Sources of Carotenoids in Amazonian Fruits
Abstract
1. Introduction
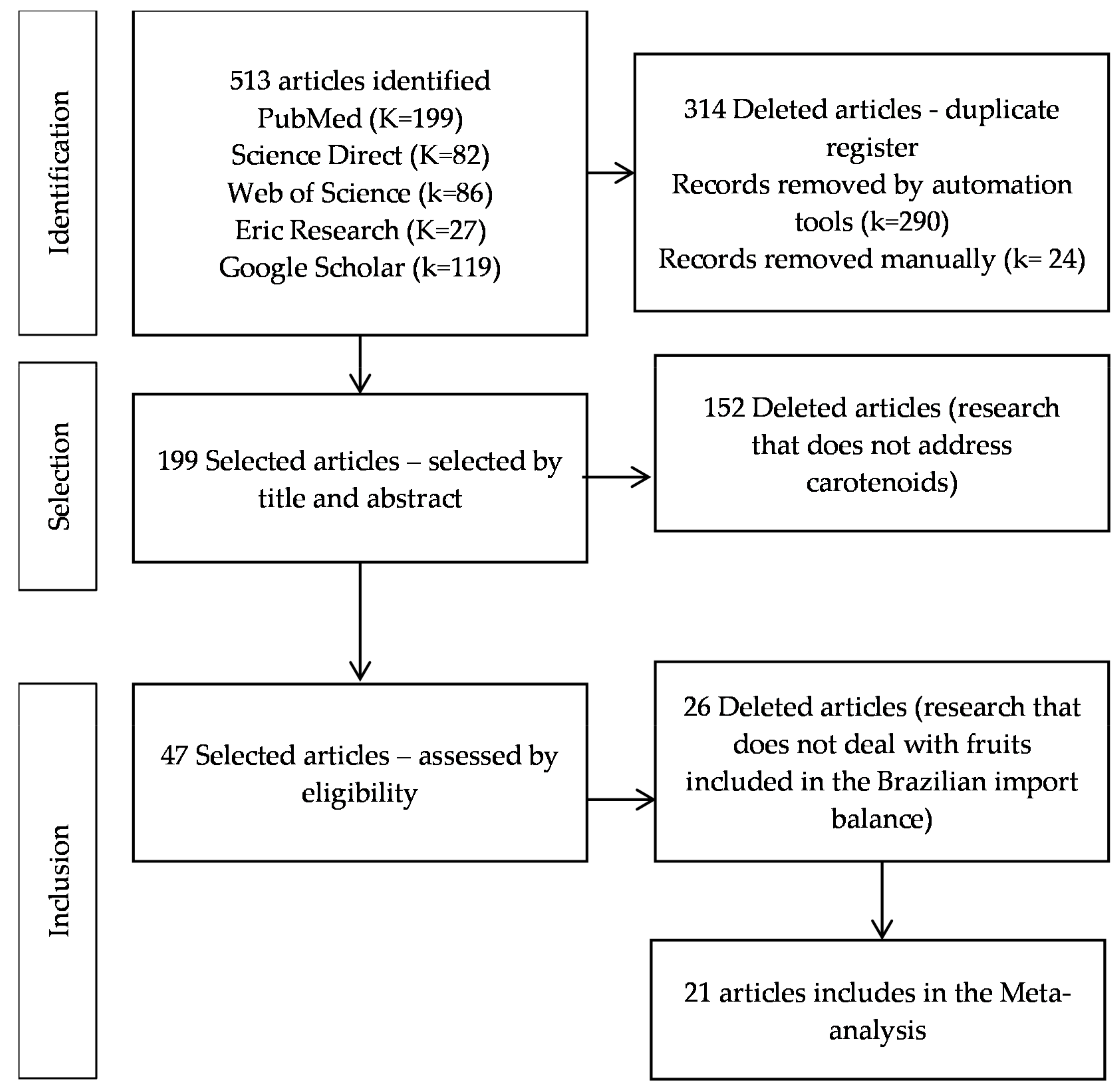
2. Methods
2.1. Study Selection Process
2.2. Data Extraction
3. Results
4. Properties of Some Food Compounds in the Prevention of Etiological Diseases
5. Bioactive Compounds
6. Carotenoids
7. Research on Products Derived from Fruits Rich in Carotenoids: Flour and Oil
8. Research Using Parts of Rich-Carotenoids Fruits
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy Aging and Dietary Patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Santhakumar, A.B.; Battino, M.; Alvarez-suarez, J.M. Dietary polyphenols: Structures, bioavailability and protective effects against atherosclerosis. Food Chem. Toxicol. 2018, 113, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef] [PubMed]
- Marhuenda, J.; Villaño, D.; Cerdá, B.; Zafrilla, M.P. Cardiovascular Disease and Nutrition. Nutrition in Health and Disease—Our Challenges Now and Forthcoming Time, 1st ed.; IntechOpen: Vienna, Austria, 2019; pp. 90–150. [Google Scholar] [CrossRef]
- Wastesson, J.W.; Morin, L.; Tan, E.C.; Johnell, K. An update on the clinical consequences of polypharmacy in older adults: A narrative review. Expert Opin. Drug Saf. 2018, 17, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Septembre-malaterre, A.; Remize, F.; Poucheret, P. Fruits and vegetables, as a source of nutritional compounds and phytochemicals: Changes in bioactive compounds during lactic fermentation. Food Res. Int. 2018, 104, 86–99. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Kazemi, M.; Samani, S.A.; Simal-Gandara, J. Bioactive compounds from by-products of eggplant: Functional properties, potential applications and advances in valorization methods. Trends Food Sci. Technol. 2021, 112, 518–531. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística. Pesquisas de Orçamento Familiares. Available online: https://www.ibge.gov.br/estatisticas/sociais/saude.html (accessed on 15 September 2022).
