In Situ Carbon-Confined MoSe2 Catalyst with Heterojunction for Highly Selective CO2 Hydrogenation to Methanol
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
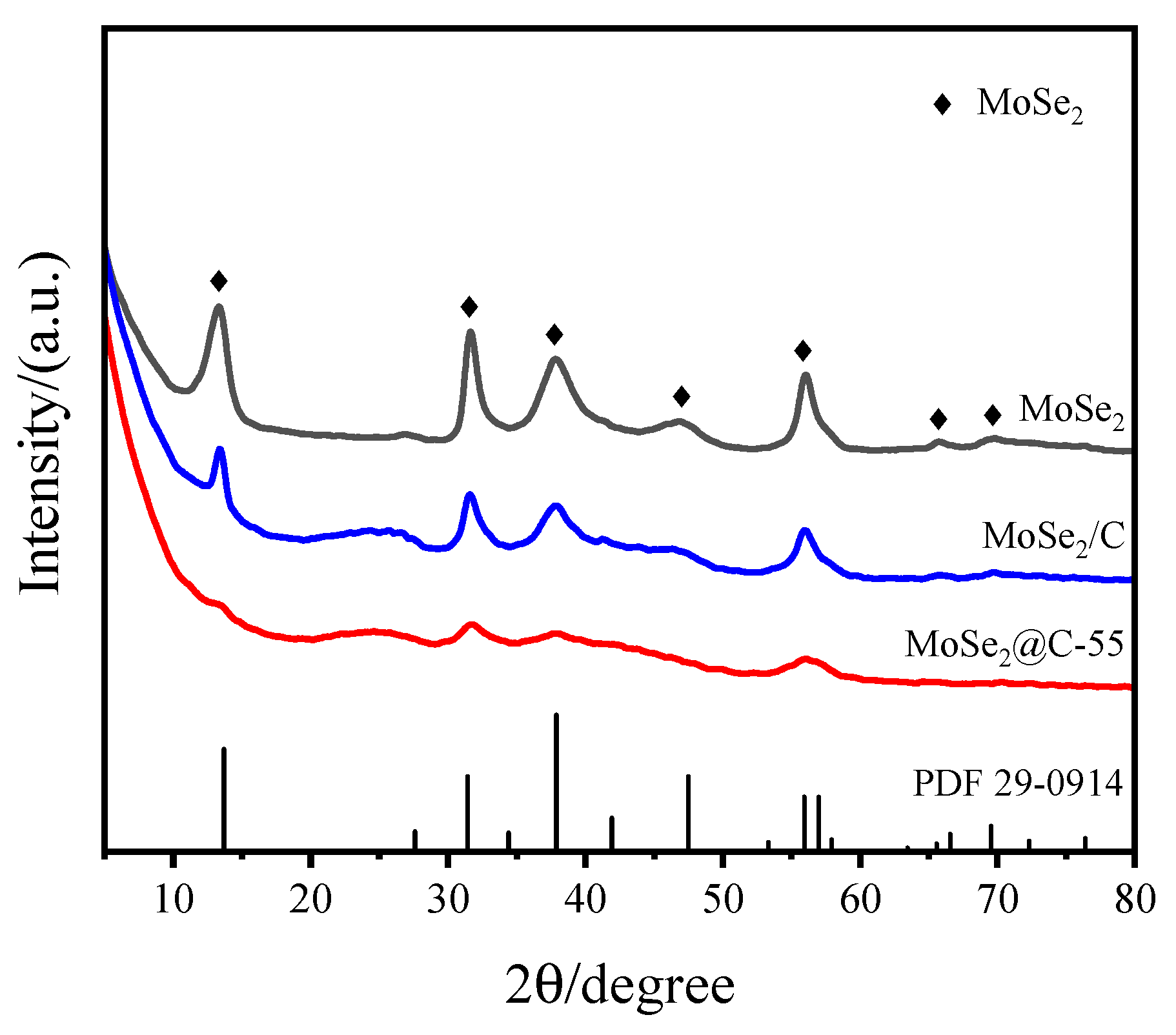
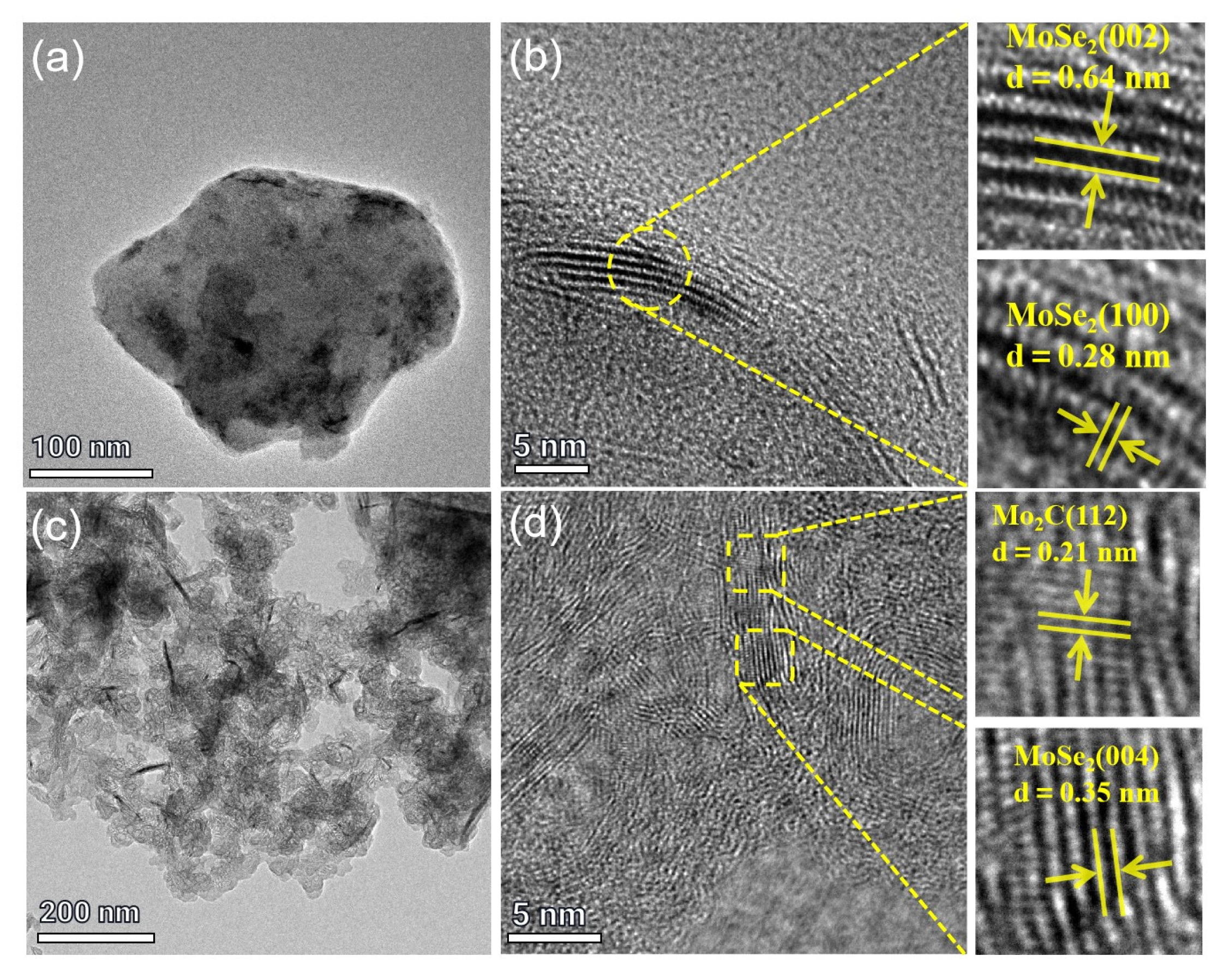
2.1.1. Structural Characterizations
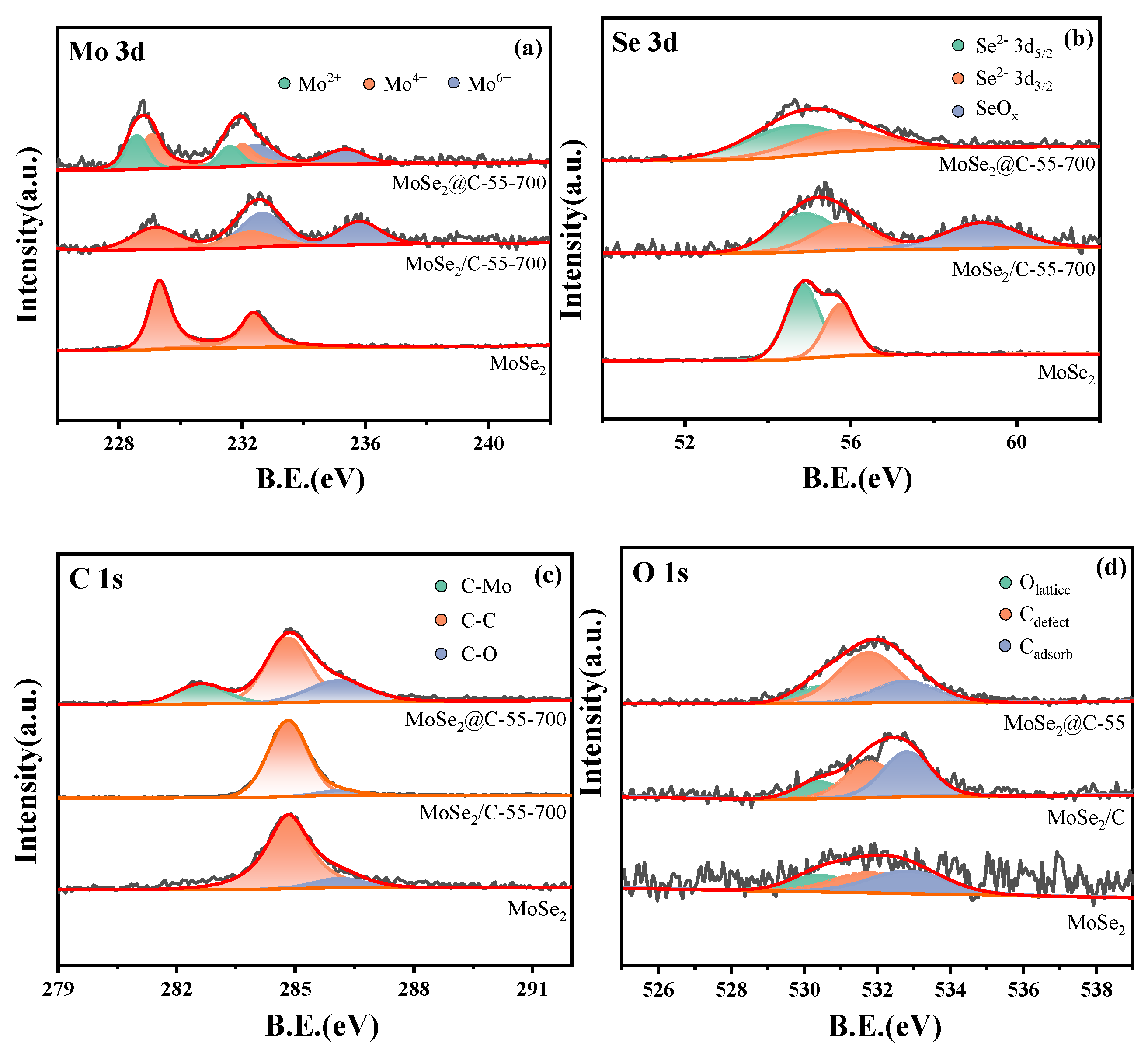
2.1.2. XPS Analysis of Catalysts
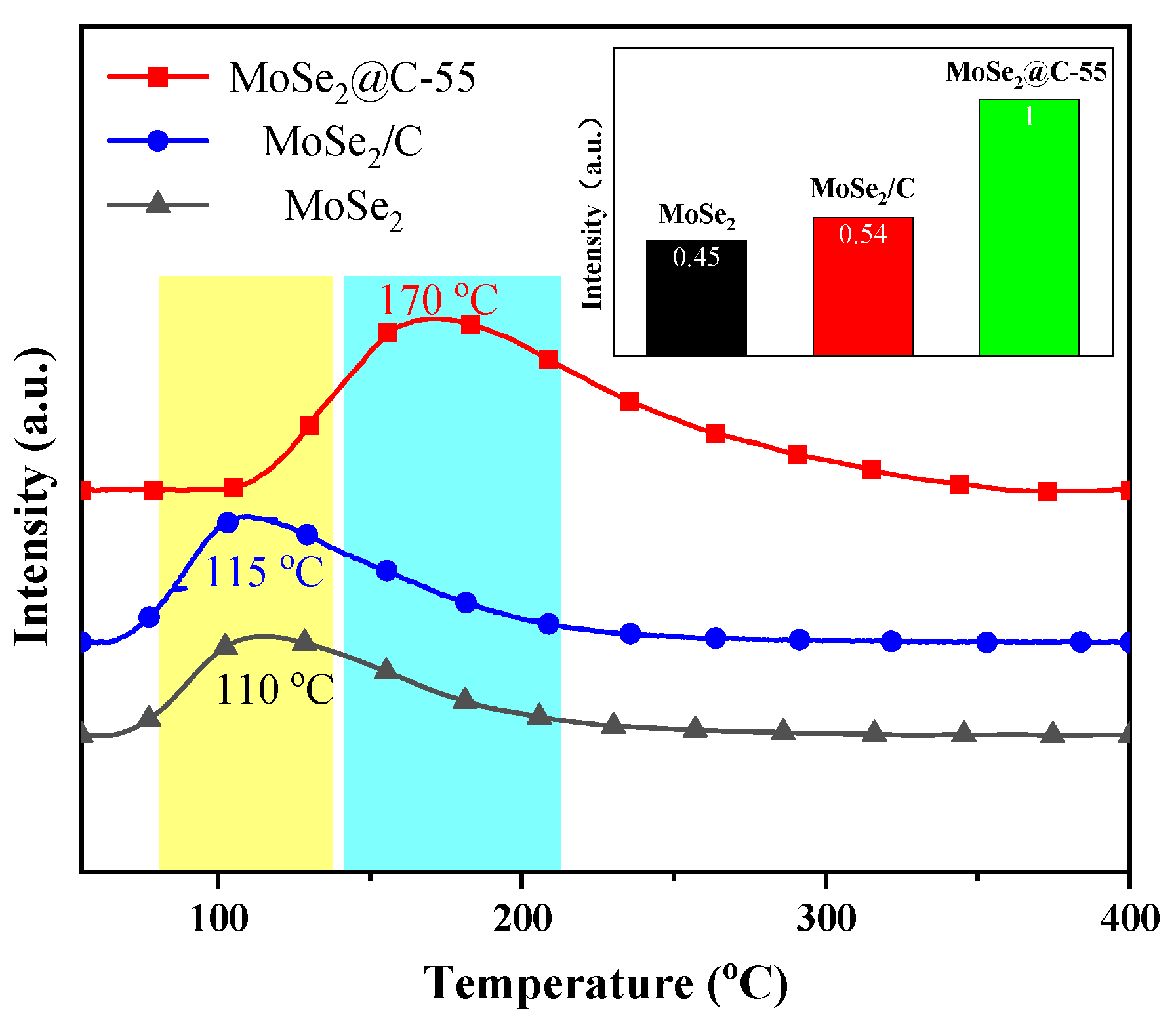
2.1.3. Characterization of CO2 Adsorption Capacity
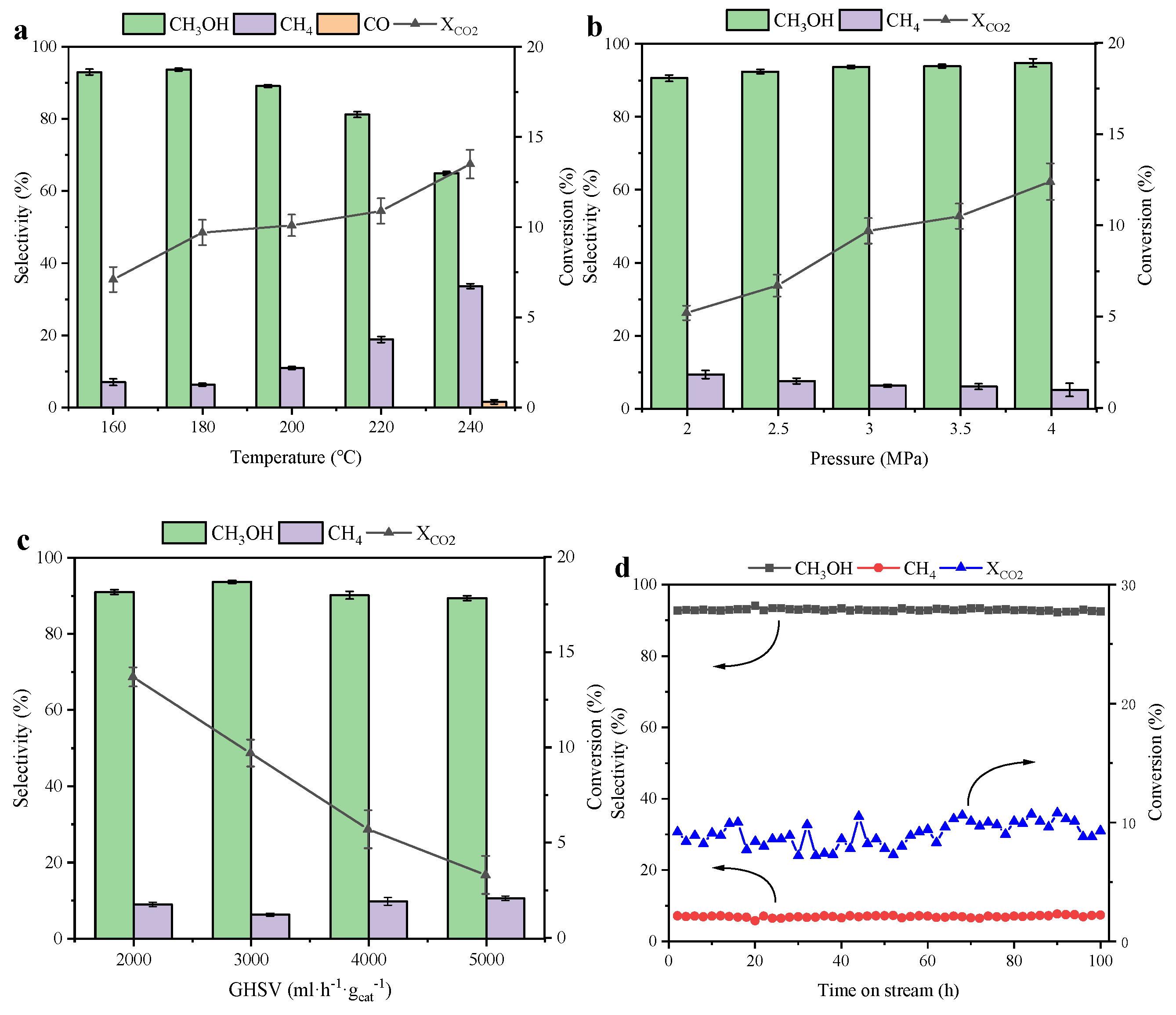
2.2. Catalytic Performance in CO2 Hydrogenation to Yield Methanol
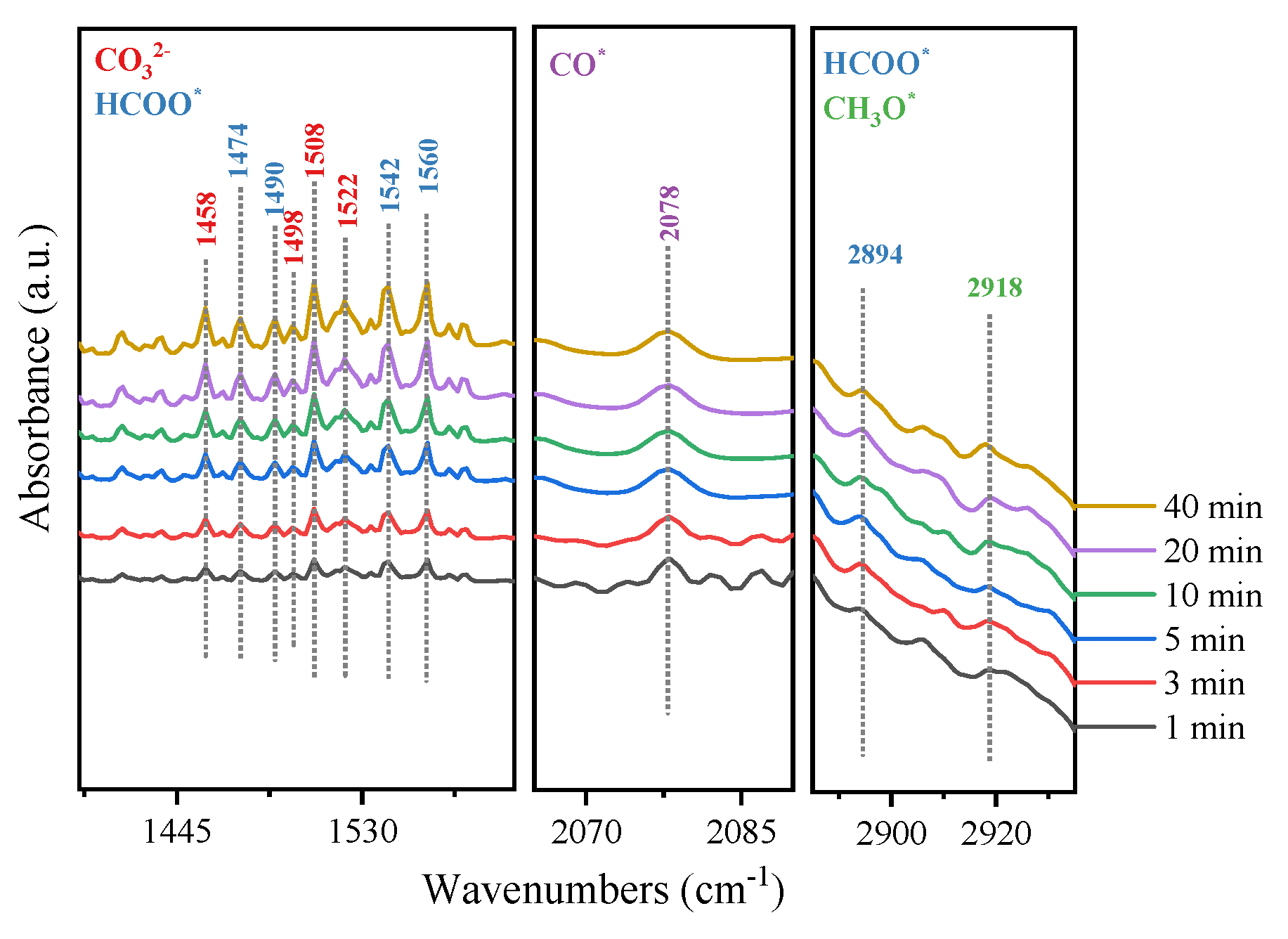
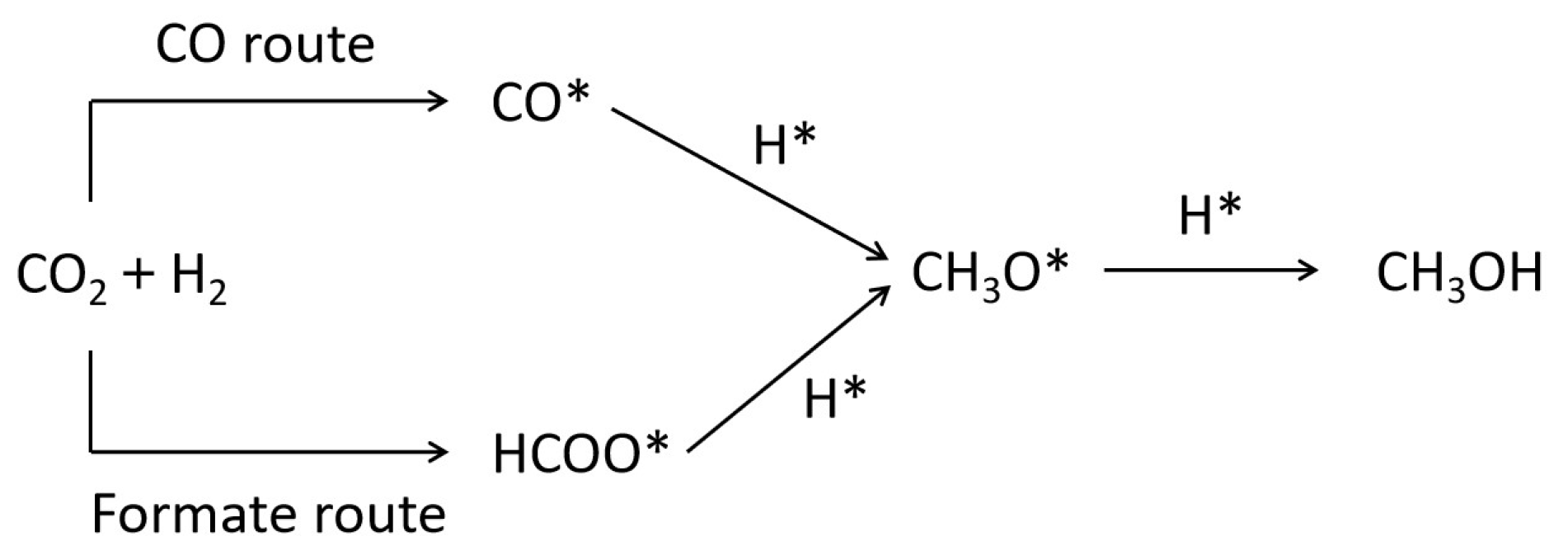
2.3. Reaction Mechanism
3. Experiment
3.1. Materials
3.2. Preparation of Catalysts
3.2.1. Preparing the MoSe2@C-55 Catalyst
3.2.2. Preparing the MoSe2/C Catalyst
3.2.3. Preparing the MoSe2 Catalyst
3.3. Characterization
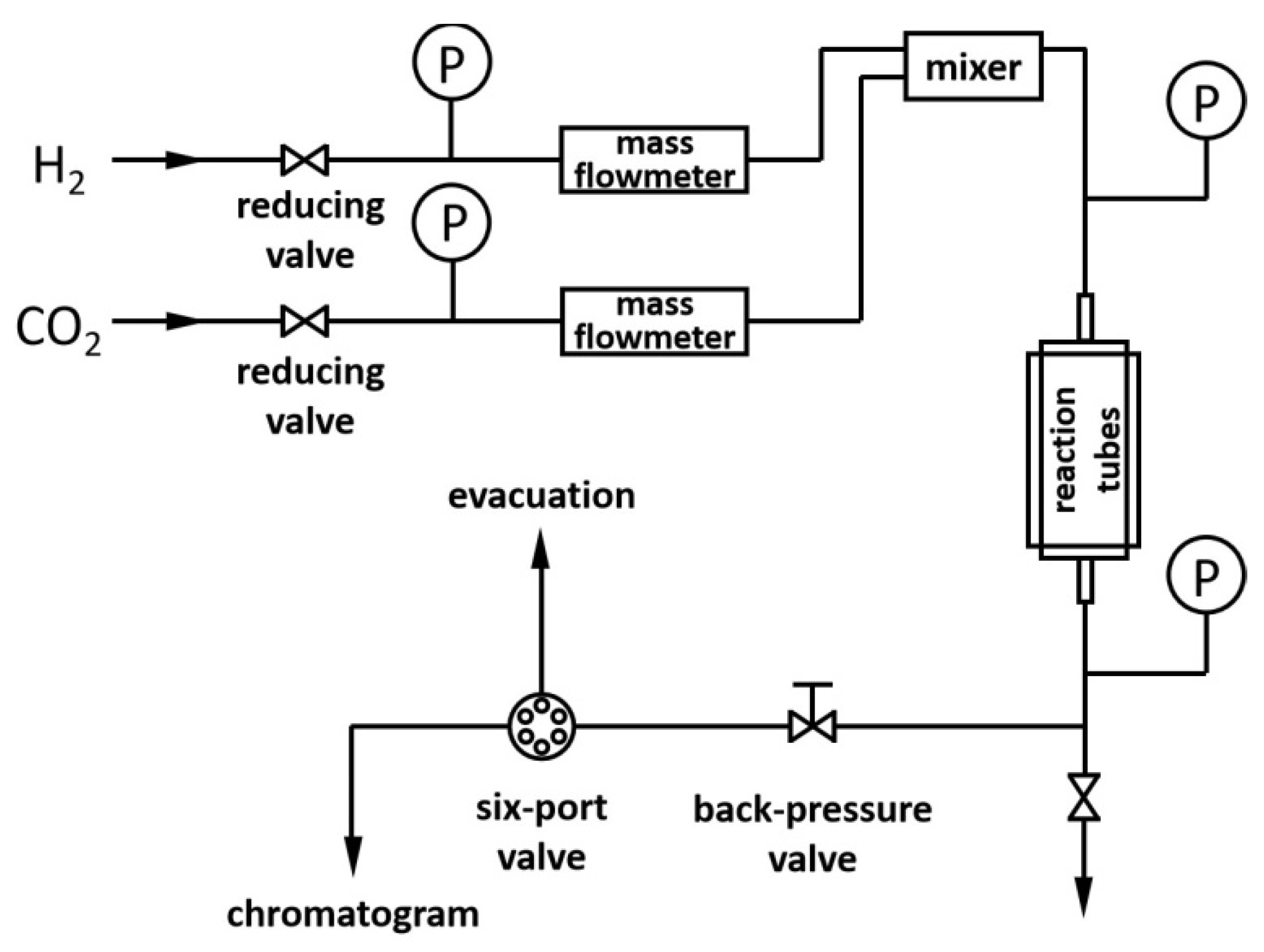
3.4. Catalytic Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhong, S.; Guo, X.; Zhou, A.; Chen, Z.; Jin, D.; Fan, M.; Ma, T. Fundamentals and recent progress in magnetic field assisted CO2 capture and conversion. Small 2024, 20, 2305533. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- MacDowell, N.; Florin, N.; Buchard, A.; Hallett, J.; Galindo, A.; Jackson, G.; Adjiman, C.S.; Williams, C.K.; Shah, N.; Fennell, P. An overview of CO2 capture technologies. Energy Environ. Sci. 2010, 3, 1645–1669. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Li, L.; Feng, J.; Li, K.; Lin, H. Integrated CO2 capture and utilization: A review of the synergistic effects of dual function materials. Catal. Sci. Technol. 2024, 14, 790–819. [Google Scholar] [CrossRef]
- Zhang, X.G.; Buthiyappan, A.; Jewaratnam, J.; Metselaar, H.S.C.; Raman, A.A.A. Bifunctional materials for integrated CO2 capture and conversion: Review on adsorbent and catalyst types, recent advances, and challenges. J. Environ. Chem. Eng. 2024, 12, 111799. [Google Scholar] [CrossRef]
- Zhang, Z.; Pan, S.-Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Al-Rowaili, F.N.; Zahid, U.; Onaizi, S.; Khaled, M.; Jamal, A.; AL-Mutairi, E.M. A review for Metal-Organic Frameworks (MOFs) utilization in capture and conversion of carbon dioxide into valuable products. J. CO2 Util. 2021, 53, 101715. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Zhang, Q.; Teng, F.; McLellan, B.C. A critical review on deployment planning and risk analysis of carbon capture, utilization, and storage (CCUS) toward carbon neutrality. Renew. Sustain. Energy Rev. 2022, 167, 112537. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Guo, X.; Song, C.; Guo, X. Recent advances in application of iron-based catalysts for COx hydrogenation to value-added hydrocarbons. Chin. J. Catal. 2022, 43, 731–754. [Google Scholar] [CrossRef]
