Nanostructure Lipid Carrier of Curcumin Co-Delivered with Linalool and Geraniol Monoterpenes as Acetylcholinesterase Inhibitor of Culex pipiens
Abstract
1. Introduction
2. Results
2.1. Nanodrug Delivery
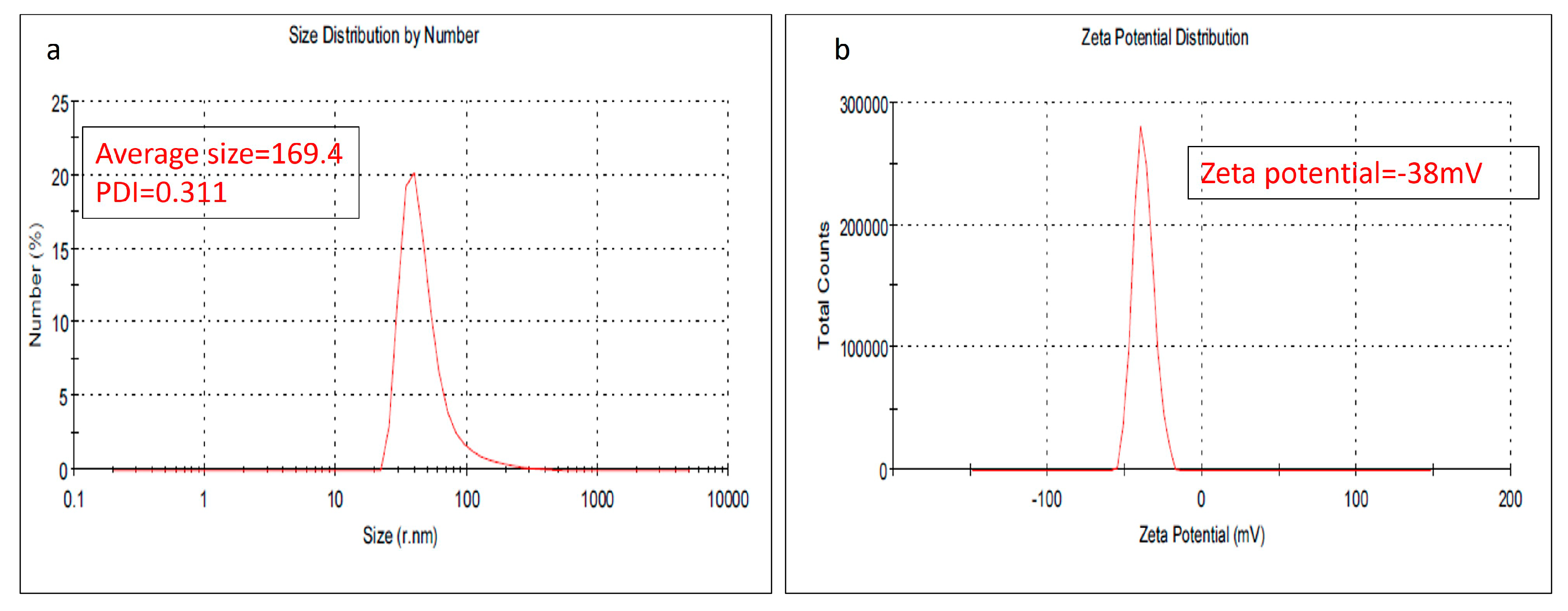
2.1.1. Particle Size (DLS) and Zeta Potential and Stability (Z.P)
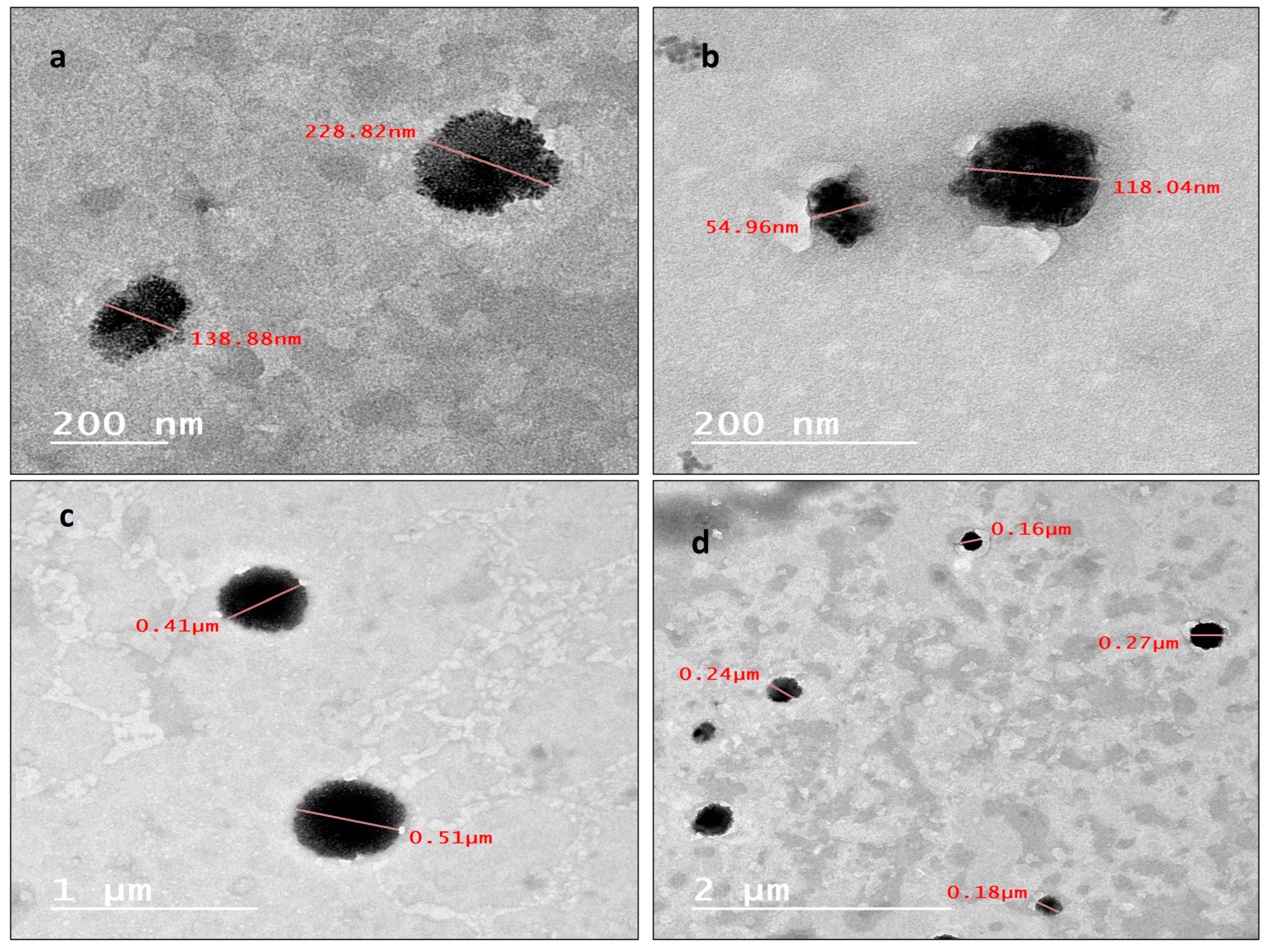
2.1.2. Transmission Electron Microscope (TEM)
2.2. Insecticidal Evaluation
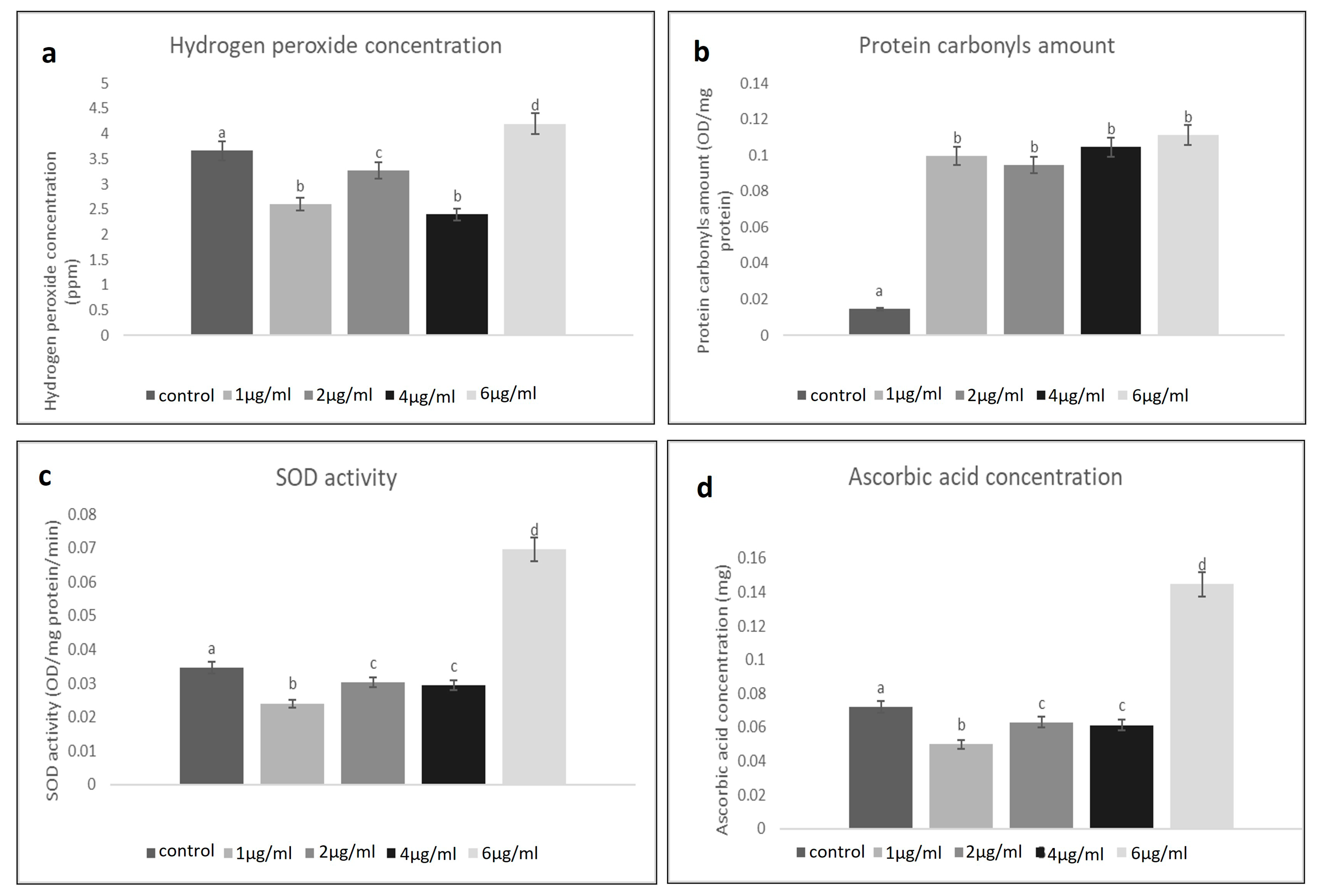
2.2.1. H2O2 Concentration
2.2.2. Protein Carbonyl Amounts
2.2.3. Ascorbic Acid Concentration and SOD Activity
2.2.4. Effect of Nanoparticles on the Fourth Instar C. pipiens
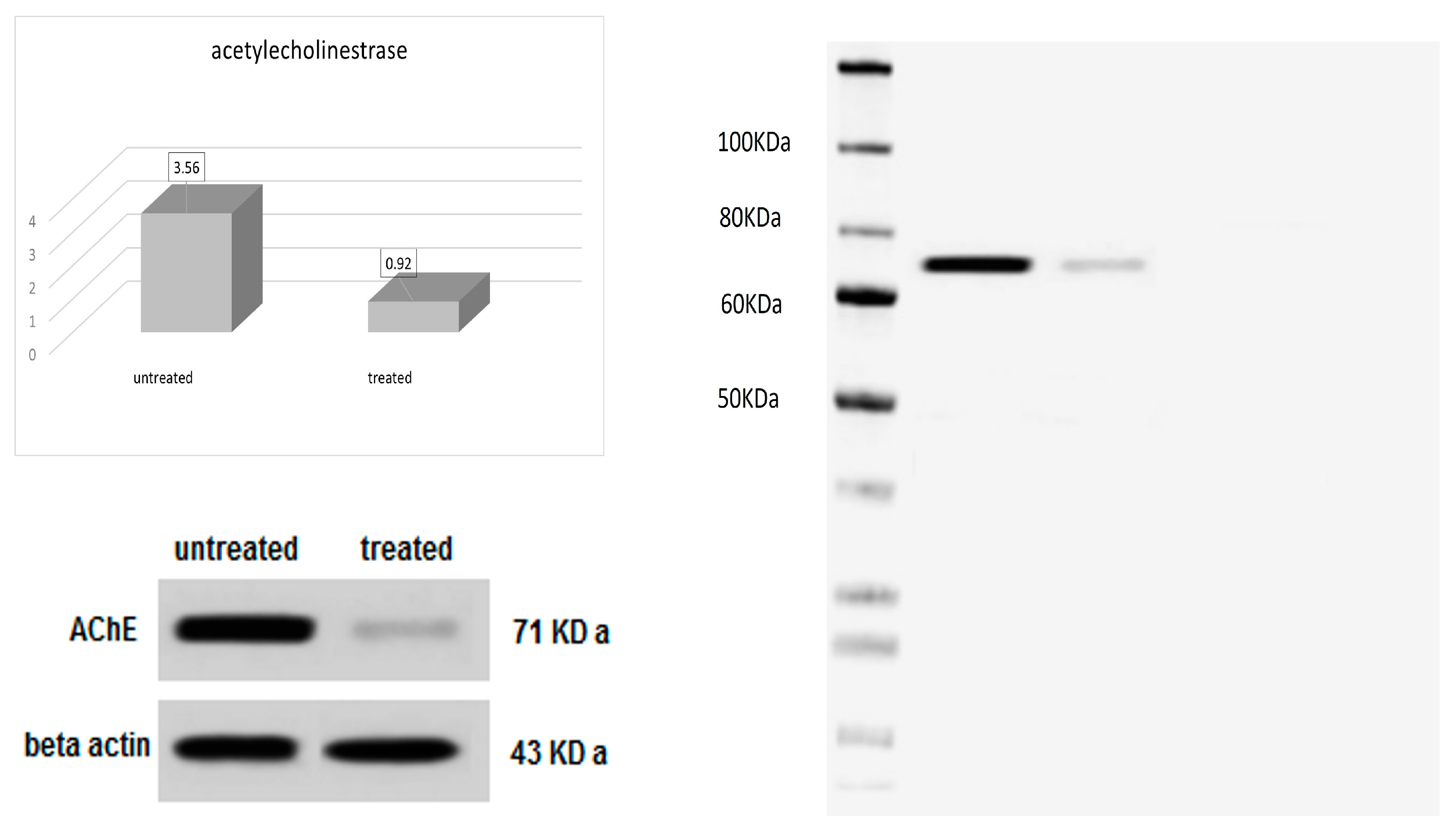
2.2.5. Acetylcholinesterase Enzyme Inhibition Assay
2.2.6. Acetylcholinesterase Activity by Western Blotting
2.2.7. Cytotoxic Effect of NLC-Cur-LG Nanoparticles against Vero and WI38 Normal Cell Lines
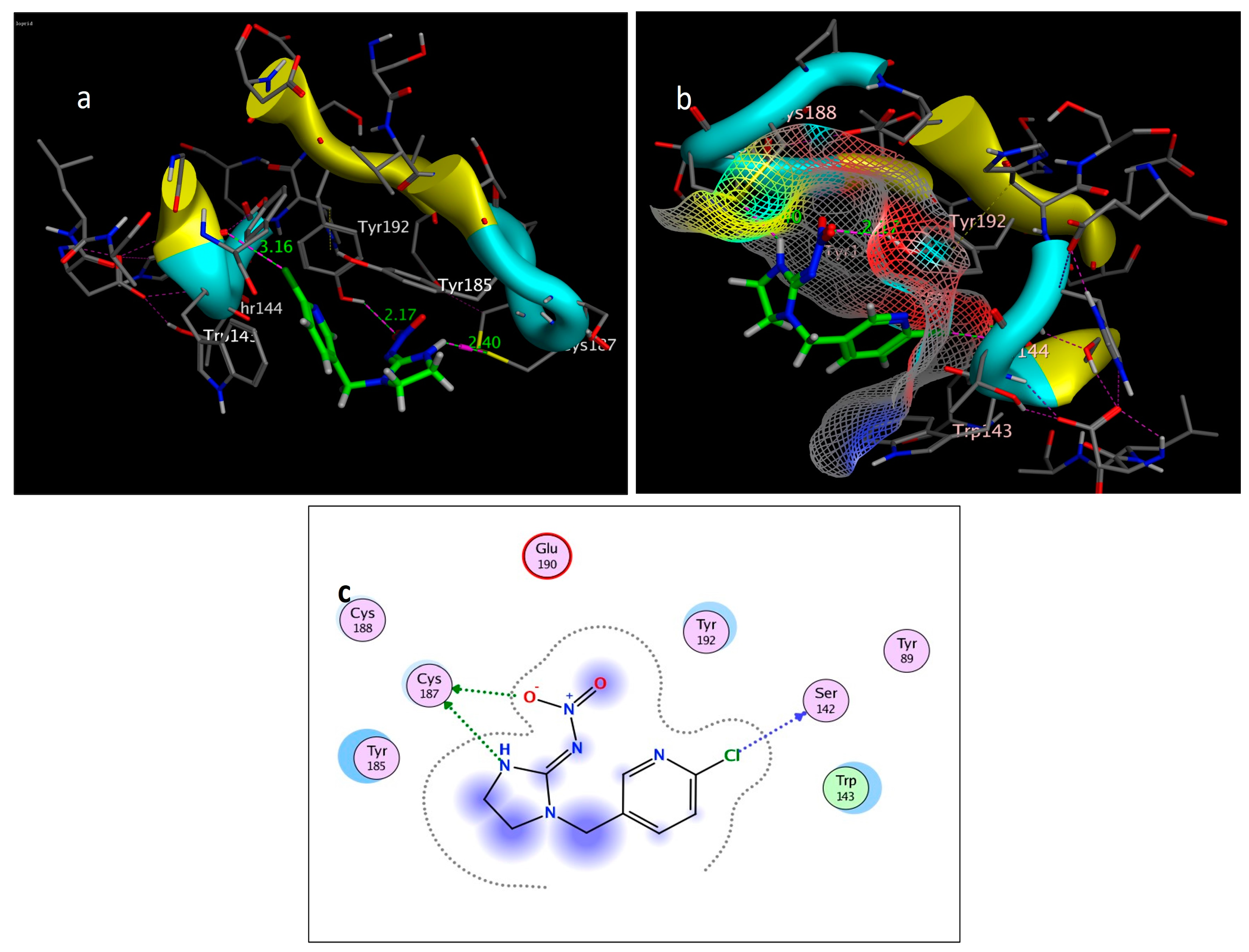
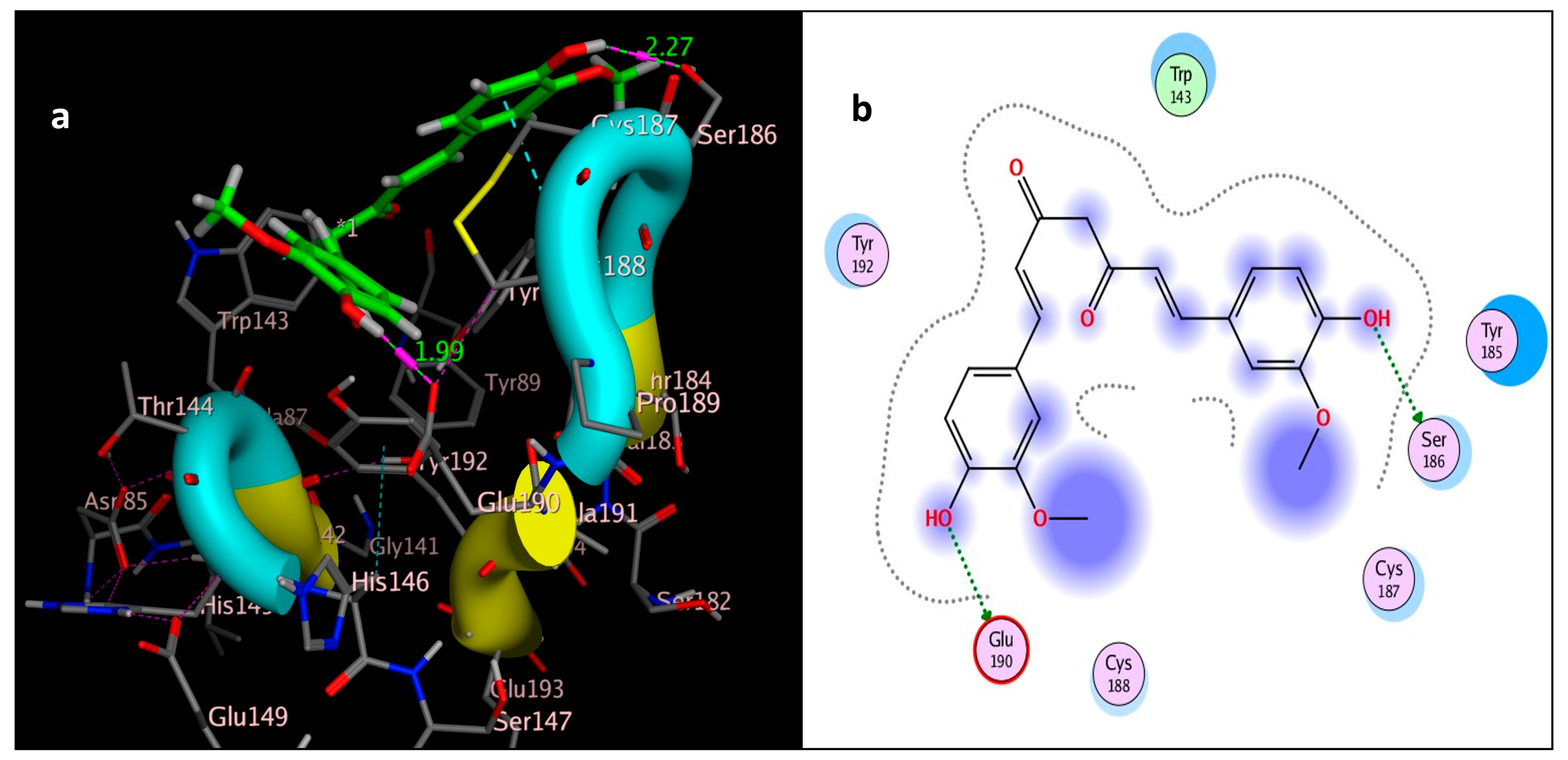
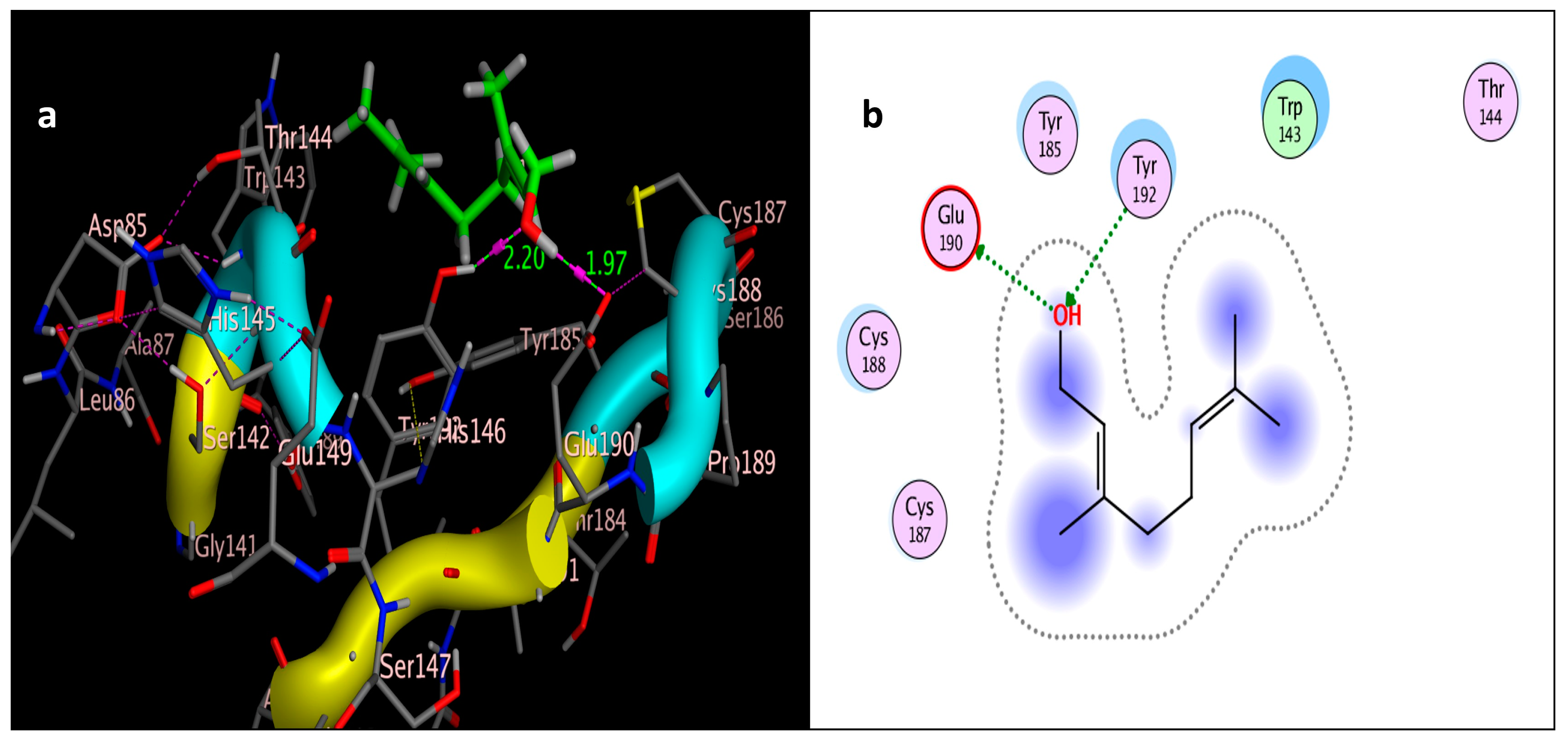
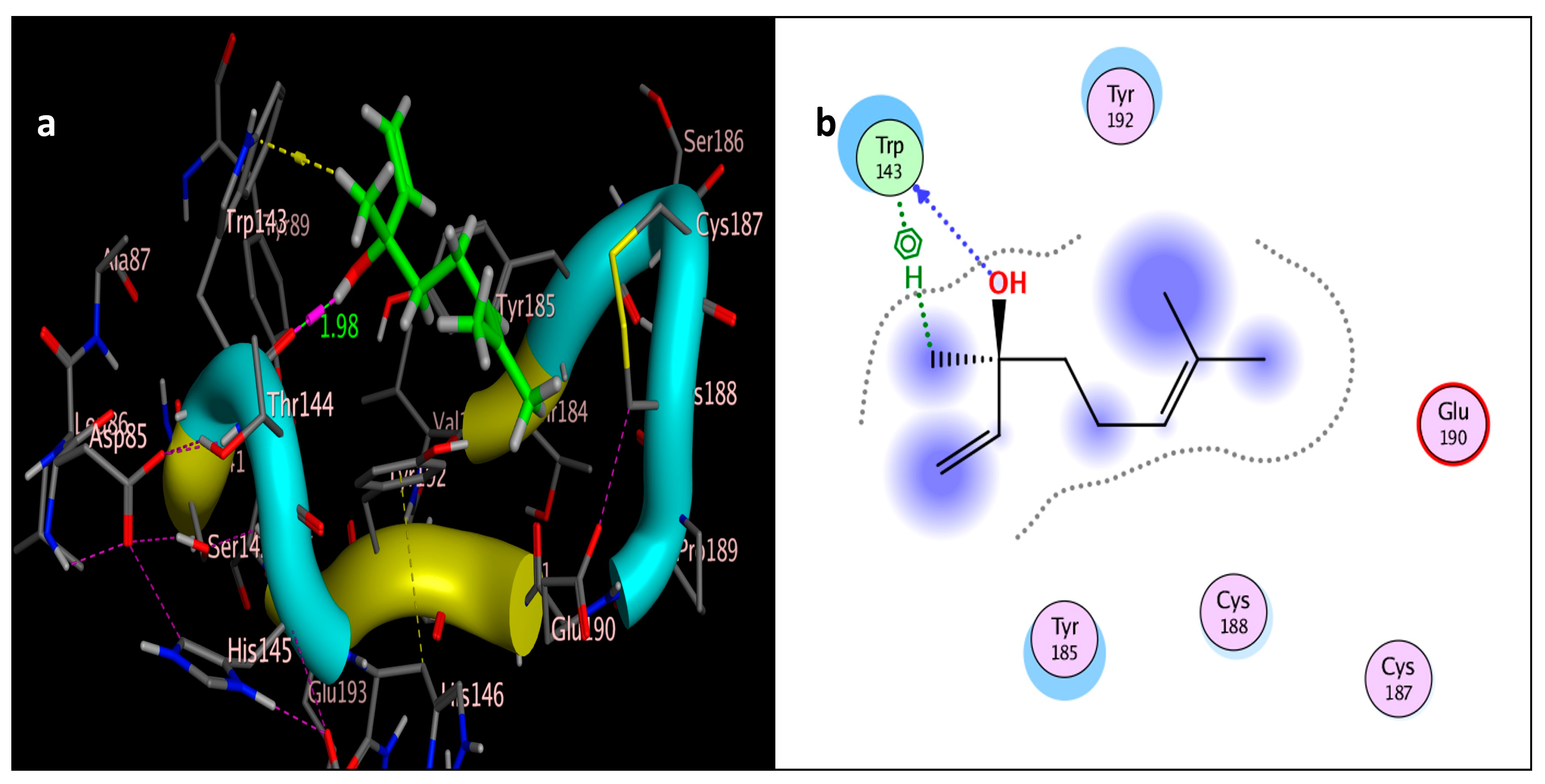
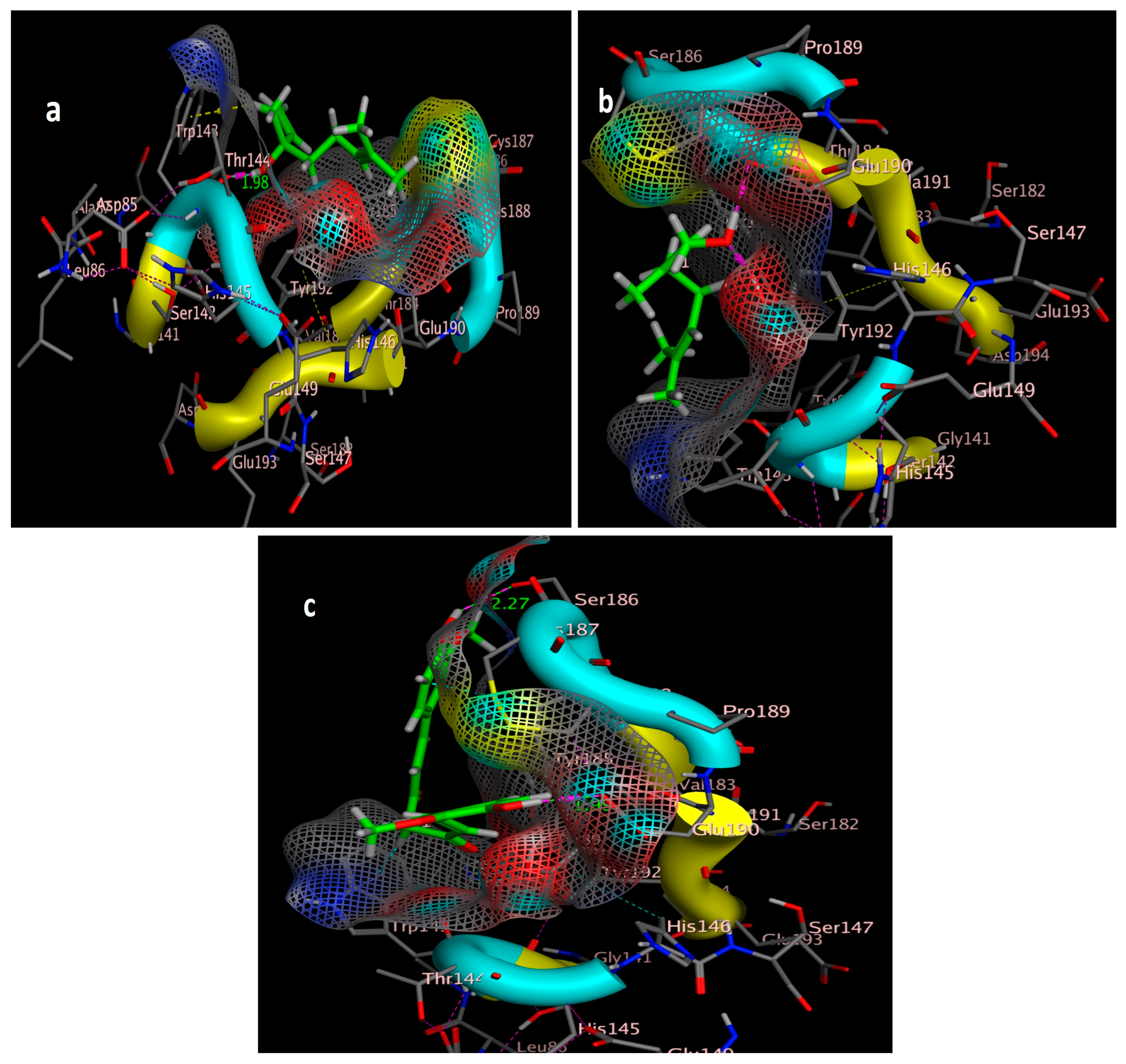
2.3. Molecular Simulation Docking
3. Discussion
4. Materials and Methods
4.1. Synthesis of Nanostructure Lipid Carrier Encapsulated Curcumin (NLC-Cur-LG)
4.2. Biochemical Assays
4.3. Synthesis of Encapsulated Nanostructure Lipid Carrier
4.4. Characterization of Nanostructure Lipid Carrier
4.4.1. The Particle Size (DLS), Polydispersity (PDI), and Zeta Potential
4.4.2. Transmission Electron Microscope (TEM)
4.5. In Vitro Cytotoxicity Effect of NLC-Cur-LG
4.6. Acetylcholinesterase Inhibition Assay
4.7. Western Blotting
4.8. Molecular Docking of Acetylcholineesterase Enzyme
4.8.1. Source of the Objective Protein
4.8.2. Energy Minimization
4.8.3. Docking Procedure
4.9. Insecticidal Evaluation
4.9.1. Laboratory Rearing of C. pipiens
4.9.2. Insecticidal Effect and Enzyme Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Idamokoro, E.M.; Hosu, Y.S. Out-Look on Worldwide Trends of Related Studies on Citrus Waste as Feed for Livestock Production: A Scientometric Analysis. Front. Res. Metr. Anal. 2022, 7, 869974. [Google Scholar] [CrossRef] [PubMed]
- Purba, R.A.P.; Suong, N.T.M.; Paengkoum, S.; Schonewille, J.T.; Paengkoum, P. Dietary inclusion of anthocyanin-rich black cane silage treated with ferrous sulfate heptahydrate reduces oxidative stress and promotes tender meat production in goats. Front. Vet. Sci. 2022, 9, 969321. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef]
