Enhanced Production of Erythritol from Glucose by the Newly Obtained UV Mutant Yarrowia lipolytica K1UV15
Abstract
1. Introduction
2. Results and Discussion
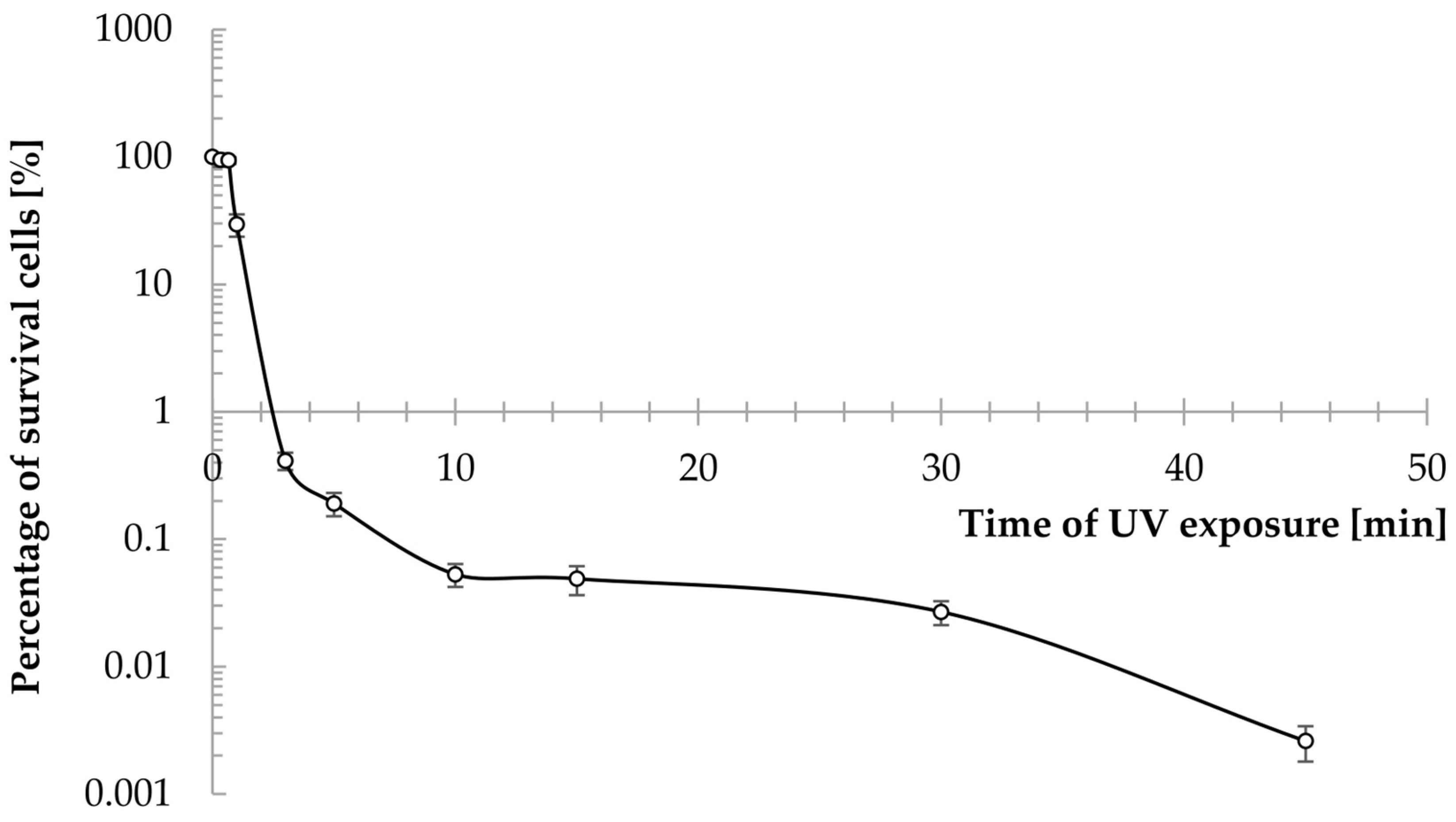
2.1. UV Mutagenesis and Preliminary Characterization of New Strains
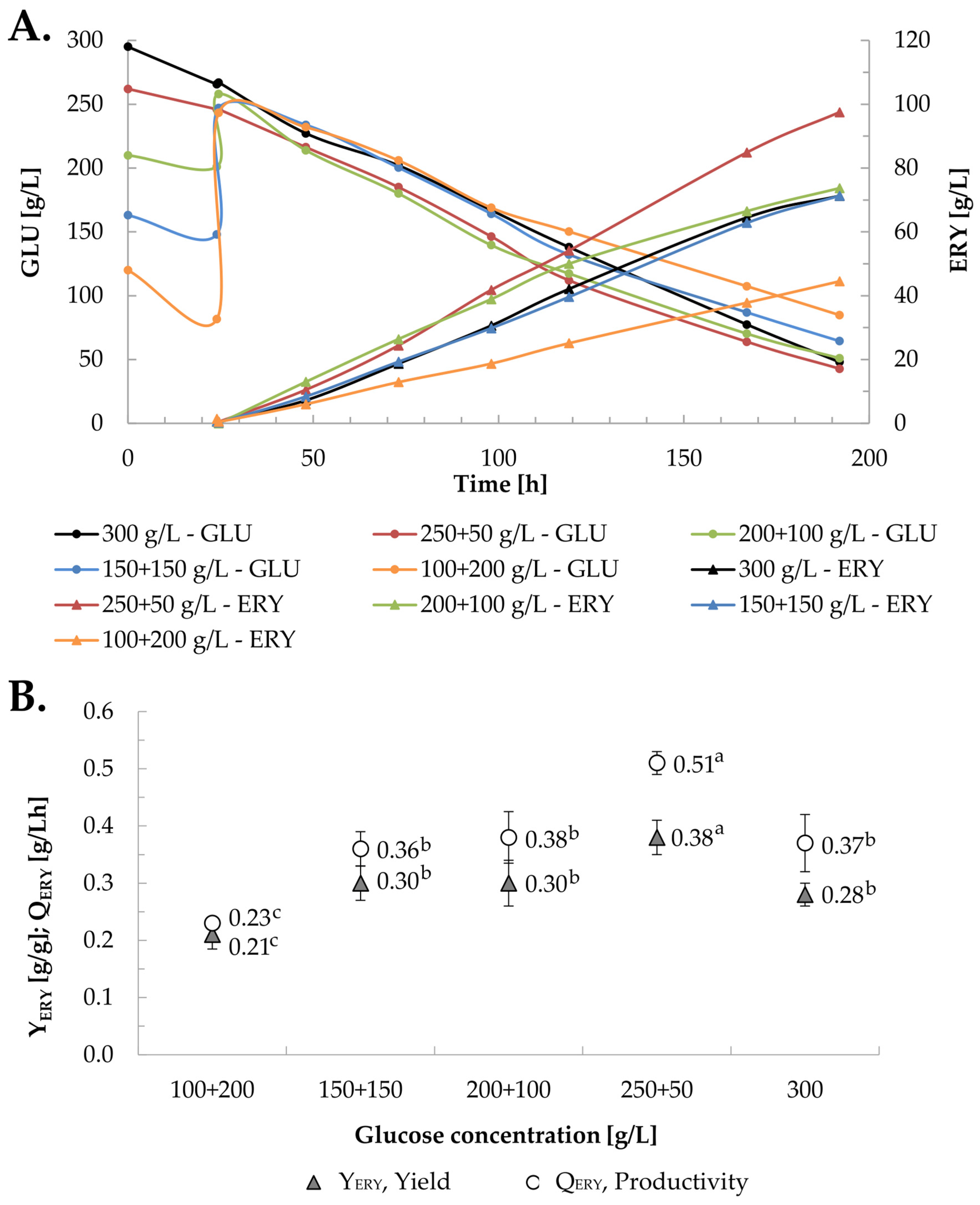
2.2. Selection of the Initial Glucose Concentration for Erythritol Biosynthesis
2.3. Erythritol Production Using UV Mutants
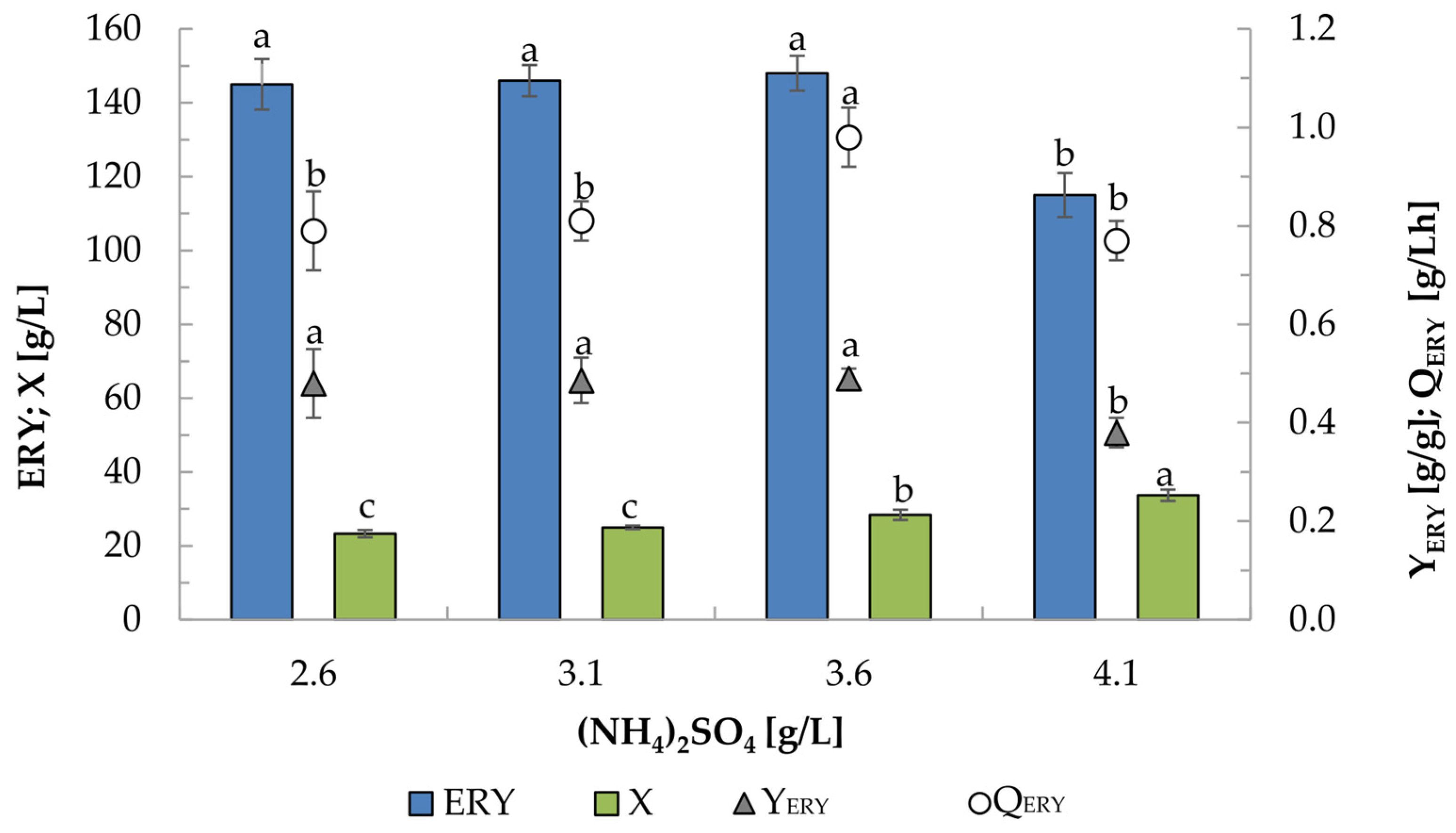
2.4. Effect of (NH4)2SO4 Concentration on Erythritol Production by K1UV15 Strain
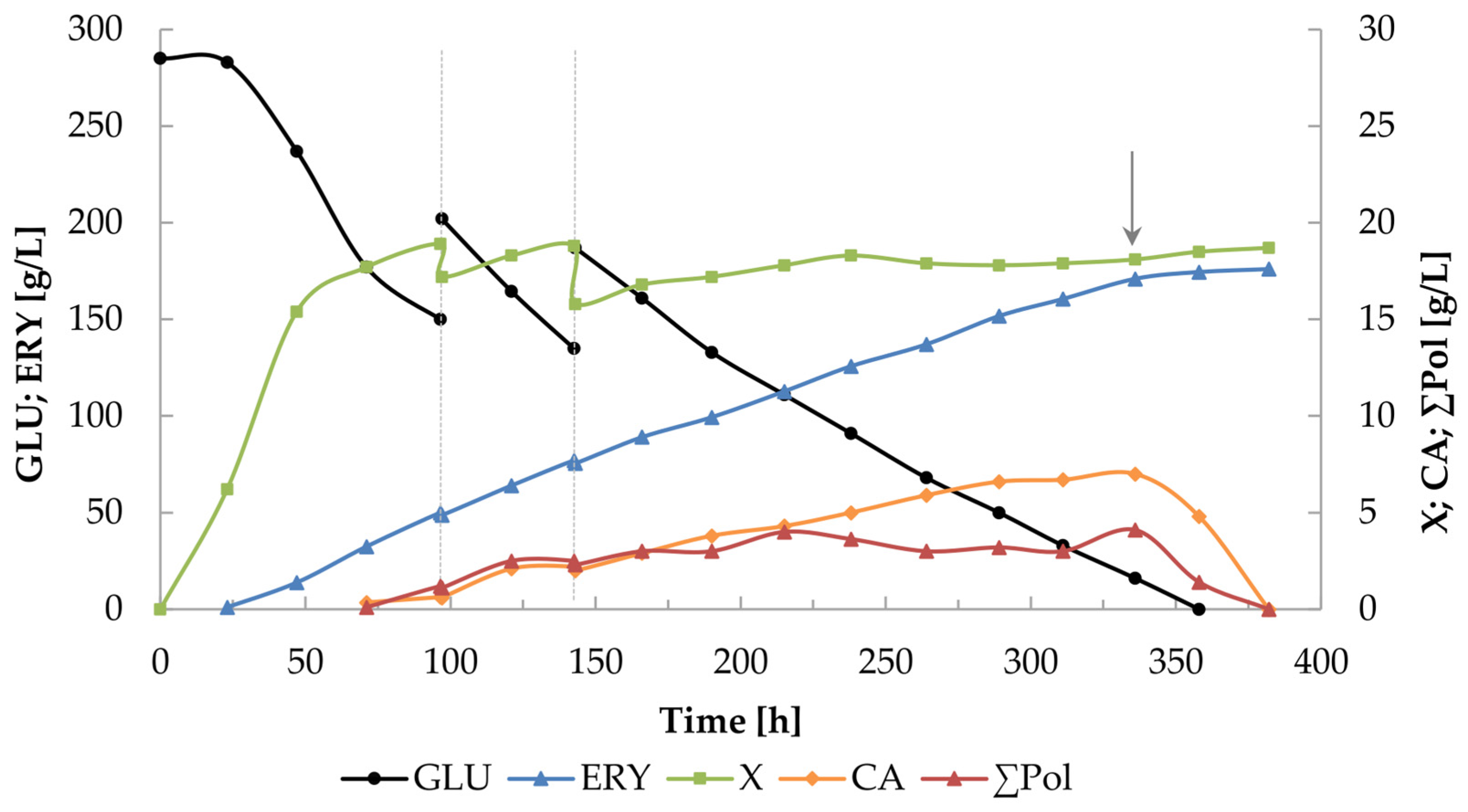
2.5. Intensification of the Erythritol Production Process Using the K1UV15 Strain
3. Materials and Methods
3.1. Microorganisms
3.2. Procedure of Mutagenization
3.3. Media
3.4. Culture Conditions
3.5. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, H.J.; Jeya, M.; Kim, I.W.; Lee, J.K. Biotechnological production of erythritol