
3. Discussion
Alzheimer’s disease (AD), a type of dementia, is projected to become the primary cause of global mortality [
1]; therefore, comprehensive studies are investigating novel pharmaceuticals to treat AD. Therapeutic agents, including cholinesterase (AChE and BChE) and BACE-1 inhibitors as well as antioxidants, are among those undergoing investigation for AD treatment. Previous reports revealed that
Diplazium esculentum (DE) extract suppressed the key enzymes involved in AD pathogenesis, including AChE, BChE, and BACE-1. Particularly, the extract inhibited BACE-1 activities and subsequently decreased amyloid peptides in the brain of
Drosophila expressing APPs and BACE-1 (a model for the amyloid pathway) [
16]. The DE extract utilized in the previous study was derived under suboptimal extraction conditions and the optimal BACE-1 inhibition from the DE extract remains unclear. Non-optimized extraction may lead to several issues, such as reductions in extraction efficiency and increased time and plant materials. In this study, the extraction conditions were optimized to achieve the appropriate BACE-1 inhibitor from DE, with BBD and RSM employed in the experimental design and data interpretation. The optimized DE extract was then subjected to phytochemical profile analysis, antioxidant activities, BACE-1 inhibitory activities, and anti-AD activities in the
Drosophila model of the amyloid pathway.
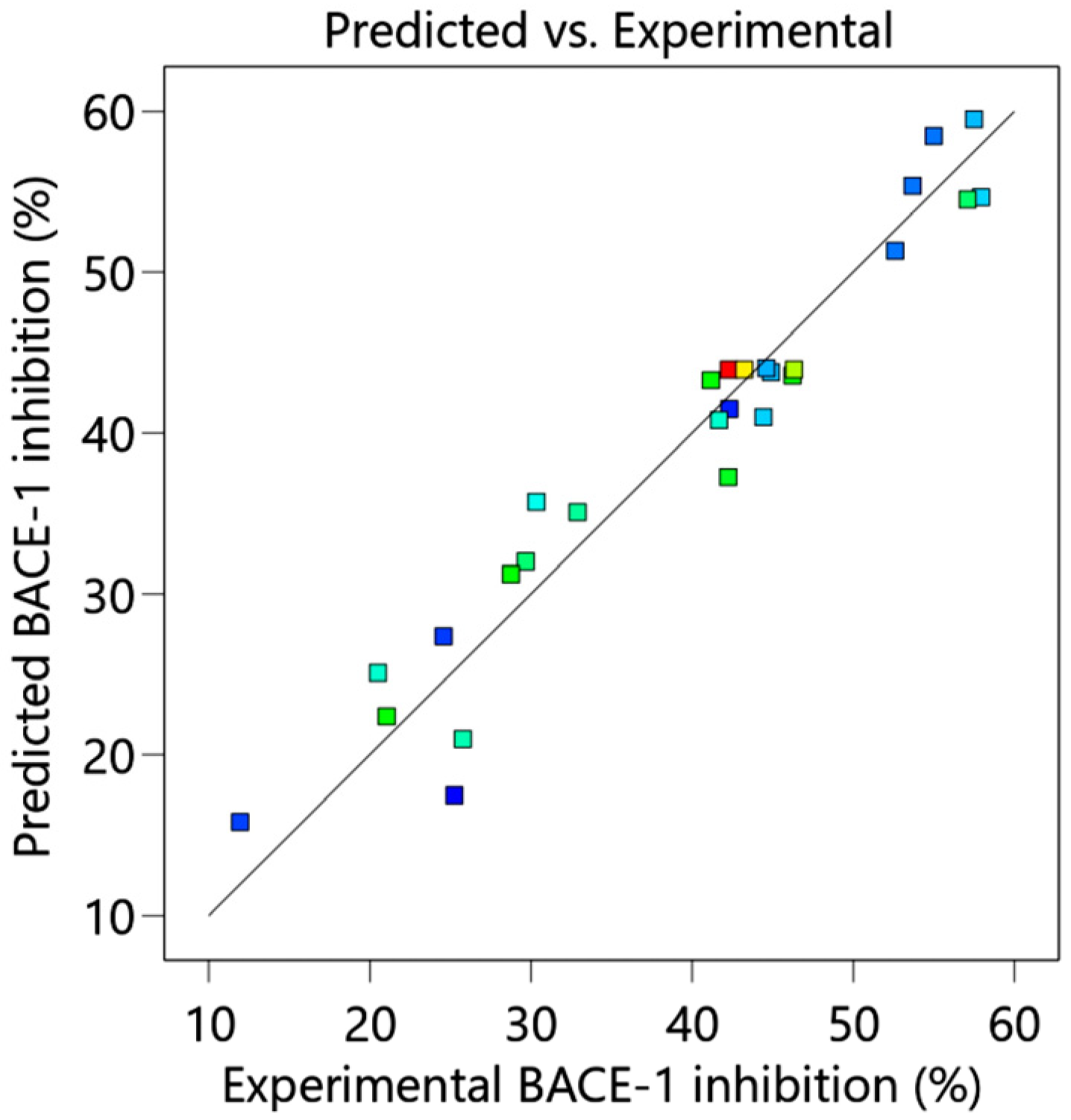
The optimized extraction condition for maximal BACE-1 inhibition was 70% (
v/
v) aqueous ethanol, 50 min extraction time, 30 °C extraction temperature, and 1:30 g/mL SLR. Under this condition, 56.33% of BACE-1 inhibition was observed. The optimized extraction condition was quite similar to the non-optimized extraction [
16], which was 70% (
v/
v) aqueous ethanol, 120 min extraction time, 30 °C extraction temperature, and 1:10 g/mL SLR [
16], but the optimized condition resulted in a reduction of 70 min of extraction time. In addition, compared to Inthachat et al. (2024) [
18], the optimized condition consumed less extraction time by almost 300 min. Compared to the non-optimized extraction, the optimized extraction condition also yielded significantly higher levels of TPCs, TFCs, BACE-1 inhibitory activities, and antioxidant activities (DPPH, FRAP, and ORAC) (
Table 6). The results suggested that the optimized extraction condition increased phytochemical contents and bioactivities while reducing the extraction time. Four extraction factors, including ethanol concentration, extraction time, extraction temperature, and SLR, were studied. The data revealed that none of these factors were associated with TPCs. Ethanol concentration was significantly correlated with BACE-1 inhibition, with an increasing ethanol concentration resulting in higher BACE-1 inhibition (