- Moura de Oliveira, B.D.; Neves Soares Oliveira, C.; Borelli, T.; Andrade Cardoso Santiago, R.; Coradin, L.; Hunter, D. Brazilian underutilised species to promote dietary diversity, local food procurement, and biodiversity conservation: A food composition gap analysis. Lancet Planet. Health 2018, 2, S22. [Google Scholar] [CrossRef]
- Costa, J.C.; Canella, D.S.; Martins, A.P.B.; Levy, R.B.; Andrade, G.C.; Louzada, M.L.C. Consumo de frutas e associação com a ingestão de alimentos ultraprocessados no Brasil em 2008–2009. Cien. Saúde Colet. 2021, 26, 1233–1244. [Google Scholar] [CrossRef]
- Serra, J.L.; Rodrigues, A.M.C.; Freitas, R.A.; Meirelles, A.J.A.; Darnet, S.H.; Silva, L.H.M. Alternative sources of oils and fats from Amazonian plants: Fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res. Int. 2018, 116, 12–19. [Google Scholar] [CrossRef]
- Berni, P.; Campoli, S.S.; Negri, T.C.; Toledo, N.M.V.; Canniatti-Brazaca, S.G. Non-conventional tropical fruits: Characterization, antioxidant potential and carotenoid bioaccessibility. Plant Foods Hum. Nutr. 2019, 74, 141–148. [Google Scholar] [CrossRef]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Galiazzi, M.C.; Sousa, R.S. O que é isso que se mostra: O fenômeno na análise textual discursiva? Atos Pesqui. Educ. 2020, 15, 1167–1184. [Google Scholar] [CrossRef]
- Ministério do Desenvolvimento, Indústria e Comércio, Brasil; Secretaria de Comércio Exterior—SECEX. Base de dados Comex Stat. Available online: http://comexstat.mdic.gov.br/pt/home (accessed on 15 September 2022).
- Sanches, J.L.S.; Martins, J.P.; Conceição, A.F.D.; Matoso, A.O.; Lapiccirella, J.N. Food (In)security and the Possibilities to Minimize Malnutrition from the PANC. Cad. Agroecol. 2022, 17, 1–12. Available online: http://cadernos.aba-agroecologia.org.br/cadernos/article/view/6943 (accessed on 15 September 2022).
