1. Introduction
1,2-Oxazines and their benzo-fused derivatives are embedded in many drugs and bioactive natural products. Their synthetic strategies, which are used as intermediates in synthesis due to their biological potential, have been comprehensively reported [
1,
2,
3,
4,
5,
6]. Analogues of 1,2-oxazine,
peri-annelated to naphthalene, are not abundant in the literature. The first naphtho[1,8-
de][
1,
2]oxazine to be synthesized was produced by the cyclodehydration of 6,6′,7,7′-tetramethoxygossypol dioxime [
7]. Analogous reactions produced more derivatives [
8,
9]. Varvounis and co-workers oxidized 2-hydroxy-1-naphthaldehyde oxime (
1) with lead(IV) acetate (LTA) in THF, and obtained, for the first time, naphtho[1,8-
de][1,2]oxazin-4-ol (
2) and naphtho[1,2-
d]isoxazole 2-oxide (
4); this was a reaction that never gave
4 again but instead gave
2 together with the spiro adduct-dimer
3 [
10,
11]. Applying the same reaction conditions to (2-hydroxy-1-naphthyl)ketoximes gave the appropriate 3-alkyl-4-hydroxynaphtho[1,8-
de][1,2]oxazines together with the corresponding 2-alkyl-1-hydroxybenzo[
cd]indol-3(1
H)-one side product. (2-Hydroxynaphthalen-1-yl)(phenyl)methanone oxime, on the other hand, gave solely 3-phenyl-4-hydroxynaphtho[1,8-
de][1,2]oxazine [
11]. A high yield of
2 was obtained from the oxidation of
1 with phenyliodonium diacetate (PIDA) in
t-BuOH [
12].
Naphthalenediols have applications in dyes, medicines, catalysis, and batteries [
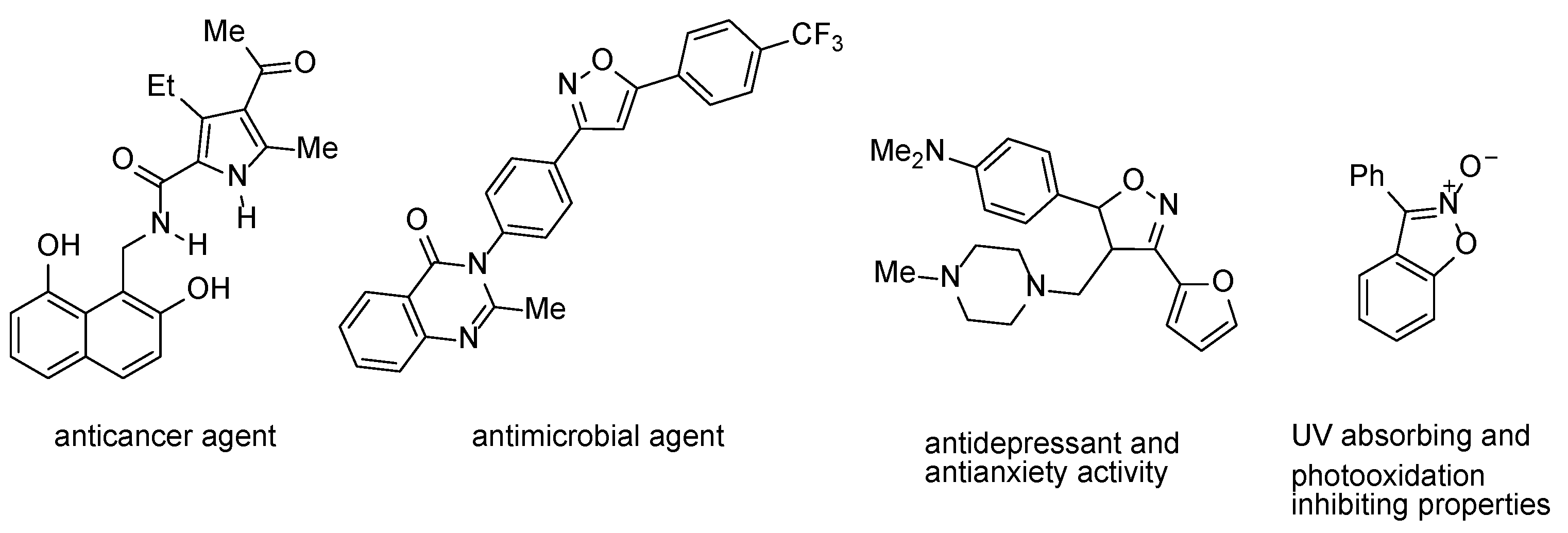
13]. For example, the naphthalenediol derivative with an alkylamide arm (
Figure 1) is an inhibitor of malignant melanoma, breast cancer, and leukemia [
14]. The
ortho-CH acetoxylation and
peri-CH hydroxylation [
15], as well as the
ortho- and
peri-CH methoxylation [
16], of substituted 1-naphthaldehydes are important chemical processes for the preparation of useful structures in synthesis and applications.
Isoxazole and 4,5-dihydroisoxazole (isoxazoline) have long been known as privileged core structures of the family of 5-membered aromatic
N,
O-heterocycles. Their commonly reported di- and trisubstituted derivatives have shown a wide spectrum of applications in medicinal chemistry, marketed drugs, organic materials, and agriculture, and they are versatile building blocks in organic synthesis. These aspects of isoxazoles and isoxazolines have been extensively reviewed [
17,
18,
19,
20,
21]. For example, the depicted 3,5-diarylisoxazole derivative (
Figure 1) has shown excellent antimicrobial activity against all pathogenic strains [
20], while the 3,4,5-trisubstituted 4,5-dihydroisoxazole exhibited antidepressant activity and antianxiety activity [
22]. Dialkyl 3-(napththalen-1-yl)isoxazole-4,5-dicarboxylates were obtained by a 1,3-dipolar cycloaddition of a (naphthalene-1-yl)nitrile oxide in situ, with the appropriate dialkyl acetylenedicarboxylate. The reactions differ by the generation mode of the nitrile oxide from the 1-naphthaldehyde oxime, by the use of PIDA [
23] and oxone
® [
24,
25,
26,
27], or by chlorination and then dehydrochlorination [
28]. 5-Aryl-3-(naphthalene-1- or 2-yl)isoxazoles have been prepared by the oxidative aromatization of 5-aryl-4,5-dihydro-3-(naphthalene-1 or 2-yl)isoxazoles [
29,
30]. 3,5-Disubstituted isoxazoles have been assembled by the 1,3-dipolar cycloaddition of appropriate nitrile oxides and arylacetylenes [
31,
32,
33]. There are only two published reports on the synthesis of ethyl 4,5-dihydro-3-(naphthalen-1- or 2-yl)isoxazole-5-carboxylates [
34]. Both naphthalen-1- and 2-yl derivatives have been synthesized by [2 + 2 + 1] cycloaddition reactions [
35]. 5-Aryl-5-(phenyl or 4-chlorophenyl)-4,5-dihydro-3-(naphthalene-1-yl)isoxazoles [
36,
37] and a few more that have recently been reported have been synthesized [
38,
39].
A series of 3-(methyl or phenyl)benzo[
d]isoxazole-2-oxide derivatives have been studied for their use as novel UV absorbers and photooxidation inhibitors of polystyrene, the most effective being 3-phenylbenzo[
d]isoxazole 2-oxide (
Figure 1) [
40]. The first 3-(methyl, ethyl or phenyl)benzo[
d]isoxazole-2-oxide derivatives were synthesized by Boulton and Tsoungas, using the oxidative cyclization of (2-hydroxy-1-phenyl)ketoximes with LTA or sodium hypochlorite [
41,
42,
43]. The reaction has worked equally efficiently with sodium perborate [
44], PIDA [
40,
45,
46], HTIB [
47], and NCS [
46]. The parent C-3 unsubstituted benzo[
d]isoxazole-2-oxide and the C-1 unsubstituted naphtho[1,2-
d]isoxazole 2-oxide (
4) (vide infra) have so far not been isolated, which is a result of their susceptibility to ring-opening or hydrolysis. In line with stable 3-alkyl benzo[
d]isoxazole 2-oxides, the oxidative cyclization of (
E,
Z)-1-(2-hydroxynaphthalen-1-yl)propan-1-one oxime produced 1-ethylnaphtho[1,2-
d]isoxazole 2-oxide [
11]. The oxidative cyclization of 1-(2-hydroxynaphthalen-1-yl)ethan-1-one oxime with NCS gave stable 1-methylnaphtho[1,2-
d]isoxazole 2-oxide [
46].
Nitrile oxides are highly reactive; they are mostly non-isolable structures, generated in situ from various precursors [
48]. In turn, they, as ambiphilic dipoles with a low HOMO-LUMO energy difference [
49], serve as precursors to diverse heterocycles, mainly the 5-membered ones [
50,
51,
52]. Relatively few substituted arylnitrile oxides stable enough to be isolable, such as those bearing a bulky
t-butyl or mesityl group, an alkoxy, and/or a dimethyl acetal group, have been reported [
53,
54,
55,
56]. Moreover, several stable (naphthalen-1-yl)nitrile oxide derivatives have been found in the literature [
33,
53,
57,
58,
59].