- Zhang, H.A.; Kitts, D.D. Turmeric and its bioactive constituents trigger cell signaling mechanisms that protect against diabetes and cardiovascular diseases. Mol. Cell. Biochem. 2021, 476, 3785–3814. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Huebbe, P.; Ernst, I.M.; Chin, D.; Wagner, A.E.; Rimbach, G. Curcumin—From molecule to biological function. Angew. Chem. 2012, 51, 5308–5332. [Google Scholar] [CrossRef] [PubMed]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of curcumin: An emerging paradigm for improved remedial application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Young, K.N.; Moniruzzaman, M.; Beyene, A.M.; Do, K.; Kalaiselvi, S.; Min, T. Curcumin and Its Modified Formulations on Inflammatory Bowel Disease (IBD): The Story So Far and Future Outlook. Pharmaceutics 2021, 13, 484. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Wu, G.Q.; Chai, K.Q.; Zhu, X.M.; Jiang, H.; Wang, X.; Xue, Q.; Zheng, A.H.; Zhou, H.Y.; Chen, Y.; Chen, X.C.; et al. Anti-cancer effects of curcumin on lung cancer through the inhibition of EZH2 and NOTCH1. Oncotarget 2016, 7, 26535–26550. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Z.; Wu, Z.; Jin, M.; An, L.; Xue, F. Curcumin Improves Chronic Pain Induced Depression Through Regulating Serum Metabolomics in a Rat Model of Trigeminal Neuralgia. J. Pain Res. 2020, 13, 3479–3492. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shen, J.; Pan, H.; Xu, L.; Sheng, H.; Liu, B.; Yao, M. Curcumin analog B14 has high bioavailability and enhances the effect of anti-breast cancer cells in vitro and in vivo. Cancer Sci. 2021, 112, 815–827. [Google Scholar] [CrossRef] [PubMed]
- De Guzman, A.C.V.; Razzak, M.A.; Cho, J.H.; Kim, J.Y.; Choi, S.S. Curcumin-Loaded Human Serum Albumin Nanoparticles Prevent Parkinson’s Disease-like Symptoms in C. elegans. Nanomaterials 2022, 12, 758. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, S.; Lu, W.; Zhang, P. Fabrication of Curcumin@Ag Loaded Core/Shell Nanofiber Membrane and its Synergistic Antibacterial Properties. Front. Chem. 2022, 10, 870666. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, H.; Abdolmohammadi-Vahid, S.; Danshina, S.; Ziya Gencer, M.; Ammari, A.; Sadeghi, A.; Roshangar, L.; Aslani, S.; Esmaeilzadeh, A.; Ghaebi, M.; et al. Nano-curcumin therapy, a promising method in modulating inflammatory cytokines in COVID-19 patients. Int. Immunopharmacol. 2020, 89, 107088. [Google Scholar] [CrossRef] [PubMed]
- Paulraj, F.; Abas, F.; Lajis, N.H.; Othman, I.; Naidu, R. Molecular Pathways Modulated by Curcumin Analogue, Diarylpentanoids in Cancer. Biomolecules 2019, 9, 270. [Google Scholar] [CrossRef]