and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.; Zannini, E.; Arendt, E.K.; Coffey, A. A review of polyols—Biotechnological production, food applications, regulation, labeling and health effects. Crit. Rev. Food Sci. Nutr. 2020, 60, 2034–2051. [Google Scholar] [CrossRef] [PubMed]
- Wölnerhanssen, B.K.; Meyer-Gerspach, A.C.; Beglinger, C.; Islam, M.S. Metabolic effects of the natural sweeteners xylitol and erythritol: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1986–1998. [Google Scholar] [CrossRef] [PubMed]
- Mazi, T.A.; Stanhope, K.L. Elevated erythritol: A marker of metabolic dysregulation or contributor to the pathogenesis of cardio metabolic disease? Nutrients 2023, 15, 4011. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Bernt, W.O.; Borzelleca, J.F.; Flamm, G.; Lynch, B.S.; Kennepohl, E.; Modderman, J. Erythritol: An interpretive summary of biochemical, metabolic, toxicological and clinical data. Food Chem. Toxicol. 1998, 36, 1139–1174. [Google Scholar] [CrossRef] [PubMed]
- Bordier, V.; Teysseire, F.; Drewe, J.; Madörin, P.; Bieri, O.; Schmidt-Trucksäss, A.; Hanssen, H.; Beglinger, C.; Meyer-Gerspach, A.C.; Wölnerhanssen, B.K. Effects of a 5-week intake of erythritol and xylitol on vascular function, abdominal fat and glucose tolerance in humans with obesity: A pilot trial. BMJ Nutr. Prev. Health 2023, e000764. [Google Scholar] [CrossRef] [PubMed]
- BeMiller, J.N. Carbohydrate reactions. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; BeMiller, J.N., Ed.; AACC International Press, Elsevier: Amsterdam, The Netherlands, 2019; Volume 2, pp. 25–48. [Google Scholar]
- Mäkinen, K.K.; Isotupa, K.P.; Mäkinen, P.L.; Söderling, E. Six-month polyol chewing-gum programme in kindergarten-age children: A feasibility study focusing on mutans streptococci and dental plaque. Int. Dent. J. 2005, 55, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rzechonek, D.; Dobrowolski, A.; Rymowicz, W.; Mirończuk, A. Recent advances in biological production of erythritol. Crit. Rev. Biotechnol. 2018, 38, 620–633. [Google Scholar] [CrossRef] [PubMed]
- EUR-Lex. COMMISSION REGULATION (EU) 2015/1832 of 12 October 2015. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32015R1832 (accessed on 25 April 2024).
- Bernt, W.O.; Borzelleca, J.F.; Lamm, G.F.; Munro, I.C. Erythritol: A review of biological and toxicological studies. Regul. Toxicol. Pharmacol. 1996, 24, 191–197. [Google Scholar] [CrossRef]
- Ibrahim, O. Erythritol chemical structure, biosynthesis pathways, properties, applications and production. Int. J. Microbiol. Biotechnol. 2021, 6, 59–70. [Google Scholar] [CrossRef]
- Future Market Insights. Erythritol Market Snapshot (2023 to 2033). Available online: https://www.futuremarketinsights.com/reports/erythritol-market (accessed on 25 April 2024).