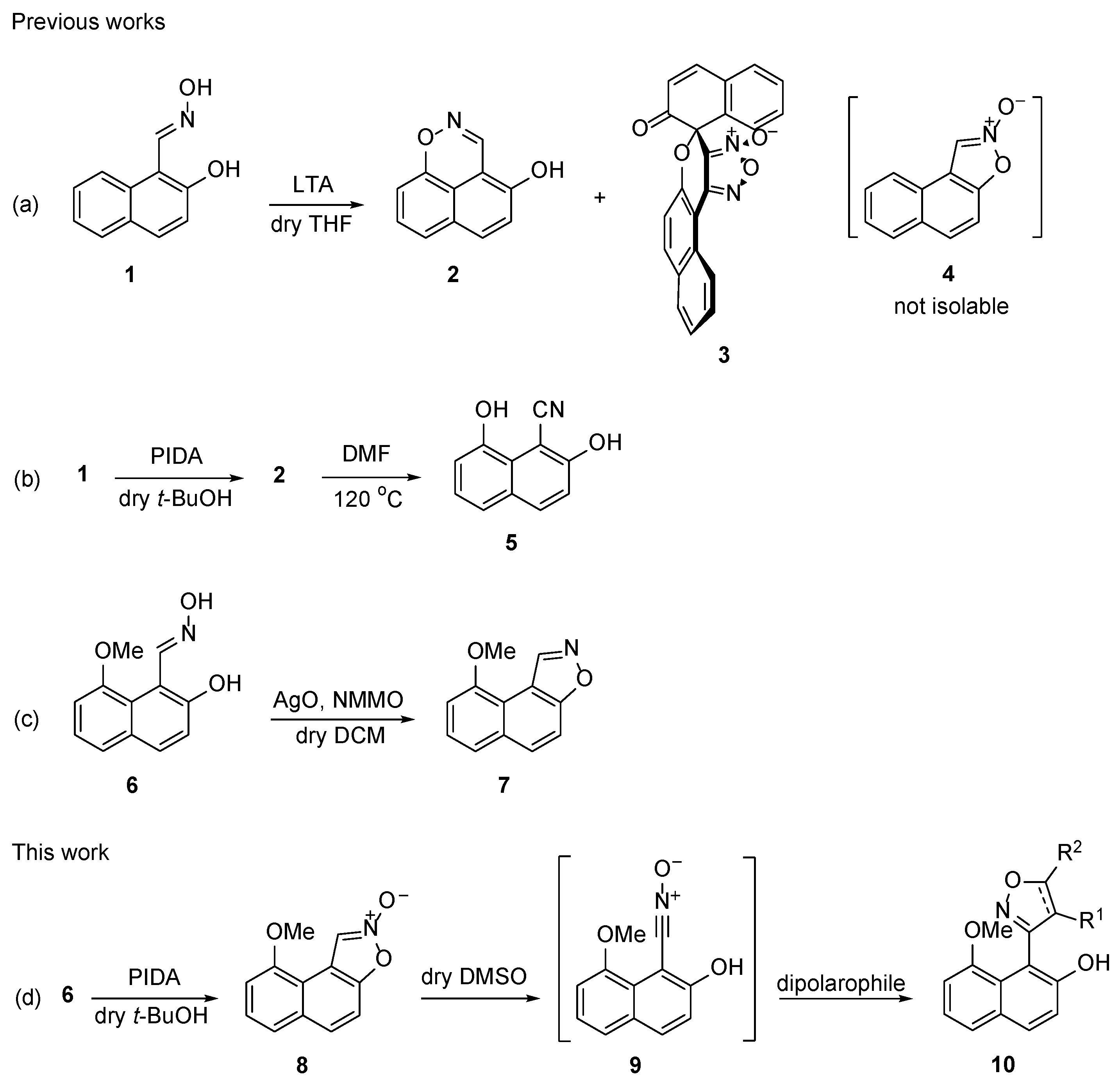
In our previous works, we demonstrated that the oxidation of oxime
1 with LTA, a one-electron oxidant, gave rise to intermediate
o-naphthoquinone nitrosomethide, which then underwent
peri-cyclization and intermolecular cyclodimerization to naphtho[1,8-
de]-[1,2]oxazine (
2) and (±)-
spiro adduct-dimer
3, respectively (
Scheme 1a) [
10,
11]. Later, we established that when
1 was oxidized with PIDA, a two-electron oxidant, in non-nucleophilic
t-BuOH, oxidative
peri-cyclization occurred exclusively and provided
2 in an 80% yield [
12] (
Scheme 1b). More recently,
1 was oxidized by AgO, in the presence of
N-methyl morpholine
N-oxide (NMMO), to afford the
spiro adduct-dimer
3 (
Scheme 1a) as the sole product. Under the same reaction conditions, oxime
6 afforded 9-methoxynaphtho[1,2-
d]isoxazole (
7), instead of the corresponding
spiro adduct-dimer [
60] (
Scheme 1c). We therefore conclude that the formation of the
spiro adduct-dimer is sterically impeded by the
peri-OMe substituents.
Herein, we report an optimized synthetic protocol for 2-hydroxy-8-methoxy-1-naphthaldehyde (
14) from oxazine
2, which provides access to a variety of 1,2,8-trisubstituted naphthalenes. The transformation of the pivotal aldehyde to oxime
6; oxidative cyclization to 9-methoxynaphtho[1,2-
d]isoxazole 2-oxide (
8); isomerization in DMSO to 2-hydroxy-8-methoxy(naphthalen-1-yl)nitrile oxide (
9); and the reaction with various dipolarophiles afforded substituted isoxazoles
10 (
Scheme 1d).
2. Results and Discussion
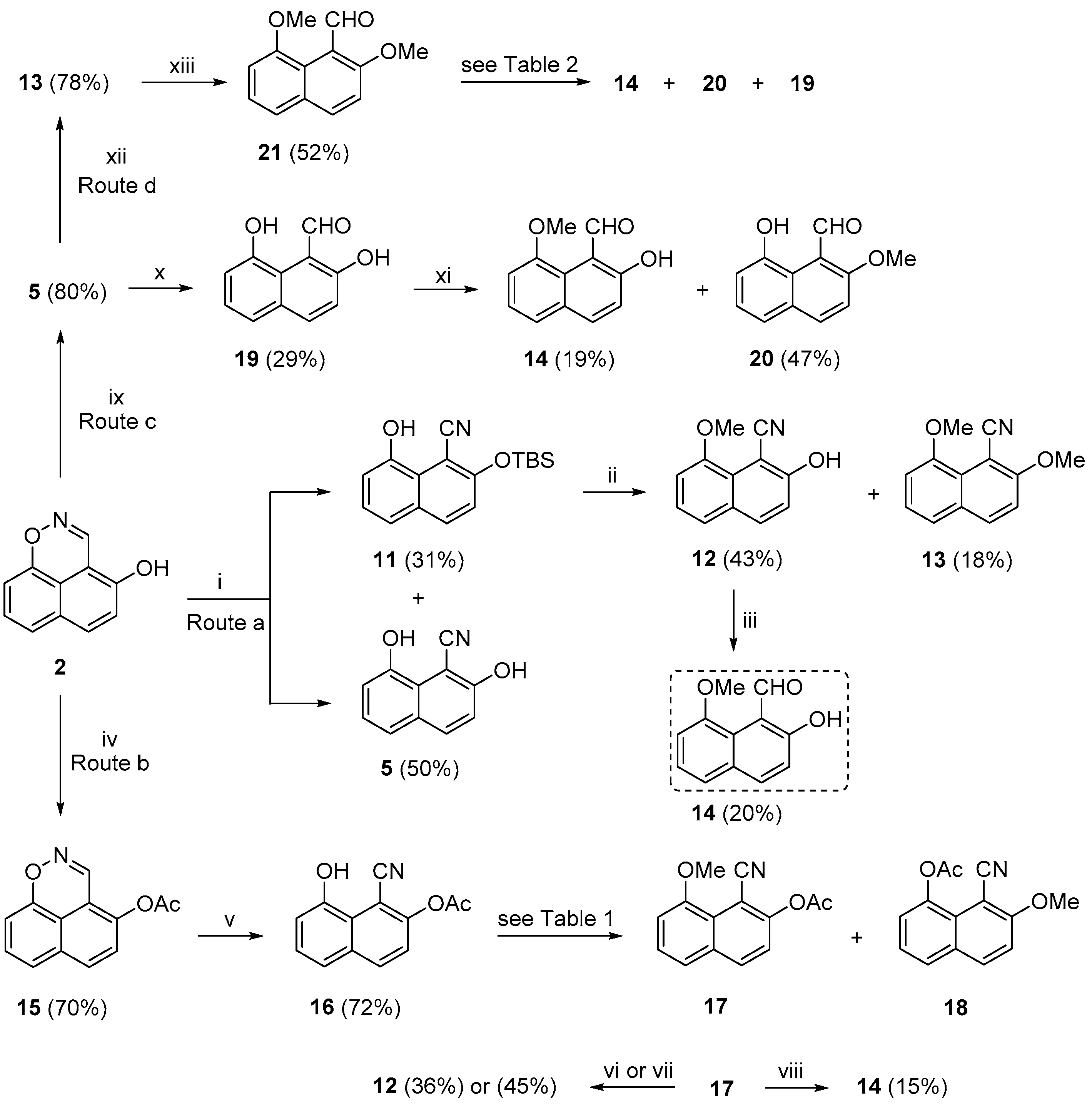
Following up our previous observations about the instability of unsubstituted naphtho[1,2-
d]isoxazole 2-oxide (
4) [
11] (
Scheme 1a) and the effect of the C-8 OMe group of (
E)-oxime (
6) on its AgO (or Ag
2O) selective oxidation to 9-methoxynaphtho[1,2-
d]isoxazole (
7) [
60] (
Scheme 1c), we investigated the outcome of a PIDA oxidation of that oxime, a reagent used successfully for the oxidative cyclization of 2-hydroxyaryl ketoximes into 3-arylbenzo[
d]isoxazole 2-oxides [
46]. Our recently reported [
60] key structure aldehyde
14 (
Scheme 2) was prepared by an
o- and
peri-methoxylation of 1-naphthaldehyde with Pd(OAc)
2, K
2S
2O
8, and 3-(trifluoromethyl)aniline in a closed vessel to 2,8-dimethoxy-1-naphthaldehyde in a 30% yield and was further selectively demethylated by AlCl
3 to
14 in a 15% yield. The low overall yield (4.5%) and the use of a sealed vessel steered us towards an alternative route to
14. The high-yielding (80%) synthesis of
2, followed by its equally high-yielding (80%) ring-opening to
5 (
Scheme 1b), provided relevant and attractive starting materials.
Thus, compound
2 was reacted with TBSCl and imidazole in dry DMF at room temperature (
Scheme 2, Route a), following a relevant report by Bencivenni and co-workers [
61], then heated at 120 °C for a short period of time, under the conditions used in our previous report (
Scheme 1b) [
10]. The expected ring-opened 2-TBS protected 1-naphthonitrile
11 and the unprotected 1-naphthonitrile
5 were produced in 31% and 50% yields, respectively. Compound
5 most likely resulted from
11 by the cleavage of its TBS group. The methylation of
11 (MeI and K
2CO
3 in dry acetone), under the conditions used by Bencivenni and co-workers [
61] on 7-[(
tert-butyldimethylsilyl)oxy]naphthalen-1-ol, furnished the selectively methylated nitrile
12 in a 43% yield and dimethylated nitrile
13 in an 18% yield. Nitrile
12 was then reduced to aldehyde
14 with DIBAL in toluene at 0 °C [
62] and then at room temperature, in our case. The overall yield of
14 from
2, as described in
Scheme 2, was only 2.8%.
In our next attempt (
Scheme 2, Route b), we acetylated
2 with acetic anhydride, according to an old, published procedure [
8], and obtained acetate
15 in a 70% yield. The ring-opening of
15 in DMF at 120 °C, under conditions used previously [
10], afforded 1-naphthonitrile
16 in a 72% yield. We then conducted a search for methylation of the OH group of
16 without cleaving the acetyl group (
Table 1). For a detailed description of the procedures, see Materials and Methods (
Section 3) [
63]. The origin of
18 may well have been derived from an intermolecular acetylation between two molecules of
16, followed by the methylation of the resulting 8-acetoxy-1-cyanonaphthalen-2-olate. The methylation outcome of
16 remained the same, under different conditions but with varying yields of
17 and
18. It was interesting to note that the solvent effect in the last two attempts apparently favored
17 (41% yield) compared to
18 (9% yield). Moreover, the OMe group in
17 was properly placed for further deprotection and reduction to the target
16. Thus, by reducing
17 with either DIBAL in dry THF at −78 °C [
62] or with PtO
2 in an equal volume of HCO
2H/H
2O at 55–60 °C [
64],
12 was obtained in 36% and 45% yields, respectively. The reduction of
17 to
14 proved difficult, under either the standard DIBAL conditions [
62] or an attempted modification (initiating the reaction at 0 °C and allowing a long period at room temperature), and gave a disappointing 15% yield and an overall 3% yield from
2.