- Navarro-Jaén, S.; Virginie, M.; Bonin, J.; Robert, M.; Wojcieszak, R.; Khodakov, A.Y. Highlights and challenges in the selective reduction of carbon dioxide to methanol. Nat. Rev. Chem. 2021, 5, 564–579. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Sen, R.; Goeppert, A.; Prakash, G.K.S. Homogeneous hydrogenation of CO2 and CO to methanol: The renaissance of low-temperature catalysis in the context of the methanol economy. Angew. Chem. Int. Ed. 2022, 61, e202207278. [Google Scholar] [CrossRef] [PubMed]
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol fuel production, utilization, and techno-economy: A review. Environ. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Onishi, N.; Himeda, Y. Homogeneous catalysts for CO2 hydrogenation to methanol and methanol dehydrogenation to hydrogen generation. Coord. Chem. Rev. 2022, 472, 214767. [Google Scholar] [CrossRef]
- Sheetal; Mehara, P.; Das, P. Methanol as a greener C1 synthon under non-noble transition metal-catalyzed conditions. Coord. Chem. Rev. 2023, 475, 214851. [Google Scholar] [CrossRef]
- Bowker, M. Methanol synthesis from CO2 hydrogenation. ChemCatChem 2019, 11, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- LBobadilla, F.; Azancot, L.; González-Castaño, M.; Ruíz-López, E.; Pastor-Pérez, L.; Durán-Olivencia, F.-J.; Odriozola, J.-A. Biomass gasification, catalytic technologies and energy integration for production of circular methanol: New horizons for industry decarbonisation. J. Environ. Sci. 2024, 140, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.C.; Liu, X.Y.; Zhu, R.R.; Xue, D.M.; Liu, X.Q.; Sun, L.B. Causation of catalytic activity of Cu-ZnO for CO2 hydrogenation to methanol. Chem. Eng. J. 2022, 430, 132784. [Google Scholar] [CrossRef]
- Qi, T.; Li, W.; Li, H.; Ji, K.; Chen, S.; Zhang, Y. Yttria-doped Cu/ZnO catalyst with excellent performance for CO2 hydrogenation to methanol. Mol. Catal. 2021, 509, 111641. [Google Scholar] [CrossRef]
- Guo, H.; Li, Q.; Zhang, H.; Peng, F.; Xiong, L.; Yao, S.; Huang, C.; Chen, X. CO2 hydrogenation over acid-activated Attapulgite/Ce0.75Zr0.25O2 nanocomposite supported Cu-ZnO based catalysts. Mol. Catal. 2019, 476, 110499. [Google Scholar] [CrossRef]
