Recent Advances in the Synthesis of Rosettacin
Abstract
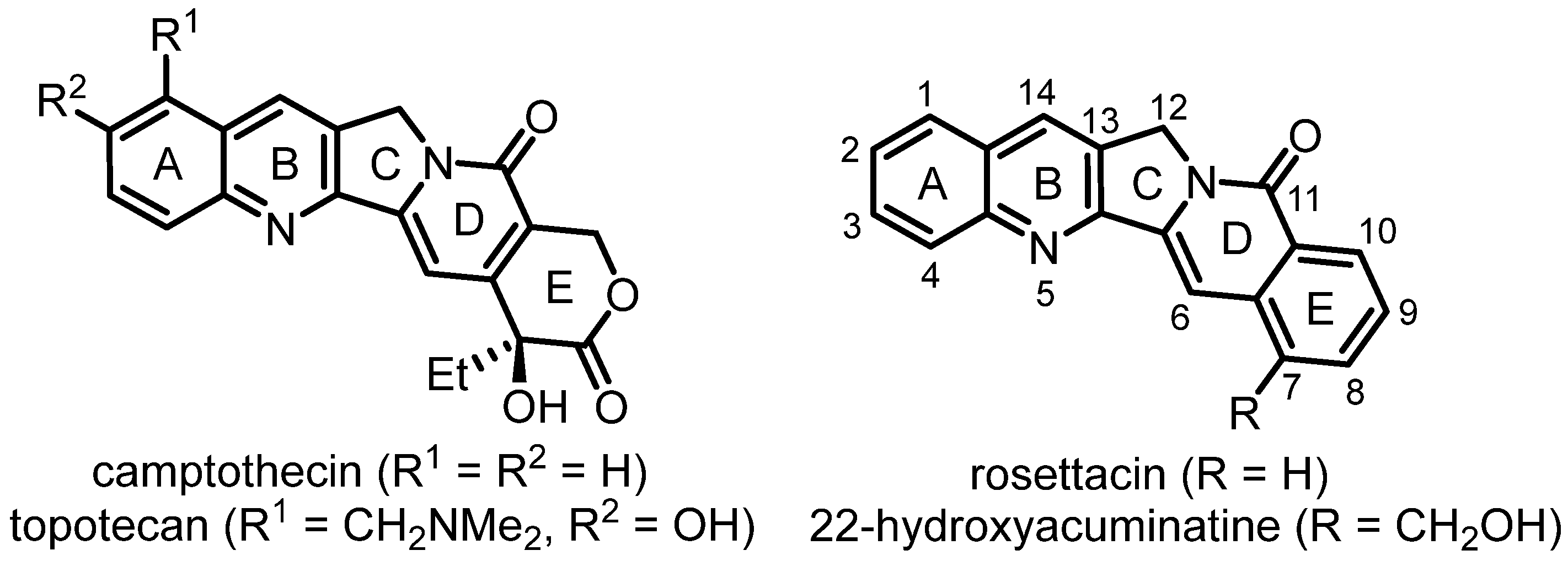
1. Introduction
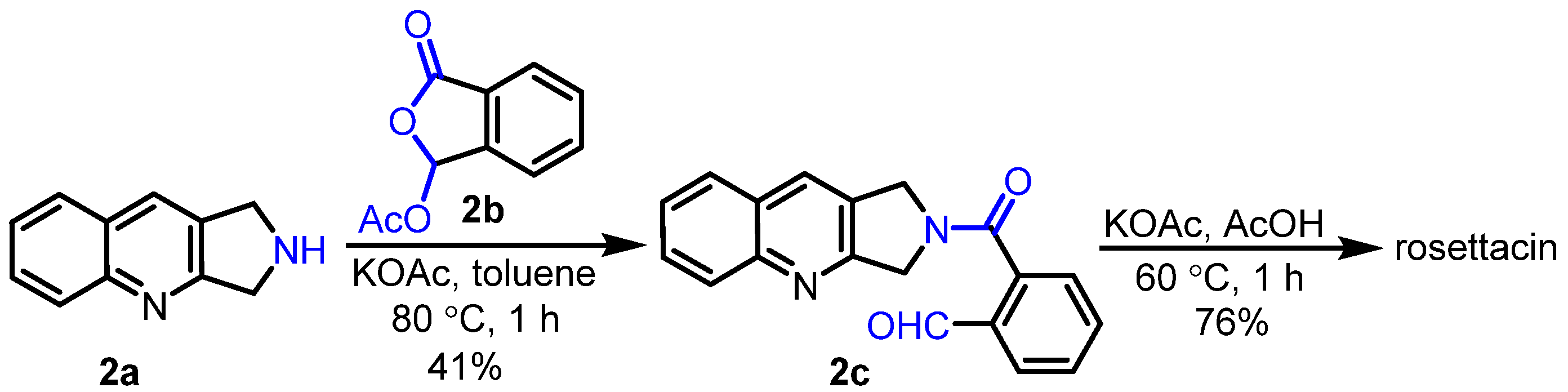
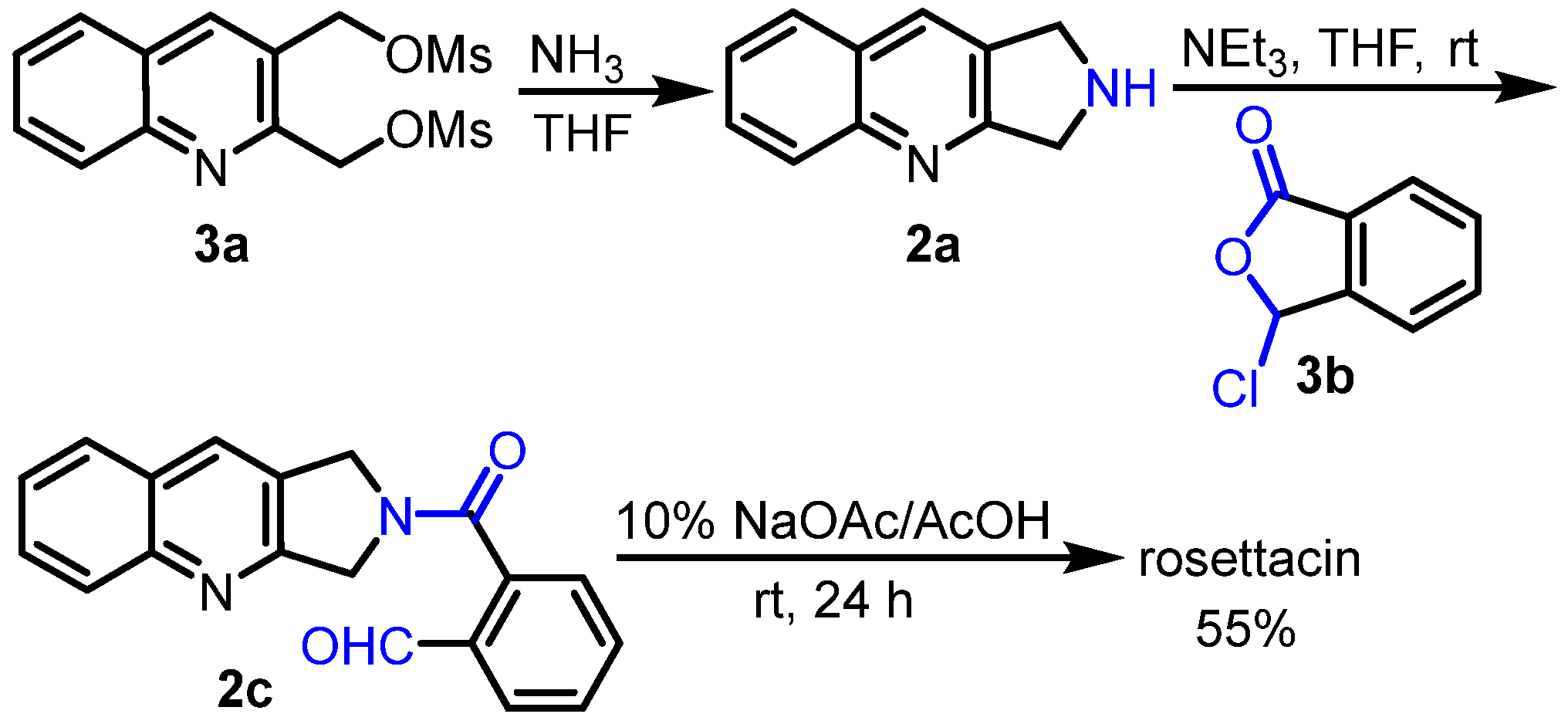
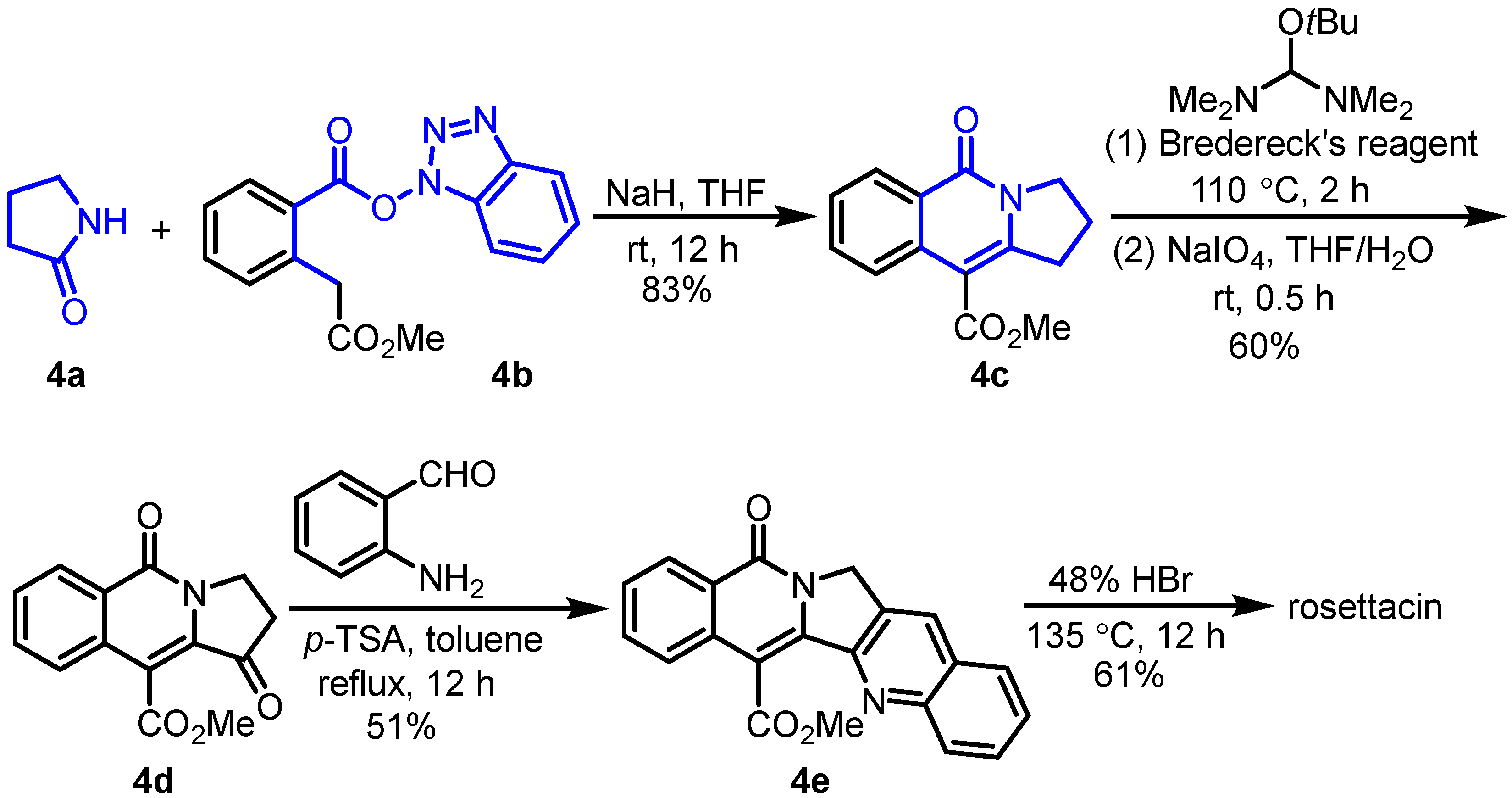
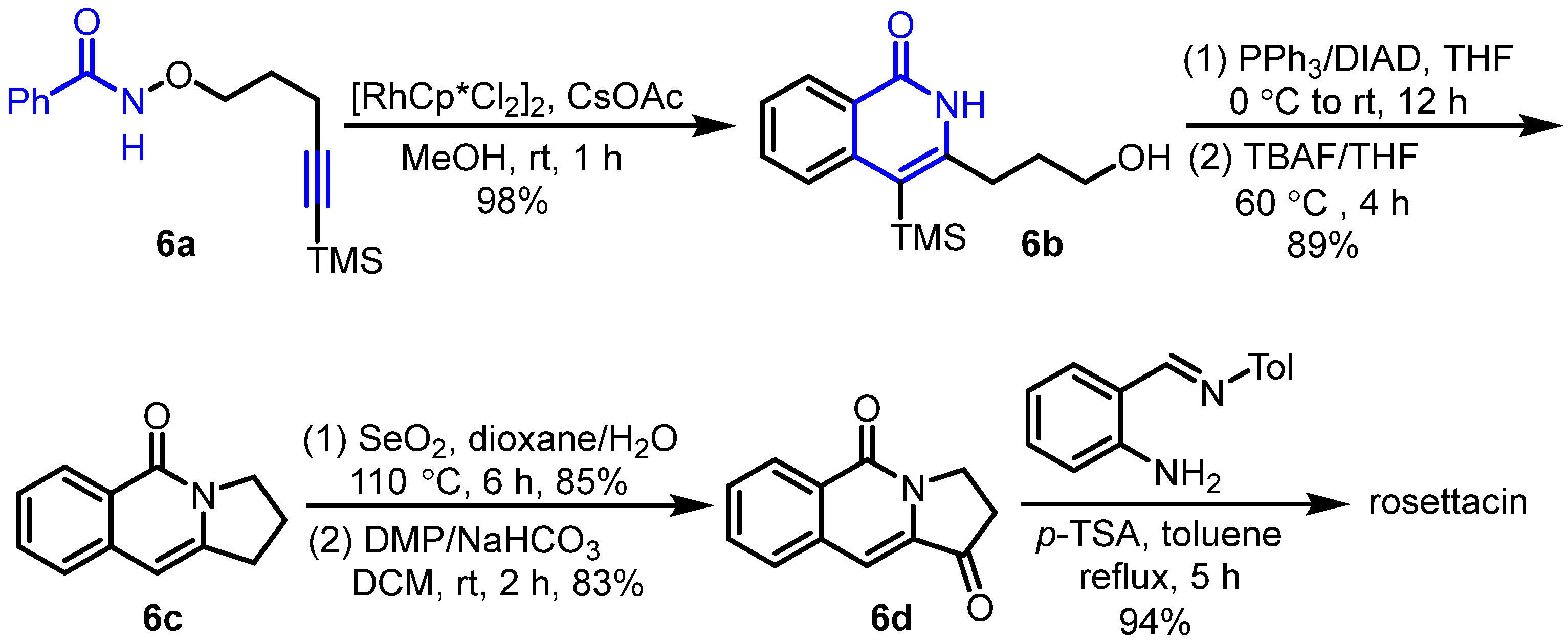
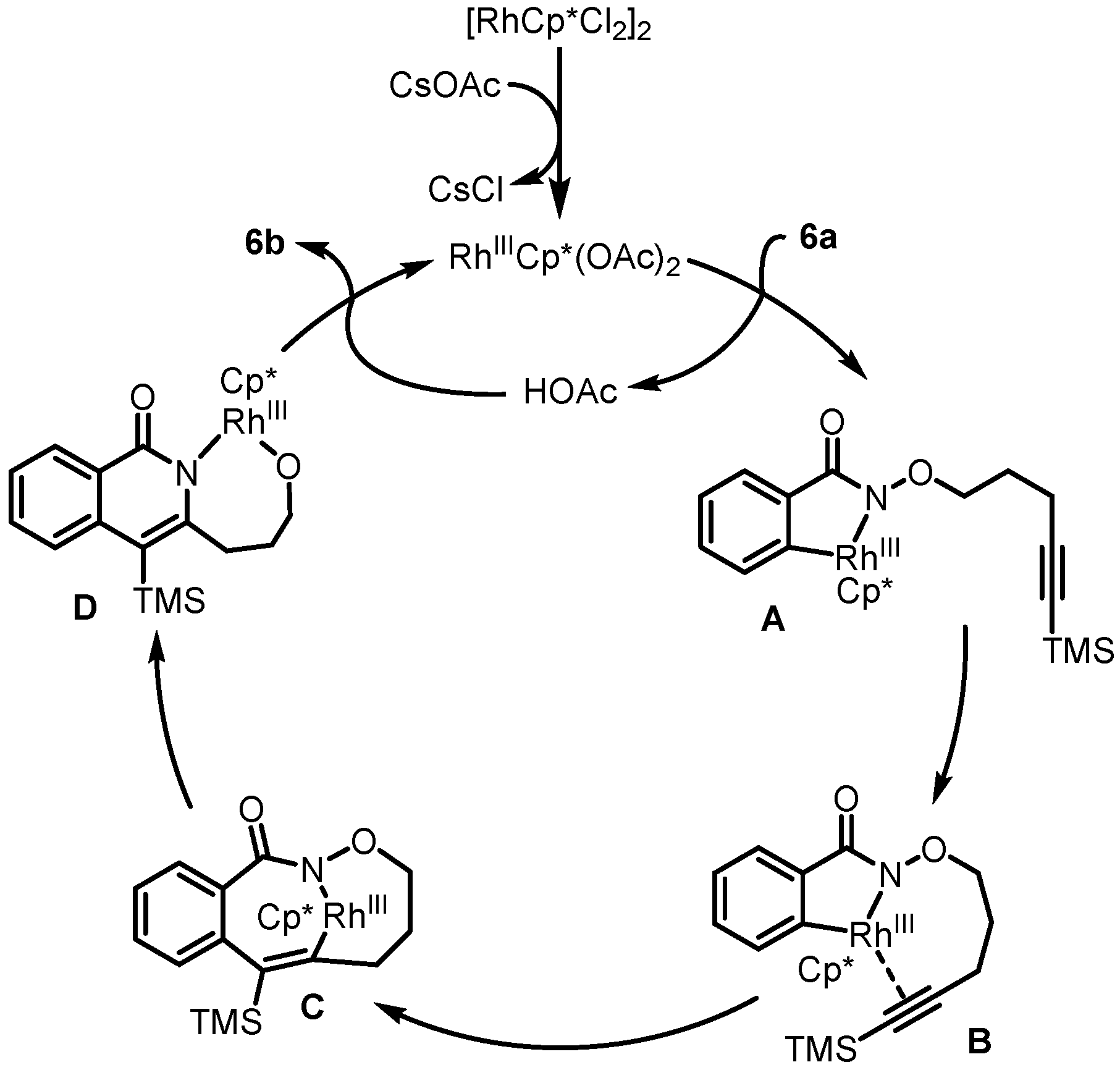
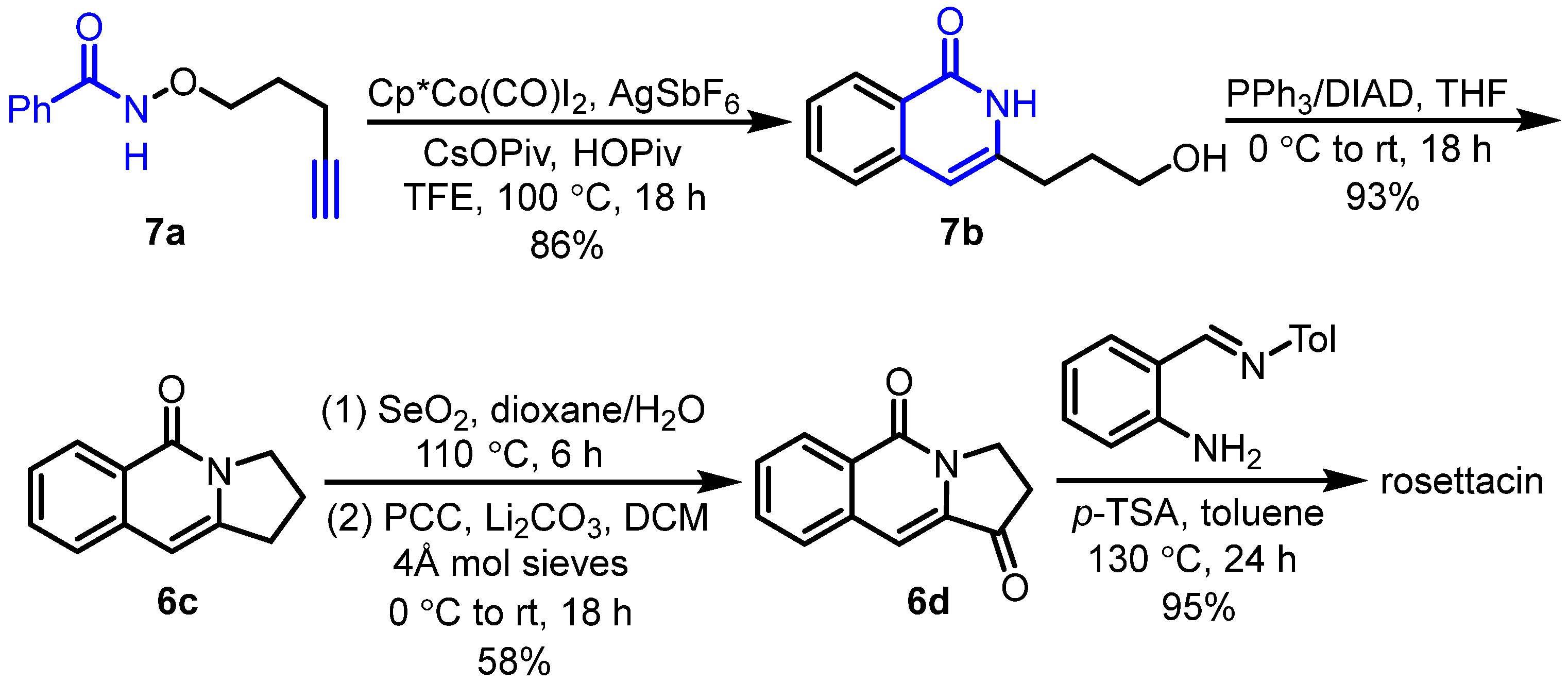
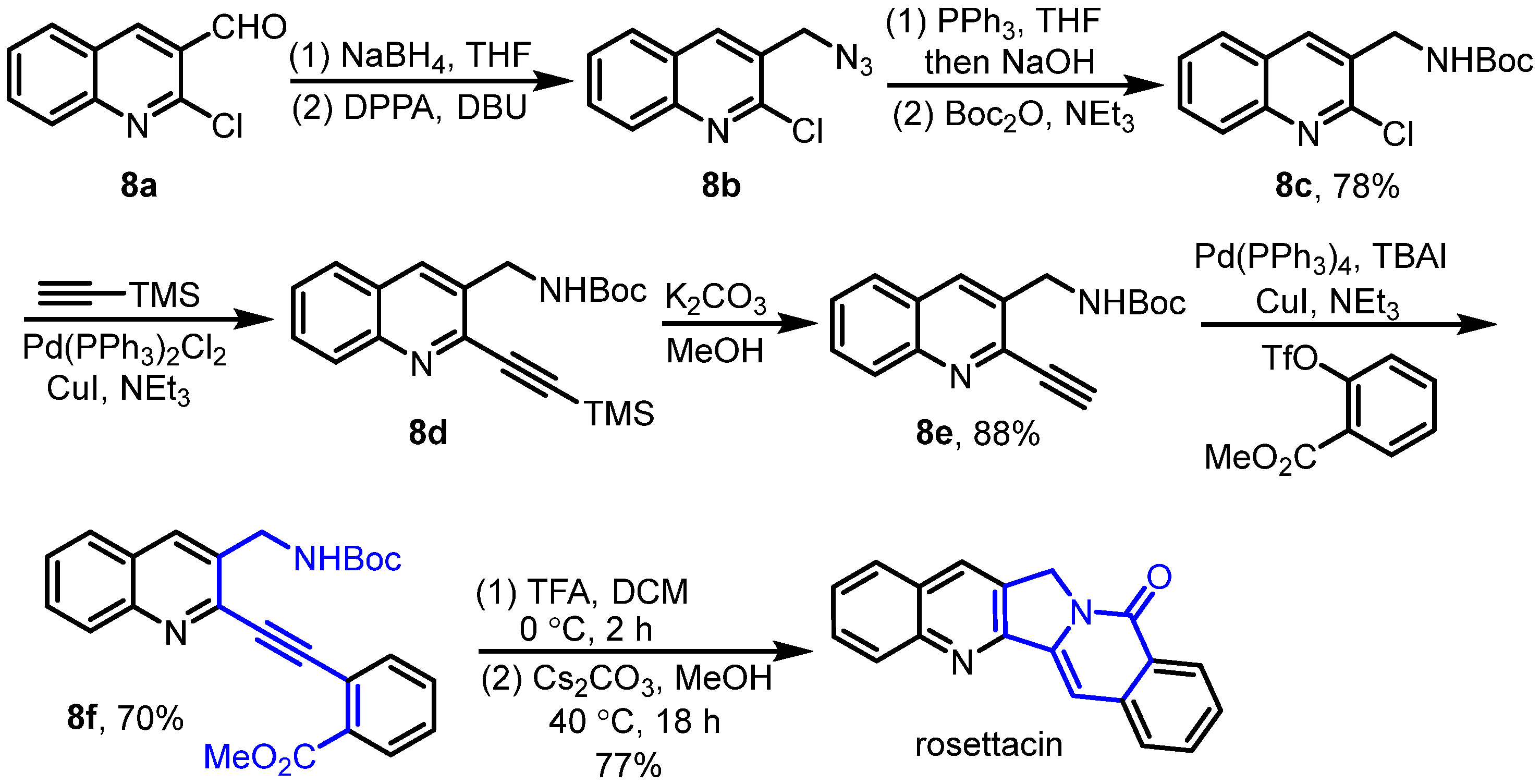
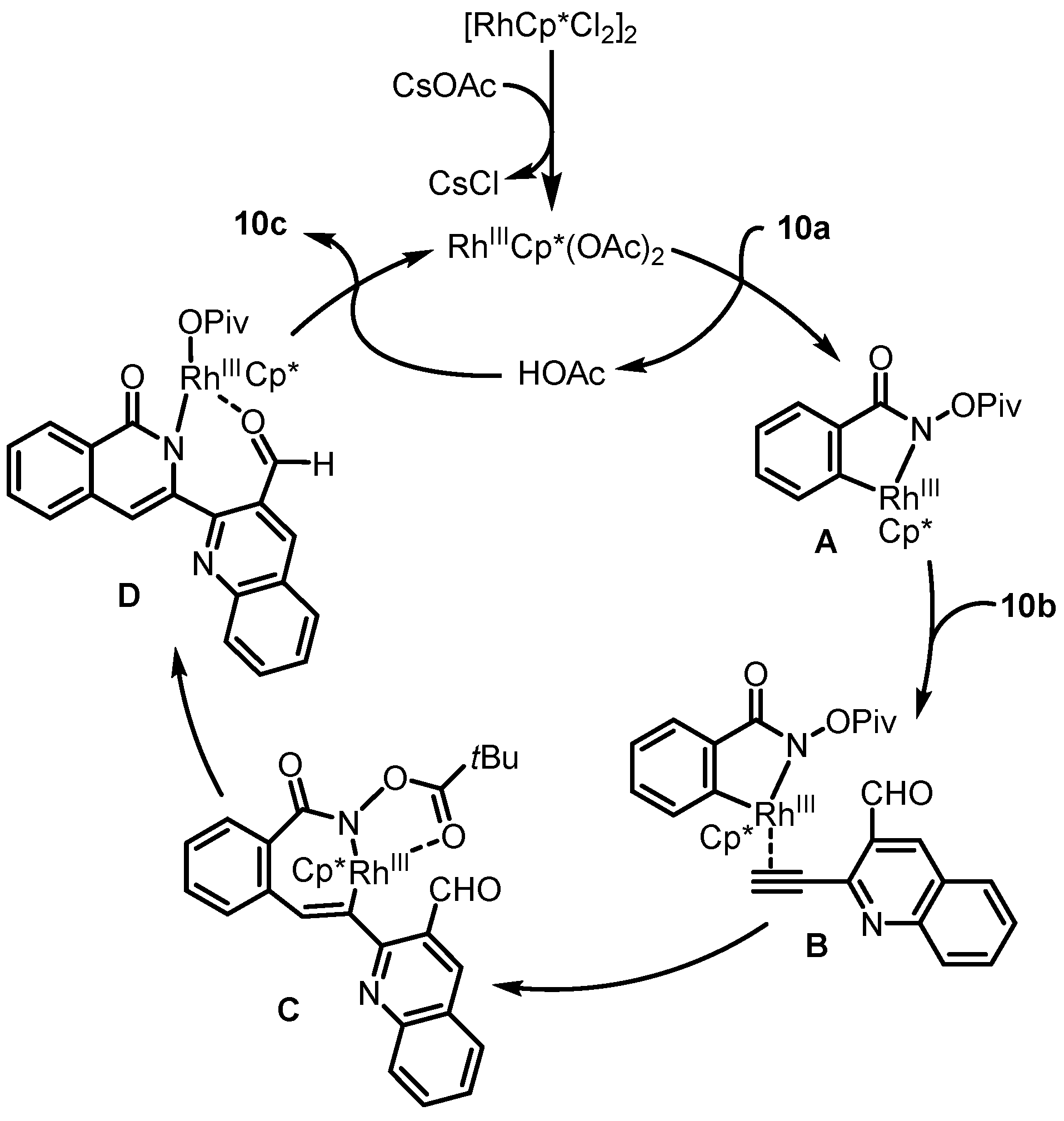
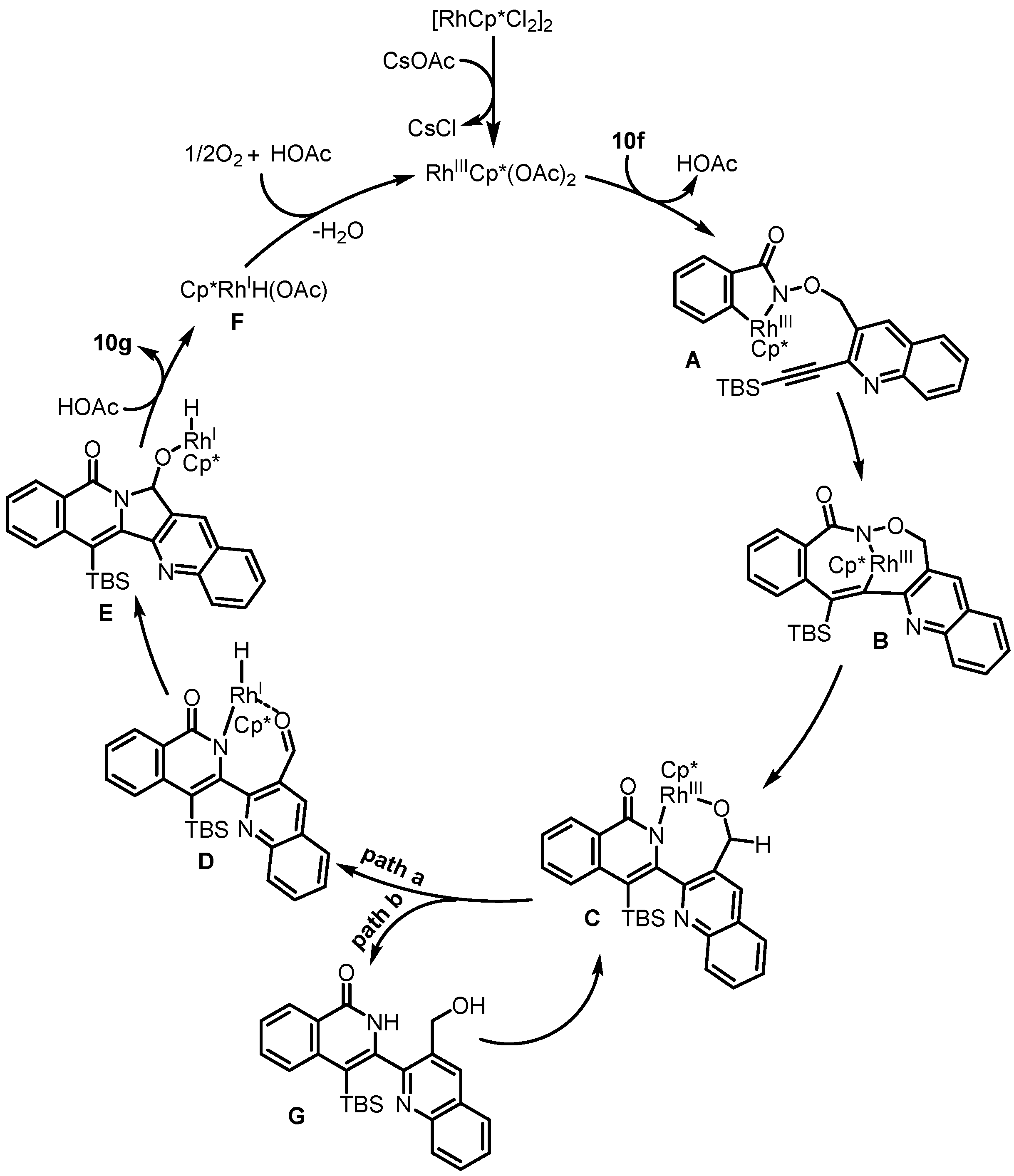
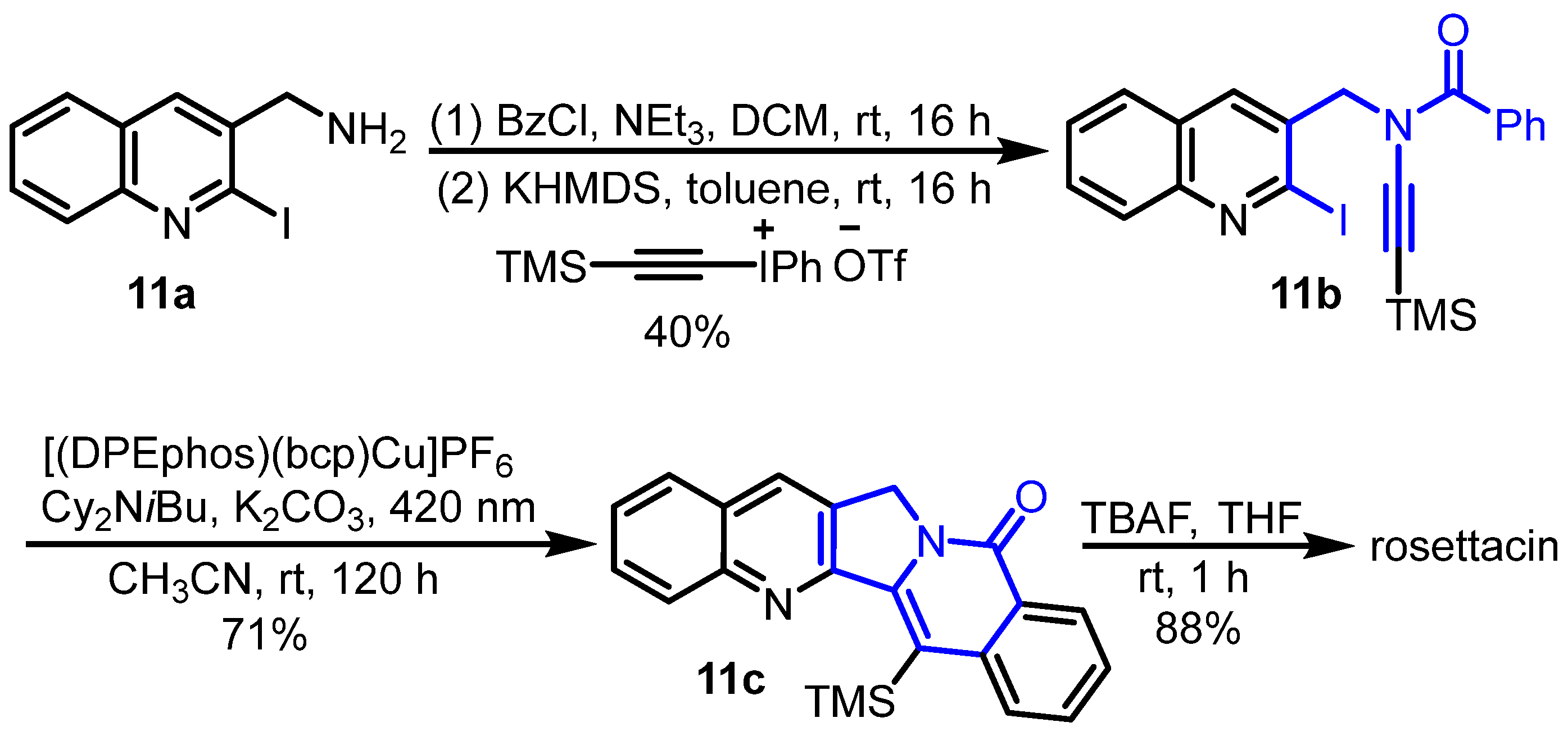
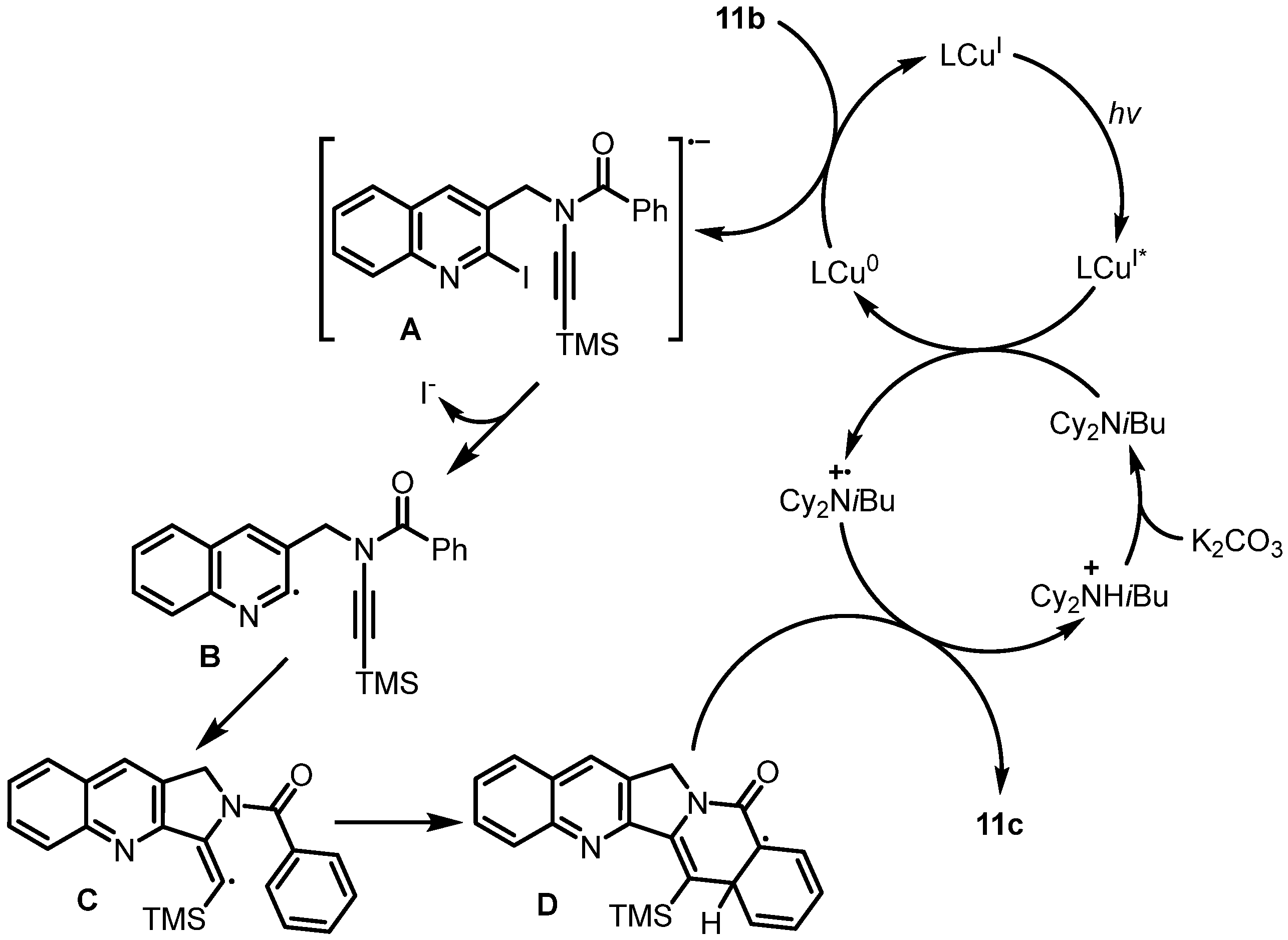
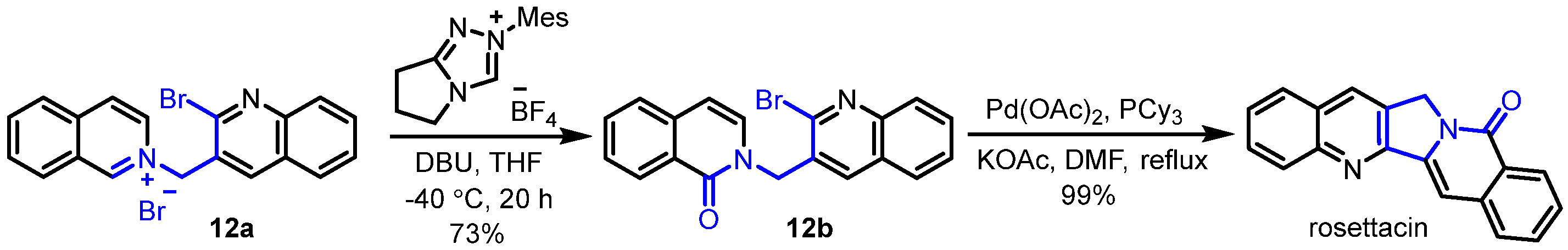
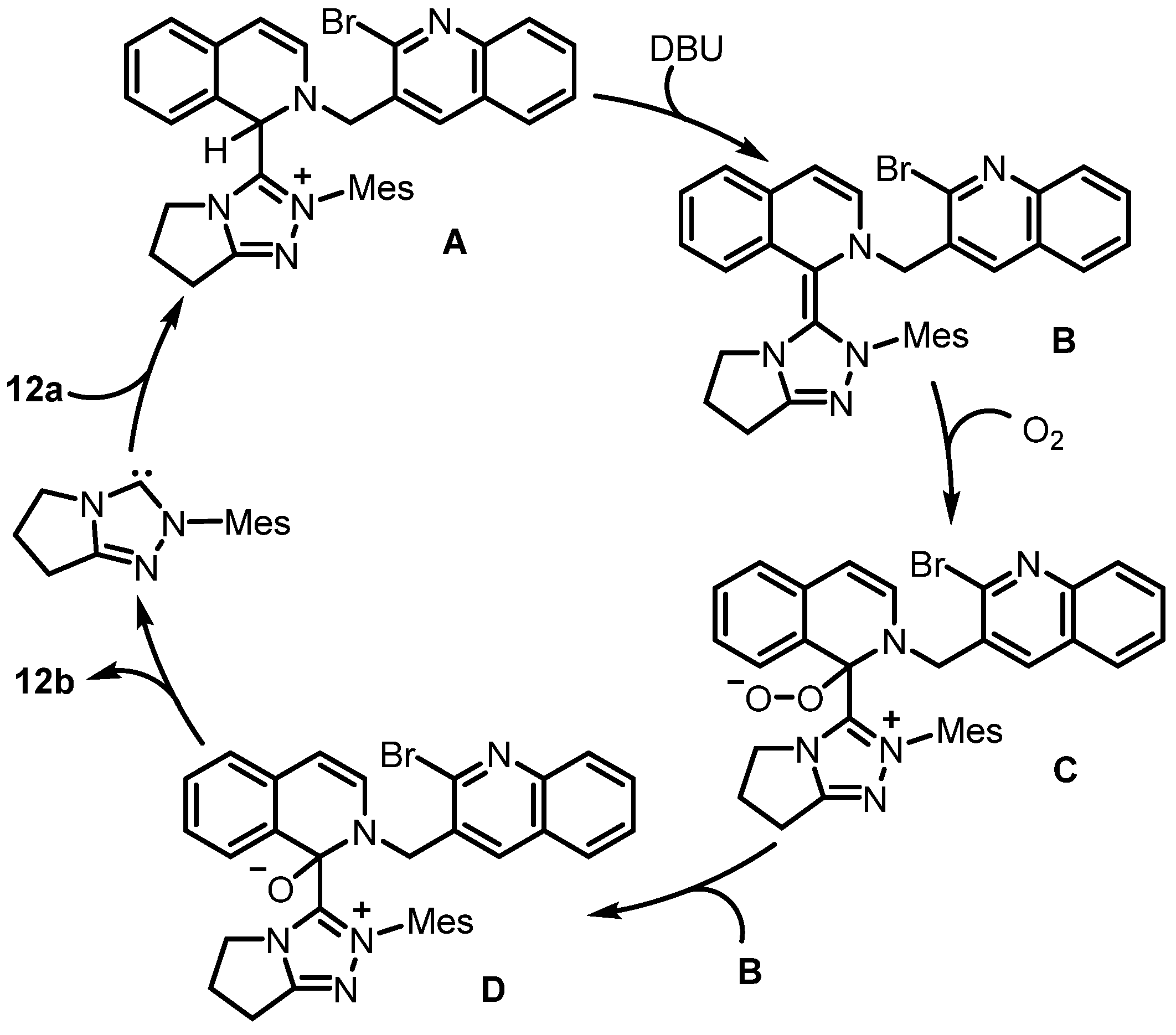
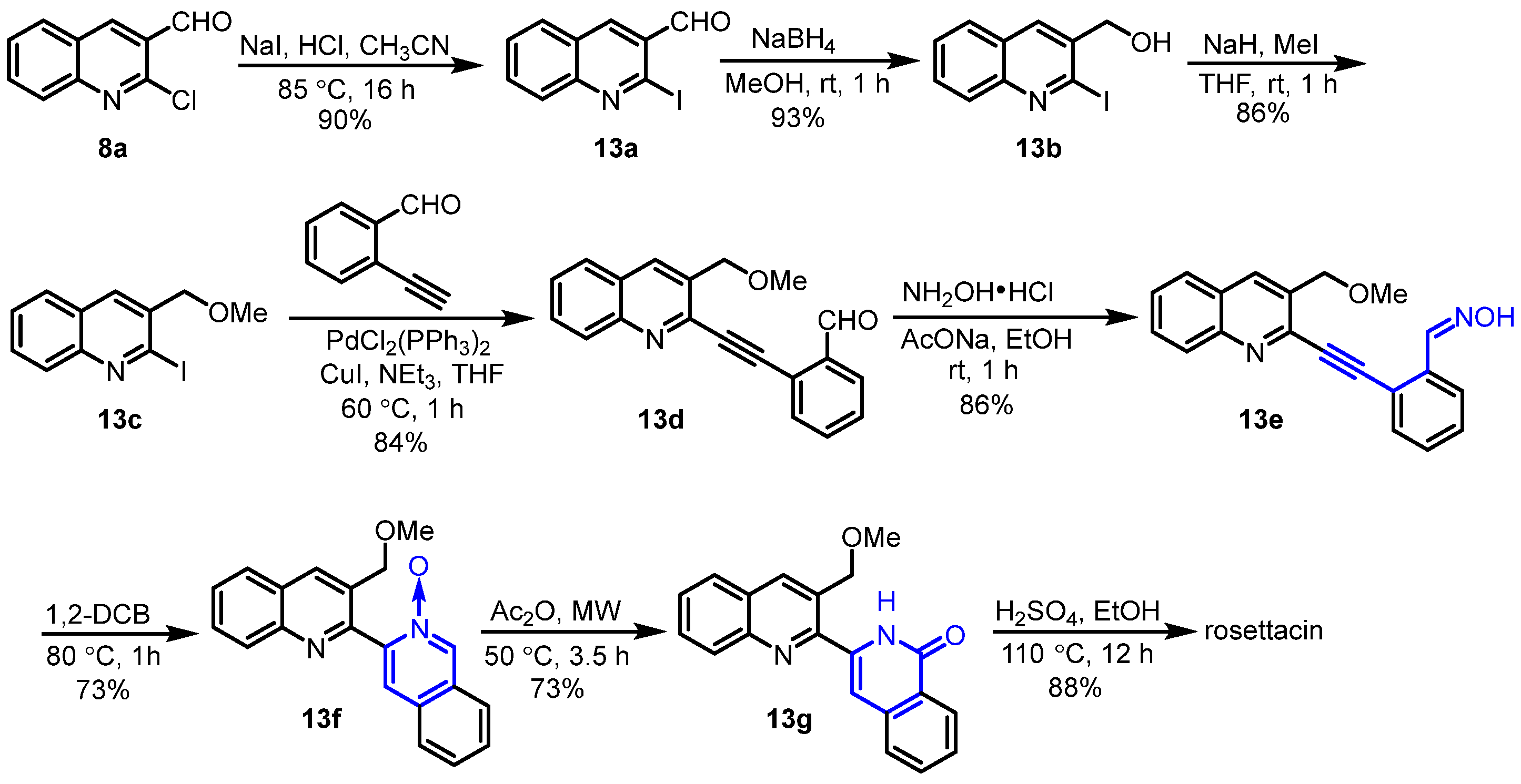
2. Synthesis of Rosettacin
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bur, S.K.; Padwa, A. The Pummerer Reaction: Methodology and Strategy for the Synthesis of Heterocyclic Compounds. Chem. Rev. 2004, 104, 2401–2432. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Buriol, L.; Machado, P. Solvent-Free Heterocyclic Synthesis. Chem. Rev. 2009, 109, 4140–4182. [Google Scholar] [CrossRef] [PubMed]
- Godoi, B.; Schumacher, R.F.; Zeni, G. Synthesis of Heterocycles via Electrophilic Cyclization of Alkynes Containing Heteroatom. Chem. Rev. 2011, 111, 2937–2980. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Ding, S.; Song, L.; Van der Eycken, E.V. Transition Metal-Catalyzed C−H Activation/Annulation Approaches to Isoindolo [2,1-b]isoquinolin-5(7H)-ones. Chem. Rec. 2023, 23, e202200255. [Google Scholar] [CrossRef]
