Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone
Abstract
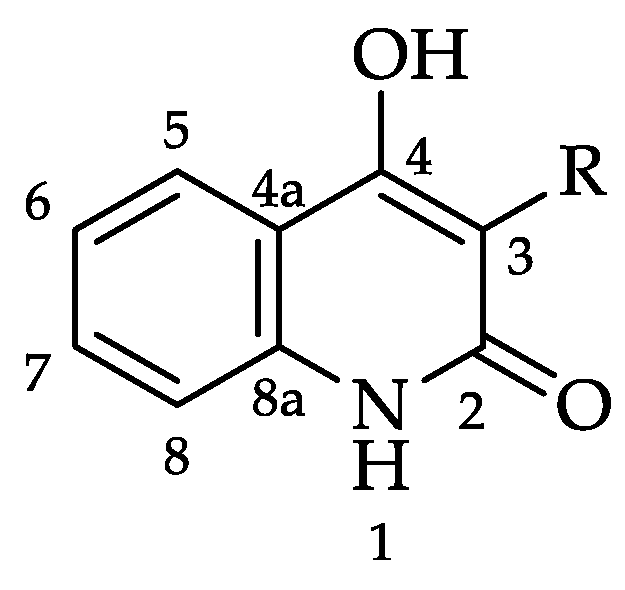
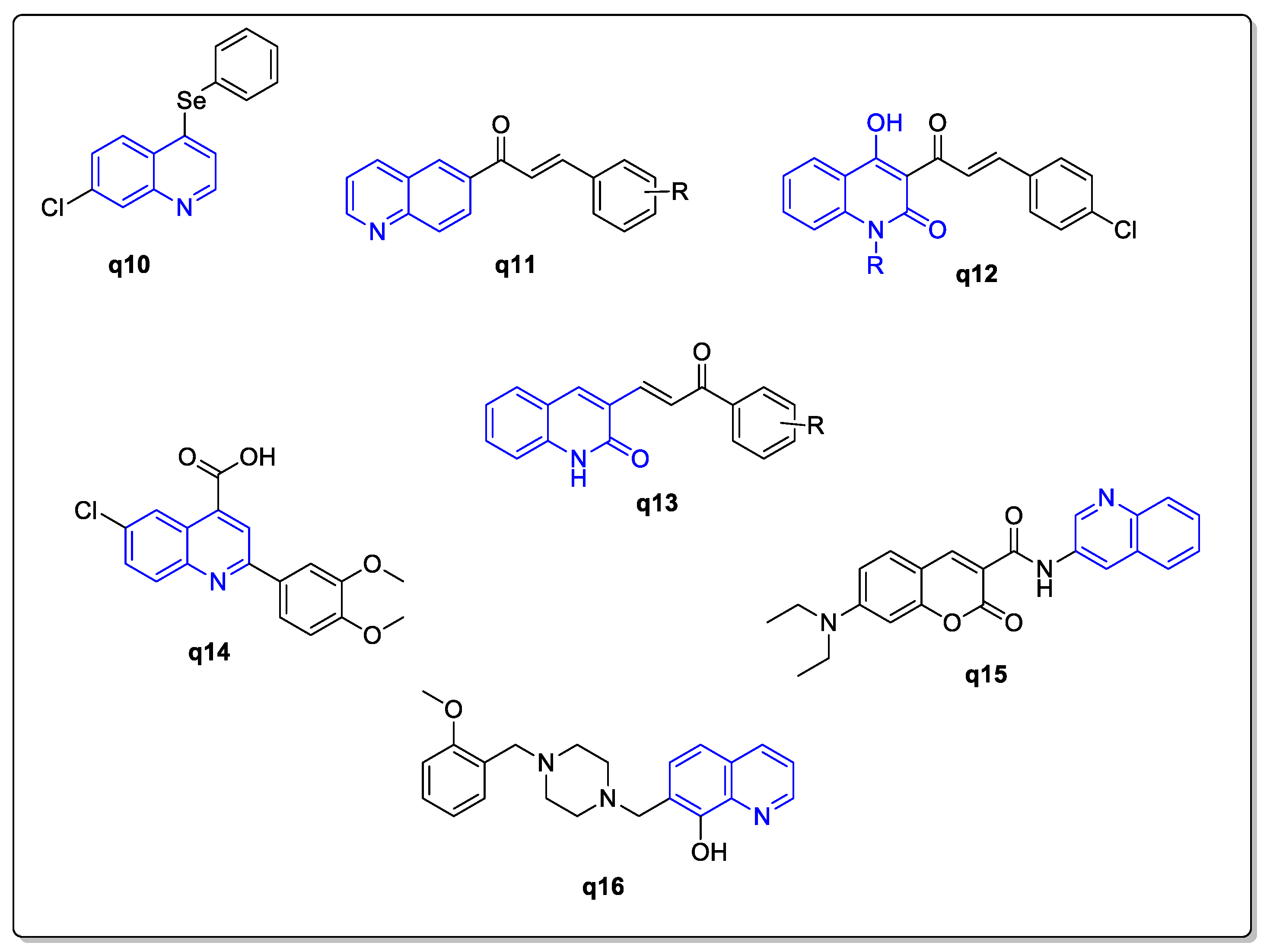
:1. Introduction
2. Results and Discussion
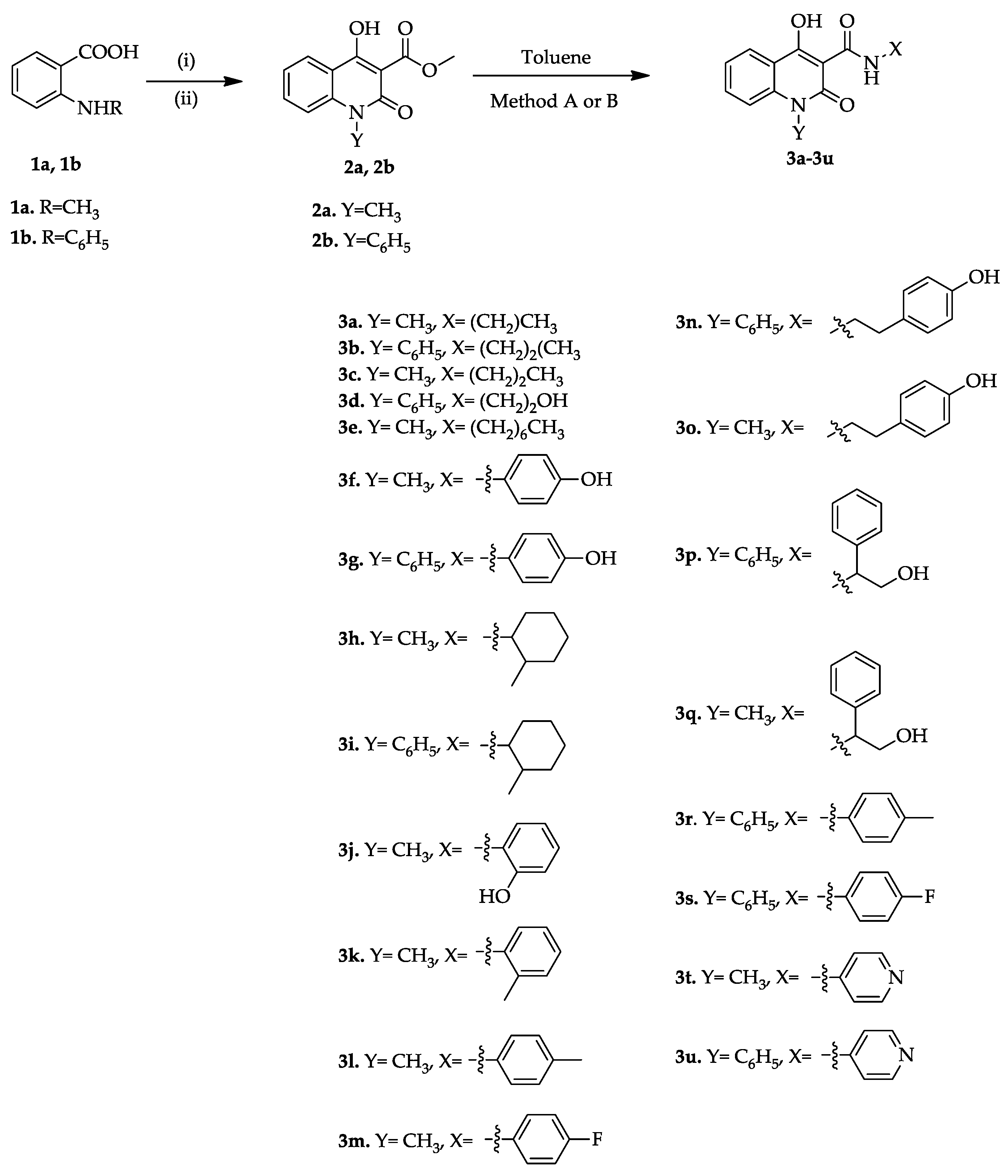
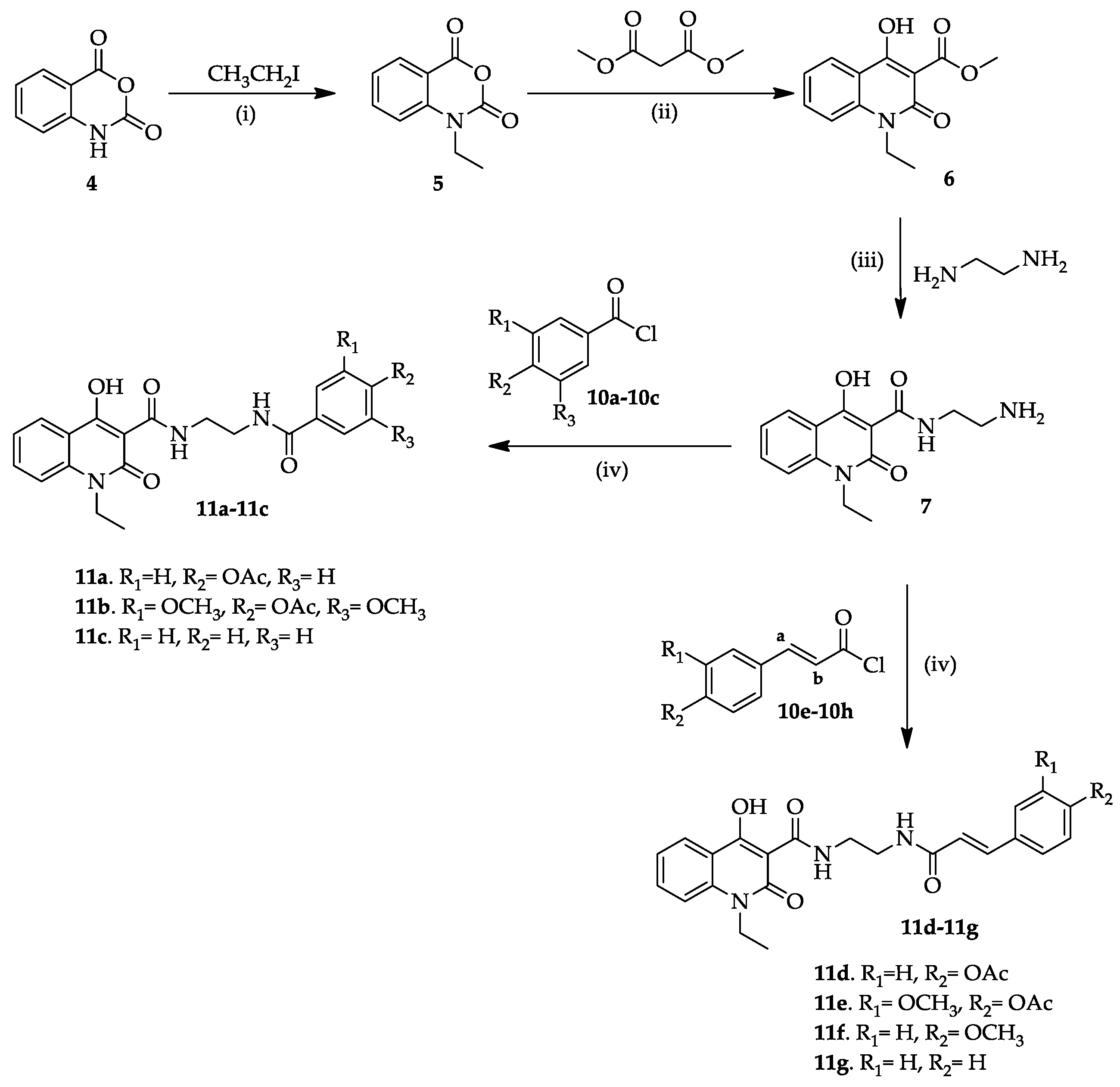
2.1. Chemistry
2.2. Bioactivity Assays
2.2.1. DPPH Assay
