Structural Studies of Mexican Husk Tomato (Physalis ixocarpa) Fruit Cutin
Abstract
:1. Introduction
2. Results and Discussion
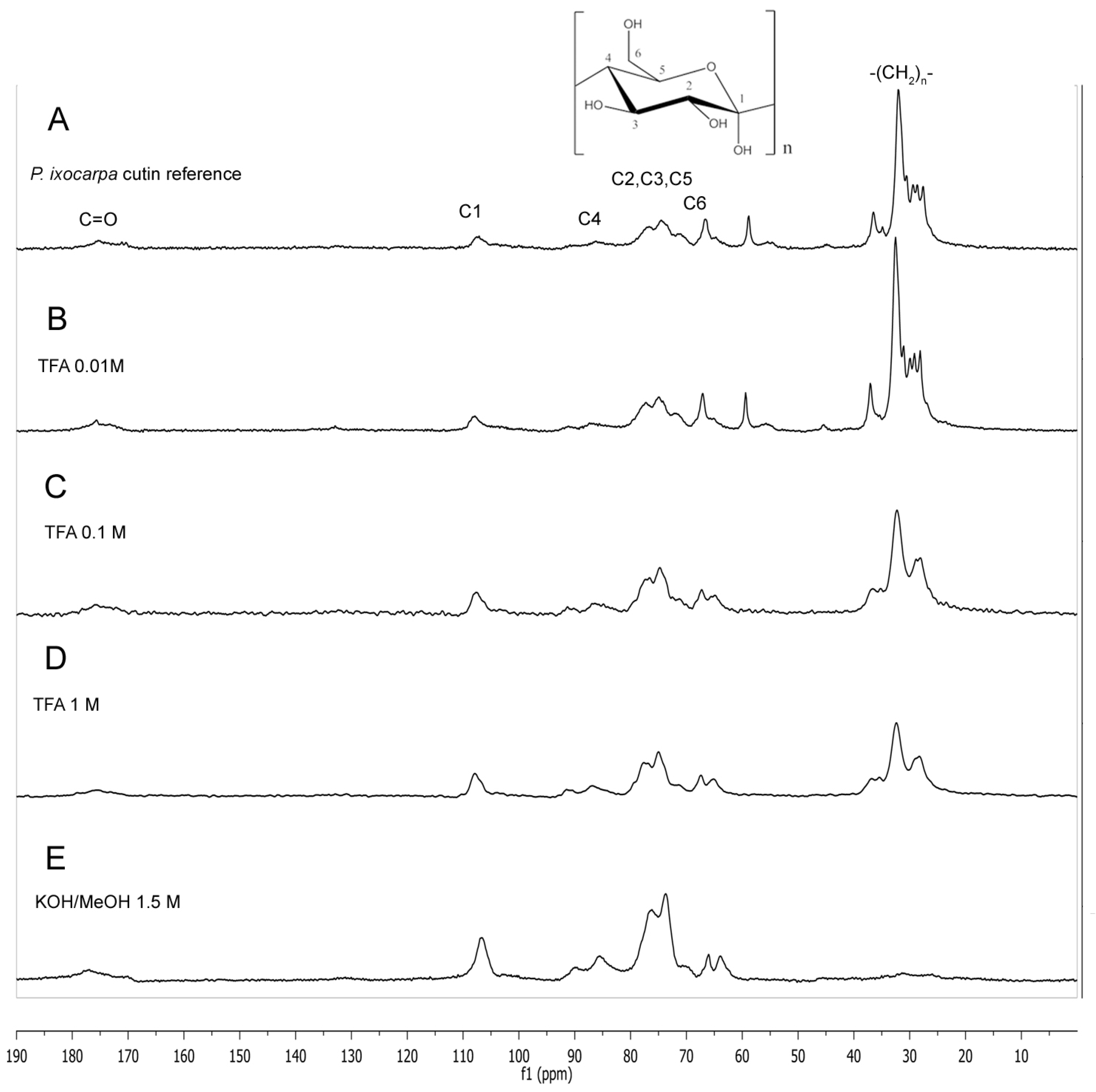
2.1. Cross Polarization Magic Angle Spinning 13C Nuclear Magnetic Resonance (CPMAS 13C NMR) of Mexican Husk Tomato Cutin
2.2. Ultra-High Performance Liquid Chromatographic (UHPLC-MS) and Direct Injection Electrospray Mass Spectrometry (DIESI-MS) Analysis of the Physalis ixocarpa Cutin TFA and ALKaline Hydrolysis (KOH/MeOH) Products