Figure 2), implying that BACE-1 inhibitors from DE may have a low polarity index (PI) since 30% aqueous ethanol has a PI higher than 70% ethanol [
30]. Likewise, BACE-1 inhibition did not decrease at an extraction temperature of 60 °C based on the absence of a decrease in BACE-1 inhibition at an extraction temperature of 60 °C. The data suggested that the BACE-1 inhibitors present in the DE extract were heat-stable (
Figure 2D). Interestingly, the interaction between ethanol concentration and the other extraction factors was insignificant, suggesting the importance of ethanol as a single factor to acquire optimal BACE-1 inhibition. Surprisingly, none of the four extraction factors contributed to TPCs. Previous studies produced contradictory results. Azahar et al. (2017) [
31] reported that extraction time, SLR, and temperature contributed to TPCs from
Curcuma zedoaria leaves, while Zhang et al. (2021) [
32] also found that all four extraction factors contributed to TPCs of
Empetrum nigrum fruits. Compared with other organic solvents, ethanol is commonly used in food and nutraceutical sectors because of its safety and edibility [
33], with aqueous ethanol the most appropriate solvent to extract phenolics from plant samples [
34]. Because soluble phenolics are predominantly accumulated in the epidermal and sub-epidermal (outer layers) [
35], we hypothesized that a low concentration of ethanol (30%) might be strong enough to recover most of the phenolics from DE, an edible fern with a soft and thin wall. Thus, increasing the ethanol concentration to 70%, even in combination with other extraction factors, may not increase the TPC since it may have already reached saturation.
The optimized DE extract was subjected to metabolomic phytochemical analysis to detect the abundance of tentative compounds. To the best of our knowledge, this is the first study investigating the metabolomic phytochemical profiles of DE. Phytosterols, phenolics, flavonoids, amino acids, and fatty acids comprised the majority of the identified compounds in DE extract, in accordance with previous reports [
36]. For phytochemicals, the DE extract contained rosmarinic acid, kaempferol glucosides, quercetin, quercetin derivatives, rutin, ferulic acid, piperine, capsaicin, campesterol, and mangostin. The targeted LC-ESI-MS/MS confirmed kaempferol, quercetin, rutin, and rosmarinic acid. Our previous study also identified kaempferol, quercetin, rosmarinic acid, and rutin in DE samples collected in the same area but in a different collecting year [
18]. Unfortunately, the metabolic study failed to detect morin, cinnamic acid, eriodictyol, 5-O-methyl ether, 7-O-β-D-xylosylgalactoside, syringic acid, protocatechuic acid, myricetin, and three ecdysteroids (amarasterone A1, makisterone C, and ponasterone A), as formerly documented [
37,
38,
39,
40], possibly due to different planting areas and extraction methods.