- Sha, F.; Han, Z.; Tang, S.; Wang, J.; Li, C. Hydrogenation of carbon dioxide to methanol over Non-Cu-based heterogeneous catalysts. ChemSusChem 2020, 13, 6160–6181. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Martín, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferré, D.; Pérez-Ramírez, J. Indium oxide as a superior catalyst for methanol synthesis by CO2 hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Li, Z.; Tang, C.; Feng, Z.; An, H.; Liu, H.; Liu, T.; Li, C. A highly selective and stable ZnO-ZrO2 solid solution catalyst for CO2 hydrogenation to methanol. Sci. Adv. 2017, 3, e1701290. [Google Scholar] [CrossRef] [PubMed]
- Levy, R.B.; Boudart, M. Platinum-like behavior of tungsten carbide in surface catalysis. Sci. New Ser. 1973, 181, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Wu, H.B.; Xia, B.Y.; Xu, C.; Lou, X.W. Hierarchical β-Mo2C nanotubes organized by ultrathin nanosheets as a highly efficient electrocatalyst for hydrogen production. Angew. Chem. Int. Ed. 2015, 54, 15395–15399. [Google Scholar] [CrossRef] [PubMed]
- Akmach, D.; Bathla, S.; Tran, C.C.; Kaliaguine, S.; Mushrif, S.H. Transition metal carbide catalysts for Upgrading lignocellulosic biomass-derived oxygenates: A review of the experimental and computational investigations into structure-property relationships. Catal. Today 2023, 423, 114285. [Google Scholar] [CrossRef]
- Jujjuri, S.; Cárdenas-Lizana, F.; Keane, M.A. Synthesis of group VI carbides and nitrides: Application in catalytic hydrodechlorination. J. Mater. Sci. 2014, 49, 5406–5417. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, Y.; Li, Z.; Cao, Y.; Li, Q. Hydrodenitrogenation of Quinoline with high selectivity to aromatics over α-MoC1-x. Mol. Catal. 2021, 516, 112002. [Google Scholar] [CrossRef]
- Dubois, J.-L.; Sayama, K.; Arakawa, H. CO2 hydrogenation over carbide catalysts. Chem. Lett. 1992, 21, 5–8. [Google Scholar] [CrossRef]
- Posada-Pérez, S.; Viñes, F.; Ramirez, P.J.; Vidal, A.B.; Rodriguez, J.A.; Illas, F. The bending machine: CO2 activation and hydrogenation on δ-MoC(001) and β-Mo2C(001) surfaces. Phys. Chem. Chem. Phys. 2014, 16, 14912–14921. [Google Scholar] [CrossRef]
- Han, H.; Cui, P.; Xiao, L.; Wu, W. MoCS@NSC with interfacial heterostructure nanostructure: A highly selective catalyst for synthesizing methanol from CO2 at low temperature. J. Environ. Chem. Eng. 2021, 9, 106354. [Google Scholar] [CrossRef]