- Nakagawa, Y.; Kasumi, T.; Ogihara, J.; Tamura, M.; Arai, T.; Tomishige, K. Erythritol: Another C4 platform chemical in biomass refinery. ACS Omega 2020, 5, 2520–2530. [Google Scholar] [CrossRef] [PubMed]
- Khatape, A.B.; Dastager, S.G.; Rangaswamy, V. An overview of erythritol production by yeast strains. FEMS Microbiol. Lett. 2022, 369, 1. [Google Scholar] [CrossRef]
- Krzyczkowska, J.; Fabiszewska, A.U. Yarrowia lipolytica—Non-conventional yeast in biotechnology. Post. Mikrobiol. 2015, 54, 33–43. (In Polish) [Google Scholar]
- Bilal, M.; Xu, S.; Iqbal, H.M.N.; Cheng, H. Yarrowia lipolytica as an emerging biotechnological chassis for functional sugars biosynthesis. Crit. Rev. Food Sci. Nutr. 2021, 61, 535–552. [Google Scholar] [CrossRef]
- Diamantopoulou, P.; Papanikolaou, S. Biotechnological production of sugar-alcohols: Focus on Yarrowia lipolytica and edible/medicinal mushrooms. Process Biochem. 2023, 124, 113–131. [Google Scholar] [CrossRef]
- Deshpande, M.S.; Kulkarni, P.P.; Kumbhar, P.S.; Ghosalkar, A.R. Erythritol production from sugar based feedstocks by Moniliella pollinis using lysate of recycled cells as nutrients source. Process Biochem. 2022, 112, 45–52. [Google Scholar] [CrossRef]
- Rymowicz, W.; Rywińska, A.; Marcinkiewicz, M. High-yield production of erythritol from raw glycerol in fed-batch cultures of Yarrowia lipolytica. Biotechnol. Lett. 2009, 31, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Ruy, A.D.d.S.; Ferreira, A.L.F.; Bresciani, A.E.; Alves, R.M.d.B.; Pontes, L.A.M. Market Prospecting and Assessment of the Economic Potential of Glycerol from Biodiesel. In Biotechnological Applications of Biomass; Basso, T.P., Basso, T.O., Basso, L.C., Eds.; IntechOpen Limited: London, UK, 2021; Volume 11. [Google Scholar] [CrossRef]
- Kenar, J.A. Glycerol as a platform chemical: Sweet opportunities on the horizon? Lipid Technol. 2007, 19, 249–253. [Google Scholar] [CrossRef]
- Global Market Insights. Available online: https://www.gminsights.com/industry-analysis/industrial-glucose-market (accessed on 25 April 2024).
- Karamerou, E.E.; Parsons, S.; Marcelle, C.; McManus, M.M.; Chuck, J.C. Using techno-economic modelling to determine the minimum cost possible for a microbial palm oil substitute. Biotechnol. Biofuels 2021, 14, 57. [Google Scholar] [CrossRef]
- Humbird, D. Scale-up economics for cultured meat. Biotechnol. Bioeng. 2021, 118, 3239–3250. [Google Scholar] [CrossRef]
- Ferreira, R.D.G.; Azzoni, A.R.; Freitas, S. Techno-economic analysis of the industrial production of a low-cost enzyme using E. coli: The case of recombinant β-glucosidase. Biotechnol. Biofuels 2018, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Rakicka, M.; Lazar, Z.; Rywińska, A.; Rymowicz, W. Efficient utilization of inulin and glycerol as fermentation substrates in erythritol and citric acid production using Yarrowia lipolytica expressing inulinase. Chem. Pap. 2016, 70, 1452–1459. [Google Scholar] [CrossRef]
- Wang, N.; Chi, P.; Zou, Y.; Xu, Y.; Xu, S.; Bilal, M.; Fickers, P.; Cheng, H. Metabolic engineering of Yarrowia lipolytica for thermoresistance and enhanced erythritol productivity. Biotechnol. Biofuels 2020, 13, 176. [Google Scholar]
- Zhang, Y.; Zhang, X.; Xu, Y.; Xu, S.; Bilal, M.; Cheng, H. Engineering thermotolerant Yarrowia lipolytica for sustainable biosynthesis of mannitol and fructooligosaccharides. Biochem. Eng. J. 2022, 187, 108604. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Kong, H.; Wang, Z. Screening of ultraviolet-induced thermotolerant yeast mutants and their performance. Fermentation 2023, 9, 608. [Google Scholar] [CrossRef]
