Abstract
An efficient and convenient method for the synthesis of 1-hydroxyalkylphosphonium salts is described. Reactions were carried out at room temperature, in a short time, and without chromatography for product isolation. The properties of the obtained phosphonium salts were examined and discussed. In this paper, primary attention was paid to the stability of phosphonium salts, depending on the structure of the aldehydes used as substrates in their preparation. Other conditions such as the type of solvent, temperature, and molar ratio of the substrates were also investigated. Finally, the high reactivity of 1-hydroxyalkylphosphonium salts was demonstrated in reactions with amide-type substrates and (hetero)aromatic compounds. The developed step-by-step procedure (with the isolation of 1-hydroxyphosphonium salts) was compared to the one-pot protocol (in situ formation of such phosphonium salts).
1. Introduction
Chemical compounds containing a phosphonium moiety play an increasingly important role in organic synthesis. They are already known not only for their use in the Wittig reaction as ylide precursors [1,2,3], but also as convenient building blocks used in many other reactions, especially various types of couplings [4,5,6,7,8]. Moreover, they are successfully used as solvents and catalysts (PILs—phosphonium ionic liquids are good examples here) [9,10,11,12,13]. Due to some specific properties, the presence of a phosphonium group can also influence the biological properties of the whole molecule (e.g., (TPP+)-based mitochondria-targeted compounds) [14].
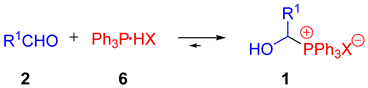
One of the relatively poorly known classes of phosphonium salts is 1-hydroxyalkylphosphonium salts 1. Although such phosphonium salts were already described in the 1960s [15,16,17], their properties (apart from spectroscopic ones [18,19]) have not been studied in detail thus far. However, there have been some references to the synthesis of these compounds based on the three-component reaction of an aldehyde with phosphine in the presence of HX (aqueous or ethereal HBF4 solution, concentrated hydrochloric acid, etc., Scheme 1/Method A) [15,16,17,18,20,21,22] or the two-component reaction in the presence of complex triphenylphosphonium salts 5 (Scheme 1/Method B) [23]. Each time, they refer to individual examples—most often the synthesis of hydroxymethylphosphonium salts (R1 = H, from paraformaldehyde or formalin). In many cases, phosphonium salts 1 were further processed without isolation and purification [6,24,25]. They perfectly fulfill the role of building blocks in Wittig reactions (e.g., the Anders-Gassner variant) [20,26,27], Friedel–Crafts reactions, or other couplings characterized by high chemo- and regioselectivity [6,24,25].
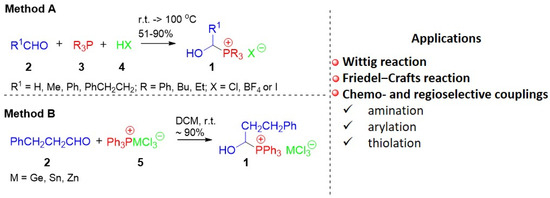
Scheme 1.
1-hydroxyalkylphosphonium salts 1: synthesis and applications.
Therefore, we present our research related to the synthesis and isolation of 1-hydroxyphosphonium salts as well as their properties, with particular emphasis on their stability, and reactivity with selected reagents.
2. Results and Discussion
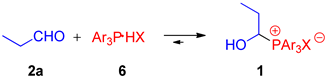
Our journey to discover the properties of 1-hydroxyalkylphosphonium salts began with the synthesis of a wide range of such salts by slightly modifying the methods already described in the literature [6,24]. To this end, we conducted two-component couplings of an aldehyde (aliphatic, aromatic, simple, and more complex) with triarylphosphonium salts in a molar ratio of 1:1 (see Table 1). We decided to use previously synthesized [24] or commercially available triarylphosphonium salts Ar3P∙HX (HP+Ar3 X−) in the reaction, rather than generating them in situ from the appropriate phosphines and acids.

Table 1.
Synthesis of 1-hydroxyalkylphosphonium salts 1—optimization of the reaction conditions.
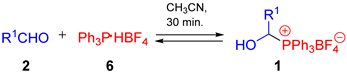
In general, the reactions were carried out at room temperature, but in some cases (see paraformaldehyde) raising the temperature was necessary. The type of solvent affects the course of the reaction. Acetonitrile, chloroform, and dichloromethane (DCM) turned out to be the most effective. However, we also noticed that the use of THF leads to a complex reaction mixture (Table 1, entries 1–3). We demonstrated that the described procedure can be applied to a variety of phosphonium salts Ar3P∙HX (HP+Ar3 X−). The anion (X−) does not play a significant role, while the triarylphosphonium group is crucial here. So far, we have not been able to obtain phosphonium salt derivatives of triarylphosphines substituted with electron-withdrawing substituents (e.g., P(3-C6H4Cl)3). Triphenylphosphonium salts and derivatives of triarylphosphines substituted with electron-donating substituents (PPh3 or P(4-C6H4OMe)3) can be synthesized without any problems (compare entries 5–7, Table 1). The reaction in which Ph3P·HBF4 was generated in situ using PPh3 and HBF4 (tetrafluoroboric acid diethyl ether complex) was also performed, but it turned out to be less efficient (92 vs. 86%—Table 1, entry 1).