- Hu, J.; Yu, L.; Deng, J.; Wang, Y.; Cheng, K.; Ma, C.; Zhang, Q.; Wen, W.; Yu, S.; Pan, Y.; et al. Sulfur vacancy-rich MoS2 as a catalyst for the hydrogenation of CO2 to methanol. Nat. Catal. 2021, 4, 242–250. [Google Scholar] [CrossRef]
- Sharma, M.D.; Mahala, C.; Basu, M. 2D thin sheet heterostructures of MoS2 on MoSe2 as efficient electrocatalyst for hydrogen evolution reaction in wide ph range. Inorg. Chem. 2020, 59, 4377–4388. [Google Scholar] [CrossRef] [PubMed]
- Wazir, M.B.; Daud, M.; Safeer, S.; Almarzooqi, F.; Qurashi, A. Review on 2D molybdenum diselenide (MoSe2) and its hybrids for green hydrogen (H2) generation applications. ACS Omega 2022, 7, 16856–16865. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, X.; Liu, W.; Sun, Y.; Ju, Z.; Yao, T.; Wang, C.; Ju, H.; Zhu, J.; Wei, S.; et al. Carbon Dioxide electroreduction into syngas boosted by a partially delocalized charge in molybdenum sulfide selenide alloy monolayers. Angew. Chem. Int. Ed. 2019, 56, 9121–9125. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; He, S.; Su, Y.; Zhang, Y.; Su, H. MoSe2: A promising non-noble metal catalyst for direct ethanol synthesis from syngas. Fuel 2020, 281, 118760. [Google Scholar] [CrossRef]
- Zhao, G.-Q.; Long, X.; Zou, J.; Hu, J.; Jiao, F.-P. Design of hollow nanostructured photocatalysts for clean energy production. Coord. Chem. Rev. 2023, 477, 214953. [Google Scholar] [CrossRef]
- Dai, M.; Wang, R. Synthesis and applications of nanostructured hollow transition metal chalcogenides. Small 2021, 17, 2006813. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, M.; Chen, D. Sheet-like MoSe2/C composites with enhanced Li-ion storage properties. J. Mater. Chem. A 2015, 3, 11857–11862. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, Y.; Dong, M.; Hou, Z.; Qian, Y. Construction of hierarchical MoSe2@C hollow nanospheres for efficient lithium/sodium ion storage. Inorg. Chem. Front. 2020, 7, 1691–1698. [Google Scholar] [CrossRef]
- Liu, H.; Liu, B.; Guo, H.; Liang, M.; Zhang, Y.; Borjigin, T.; Yang, X.; Wang, L.; Sun, X. N-doped C-encapsulated scale-like yolk-shell frame assembled by expanded planes few-layer MoSe2 for enhanced performance in sodium-ion batteries. Nano Energy 2018, 51, 639–648. [Google Scholar] [CrossRef]