In our next attempt (
Scheme 2, Route c), we anticipated that the 6-membered intramolecular H bond in aldehyde
19 between the CHO oxygen atom and the OH hydrogen atom would be strong enough to survive in DMF and would thus encourage selective methylation at the
peri position. Thus, the ring-opening of
2 to
5 [
10] and the reduction of the CN group in
5 with calcium hypophosphite, in the presence of a base and nickel(II) acetate tetrahydrate, according to Estelle Métay, Marc Lemaire, and co-workers [
65], who reduced 1-naphthonitrile to 1-naphthaldehyde in an 85% yield, led to a disappointing 29% yield of
19. Aryl nitriles bearing OH groups are tolerant to these conditions. The methylation of
19 (dry DMF and at room temperature, in the presence of MeI and K
2CO
3) afforded the targets
14 and
20 in 19% and 47% yields, respectively [
15]. The formation of
20 implies that in a DMF solution of
19, the intramolecular H bond between the CHO group and the
peri-OH group survives, whereas that between the CHO group and the
ortho OH group is disrupted by intermolecular H bonding with the solvent. The outcome of this effort was a 4% overall yield of
6, which was more or less the same as in
Scheme 2, Route b.
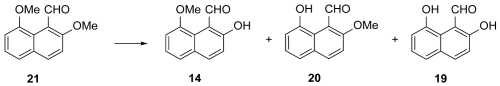
The next plan was to alkylate
5, reduce the resultant nitrile
13 to the corresponding aldehyde
21, and then selectively demethylate to the target
14 (
Scheme 2, Route d). Accordingly, the dimethylation of
5 (MeI and Na
2CO
3, in aqueous acetone, under mild heating) [
61], gave
13 in a satisfying 78% yield. The latter was reduced to the aldehyde
21 in a moderate yield by a modification of the standard DIBAL conditions [
62], as described earlier. Aldehyde
21 was then subjected to what we hoped would be the selective deprotection of the 2-OMe group to the target
14 in a useful yield. Thus, the selective demethylation of
21 to
14 entailed various reagents and conditions, as depicted in
Table 2. For a detailed description of the procedures, see Materials and Methods (
Section 3) [
66,
67]. We can assume that at low temperature, the
peri-OMe group in
21 is intramolecularly engaged in a H bond with the CHO group for most of the time. Consequently, this H bonding interaction allows a relatively easier demethylation of its more available
o-OMe counterpart. The moderate yields of the last two steps of this reaction sequence towards
14 diminished its value, despite an increase in the overall yield to 18%.
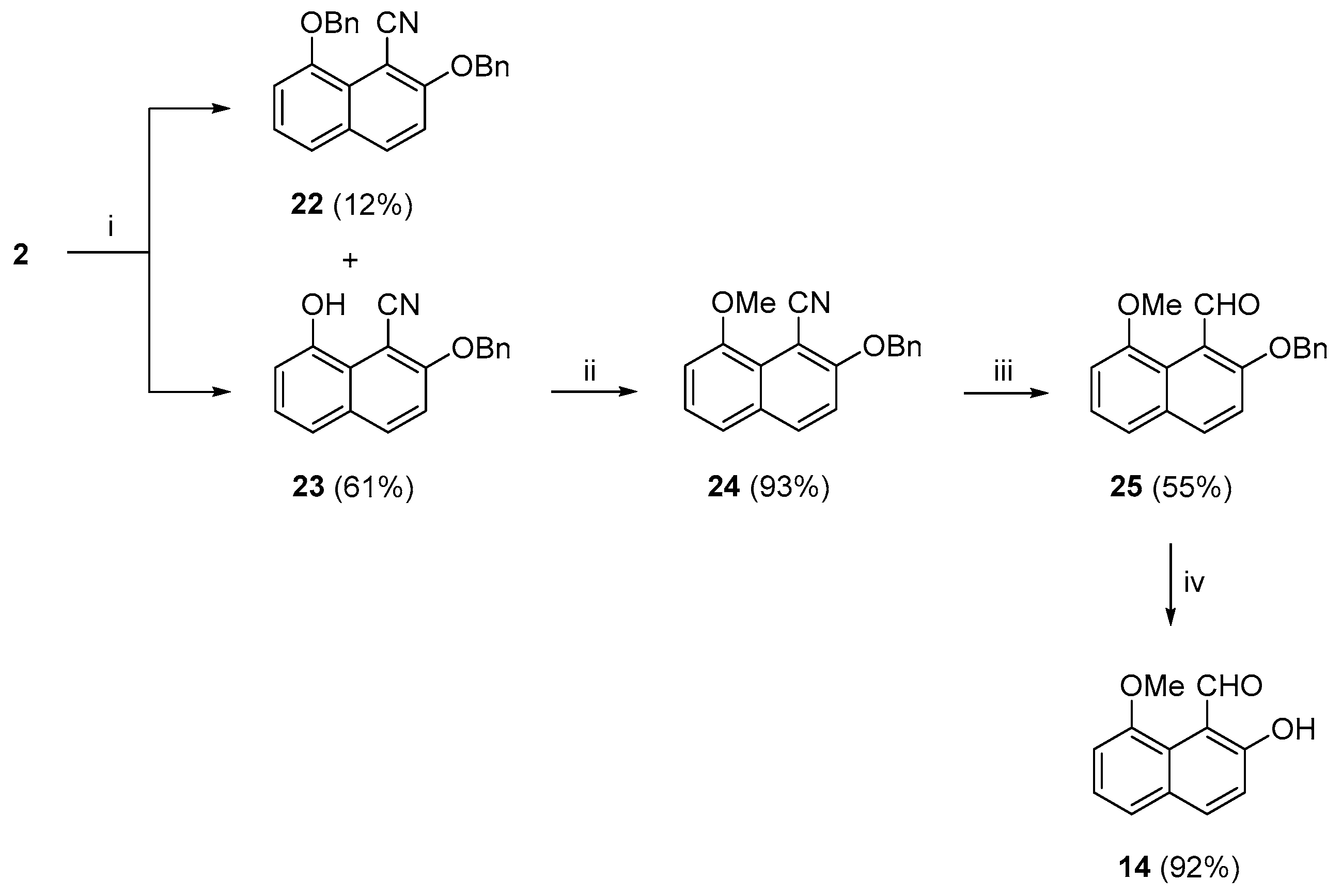
The final attempt to synthesize
14 is delineated in
Scheme 3. Accordingly, we started with an
O-benzylation of
2 and then a ring-opening of the resulting 4-(benzyloxy)naphtho[1,8-
de][1,2]oxazine (by heating in DMF) to the target precursor
23. Further, we envisaged the methylation of the
peri-OH group, the reduction of nitrile to aldehyde, and the debenzylation of the OBn group. For the benzylation step, we slightly modified the conditions applied by Luo and Zheng and co-workers [
68] to convert 2-hydroxy-1-naphthaldehyde to 2-(benzyloxy)-1-naphthaldehyde. Starting from
2, we used benzyl bromide, instead of benzyl chloride, in the presence of K
2CO
3 and KI and stirred the reaction in acetone at room temperature for 18 h. Compounds
22 and
23 were identified in 12% and 61% yields, respectively. To our surprise, the intermediate 4-(benzyloxy)naphtho[1,8-
de][1,2]oxazine was not detected. Apparently, the oxazine suffered ring-opening in the presence of the base. Indeed, this result was experimentally verified by stirring
2 with K
2CO
3 and KI in acetone, and after 4 h,
5 was obtained as a single product. It may be argued that benzylation could take place either sequentially, first on
2 and then on ring-opened
22 to
23, or on the ring-opened
5. The higher yield of
23 points to the former process. In the second step of this reaction sequence,
23 was methylated (MeI and K
2CO
3 in dry acetone under reflux) [
61] to afford the 8-OMe derivative
24 in an excellent 94% yield. The reduction of the nitrile group of
24 was accomplished under conditions described earlier [
62], and aldehyde
25 was thus obtained in a moderate 55% yield. The debenzylation of
25 took place with H
2 and Pd/C as catalysts, according to an analogously reported procedure [
69] that gave
14 in an excellent 92% yield. The overall yield of
14 from
2 by this route (
Scheme 3) was increased to 29%.
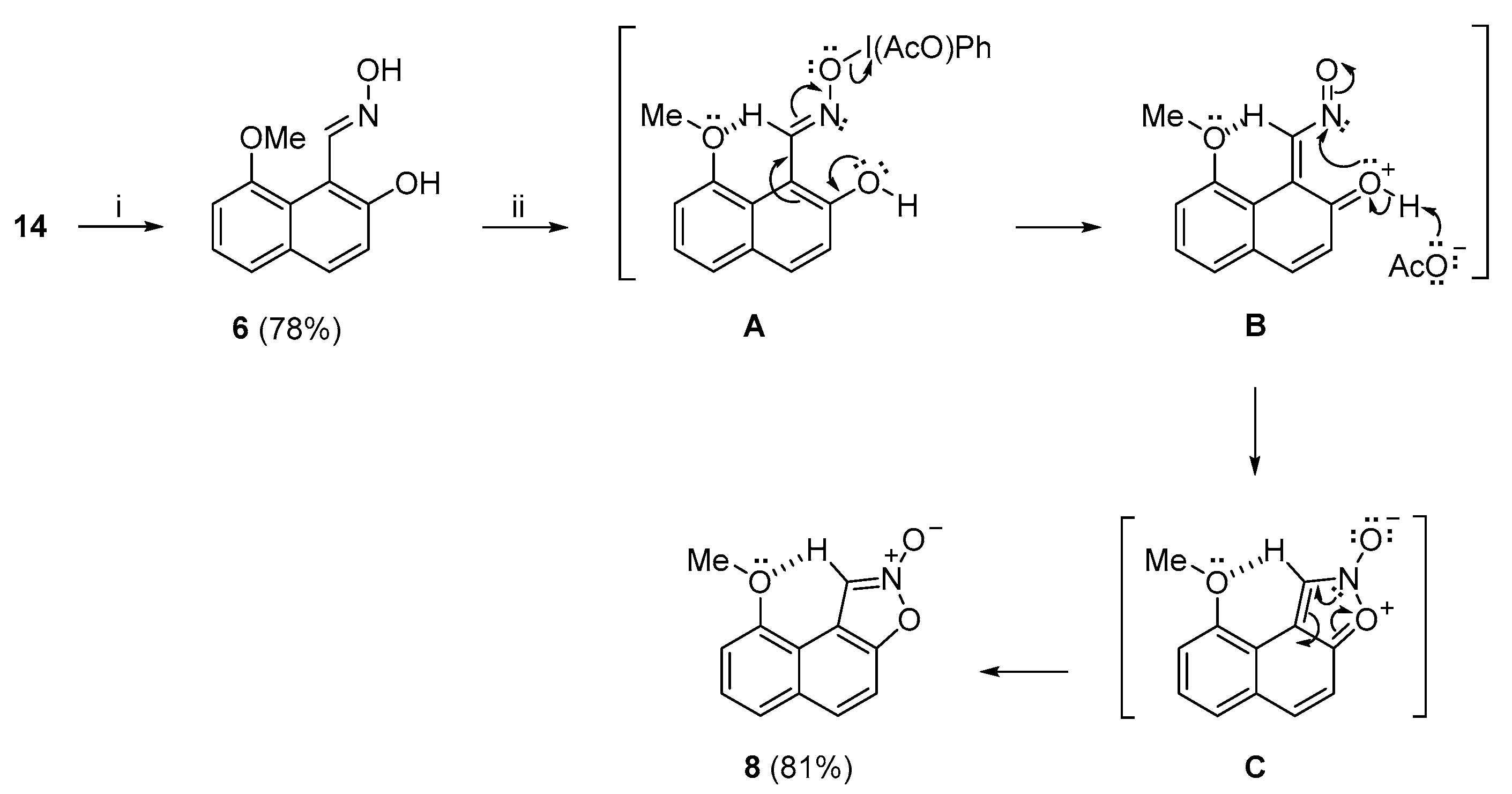
Having established a viable route to target
14, we moved to the synthesis of (
E)-oxime
6 and its reaction with PIDA (
Scheme 4). Compound
6 was accordingly obtained in a 78% yield by a method in a recent report by Tzeli, Tsoungas, and co-workers [
60]. Compound
6 was then subjected to oxidation with PIDA in non-nucleophilic dry
t-BuOH at room temperature, to afford isoxazole-2-oxide
8 in a very good yield (81%). We propose that the initially formed organoiodo complex
A by the ligand coupling mechanism [
70] collapses to
o-naphthoquinone nitrosomethide intermediate
B; the driving force is the rupture of the weaker O–I bond compared to the N–O bond.