2.2.2. Lipid Peroxidation of Linoleic Acid Induced by AAPH Radical
2.2.3. Competition with DMSO for Hydroxyl Radicals
2.2.4. ABTS Radical Cation Decolorization Assay
2.2.5. Superoxide Anion Radical Scavenging Ability
2.2.6. Soybean LOX Inhibitory Activity
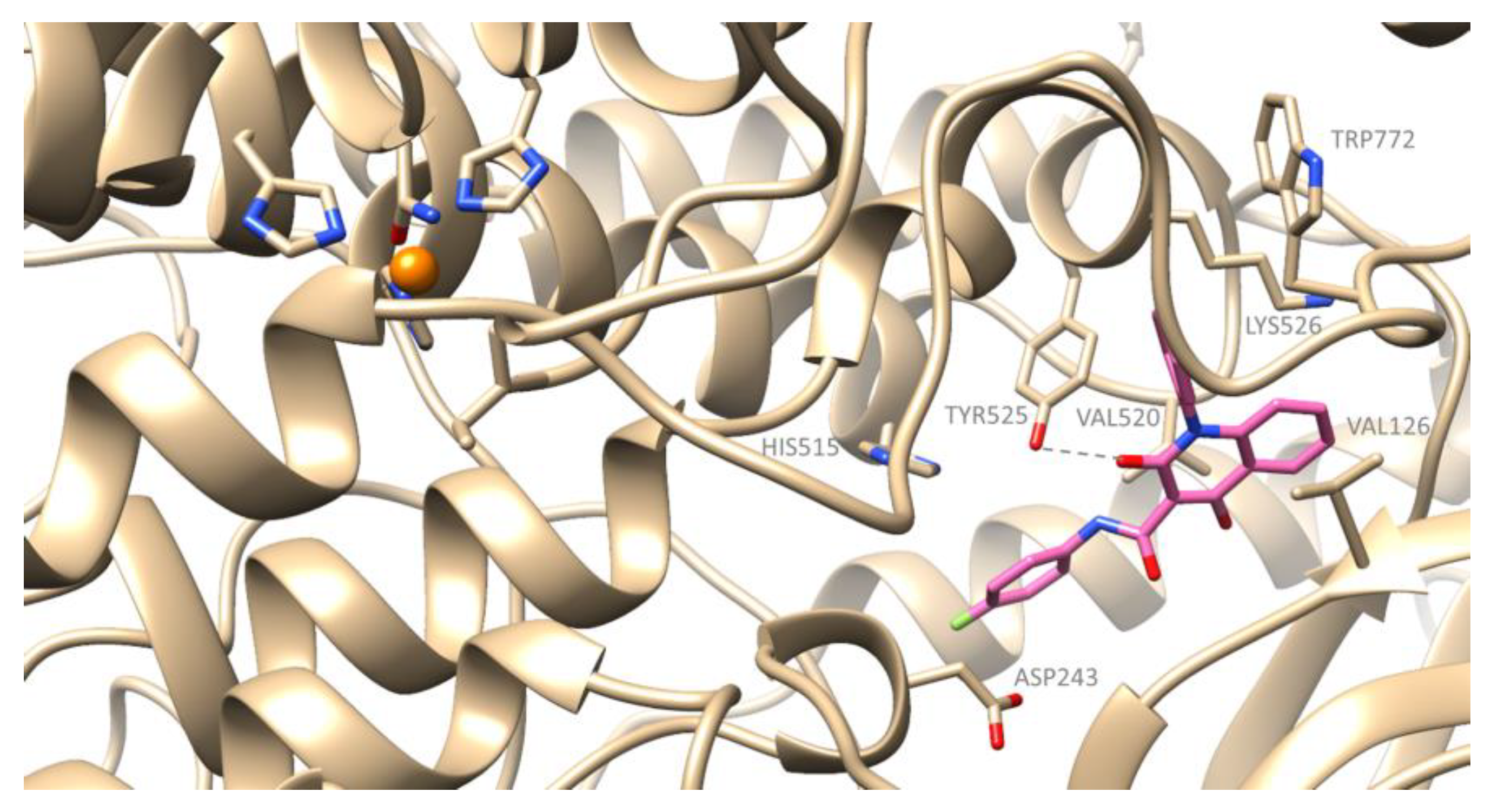
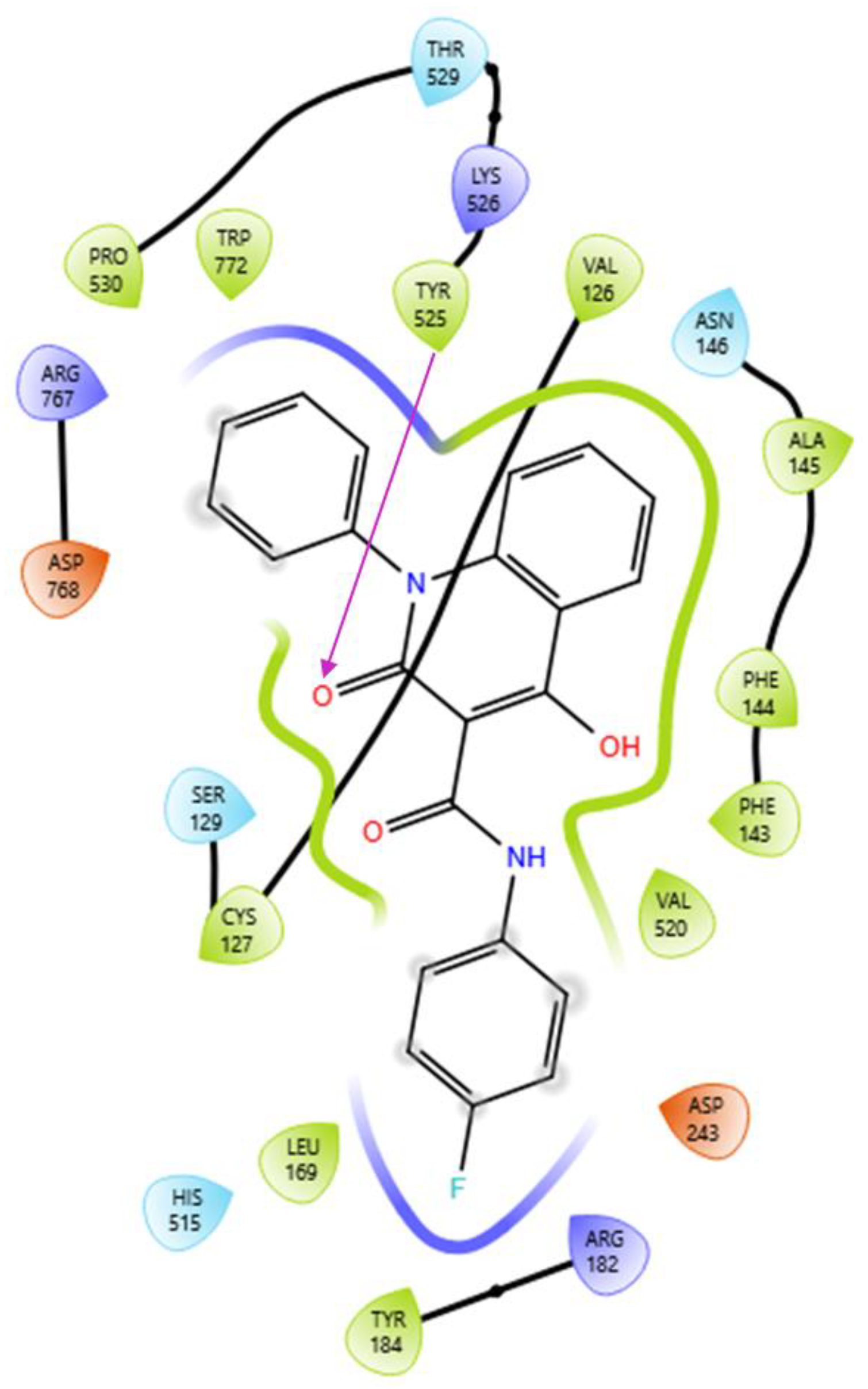