- Tang, H.; Dou, K.; Kaun, C.C.; Kuang, Q.; Yang, S. MoSe2 nanosheets and their graphene hybrids: Synthesis, characterization and hydrogen evolution reaction studies. J. Mater. Chem. A 2014, 2, 360–364. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Chen, B.; Meng, Y.; He, F.; Liu, E.; Shi, C.; He, C.; Ma, L.; Li, Q.; Li, J.; Zhao, N. Thermal decomposition-reduced layer-by-layer nitrogen-doped graphene/MoS2/nitrogen-doped graphene heterostructure for promising lithium-ion batteries. Nano Energy 2017, 41, 154–163. [Google Scholar] [CrossRef]
- Niu, F.; Yang, J.; Wang, N.; Zhang, D.; Fan, W.; Yang, J.; Qian, Y. MoSe2-covered n, p-doped carbon nanosheets as a long-life and high-rate anode material for sodium-ion batteries. Adv. Funct. Mater. 2017, 27, 1700522. [Google Scholar] [CrossRef]
- Balati, A.; Bazilio, A.; Shahriar, A.; Nash, K.; Shipley, H.J. Simultaneous formation of ultra-thin MoSe2 nanosheets, inorganic fullerene-like MoSe2 and MoO3 quantum dots using fast and ecofriendly pulsed laser ablation in liquid followed by microwave treatment. Mater. Sci. Semicond. Process. 2019, 99, 68–77. [Google Scholar] [CrossRef]
- Qu, B.; Li, C.; Zhu, C.; Wang, S.; Zhang, X.; Chen, Y. Growth of MoSe2 nanosheets with small size and expanded spaces of (002) plane on the surfaces of porous N-doped carbon nanotubes for hydrogen production. Nanoscale 2016, 8, 16886–16893. [Google Scholar] [CrossRef] [PubMed]
- Vikraman, D.; Hussain, S.; Karuppasamy, K.; Feroze, A.; Kathalingam, A.; Sanmugam, A.; Chun, S.H.; Jung, J.; Kim, H.S. Engineering the novel MoSe2-Mo2C hybrid nanoarray electrodes for energy storage and water splitting applications. Appl. Catal. B Environ. 2020, 264, 118531. [Google Scholar] [CrossRef]
- Ge, P.; Hou, H.; Banks, C.E.; Foster, C.W.; Li, S.; Zhang, Y.; He, J.; Zhang, C.; Ji, X. Binding MoSe2 with carbon constrained in carbonous nanosphere towards high-capacity and ultrafast Li/Na-ion storage. Energy Storage Mater. 2018, 12, 310–323. [Google Scholar] [CrossRef]
- Liu, H.; Guo, H.; Liu, B.; Liang, M.; Lv, Z.; Adair, K.R.; Sun, X. Few-layer MoSe2 nanosheets with expanded (002) planes confined in hollow carbon nanospheres for ultrahigh-performance Na-ion batteries. Adv. Funct. Mater. 2018, 28, 1707480. [Google Scholar] [CrossRef]
- Li, J.; Hong, W.; Jian, C.; Cai, Q.; He, X.; Liu, W. High-performance hydrogen evolution at a MoSe2–Mo2C seamless heterostructure enabled by efficient charge transfer. J. Mater. Chem. A 2020, 8, 6692–6698. [Google Scholar] [CrossRef]
- Su, J.; Nie, Z.; Feng, Y.; Hu, X.; Li, H.; Zhao, Z.; Zan, S.; Qi, S. Hollow core-shell structure Co/C@MoSe2 composites for high-performance microwave absorption. Compos. Part A Appl. Sci. Manuf. 2022, 162, 107140. [Google Scholar] [CrossRef]