B underwent a 6π-electrocyclization to the non-aromatic naphthoisoxazole-2-oxide
C, which aromatized to stable 2-oxide
8. The stability of
8 was apparently due to the intramolecular H bonding between the OMe
peri substituent and the sp
2 C–1 hydrogen atom (2.006 Å) [
60], which impeded the ring-opening of the isoxazole ring. This result could well serve as a rationale for the non-isolable naphtho[1,2-
d]isoxazole 2-oxide (
4), which lacks this particular stabilizing factor (
Scheme 1a) [
11].
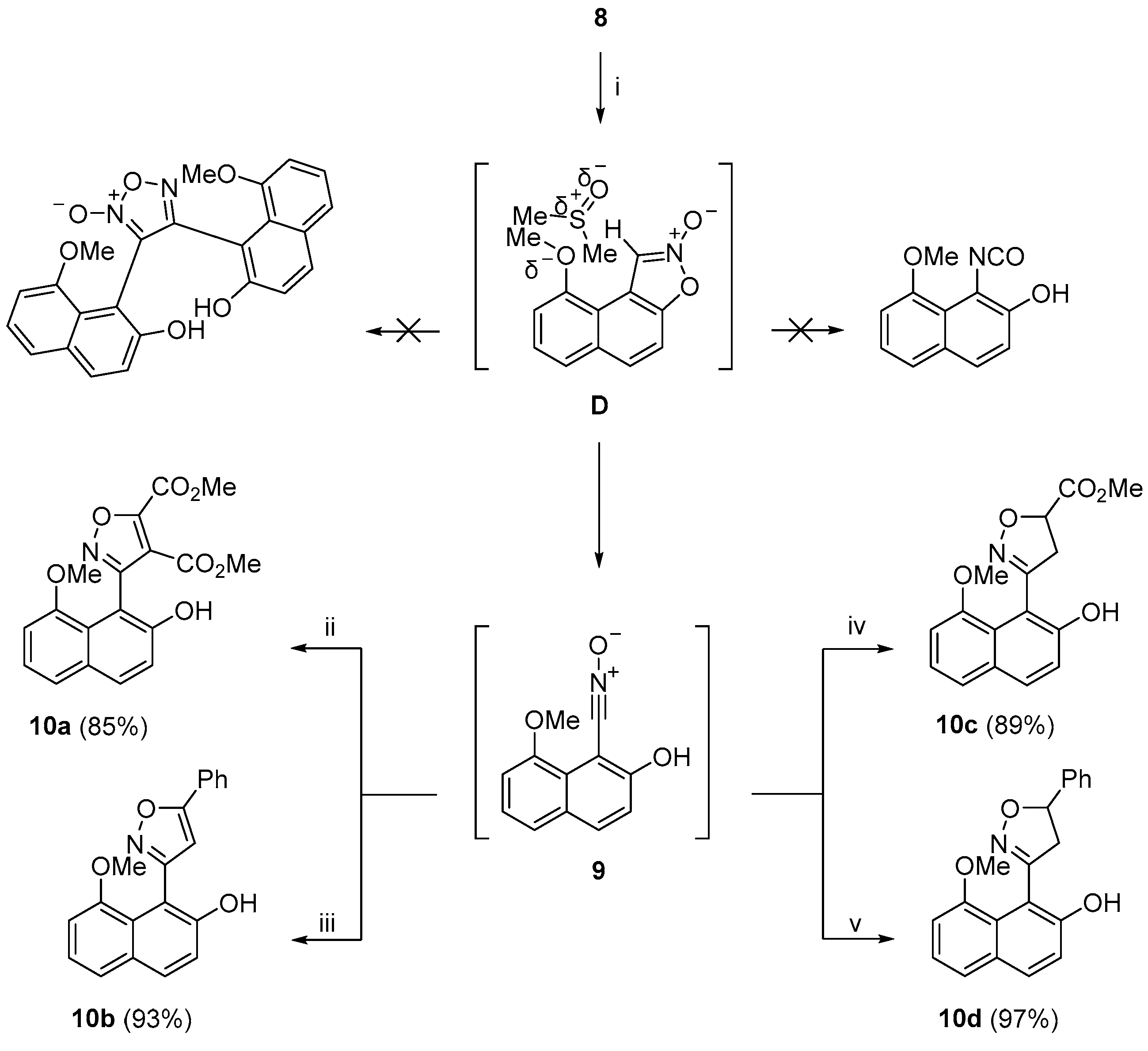
When first recorded, the
1H NMR spectrum of
8 in DMSO-
d6 did show the peaks corresponding to its structure. When recorded again the next day, its
1H NMR spectrum was identified as that of the nitrile oxide
9 (see
Supplementary Figure S59 for superimposed and
Figure S60 for stacked
1H NMR spectra of
8 and
9). This novel isomerization of
8 to
9 was also detected by
1H NMR spectroscopy, which was measured by a time-course plot (see
Supplementary Figure S61). When recorded in CDCl
3, the
1H and
13C NMR spectra of
8 confirmed that the compound is stable in this solvent for at least 18 h. We stirred a sample of
8 in DMSO-
d6 and after 6 h recorded the
1H and
13C NMR spectra. As with the previously recorded NMR spectra of nitrile oxide
9, in the
1H NMR spectrum in DMSO-
d6, the OH proton at a high field was not visible, and there was a total of five aromatic protons belonging to the naphthalen-2-ol ring in the range of 7.90–7.02 ppm. The characteristic singlet of the methyl group was found at 3.92 ppm. The
13C NMR spectrum in DMSO-
d6 showed 11 signals. According to Koyama, Takata, and co-workers, [
71] quaternary signals of nitrile groups usually turn up under the residual DMSO signal so that for nitrile oxide
9 the total number of carbon atoms was 12, as expected. High-resolution mass spectrometry (HRMS) analysis confirmed the expected molecular ion at
m/
z = 216.0661 [M + H]
+ (ESI), which took up a proton and was calculated for C
12H
10NO
3 m/
z = 216.0661. There was no peak at
m/
z = 431.1243 [M + H]
+ corresponding to the dimerization of
9 to its 1,2,5-oxadiazole 2-oxide (
Scheme 5). The confirmation of the nitrile oxide structure of
9 and not its isocyanate isomer came from 1,3-dipolar cycloaddition reactions of in situ generated nitrile oxide
9 with various dipolarophiles, which produced substituted isoxazoles
10a–
d (
Scheme 5). The reactions took place by dissolving isoxazole 2-oxide
8 in DMSO, under an atmosphere of nitrogen, followed by the addition of the appropriate dipolarophile (DMAD, phenylacetylene, methyl acrylate, or styrene, stirred at room temperature for 18 h). The substituted isoxazoles
10a–
d were produced in 85%, 93%, 89%, and 97% yields, respectively. It was suggested that when 2-oxide
8 was dissolved in DMSO, the solvated species
D could suffer disruption of the intramolecular H bond between the MeO group and the C-1 hydrogen atom, allowing for the ring-opening of the isoxazole ring to its nitrile oxide isomer
9, which then underwent 1,3-dipolar cycloaddition with the dipolarophiles (
Scheme 5).
3. Materials and Methods
The organic solutions were concentrated by rotary evaporation at 40 °C under 15 Torr. Melting points were taken using a Büchi 510 apparatus (Büchi Labortechnik AG, Flawil, Switzerland) and are uncorrected. 1H and 13C NMR spectra were measured in CDCl3 or DMSO-d6 on a 400 MHz Brüker Avance spectrometer (Brüker BioSpin GmbH, Rheinstetten, Germany). 1H chemical shifts are reported in ppm from an internal standard TMS, residual CHCl3 (7.26 ppm), or DMSO (2.50 ppm). 13C NMR chemical shifts are reported in ppm from an internal standard TMS, residual CHCl3 (77.00 ppm), or DMSO (39.43 ppm). High resolution ESI mass spectra were measured on a Thermo Fisher Scientific Orbitrap XL system (Thermo Fisher Scientific, Waltham, MA, USA). IR spectra were acquired on an Agilent Cary 630 FTIR spectrophotometer (Agilent Technologies, 5301 Stevens Creek Blvd. CA, USA) as solids and are reported in wave numbers (cm−1). Analytical thin layer chromatography (TLC) was performed with TLC plates (Merck 70–230 mesh silica gel). TLC visualization took place under a 254 nm UV light source. Purification of the reaction products was generally conducted by flash column chromatography using Carlo Erba Reactifs-SDS silica gel 60. Solvents, reagents, and catalysts were used as received from the manufacturers, Thermo Scientific™ (Paisely, U.K.), Sigma-Aldrich (St. Louis, MO, USA), and Merck Chemicals GmbH (Darmstadt, Germany), except for DCM, EtOAc, and hexane, which were dried and purified according to the recommended procedures.
3.1. Synthesis of 2-[(Tert-butyldimethylsilyl)oxy]-8-hydroxy-1-naphthonitrile (11) and 2,8-Dihydroxy-1-naphthonitrile (5)
To a solution of compound 2 (500 mg, 2.2 mmol, 1 equiv) in dry DMF (15 mL), under an atmosphere of N2, was added imidazole (374 mg, 5.5 mmol, 2.5 equiv) and TBSCl (398 mg, 2.64 mmol, 1.2 equiv), and the reaction was left stirring at room temperature for 2 h (TLC analysis showed the absence of the starting material spot and the presence of a new spot). The reaction mixture was then heated at 120 °C for 0.5 h. TLC examination revealed the absence of the starting material spot and the presence of two new spots. To the cooled reaction mixture, water (100 mL) was added and extracted with EtOAc (3 × 20 mL), and the combined organic extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated in a vacuum. The acquired crude residue was purified by flash column chromatography (25% EtOAc in hexane) to give the title compounds.
Compound 11: (204 mg, 31%) as a yellow oil; Rf = 0.61 (25% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: a broad singlet corresponding to OH is not visible, 7.76 (d, J = 8.9 Hz, 1H), 7.26 (d, J = 8.2 Hz, 1H), 7.18 (t, J = 7.5 Hz, 1H), 7.00–6.90 (m, 2H), 1.01 (s, 9H), 0.25 (s, 6H); 13C NMR (100.6 MHz, CDCl3) δ: 160.0, 150.9, 134.9, 130.4, 125.8, 123.7, 121.1, 120.3, 118.7, 113.2, 95.2, 25.8, 18.5; IR (solid): 3161, 3060, 2955, 2922, 2855, 2215, 2118, 1729 cm−1; HRMS (ESI): m/z [M–H]− calcd. for C17H21NO2Si: 298.1263, found: 298.1264.