- Brandi, A.; Cicchi, S.; Cordero, F.M.; Goti, A. Heterocycles from Alkylidenecyclopropanes. Chem. Rev. 2003, 103, 1213–1270. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.T.; Yamamoto, Y. Coinage Metal-Assisted Synthesis of Heterocycles. Chem. Rev. 2008, 108, 3395–3442. [Google Scholar] [CrossRef]
- Tang, X.; Song, L.; Van der Eycken, E.V. Post-Ugi Cyclizations towards Polycyclic N-Heterocycles. Chem. Rec. 2023, 23, e202300095. [Google Scholar] [CrossRef]
- Wang, Y.; Cobo, A.A.; Franz, A.K. Recent advances in organocatalytic asymmetric multicomponent cascade reactions for enantioselective synthesis of spirooxindoles. Org. Chem. Front. 2021, 8, 4315–4348. [Google Scholar] [CrossRef]
- St. Jean, D.J., Jr.; Fotsch, C. Mitigating Heterocycle Metabolism in Drug Discovery. J. Med. Chem. 2012, 55, 6002–6020. [Google Scholar] [CrossRef]
- Yamamoto, Y. Synthesis of heterocycles via transition-metal-catalyzed hydroarylation of alkynes. Chem. Soc. Rev. 2014, 43, 1575–1600. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.-X.; Xi, Z. Carbodiimide-based synthesis of N-heterocycles: Moving from two classical reactive sites to chemical bond breaking/forming reaction. Chem. Soc. Rev. 2020, 49, 5810–5849. [Google Scholar] [CrossRef]
- Xue, W.; Warshawsky, D. Metabolic activation of polycyclic and heterocyclic aromatic hydrocarbons and DNA damage: A review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef]
- Meade, J.D.; Hellou, J.; Patel, T.R. Aerobic co-metabolism of sulfur, nitrogen and oxygen heterocycles by three marine bacterial consortia. J. Basic Microbiol. 2002, 42, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Turesky, R.J.; Le Marchand, L. Metabolism and Biomarkers of Heterocyclic Aromatic Amines in Molecular Epidemiology Studies: Lessons Learned from Aromatic Amines. Chem. Res. Toxicol. 