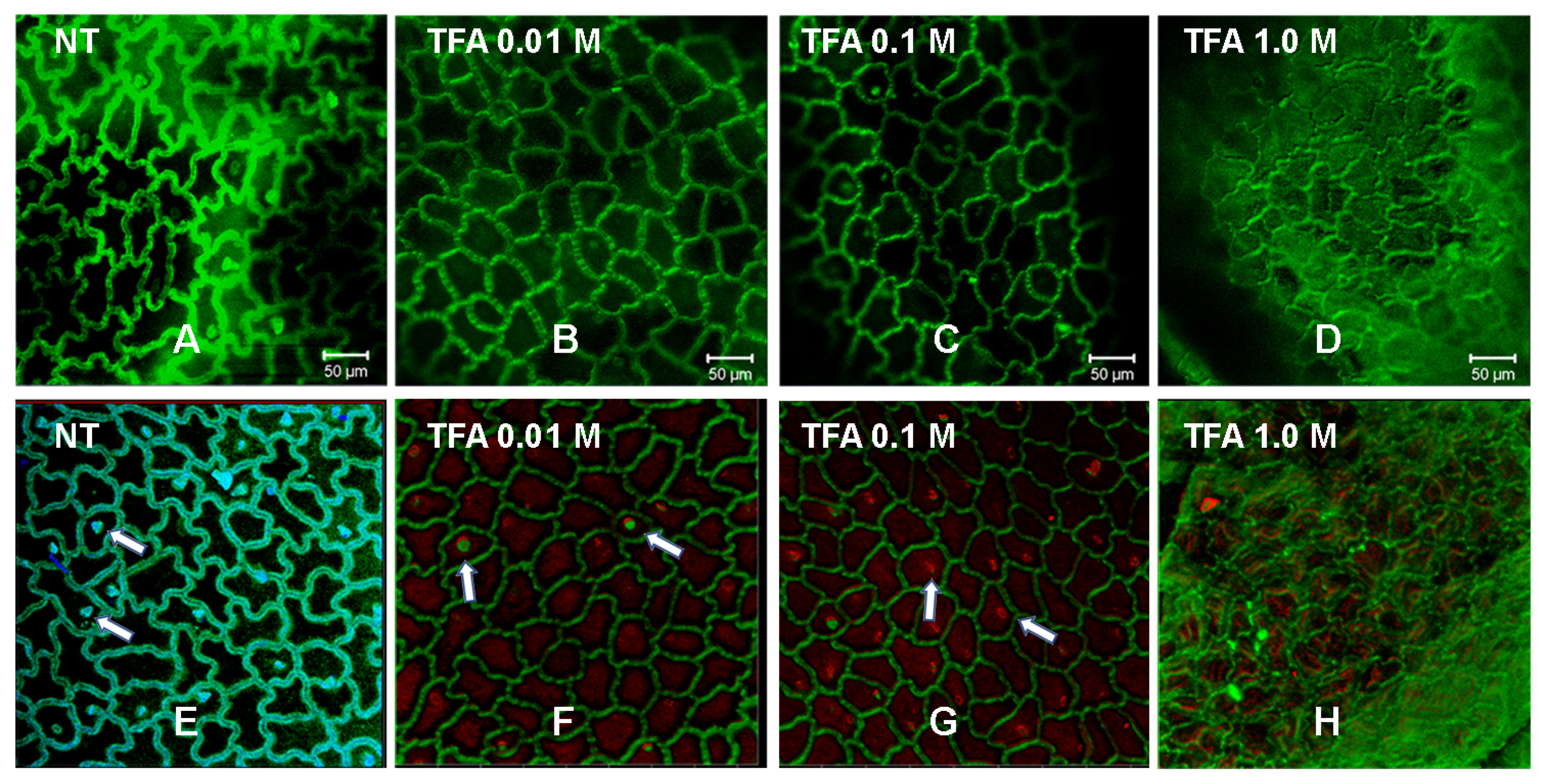
2.3. Confocal Laser Scanning Microscopy (CLSM) of Mexican Husk Tomato (Physalis ixocarpa) Cutin
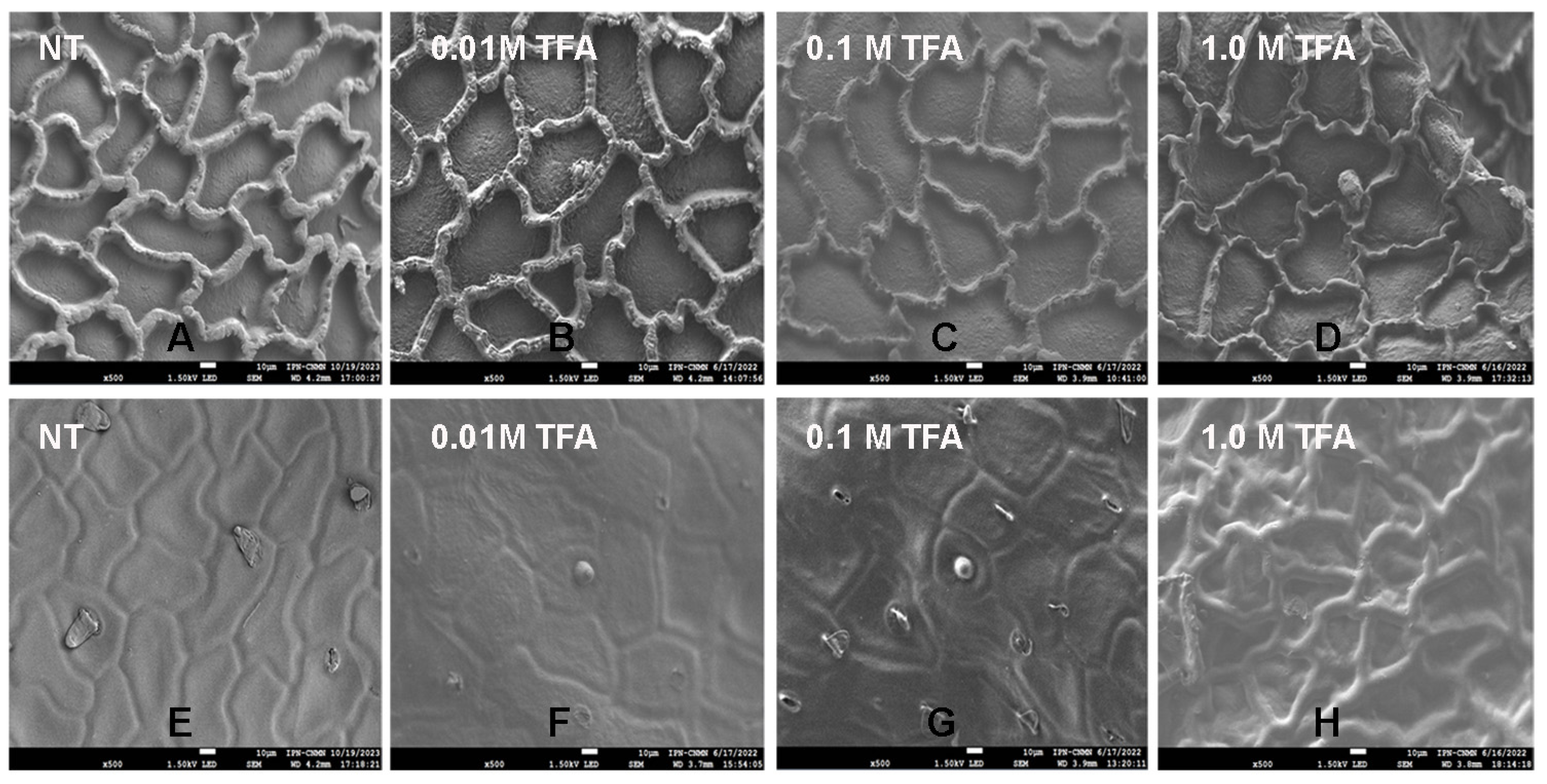
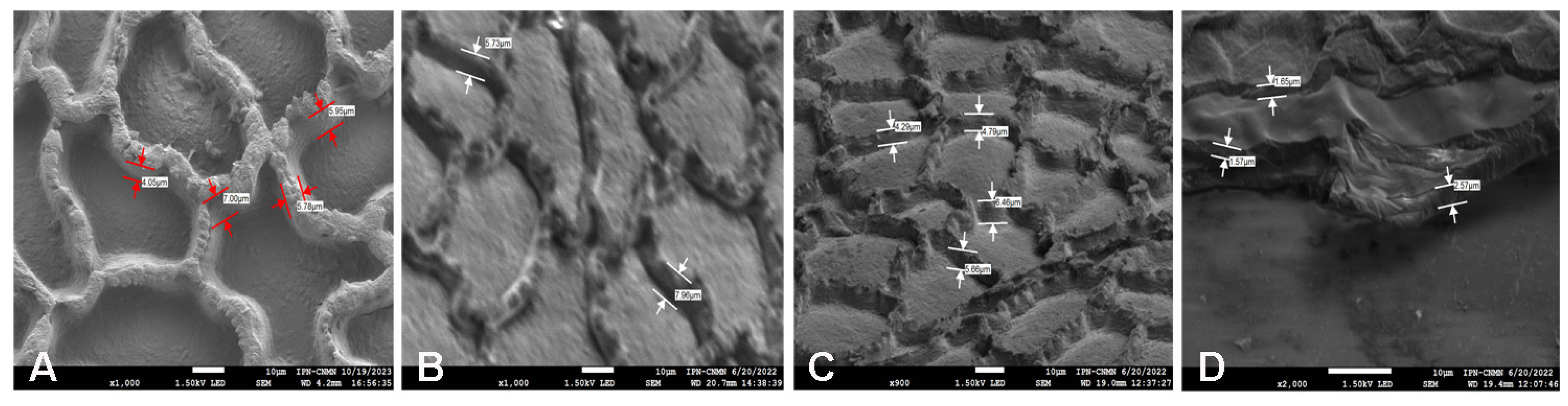
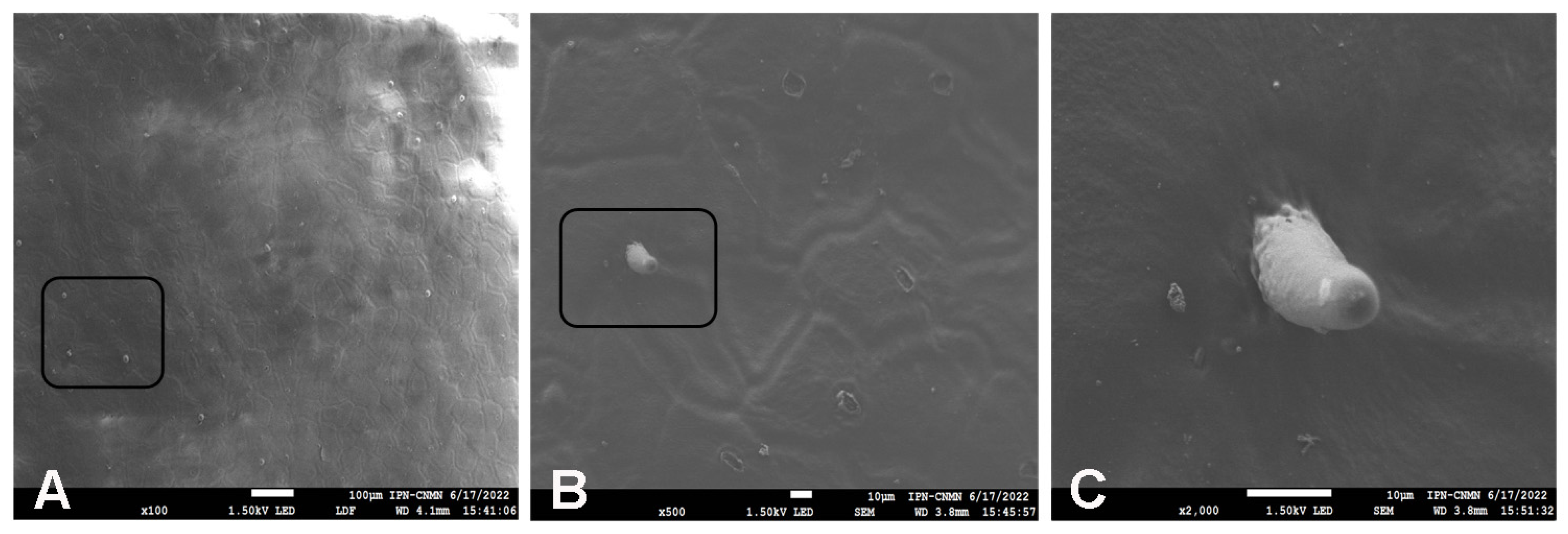
2.4. Scanning Electron Microscopy (SEM) Analysis of Mexican Husk Tomato (Physalis ixocarpa) Cutin
3. Materials and Methods
3.1. Chemicals
3.2. Isolation of Cutin
3.3. Treatment of Cutin with Trifluoroacetic Acid [42]
3.4. Alkaline Hydrolysis of CUTIN and 0.1 M TFA Cutin with KOH [23]
3.5. CPMAS 13C NMR of Mexican Husk Tomato Cutin Analysis
3.6. Ultra-High Performance Liquid Chromatography Mass Spectrometry (UHPLC-MS) and Direct Injection Electrospray Mass Spectrometry (DIESI-MS) Analysis
3.7. Confocal Laser Scanning Microscopy Analysis
3.8. Scanning Electron Microscopy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robledo-Torres, V.; Ramírez-Godina, F.; Foroughbakhch-Pournavab, R.; Benavides-Mendoza, A.; Hernandez-Guzman, G.; Reyes-Valdes, M.H. Development of tomatillo (Physalis ixocarpa Brot.) autotetraploids and their chromosome and phenotypic characterization. Breed. Sci. 2011, 61, 288–293. [Google Scholar] [CrossRef]
- Small, E. Top 100 Exotic Food Plants; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Servicio de Información Agroalimentaria y Pesquera. Available online: https://www.gob.mx/siap/articulos/tomate-verde-ingrediente-esencial-de-la-comida-mexicana?idiom=es (accessed on 12 November 2023).