- Tang, J.; Huang, C.; Wu, Q.; Cui, A.; Li, W. Atomic-scale intercalation of N-doped carbon into monolayered MoSe2-Mo2C heterostructure as a highly efficiency hydrogen evolution reaction catalyst. J. Electroanal. Chem. 2022, 906, 115897. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, X.; Feng, C.; Zhang, W.; Wang, Z.; Feng, S.; Huang, Z.; Lu, X.; Dai, F. Interfacial Mo-N-C bond endowed hydrogen evolution reaction on MoSe2@N-Doped carbon hollow nanoflowers. Inorg. Chem. 2021, 60, 12377–12385. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cui, J.; Liu, G.; Bi, R.; Zhang, L. Carbon-coated MoSe2/MXene hybrid nanosheets for superior potassium storage. ACS Nano 2019, 13, 3448–3456. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Guan, D.; Gu, Y.; Xu, H.; Wang, C.; Shao, Z.; Guo, Y. Tuning synergy between nickel and iron in Ruddlesden-Popper perovskites through controllable crystal dimensionalities towards enhanced oxygen-evolving activity and stability. Carbon Energy 2024, 6, e465. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Cao, M.; Liu, B.; Li, J. High selective methanol synthesis from CO2 hydrogenation over Mo-Co-C-N catalyst. Fuel 2022, 325, 124854. [Google Scholar] [CrossRef]
- Ma, Z.-Y.; Yang, C.; Wei, W.; Li, W.-H.; Sun, Y.-H. Surface properties and CO adsorption on zirconia polymorphs. J. Mol. Catal. A Chem. 2005, 227, 119–124. [Google Scholar] [CrossRef]
- Dong, X.; Li, F.; Zhao, N.; Xiao, F.; Wang, J.; Tan, Y. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Appl. Catal. B Environ. 2016, 191, 8–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, F.; Geng, S.; Yao, M.; Ma, J.; Cao, J. Tuning the content of S vacancies in MoS2 by Cu doping for enhancing catalytic hydrogenation of CO2 to methanol. Mol. Catal. 2023, 547, 113288. [Google Scholar] [CrossRef]
- Yang, Z.; He, Y.; Tang, P.; Xu, C.; Zhang, G.; He, J. Oxygen-incorporated 3D flower-like MoS2 microsphere as a bifunctional catalyst for effective synthesis of 2,5-diformyfuran from fructose. Catal. Sci. Technol. 2023, 13, 2340–2348. [Google Scholar] [CrossRef]
- Zhang, P.; Na, W.; Zuo, J.; Wen, J.; Huang, Z.; Huang, H.; Gao, W.; Qi, X.; Zheng, M.; Wang, H. CO2 hydrogenation to methanol over hydrothermally synthesized Inx-Zry catalysts. Mol. Catal. 2023, 538, 112977. [Google Scholar] [CrossRef]
- Heracleous, E.; Koidi, V.; Lappas, A.A. Experimental Investigation of Sorption-Enhanced CO2 Hydrogenation to Methanol. ACS Sustain. Chem. Eng. 2023, 11, 9684–9695. [Google Scholar]