- Wan, Z.; Hu, H.; Liu, K.; Qiao, Y.; Guo, F.; Wang, C.; Xin, F.; Zhang, W.; Jiang, M. Engineering industrial yeast for improved tolerance and robustness. Crit. Rev. Biotechnol. 2024, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Rywińska, A.; Bąk, M.; Rakicka, M.; Tomaszewska, L.; Boruczkowski, T.; Lazar, Z.; Musiał, I.; Rymowicz, W. Selection of the UV mutants of Yarrowia lipolytica yeast for erythritol biosynthesis from glycerol. Acta Sci. Pol. Biotechnol. 2012, 11, 23–38. (In Polish) [Google Scholar]
- Ghezelbash, G.R.; Nahvi, I.; Malekpor, A. Erythritol production with minimum By-product using Candida magnoliae mutant. Appl. Biochem. Microbiol. 2014, 50, 292–296. [Google Scholar] [CrossRef]
- Mirończuk, A.M.; Dobrowolski, A.; Rakicka, M.; Rywińska, A.; Rymowicz, W. Newly isolated mutant of Yarrowia lipolytica MK1 as a proper host for efficient erythritol biosynthesis from glycerol. Process Biochem. 2015, 50, 61–68. [Google Scholar] [CrossRef]
- Rywińska, A.; Wojtatowicz, M.; Wielebińska, A. Obtaining of FIL- mutants in Yarrowia lipolytica yeasts for citric acid production. Acta Sci. Pol. Biotechnol. 2003, 2, 11–20. (In Polish) [Google Scholar]
- Rywińska, A.; Rymowicz, W.; Żarowska, B.; Skrzypinski, A. Comparison of citric acid production from glycerol and glucose by different strains of Yarrowia lipolytica. World J. Microbiol. Biotechnol. 2010, 26, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, L.; Rywińska, A.; Rymowicz, W. High selectivity of erythritol production from glycerol by Yarrowia lipolytica. Biomass Bioenergy 2014, 64, 309–320. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Rywińska, A. Erythritol biosynthesis from glycerol by Yarrowia lipolytica yeast: Effect of osmotic pressure. Chem. Pap. 2016, 70, 272–283. [Google Scholar] [CrossRef]
- Tomaszewska-Hetman, L.; Cybulski, K.; Gryszkin, M.; Rymowicz, W.; Rywińska, A. Erythritol biosynthesis from glycerol by Yarrowia lipolytica in the presence of different salts. Chem. Eng. Equip. 2015, 54, 123–125. (In Polish) [Google Scholar]
- Tomaszewska, L.; Rakicka, M.; Rymowicz, W.; Rywińska, A. A comparative study on glycerol metabolism to erythritol and citric acid in Yarrowia lipolytica yeast cells. FEMS Yeast Res. 2014, 14, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Xiaoyan, L.; Jinshun, L.; Jiaxing, X.; Xia, J.; He, A.; Zhang, T.; Xiangqian, L.; Xu, J. Effects of osmotic pressure and pH on citric acid and erythritol production from waste cooking oil by Yarrowia lipolytica. Eng. Life Sci. 2018, 18, 344–352. [Google Scholar]
- Rywińska, A.; Marcinkiewicz, M.; Cibic, E.; Rymowicz, W. Optimization of medium composition for erythritol production from glycerol by Yarrowia lipolytica using Response Surface Methodology. Prep. Biochem. Biotechnol. 2015, 45, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Jeya, M.; Lee, K.M.; Tiwari, M.K.; Kim, J.S.; Gunasekaran, P.; Kim, S.Y.; Lee, J.K. Isolation of a novel high erythritol-producing Pseudozyma tsukubaensis and scale-up of erythritol fermentation to industrial level. Appl. Microbiol. Biotechnol. 2009, 83, 225–231. [Google Scholar] [CrossRef]
- Suwanapetch, C.; Vanichsriratana, W. Media optimization for erythritol production by Moniliella sp. BCC25224. Sugar Tech. 2023, 25, 257–261. [Google Scholar] [CrossRef]
- Saran, S.; Mukherjee, S.; Dalal, J.; Saxena, R.K. High production of erythritol from Candida sorbosivorans SSE-24 and its inhibitory effect on biofilm formation of Streptococcus mutans. Bioresour. Technol. 2015, 198, 31–38. [Google Scholar] [CrossRef]
- Lin, S.J.; Wen, C.Y.; Liau, J.C.; Chu, W.S. Screening and production of erythritol by newly isolated osmophilic yeast-like fungi. Process Biochem. 2001, 36, 1249–1258. [Google Scholar] [CrossRef]
- Burschapers, J.D.; Schustolla, D.K.; Schügerl, K.; Röper, H.; de Troostembergh, J.C. Engineering aspects of the production of sugar alcohols with the osmophilic yeast Moniliella tomentosa var pollinis. Part I. Batch and fed-batch operation in stirred tank. Process Biochem. 2002, 38, 497–506. [Google Scholar] [CrossRef]