- Marcon, H.; Griss, L.G.; Molosse, V.L.; Cecere, B.G.O.; Alba, D.F.; Leal, K.W.; Galli, G.M.; Souza, C.F.; Baldissera, M.D.; Gundel, S.; et al. Dietary supplementation with curcumin-loaded nanocapsules in lambs: Nanotechnology as a new tool for nutrition. Anim. Nutr. 2021, 7, 521–529. [Google Scholar] [CrossRef]
- Yadav, S.; Teng, P.Y.; Souza Dos Santos, T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A.; Pazdro, R.; Kim, W.K. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar] [CrossRef]
- Veeran, S.; Cui, G.; Shu, B.; Yi, X.; Zhong, G. Curcumin-induced autophagy and nucleophagy in Spodoptera frugiperda Sf9 insect cells occur via PI3K/AKT/TOR pathways. J. Cell. Biochem. 2019, 120, 2119–2137. [Google Scholar] [CrossRef]
- Matiadis, D.; Liggri, P.G.V.; Kritsi, E.; Tzioumaki, N.; Zoumpoulakis, P.; Papachristos, D.P.; Balatsos, G.; Sagnou, M.; Michaelakis, A. Curcumin Derivatives as Potential Mosquito Larvicidal Agents against Two Mosquito Vectors, Culex pipiens and Aedes albopictus. Int. J. Mol. Sci. 2021, 22, 8915. [Google Scholar] [CrossRef]
- Nandakumar, D.N.; Nagaraj, V.A.; Vathsala, P.G.; Rangarajan, P.; Padmanaban, G. Curcumin-artemisinin combination therapy for malaria. Antimicrob. Agents Chemother. 2006, 50, 1859–1860. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.; Megahed, A.; Kandeel, S.; Alouffi, A.; Almutairi, M.M. West Nile virus seroprevalence and associated risk factors among horses in Egypt. Sci. Rep. 2021, 11, 20932. [Google Scholar] [CrossRef]
- Marzok, M.; Alkashif, K.; Kandeel, M.; Salem, M.; Sayed-Ahmed, M.Z.; Selim, A. Seroprevalence of Rift Valley Fever virus in one-humped camels (Camelus dromedaries) in Egypt. Trop. Anim. Health Prod. 2023, 55, 345. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.E.-D.M.; Abou-Taleb, H.K.; Abdelgaleil, S.A.M. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J. Asia-Pac. Entomol. 2017, 20, 133–139. [Google Scholar] [CrossRef]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. BioMed Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef] [PubMed]
- Baz, M.M.; Selim, A.; Radwan, I.T.; Alkhaibari, A.M.; Khater, H.F. Larvicidal and adulticidal effects of some Egyptian oils against Culex pipiens. Sci. Rep. 2022, 12, 4406. [Google Scholar] [CrossRef]
- Benelli, G. Research in mosquito control: Current challenges for a brighter future. Parasitol. Res. 2015, 114, 2801–2805. [Google Scholar] [CrossRef]