Compound
5: (203 mg, 50%) as a yellow solid, m.p. = 166–167 °C (lit. [
10], m.p. = 167–168 °C); R
f = 0.1 (20% ethyl acetate in hexane);
1H NMR (400 MHz, DMSO-
d6)
δ: two broad singlets corresponding to OH are not visible, 11.24 (s, 1H), 10.31 (s, 1H), 8.20 (d,
J = 8.7 Hz, 1H), 7.66 (d,
J = 8.3 Hz, 1H), 7.42 (t,
J = 7.8 Hz, 1H), 7.34 (d,
J = 8.7 Hz, 1H), 7.25 (d,
J = 7.3 Hz, 1H) (in agreement with the
1H NMR data that were previously reported for this compound) [
10].
3.2. Synthesis of 2-Hydroxy-8-methoxy-1-naphthonitrile (12) and 2,8-Dimethoxy-1-naphthonitrile (13)
To a solution of compound 2 (100 mg, 0.334 mmol, 1 equiv) in dry acetone (10 mL), under an atmosphere of N2, was added oven-dried K2CO3 (50 mg, 0.367 mmol, 1.1 equiv) and MeI (70 mg, 0.501 mmol, 1.5 equiv), and the reaction was left stirring at room temperature for 1 h (TLC analysis showed complete conversion of the starting material and the presence of two new spots). Water (20 mL) was added, the reaction mixture was extracted with EtOAc (3 × 10 mL), and the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (11% EtOAc in hexane) to give the title compounds.
Compound 12: (28.6 mg, 43%) as a yellow solid, m.p. = 172–174 °C; Rf = 0.09 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: a broad singlet corresponding to OH is not visible, 7.90 (d, J = 9.0 Hz, 1H), 7.42–7.33 (m, 2H), 7.22 (d, J = 9.0 Hz, 1H), 6.97 (d, J = 7.3 Hz, 1H), 4.05 (s, 3H); 13C NMR (100.6 MHz, CDCl3) δ: 160.4, 153.9, 135.2, 129.8, 125.5, 123.6, 121.2, 117.5, 117.4, 107.9, 89.4, 55.9; IR (solid): 3072, 2920, 2848, 2216, 1705, 1600, 1513 cm−1; HRMS (ESI): m/z [M + Na]+ calcd. for C12H9NO3Na: 222.0531, found: 222.0528.
Compound 13: (12.8 mg, 18%) as a colorless amorphous solid (hexane), m.p. = 147–149 °C; Rf = 0.21 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: 7.83 (d, J = 9.2 Hz, 1H), 7.25 (d, J = 8.2 Hz, 1H), 7.19 (t, J = 7.9 Hz, 1H), 7.13 (d, J = 9.3 Hz, 1H), 6.80 (dd, J = 7.5, 1.2 Hz, 1H), 3.93 (s, 3H), 3.89 (s, 3H); 13C NMR (100.6 MHz, CDCl3) δ: 162.9, 154.3, 134.8, 129.7, 125.2, 123.8, 121.1, 117.0, 112.4, 107.7, 92.6, 56.8, 55.8; IR (solid): 2921, 2848, 2218, 2091, 1915, 1826, 1593 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C13H12NO2: 214.0863, found: 214.0858.
3.3. Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14)
To a stirred solution of
12 (200 mg, 1 mmol, 1 equiv) in dry toluene (10 mL), under an atmosphere of N
2 at 0 °C, was added DIBAL (1.67 mL of 1.2 M in toluene, 2 mmol, 2 equiv), and the reaction mixture was left stirring for 0.5 h and then for 18 h at room temperature (TLC analysis showed the absence of a starting material spot and the presence of a new spot). The solvent was removed under reduced pressure, water (20 mL) was carefully added to the residue, and the resulting mixture was cooled to 0 °C, followed by the dropwise addition of 1 M aqueous HCl until pH = 1. The aqueous solution was extracted with EtOAc (3 × 10 mL), and the combined organic extracts were washed with brine (20 mL), dried over anhydrous Na
2SO
4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compound (40 mg, 20%) as a yellow solid, m.p. = 66–69 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% ethyl acetate in hexane);
1H NMR (400 MHz, CDCl
3)
δ: 14.15 (s, 1H), 11.22 (s, 1H), 7.87 (d,
J = 9.0 Hz, 1H), 7.38 (dd,
J = 8.0, 1.3 Hz, 1H), 7.31 (t,
J = 7.9 Hz, 1H), 7.10 (d,
J = 9.0 Hz, 1H), 7.04 (dd,
J = 7.8, 1.2 Hz, 1H), 3.99 (s, 3H) (in agreement with the
1H NMR data that were previously reported for this compound) [
60].
3.4. Synthesis of Naphtho[1,8-de][1,2]oxazin-4-yl Acetate (15)
A solution of 2 (500 mg, 2.7 mmol, 1 equiv) in freshly distilled acetic anhydride (10 mL, 41 equiv), under an atmosphere of N2, was stirred at room temperature for 18 h (TLC showed the absence of a starting material spot and the presence of one new spot). Ice water (30 mL) was added, and the reaction mixture was stirred for 0.5 h at room temperature. The reaction mixture was then extracted with EtOAc (3 × 15 mL), and the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compound (429 mg, 70%) as a brown oil; Rf = 0.05 (20% EtOAc in hexane); 1H NMR (400 MHz, DMSO-d6) δ: 8.71 (s, 1H), 7.99 (d, J = 9.1 Hz, 1H), 7.58–7.51 (m, 2H), 7.41 (d, J = 9.1 Hz, 1H), 7.06–6.97 (m, 1H), 2.33 (s, 3H); 13C NMR (100.6 MHz, DMSO-d6) δ: 169.3, 167.0, 151.3, 144.2, 140.4, 130.8, 128.3, 124.1, 119.9, 119.2, 107.9, 105.7, 22.0; IR (solid): 2924, 2682, 2217, 1926, 1749, 1510 cm−1; HRMS (ESI): m/z [M + Na]+ calcd. for C13H9NO3Na: 250.0480, found: 250.0477.
3.5. Synthesis of 1-Cyano-8-hydroxynaphthalen-2-yl Acetate (16)
A solution of 15 (400 mg, 2.16 mmol) in DMF (8 mL) was heated at 120 °C for 45 min. (TLC analysis showed the absence of a starting material spot and the presence of a new spot). Water (30 mL) was added, and the reaction mixture was extracted with Et2O (3 × 15 mL). The combined organic extracts were dried (Na2SO4), and the solvent was removed under vacuum. The residue was purified by flash column chromatography (50% EtOAc in hexane) to give the title compound (267 mg, 72%) as a brown solid; m.p. = 165–167 °C; Rf = 0.13 (20% ethyl acetate in hexane); 1H NMR (400 MHz, DMSO-d6) δ: 10.85 (s, 1H), 8.22 (d, J = 9.0 Hz, 1H), 7.55–7.42 (m, 3H), 7.07 (dd, J = 7.5, 1.3 Hz, 1H), 2.41 (s, 3H); 13C NMR (100.6 MHz, DMSO-d6) δ: 168.7, 153.8, 152.4, 134.8, 132.69, 127.9, 122.2, 121.7, 119.4 (2C), 115.4, 111.7, 99.0, 20.7; IR (solid): 3338, 2921, 2855, 2229, 2070, 1906, 1753, 1583 cm−1; HRMS (ESI): m/z [M + Na]+ calcd. for C13H9NO3Na: 250.0480, found: 250.0471.
3.6. Procedure A for the Synthesis of 1-Cyano-8-methoxynaphthalen-2-yl Acetate (17) and 8-Cyano-7-methoxynaphthalen-1-yl Acetate (18)
To a solution of 16 (50 mg, 0.22 mmol, 1 equiv) in dry acetone (5 mL), under an atmosphere of N2, was added oven-dried K2CO3 (34 mg, 0.24 mmol, 1.1 equiv) and dimethyl sulfate (31 mg, 0.24 mmol, 1.1 equiv), and the reaction was left stirring for 24 h at room temperature. (TLC showed complete conversion of the starting material and the presence of two new spots (visualized under a UV lamp). Water (20 mL) was added; the reaction mixture was extracted with EtOAc (3 × 10 mL); and the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated in vacuo. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give the title compounds.
Compound 17: (5.3 mg, 10%) as a yellow solid, m.p. = 94–96 °C; Rf = 0.25 (20% EtOAc in hexane); 1H NMR (400 MHz, DMSO-d6) δ: 8.30 (d, J = 8.9 Hz, 1H), 7.66 (dd, J = 8.3, 1.2 Hz, 1H), 7.64–7.52 (m, 2H), 7.24 (dd, J = 7.8, 1.1 Hz, 1H), 4.00 (s, 3H), 2.42 (s, 3H); 13C NMR (100.6 MHz, DMSO-d6) δ: 168.6, 154.2, 153.8, 135.0, 132.2, 127.8, 122.8, 122.1, 121.1, 115.2, 108.5, 98.8, 56.0, 20.6; IR (solid): 2924, 2855, 2223, 1578 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C14H12NO3: 242.0812, found: 242.0813.
Compound 18: (26.5 mg, 50%) as a yellow solid, m.p. = 132–134 °C; Rf = 0.11 (20% EtOAc in hexane); 1H NMR (400 MHz, DMSO-d6) δ: 8.36 (d, J = 9.3 Hz, 1H), 7.95 (dd, J = 8.2, 1.3 Hz, 1H), 7.66 (d, J = 9.3 Hz, 1H), 7.54–7.50 (m, 1H), 7.43 (dd, J = 7.6, 1.2 Hz, 1H), 4.08 (s, 3H), 2.42 (s, 3H); 13C NMR (100.6 MHz, DMSO-d6) δ: 169.9, 163.7, 144.0, 136.2, 129.3, 127.4, 124.9 (2C), 123.0, 116.1, 113.7, 88.912, 57.1, 20.9; IR (solid): 2942, 2850, 2219, 2101, 1747, 1592 cm−1; HRMS (ESI): m/z [M + Na]+ calcd. for C14H11NO3Na: 264.0631, found: 264.0625.
3.7. Procedure B for the Synthesis of Compounds 17 and 18
To a solution of 8 (50 mg, 0.22 mmol, 1 equiv) in dry THF (5 mL), under an atmosphere of nitrogen, was added oven-dried K2CO3 (34 mg, 0.24 mmol, 1.1 equiv) and MeI (34 mg, 0.24 mmol, 1.1 equiv), and the reaction mixture was left stirring for 18 h at room temperature (TLC showed complete conversion of the starting material and the presence of two new spots). Water (20 mL) was added, and the reaction mixture was extracted with EtOAc (3 × 10 mL); the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give the title compounds.
Compound 17: (6.9 mg, 13%) as a yellow solid, m.p. = 94–96 °C; Rf = 0.25 (20% EtOAc in hexane); (the 1H NMR data were in agreement with those reported in Procedure A for this compound).
Compound 18: (25 mg, 50%) as a yellow solid, m.p. = 132–134 °C; Rf = 0.11 (20% EtOAc in hexane); (the 1H NMR data were in agreement with those reported in Procedure A for this compound).