Synergistic use with approved medications is an alternative way of treating ailments. Phytochemicals are the subject of extensive study for this approach due to their low cost and safety. An ethanolic extract of propolis combined with donepezil improved memory better than monotherapy [
41], while the combination of gallic acid with donepezil synergistically inhibited AChE activities in the brain of aluminum chloride-induced neurotoxicity in rats [
42]. In this study, we found that the optimized DE extract acted synergistically with donepezil to impede BACE-1 activities, especially when low doses of donepezil and DE extract were combined, suggesting that low doses of donepezil can be used in combination with DE extract to attain optimal BACE-1 inhibition (
Table 7). Quercetin may also inhibit BACE-1 when combined with donepezil in a synergistic manner, whereas kaempferol may have a slight antagonistic effect with BACE-1 [
18]. Regarding BACE-1 inhibition, molecular docking showed that donepezil interacts with the active site of BACE-1 with a half-maximal inhibitory concentration (IC
50) of 1.5 µM [
17], while an in vitro study and molecular docking demonstrated that quercetin and kaempferol bind directly to the active site of BACE-1, albeit with different binding capacity, leading to IC
50 values of 5.4 µM and 14.7 µM, respectively. A slight antagonistic effect of kaempferol against donepezil may result from competition for the active site of BACE-1 or from kaempferol binding, causing a minor conformational change in BACE-1, which hinders donepezil binding. In contrast, the inhibitory effects of BACE-1 and the reduction in amyloid peptides Aβ
1-40 and Aβ
1-42 in neuronal cells were observed exclusively with quercetin [
43], suggesting that while kaempferol was abundant in the optimized DE extract, quercetin may possess bioactive anti-AD properties in vivo. No experimental data were available for fisetin and BACE-1 but the molecular docking study showed that fisetin can bind to the active site of BACE-1 with a significant ligand-binding complex [
44]. Rosmarinic acid, a polyphenol compound, showed non-competitively inhibited BACE-1 activities with IC
50 at 21 µM, suggesting that rosmarinic acid interacted with BACE-1 in other areas but not at the enzyme active site [
45]. Thus, rosmarinic acid might function together with quercetin to inhibit BACE-1. In summary, with the possible exception of kaempferol, three compounds (quercetin, fisetin, and rosmarinic acid) might contribute to BACE-1 inhibition in the optimized DE extract.
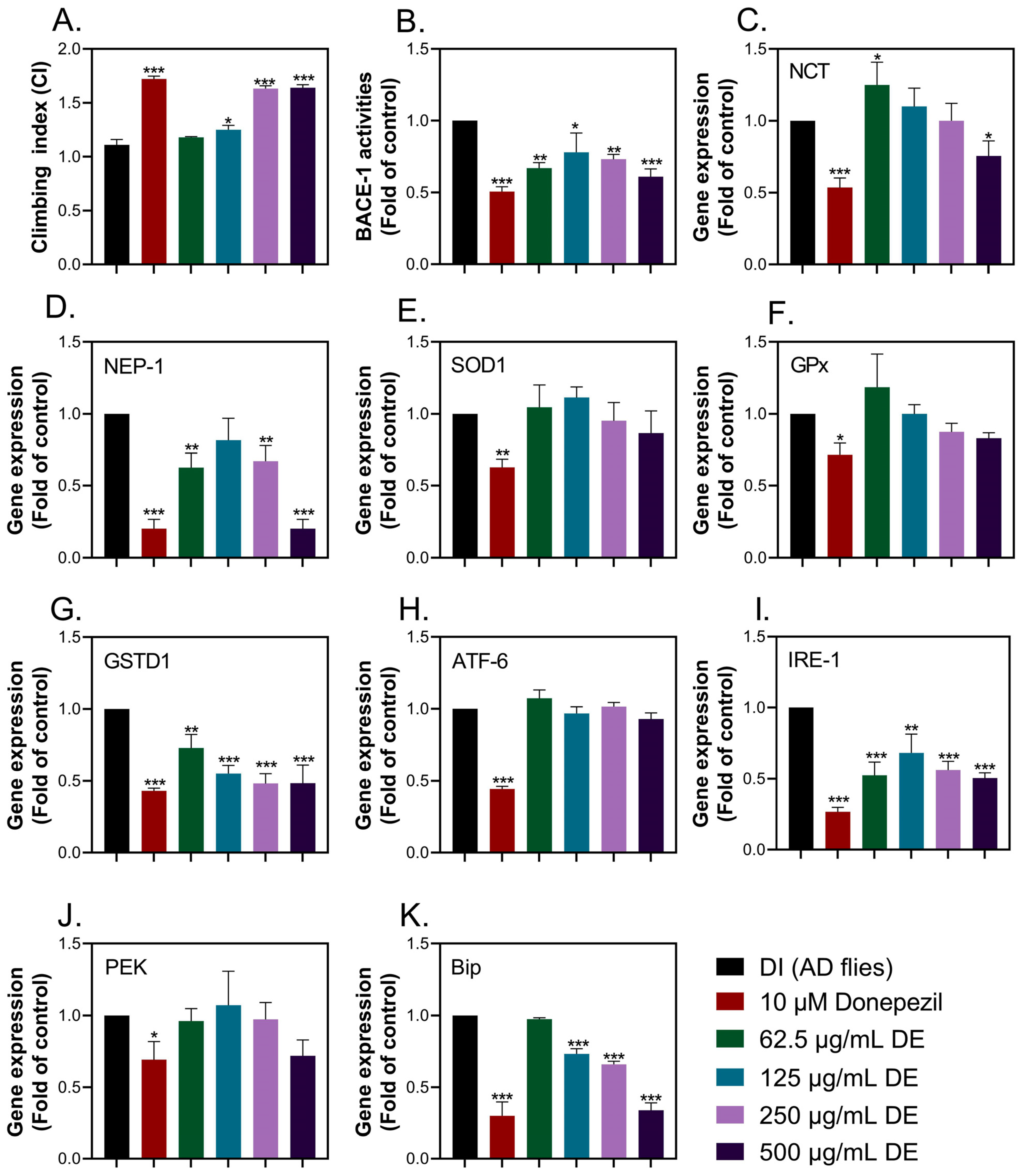
The cleavage of APPs by BACE-1 leads to the formation of amyloid peptides, which are eventually aggregated and accumulated. Aggregated amyloid peptides are neurotoxic and are capable of activating various cellular pathways, including oxidative stress and ER stress responses [