- Koureas, M.; Tsakalof, A.; Tsatsakis, A.; Hadjichristodoulou, C. Systematic review of biomonitoring studies to determine the association between exposure to organophosphorus and pyrethroid insecticides and human health outcomes. Toxicol. Lett. 2012, 210, 155–168. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. A Review of Resistance Mechanisms of Synthetic Insecticides and Botanicals, Phytochemicals, and Essential Oils as Alternative Larvicidal Agents Against Mosquitoes. Front. Physiol. 2019, 10, 1591. [Google Scholar] [CrossRef]
- Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Use of hydrogen peroxide as a biocide: New consideration of its mechanisms of biocidal action. J. Antimicrob. Chemother. 2012, 67, 1589–1596. [Google Scholar] [CrossRef]
- Ahmad, S.; Duval, D.L.; Weinhold, L.C.; Pardini, R.S. Cabbage looper antioxidant enzymes: Tissue specificity. Insect Biochem. 1991, 21, 563–572. [Google Scholar] [CrossRef]
- Renault, D.; Dorrah, M.A.; Mohamed, A.A.; Abdelfattah, E.A.; Bassal, T.T. Assessment of oxidative stress and activities of antioxidant enzymes depicts the negative systemic effect of iron-containing fertilizers and plant phenolic compounds in the desert locust. Environ. Sci. Pollut. Res. Int. 2016, 23, 21989–22000. [Google Scholar] [CrossRef]
- Bi, J.L.; Felton, G.W. Foliar oxidative stress and insect herbivory: Primary compounds, secondary metabolites, and reactive oxygen species as components of induced resistance. J. Chem. Ecol. 1995, 21, 1511–1530. [Google Scholar] [CrossRef]
- Summers, C.B.; Felton, G.W. Prooxidant effects of phenolic acids on the generalist herbivore Helicoverpa zea (Lepidoptera: Noctuidae): Potential mode of action for phenolic compounds in plant anti-herbivore chemistry. Insect Biochem. Mol. Biol. 1994, 24, 943–953. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Abdelfattah, E.A.; Augustyniak, M.; Yousef, H.A. Biomonitoring of genotoxicity of industrial fertilizer pollutants in Aiolopus thalassinus (Orthoptera: Acrididae) using alkaline comet assay. Chemosphere 2017, 182, 762–770. [Google Scholar] [CrossRef]
- Singh, K.D.; Labala, R.K.; Devi, T.B.; Singh, N.I.; Chanu, H.D.; Sougrakpam, S.; Nameirakpam, B.S.; Sahoo, D.; Rajashekar, Y. Biochemical efficacy, molecular docking and inhibitory effect of 2, 3-dimethylmaleic anhydride on insect acetylcholinesterase. Sci. Rep. 2017, 7, 12483. [Google Scholar] [CrossRef]
- Siegfried, B.D.; Scott, J.G. Properties and inhibition of acetylcholinesterase in resistant and susceptible German cockroaches (Blattella germanica L.). Pestic. Biochem. Physiol. 1990, 38, 122–129. [Google Scholar] [CrossRef]