3.8. Procedure C for the Synthesis of Compounds 17 and 18
To a solution of 16 (50 mg, 0.22 mmol, 1 equiv) in dry THF (5 mL), under an atmosphere of N2, was added NaH (5.8 mg, 0.24 mmol, 1.1 equiv) and MeI (34 mg, 0.24 mmol, 1.1 equiv), and the reaction mixture was left stirring for 18 h at room temperature (TLC analysis showed the absence of starting material and the presence of two new spots). Water (20 mL) was added to the reaction mixture and then extracted with EtOAc (3 × 10 mL). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and removed under reduced pressure. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give the title compounds.
Compound 17: (11.7 mg, 22%) as a yellow solid, m.p. = 94–96 °C; Rf = 0.25 (20% EtOAc in hexane); (the 1H NMR data were in agreement with those reported in Procedure A for this compound).
Compound 18: (21 mg, 40%) as a yellow solid, m.p. = 132–134 °C; Rf = 0.11 (20% EtOAc in hexane); (the 1H NMR data were in agreement with those reported in Procedure A for this compound).
3.9. Procedure D for the Synthesis of Compounds 17 and 18
To a solution of 16 (50 mg, 0.22 mmol, 1 equiv) in dry THF (50 mL), under an atmosphere of N2, was added NaH (5.8 mg, 0.24 mmol, 1.1 equiv) and MeI (34 mg, 0.24 mmol, 1.1 equiv), and the reaction was left stirring for 18 h at room temperature (TLC analysis showed the absence of the starting material spot and the presence of two new spots). Water (20 mL) was added to the reaction mixture and extracted with EtOAc (3 × 10 mL); the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and evaporated under vacuum. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give the title compounds.
Compound 17: (21.8 mg, 41%) as a yellow solid, m.p. = 94–96 °C; Rf = 0.25 (20% EtOAc in hexane); (the 1H NMR data were in agreement with those reported in Procedure A for this compound).
Compound 18: (4.8 mg, 9%) as a yellow solid, m.p. = 132–134 °C; Rf = 0.11 (20% EtOAc in hexane); (the 1H NMR data were in agreement with those reported in Procedure A for this compound).
3.10. Attempted Reduction of 1-Cyano-8-methoxynaphthalen-2-yl Acetate (17) with DIBAL
To a solution of
17 (50 mg, 0.27 mmol, 1 equiv) in dry THF, under an atmosphere of N
2 that was cooled to −78 °C, DIBAL (0.86 mL of 1.2 M in toluene, 0.864 mmol, 3.2 equiv) was added dropwise, and the reaction mixture was left stirring at that temperature for 0.5 h and then for 1 h at room temperature (TLC analysis showed the absence of the starting material spot and the presence of one new spot). A saturated aq. solution of NH
4Cl (5 mL) was added dropwise, followed by 1 N HCl aq. (25 mL) and EtOAc (25 mL), and the reaction mixture was left stirring for 1 h. The organic phase was separated, and the aqueous phase was extracted with EtOAc (3 × 10 mL); the combined organic phases were washed with brine (20 mL), dried over anhydrous Na
2SO
4, and evaporated under vacuum. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give 2-hydroxy-8-methoxy-1-naphthonitrile (
12) (23 mg, 36%) as a yellow solid, m.p. = 172–174 °C; R
f = 0.09 (20% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound
2 (
Scheme 2)).
3.11. Attempted Reduction of 1-Cyano-8-methoxynaphthalen-2-yl Acetate (17) with PtO2
Compound
17 (30 mg, 0.16 mmol, 1 equiv) was dissolved in a stirred 1/1 solution of HCOOH/H
2O (4 mL), under an atmosphere of N
2. PtO
2 (3.6 mg, 0.016 mmol, 0.1 equiv) was added and the reaction mixture was heated at 55 °C for 2 h (TLC analysis showed the absence of the starting material spot and the presence of one new spot). The cooled reaction mixture was filtered through celite, water (10 mL) was added to the filtrate, and the aqueous solution was extracted with EtOAc (3 × 10 mL). The combined organic phases were washed with brine (20 mL), dried over anhydrous Na
2SO
4, and evaporated under vacuum. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give 2-hydroxy-8-methoxy-1-naphthonitrile (
12) (29 mg, 45%) as a yellow solid, m.p. = 172–174 °C; R
f = 0.09 (20% EtOAc in hexane) (the
1H NMR data were in agreement with those reported for this compound synthesized from compound
11 (
Scheme 2)).
3.12. Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14)
To a solution of
17 (250 mg, 1 mmol, 1 equiv) in dry toluene (10 mL) at 0 °C, under an atmosphere of N
2, was added DIBAL (1.67 mL of 1.2 M in toluene, 2 mmol, 2 equiv), and the reaction mixture was stirred for 0.5 h and then left stirring for 18 h at room temperature. Upon completion of the reaction (TLC examination), the solvent was removed under vacuum, and water (20 mL) was carefully added to the residue. The resulting mixture was cooled to 0 °C, followed by the dropwise addition of 1 M HCl aq. until pH = 1. The aqueous solution was extracted with EtOAc (3 × 10 mL), and the combined organic extracts were washed with brine (20 mL) and dried over anhydrous Na
2SO
4; the solvent was removed under vacuum. The acquired crude residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compound (31.5 mg, 15%) as a yellow solid, m.p. = 67–69 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% ethyl acetate in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from
12 (
Scheme 2) and with those previously reported) [
60].
3.13. Synthesis of 2,8-Dihydroxy-1-naphthonitrile (5)
A solution of
2 (500 mg, 2.7 mmol) in dry DMF (15 mL) was heated at 120 °C for 0.5 h (TLC analysis showed the absence of starting material and the presence of one new spot). The reaction was left to cool to room temperature; then, water (100 mL) was added. The reaction mixture was extracted with EtOAc (3 × 20 mL), and the combined organic extracts were washed with brine (20 mL), dried over anhydrous Na
2SO
4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (25% EtOAc in hexane) to give the title compound as a yellow solid (400 mg, 80%), m.p. = 166–167 °C (lit. [
10], m.p. = 167–168 °C); R
f = 0.1 (20% ethyl acetate in hexane);
1H NMR (400 MHz, DMSO-
d6)
δ: 11.24 (s, 1H), 10.31 (s, 1H), 7.93 (d,
J = 9.0 Hz, 1H), 7.32 (d,
J = 7.9 Hz, 1H), 7.25–7.15 (m, 2H), 6.92 (dd,
J = 7.6, 1.1 Hz, 1H) (in agreement with the
1H NMR data that were previously reported for this compound) [
10].
3.14. Synthesis of 2,8-Dihydroxy-1-naphthaldehyde (19)
A closed vessel with a magnetic stir bar was charged with Ni(OAc)
2·4H
2O (20 mg, 0.11 mmol, 0.2 equiv), followed by water (1 mL), and the mixture was stirred at room temperature for a few minutes. Ca(H
2PO
2)
2 (90 mg, 0.54 mmol, 1 equiv) was then added, followed by Ca(OAc)
2·H
2O (40 mg, 0.22 mmol, 0.4 equiv), compound
5 (100 mg, 0.54 mmol, 1 equiv), and EtOH (1 mL). The reaction mixture was heated at 100 °C for 24 h. Upon completion (TLC analysis), the reaction was left to cool to room temperature, and water (10 mL) was added. The reaction mixture was then extracted with EtOAc (3 × 10 mL), and the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na
2SO
4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compound (29 mg, 29%) as a yellow solid, m.p. = 194–196 °C (lit. [
14], m.p. = 195–197 °C); R
f = 0.51 (33% EtOAc in hexane);
1H NMR (400 MHz, CDCl
3)
δ: a broad singlet corresponding to OH is not visible, 11.33 (s, 1H), 7.92 (d,
J = 9.1 Hz, 1H), 7.42 (d,
J = 7.9 Hz, 1H), 7.23 (d,
J = 7.7 Hz, 1H), 7.14 (d,
J = 9.0 Hz, 1H), 6.98 (d,
J = 7.4 Hz, 1H) (in agreement with the
1H NMR data that were previously reported for this compound [
14].
3.15. Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14) and 8-Hydroxy-2-methoxy-1-naphthaldehyde (20)
To a solution of 19 (25 mg, 0.133 mmol, 1 equiv) in dry DMF (5 mL), under an atmosphere of N2, oven-dried K2CO3 (19 mg, 0.134 mmol, 1.05 equiv) was added, followed by MeI (19 mg, 0.134 mmol, 1.05 equiv), and the reaction was stirred at room temperature for 2 h (TLC showed the absence of the starting material and the presence of two new spots). Water (50 mL) was added; the reaction mixture was extracted with EtOAc (3 × 20 mL); and the combined organic extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (11% EtOAc in hexane) to give the title compounds.
Compound
14: (5.1 mg, 19%) as a yellow solid, m.p. = 67–68 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% ethyl acetate in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from
4 (
Scheme 2) and with those previously reported) [
60].
Compound
20: (12.5 mg, 47%) as a yellow solid, m.p. = 110–112 °C); R
f = 0.28 (20% ethyl acetate in hexane);
1H NMR (400 MHz, CDCl
3)
δ: 12.00 (s, 1H), 10.59 (s, 1H), 8.12 (d,
J = 9.3 Hz, 1H), 7.35 (d,
J = 7.7 Hz, 1H), 7.29–7.26 (m, 1H), 7.22 (d,
J = 9.2 Hz, 1H), 7.14 (dd,
J = 7.6, 1.4 Hz, 1H), 4.08 (s, 3H) (in agreement with the
1H NMR data that were previously reported for this compound) [
15].
3.16. Synthesis of 2,8-Dimethoxy-1-naphthonitrile (13)
To a stirred solution of
5 (300 mg, 1.62 mmol, 1 equiv) in acetone (10 mL) was added MeI (460 mg, 3.28 mmol, 2.02 equiv), followed by Na
2CO
3 (175 mg, 1.65 mmol, 1.02 equiv) and H
2O (2 mL). The reaction mixture was gently heated for 3 h, during which time TLC analysis showed complete conversion of the starting material and the presence of one new spot. The solvents were removed under reduced pressure and the oily residue was triturated with hexane to give the title compound (270 mg, 78%) as a colorless solid (hexane), m.p. = 147–149 °C; R
f = 0.21 (20% ethyl acetate in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound 2 (
Scheme 2)).
3.17. Synthesis of 2,8-Dimethoxy-1-naphthaldehyde (21)
To a stirred solution of
13 (210 mg, 1 mmol, 1 equiv) in dry toluene (10 mL) at 0 °C, under an atmosphere of N
2, was added DIBAL (1.67 mL of 1.2 M in toluene, 2 mmol, 2 equiv), and the reaction mixture stirred for 0.5 h and then left stirring at room temperature for 18 h. Upon completion of the reaction (TLC analysis), the solvent was removed under reduced pressure, cold water (20 mL) was carefully added to the residue, and the resulting mixture was cooled to 0 °C, followed by the dropwise addition of 1 M HCl aq. until pH = 1. The aqueous solution was extracted with EtOAc (3 × 10 mL), and the combined organic extracts were washed with brine (20 mL), dried over anhydrous Na
2SO
4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compound as a light brown oil (112 mg, 52%), R
f = 0.37 (20% ethyl acetate in hexane);
1H NMR (400 MHz, CDCl
3) δ: 10.75 (s, 1H), 7.85 (d,
J = 9.1 Hz, 1H), 7.40 (dd,
J = 8.2, 1.0 Hz, 1H), 7.34–7.25 (m, 2H), 6.86 (dd,
J = 7.6, 1.0 Hz, 1H), 3.93 (s, 3H), 3.92 (s, 3H) (in agreement with the
1H NMR data that were previously reported for this compound) [
16].