2011, 24, 1169–1214. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Lv, Z.; Zhang, K.; Wu, Y.; Van der Eycken, E.V.; Cai, L. Recent Advances in the Asymmetric Total Synthesis of Camptothecin. Asian J. Org. Chem. 2022, 11, e202200515. [Google Scholar] [CrossRef]
- Yuan, J.-M.; Wei, K.; Zhang, G.-H.; Chen, N.-Y.; Wei, X.-W.; Pan, C.-X.; Mo, D.-L.; Su, G.-F. Cryptolepine and aromathecin based mimics as potent G-quadruplex-binding, DNA-cleavage and anticancer agents: Design, synthesis and DNA targeting-induced apoptosis. Eur. J. Med. Chem. 2019, 169, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from camptotheca acuminata1, 2. J. Am. Chem. Soc. 1966, 88, 3888–3890. [Google Scholar] [CrossRef]
- Thomas, C.J.; Rahier, N.J.; Hecht, S.M. Camptothecin: Current perspectives. Bioorg. Med. Chem. 2004, 12, 1585–1604. [Google Scholar] [CrossRef]
- Martino, E.; Volpe, S.D.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorg. Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef]
- Chen, L.; Chen, F.-E. Total Synthesis of Camptothecins: An Update. Synlett 2017, 28, 1134–1150. [Google Scholar] [CrossRef]
- Adams, D.J.; Dewhirst, M.W.; Flowers, J.L.; Gamcsik, M.P.; Colvin, O.M.; Manikumar, G.; Wani, M.C.; Wall, M.E. Camptothecin analogues with enhanced antitumor activity at acidic pH. Cancer Chemother. Pharmacol. 2000, 46, 263–271. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Morrell, A.E.; Dexheimer, T.S.; Agama, K.; Agrawal, S.; Pommier, Y.; Cushman, M. The structure–activity relationships of A-ring-substituted aromathecin topoisomerase I inhibitors strongly support a camptothecin-like binding mode. Bioorg. Med. Chem. 2010, 18, 5535–5552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Liu, G.; Yao, Z.-J. Short and Efficient Total Synthesis of Luotonin A and 22-Hydroxyacuminatine Using A Common Cascade Strategy. J. Org. Chem. 2007, 72, 6270–6272. [Google Scholar] [CrossRef]
- Cheng, K.; Rahier, N.J.; Eisenhauer, B.M.; Gao, R.; Thomas, S.J.; Hecht, S.M. 14-Azacamptothecin: A Potent Water-Soluble Topoisomerase I Poison. J. Am. Chem. Soc. 2005, 127, 838–839. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Morrell, A.; Dexheimer, T.S.; Scher, E.S.; Pommier, Y.; Cushman, M. Design, Synthesis, and Biological Evaluation of 14-Substituted Aromathecins as Topoisomerase I Inhibitors. J. Med. Chem. 2008, 51, 4609–4619. [Google Scholar] [CrossRef]
- Hamid, A.; Souizi, A.; Lawson, A.M.; Othman, M.; Ghinet, A.; Rigo, B.; Daïch, A. Benzo[7,8]indolizinoquinoline scaffolds based on Mg(ClO4)2-promoted regiospecific imide reduction and π-cyclization of N-acyliminium species. Analogues of the topo-1 poison rosettacin and 22-hydroxyacuminatine alkaloids. Arab. J. Chem. 2019, 12, 680–693. [Google Scholar] [CrossRef]
- Warneke, J.; Winterfeldt, E. Reaktionen an Indolderivaten, XVI. Die autoxydative Indol-Chinolon-Umwandlung eines Camptothecin-Modells. Chem. Ber. 1972, 105, 2120–2125. [Google Scholar] [CrossRef] [PubMed]
- Walraven, H.G.M.; Pandit, U.K. A facile two synthon approach to the camptothecin skeleton. Tetrahedron 1980, 36, 321–327. [Google Scholar] [CrossRef]