- Pascual-Villalobos, M.; Ballesta-Acosta, M. Chemical variation in an Ocimum basilicum germplasm collection and activity of the essential oils on Callosobruchus maculatus. Biochem. Syst. Ecol. 2003, 31, 673–679. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Hamraoui, A. Inhibition of reproduction of Acanthoscelides obtectus Say (Coleoptera), a kidney bean (Phaseolus vulgaris) bruchid, by aromatic essential oils. Crop Prot. 1994, 13, 624–628. [Google Scholar] [CrossRef]
- Kovacevic, A.; Savic, S.; Vuleta, G.; Mueller, R.H.; Keck, C.M. Polyhydroxy surfactants for the formulation of lipid nanoparticles (SLN and NLC): Effects on size, physical stability and particle matrix structure. Int. J. Pharm. 2011, 406, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Xu, S.; Li, J.; Pan, S.; Miao, X. Particle size effect of curcumin nanocrystals on transdermal and transfollicular penetration by hyaluronic acid-dissolving microneedle delivery. Pharmaceuticals 2022, 15, 206. [Google Scholar] [CrossRef] [PubMed]
- Radwan, I.T.; Eltaly, R.I.; Baz, M.M.; Yousif, M.; Selim, A.; Taie, H.A.; Manaa, E.A.; Khater, H.F. Novel acaricidal and growth-regulating activity of Aloe vera and Rheum rhabarbarum extracts and their oil/water nanoemulsions against the camel tick, Hyalomma dromedarii. Sci. Rep. 2023, 13, 16802. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Rostamizadeh, K.; Shademani, N.; Parsa, M. Synthesis and investigation of the curcumin-loaded magnetic lipid nanoparticles and their cytotoxicity assessment on human breast carcinoma cell line. Jundishapur J. Nat. Pharm. Prod. 2020, 15, e91886. [Google Scholar] [CrossRef]
- López, M.; Pascual-Villalobos, M. Mode of inhibition of acetylcholinesterase by monoterpenoids and implications for pest control. Ind. Crop. Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Ganguli, A.; Choudhury, D.; Chakrabarti, G. 2, 4-Dichlorophenoxyacetic acid induced toxicity in lung cells by disruption of the tubulin-microtubule network. Toxicol. Res. 2014, 3, 118–130. [Google Scholar] [CrossRef]
- Ghazawy, N.A.R.; Afify, A.; Radwan, I.T.; Ghabban, H.; Alkhaibari, A.M.; Gattan, H.S.; Alruhaili, M.H.; Selim, A.; Saad, M.M.A. The Effect of Abamectin on Locusta Migratoria Neurosecretory Cells and Mid Gut, Using Ultrastructure Examination, Oxidative Stress Study, and In-Silico Molecular Docking. Molecules 2023, 28, 6956. [Google Scholar] [CrossRef]
- Radwan, I.T.; Sayed-Ahmed, M.Z.; Ghazawy, N.A.; Alqahtani, S.S.; Ahmad, S.; Alam, N.; Alkhaibari, A.M.; Ali, M.S.; Selim, A.; AbdelFattah, E.A. Effect of nanostructure lipid carrier of methylene blue and monoterpenes as enzymes inhibitor for Culex pipiens. Sci. Rep. 2023, 13, 12522. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Marí, M.; Morales, A.; Colell, A.; García-Ruiz, C.; Fernández-Checa, J.C. Mitochondrial glutathione, a key survival antioxidant. Antioxid. Redox Signal. 2009, 11, 2685–2700. [Google Scholar] [CrossRef]