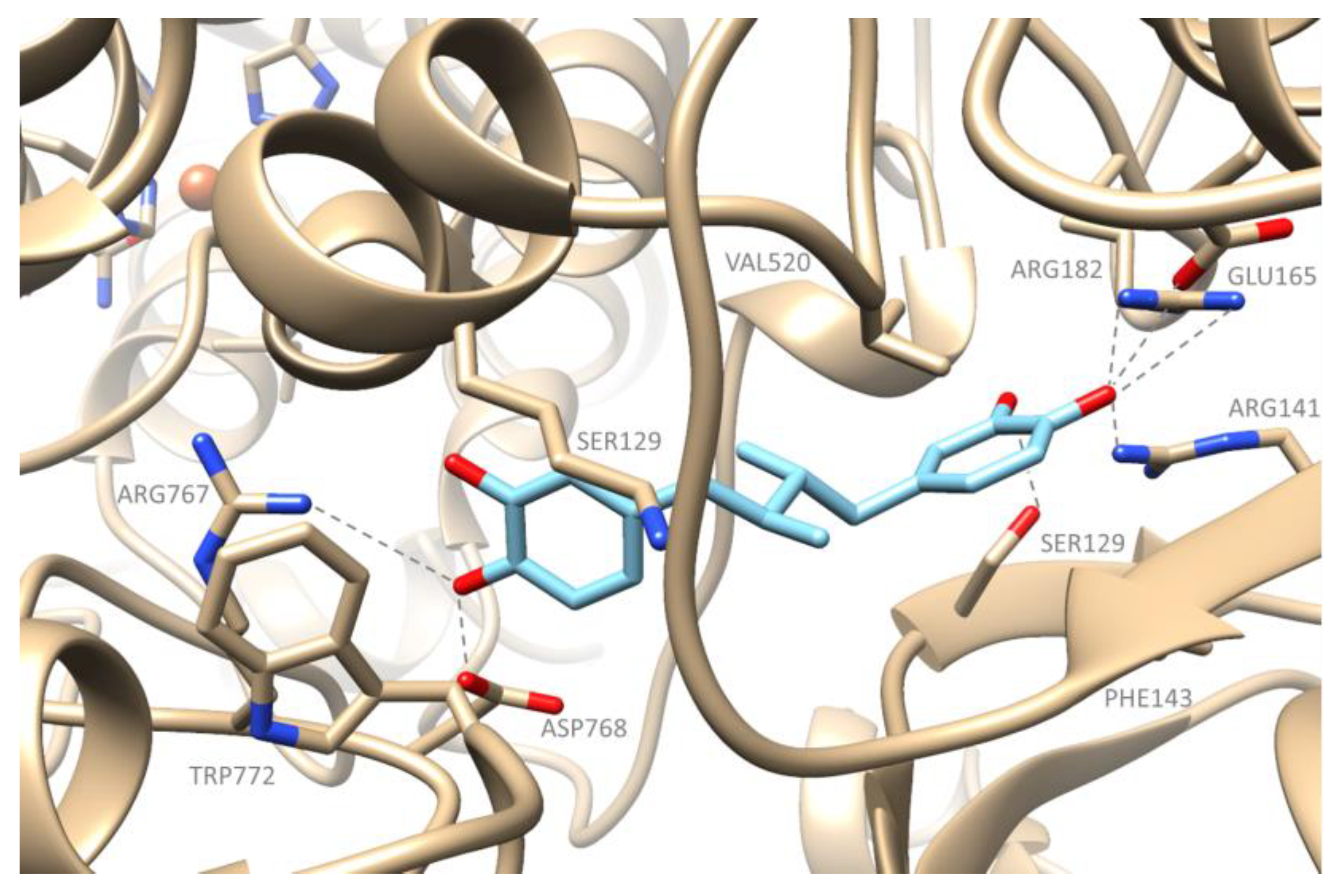
2.3. Computational Studies–Docking Simulation Soybean Lipoxygenase
Docking Studies of the Synthesized Derivatives in Soybean LOX
3. Materials and Methods
3.1. Synthesis and General Procedures
3.1.1. General Procedure for the Synthesis of Quinolinone–Carboxamides 3a–3x
- Method A:
- 2.