- Santos, O.V.; Cunha, N.S.R.; Duarte, S.P.A.; Soares, S.D.; Costa, R.S.; Mendes, P.; Nascimento, F.C.A.; Figueira, M.S.; Costa, B.E.T. Determination of bioactive compounds obtained by the green extraction of taioba leaves (Xanthosoma taioba) on hydrothermal processing. Food Sci. Technol. 2022, 42, e22422. [Google Scholar] [CrossRef]
- Bamigboye, C.O.; Omomowo, I.O.; Alao, M.B.; Elegbede, J.A.; Adebayo, E.A. Free radical scavenging ability, mechanisms of action and health implications of oyster mushrooms (Pleurotus species). J. Microbiol. Biotechnol. Food Sci. 2021, 10, 636–647. [Google Scholar] [CrossRef]
- Vieira, L.A.S.L.; Souza, R.B.A. Ação dos Antioxidantes no Combate aos Radicais Livres e na Prevenção do Envelhecimento Cutâneo. Id Line Rev. Psicol. 2019, 13, 408–418. [Google Scholar] [CrossRef]
- Verruck, S.; Prudencio, E.S.; Silveira, S.M.D.A. Compostos bioativos com capacidade antioxidante e antimicrobiana em frutas. Rev. Congr. Bras. Eng. Aliment. 2019, 4, 111–124. [Google Scholar] [CrossRef]
- Mendes, A.P.A.; Pereira, R.C.; Angelis-pereira, M.C. Estresse oxidativo e sistemas antioxidantes: Conceitos fundamentais sob os aspectos da nutrição e da ciência dos alimentos. In Tecnologia de Alimentos: Tópicos Físicos, Químicos e Biológicos, 1st ed.; Cordeiro, C.A.M., Ed.; Científica: São Paulo, Brazil, 2020; Volume 2, pp. 296–312. [Google Scholar] [CrossRef]
- Henrique, V.A.; Nunes, C.R.; Azevedo, F.T.; Pereira, S.M.F.; Barbosa, J.B.; Talma, S.V. Alimentos Funcionais: Aspectos Nutricionais na Qualidade de Vida, 1st ed.; Edifs: Aracajú, Brazil, 2018; pp. 24–37. Available online: https://www.ifs.edu.br/images/EDIFS/ebooks/2019/E-book_-_alimentos_funcionais.pdf (accessed on 15 September 2022).
- Oliveira, R.S.; Lucas, C.P.; Antonucci, G.; Silva, F.C. Compostos bioativos naturais: Agentes promissores na redução do extresse oxidativo e processos inflamatórios. S. Am. J. Basic Educ. Tech. Technol. 2018, 5, 273–285. [Google Scholar]
- Ministério da Saúde, Brasil; Agência Nacional de Vigilância Sanitária. Alimentos com Alegações de Propriedades Funcionais e/ou de Saúde. 2016. Available online: http://portal.anvisa.gov.br/alimentos/alegacoes (accessed on 15 September 2022).
- Oliveira, J.L.; Almeida, C.; Silva, B.N. A importância do uso de probióticos na saúde humana. Unoesc Ciência-ACBS 2017, 8, 7–12. [Google Scholar]
- Monsalve, B.; Concha-Meyer, A.; Palomo, I.; Fuentes, E. Mechanisms of Endothelial Protection by Natural Bioactive Compounds from Fruit and Vegetables. An. Acad. Bras. Ciênc. 2017, 89, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Schiassi, M.C.E.V.; Souza, V.R.; Lago, A.M.T.; Campos, L.G.; Queiroz, F. Fruits from the Brazilian Cerrado region: Physico-chemical characterization, bioactive compounds, antioxidant activities, and sensory evaluation. Food Chem. 2018, 245, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Giuntini, E.B.I. Alimentos Funcionais, 1st ed.; Distribuidora Educacional, S.A.: Londrina, Brazil, 2018; pp. 7–100. Available online: https://cm-kls-content.s3.amazonaws.com/201801/INTERATIVAS_2_0/ALIMENTOS_FUNCIONAIS/U1/LIVRO_UNICO.pdf (accessed on 15 September 2022).
- Bento-silva, A.; Koistinen, V.M.; Mena, P.; Bronze, M.R.; Hanhineva, K.; SAhlstrøm, S.; Kitrytė, V.; Moco, S.; Aura, A.M. Factors affecting intake, metabolism and health benefits of phenolic acids: Do we understand individual variability? Eur. J. Nutr. 2019, 59, 1275–1293. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, W. The delivery of sensitive food bioactive ingredients: Absorption mechanisms, influencing factors, encapsulation techniques and evaluation models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef]
- Morand, C.; Tomás-Barberán, F.A. Contribution of plant food bioactives in promoting health effects of plant foods: Why look at interindividual variability? Eur. J. Nutr. 2019, 58, 13–19. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Ortega, A.M.M.; Campos, M.R.S. Bioactive Compounds as Therapeutic Alternatives. In Bioactive Compounds, Health Benefits and Potential Applications, 1st ed.; Campos, M.R.S., Ed.; Woodhead Publishing: Cambridge, UK, 2019; pp. 247–264. [Google Scholar] [CrossRef]
- Abreu, L.F.; Cardoso, T.N.; Dantas, K.G.F.; De Oliveira, M.S.P. Prospecção e quantificação de carotenoides em frutos de tucumã-do-Pará. Bol. Pesqui. Desenvolv. 2020, 39, 5–21. [Google Scholar]
- Hashimoto, H.; Uragami, C.; Cogdell, R.J. Carotenoids and photosynthesis. Subcell. Biochem. 2016, 79, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B.A.; Kimura, M.; Amaya-Farfan, J. Fontes Brasileiras de Carotenoides: Tabela Brasileira de Composição de Carotenoides em Alimentos. Brasília, DF: MMA/SBF. Available online: http://www.mma.gov.br/estruturas/sbf_agrobio/_publicacao/89_publicacao09032009113306.pdf (accessed on 16 June 2022).