3.18. Procedure F for the Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14), 8-Hydroxy-2-methoxy-1-naphthaldehyde (12), and 2,8-Dihydroxy-1-naphthaldehyde (19)
To an oven-dried closed vessel with a magnetic stirrer bar, under an atmosphere of N
2, was added
13 (50 mg, 0.231 mmol, 1 equiv), MgBr
2 diethyl etherate (119 mg, 0.462 mmol, 2 equiv), KI (76.5 mg, 0.642 mmol, 2 equiv), and MeCN (10 mL). The vessel was sealed and then heated with stirring at 150 °C for 2 h. TLC analysis of the cooled reaction mixture showed the absence of the starting material and the presence of three new spots, one intense and two very faint (visualized under a UV lamp). The solvent was removed under vacuum, cooled water (20 mL) was carefully added to the residue, and the resulting cooled mixture was acidified by adding dropwise 1 M HCl aq. to pH = 1. The aqueous mixture was extracted with EtOAc (3 × 10 mL); the combined organic extracts were washed with brine (10 mL), dried over anhydrous Na
2SO
4, and concentrated under reduced pressure. The acquired crude residue was purified by flash column chromatography (11% EtOAc in hexane) to give the title compound (4.6 mg, 10%) as a yellow solid, m.p. = 67–69 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound (
12) (
Scheme 2) and with those previously reported) [
60].
3.19. Procedure G for the Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14), 8-Hydroxy-2-methoxy-1-naphthaldehyde (20), and 2,8-Dihydroxy-1-naphthaldehyde (19)
A solution of 21 (50 mg, 0.231 mmol, 1 equiv) in dry DCM (10 mL), over an atmosphere of N2, was cooled to 0 °C; then, BBr3 (690 μL of a 1 M solution in DCM, 0.693 mmol, 3 equiv) was added dropwise. The reaction was left stirring at room temperature for 18 h (TLC analysis showed complete conversion of the starting material and the presence of three new spots) and then cooled to 0 °C, quenched slowly with cold water (30 mL), and extracted with DCM (3 × 50 mL). The combined organic extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The acquired crude residue was purified by flash column chromatography (11% EtOAc in hexane) to give the title compounds.
Compound
14: (3.7 mg, 8%) as a yellow solid, m.p. = 67–68 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from (
12) (
Scheme 2) and with those previously reported) [
60].
Compound
20: (3.2 mg, 7%) as a yellow solid, m.p. = 110–112 °C); R
f = 0.28 (20% ethyl acetate in hexane);
1H NMR (400 MHz, CDCl
3)
δ: 12.00 (s, 1H), 10.59 (s, 1H), 8.12 (d,
J = 9.3 Hz, 1H), 7.35 (d,
J = 7.7 Hz, 1H), 7.29–7.26 (m, 1H), 7.22 (d,
J = 9.2 Hz, 1H), 7.14 (dd,
J = 7.6, 1.4 Hz, 1H), 4.08 (s, 3H) (in agreement with the
1H NMR data that were previously reported for this compound) [
15].
Compound
19: (25.6 mg, 59%) as a yellow solid, m.p. = 194–196 °C (lit. [
14], m.p. = 195–197 °C); R
f = 0.51 (33% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound (
5) (
Scheme 2) and with those previously reported) [
14].
3.20. Procedure H for the Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14), 8-Hydroxy-2-methoxy-1-naphthaldehyde (20), and 2,8-Dihydroxy-1-naphthaldehyde (19)
A solution of 21 (50 mg, 0.231 mmol, 1 equiv) in dry DCM (10 mL), over an atmosphere of N2, was cooled to 0 °C; then, BBr3 (230 μL of a 1M solution in DCM, 0.231 mmol, 1 equiv) was added dropwise, and the reaction was left stirring at room temperature for 1 h. TLC analysis showed complete conversion of the starting material and the presence of three new spots (visualized under a UV lamp). The reaction mixture was cooled to 0 °C, quenched slowly with cold water (30 mL), and extracted with DCM (3 × 20 mL); the combined organic extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The acquired crude residue was purified by flash column chromatography (11% EtOAc in hexane) to give the title compounds.
Compound
14: (3.7 mg, 19%) as a yellow solid, m.p. = 67–68 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from (
12) (
Scheme 2) and with those previously reported) [
60].
Compound
20: (5 mg, 11%) as a yellow solid, m.p. = 110–112 °C; R
f = 0.28 (20% ethyl acetate in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound (
21) (
Scheme 2) and with those previously reported) [
15].
Compound
19: (14.3 mg, 33%) as a yellow solid, m.p. = 194–196 °C (lit. [
14], m.p. = 195–197 °C); R
f = 0.51 (33% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound (
13) (
Scheme 2) and with those previously reported) [
14].
3.21. Procedure I for the Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14), 8-Hydroxy-2-methoxy-1-naphthaldehyde (20), and 2,8-Dihydroxy-1-naphthaldehyde (19)
A solution of 21 (50 mg, 0.231 mmol, 1 equiv) in dry DCM (10 mL), over an atmosphere of N2, was cooled to −15 °C; then, BBr3 (230 μL of a 1 M solution in DCM, 0.231 mmol, 1 equiv) was added dropwise over a period of 10 min. The reaction was allowed to slowly reach room temperature and was left stirring for 1 h, after which TLC analysis showed complete conversion of the starting material and the presence of three new spots (visualized under a UV lamp). The reaction mixture was cooled to 0 °C, quenched slowly with cold water (30 mL), and extracted with DCM (3 × 20 mL). The combined organic extracts were washed with brine (20 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The acquired crude residue was purified by flash column chromatography (11% EtOAc in hexane) to give the title compounds.
Compound
14: (25.9 mg, 56%) as a yellow solid, m.p. = 67–68 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from (
12) (
Scheme 2) and with those previously reported) [
60].
Compound
20: (10 mg, 22%) as a yellow solid, m.p. = 110–112 °C); R
f = 0.28 (20% ethyl acetate in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound (
21) (
Scheme 2) and with those previously reported) [
15].
Compound
19: (4.3 mg, 10%) as a yellow solid, m.p. = 194–196 °C (lit. [
14], m.p. = 195–197 °C); R
f = 0.51 (33% EtOAc in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound (
5) (
Scheme 2) and with those previously reported) [
14].
3.22. Procedure J for the Synthesis of 2,8-Bis(benzyloxy)-1-naphthonitrile (22) and 2-(Benzyloxy)-8-hydroxy-1-naphthonitrile (23)
To a stirred solution of 2 (500 mg, 2.7 mmol, 1 equiv) in dry acetone (20 mL), under an atmosphere of N2, was added benzyl bromide (462 mg, 2.7 mmol, 1 equiv), oven-dried K2CO3 (391 mg, 2.83 mmol, 1.05 equiv), and KI (89 mg, 0.54 mmol, 0.2 equiv). The reaction was left stirring at room temperature for 18 h, after which TLC analysis indicated the absence of the starting material and the presence of two new spots. The solvent was removed under vacuum, the residue was dissolved in EtOAc (30 mL) and washed with water (3 × 10 mL). The organic extract was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The acquired crude residue was purified by flash column chromatography (20% EtOAc in hexane) to give the title compounds 22 and 23.
Compound 22: (118 mg, 12%) as an orange oil; Rf = 0.38 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: 7.97 (d, J = 9.1 Hz, 1H), 7.73–7.66 (m, 2H), 7.66–7.57 (m, 2H), 7.53–7.31 (m, 9H), 7.06 (dd, J = 7.6 Hz, 1.2 Hz, 1H), 5.47 (s, 4H); 13C NMR (100.6 MHz, CDCl3) δ: 162.1, 153.1, 136.4, 136.1, 134.6, 130.0, 128.8 (2C), 128.6 (2C), 128.2, 128.1, 127.8 (2C), 127.0 (2C), 125.3, 125.3, 121.3, 116.8, 114.1, 109.6, 93.8, 71.3, 71.1.; IR (solid): 3067, 3027, 2922, 2887, 2214, 2094, 1737, 1677, 1591 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C25H20NO2: 366.1489, found: 366.1485.
Compound 23: (450 mg, 61%) as a colorless solid, m.p. = 136–138 °C; Rf = 0.49 (20% ethyl acetate in hexane); 1H NMR (400 MHz, CDCl3) δ: 7.82–7.75 (m, 2H), 7.57 (d, J = 8.8 Hz, 1H), 7.41 (d, J = 8.8 Hz, 1H), 7.33–7.12 (m, 6H), 5.44 (s, 2H); 13C NMR (100.6 MHz, CDCl3) δ: 156.0, 139.6, 135.7, 131.3, 129.7, 129.3 (2C), 128.1, 127.0, 126.3 (2C), 124.9, 124.4, 123.9, 120.3, 120.2, 110.8, 47.8; IR (solid): 3052, 2920, 2845, 2216, 2117, 1886, 1752 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C18H14NO2: 276.1019, found: 216.1018.
3.23. Synthesis of 2-(Benzyloxy)-8-methoxy-1-naphthonitrile (24)
This was prepared according to the experimental procedure for the synthesis of (
13) from (
5) (
Scheme 2). Used as starting material of compound (
23) (400 mg, 5 mmol, 1 equiv), MeI (724 mg, 5.1 mmol, 1.02 equiv) Na
2CO
3 (540 mg, 5.1 mmol, 1.02 equiv), acetone (15 mL) and H
2O (3 mL), which gave the title compound (384 mg, 93%) as a yellow solid, m.p. = 100–103 °C; R
f = 0.32 (20% EtOAc in hexane);
1H NMR (400 MHz, CDCl
3)
δ: 7.80 (d,
J = 9.2 Hz, 1H), 7.57–7.39 (m, 2H), 7.36–7.13 (m, 6H), 6.86 (dd,
J = 6.3, 2.5 Hz, 1H), 5.31 (s, 2H), 3.97 (s, 3H);
13C NMR (100.6 MHz, CDCl
3)
δ: 162.0, 154.3, 136., 134.5, 129.9, 128.8 (2C), 128.2, 127.0 (2C), 125.4, 125.2, 121.0, 116.8, 114.1, 107.7, 93.7, 71.3, 55.8; IR (solid): 2919, 2844, 2212, 2101, 1737, 1595 cm
−1; HRMS (ESI):
m/
z [M + H]
+ calcd. for C
19H
16NO
2: 290.1176, found: 290.1177.
3.24. Synthesis of 2-(Benzyloxy)-8-methoxy-1-naphthaldehyde 25
This was prepared according to the experimental procedure for the synthesis of compound
21 from
13 (
Scheme 2). Used as starting material of compound
24 (100 mg, 0.34 mmol, 1 equiv), DIBAL (580 μL of 1.2 M in toluene, 0.68 mmol, 2 equiv) and dry toluene (10 mL), which gave the title compound (55.6 mg, 55%) as a yellow oil; R
f = 0.46 (20% EtOAc in hexane);
1H NMR (400 MHz, CDCl
3)
δ: 10.76 (s, 1H), 7.81 (d,
J = 9.0 Hz, 1H), 7.46–7.27 (m, 8H), 6.86 (dd,
J = 7.7, 1.1 Hz, 1H), 5.23 (s, 2H), 3.94 (s, 3H);
13C NMR (100.6 MHz, CDCl
3) δ: 195.0, 155.0, 153.7, 136.9, 131.7, 130.5, 128.7 (2C), 128.1, 127.4 (2C), 124.8, 124.1, 123.4, 121.1, 116.4, 106.62, 72.3, 56.0; IR (solid): 2924, 2843, 2210, 1711, 1596 cm
−1; HRMS (ESI):
m/
z [M + H]
+ calcd. for C
19H
17O
3: 293.1172, found: 293.1168.