- Fox, B.M.; Xiao, X.; Antony, S.; Kohlhagen, G.; Pommier, Y.; Staker, B.L.; Stewart, L.; Cushman, M. Design, Synthesis, and Biological Evaluation of Cytotoxic 11-Alkenylindenoisoquinoline Topoisomerase I Inhibitors and Indenoisoquinoline−Camptothecin Hybrids. J. Med. Chem. 2003, 46, 3275–3282. [Google Scholar] [CrossRef]
- Pin, F.; Comesse, S.; Sanselme, M.; Daïch, A. A Domino N-Amidoacylation/Aldol-Type Condensation Approach to the Synthesis of the Topo-I Inhibitor Rosettacin and Derivatives. J. Org. Chem. 2008, 73, 1975–1978. [Google Scholar] [CrossRef]
- El Blidi, L.; Namoune, A.; Bridoux, A.; Nimbarte, V.D.; Lawson, A.M.; Comesse, S.; Daïch, A. Expeditious Synthesis of the Topoisomerase I Inhibitors Isoindolo [2,1-b]isoquinolin-7(5H)-one and the Alkaloid Rosettacin Based on Aryl Radical Cyclization of Enamide Generated by Using N-Acyliminium Chemistry. Synthesis 2015, 47, 3583–3592. [Google Scholar]
- Xu, X.; Liu, Y.; Park, C.-M. Rhodium(III)-Catalyzed Intramolecular Annulation through C-H Activation: Total Synthesis of (±)-Antofine, (±)-Septicine, (±)-Tylophorine, and Rosettacin. Angew. Chem. Int. Ed. 2012, 51, 9372–9376. [Google Scholar] [CrossRef] [PubMed]
- Lerchen, A.; Knecht, T.; Koy, M.; Daniliuc, C.G.; Glorius, F. A General Cp*CoIII-Catalyzed Intramolecular C−H Activation Approach for the Efficient Total Syntheses of Aromathecin, Protoberberine, and Tylophora Alkaloids. Chem. Eur. J. 2017, 23, 12149–12152. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ou, J.; Gao, S. Total Synthesis of Camptothecin and Related Natural Products by a Flexible Strategy. Angew. Chem. Int. Ed. 2016, 55, 14778–14783. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Tian, G.; He, Y.; Van der Eycken, E.V. Rhodium(iii)-catalyzed intramolecular annulation through C–H activation: Concise synthesis of rosettacin and oxypalmatime. Chem. Commun. 2017, 53, 12394–12397. [Google Scholar] [CrossRef]
- Raji Reddy, C.; Mallesh, K. Rh(III)-Catalyzed Cascade Annulations To Access Isoindolo [2,1-b]isoquinolin-5(7H)-ones via C–H Activation: Synthesis of Rosettacin. Org. Lett. 2018, 20, 150–153. [Google Scholar] [CrossRef]
- Song, L.; Tian, G.; Van der Eycken, E.V. Rhodium(III)-catalyzed intermolecular cascade annulation through C-H activation: Concise synthesis of rosettacin. Mol. Catal. 2018, 459, 129–134. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Tian, G.; Robeyns, K.; Van Meervelt, L.; Harvey, J.N.; Van der Eycken, E.V. Intramolecular cascade annulation triggered by CH activation via rhodium hydride intermediate. Mol. Catal. 2019, 463, 30–36. [Google Scholar] [CrossRef]
- Baguia, H.; Deldaele, C.; Romero, E.; Michelet, B.; Evano, G. Copper-Catalyzed Photoinduced Radical Domino Cyclization of Ynamides and Cyanamides: A Unified Entry to Rosettacin, Luotonin A, and Deoxyvasicinone. Synthesis 2018, 50, 3022–3030. [Google Scholar]
- Wang, G.; Hu, W.; Hu, Z.; Zhang, Y.; Yao, W.; Li, L.; Fu, Z.; Huang, W. Carbene-catalyzed aerobic oxidation of isoquinolinium salts: Efficient synthesis of isoquinolinones. Green Chem. 2018, 20, 3302–3307. [Google Scholar] [CrossRef]