- Ryu, Y.W.; Park, C.Y.; Park, J.B.; Kim, S.Y.; Seo, J.H. Optimization of erythritol production by Candida magnoliae in fed-batch culture. J. Ind. Microbiol. Biotechnol. 2000, 25, 100–103. [Google Scholar] [CrossRef]
- Lin, S.J.; Wen, C.Y.; Wang, P.M.; Huang, J.C.; Wei, C.L.; Chang, J.W.; Chu, W.S. High-level production of erythritol by mutants of osmophilic Moniliella sp. Process Biochem. 2010, 45, 973–979. [Google Scholar] [CrossRef]
- Ishizuka, H.; Wako, K.; Kasumi, T.; Sasaki, T. Breeding of a mutant of Aureobasidium sp. with high erythritol production. J. Ferment. Bioeng. 1989, 68, 310–314. [Google Scholar] [CrossRef]
- Park, J.; Seo, B.; Kim, J.; Park, Y. Production of erythritol in fed-batch cultures of Trichosporon sp. J. Ferment. Bioeng. 1998, 86, 577–580. [Google Scholar] [CrossRef]
- Lee, J.K.; Ha, S.J.; Kim, S.Y.; Oh, D.K. Increased erythritol production in Torula sp. with inositol and phytic acid. Biotechnol. Lett. 2001, 23, 497–500. [Google Scholar] [CrossRef]
- Oh, D.K.; Cho, C.H.; Lee, J.K.; Kim, S.Y. Increased erythritol production in fed-batch culture of Torula sp. by controlling glucose concentration. J. Ind. Microbiol. Biotechnol. 2001, 26, 248–252. [Google Scholar] [CrossRef]

| Strain | Substrate Consumption Rate | Erythritol Production from Glucose | |||
|---|---|---|---|---|---|
| qS(ERY) | qS(GLU) | ERY * | YERY | qERY | |
| [mg/Lh] | [g/Lh] | [g/L] | [g/g] | [mg/gh] | |
| K1UV1 | 0.00 ± 0.00 f ** | 1.022 ± 0.034 a | 24.9 ± 1.3 c | 0.25 ± 0.02 bcd | 31.25 ± 2.55 a |
| K1UV3 | 69.4 ± 6.4 d | 0.881 ± 0.040 c | 22.1 ± 1.4 e | 0.26 ± 0.02 bc | 17.17 ± 1.18 d |
| K1UV11 | 313.9 ± 17.5 a | 0.906 ± 0.046 bc | 23.0 ± 0.6 d | 0.26 ± 0.00 bc | 26.62 ± 4.36 c |
| K1UV15 | no growth | 1.041 ± 0.040 a | 26.5 ± 1.9 b | 0.27 ± 0.02 b | 28.75 ± 2.95 ab |
| K1UV16 | 125.0 ± 14.0 b | 0.893 ± 0.026 bc | 20.3 ± 0.8 f | 0.24 ± 0.02 d | 14.09 ± 0.93 d |
| K1UV17 | 90.3 ± 3.8 c | 0.932 ± 0.020 bc | 26.3 ± 1.8 b | 0.29 ± 0.02 a | 29.14 ± 1.84 a |
| K1UV18 | 34.7 ± 1.8 e | 1.030 ± 0.032 a | 25.3 ± 0.6 c | 0.26 ± 0.00 bc | 25.09 ± 1.88 bc |
| K1UV20 | 95.8 ± 5.6 c | 0.947 ± 0.036 b | 27.4 ± 2.1 a | 0.30 ± 0.02 a | 29.42 ± 1.79 a |
| Wratislavia K1 | 0.00 ± 0.00 f ** | 0.897 ± 0.026 bc | 21.6 ± 0.4 e | 0.25 ± 0.01 cd | 22.95 ± 1.07 c |
| Strain | Time [h] | ERY * [g/L] | YERY [g/g] | QERY [g/Lh] | qERY [g/gh] | S [%] | Foam ** |
|---|---|---|---|---|---|---|---|
| K1UV1 | 196 | 127.0 c | 0.42 ± 0.02 bc | 0.65 ± 0.01 d | 0.026 ± 0.001 d | 83 | + |
| K1UV3 | 196 | 134.6 b | 0.45 ± 0.02 ab | 0.69 ± 0.01 c | 0.036 ± 0.003 b | 84 | + |
| K1UV11 | 145 | 106.4 e | 0.35 ± 0.01 ef | 0.73 ± 0.00 b | 0.031 ± 0.002 c | 78 | − |
| K1UV15 | 183 | 145.0 a | 0.48 ± 0.03 a | 0.79 ± 0.09 a | 0.034 ± 0.002 bc | 88 | − |
| K1UV16 | 196 | 98.5 f | 0.33 ± 0.01 f | 0.50 ± 0.00 e | 0.025 ± 0.003 d | 74 | + |
| K1UV17 | 168 | 124.1 c | 0.41 ± 0.02 cd | 0.74 ± 0.01 b | 0.035 ± 0.003 bc | 77 | + |
| K1UV18 | 250 | 115.0 d | 0.38 ± 0.02 de | 0.46 ± 0.01 f | 0.020 ± 0.001 e | 86 | + |
| K1UV20 | 167 | 124.0 c | 0.41 ± 0.02 cd | 0.74 ± 0.01 b | 0.042 ± 0.003 a | 86 | + |
| Wratislavia K1 | 222 | 112.0 d | 0.37 ± 0.02 ef | 0.50 ± 0.01 e | 0.022 ± 0.001 ed | 82 | − |
| Microorganism | Cultivation System; Tank Volume; Working Volume | GLU [g/L]; Nitrogen Source [g/L]; pH | ERY [g/L] | QERY [g/L/h] | YERY [g/g] | Ref. | |
|---|---|---|---|---|---|---|---|
| Candida sorbosivorans SSE-24 | Batch; 30 L; 22.5 L | 160; yeast extract—12; 5.0 | 60.2 | 0.5 | 0.38 | [45] | |
| Moniliella sp. BCC25224 | Batch, stirred tank, 10 L; not specified | 200; soybean flour—13; 6.0 | 86.6 | 0.4 | 0.47 | [44] | |
| Moniliella sp. 440 | Batch, stirred tank; 5 L; 2 L | 300; yeast extract—10; 5.3 | 116 | 0.81 | 0.39 | [46] | |