- Brown, C.H. Development of agriculture in prehistoric mesoamerica: The linguistic evidence. In Pre-Columbian Foodways; Staller, J., Carrasco, M., Eds.; Springer: New York, NY, USA, 2010; pp. 71–107. [Google Scholar]
- Gollapudi, R.; Motohashi, N. The Health Benefits of Tomatillo Berries. Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits; Nova Science Publishers: New York, NY, USA, 2013; pp. 123–139. [Google Scholar]
- González-Mendoza, D.; Grimaldo-Juarez, O.; Soto-Ortiz, R.; Escoboza-García, F.; Santiguillo-Hern’andez, J.F. Evaluation of total phenolics, anthocyanins and antioxidant capacity in purple tomatillo (Physalis ixocarpa) genotypes. Afr. J. Biotechnol. 2010, 9, 5173–5176. [Google Scholar]
- Maldonado, E.; Pérez-Castorena, A.L.; Garcés, C.; Martínez, M. Philadelphicalactones C and D and other cytotoxic compounds from Physalis philadelphica. Steroids 2011, 76, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Cortés, L.A.; Moncayo, D.C.; Castellanos, D.A. Development of an antimicrobial packaging system for fresh cape gooseberry (Physalis peruviana L.) fruits. Food Pack. Shelf Life 2023, 38, 101113. [Google Scholar] [CrossRef]
- Chand, S.; Devi, S.; Devi, D.; Arya, P.; Manorma, K.; Kesta, K.; Sharma, M.; Bishist, R.; Tomar, M. Microbial and physico-chemical dynamics associated with chicken feather compost preparation vis-à-vis its impact on the growth performance of tomato crop. Biocat. Agr. Biotech. 2023, 54, 102885. [Google Scholar] [CrossRef]
- Martin, L.B.B.; Rose, J.K.C. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Heredia-Guerrero, J.A.; Heredia, A. The biophysical design of plant cuticles: An overview. New Phytol. 2011, 189, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Nawrath, C. Unraveling the complex network of cuticular structure and function. Curr. Opin. Plant Biol. 2006, 9, 281–287. [Google Scholar] [CrossRef]
- Diarte, C.; de Souza, A.X.; Staiger, S.; Deininger, A.C.; Bueno, A.; Burghardt, M. Compositional, structural and functional cuticle analysis of Prunus laurocerasus L. sheds light on cuticular barrier plasticity. Plant Physiol. Biochem. 2021, 158, 434–445. [Google Scholar] [CrossRef]
- Xu, X.; Feng, J.; Lü, S.; Lohrey, G.T.; An, H.; Zhou, Y. Leaf cuticular lipids on the Shandong and Yukon ecotypes of saltwater cress, Eutrema salsugineum, and their response to water deficiency and impact on cuticle permeability. Physiol. Plant. 2014, 151, 446–458. [Google Scholar] [CrossRef]
- Gómez-Patiño, M.B.; Estrada-Reyes, R.; Vargas-Diaz, M.E.; Arrieta-Baez, D. Cutin from Solanum myriacanthum Dunal and Solanum aculeatissimum Jacq. as a potential raw material for biopolymers. Polymers 2020, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Walton, T.J. Waxes, cutin and suberin. Methods Plant Biochem. 1990, 4, 105–158. [Google Scholar]
- Kolattukudy, P.E. Polyesters in higher plants. Adv. Biochem. Eng. Biotechnol. 2001, 71, 1–49. [Google Scholar] [PubMed]