- Method B:
3.1.2. Synthesis of 1-Ethyl-2,4-dihydro-1H-3,1-benzoxazine-2,4-dione (5)
3.1.3. Synthesis of Methyl 1-Ethyl-4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxylate (6)
3.1.4. Synthesis of the N-(2-Aminoethyl)-1-ethyl-4-hydroxy-2-oxo-1,2-dihydroquinoline-3-carboxamide (7)
3.1.5. General Procedure for the Synthesis of Acetyl Phenolic Acid Derivatives (9a, 9b, 9e, and 9f)
3.1.6. General Procedure for the Synthesis of the Acetyl Chlorides 10a–10g
3.1.7. General Procedure for the Synthesis of Hybrid Compounds 11a–11g
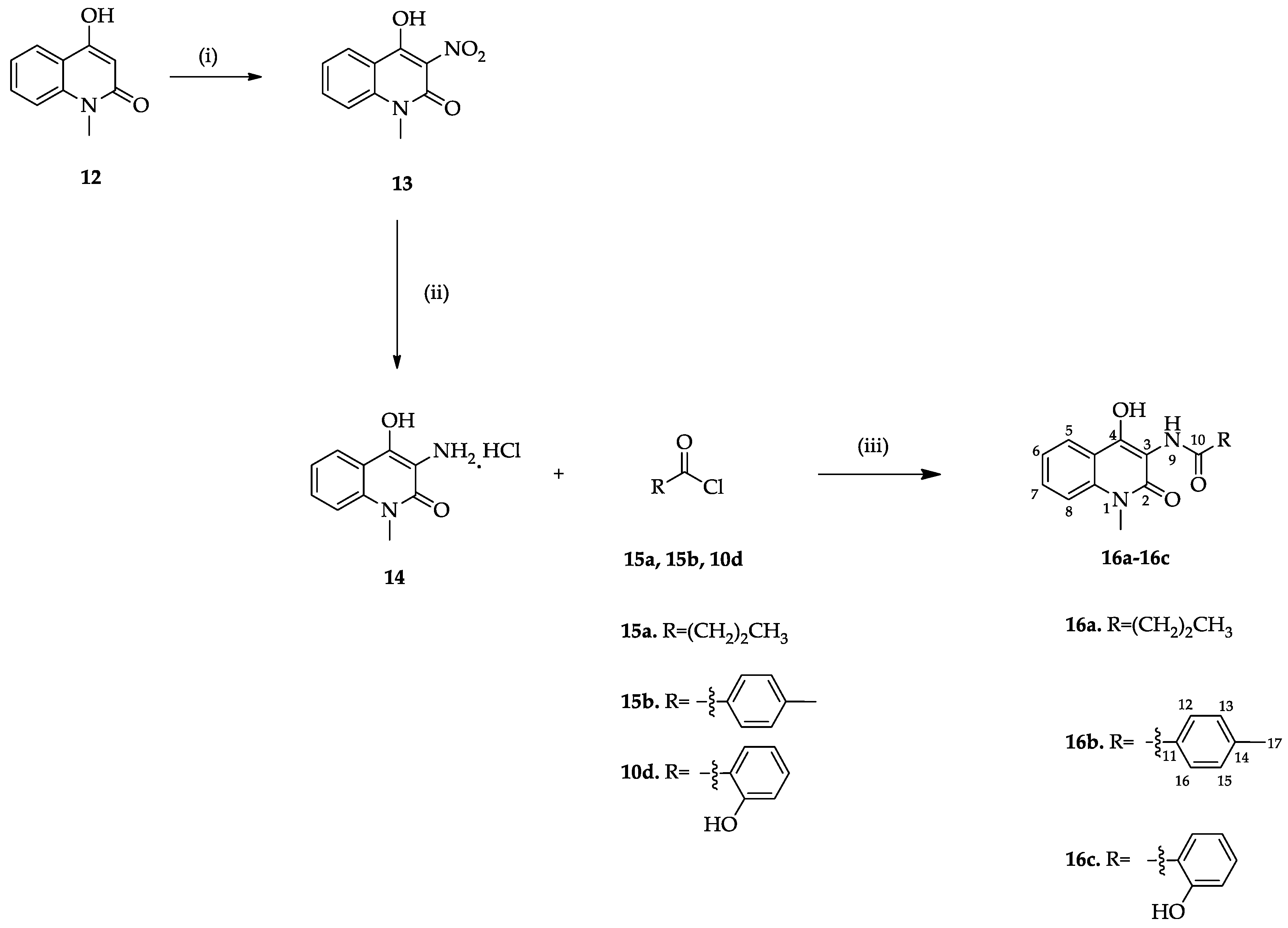
3.1.8. Synthesis of 4-Hydroxy-1-methyl-3-nitroquinolin-2(1H)-one (13)
3.1.9. Synthesis of the 3-Amino-4-hydroxy-1-methylquinolin-2(1H)-one Hydrochloride (14)
3.1.10. General Procedure for the Preparation of Reverse Amides 16a–16d
3.2. Biological In Vitro Assays
3.2.1. Determination of the Reducing Activity of DPPH Radical
3.2.2. Inhibition of Linoleic Acid Lipid Peroxidation
3.2.3. Competition of the Tested Compounds with DMSO for Hydroxyl Radicals
3.2.4. ABTS+—Decolorization Assay for Antioxidant Activity
3.2.5. Non-Enzymatic Assay of Superoxide Radicals Measurement of Superoxide Radical Scavenging Activity
3.2.6. Soybean LOX Inhibition Study In Vitro
3.2.7. Computational Methods–Molecular Docking Studies on Soybean Lipoxygenase
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajesh, Y.B. Quinoline heterocycles: Synthesis and bioactivity. In Heterocycles-Synthesis and Biological Activities; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Zeleke, D.; Eswaramoorthy, R.; Belay, Z.; Melaku, Y. Synthesis and antibacterial, antioxidant, and molecular docking analysis of some novel quinoline derivatives. J. Chem. 2020, 2020, 1324096. [Google Scholar] [CrossRef]
- Chu, X.M.; Wang, C.; Liu, W.; Liang, L.L.; Gong, K.K.; Zhao, C.Y.; Sun, K.L. Quinoline and quinolone dimers and their biological activities: An overview. Eur. J. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological effects of quinolones: A family of broad-spectrum antimicrobial agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; Ramadan, M.; Abuo-Rahma, G.E.D.A.; Elshaier, Y.A.; Elbastawesy, M.A.; Brown, A.B.; Braese, S. Quinolones as prospective drugs: Their syntheses and biological applications. Adv. Heterocycl. Chem. 2021, 135, 147–196. [Google Scholar]
- Fu, J.; Bao, F.; Gu, M.; Liu, J.; Zhang, Z.; Ding, J.; Xie, S.S.; Ding, J. Design, synthesis and evaluation of quinolinone derivatives containing dithiocarbamate moiety as multifunctional AChE inhibitors for the treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2020, 35, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Shiro, T.; Fukaya, T.; Tobe, M. The chemistry and biological activity of heterocycle-fused quinolinone derivatives: A review. Eur. J. Med. Chem. 2015, 97, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Hradil, P.; Hlavac, J.; Soural, M.; Hajduch, M.; Kolar, M.; Vecerova, R. 3-Hydroxy-2-phenyl-4 (1H)-quinolinones as promising biologically active compounds. Mini-Rev. Med. Chem. 2009, 9, 696–702. [Google Scholar] [CrossRef]
- Angeleska, S.; Kefalas, P.; Detsi, A. Crude peroxidase from onion solid waste as a tool for organic synthesis. Part III: Synthesis of tetracyclic heterocycles (coumestans and benzofuroquinolinones). Tetrahedron Lett. 2013, 54, 2325–2328. [Google Scholar] [CrossRef]
- Mostafa, M.A.; Ismail, M.M.; Morsy, J.M.; Hassanin, H.M.; Abdelrazek, M.M. Synthesis, characterization, anticancer, and antioxidant activities of chitosan Schiff bases bearing quinolinone or pyranoquinolinone and their silver nanoparticles derivatives. Polym. Bull. 2023, 80, 4035–4059. [Google Scholar] [CrossRef]
- Laiolo, J.; Lanza, P.A.; Parravicini, O.; Barbieri, C.; Insuasty, D.; Cobo, J.; Vera, M.A.; Enriz, R.D.; Carpinella, M.C. Structure activity relationships and the binding mode of quinolinone-pyrimidine hybrids as reversal agents of multidrug resistance mediated by P-gp. Sci. Rep. 2021, 11, 16856. [Google Scholar] [CrossRef]
- Beker, H.K.; Yıldırım, I. Anticancer Activity–Structure Relationship of Quinolinone-Core Compounds: An Overall Review. Pharm. Chem. J. 2023, 56, 1333–1343. [Google Scholar] [CrossRef]
- Hassan, A.H.; Mahmoud, K.; Phan, T.N.; Shaldam, M.A.; Lee, C.H.; Kim, Y.J.; Cho, S.B.; Bayoumi, W.A.; El-Sayed, S.M.; Choi, Y.; et al. Bestatin analogs-4-quinolinone hybrids as antileishmanial hits: Design, repurposing rational, synthesis, in vitro and in silico studies. Eur. J. Med. Chem. 2023, 250, 115211. [Google Scholar] [CrossRef] [PubMed]
- Aly, A.A.; El-Sheref, E.M.; Mourad, A.F.E.; Bakheet, M.E.; Bräse, S. 4-Hydroxy-2-quinolones: Syntheses, reactions and fused heterocycles. Mol. Divers. 2020, 24, 477–524. [Google Scholar] [CrossRef] [PubMed]
- Arya, K.; Agarwal, M. Microwave prompted multigram synthesis, structural determination, and photo-antiproliferative activity of fluorinated 4-hydroxyquinolinones. Bioorg. Med. Chem. Lett. 2007, 17, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Musiol, R.; Pesko, M.; Kralova, K.; Vejsova, M.; Carroll, J.; Coffey, A.; Finster, J.; Tabak, D.; Niedbala, H.; et al. Ring-substituted 4-hydroxy-1H-quinolin-2-ones: Preparation and biological activity. Molecules 2009, 14, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.T.; Hou, W.D.; Zheng, M.R.; Tang, L.J. Clean and convenient one-pot synthesis of 4-hydroxycoumarin and 4-hydroxy-2-quinolinone derivatives. Synth. Commun. 2010, 40, 732–738. [Google Scholar] [CrossRef]