The structure of the aldehyde appears to be most important to the success of the reaction. The synthesis of pure products from aliphatic aldehydes does not cause any significant problems (see Table 2). Even the use of hydrated chloroacetaldehyde allowed the expected product to be obtained with a high yield (Table 2, entry 8). The reactions can also be scaled up and conducted at a gram-scale. We demonstrated this for the reaction of paraformaldehyde with Ph3P·HBr (Table 2, entry 5), obtaining 2.6 g of product 1h with a yield of 87%.

Table 2.
Synthesis of 1-hydroxyalkylphosphonium salts 1—scope of application.
Interestingly, we also managed to obtain hydroxyalkylphosphonium salts from bis-aldehyde systems (e.g., bromomalonaldehyde; see compound 1l) or unsaturated aldehydes (e.g., (E)-cinnamaldehyde; see compounds 1m and 1n). In both cases, we had to use an excess of triphenylphosphonium salt (the molar ratio of substrates 2:6 was 1:2); otherwise, complex reaction mixtures were obtained. Furthermore, in the reaction of bromomalonoaldehyde, we isolated only compound 1l, most likely due to the reductive dehalogenation that occurred (Scheme 2).
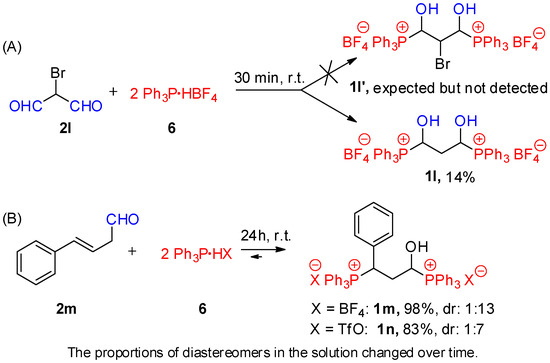
Scheme 2.
Hydroxyalkylphosphonium salts from bromomalonaldehyde (A) or (E)-cinnamaldehyde (B): conditions and results.
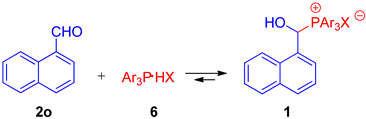
On the other hand, in the case of bulky aldehydes (R1 = i-Pr) and especially aromatic aldehydes, we observed the incomplete conversion of substrates (see Figure 1, Table 3 and Table 4). We assumed that equilibrium was reached and decided to investigate this phenomenon closely (see also Table S1, Supporting Information). Reactions between 1-naphthaldehyde and triarylphosphonium salts Ar3P∙HX (HP+Ar3 X−) were chosen as model reactions (see Figure 1 and Table 3). As we checked (1H NMR and 31P{1H} NMR), the reaction initially proceeds very quickly (until about 5 min), after which the composition of the reaction mixture does not change at a given temperature (Table 3, entries 1–3).
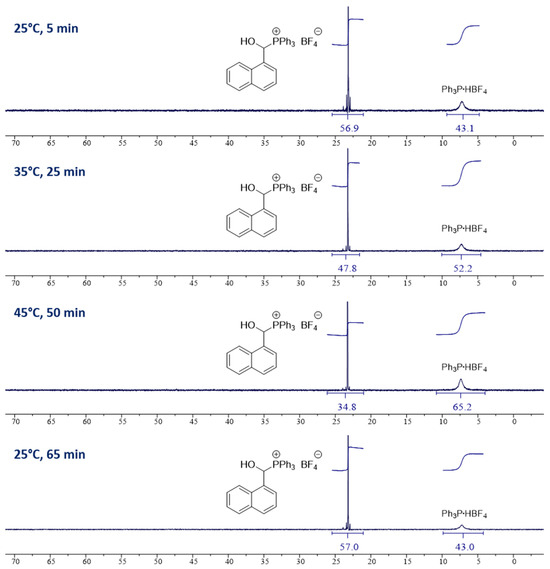
Figure 1.
31P{1H} NMR spectra (162 MHz/CD3CN) of reaction mixtures (1-naphthaldehyde with triphenylphosphonium tetrafluoroborate) recorded at 25 °C, 35 °C, 45 °C, and back to 25 °C.

Table 3.
Equilibrium in the reaction of naphthaldehyde 2o and triarylphosphonium salts 6.

Table 4.
Equilibrium in the synthesis of 1-hydroxyalkylphosphonium salts 1.
Next, we performed some temperature tests using NMR (VT techniques) and determined the composition of the reaction mixture after increasing the temperature every 10 degrees (from 25 °C to 45 °C and back to 25 °C, see Figure 1 and Table 3). Regardless of the type of phosphonium salt used (anion X or phosphonium moiety Ar3P), increasing the temperature shifts the equilibrium towards the substrates (see entries 4–15 and Figure 1). Coming back to the initial temperature (25 °C) returns it to the starting composition of the reaction mixture (e.g., compare entries 4–6, Table 3). The equilibrium state itself is slightly affected by the type of solvent used (see CD3CN vs. CDCl3, entries 4–6 and 13–15) and the counteranion of the phosphonium salt 6 (compare BF4 vs. TfO, entries 4–9). We also observed that the use of excess aldehyde 2o (2:1, 3:1, and 5:1; entries 16–19, Table 3) moves the reaction equilibrium toward product 1.