- Wei, Y.; Liu, F.; Ma, J.; Yang, C.; Wang, X.; Cao, J. Catalytic roles of In2O3 in ZrO2-based binary oxides for CO2 hydrogenation to methanol. Mol. Catal. 2022, 525, 112354. [Google Scholar] [CrossRef]
- Daifeng, L.; Zhen, Z.; Yinye, C.; Lingxing, Z.; Xiaochuan, C.; Xuhui, Y.; Baoquan, H.; Yongjin, L.; Qingrong, Q.; Qinghua, C. The Co-In2O3 interaction concerning the effect of amorphous Co metal on CO2 hydrogenation to methanol. J. CO2 Util. 2022, 65, 102209. [Google Scholar] [CrossRef]
- Rasteiro, L.F.; De Sousa, R.A.; Vieira, L.H.; Ocampo-Restrepo, V.K.; Verga, L.G.; Assaf, J.M.; Da Silva, J.L.F.; Assaf, E.M. Insights into the alloy-support synergistic effects for the CO2 hydrogenation towards methanol on oxide-supported Ni5Ga3 catalysts: An experimental and DFT study. Appl. Catal. B Environ. 2022, 302, 120842. [Google Scholar] [CrossRef]
- Alabsi, M.H.; Chen, X.; Wang, X.; Zhang, M.; Ramirez, A.; Duan, A.; Xu, C.; Cavallo, L.; Huang, K.W. Highly dispersed Pd nanoparticles supported on dendritic mesoporous CeZrZnOx for efficient CO2 hydrogenation to methanol. J. Catal. 2022, 413, 751–761. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Hu, Q.; Huang, Y.; Wang, X.; Wang, Y.; Wang, F. Application of microimpinging stream reactor coupled with ultrasound in Cu/CeZrOx solid solution catalyst preparation for CO2 hydrogenation to methanol. Renew. Energy 2023, 202, 834–843. [Google Scholar] [CrossRef]
- Zhou, S.; Zeng, H.C. Boxlike Assemblages of few-layer MoS2 nanosheets with edge blockage for high-efficiency hydrogenation of CO2 to methanol. ACS Catal. 2022, 12, 9872–9886. [Google Scholar] [CrossRef]
- Chen, G.; Yu, J.; Li, G.; Zheng, X.; Mao, H.; Mao, D. Cu+-ZrO2 interfacial sites with highly dispersed copper nanoparticles derived from Cu@UiO-67 hybrid for efficient CO2 hydrogenation to methanol. Int. J. Hydrogen Energy 2023, 48, 2605–2616. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Hong, X.; Liu, G. Sulfate-promoted higher alcohol synthesis from CO2 hydrogenation. ACS Sustain. Chem. Eng. 2022, 10, 8980–8987. [Google Scholar]
- Marcos, F.C.F.; Alvim, R.S.; Lin, L.; Betancourt, L.E.; Petrolini, D.D.; Senanayake, S.D.; Alves, R.M.B.; Assaf, J.M.; Rodriguez, J.A.; Giudici, R.; et al. The role of copper crystallization and segregation toward enhanced methanol synthesis via CO2 hydrogenation over CuZrO2 catalysts: A combined experimental and computational study. Chem. Eng. J. 2023, 452, 139519. [Google Scholar] [CrossRef]