- Atalay, S.S.; Assad, M.Y.; Amagata, T.; Wu, W. Mild, efficient, and solvent-free synthesis of 4-hydroxy-2-quinolinones. Tetrahedron Lett. 2020, 61, 151778. [Google Scholar] [CrossRef]
- Yokoo, K.; Mori, K. Expeditious Synthesis of Multisubstituted Quinolinone Derivatives Based on Ring Recombination Strategy. Org. Lett. 2019, 22, 244–248. [Google Scholar] [CrossRef]
- Ishida, T.; Kikuchi, S.; Yamada, T. Efficient preparation of 4-hydroxyquinolin-2 (1 H)-one derivatives with silver-catalyzed carbon dioxide incorporation and intramolecular rearrangement. Org. Lett. 2013, 15, 3710–3713. [Google Scholar] [CrossRef]
- Matiadis, D.; Stefanou, V.; Athanasellis, G.; Hamilakis, S.; McKee, V.; Igglessi-Markopoulou, O.; Markopoulos, J. Synthesis, X-ray crystallographic study, and biological evaluation of coumarin and quinolinone carboxamides as anticancer agents. Monatsh. Chem. 2013, 144, 1063–1069. [Google Scholar] [CrossRef]
- Teli-Kokalari, E.; Stefanou, V.; Matiadis, D.; Athanasellis, G.; Igglessi-Markopoulou, O.; Hamilakis, S.; Markopoulos, J. Synthesis of six membered fused and five membered heterocycles, possessing the beta, beta’-tricarbonylfunctionality: Coordination mode against selected environmental ions. Fresenius Environ. Bull. 2012, 21, 3215–3223. [Google Scholar]
- Jönsson, S.; Andersson, G.; Fex, T.; Fristedt, T.; Hedlund, G.; Jansson, K.; Abramo, L.; Fritzson, I.; Pekarski, O.; Runström, A.; et al. Synthesis and biological evaluation of new 1, 2-Dihydro-4-hydroxy-2-oxo-3-quinoline carboxamides for treatment of autoimmune disorders: Structure—Activity relationship. J. Med. Chem. 2004, 47, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kalamuddin, M.; Maqbool, M.; Mohmmed, A.; Malhotra, P.; Hoda, N. Quinoline carboxamide core moiety-based compounds inhibit P. falciparum falcipain-2: Design, synthesis and antimalarial efficacy studies. Bioorg. Chem. 2021, 108, 104514. [Google Scholar] [CrossRef] [PubMed]
- Govender, H.; Mocktar, C.; Koorbanally, N.A. Synthesis and Bioactivity of Quinoline-3-carboxamide Derivatives. J. Heterocycl. Chem. 2018, 55, 1002–1009. [Google Scholar] [CrossRef]
- Boros, F.; Vécsei, L. Progress in the development of kynurenine and quinoline-3-carboxamide-derived drugs. Expert Opin. Investig. Drugs. 2020, 29, 1223–1247. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Sun, X.; Jin, S.; Yang, L.; Xu, H.; Rao, Y. Discovery of 4-aminoquinoline-3-carboxamide derivatives as potent reversible Bruton’s tyrosine kinase inhibitors for the treatment of rheumatoid arthritis. J. Med. Chem. 2019, 62, 6561–6574. [Google Scholar] [CrossRef] [PubMed]
- He, J.F.; Yun, L.H.; Yang, R.F.; Xiao, Z.Y.; Cheng, J.P.; Zhou, W.X.; Zhang, Y.X. Design, synthesis, and biological evaluation of novel 4-hydro-quinoline-3-carboxamide derivatives as an immunomodulator. Bioorg. Med. Chem. Lett. 2005, 15, 2980–2985. [Google Scholar] [CrossRef]
- Joseph, I.B.; Vukanovic, J.; Isaacs, J.T. Antiangiogenic treatment with linomide as chemoprevention for prostate, seminal vesicle, and breast carcinogenesis in rodents. Cancer Res. 1996, 56, 3404–3408. [Google Scholar]
- Shi, J.; Xiao, Z.; Ihnat, M.A.; Kamat, C.; Pandit, B.; Hu, Z.; Li, P.K. Structure–activity relationships studies of the anti-angiogenic activities of linomide. Bioorg. Med. Chem. Lett. 2003, 13, 1187–1189. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T. Rebamipide: A gastrointestinal protective drug with pleiotropic activities. Expert Rev. Gastroenterol. Hepatol. 2010, 4, 261–270. [Google Scholar] [CrossRef]
- Hong, W.S.; Jung, H.Y.; Yang, S.K.; Myung, S.J.; Kim, J.H.; Min, Y.I.; Chung, M.H.; Lee, H.S.; Kim, H.W. The antioxidant effect of rebamipide on oxygen free radical production by H. pylori-activated human neutrophils: In comparison with N-acetylcysteine, ascorbic acid and glutathione. Pharmacol. Res. 2001, 44, 293–297. [Google Scholar] [CrossRef]
- Isaacs, J.T.; Pili, R.; Qian, D.Z.; Dalrymple, S.L.; Garrison, J.B.; Kyprianou, N.; Björk, A.; Olsson, A.; Leanderson, T. Identification of ABR-215050 as lead second generation quinoline-3-carboxamide anti-angiogenic agent for the treatment of prostate cancer. Prostate Int. 2006, 66, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.R.; Armstrong, A.J. Tasquinimod in the treatment of castrate-resistant prostate cancer–current status and future prospects. Ther. Adv. Urol. 2016, 8, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Mugnaini, C.; Brizzi, A.; Ligresti, A.; Allarà, M.; Lamponi, S.; Vacondio, F.; Silva, C.; Mor, M.; Di Marzo, V.; Corelli, F. Investigations on the 4-quinolone-3-carboxylic acid motif. 7. Synthesis and pharmacological evaluation of 4-quinolone-3-carboxamides and 4-hydroxy-2-quinolone-3-carboxamides as high affinity cannabinoid receptor 2 (CB2R) ligands with improved aqueous solubility. J. Med. Chem. 2016, 59, 1052–1067. [Google Scholar] [PubMed]