- Milanez, J.T.; Neves, L.C.; Colombo, R.C.; Shahab, M.; Roberto, S.R. Bioactive compounds and antioxidant activity of buriti fruits, during the postharvest, harvested at different ripening stages. Sci. Hortic. 2018, 227, 10–21. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Sancho, R.A.S.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian wild fruits: Nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.N.M.; Arruda, H.S.; Marques, D.R.P.; de Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and Nutritional Properties of Selected Amazon Fruits: A Review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.; Oliveira, T.; Mattietto, R.; Nascimento, W.; Lopes, A. Bioactive compounds in the peel of camu-camu genotypes from Embrapa’s active germplasm bank. Food Sci. Technol. 2018, 38, 67–71. [Google Scholar] [CrossRef]
- Anunciação, P.C.; Giuffrida, D.; Murador, D.C.; Paula Filho, G.F.; Dugo, G.; Pinheiro-Sant’Ana, H.M. Identification and quantification of the native carotenoid composition in fruits from the Brazilian Amazon by HPLC–DAD–APCI/MS. J. Food Compos. Anal. 2019, 83, 103296. [Google Scholar] [CrossRef]
- Belisário, C.M.; Soares, A.G.; Coneglian, R.C.C.; Plácido, G.R.; Castro, C.F.D.S.; Rodrigues, L.A.N. Carotenoids, sugars, ascorbic acid, total phenolics, and antioxidant activity of murici from Brazilian Cerrado during refrigerated storage. Cienc. Rural 2020, 50, e20180620. [Google Scholar] [CrossRef]
- Otero, D.; Antunes, B.; Bohmer, B.; Jansen, C.; Crizel, M.; Lorini, A.; Krumreich, F.; Zambiazi, R.C. Bioactive compounds in fruits from different regions of Brazil. Rev. Chil. Nutr. 2020, 47, 31–40. [Google Scholar] [CrossRef]
- Assis, R.C.; Soares, R.D.L.G.; Siqueira, A.C.P.; de Rosso, V.V.; de Sousa, P.H.M.; Mendes, A.E.P.; Costa, E.A.; Carneiro, A.P.G.; Maia, C.S.C. Determination of water-soluble vitamins and carotenoids in Brazilian tropical fruits by High Performance Liquid Chromatography. Heliyon 2020, 6, e05307. [Google Scholar] [CrossRef]
- Matos, K.A.N.; Lima, D.P.; Barbosa, A.P.P.; Mercadante, A.Z.; Chisté, R.C. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2018, 272, 216–221. [Google Scholar] [CrossRef]
- Bernardina, R.G.D.; Holtz, S.G.; Pretti, I.R.; Oliveira, D.B.; Cruz, L.L. Aproveitamento tecnológico do araçá-boi (Eugenia stipitata) como farinha para a alimentação. In Tecnologia de Alimentos: Tópicos Físicos, Químicos e Biológicos, 3rd ed.; Cordeiro, C.A.M., Ed.; Editora Científica Digital: São Paulo, Brazil, 2020; pp. 54–62. [Google Scholar] [CrossRef]
- Santos, O.V.; Viana, A.A.; Soares, S.D.; Vieira, E.L.S.; Martins, M.G.; Nascimento, F.C.A.; Teixeira-Costa, B.E. Industrial potential of Bacaba (Oenocarpus bacaba) in powder: Antioxidant activity, spectroscopic and morphological behavior. Food Sci. Technol. 2021, 42, e62820. [Google Scholar] [CrossRef]