The presence of equilibria was confirmed for the reactions of structurally diverse aldehydes, mostly aromatic but sometimes also aliphatic (e.g., isobutyraldehyde, cyclohexanecarboaldehyde; see Table 4). Generally, in CD3CN the equilibrium is clearly shifted toward the 1-hydroxyalkylphosphonium salts. In CDCl3, it looks less favorable, most likely due to the acidic nature of this solvent. Furthermore, the (hetero)aromatic systems react less willingly, giving reaction mixtures with lower contents of 1-hydroxyalkylphosphonium salts 1.
Attempts to isolate pure 1-aryl-1-hydroxyalkylphosphonium salts, e.g., by crystallization from the CH2Cl2/Et2O or CD3CN/Et2O, failed (the crystallization leads to the precipitation of Ar3P∙HX rather than to 1-aryl-1-hydroxyalkylphosphonium salts).
Masarwa et al. reported similar observations for many aromatic aldehydes. They were also unable to isolate 1-hydroxyalkylphosphonium salts either through crystallization or flash chromatography, but they did not provide any explanation for this problem [6].
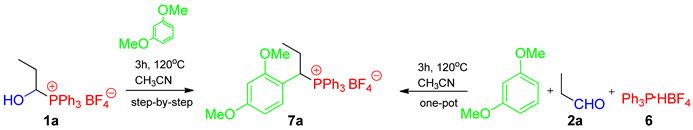
The high reactivity of the 1-hydroxyalkylphosphonium salts generated in situ (one-pot method) toward aromatic compounds or heteronucleophiles is well documented [6,24]. Therefore, we decided to examine the reactivity of the isolated 1-hydroxyalkylphosphonium salts (step-by-step procedure); however, some in-house results for one-pot reactions are also presented for comparison (see Table 5, Scheme 3, and Table S2, Supporting Information). To this end, we used the reaction with (hetero)aromatic systems or amide-type substrates (amides/carbamates/lactams).

Table 5.
The reaction of 1-hydroxyalkylphosphonium salts 1 with aromatic compounds—optimization of the conditions (step-by-step vs. one-pot protocol).
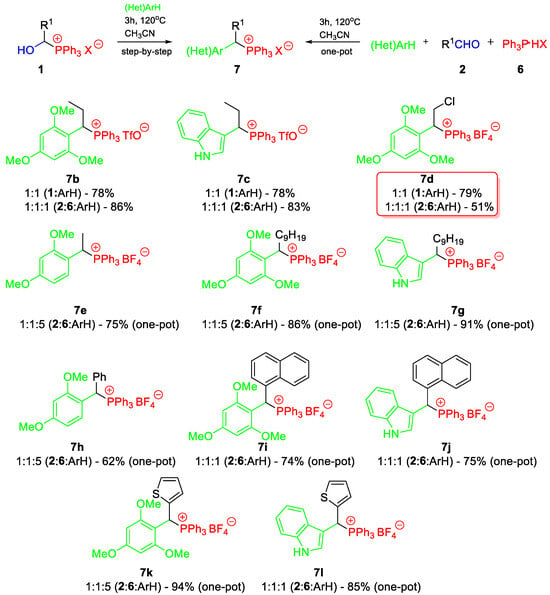
Scheme 3.
Conditions and results for the reaction of 1-hydroxyalkylphosphonium salts 1 with (hetero)aromatic compounds (step-by-step vs. one-pot protocol).
Both transformations required an elevated temperature of 120 °C and 50 °C, respectively.
In the case of the less activated aromatic systems (e.g., 1,3-dimethoxybenzene), their excess (1:2 or 1:5) had a positive effect on the reaction yields but made the work-up procedure more difficult (sometimes one crystallization was not enough). Our research showed that the one-pot methodology (without the isolation of 1-hydroxyalkylphosphonium salts) is slightly more efficient (Table 5, entries 1, 4, and 5).
For more reactive substrates (1,3,5-trimethoxybenzene and indole), the stoichiometric ratio (1:1) is optimal, as it ensures high yields and an easier purification of the crude product (see Scheme 3). In special cases, e.g., when aqueous solutions of aldehydes are used (see chloroacetaldehyde, Scheme 3/compound 7d), the step-by-step procedure gives much better results. Water does not affect the formation of 1-hydroxyalkylphosphonium salts, but it hinders reactions with arenes. All in all, both methods allowed the synthesis of 1-arylalkylphosphonium salts 7a–g (R1 = alkyl) at good to very good yields, and such compounds had not been synthesized in this way before. The only option for the synthesis of compounds 7h–l is the one-pot protocol because the isolation of the expected 1-hydroxyalkylphosphonium salt is not possible.