- Schouten, A.; Tenberge, K.B.; Vermeer, J.; Stewart, J.; Wagemakers, L.; Williamson, B.; Van Kan, J.A. Functional analysis of an extracellular catalase of Botrytis cinerea. Mol. Plant Pathol. 2002, 3, 227–238. [Google Scholar] [CrossRef]
- Mecdad, A.A.; Ahmed, M.H.; ElHalwagy, M.E.; Afify, M.M. A study on oxidative stress biomarkers and immunomodulatory effects of pesticides in pesticide-sprayers. Egypt. J. Forensic Sci. 2011, 1, 93–98. [Google Scholar] [CrossRef]
- Da Silva, F.R.; Da Silva, J.; Allgayer, M.d.C.; Simon, C.F.; Dias, J.F.; dos Santos, C.E.; Salvador, M.; Branco, C.; Schneider, N.B.; Kahl, V. Genotoxic biomonitoring of tobacco farmers: Biomarkers of exposure, of early biological effects and of susceptibility. J. Hazard. Mater. 2012, 225, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide radical: An endogenous toxicant. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Jiravanichpaisal, P.; Lee, B.L.; Söderhäll, K. Cell-mediated immunity in arthropods: Hematopoiesis, coagulation, melanization and opsonization. Immunobiology 2006, 211, 213–236. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E. Quantification and significance of protein oxidation in biological samples. Drug Metab. Rev. 2000, 32, 307–326. [Google Scholar] [CrossRef]
- Arakane, Y.; Muthukrishnan, S. Insect chitinase and chitinase-like proteins. Cell. Mol. Life Sci. 2010, 67, 201–216. [Google Scholar] [CrossRef]
- Lalouette, L.; Williams, C.; Hervant, F.; Sinclair, B.J.; Renault, D. Metabolic rate and oxidative stress in insects exposed to low temperature thermal fluctuations. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 158, 229–234. [Google Scholar] [CrossRef]
- Kregel, K.C.; Zhang, H.J. An integrated view of oxidative stress in aging: Basic mechanisms, functional effects, and pathological considerations. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R18–R36. [Google Scholar] [CrossRef]
- Gutierrez-Correa, J.; Stoppani, A. Inactivation of yeast glutathione reductase by Fenton systems: Effect of metal chelators, catecholamines and thiol compounds. Free Radic. Res. 1997, 27, 543–555. [Google Scholar] [CrossRef]
- Costa, V.; Quintanilha, A.; Moradas-Ferreira, P. Protein oxidation, repair mechanisms and proteolysis in Saccharomyces cerevisiae. IUBMB Life 2007, 59, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Łukasik, I.; Goławska, S.; Wójcicka, A. Antioxidant defense mechanisms of cereal aphids based on ascorbate and ascorbate peroxidase. Biologia 2009, 64, 994–998. [Google Scholar] [CrossRef]