- Kwak, S.H.; Kang, J.A.; Kim, M.; Lee, S.D.; Park, J.H.; Park, S.G.; Ko, H.; Kim, Y.C. Discovery and structure–activity relationship studies of quinolinone derivatives as potent IL-2 suppressive agents. Bioorg. Med. Chem. 2016, 24, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Huddar, S.; Park, C.M.; Kim, H.J.; Jang, S.; Lee, S. Discovery of 4-hydroxy-2-oxo-1, 2-dihydroquinolines as potential inhibitors of Streptococcus pneumoniae, including drug-resistant strains. Bioorg. Med. Chem. Lett. 2020, 30, 127071. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Li, X.; Ma, G.; Zhang, H.; Chen, Y.; Kirchmair, J.; Xia, J.; Wu, S. N-thiadiazole-4-hydroxy-2-quinolone-3-carboxamides bearing heteroaromatic rings as novel antibacterial agents: Design, synthesis, biological evaluation and target identification. Eur. J. Med. Chem. 2020, 188, 112022. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Samarat, H.H.; Al-Shalabi, E.; Bardaweel, S.K.; Hajjo, R.; Sweidan, K.; Khalaf, R.A.; Al-Zuheiri, A.M.; Abushaikha, G. Design, Synthesis, and Biological Examination of N-Phenyl-6-fluoro-4-hydroxy-2-quinolone-3-carboxamides as Anticancer Agents. ChemistrySelect 2022, 7, e202200662. [Google Scholar] [CrossRef]
- Sweidan, K.; Elfadel, H.; Sabbah, D.A.; Bardaweel, S.K.; Hajjo, R.; Anjum, S.; Sinoj, J.; Nair, V.A.; Abu-Gharbieh, E.; El-Huneidi, W. Novel Derivatives of 4, 6-Dihydroxy-2-Quinolone-3-Carboxamides as Potential PI3Kα Inhibitors. ChemistrySelect 2022, 7, e202202263. [Google Scholar] [CrossRef]
- Reis, A.S.; Pinz, M.; Duarte, L.F.B.; Roehrs, J.A.; Alves, D.; Luchese, C.; Wilhelm, E.A. 4-phenylselenyl-7-chloroquinoline, a novel multitarget compound with anxiolytic activity: Contribution of the glutamatergic system. J. Psychiatr. Res. 2017, 84, 191–199. [Google Scholar] [CrossRef]
- Ismail, E.M.; Shantier, S.W.; Mohammed, M.S.; Musa, H.H.; Osman, W.; Mothana, R.A. Quinoline and quinazoline alkaloids against COVID-19: An in silico multitarget approach. J. Chem. 2021, 2021, 3613268. [Google Scholar] [CrossRef]
- Mohamed, M.F.; Abuo-Rahma, G.E.D.A. Molecular targets and anticancer activity of quinoline–chalcone hybrids: Literature review. RSC Adv. 2020, 10, 31139–31155. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Khan, I.; Abid, S.; Zaib, S.; Ibrar, A.; Andleeb, H.; Hameed, S.; Iqbal, J. Quinolinic carboxylic acid derivatives as potential multi-target compounds for neurodegeneration: Monoamine oxidase and cholinesterase inhibition. Med. Chem. 2018, 14, 74–85. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Kovaleva, N.V.; Boltneva, N.P.; Lushchekina, S.V.; Astakhova, T.Y.; Rudakova, E.V.; Proshin, A.N.; Serkov, I.V.; Radchenko, E.V.; Palyulin, V.A.; et al. New hybrids of 4-amino-2, 3-polymethylene-quinoline and p-tolylsulfonamide as dual inhibitors of acetyl-and butyrylcholinesterase and potential multifunctional agents for Alzheimer’s disease treatment. Molecules 2020, 25, 3915. [Google Scholar] [CrossRef] [PubMed]
- Duarte, Y.; Fonseca, A.; Gutiérrez, M.; Adasme-Carreño, F.; Muñoz-Gutierrez, C.; Alzate-Morales, J.; Santana, L.; Uriarte, E.; Álvarez, R.; Matos, M.J. Novel Coumarin-Quinoline Hybrids: Design of Multitarget Compounds for Alzheimer’s Disease. ChemistrySelect 2019, 4, 551–558. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Yang, X.; Feng, X.; Li, X.; Huang, L.; Chan, A.S. Design, synthesis, and evaluation of orally bioavailable quinoline–indole derivatives as innovative multitarget-directed ligands: Promotion of cell proliferation in the adult murine Hippocampus for the treatment of alzheimer’s disease. J. Med. Chem. 2018, 61, 1871–1894. [Google Scholar] [CrossRef] [PubMed]
- Prati, F.; Bergamini, C.; Fato, R.; Soukup, O.; Korabecny, J.; Andrisano, V.; Bolognesi, M.L. Novel 8-hydroxyquinoline derivatives as multitarget compounds for the treatment of Alzheimer′ s disease. ChemMedChem 2016, 11, 1284–1295. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, R.R.; Popovic-Nikolic, M.R.; Nikolic, K.; Uliassi, E.; Bolognesi, M.L. A perspective on multi-target drug discovery and design for complex diseases. Clin. Transl. Med. 2018, 7, 3. [Google Scholar] [CrossRef]
- Sánchez-Tejeda, J.F.; Sánchez-Ruiz, J.F.; Salazar, J.R.; Loza-Mejía, M.A. A definition of “multitargeticity”: Identifying potential multitarget and selective ligands through a vector analysis. Front. Chem. 2020, 8, 176. [Google Scholar] [CrossRef]
- Feldmann, C.; Bajorath, J. Compounds with multitarget activity: Structure-based analysis and machine learning. Future Drug Discov. 2020, 2, FDD44. [Google Scholar] [CrossRef]
- Bajorath, J. Structural characteristics of compounds with multitarget activity. Future Drug Discov. 2021, 3, FDD60. [Google Scholar] [CrossRef]