- Zhou, S.; Kosari, M.; Zeng, H.C. Boosting CO2 hydrogenation to methanol over monolayer MoS2 nanotubes by creating more strained basal planes. J. Am. Chem. Soc. 2024, 146, 10032–10043. [Google Scholar] [CrossRef]
- Zhou, S.; Ma, W.; Anjum, U.; Kosari, M.; Xi, S.; Kozlov, S.M.; Zeng, H.C. Strained few-layer MoS2 with atomic copper and selectively exposed in-plane sulfur vacancies for CO2 hydrogenation to methanol. Nat. Commun. 2023, 14, 5872. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Qi, L.; Gao, Z.; Guo, T.; Zhai, D.; He, Y.; Guo, Q. Performance exploration of Ni-doped MoS2 in CO2 hydrogenation to methanol. Molecules 2023, 28, 5796. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Shen, C.; Sun, K.; Ma, X.; Ma, X.; Li, Z.; Li, M.; Liu, C.J. CO2 hydrogenation to methanol over the copper promoted In2O3 catalyst. J. Energy. Chem. 2024, 93, 135–145. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Li, J.; Liu, F.; Wang, J.; Lv, J.; Ma, X. Modulation of Al2O3 and ZrO2 composite in Cu/ZnO-based catalysts with enhanced performance for CO2 hydrogenation to methanol. Appl. Catal. A 2024, 674, 119618. [Google Scholar] [CrossRef]
| Catalyst | Conversion (%) | Selectivity (%) | |||
|---|---|---|---|---|---|
| CH3OH | CH4 | CO | CH3OCH3 | ||
| MoSe2 | 2.4 | 52.5 | 10.2 | 33.1 | 4.2 |
| MoSe2/C | 4.3 | 89.9 | 10.1 | - | - |
| MoSe2@C-55 | 9.7 | 93.7 | 6.3 | - | - |
| MoSe2@C-15 | 2.8 | 72.3 | 27.7 | - | - |
| MoSe2@C-35 | 3.3 | 89.5 | 10.5 | - | - |
| MoSe2@C-75 | 4.4 | 94.3 | 5.7 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Xiao, L.; Wu, W. In Situ Carbon-Confined MoSe2 Catalyst with Heterojunction for Highly Selective CO2 Hydrogenation to Methanol. Molecules 2024, 29, 2186. https://doi.org/10.3390/molecules29102186
Sun Y, Xiao L, Wu W. In Situ Carbon-Confined MoSe2 Catalyst with Heterojunction for Highly Selective CO2 Hydrogenation to Methanol. Molecules. 2024; 29(10):2186. https://doi.org/10.3390/molecules29102186
Chicago/Turabian StyleSun, Yanyang, Linfei Xiao, and Wei Wu. 2024. "In Situ Carbon-Confined MoSe2 Catalyst with Heterojunction for Highly Selective CO2 Hydrogenation to Methanol" Molecules 29, no. 10: 2186. https://doi.org/10.3390/molecules29102186
APA StyleSun, Y., Xiao, L., & Wu, W. (2024). In Situ Carbon-Confined MoSe2 Catalyst with Heterojunction for Highly Selective CO2 Hydrogenation to Methanol. Molecules, 29(10), 2186. https://doi.org/10.3390/molecules29102186