- Heredia, A. Biophysical and biochemical characteristics of cutin, a plant barrier biopolymer. Biochim. Biophys. Acta 2003, 1620, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Espelié, K.E.; Davis, R.W.; Kolattukudy, P.E. Composition, ultrastructure and function of the cutin-and suberin-containing layers in the leaf, fruit peel, juice sac and inner seed coat of grapefruit (Citrus paradise Macfed.). Planta 1980, 149, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Gérard, H.C.; Osman, S.F.; William, F.F.; Moreau, R.A. Separation, Identification and Quantification of Monomers from Cutin Polymers by High Performance Liquid Chromatography and Evaporative Light Scattering Detection. Phytochem. Anal. 1992, 3, 139–142. [Google Scholar] [CrossRef]
- Osman, S.F.; Gerard, H.C.; Fett, W.F.; Moreau, R.A.; Dudley, R.L. Method for the production and characterization of tomato cutin oligomers. J. Agric. Food Chem. 1995, 43, 2134–2137. [Google Scholar] [CrossRef]
- Osman, S.F.; Irwin, P.; Fett, W.F.; O’Connor, J.V.; Parris, N. Preparation, isolation and characterization of cutin monomers and oligomers from tomato peels. J. Agric. Food Chem. 1999, 47, 799–802. [Google Scholar] [CrossRef]
- Ray, A.K.; Chen, Z.J.; Stark, R.E. Chemical Depolymerization studies of the Molecular Architecture of lime fruit cuticle. Phytochemistry 1998, 1, 65–70. [Google Scholar] [CrossRef]
- Hernández Velasco, B.L.; Arrieta-Baez, D.; Cortez Sotelo, P.I.; Méndez-Méndez, J.V.; Berdeja Martínez, B.M.; Gómez-Patiño, M.B. Comparative studies of cutins from lime (Citrus aurantifolia) and grapefruit (Citrus paradisi) after TFA hydrolysis. Phytochemistry 2017, 144, 78–86. [Google Scholar] [CrossRef]
- Gérard, H.C.; Pfeffer, P.E.; Osman, S.F. 8,16-dihidroxyhexadecanoic acid, a mayor component from cucumber cutin. Phytochemistry 1994, 33, 818–819. [Google Scholar] [CrossRef]
- Kallio, H.; Nieminen, R.; Tuomasjukka, S.; Hakala, M. Cutin composition of five Finnish berries. J. Agric. Food Chem. 2006, 54, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, R.; Kaimainen, M.; Kallio, H. Cutin composition of selected northernberries and seeds. Food Chem. 2010, 122, 137–144. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Perea Flores, M.D.J.; Méndez-Méndez, J.V.; Mendoza León, H.F.; Gómez-Patiño, M.B. Structural Studies of the Cutin from Two Apple Varieties: Golden Delicious and Red Delicious (Malus domestica). Molecules 2020, 25, 5955. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Fang, X.; Wang, W.; Yu, B.; Cheng, X.; Qiu, F.; Mort, A.J.; Stark, R.E. Isolation and identification of oligomers from partial degradation of lime fruit cutin. J. Agric. Food Chem. 2008, 56, 10318–10325. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Qiu, F.; Yan, B.; Wang, H.; Mort, A.J.; Stark, R.E. NMR studies of molecular structure in fruit cuticle polyesters. Phytochemistry 2001, 57, 1035–1042. [Google Scholar] [CrossRef]