In comparison to the one-pot procedure proposed by Masarwa et al. (PPh3:TfOH:2:(Het)ArH = 1.1:1.2:1:1, CH3CN, 45–80 °C, 15–24 h) [6], we used a different ratio of substrates (the lack of excess acid is crucial here; its excess can catalyze the reaction). Therefore, we had to increase the reaction temperature, but its duration was shortened, and the product purification became easier (simple crystallization, without chromatography). Furthermore, the use of Ar3P·HX seems to be safer and more convenient (solid and less aggressive reagents).
For reactions with amide-type substrates, we used analogous conditions to those in the case of the one-pot procedure we recently described [24]. Transformations occurred similarly, with good yields. It is easy to notice that hydroxymethylphosphonium salts 1 (R1 = H) are less reactive compared to phosphonium salts with a longer alkyl chain (e.g., R1 = Et), which also is in agreement with the observations made previously (Scheme 4, see also Table S2, Supporting Information) [24].
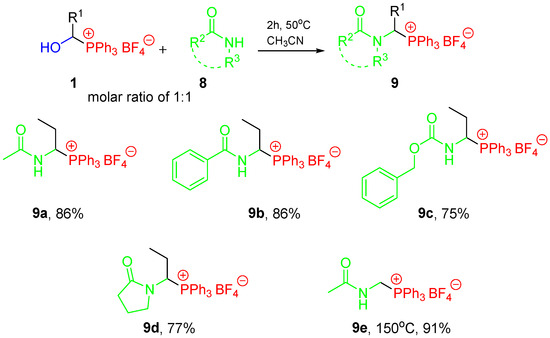
Scheme 4.
Conditions and results for the reaction of 1-hydroxyalkylphosphonium salts 1 with amide-type substrates.
Interestingly, the conducted research provided further evidence for the presence of equilibrium in the examined systems. The reactions of phosphonium salt 1a with acrylamide did not lead to the expected 1-(N-acylamino)alkylphosphonium salt 9f, but rather a complex mixture in which we identified the phosphonium salt 10 as one of the main products (about 30%). In turn, the phosphonium salt 1o (generated in situ) reacts under the same conditions to give 10 as the only reaction product. This can be explained by the course of the reaction proposed in Scheme 5. The addition to the double bond appears to occur more readily, causing the equilibrium to be shifted, limiting the formation of the desired product 9f. The 1-aryl-1-hydroxyalkylphosphonium salts (e.g., 1o) are much less stable, which means that there is more Ph3P·HBF4 in the system and thus the addition to the double bond is even more privileged.
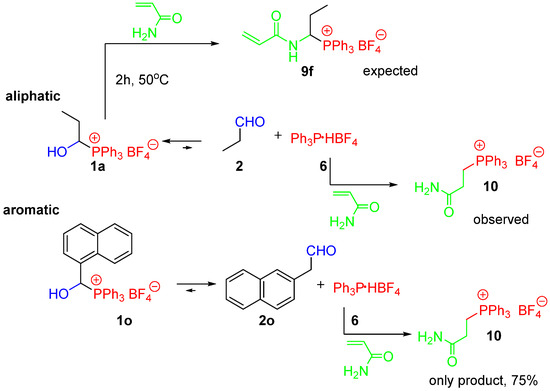
Scheme 5.
The reaction of 1-hydroxyalkylphosphonium salts 1a and 1o with acrylamide—possible pathways.
3. Materials and Methods
3.1. General Methods
Melting points were determined in capillaries and were uncorrected. The 1H- and 13C-NMR spectra were recorded at operating frequencies of 400 and 100 MHz, respectively, using TMS (tetramethylsilane) as the internal resonance shift standard. The 31P-NMR spectra were recorded at an operating frequency of 161.9 MHz, with respect to H3PO4 at zero ppm. All chemical shifts (δ) are reported in ppm and coupling constants (J) in Hz. Infrared (IR) spectra were measured on a Fourier transform (FT)-IR spectrophotometer (Attenuated Total Reflectance—ATR method). High-resolution mass spectrometry (HR-MS) analyses were performed using a Waters Xevo G2 Q-TOF mass spectrometer equipped with an ESI source operating in positive ion mode. The accurate mass and composition of the molecular ion adducts were calculated using the MassLynx 4.1 software incorporated within the instrument. Solvents (ACS grade) were stored over molecular sieves prior to use. All other commercially available reagents were used as received, without further purification or modifications.
3.2. Synthesis of 1-Hydroxyalkylphosphonium Salts
To an aldehyde (1.0 mmol) and a solvent (1 cm3) placed in a glass vial sealed with a screw-cap, triarylphosphonium salt HP+Ar3 X− (1 mmol) was added. The reaction was carried out under the conditions given in Table 1 and 2. Then, 1-hydroxyalkylphosphonium salts 1 were precipitated using Et2O. The crude 1-hydroxyalkylphosphonium salts 1 can also be obtained only via the evaporation of volatile components.