- Resende, L.M.; Franca, A.S.; Oliveira, L.S. Buriti (Mauritia flexuosa L. f.) fruit by-products flours: Evaluation as source of dietary fibers and natural antioxidants. Food Chem. 2019, 270, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.A.; Oliveira, T.T.S.; Santos, J.G.S.; Gonçalves, M.R.; Gaspar, R.C.; Vieira, V.A.; Rodrigues, E.C.; Nascimento, E.; Faria, P.B.; Faria, R.A.P.G. Fatty acid profile and physicochemical characterization of buriti oil during storage. Cienc. Rural 2020, 50, e20190997. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; Hornung, P.S.; Ávila, S.; Da Silva Bambirra Alves, F.E.; Beta, T.; Ribani, R.H. Ripe and unripe inajá (Maximilia maripa) fruit: A new high source of added value bioactive compounds. Food Chem. 2020, 331, 127333. [Google Scholar] [CrossRef] [PubMed]
- Barbi, R.C.T.; Souza, A.R.C.; Hamerski, F.; Teixeira, G.L.; Corazza, M.L.; Ribani, R.H. Subcritical propane extraction of high-quality inajá (Maximiliana maripa) pulp oil. J. Supercrit. Fluids 2019, 153, 104576. [Google Scholar] [CrossRef]
- Santos, O.V.; Soares, S.D.; Dias, P.C.S.; Duarte, S.P.A.; Santos, M.P.L.; Nascimento, F.C.A. Chromatographic profile and bioactive compounds found in the composition of pupunha oil (Bactris gasipaes Kunth): Implications for human health. Rev. Nutr. 2020, 33, e190146. [Google Scholar] [CrossRef]
- Leão, D.P.; Franca, A.S.; Oliveira, L.S.; Bastos, R.; Coimbra, M.A. Physicochemical characterization, antioxidant capacity, total phenolic and proanthocyanidin content of flours prepared from pequi (Caryocar brasilense Camb.) fruit by-products. Food Chem. 2017, 225, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Costa, A.P.S.; Crispino, A.C.S.; Silva, A.P.R.; Oliveira, J.A.R. Physicochemical characterization, bioactive compounds and antioxidant activity of pulp, peel, endocarp and food paste developed with buriti pulp and waste (Mauritia flexuosa L.). Sci. Plena 2020, 16, 111501. [Google Scholar] [CrossRef]
- Silva, C.M.; Zanqui, A.B.; Visentainer, J.V.; Cardozo-Filho, L.; Bittencourt, P.R.S.; Morais, D.R.; Santos, J.M.; Eberlin, M.N.; Matsushita, M. Quality and composition of three palm oils isolated by clean and sustainable process. J. Clean. Prod. 2020, 259, 120905. [Google Scholar] [CrossRef]
- Amorim-Carrilho, K.T.; Celeda, A.; Gente, C.; Regal, P. Review of methods for analysis of carotenoids. Trends Anal. Chem. 2014, 56, 49–73. [Google Scholar] [CrossRef]
- Speranza, P.; Falcão, A.O.; Macedo, J.A.; Silva, L.H.M.; Rodrigues, A.M.C.; Macedo, G.A. Amazonian Buriti oil: Chemical characterization and antioxidant potential. Grasas Aceites 2016, 67, e135. [Google Scholar] [CrossRef]
- Cuco, R.P.; Massa, T.B.; Postaue, N.; Cardozo-Filho, L.; Silva, C. Oil extraction from structured bed of pumpkin seeds and peel using compressed propane as solvente. J. Supercrit. Fluids 2019, 152, 104568. [Google Scholar] [CrossRef]
- Barros, R.G.C.; Andrade, J.K.S.; Denadai, M.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity in some Brazilian exotic fruit residues. Food Res. Int. 2017, 102, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Amariz, A.; Lima, M.A.C.; Alves, R.E. Quality and antioxidant potential of byproducts from refining of fruit pulp. Food Sci. Technol. 2018, 38, 203–209. [Google Scholar] [CrossRef]