- Benítez, J.J.; Matas, A.J.; Heredia, A. Molecular characterization of the plant biopolyester cutin by AFM and spectroscopic techniques. J. Struct. Biol. 2004, 147, 179–184. [Google Scholar] [CrossRef]
- Baker, E.A.; Bukovac, M.J.; Hunt, G.M. Composition of Tomato FRUIT Cuticle as Related to Fruit Growth and Development. In The Plant Cuticle; Cutler, D., Alvin, K., Price, C., Eds.; Academic Press: Cambridge, MA, USA, 1982; pp. 33–44. [Google Scholar]
- Espelie, K.E.; Dean, B.B.; Kolattukudy, P.E. Composition of lipid-derived polymers from different anatomical regions of several plant species. Plant Phys. 1979, 64, 1089–1093. [Google Scholar] [CrossRef]
- Wang, C.; Chin, C.-K.; Gianfagna, T. Relationship between cutin monomers and tomato resistance to powdery mildew infection. Physiol. Mol. Plant Pathol. 2000, 57, 55–61. [Google Scholar] [CrossRef]
- Serrano, M.; Coluccia, F.; Torres, M.; L’Haridon, F.; Métraux, J.-P. The cuticle and plant defense to pathogens. Front. Plant Sci. 2014, 5, 274. [Google Scholar] [CrossRef]
- Riederer, M.; Schönherr, J. Covalent binding of chlorophenoxyacetic acids to plant cuticles. Arch. Environ. Contam. Toxicol. 1986, 15, 97–105. [Google Scholar] [CrossRef]
- Marga, F.; Pesacreta, T.C.; Hasenstein, K.H. Biochemical analysis of elastic and rigid cuticles of Cirsium horridulum. Planta 2001, 213, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Bargel, H.; Neinhuis, C. Biomechanical properties of tomato (Lycopersicon esculentum Mill.) fruit skin and isolated cuticle during fruit growth and ripening. J. Exp. Bot. 2005, 56, 1049–1060. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, G.; Beisson, F.; Ohlrogge, J.; Pollard, M. Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: Occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J. 2004, 40, 920–930. [Google Scholar] [CrossRef] [PubMed]
- Morrison, I.M.; Stewart, D. Plant cell wall fragments released on solubilisation in trifluoroacetic acid. Phytochemistry 1998, 49, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Fanta, G.F.; Abbott, T.P.; Herman, A.I.; Burr, R.C.; Doane, W.M. Hydrolysis of wheat straw hemicellulose with trifluoroacetic acid. Fermentation of xylose with Pachysolen tannophilus. Biotechnol. Bioeng. 2004, 26, 1122–1125. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Stark, R.E. Using Trifluoroacetic Acid to Augment Studies of Potato Suberin Molecular Structure. J. Agric. Food Chem. 2006, 54, 9636–9641. [Google Scholar] [CrossRef]
- Arrieta-Baez, D.; Cruz-Carrillo, M.; Gómez-Patiño, M.B.; Zepeda-Vallejo, L.G. Derivatives of 10,16-dihydroxyhexadecanoicacid isolated from tomato (Solanum lycopersicum) as potential material for aliphatic polyesters. Molecules 2011, 16, 4923–4936. [Google Scholar] [CrossRef]