- Chedea, V.S.; Jisaka, M. Lipoxygenase and carotenoids: A co-oxidation story. Afr. J. Biotechnol. 2013, 12, 2786–2791. [Google Scholar]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci. 2015, 24, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Son, B.; Lee, S.; Do, H.; Youn, B. Targeting the enzymes involved in arachidonic acid metabolism to improve radiotherapy. Cancer Metastasis Rev. 2018, 37, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Lötzer, K.; Funk, C.D.; Habenicht, A.J. The 5-lipoxygenase pathway in arterial wall biology and atherosclerosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2005, 1736, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Merchant, N.; Bhaskar, L.V.; Momin, S.; Sujatha, P.; Reddy, A.B.; Nagaraju, G.P. 5-Lipoxygenase: Its involvement in gastrointestinal malignancies. Crit. Rev. Oncol. Hematol. 2018, 127, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.L.; Ding, Y.; Alexander, L.D.; Bao, C.; Al-Khalili, O.K.; Simonson, M.; Eaton, D.C.; Douglas, J.G. Oxidative signaling in renal epithelium: Critical role of cytosolic phospholipase A2 and p38SAPK. Free Radic. Biol. Med. 2006, 41, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Chen, Y.; Gibson, S.B. Regulation of autophagy by reactive oxygen species (ROS): Implications for cancer progression and treatment. Antioxid Redox Signal. 2009, 11, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Patsilinakos, A.; Tran, T.M.; Marson, C.M. Pteridine-2,4-diamine derivatives as radical scavengers and inhibitors of lipoxygenase that can possess anti-inflammatory properties. Future Med. Chem. 2015, 7, 1937–1951. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Majdalani, M.; Kontogiorgis, C.A.; Hadjipavlou-Litina, D.; Kefalas, P. Natural and synthetic 2′-hydroxy-chalcones and aurones: Synthesis, characterization and evaluation of the antioxidant and soybean lipoxygenase inhibitory activity. Bioorg. Med. Chem. 2009, 17, 8073–8085. [Google Scholar] [CrossRef]
- Detsi, A.; Bardakos, V.; Markopoulos, J.; Igglessi-Markopoulou, O. Reactions of 2-methyl-3, 1-benzoxazin-4-one with active methylene compounds: A new route to 3-substituted 4-hydroxyquinolin-2 (1H)-ones. J. Chem. Soc. Perkin Trans. 1996, 1, 2909–2913. [Google Scholar] [CrossRef]
- Zikou, L.; Athanasellis, G.; Detsi, A.; Zografos, A.; Mitsos, C.; Igglessi-Markopoulou, O. A novel short-step synthesis of functionalized 4-hydroxy-2-quinolones using a 1-hydroxybenzotriazole methodology. Bull. Chem. Soc. Jpn. 2004, 77, 1505–1508. [Google Scholar] [CrossRef]
- Detsi, A.; Bouloumbasi, D.; Prousis, K.C.; Koufaki, M.; Athanasellis, G.; Melagraki, G.; Afantitis, A.; Igglessi-Markopoulou, O.; Kontogiorgis, C.; Hadjipavlou-Litina, D.J. Design and synthesis of novel quinolinone-3-aminoamides and their α-lipoic acid adducts as antioxidant and anti-inflammatory agents. J. Med. Chem. 2007, 50, 2450–2458. [Google Scholar] [CrossRef]
- Kostopoulou, I.; Diassakou, A.; Kavetsou, E.; Kritsi, E.; Zoumpoulakis, P.; Pontiki, E.; Hadjipavlou-Litina, D.J.; Detsi, A. Novel quinolinone–pyrazoline hybrids: Synthesis and evaluation of antioxidant and lipoxygenase inhibitory activity. Mol. Divers. 2021, 25, 723–740. [Google Scholar] [CrossRef] [PubMed]
- Prousis, K.C.; Tzani, A.; Avlonitis, N.; Calogeropoulou, T.; Detsi, A. Reactivity of 2-Methyl-4H-3, 1-benzoxazin-4-ones and 2-Methyl-4H-pyrido [2, 3-d][1, 3] oxazin-4-one under Microwave Irradiation Conditions. J. Heterocycl. Chem. 2013, 50, 1313–1321. [Google Scholar] [CrossRef]
- Hadjipavlou-Litina, D.; Garnelis, T.; Athanassopoulos, C.M.; Papaioannou, D. Kukoamine A analogs with lipoxygenase inhibitory activity. J. Enzyme Inhib. Med. Chem. 2009, 24, 1188–1193. [Google Scholar] [CrossRef] [PubMed]
- Kontogiorgis, C.; Hadjipavlou-Litina, D. Biological evaluation of several coumarin derivatives designed as possible anti-inflammatory/antioxidant agents. J. Enzyme Inhib. Med. Chem. 2003, 18, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Multi-target cinnamic acids for oxidative stress and inflammation: Design, synthesis, biological evaluation and modeling studies. Molecules 2018, 24, 12. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D. Synthesis and pharmacochemical evaluation of novel aryl-acetic acid inhibitors of lipoxygenase, antioxidants, and anti-inflammatory agents. Bioorg. Med. Chem. 2007, 15, 5819–5827. [Google Scholar] [CrossRef]
- Minor, W.; Steczko, J.; Bolin, J.T.; Otwinowski, Z.; Axelrod, B. Crystallographic determination of the active site iron and its ligands in soybean lipoxygenase L-1. Biochemistry 1993, 32, 6320–6323. [Google Scholar] [CrossRef]
- Skrzypczak-Jankun, E.; Amzel, L.M.; Kroa, B.A.; Funk Jr, M.O. Structure of soybean lipoxygenase L3 and a comparison with its L1 isoenzyme. Proteins Struct. Funct. Genet. 1997, 29, 15–31. [Google Scholar] [CrossRef]
- Taraporewala, I.B.; Kauffman, J.M. Synthesis and Structure–Activity Relationships of Anti-inflammatory 9, lO-Dihydro-9-oxo-2-Acridine-alkanoic Acids and 4-(2-Carboxyphenyl) aminobenzenealkanoic Acids. J. Pharm. Sci. 1990, 79, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Kasthuri, J.K.; Singh Jadav, S.; Thripuram, V.D.; Gundabolu, U.R.; Babu, A.V.; Kolla, J.N.; Jayaprakash, V.; Ahsan, M.J.; Bollikolla, H.B. Synthesis, Characterization, Docking and Study of Inhibitory Action of Some Novel C-Alkylated Chalcones on 5-LOX Enzyme. ChemistrySelect 2017, 2, 8771–8778. [Google Scholar] [CrossRef]
- Schrödinger Release 2023-4: Maestro, Schrödinger. Biomedicines 2024, 12, 50. [CrossRef]