3.3. Synthesis of 1-Hydroxyalkylphosphonium Salts on a Gram-Scale
The reaction was carried out in a 25 cm3 round-bottom flask equipped with a reflux condenser. Triphenylphosphonium bromide (2.74 g, 8 mmol) was added to a solution of paraformaldehyde (0.24 g, 8 mmol) in CH3CN (8 cm3). Then, the obtained mixture was stirred vigorously and heated at 50 °C for 2 h using an oil bath. After cooling to room temperature, the product was precipitated with diethyl ether (10 cm3), separated via vacuum filtration, washed on a Büchner funnel with CH3CN/Et2O (10 cm3, 1:3 [v/v]), and dried to afford pure hydroxymethyltriphenylphosphonium bromide 1h at an 87% yield.
3.4. Synthesis of 1-Hydroxyalkylphosphonium Salts in the Presence of PPh3 and HBF4·Et2O
To a solution of propionaldehyde (67 µL, 58.1 mg, 1.0 mmol) in CH3CN (1 cm3) placed in a glass vial sealed with a screw-cap, PPh3 (262.2 mg, 1.0 mmol) and HBF4·Et2O (136 µL, 161.9 mg, 1.0 mmol) were added. The reaction was carried out under the conditions given in Table 1. Then, 1-hydroxyalkylphosphonium salt 1a was precipitated using Et2O.
- 1-hydroxypropyltriphenylphosphonium tetrafluoroborate (1a). White crystals (379.6 mg, 93% yield), mp 125–127 °C. 1H NMR (400 MHz, CDCl3) δ 7.83–7.62 (m, 15H, 3×Ph), 5.64 (dd, J = 10.2, 3.0 Hz, 1H, CαH), 5.31 (br s, 1H, OH), 1.91–1.80 (m, 2H, CH2), and 1.20 (td, J = 7.2, 0.9 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.2, 134.3 (d, J = 8.5 Hz), 130.5 (d, J = 12.0 Hz), 117.2 (d, J = 81.1 Hz), 69.9 (d, J = 60.9 Hz, CαH), 25.7 (d, J = 4.9 Hz, CH2), and 10.3 (d, J = 14.4 Hz, CH3); 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.5 ppm; IR (ATR) 3407, 1438, 1110, 1088, 1066, 996, and 976 cm−1. HRMS (TOF-ESI) calcd. for C21H22OP+ [M+] 321.1408, found 321.1422.
- 1-hydroxypropyltriphenylphosphonium bromide (1b) [24]. White crystals (353.1 mg, 88% yield), mp 150–152 °C (lit.: mp 157–159 °C [24]). 1H NMR (400 MHz, CDCl3) δ 7.95–7.71 (m, 9H, Ph), 7.70–7.58 (m, 6H, Ph), 5.96–5.88 (m, 1H, CαH), 1.91–1.77 (m, 2H, CH2), and 1.24 (td, J = 7.2, 1.1 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 134.9 (d, J = 3.0 Hz), 134.4 (d, J = 9.0 Hz), 130.3 (d, J = 12.1 Hz), 117.7 (d, J = 80.6 Hz), 68.4 (d, J = 60.4 Hz, CαH), 25.7 (d, J = 6.2 Hz, CH2), and 10.7 (d, J = 14.5 Hz, CH3); 31P{1H} NMR (161.9 MHz, CDCl3) δ 20.9 ppm; IR (ATR) 3072, 1438, 1111, 754 cm−1.
- 1-hydroxypropyltriphenylphosphonium triflate (1c). White crystals (371.6 mg, 79% yield), mp 132–134 °C. 1H NMR (400 MHz, CDCl3) δ 7.84–7.70 (m, 9H, Ph), 7.70–7.62 (m, 6H, Ph), 5.76–5.67 (m, 1H, CαH), 1.91–1.76 (m, 2H, CH2), and 1.21 (td, J = 7.4, 1.2 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.1 (d, J = 3.0 Hz), 134.4 (d, J = 8.9 Hz), 130.5 (d, J = 12.0 Hz), 120.7 (q, J = 319.6 Hz, CF3), 117.5 (d, J = 80.7 Hz), 69.7 (d, J = 60.4 Hz, CαH), 25.8 (d, J = 5.4 Hz, CH2), and 10.4 (d, J = 14.7 Hz, CH3); 31P{1H} NMR (161,9 MHz, CDCl3) δ 21.0 ppm; IR (ATR) 3266, 1438, 1292, 1244, 1224, 1155, 1109, and 1028 cm−1. HRMS (TOF-ESI) calcd. For C21H22OP+ [M+] 321.1408, found 321.1407.
- 1-hydroxypropyltris(4-methoxyphenyl)phosphonium tetrafluoroborate (1d). Resin (328.8 mg, 66% yield). 1H NMR (400 MHz, CDCl3) δ 7.70–7.53 (m, 6H, aromatic), 7.20–7.07 (m, 6H, aromatic), 5.42 (dd, J = 10.5, 3.0 Hz, 1H, CαH), 3.90 (s, 9H, 3xOCH3), 1.87–1.68 (m, 2H, CH2), and 1.17 (td, J = 7.2, 0.4 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 164.7 (d, J = 3.0 Hz), 136.1 (d, J = 10.5 Hz), 116.1 (d, J = 13.2 Hz), 108.0 (d, J = 89.2 Hz), 70.1 (d, J = 65.2 Hz, CαH), 55.9 (OCH3), 25.4 (CH2), and 10.3 (d, J = 14.3 Hz, CH3); 31P{1H} NMR (161.9 MHz, CDCl3) δ 19.9 ppm; IR (ATR) 3442, 1593, 1503, 1263, 1110, 1055, and 1016 cm−1. HRMS (TOF-ESI) calcd. for C24H28O4P+ [M+] 411.1725, found 411.1721.