- Luque, P.; Ramírez, F.J.; Heredia, A.; Bukovac, M.J. Fourier Transform IR Studies on the Interaction of Selected Chemicals with Isolates Cuticles. In Air Pollutants and the Leaf Cuticle; NATO ASI Series, G36; Percy, K.E., Cape, J.N., Jagels, R., Simpson, C.J., Eds.; Springer: Berlin/Heidelberg, Germany, 1994; pp. 217–223. [Google Scholar]
- Veraverbeke, E.A.; Bruaene, N.V.; Oostveldt, P.V.; Nicolaï, B.M. Non destructive analysis of wax layer of apple (Malus domestica Borkh.) by mean of confocal laser scanning microscopy. Planta 2001, 213, 525–533. [Google Scholar] [CrossRef]
- España, L.; Heredia-Guerrero, J.A.; Segado, P.; Benítez, J.J.; Heredia, A.; Domínguez, E. Biomechanical properties of the tomato (Solanum lycopersicum) fruit cuticle during development are modulated by changes in the relative amounts of its components. New Phytol. 2014, 202, 790–802. [Google Scholar] [CrossRef]

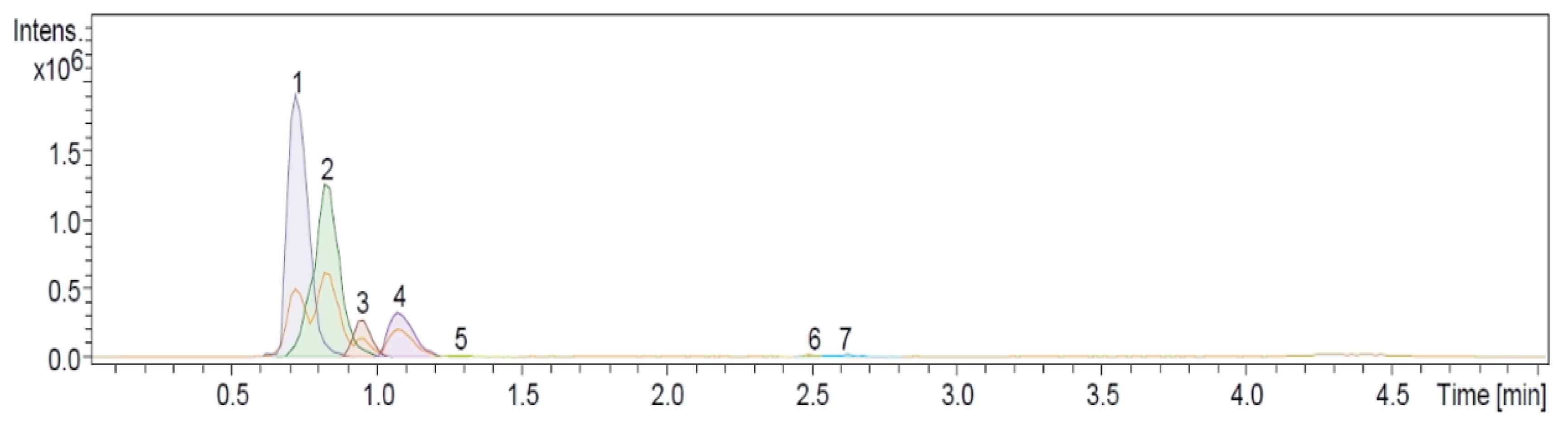
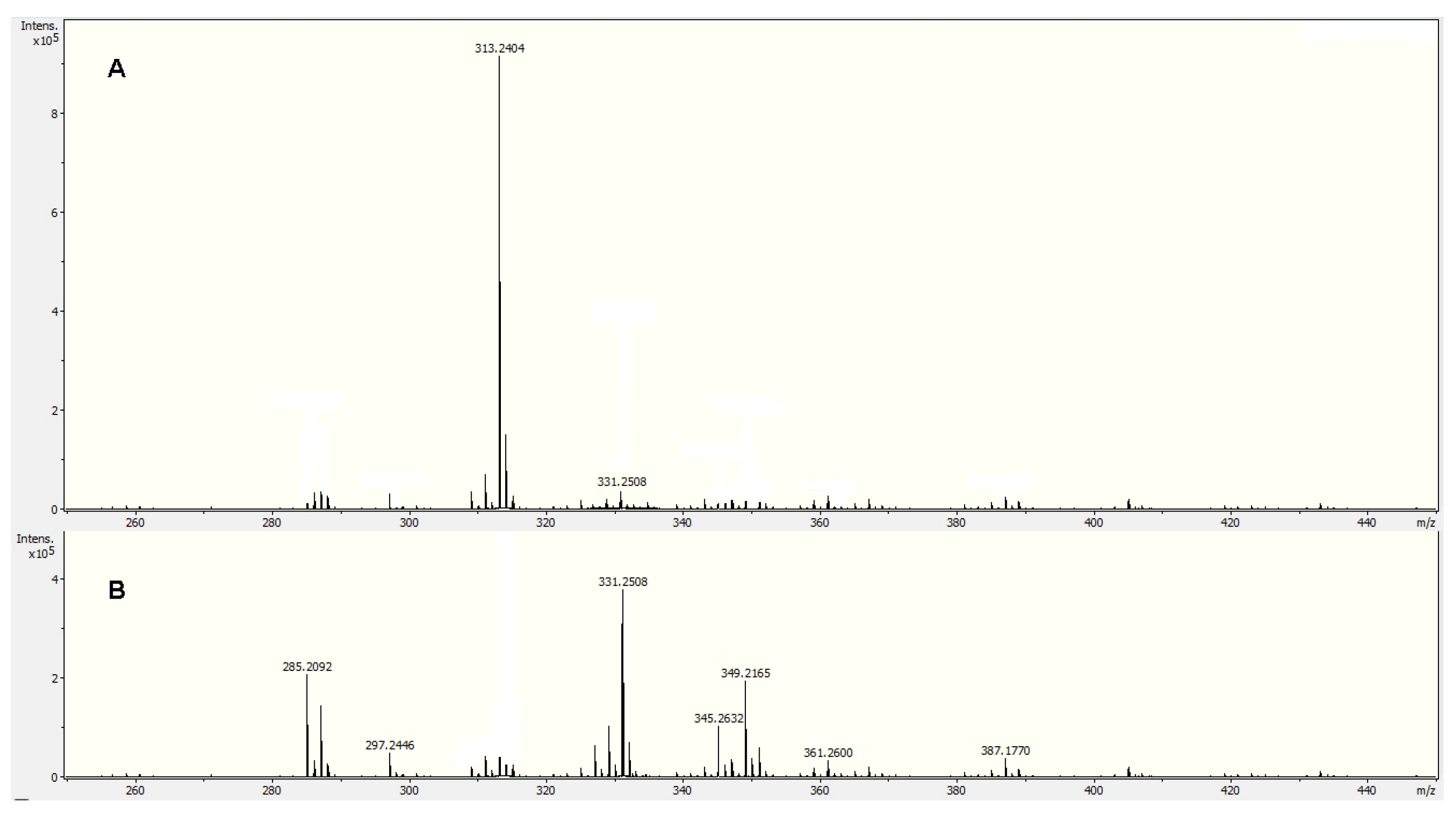
| # | Rt | Name | Fragments | [M-H]−obs | [M-H]−exact | Formula | Error | % RA |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.7 | 9,10,18-trihydroxy-octadecanoic acid | 172, 189, 202, 287 | 331.2526 | 331.2490 | C18H36O5 | −10.7 | 44.7 |
| 2 | 0.8 | 9,10-epoxy-18-hydroxy-octadecanoic acid | 171, 184, 269 | 313.2424 | 313.2384 | C18H34O4 | −12.7 | 37.5 |
| 3 | 0.9 | 9,10-epoxy-12Z-18-hydroxy-octadecenoic acid | 142, 184, 210, 267 | 311.2267 | 311.2216 | C18H32O4 | −12.5 | 5.7 |
| 4 | 1.1 | 18-hydroxyoleic acid | 155, 253 | 297.2435 | 297.2424 | C18H34O3 | 12.1 | 9.7 |
| 5 | 1.3 | 18-hydroxyoctadecanoic acid | 255, 282 | 299.2630 | 299.2580 | C18H36O3 | 12.8 | 0.6 |
| 6 | 2.5 | Oleic acid | 142, 237 | 281.2511 | 281.2475 | C18H34O2 | −9.0 | 0.8 |
| 7 | 2.6 | Palmitic acid | 211 | 255.2326 | 255.2330 | C16H32O2 | −0.8 | 0.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arrieta-Baez, D.; Quezada Huerta, C.; Rojas-Torres, G.S.; Perea-Flores, M.d.J.; Mendoza-León, H.F.; Gómez-Patiño, M.B. Structural Studies of Mexican Husk Tomato (Physalis ixocarpa) Fruit Cutin. Molecules 2024, 29, 184. https://doi.org/10.3390/molecules29010184
Arrieta-Baez D, Quezada Huerta C, Rojas-Torres GS, Perea-Flores MdJ, Mendoza-León HF, Gómez-Patiño MB. Structural Studies of Mexican Husk Tomato (Physalis ixocarpa) Fruit Cutin. Molecules. 2024; 29(1):184. https://doi.org/10.3390/molecules29010184
Chicago/Turabian StyleArrieta-Baez, Daniel, Camila Quezada Huerta, Giovana Simone Rojas-Torres, María de Jesús Perea-Flores, Héctor Francisco Mendoza-León, and Mayra Beatriz Gómez-Patiño. 2024. "Structural Studies of Mexican Husk Tomato (Physalis ixocarpa) Fruit Cutin" Molecules 29, no. 1: 184. https://doi.org/10.3390/molecules29010184
APA StyleArrieta-Baez, D., Quezada Huerta, C., Rojas-Torres, G. S., Perea-Flores, M. d. J., Mendoza-León, H. F., & Gómez-Patiño, M. B. (2024). Structural Studies of Mexican Husk Tomato (Physalis ixocarpa) Fruit Cutin. Molecules, 29(1), 184. https://doi.org/10.3390/molecules29010184