- Mizuno, S.; Nishiyama, T.; Endo, M.; Sakoguchi, K.; Yoshiura, T.; Bessho, H.; Motoyashiki, T.; Hatae, N.; Choshi, T. Novel Approach to the Construction of Fused Indolizine Scaffolds: Synthesis of Rosettacin and the Aromathecin Family of Compounds. Molecules 2023, 28, 4059. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.; Crouse, D.N.; Anderson, J.E. Total synthesis of natural 20 (S)-camptothecin. J. Org. Chem. 1975, 40, 2140–2141. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Ives, J.L. Reaction of singlet oxygen with enamino carbonyl systems. A general method for the synthesis of. alpha.-keto derivatives of lactones, esters, amides, lactams, and ketones. J. Org. Chem. 1985, 50, 3573–3580. [Google Scholar] [CrossRef]
- Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M.d.C.; Soriano, E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef]
- Deniau, E.; Enders, D. Synthesis of 3-alkyl-1-isoindolinones by alkylation of a benzotriazolyl substituted N-dimethylamino-phthalimidine. Tetrahedron 2001, 57, 2581–2588. [Google Scholar] [CrossRef]
- Witulski, B.; Stengel, T. N-Functionalized 1-Alkynylamides: New Building Blocks for Transition Metal Mediated Inter- and Intramolecular [2+2+1] Cycloadditions. Angew. Chem. Int. Ed. 1998, 37, 489–492. [Google Scholar] [CrossRef]



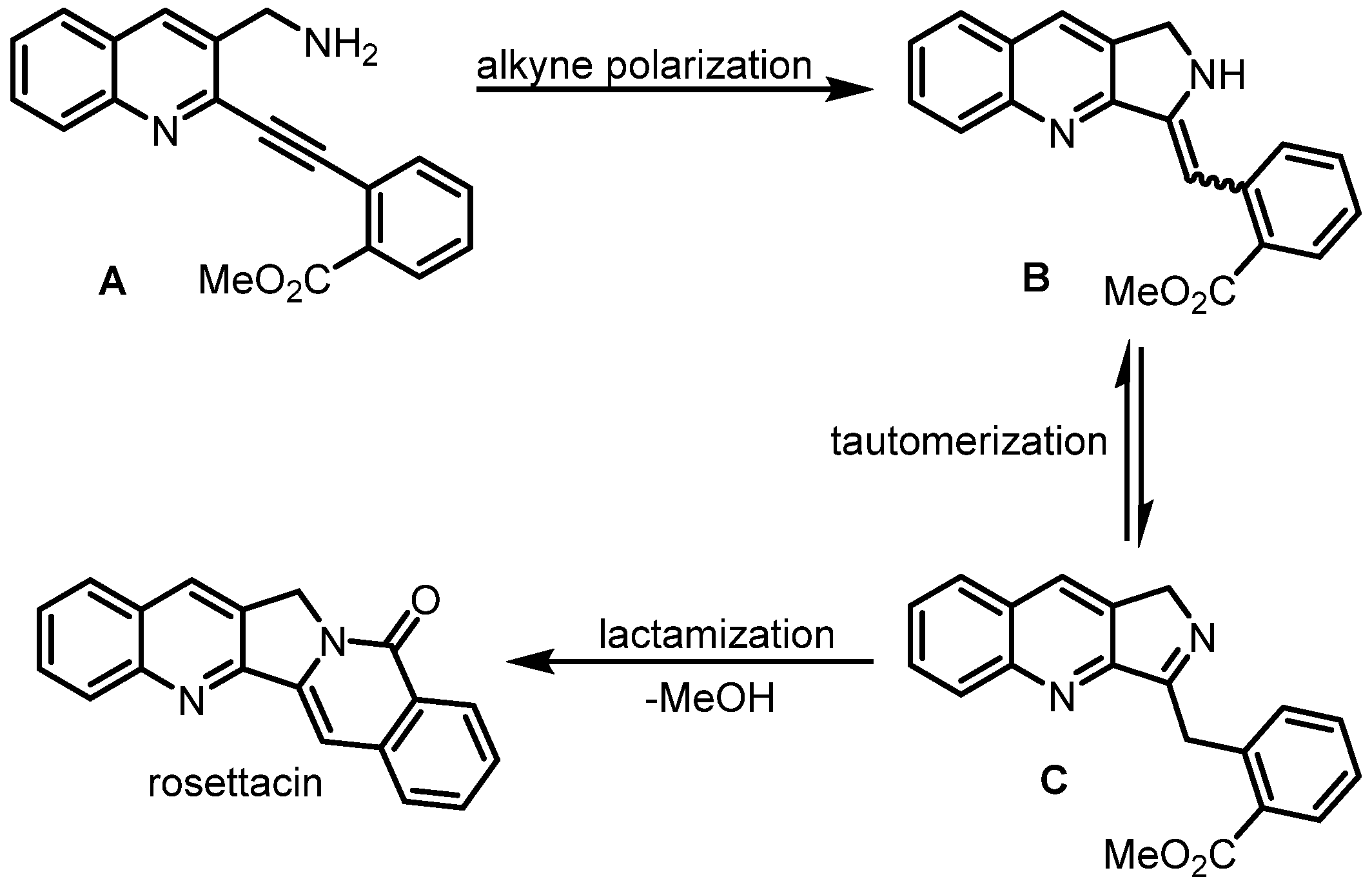
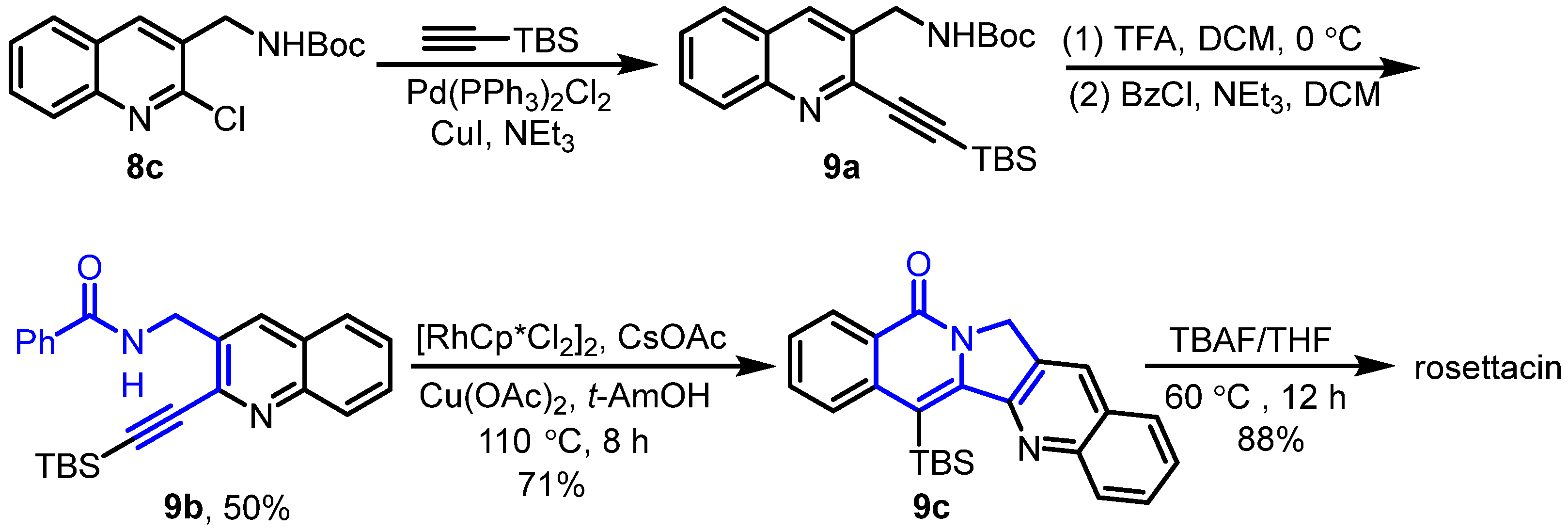
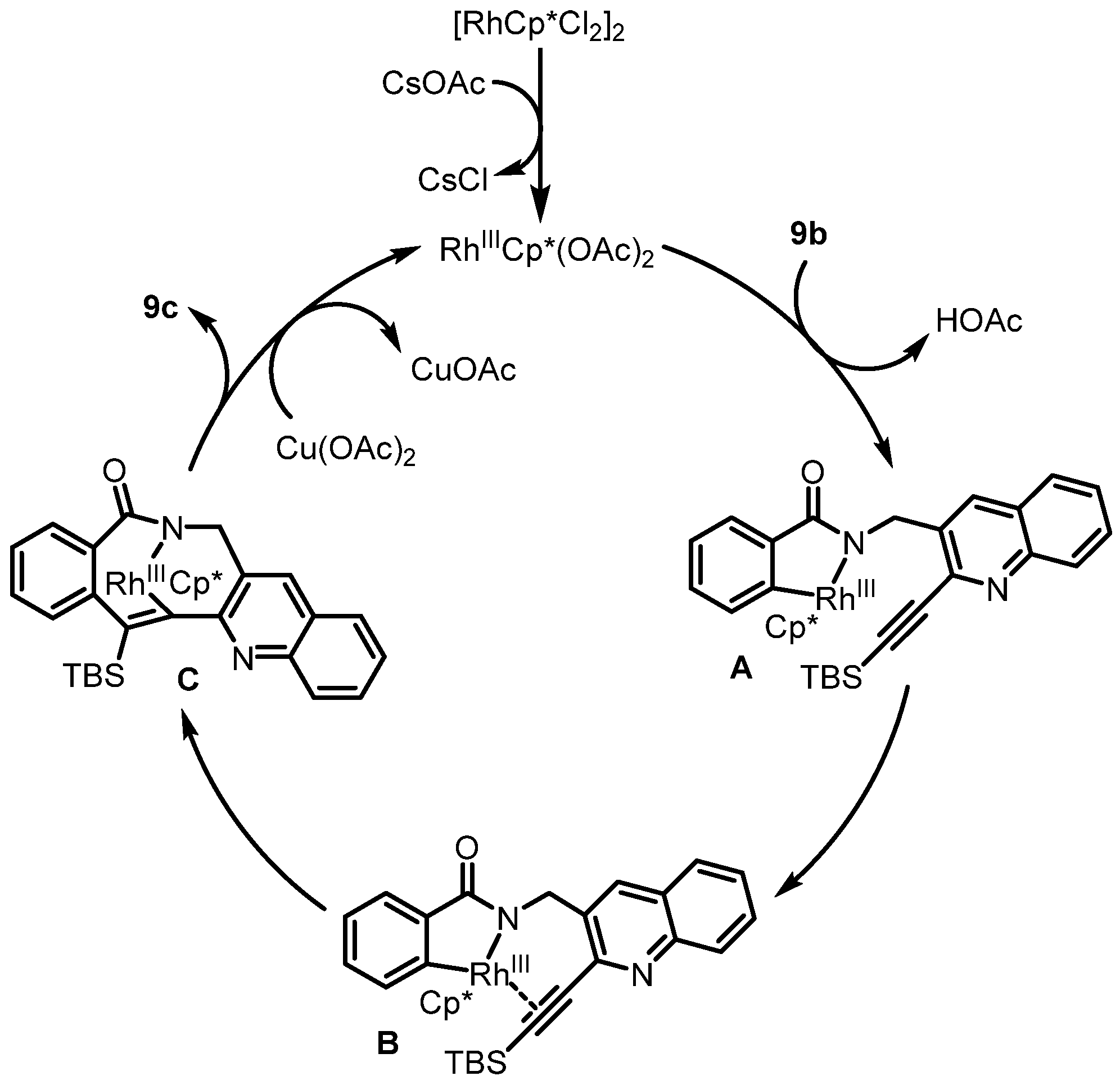
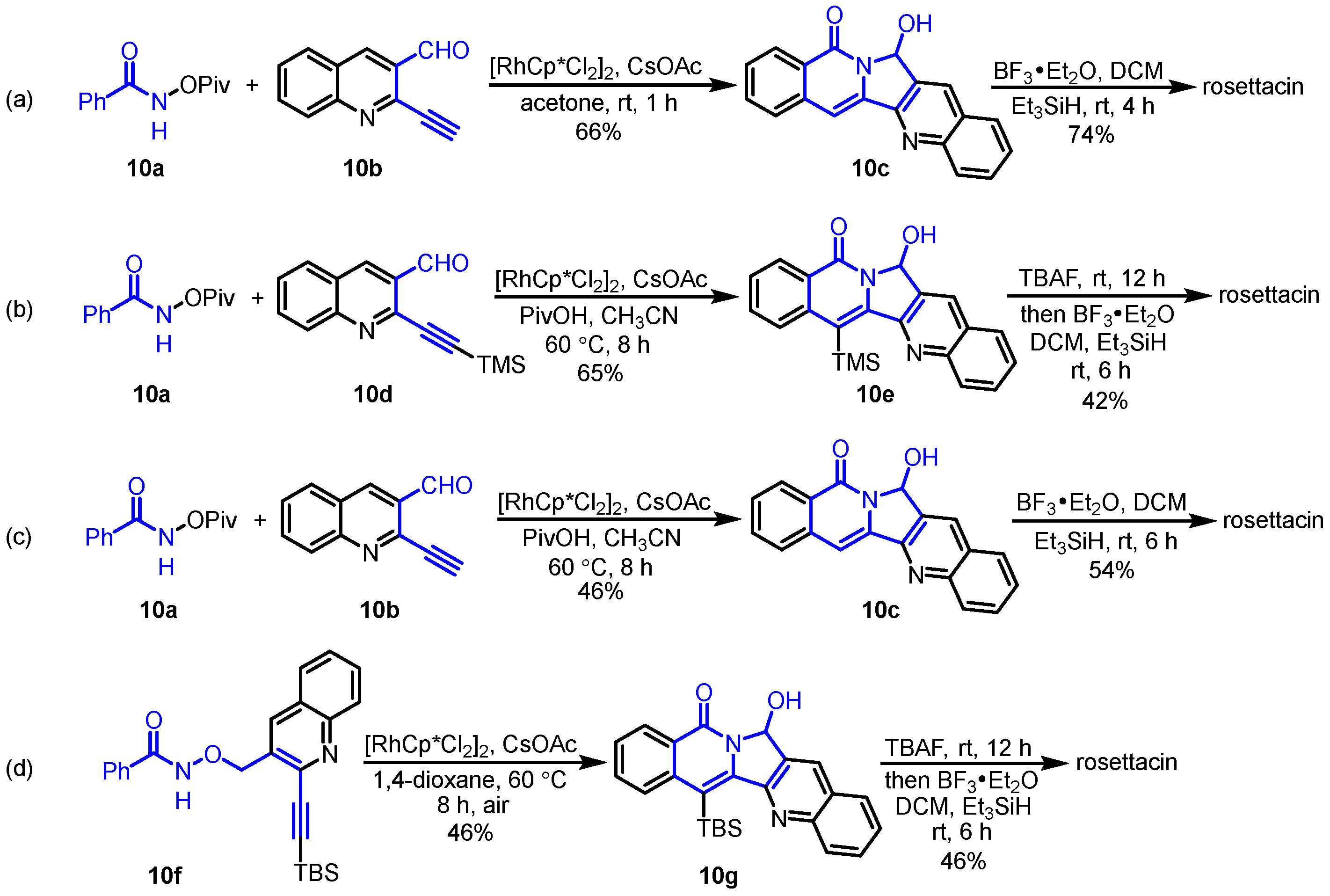
| Year | Author | Key Step | Formed Ring | Reference |
|---|---|---|---|---|
| 1972 | Warneke and Winterfeldt | Oxidative rearrangement | B and C | [27] |
| 1980 | Walraven and Pandit | Aminolysis and aldol condensation | D | [28] |
| 2003 | Cushman | Aminolysis and aldol condensation | D | [29] |
| 2008 | Daïch | N-Amidoacylation/aldol condensation | D | [30] |