- hydroxymethyltriphenylphosphonium tetrafluoroborate (1f) [16,17]. White crystals (361.1 mg, 95% yield), mp 132–134 °C C (lit.: mp 128–130 °C [16], 130–131 °C [17]). 1H NMR (400 MHz, CDCl3) δ 7.85–7.64 (m, 15H, 3xPh), 5.47 (s, 2H, CH2); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.4 (d, J = 3.0 Hz), 134.1 (d, J = 9.5 Hz), 130.5 (d, J = 12.3 Hz), 117.3 (d, J = 83.9 Hz), and 57.5 (d, J = 65.2 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 17.1 ppm; IR (ATR) 3073, 1439, 1076, 1025, and 998 cm−1. HRMS (TOF-ESI) calcd. for C19H18OP+ [M+] 293.1095, found 293.1085.
- hydroxymethyltriphenylphosphonium triflate (1g) [28]. White crystals (411.4 mg, 93% yield), mp 134–135 °C. 1H NMR (400 MHz, CDCl3) δ 7.89–7.78 (m, 3H, Ph), 7.75–7.63 (m, 12H, Ph), 5.41 (s, 2H, CH2); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.5 (d, J = 3.0 Hz), 134.0 (d, J = 9.5 Hz), 130.6 (d, J = 12.4 Hz), 120.6 (q, J = 319.6 Hz, CF3), 116.7 (d, J = 84.0 Hz), and 58.1 (d, J = 65.1 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 17.2 ppm; IR (ATR) 3313, 1438, 1281, 1249, 1228, 1158, 1113, and 1027 cm−1. HRMS (TOF-ESI) calcd. for C19H18OP+ [M+] 293.1095, found 293.1096.
- hydroxymethyltriphenylphosphonium bromide (1h) [29]. White crystals (354.5 mg, 95% yield), mp 191–193 °C (lit.: mp 203 °C [29]). 1H NMR (400 MHz, CDCl3) δ 7.85–7.61 (m, 15H, 3xPh), 5.47 (s, 2H, CH2); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.4 (d, J = 3.0 Hz), 134.1 (d, J = 9.5 Hz), 130.5 (d, J = 12.3 Hz), 117.3 (d, J = 83.6 Hz), and 57.6 (d, J = 65.1 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 17.1 ppm; IR (ATR) 3087, 1435, 1115, and 1051 cm−1. HRMS (TOF-ESI) calcd. For C19H18OP+ [M+] 293.1095, found 293.1096.
- 1-hydroxyethyltriphenylphosphonium tetrafluoroborate (1i). White crystals (390.3 mg, 99% yield), mp 114–116 °C. 1H NMR (400 MHz, CDCl3) δ 7.90–7.55 (m, 15H, 3xPh), 5.96–5.86 (m, 1H, CαH), 5.29 (dd, J = 13.1, 6.8 Hz, 1H, OH), and 1.70 (dd, J = 18.2, 6.8 Hz, 3H, CH3); 13C{1H} NMR (150 MHz, CDCl3) δ aromatic carbons: 135.3 (br s), 134.2 (d, J = 8.9 Hz), 130.5 (d, J = 12.1 Hz), 116.8 (d, J = 82.1 Hz), 65.4 (d, J = 65.1 Hz, CαH), and 18.5 (br s, CH3); 31P{1H} NMR (161.9 MHz, CDCl3) δ 17.5 ppm; IR (ATR) 3451, 1488, 1439, 1108, 1060, 996, and 976 cm−1. HRMS (TOF-ESI) calcd. for C20H20OP+ [M+] 307.1252, found 307.1251.
- 2-chloro-1-hydroxyethyltriphenylphosphonium tetrafluoroborate (1j). White crystals (369.0 mg, 89% yield), mp 111.5–113.5 °C. 1H NMR (400 MHz, CDCl3) δ 7.90–7.74 (m, 9H, Ph), 7.70–7.59 (m, 6H, Ph), 6.11 (br s, 1H, OH), 5.73 (dd, J = 13.2, 6.2 Hz, 1H, CαH), 4.03 (ddd, J = 19.9, 12.8, 4.3 Hz, 1H, CHH), and 3.88 (ddd, J = 12.9, 8.2, 5.0 Hz, 1H, CHH); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.6 (d, J = 3.1 Hz), 134.3 (d, J = 9.5 Hz), 130.6 (d, J = 12.5 Hz), 116.5 (d, J = 82.7 Hz), 69.8 (d, J = 67.3 Hz, CαH), and 44.4 (d, J = 7.3 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 20.8 ppm; IR (ATR) 3337, 1586, 1483, 1436, 1061, 1023, 995, and 973 cm−1. HRMS (TOF-ESI) calcd. for C20H19ClOP+ [M+] 341.0862, found 341.0861.