- Audisio, D.; Messaoudi, S.; Cegielkowski, L.; Peyrat, J.F.; Brion, J.D.; Methy, D.; Radanyi, C.; Renoir, J.-M.; Alami, M. Discovery and biological activity of 6BrCaQ as an inhibitor of the Hsp90 protein folding machinery. ChemMedChem 2011, 6, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, I.; Tzani, A.; Polyzos, N.I.; Karadendrou, M.A.; Kritsi, E.; Pontiki, E.; Liargkova, T.; Hadjipavlou-Litina, D.; Zoumpoulakis, P.; Detsi, A. Exploring the 2′-hydroxy-chalcone framework for the development of dual antioxidant and soybean lipoxygenase inhibitory agents. Molecules 2021, 26, 2777. [Google Scholar] [CrossRef] [PubMed]
- Pontiki, E.; Hadjipavlou-Litina, D.; Litinas, K.; Nicolotti, O.; Carotti, A. Design, synthesis and pharmacobiological evaluation of novel acrylic acid derivatives acting as lipoxygenase and cyclooxygenase-1 inhibitors with antioxidant and anti-inflammatory activities. Eur. J. Med. Chem. 2011, 46, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Fiser, A.; Šali, A. Modeller: Generation and refinement of homology-based protein structure models. Methods Enzymol. 2003, 374, 461–491. [Google Scholar]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Vranken, W.F. ACPYPE-Antechamber python parser interface. BMC Res. Notes 2012, 5, 367. [Google Scholar] [CrossRef]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Automatic atom type and bond type perception in molecular mechanical calculations. J. Mol. Graph. Model. 2006, 25, 247–260. [Google Scholar] [CrossRef]
- Lindorff-Larsen, K.; Piana, S.; Palmo, K.; Maragakis, P.; Klepeis, J.L.; Dror, R.O.; Shaw, D.E. Improved side-chain torsion potentials for the Amber ff99SB protein force field. Proteins Struct. Funct. Genet. 2010, 78, 1950–1958. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]





| Compound | Interaction with the Free Radical DPPH (%) | Inhibition of Lipid Peroxidation of Linoleic Acid Induced by AAPH Radical (%) 100 μM | |
|---|---|---|---|
| 100 μM 20 min | 100 μM 60 min | ||
| 3a | 2.4 | 12.4 | 100 |
| 3b | 2.7 | 11.8 | 100 |
| 3c | 3.3 | 10.7 | 100 |
| 3d | 15.9 | 20.8 | 32.8 |
| 3e | 6.6 | 12.2 | no |
| 3f | 39.3 | 48.6 | 100 |
| 3g | 40.7 | 51.7 | 100 |
| 3h | 6.0 | 8.1 | 31 |
| 3i | 5.2 | 3.7 | 36 |
| 3j | 32.5 | 46.5 | 32.8 |
| 3k | 1.4 | 11.8 | 41.4 |
| 3l | 2.6 | 2.6 | no |
| 3m | 3.1 | 1.2 | 11.3 |
| 3n | 13.9 | 19.2 | 100 |
| 3o | 20.8 | 8.0 | 100 |
| 3p | 19.5 | 6.6 | 100 |
| 3q | 23.8 | 10.0 | 100 |
| 3r | 12.1 | 1.2 | 17.1 |
| 3s | 21.2 | 8.1 | 28.9 |
| 3t | 19.7 | 9.5 | 100 |
| 3u | 19.3 | 8.3 | 16.4 |
| 11a | 1.0 | 4.4 | 89.0 |
| 11b | 3.0 | no | 93.8 |
| 11c | 2.0 | 2.0 | 91.0 |
| 11d | 3.0 | 2.0 | 94.0 |
| 11e | 3.0 | 5.0 | 97.0 |
| 11f | 2.0 | 4.0 | 93.0 |
| 11g | no | 2.0 | 97.0 |
| 16a | 24.0 | 31.0 | 83.0 |
| 16b | 27.0 | 32.0 | 88.0 |
| 16c | 25.0 | 28.0 | 88.0 |
| NDGA | 83.0 | 94.0 | |
| Trolox | 88.0 | ||
| Compound | OH% 0.1 mM | ABTS% 0.1 mM | PMS% 0.1 mM |
|---|---|---|---|
| 3a | 39.3 | 33.8 | 23.1 |
| 3b | 40.8 | 35.5 | 84.6 |
| 3c | 39.8 | 25.6 | 76.9 |
| 3d | no | no | 66.6 |
| 3e | 86.1 | 2.6 | 66.6 |
| 3f | 67.7 | 77.3 | no |
| 3g | 67.7 | 72.4 | no |
| 3h | 78.6 | no | no |
| 3i | 78.1 | no | no |
| 3j | 100 | 49.7 | no |
| 3k | no | 54.5 | 61.5 |
| 3l | 53.7 | no | no |
| 3m | 94.0 | 12.4 | no |
| 3n | 14.9 | no | no |
| 3o | 44.3 | 20.6 | no |
| 3p | 16.9 | no | no |
| 3q | 98.0 | no | no |
| 3r | 50.7 | no | no |
| 3s | 100 | no | no |
| 3t | no | no | no |
| 3u | 10.3 | no | no |
| Trolox | 82.0 | 93.0 | |
| Caffeic acid | 23 |
| Compound | Inhibition of Soybean Lipoxygenase | |
|---|---|---|
| % at 0.1 mM | IC50 (μM) * | |
| 3a | 60.0 | |
| 3b | 45.0 | |
| 3c | 52.0 | |
| 3d | no | |
| 3e | no | |
| 3f | 49.0 | |
| 3g | 27.5 | |
| 3h | 10.0 | |
| 3i | no | |
| 3j | 100.0 | |
| 3k | 315.0 | |
| 3l | 45.0 | |
| 3m | 15.0 | |
| 3n | 85.0 | |
| 3o | no | |
| 3p | no | |
| 3q | 24.0 | |
| 3r | 37.4 | |
| 3s | 10.0 | |
| 3t | no | |
| 3u | no | |
| 11a | 61.0 | |
| 11b | 70.0 | |
| 11c | 57.5 | |
| 11d | 5.0 | |
| 11e | 52.5 | |
| 11f | 70.0 | |
| 11g | 85.5 | |
| 16a | 81.0 | |
| 16b | 100.0 | |
| 16c | 82.5 | |
| NDGA | 0.45 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostopoulou, I.; Tzani, A.; Chronaki, K.; Prousis, K.C.; Pontiki, E.; Hadjiplavlou-Litina, D.; Detsi, A. Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules 2024, 29, 190. https://doi.org/10.3390/molecules29010190
Kostopoulou I, Tzani A, Chronaki K, Prousis KC, Pontiki E, Hadjiplavlou-Litina D, Detsi A. Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules. 2024; 29(1):190. https://doi.org/10.3390/molecules29010190
Chicago/Turabian StyleKostopoulou, Ioanna, Andromachi Tzani, Konstantina Chronaki, Kyriakos C. Prousis, Eleni Pontiki, Dimitra Hadjiplavlou-Litina, and Anastasia Detsi. 2024. "Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone" Molecules 29, no. 1: 190. https://doi.org/10.3390/molecules29010190
APA StyleKostopoulou, I., Tzani, A., Chronaki, K., Prousis, K. C., Pontiki, E., Hadjiplavlou-Litina, D., & Detsi, A. (2024). Novel Multi-Target Agents Based on the Privileged Structure of 4-Hydroxy-2-quinolinone. Molecules, 29(1), 190. https://doi.org/10.3390/molecules29010190