| 2015 | Daïch | Aryl radical cyclization | C | [31] |
| 2012 | Park | Rh(III)-catalyzed C-H activation | D | [32] |
| 2017 | Glorius | Co(III)-catalyzed C-H activation | D | [33] |
| 2016 | Gao | exo Hydroamination and lactamization | C and D | [34] |
| 2017 | Van der Eycken | Rh(III)-catalyzed C-H activation | C and D | [35] |
| 2018 | Reddy | Rh(III)-catalyzed C-H activation | C and D | [36] |
| 2018 | Van der Eycken | Rh(III)-catalyzed C-H activation | C and D | [37] |
| 2019 | Van der Eycken | Rh(III)-catalyzed C-H activation | C and D | [38] |
| 2018 | Evano | Cu-catalyzed photoinduced radical domino cyclization | C and D | [39] |
| 2018 | Fu and Huang | Carbene-catalyzed aerobic oxidation and Pd-catalyzed cyclization | C | [40] |
| 2023 | Choshi | Thermal cyclization and Reissert–Henze-type reaction | D | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Jiang, Y.; Song, L.; Van der Eycken, E.V. Recent Advances in the Synthesis of Rosettacin. Molecules 2024, 29, 2176. https://doi.org/10.3390/molecules29102176
Tang X, Jiang Y, Song L, Van der Eycken EV. Recent Advances in the Synthesis of Rosettacin. Molecules. 2024; 29(10):2176. https://doi.org/10.3390/molecules29102176
Chicago/Turabian StyleTang, Xiao, Yukang Jiang, Liangliang Song, and Erik V. Van der Eycken. 2024. "Recent Advances in the Synthesis of Rosettacin" Molecules 29, no. 10: 2176. https://doi.org/10.3390/molecules29102176
APA StyleTang, X., Jiang, Y., Song, L., & Van der Eycken, E. V. (2024). Recent Advances in the Synthesis of Rosettacin. Molecules, 29(10), 2176. https://doi.org/10.3390/molecules29102176