- Morais, R.A.; Melo, K.K.S.; Oliveira, T.T.B.; Teles, J.S.; Peluzio, J.M.; Martins, G.A.S. Caracterização Química, física e tecnológia da farinha obtida a partir da casca de Buriti (Mauritia flexuosa L. f.). Braz. J. Dev. 2019, 5, 23307–23322. [Google Scholar] [CrossRef]
- Martínez-Girón, J.; Rodríguez-Rodríguez, X.; Pinzón-Zárate, L.X.; Ordóñez-Santos, L.E. Caracterización fisicoquímica de harina de residuos del fruto de chontaduro (Bactris gasipaes Kunth, Arecaceae) obtenida por secado convectivo. Cienc. Tecnol. Agropecu. 2017, 18, 599–613. [Google Scholar] [CrossRef]
| Bioactive Substance | Active Compounds | Biological Functions * | Foods |
|---|---|---|---|
| Phytochemicals | Phenolic compounds | Antioxidant activity; Anti-inflammatory; Contribute to balance and adequacy of intestinal functioning; Can help to reduce the absorption of fat and cholesterol. | Citrus fruits (lemon, orange and tangerine), in addition to other fruits such as cherry, grape, plum, pear, apple and papaya and vegetables (broccoli, red cabbage, onion, garlic and tomato), cereals, teas, coffee, cocoa, wine. |
| Alkaloids | Acts mainly on the nervous system, whether central or autonomic. | Vegetable alkaloids are the main sources | |
| Organosulfur compounds | Prevention of cardiovascular diseases and the reduction in blood pressure, serum lipid level, blood glucose and oxidative stress. | Garlic, onion, chestnuts and walnuts. | |
| Carotenoids | Contribute to the body’s defenses, as they have antioxidant action, in addition to being responsible for the synthesis of vitamins, being related to reducing the risk of macular degeneration, cataracts and chronic diseases. | Dark green leafy vegetables (spinach), vegetables (carrots, peppers, lettuce, broccoli, among others) and tropical fruits, such as peach palm, tucumã (Astrocaryum vulgare), mango, taperebá (Spondias mombin), murici (Byrsonima crassifolia), guava, among others. | |
| Phytosterols | Help to reduce the absorption of cholesterol. | Vegetable oils (soybean and sunflower), fruits, seeds, leaves and stems. | |
| Probiotics | Bifidobacteria and Lactobacilli | Improve balance of the intestinal microbiota; Benefits in the treatment of gastrointestinal diseases; Stimulation of the immune system. | Yogurts, fermented dairy products, kefir, kombucha and food supplements. |
| Prebiotics | Fibers, oligosaccharides, fructooligosaccharides and inulin | Collaborate for balance and adequacy function of intestines; Help to reduce the absorption of fat and cholesterol. | Fruits, oats, vegetables (chicory root and yacon potatoes), whole grains, tubers and bulbs, honey and brown sugar. |
| Polyunsaturated fatty acids | Omega 3 Omega 6 | Reduction in LDL-cholesterol; Anti-inflammatory action; Indispensable for the development of brain and retina of newborns. Helps to adjust the triglyceride’ levels. | Vegetable oil (soybean, canola, wheat germ, flaxseed), nuts and marine fish (sardines, salmon, tuna, anchovies, herring). |
| Antioxidant vitamins | A | Antioxidant activity; Important in cell growth and differentiation; Preventive action in the development of tumors. | Animal products (liver, milk, eggs, butter, cheese and fish). |