- Li, P.; Jia, J.; Zhang, D.; Xie, J.; Xu, X.; Wei, D. In vitro and in vivo antioxidant activities of a flavonoid isolated from celery (Apium graveolens L. var. dulce). Food Funct. 2014, 5, 50–56. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Bagato, N.M.A.; Radwan, I.T. Design, Synthesis, Molecular Docking, and Biological Studies of New Heterocyclic Compounds Derived from β-Diketones as Novel EGFR and Pim-1 Inhibitors Endowed with Antitumor Activity. Anti-Cancer Agents Med. Chem. 2022, 22, 2558–2576. [Google Scholar] [CrossRef] [PubMed]
- Harbach, R.E.; Knight, K.L. Taxonomists’ Glossary of Mosquito Anatomy; Plexus Publishing Inc.: Medford, NJ, USA, 1980. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.-G.; Ahn, B.-W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 186, pp. 464–478. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730. [Google Scholar] [CrossRef]



| Treatment | n a | Slope ± SE | LC50 (95% CI) | χ2 b | Df | p |
|---|---|---|---|---|---|---|
| NLC-Cur-LG | 750 | 0567 ± 0.127 | 0.649 (0.369–3.27) | 4.095 | 10 | <0.001 |
| control | 750 | 0.399 ± 0.157 | 1.411(1.051–1.812) | 3.635 | 10 | <0.001 |
| Compound | Curcumin | Linalool | Geraniol | Skweez Pesticide (Imidocloprid 34%) | NLC-Cur-LG | Donepezil |
|---|---|---|---|---|---|---|
| IC50 (µg/mL) | 14.88 | 4.34 | 2.42 | 2.11 | 1.95 | 1.8 |
| Con. | O. D | Aver O. D | St. Dev. | S. E | Viability | Toxicity | IC50 (µg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Vero | 0.694 | 0.681 | 0.689 | 0.688 | 0.0065574 | 0.003786 | 100 | 0 | 81.61 ± 3.2 | |
| NLC-Cur-LG | 1000 | 0.051 | 0.059 | 0.062 | 0.057333 | 0.0056862 | 0.003283 | 8.333333 | 91.66667 | |
| 500 | 0.042 | 0.055 | 0.056 | 0.051 | 0.0078102 | 0.004509 | 7.412791 | 92.58721 | ||
| 250 | 0.065 | 0.039 | 0.052 | 0.052 | 0.013 | 0.007506 | 7.55814 | 92.44186 | ||
| 125 | 0.14 | 0.119 | 0.156 | 0.13833333 | 0.0185562 | 0.010713 | 20.10659 | 79.89341 | ||
| 62.5 | 0.425 | 0.395 | 0.378 | 0.39933333 | 0.0237978 | 0.01374 | 58.04264 | 41.95736 | ||
| 31.25 | 0.5 | 0.535 | 0.523 | 0.51933333 | 0.0177858 | 0.010269 | 75.4845 | 24.5155 | ||
| Con. | (Optical Density) O. D | Aver O. D | St. Dev. | S. E | Viability | Toxicity | IC50 (µg/mL) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Wi38 | 0.566 | 0.559 | 0.583 | 0.56933333 | 0.0123423 | 0.007126 | 100 | 0 | 158.15 ± 5.2 | |
| NLC-Cur-LG | 1000 | 0.029 | 0.032 | 0.018 | 0.02633333 | 0.0073711 | 0.004256 | 4.625293 | 95.37471 | |