3.25. Synthesis of 2-Hydroxy-8-methoxy-1-naphthaldehyde (14)
To a stirred solution of
25 (20 mg, 0.099 mmol) in anhydrous MeOH (5 mL), under an atmosphere of N
2, 5% Pd/C (1 mg, 5%) was added, and the reaction was purged with H
2. Stirring at room temperature was continued for 18 h (TLC analysis showed complete conversion of the starting material and the presence of a new spot. The reaction mixture was filtered, and the solvent was removed under vacuum. To the remaining residue, EtOAc (20 mL) was added and then washed with brine (10 mL). The organic layer was dried over anhydrous Na
2SO
4 and concentrated under reduced pressure. The acquired crude product was purified by flash column chromatography (20% EtOAc in hexane) to give the title compound (18 mg, 92%) as a yellow solid, m.p. = 67–69 °C (lit. [
60], m.p. = 68–70 °C); R
f = 0.54 (20% ethyl acetate in hexane); (the
1H NMR data were in agreement with those reported for this compound synthesized from compound
12 (
Scheme 2) and with those previously reported) [
60].
3.26. Synthesis of (E)-2-Hydroxy-8-methoxy-1-naphthaldehyde Oxime (6)
To a stirred solution of
14 (200 mg, 0.99 mmol, 1 equiv) in MeOH (20 mL), NH
2OH.HCl (76.4 mg, 0.0011 mmol, 1.1 equiv) was added, and the resulting mixture was cooled to 0 °C, followed by the dropwise addition of an aqueous saturated Na
2CO
3 solution until pH = 8. The reaction mixture was left stirring at room temperature for 1 h. TLC analysis showed the absence of the starting material and the presence of a new spot on the baseline. The solution was cooled to 0 °C, followed by the dropwise addition of MeCO
2H until pH = 5. The solvent was evaporated under vacuum, water (25 mL) was added to the residue, and the mixture was extracted with EtOAc (3 × 10 mL). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na
2SO
4, and concentrated under reduced pressure. The acquired light yellow solid was purified by flash column chromatography (20% EtOAc in hexane) to give the title compound (164 mg, 78%) as a yellow solid, m.p. = 142–143 °C (lit. [
60], m.p. = 141–143 °C); R
f = 0.32 (20% EtOAc in hexane);
1H NMR (400 MHz, DMSO-
d6)
δ: 12.06 (s, 1H), 11.45 (s, 1H), 9.65 (s, 1H), 7.83 (d,
J = 9.0 Hz, 1H), 7.46 (d,
J = 6.8 Hz, 1H), 7.29 (t,
J = 7.9 Hz, 1H), 7.18 (d,
J = 8.9 Hz, 1H), 7.08 (d,
J = 7.9 Hz, 1H), 3.94 (s, 3H) (in agreement with the
1H NMR data that were previously reported for this compound) [
60].
3.27. Synthesis of 9-Methoxynaphtho[1,2-d]isoxazole 2-Oxide (8)
To a stirred solution of (E)-oxime 6 (150 mg, 0.70 mmol, 1 equiv) in dry t-BuOH (15 mL), under an atmosphere of N2, PIDA (450 mg, 1.40 mmol, 2 equiv) was added, and the resulting mixture was stirred at room temperature for 0.5 h. TLC analysis showed the absence of the starting material and the presence of a new spot. Water (15 mL) was added, followed by the dropwise addition of 5% NaHCO3 aq. solution until pH = 7–8. The solvents were evaporated under vacuum and water (20 mL) was added to the residue, and the resulting mixture was extracted with EtOAc (3 × 10 mL). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under reduced pressure. The acquired residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compound (120 mg, 81%) as a yellow solid, m.p. = 91–93 °C; Rf = 0.44 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: 8.10 (s, 1H), 7.89 (d, J = 8.9 Hz, 1H), 7.52 (d, J = 8.1 Hz, 1H), 7.47 (t, J = 7.9 Hz, 1H), 7.36 (d, J = 9.0 Hz, 1H), 7.02 (d, J = 7.6 Hz, 1H), 4.07 (s, 3H); 13C NMR (100.6 MHz, CDCl3) δ: 155.2, 149.7, 131.9, 129.33, 126.1, 121.2, 117.4, 112.2, 111.3, 108.4, 106.6, 55.8; IR (solid) 3098, 2919, 2840, 2365, 2209, 2107, 1743, 1568 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C12H10NO3: 216.0661, found: 216.0664.
3.28. In Situ Generation of 2-Hydroxy-8-methoxy(naphthalen-1-yl)nitrile Oxide (9)
A solution of isoxazole 2-oxide 8 (5 mg, 0.023 mmol) in dry DMSO-d6 (0.5 mL), under an atmosphere of N2, was stirred at room temperature for 6 h. TLC analysis revealed the absence of the starting material and the presence of a new spot. The solution was transferred to an NMR tube and the 1H and 13C NMR spectra of the new compound were recorded. Rf = 0.2 (20% EtOAc in hexane); 1H NMR (400 MHz, DMSO-d6) δ: 11.29 (s, 1H), 7.92 (d, J = 9.0 Hz, 1H), 7.46 (dd, J = 8.2, 1.0 Hz, 1H), 7.39–7.20 (m, 2H), 7.07 (dd, J = 7.8, 1.0 Hz, 1H), 3.98 (s, 3H).; 13C NMR (100.6 MHz, DMSO-d6) δ: 161.4, 153.3, 132.8, 128.9, 124.4, 124.4, 121.2, 117.5, 107.6, 88.7, 56.4.; HRMS (ESI): m/z [M + H]+ calcd. for C12H10NO3: 216.0661, found: 216.0661.
3.29. Procedure K for the Synthesis of Dimethyl 3-(2-Hydroxy-8-methoxynaphthalen-1-yl)isoxazole-4,5-dicarboxylate (10a), 8-Methoxy-1-(5-phenylisoxazol-3-yl)naphthalen-2-ol (10b), Methyl 3-(2-Hydroxy-8-methoxynaphthalen-1-yl)-4,5-dihydroisoxazole-5-carboxylate (10c) and 8-Methoxy-1-(5-phenyl-4,5-dihydroisoxazol-3-yl)naphthalen-2-ol (10d)
A solution of 2-oxide 8 (5 mg, 0.023 mmol, 1 equiv) in dry DMSO (1.5 mL), under an atmosphere of N2, was stirred at room temperature for 15 min. DMAD, phenylacetylene, methyl acrylate, or styrene (0.069 mmol, 3 equiv) was added, and the resulting mixture was stirred at room temperature for 18 h. TLC analysis showed the absence of the starting material and the presence of a new spot (visualized under a UV lamp). The reaction was quenched with water (15 mL), and the resulting mixture was extracted with EtOAc (3 × 10 mL). The combined organic extracts were washed with brine (10 mL), dried over anhydrous Na2SO4, and concentrated under vacuum. The acquired residue was purified by flash column chromatography (17% EtOAc in hexane) to give the title compounds.
Compound 10a: (7 mg, 85%) as a yellow solid, m.p. = 149–151 °C; Rf = 0.09 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: a singlet corresponding to OH is not visible, 7.83 (d, J = 8.9 Hz, 1H), 7.41 (d, J = 8.1 Hz, 1H), 7.32–7.21 (m, 1H), 6.79 (d, J = 7.7 Hz, 1H), 4.05 (s, 3H), 3.62 (s, 3H), 3.38 (s, 3H); 13C NMR (100.6 MHz, CDCl3) δ: 162.2, 160.5, 159.2, 157.2, 154.4, 153.4, 132.9, 130.4, 124.2, 124.0, 121.5, 118.7, 118.3, 106.6, 103.8, 55.3, 53.6, 52.2; IR (solid) 3352, 2922, 2848, 2364, 2119, 1717, 1613, 1520 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C18H16NO7: 358.0921, found: 358.0916.
Compound 10b: (6.2 mg, 93%) as a colorless solid, m.p. = 108–110 °C; Rf = 0.49 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: 8.61 (s, 1H), 7.88–7.79 (m, 3H), 7.55–7.41 (m, 4H), 7.37–7.29 (m, 2H), 6.90 (d, J = 7.6 Hz, 1H), 6.55 (s, 1H), 3.72 (s, 3H); 13C NMR (100.6 MHz, CDCl3) δ: 168.01, 167.4, 154.9, 154.0, 132.4, 130.9, 130.4, 129.2 (2C), 127.7, 126.0 (2C), 124.1, 123.5, 121.6, 118.8, 107.6, 105.2, 103.9, 55.3; IR (solid) 3205, 2919, 2845, 2363, 2123, 1732, 1606 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C20H16NO3: 318.1125, found: 318.1125.
Compound 10c: (6.2 mg, 89%) as a yellow solid, m.p. = 118–120 °C; Rf = 0.06 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: a singlet corresponding to OH is not visible, 7.76 (d, J = 8.9 Hz, 1H), 7.40 (d, J = 8.0 Hz, 1H), 7.29 (t, J = 7.9 Hz, 1H), 7.22 (d, J = 8.9 Hz, 1H), 6.90 (d, J = 7.8 Hz, 1H), 5.20 (t, J = 8.6 Hz, 1H), 3.88 (s, 3H), 3.86 (s, 3H), 3.58 (d, J = 8.6 Hz, 2H); 13C NMR (100.6 MHz, CDCl3) δ: 171.4, 158.6, 154.4, 153.3, 132.3, 130.6, 124.3, 124.1, 121.7, 118.7, 108.0, 104.7, 56.3, 53.0, 45.2; IR (solid) 3173, 2928, 2848, 2364, 2122, 1899, 1736, 1607, 1516 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C16H16NO5: 302.1023, found: 302.1028.
Compound 10d: (7.1 mg, 97%) as a colorless solid, m.p. = 205–207 °C; Rf = 0.34 (20% EtOAc in hexane); 1H NMR (400 MHz, CDCl3) δ: 8.31 (s, 1H), 7.67 (d, J = 8.9 Hz, 1H), 7.41 (d, J = 7.5 Hz, 2H), 7.36 (t, J = 7.5 Hz, 2H), 7.30 (t, J = 7.8 Hz, 2H), 7.23–7.14 (m, 2H), 6.73 (d, J = 7.7 Hz, 1H), 5.73 (dd, J = 10.4, 7.4 Hz, 1H), 3.54 (dd, J = 16.3, 10.3 Hz, 1H), 3.37–3.29 (m, 4H); 13C NMR (100.6 MHz, CDCl3) δ 159.1, 154.3, 153.4, 141.0, 132.0, 130.6, 128.7 (2C), 128.0, 125.8 (2C), 124.0, 123.9, 121.4, 118.5, 107.3, 105.3, 81.4, 55.2, 49.0; IR (solid) 3135, 2922, 2848, 2363, 2110, 1917, 1754, 1605, 1514 cm−1; HRMS (ESI): m/z [M + H]+ calcd. for C20H18NO3: 320.1281, found: 320.1279.