| C | Antioxidant activity; Decreased risk for certain types of cancer, cardiovascular disease and cataracts, as well as wound healing and immune modulation. | Fruits (orange, lemon, acerola, strawberry) and vegetables (broccoli, cabbage and spinach). | |
| E | Antioxidant activity; Anti-inflammatory; Adequacy of triglyceride levels. | Vegetable oils, wheat germ, oilseeds, dark green leafy vegetables and animal foods (egg yolks and liver). | |
| Isoflavones and soy protein | Bioactive peptides | Hormonal regulation; Antioxidant activity; Cholesterol reduction. | Soy and derivatives |
| Popular Name | Scientific Name | Carotenoids Profile | Health Benefits | References |
|---|---|---|---|---|
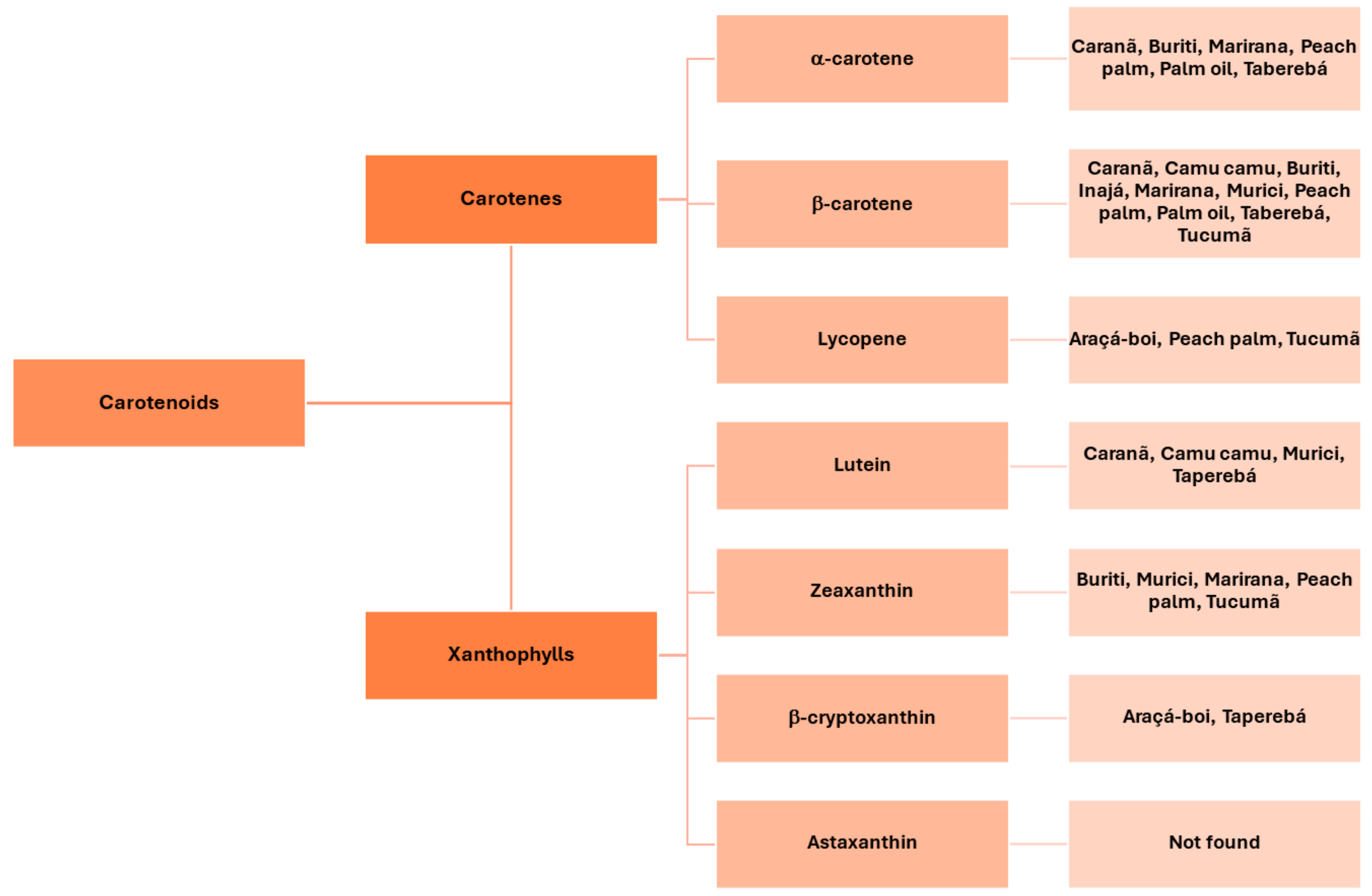
Araçá-boi | Eugenia stipitata | Lycopenes and cryptoxanthin | Antioxidant properties, anti-inflammatory and antidiabetic. | Araújo et al. (2019) [43] |
Buriti | Mauritia flexuosa Linn. F | β-carotene | Reduce the incidence of xerophthalmia; Reducing the risk of developing cardiovascular disease. | Milanez et al. (2018) [41]; Neri-Numa et al. (2018) [42] |
Camu-camu | Myrciaria dubia | Trans-lutein and β-carotene | Antioxidant capacity. | Souza et al. (2018) [44] |
Caranã | Mauritiella armata | Cis-β-carotene, trans -β-carotene,trans -α-carotene and trans-lutein | Lutein and zeaxanthin play an important role in reducing eye disorders due to their antioxidant, anti-inflammatory properties and ability to filter blue light. | Anunciação et al. (2019) [45] |
Inajá | Attalea maripa | Trans -β-carotene | Antioxidant role, protects the cell body against the excess free radicals. | Anunciação et al. (2019) [45] |
Murici | Byrsonima crassifolia | Lutein, zeaxanthin and β-carotene | High antioxidant potential; Inhibition of various degenerative processes; Source of Vitamin A. | Belisário et al. (2020) [46] |
Marirana | Couepia subcordata Benth | Trans -α-carotene, trans-β-carotene and zeaxanthin | May reduce the risk of macular degeneration and cataract formation. | Anunciação et al. (2019) [45] |
Peach palm | Bactris gasipaes | Zeaxanthin, α-carotene, β-carotene and lycopene. | Antioxidants, protecting the body against chronic diseases and certain cancers, macular degeneration, cataracts, neurological disorders, gastric anti-ulcer activity and strengthening the immune system. | Otero et al. (2020) [47] |
Taperebá | Spondias mombin | β-cryptoxanthin, α-cryptoxanthin, lutein, trans-α-carotene, cis-α-carotene and trans-β-carotene. | Strong potential in neurocognitive function together with a healthy lifestyle to promote brain health. | Assis et al. (2020) [48] |
Tucumã | Astrocaryum vulgare | β-carotene, γ-carotene and δ-carotene | Source of pro-vitamin A. | Matos et al. (2019) [49] |
| Popular Names | Scientific Names | Products | Methods | Total Carotenoids (μg/100 g) | References |
|---|---|---|---|---|---|
| Araçá-boi | Eugenia stipitata | Flour | Kiln-drying | 12.000 | Bernardina et al. (2020) [50] |
| Bacaba | Oenocarpus bacaba | Oil from pulp flour | Drying in freeze dryer | 1068.30 | Santos et al. (2021) [51] |