| 500 | 0.033 | 0.042 | 0.065 | 0.04666667 | 0.0165025 | 0.009528 | 8.196721 | 91.80328 | ||
| 250 | 0.123 | 0.114 | 0.141 | 0.126 | 0.0137477 | 0.007937 | 22.13115 | 77.86885 | ||
| 125 | 0.28 | 0.41 | 0.43 | 0.37333333 | 0.0814453 | 0.047022 | 65.57377 | 34.42623 | ||
| 62.5 | 0.392 | 0.59 | 0.48 | 0.48733333 | 0.0992035 | 0.057275 | 85.59719 | 14.40281 | ||
| 31.25 | 0.489 | 0.555 | 0.59 | 0.54466667 | 0.0512868 | 0.02961 | 95.66745 | 4.332553 | ||
| Compound | Interactions | Residue | Type | Distance (Å) | Score (kcal/mol) | RMSD (Å) |
|---|---|---|---|---|---|---|
| imidacloprid | 3 | Cl → Ser142 (Backbone) | H-bonding | 3.16 | −4.6308 | 0.8343 |
| NO2 → Cys187 | H-bonding | 2.17 | ||||
| NH → Cys187 | H-bonding | 2.40 | ||||
| curcumin | 2 | OH → Ser186 | H-bonding | 2.27 | −5.5592 | 1.5104 |
| OH → Glu190 | H-bonding | 1.99 | ||||
| Geraniol | 2 | Try192 → OH | H-bonding | 1.97 | −4.0244 | 0.8234 |
| OH → Glu190 | H-bonding | 2.20 | ||||
| linalool | 2 | OH → Trp143 (Backbone) | H-bonding | 1.98 | −4.1315 | 1.5514 |
| CH3 →Trp143 (benzene ring) | π-π interactions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radwan, I.T.; Ghazawy, N.A.R.; Alkhaibari, A.M.; Gattan, H.S.; Alruhaili, M.H.; Selim, A.; Salem, M.E.; AbdelFattah, E.A.; Hamama, H.M. Nanostructure Lipid Carrier of Curcumin Co-Delivered with Linalool and Geraniol Monoterpenes as Acetylcholinesterase Inhibitor of Culex pipiens. Molecules 2024, 29, 271. https://doi.org/10.3390/molecules29010271
Radwan IT, Ghazawy NAR, Alkhaibari AM, Gattan HS, Alruhaili MH, Selim A, Salem ME, AbdelFattah EA, Hamama HM. Nanostructure Lipid Carrier of Curcumin Co-Delivered with Linalool and Geraniol Monoterpenes as Acetylcholinesterase Inhibitor of Culex pipiens. Molecules. 2024; 29(1):271. https://doi.org/10.3390/molecules29010271
Chicago/Turabian StyleRadwan, Ibrahim Taha, Nirvina Abdel Raouf Ghazawy, Abeer Mousa Alkhaibari, Hattan S. Gattan, Mohammed H. Alruhaili, Abdelfattah Selim, Mostafa E. Salem, Eman Alaaeldin AbdelFattah, and Heba M. Hamama. 2024. "Nanostructure Lipid Carrier of Curcumin Co-Delivered with Linalool and Geraniol Monoterpenes as Acetylcholinesterase Inhibitor of Culex pipiens" Molecules 29, no. 1: 271. https://doi.org/10.3390/molecules29010271
APA StyleRadwan, I. T., Ghazawy, N. A. R., Alkhaibari, A. M., Gattan, H. S., Alruhaili, M. H., Selim, A., Salem, M. E., AbdelFattah, E. A., & Hamama, H. M. (2024). Nanostructure Lipid Carrier of Curcumin Co-Delivered with Linalool and Geraniol Monoterpenes as Acetylcholinesterase Inhibitor of Culex pipiens. Molecules, 29(1), 271. https://doi.org/10.3390/molecules29010271