| Moniliella tomentosa var. pollinis | Batch, stirred tank; 30 L; 20 L | 352; 2% CSL—20 + 0.1% urea—1; 2.5–3.4 | 90.0 | 0.59 | 0.35 | [47] | |
| Fed-batch | 170 | 1.62 | 0.38 | ||||
| Candida magnoliae | Fed-batch; 3.3 L; | 300 in the production stage; yeast extract; 4.5→3.2 | 84 | 1.4 | 0.23 | [48] | |
| 400 in the production stage; yeast extract; 4.5→3.2 | 187 | 2.8 | 0.41 | ||||
| Moniliella sp. N61188-12 (NTG mutant) | Fed-batch, 2000 L; 800 L | 400; yeast extract—10; 7.0 | 237.8 | 1.98 | 0.59 | [49] | |
| Aureobasidium sp. SN-G42 | Batch; 5 L; | 400 | 175 | 1.82 | 0.44 | [50] | |
| Trichosporon sp. | Batch; 5 L; not specified | 220; corn steep liquor—45; 3.5 | not specified | 1.51 | 0.4 | [51] | |
| 300; corn steep liquor—45; 3.5 | not specified | 1.23 | 0.46 | ||||
| Fed-batch; 5 L; 2.5→3.25 L | 220; corn steep liquor—45; 3.5 | not specified | 1.54 | 0.45 | |||
| Torula sp. | Batch; 5 L; 3 L | 400; yeast extract—20; initial 5.5 | 155 | 1.08 | 0.38 | [52] | |
| 182 1 | 1.34 | 0.45 | |||||
| Torula sp. | Batch, stirred tank, 5 L; 3 L | 400; yeast extract—20; 5.5 | 193 | 1.43 | 0.41 | [53] | |
| Fed-batch, stirred tank; 5 L; 2.4→3 L | 400; yeast extract—20; 5.5 | 192 | 2.26 | 0.48 | |||
| P. tsukubaensis | Batch; 50,000 L; 6000 L | 400; corn steep powder—15; 5.5 | 243 | 1.65 | 0.61 | [43] | |
| Fed-batch; 50,000 L; 6000 L | 400; corn steep powder—15; 5.5 | 241 | 2.84 | 0.60 | |||
| Y. lipolytica CGMCC7326 (wild type) | Batch; 30,000 L; 22,500 L | 300; yeast extract—10 + peptone—5 + ammonium citrate—3.5 + (NH4)2HPO4—0.1; 5.8–6.1 | 165 | 1.57 | 0.55 | [28] | |
| Y. lipolytica HCY118 (transformant) | 196 2 | 2.51 | 0.65 | ||||
| Y. lipolytica YALI-hsp90 | Batch, stirred tank, 5 L; 3 L | 300; yeast extract powder—8 + tryptone—2 + diammonium hydrogen phosphate—2 + ammonium citrate—2; 6.5–7.0 | 76.4 2a | 0.85 | 0.25 | [29] | |
| Y. lipolytica Wratislavia K1 | Fed-batch; stirred tank, 5 L; 2 L; | 240; (NH4)2SO4—4.6; 3.0 | 72.2 3 | 1.02 | 0.43 | [27] | |
| Y. lipolytica Wratislavia K1 | Fed-batch; stirred tank, 5 L; 2 L; | 300; NH4Cl—3 + yeast extract—1; 3.0 | 23 3 | 0.09 | 0.14 | [20] | |
| Y. lipolytica Wratislavia K1 | Batch; stirred tank, 5 L; 2 L; | 300 | 2.6 g/L (NH4)2SO4 | 71.3 | 0.33 | 0.37 | This study |
| Fed-batch, stirred tank, 5 L; 2 L; | 300 (250 + 50) | 97.5 | 0.5 | 0.39 | |||
| Y. lipolytica K1UV15 | 300 (250 + 50) | 145 | 0.79 | 0.48 | |||
| 400 (250 + 2 × 75) | 174.5 | 0.49 | 0.43 | ||||
| 400 (150 + 4 × 62.5; YE) | 3.6 g/L (NH4)2SO4 | 208 | 0.86 | 0.52 | |||
| 400 (150 + 4 × 62.5; B1) | 226 | 0.50 | 0. 565 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rywińska, A.; Tomaszewska-Hetman, L.; Juszczyk, P.; Rakicka-Pustułka, M.; Bogusz, A.; Rymowicz, W. Enhanced Production of Erythritol from Glucose by the Newly Obtained UV Mutant Yarrowia lipolytica K1UV15. Molecules 2024, 29, 2187. https://doi.org/10.3390/molecules29102187
Rywińska A, Tomaszewska-Hetman L, Juszczyk P, Rakicka-Pustułka M, Bogusz A, Rymowicz W. Enhanced Production of Erythritol from Glucose by the Newly Obtained UV Mutant Yarrowia lipolytica K1UV15. Molecules. 2024; 29(10):2187. https://doi.org/10.3390/molecules29102187
Chicago/Turabian StyleRywińska, Anita, Ludwika Tomaszewska-Hetman, Piotr Juszczyk, Magdalena Rakicka-Pustułka, Adam Bogusz, and Waldemar Rymowicz. 2024. "Enhanced Production of Erythritol from Glucose by the Newly Obtained UV Mutant Yarrowia lipolytica K1UV15" Molecules 29, no. 10: 2187. https://doi.org/10.3390/molecules29102187
APA StyleRywińska, A., Tomaszewska-Hetman, L., Juszczyk, P., Rakicka-Pustułka, M., Bogusz, A., & Rymowicz, W. (2024). Enhanced Production of Erythritol from Glucose by the Newly Obtained UV Mutant Yarrowia lipolytica K1UV15. Molecules, 29(10), 2187. https://doi.org/10.3390/molecules29102187