| Oil from pulp flour | Kiln-drying | 908.17 | Santos et al. (2021) [51] | ||
| Buriti | Mauritia flexuosa Linn. F | Defatted flour | Kiln-drying and Soxhlet (hexane) | Not detected | Resende, Franca, and Oliveira (2019) [52] |
| Oil | Not detected | 103.696 | Mesquita et al. (2020) [53] | ||
| Inajá | Maximiliana maripa | Defatted flour (mature) | Drying in freeze dryer | 125.110 | Barbi et al. (2020) [54] |
| Defatted flour (green) | Drying in freeze dryer | 42.290 | Barbi et al. (2020) [54] | ||
| Oil from pulp flour (ripe) | Soxhlet (ethanol) | 96.980 | Barbi et al. (2020) [54] | ||
| Oil from pulp flour (green) | Soxhlet (ethanol) | 76.210 | Barbi et al. (2020) [54] | ||
| Oil | Subcritical (propane) | 140.990 | Barbi et al. (2019) [55] | ||
| Peach palm | Bactris gasipaes Kunth | Oil | Soxhlet (Petroleum ether) | 832.4 | Santos et al. (2020) [56] |
| Popular Name | Scientific Name | Part of the Fruit | Carotenoids Profile | Total Carotenoids (μg 100 g−1) | References |
|---|---|---|---|---|---|
| Achachairu | Garcinia humilis | Seeds, peel and a very small amount of pulp | β-carotene | 932 | Barros et al. (2017) [63] |
| Araçá-boi | Eugenia stipitata | Seeds, peel and a very small amount of pulp | β-carotene | 3.339 | Barros et al. (2017) [63] |
| Bacaba | Oenocarpus bacaba | Seeds, peel and a very small amount of pulp | β-carotene | 1.547 | Barros et al. (2017) [63] |
| Buriti | Mauritia flexuosa Linn. F | Peel (epicarp) | β-carotene | 21.030 | Cardoso et al. (2020) [58] |
| Bleached peel (epicarp) | β-carotene | 1040.1 | Resende et al. (2019) [52] | ||
| Endocarp | β-carotene | 6.050 | Cardoso et al. (2020) [58] | ||
| Bleached endocarp | β-carotene | 150.5 | Resende et al. (2019) [50] | ||
| Camu-camu | Myrciaria dubia | Peel | Trans-luteine β-carotene | 10.588 | Souza et al. (2018) [44] |
| Cupuaçu | Theobroma grandiflorum | Fibrous material from pulp and seeds | β-carotene γ-carotene and δ-carotene | 620 | Amariz et al. (2018) [64] |
| Peach palm | Bactris gasipaes | Peel | 33.690 | Matos et al. (2019) [49] | |
| Taperebá | Spondias mombin | Fibrous material from pulp and seeds | Not determined | 7.000 | Amariz et al. (2018) [64] |
| Tucumã | Astrocaryum vulgare | Peel | β-carotene γ-carotene and δ-carotene | 18.060 | Matos et al. (2019) [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, O.V.; do Rosário, R.C.; Teixeira-Costa, B.E. Sources of Carotenoids in Amazonian Fruits. Molecules 2024, 29, 2190. https://doi.org/10.3390/molecules29102190
dos Santos OV, do Rosário RC, Teixeira-Costa BE. Sources of Carotenoids in Amazonian Fruits. Molecules. 2024; 29(10):2190. https://doi.org/10.3390/molecules29102190
Chicago/Turabian Styledos Santos, Orquidea Vasconcelos, Rosely Carvalho do Rosário, and Barbara E. Teixeira-Costa. 2024. "Sources of Carotenoids in Amazonian Fruits" Molecules 29, no. 10: 2190. https://doi.org/10.3390/molecules29102190
APA Styledos Santos, O. V., do Rosário, R. C., & Teixeira-Costa, B. E. (2024). Sources of Carotenoids in Amazonian Fruits. Molecules, 29(10), 2190. https://doi.org/10.3390/molecules29102190