- 1-hydroxydecyltriphenylphosphonium tetrafluoroborate (1k). Resin (501.3 mg, 99% yield). 1H NMR (400 MHz, CDCl3) δ 7.87–7.60 (m, 15H, 3xPh), 5.73–5.66 (m, 1H, CαH), 5.29 (br s, 1H, OH), aliphatics (8xCH2): 1.90–1.66 (m, 3H), 1.66–1.46 (m, 1H), 1.35–1.11 (m, 12H), and 0.85 (t, J = 6.9 Hz, 3H, CH3); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.2 (d, J = 3.0 Hz), 134.3 (d, J = 8.9 Hz), 130.5 (d, J = 12.0 Hz), 117.2 (d, J = 80.9 Hz), 68.9 (d, J = 60.1 Hz, CαH), 32.3 (d, J = 4.3 Hz, CH2), 31.9 (CH2), 29.5 (CH2), 29.4 (CH2), 29.3 (CH2), 29.2 (CH2), 25.7 (d, J = 13.4 Hz, CH2), 22.7 (br s, CH2), and 14.2 (CH3); 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.4 ppm; IR (ATR) 2923, 2854, 1439, 1109, 1056, and 996 cm−1. HRMS (TOF-ESI) calcd. for C28H36OP+ [M+] 419.2504, found 419.2499.
- 1,3-dihydroxypropane-1,3-bis(triphenylphosphonium) bis(tetrafluoroborate) (1l). White crystals (108.1 mg, 14% yield), mp 150–151 °C. 1H NMR (400 MHz, CDCl3) δ 7.90–7.50 (m, 30H, 6xPh), 6.64 (br s, 2H, OH), 5.94 (br s, 2H, CαH), and 2.18–2.05 (m, 2H, CH2); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.4 (br s), 134.7–134.3 (m), 130.9–130.5 (m), 116.1 (d, J = 84.2 Hz), 62.7 (dd, J = 73.2, 13.8 Hz, CαH), and 33.5 (t, J = 8.7 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 22.7 ppm; IR (ATR) 2989, 1586, 1483, 1437, 1109, 1045, and 995 cm−1. HRMS (TOF-ESI) calcd. for C39H36O2P22+ [M2+] 299.1090, found 299.1087.
- 1-hydroxy-3-phenylpropane-1,3-bis(triphenylphosphonium) bis(tetrafluoroborate) (1m). White crystals (815.8 mg, 98% yield), mp 205–207 °C. 1H NMR (400 MHz, CDCl3) δ 7.83–7.71 (m, 6H, Ph), 7.69–7.54 (m, 18H, Ph), 7.47–7.39 (m, 6H, Ph), 7.39–7.30 (m, 1H, Ph), 7.27–7.21 (m, 2H, Ph), 7.06–7.00 (m, 2H, Ph), 6.16 (t, J = 7.1 Hz, 1H, OH), 5.24–5.16 (m, 1H, CH-Ph or CαH), 5.04 (dd, J = 17.1, 10.8 Hz, 1H, CH-Ph or CαH), 2.81–2.69 (m, 1H, CHH), and 2.59–2.50 (m, 1H, CHH); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.6 (d, J = 3.0 Hz), 135.5 (d, J = 3.1 Hz), 134.4 (d, J = 9.4 Hz), 134.1 (d, J = 9.3 Hz), 131.3 (d, J = 5.8 Hz), 130.7 (d, J = 12.4 Hz), 130.6 (d, J = 12.5 Hz), 130.1 (d, J = 3.0 Hz), 129.9 (d, J = 2.2 Hz), 129.4 (d, J = 5.1 Hz), 116.1 (d, J = 83.9 Hz), 115.4 (d, J = 82.6 Hz), 66.5 (dd, J = 66.2, 14.8 Hz, CαH), 40.2 (dd, J = 47.7, 15.6 Hz, CH-Ph), and 35.2 (br d, J = 10.2 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 26.9 (d, J = 9.1 Hz), 21.8 (d, J = 9.1 Hz) ppm; IR (ATR) 3410, 1439, 1108, 1050, and 996 cm−1. HRMS (TOF-ESI) calcd. for C39H36O2P22+ [M2+] 329.1277, found 329.1265.