27,
28]. Thus, to elucidate the anti-AD properties of optimized DE extract, we employed
Drosophila-expressing human APPs and BACE-1, which represent the amyloid hypothesis. The optimized DE extract (125–500 µg/mL) reduced BACE-1 activities, leading to improvement in the locomotor function. Notably, donepezil suppressed all examined genes implicated in amyloid degradation, antioxidant enzymes, and the ER stress response, while the optimized DE extract only affected NEP-1, GSTD1, IRE-1, and Bip. Neprilysin (NEP-1) plays a crucial role in the degradation of amyloid peptides, thereby mitigating their buildup and the resulting neuronal damage linked to AD [
46]. Nevertheless, in this study, the mRNA expression of NEP-1 exhibited a decrease in flies treated with optimized DE extract. This decline in NEP-1 expression was attributed to reduced levels of aggregated amyloid peptides in fly heads, implying that diminished enzyme activity is required for the clearance of aggregated amyloid peptides. Glutathione S-transferases (GSTs) are a group of enzymes involved in the detoxification of electrophilic compounds through conjugation with glutathione, which consists of three amino acids (glycine, cysteine, and glutamic acid). Although not directly involved in amyloid peptide metabolism, GSTD1 plays a cellular defense role against oxidative stress and neuroinflammation and indirectly affects amyloid peptide levels. Elevated oxidative stress in AD flies may trigger GSTD1 upregulation to counteract damages [
46,
47]. The optimized DE extract decreased GSTD1 expression in this study, due to reduced aggregated amyloid peptide-stimulated oxidative stress. In AD, misfolded proteins like amyloid peptides accumulate in the ER of neurons, inducing ER stress and activating the unfolded protein response (UPR), including the IRE-1 signaling pathway. IRE-1 activation in AD may exacerbate neuroinflammation, synaptic dysfunction, and neuronal cell death. Bip, an ER chaperone, serves as a primary sensor for unfolded proteins. Dysregulation of Bip expression and function is implicated in AD. IRE-1 signaling and Bip dysregulation contribute to AD pathogenesis by promoting neuronal dysfunction, neuroinflammation, and neuronal death [
48,
49,
50]. Notably, a recent study demonstrated that an optimized DE extract reduced IRE-1 and Bip expression, suggesting a potential role in mitigating ER stress and decreasing AD pathogenesis. In summary, the optimized DE extract repressed not only BACE-1 activities in AD flies but also the downstream response of aggregated amyloid peptides, including oxidative stress and ER stress response.