- 1-hydroxy-3-phenylpropane-1,3-bis(triphenylphosphonium) bis(triflate) (1n). White crystals (108.1 mg, 14% yield), mp 190–192 °C. 1H NMR (400 MHz, CDCl3) δ 7.84–7.70 (m, 6H, Ph), 7.69–7.56 (m, 18H, Ph), 7.49–7.41 (m, 6H, Ph), 7.39–7.33 (m, 1H, Ph), 7.27–7.22 (m, 2H, Ph), 7.05–6.99 (m, 2H, Ph), 5.18–5.02 (m, 2H, CH-Ph and CαH), 2.76–2.64 (m, 1H, CHH), 2.59–and 2.46 (m, 1H, CHH); 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.6 (d, J = 3.0 Hz), 135.4 (d, J = 3.1 Hz), 134.6 (d, J = 9.4 Hz), 134.3 (d, J = 9.3 Hz), 131.4 (d, J = 5.8 Hz), 130.7 (d, J = 12.4 Hz), 130.7 (d, J = 12.4 Hz), 130.2 (d, J = 3.0 Hz), 129.9 (d, J = 2.3 Hz), 129.5 (d, J = 5.1 Hz), 120.6 (q, J = 320.2 Hz, CF3), 116.3 (d, J = 83.9 Hz), 115.8 (d, J = 82.5 Hz), 66.3 (dd, J = 66.8, 14.8 Hz, CαH), 40.0 (dd, J = 47.7, 15.7 Hz, CH-Ph), and 35.2 (dd, J = 9.8, 1.0 Hz, CH2); 31P{1H} NMR (161.9 MHz, CDCl3) δ 26.4 (d, J = 8.7 Hz), 21.4 (d, J = 8.7 Hz) ppm; IR (ATR) 3062, 1439, 1262, 1107, 1025, and 997 cm−1. HRMS (TOF-ESI) calcd. For C39H36O2P22+ [M2+] 329.1277, found 329.1270.
3.5. NMR Experiments (Studies of Equilibria)
A mixture of an aldehyde (0.1 mmol), a triarylphosphonium salt Ar3P∙HX (0.1 mmol), and a deuterated solvent (0.65 cm3) was placed in an NMR tube. The reaction was carried out under the conditions given in Table 3 and Table 4. Changes in substrate and/or product concentrations were monitored via 1H NMR and confirmed via 31P{1H} NMR spectroscopy (for 31P{1H} NMR: seqfil = s2pul, sw = 249 ppm, at = 0.813 s, np = 65,536, pw = 3.356 µs, d1 = 1 s, and offset = 16,377.3 Hz).
3.6. Reactions of 1-Hydroxyalkylphosphonium Salts with (hetero)arenes (Step-by-Step)
To a solution of 1-hydroxyalkylphosphonium salt (1.0 mmol) in CH3CN (1 cm3) placed in a glass vial sealed with a screw-cap, a (hetero)aromatic compound (5, 2, or 1 mmol) was added. The reaction was carried out under the conditions given in Scheme 3. Then, the 1-arylalkylphosphonium salts 7 were precipitated using Et2O.
3.7. Reactions of Aldehydes with (hetero)arenes in the Presence of Ar3P∙HX (One-Pot)
To a solution of aldehydes (1.0 mmol) in CH3CN (1.0 cm3) placed in a glass vial sealed with a screw-cap, the Ar3P∙HX (1.0 mmol) and a (hetero)aromatic compound (5.0 mmol or 1.0 mmol) were added. The reaction was carried out under the conditions given in Table 5 and Scheme 3. Then, the 1-arylalkylphosphonium salts 7 were precipitated using Et2O.
3.8. Reactions of Aldehydes with (hetero)arenes in the Presence of PPh3 and HBF4·Et2O (One-Pot)
To a solution of propionaldehyde (67 µL, 58.1 mg, 1.0 mmol) in CH3CN (1.0 cm3) placed in a glass vial sealed with a screw-cap, PPh3 (262.2 mg, 1.0 mmol), HBF4·Et2O (136 µL, 161.9 mg, 1.0 mmol), and 1,3-dimethoxybenzene (648.6 µL, 690.8 mg, 5 mmol) were added. The reaction was carried out under the conditions given in Table 5. Then, the 1-arylalkylphosphonium salt 7a was precipitated using Et2O.
- 1-(2,4-dimethoxyphenyl)propyltriphenylphosphonium tetrafluoroborate (7a). White crystals (433.2 mg, 82%), mp 174–176 °C. 1H NMR (400 MHz, CDCl3) δ 7.88–7.79 (m, 3H, Ph), 7.73–7.63 (m, 6H, Ph), 7.51–7.40 (m, 6H, Ph), 6.58 (dd, J = 9.3, 2.4 Hz, 1H, aromatic), 6.44–6.33 (m, 2H, aromatic), 4.90 (ddd, J = 15.1, 12.2, 2.6 Hz, 1H, CαH), 3.82 (s, 3H, OCH3), 3.43 (s, 3H, OCH3), 2.35–2.20 (m, 1H, CHH), 2.19–2.08 (m, 1H, CHH), and 0.95 (td, J = 7.1, 1.2 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 161.9 (d, J = 3.0 Hz), 159.4 (d, J = 5.9 Hz), 135.4 (d, J = 3.0 Hz), 134.3 (d, J = 9.0 Hz), 130.5 (d, J = 12.1 Hz), 117.7 (d, J = 82.4 Hz), 110.5 (d, J = 5.2 Hz), 105.8 (d, J = 2.6 Hz), 98.9 (d, J = 2.4 Hz), 55.7 (OCH3), 55.5 (OCH3), 36.7 (d, J = 41.6 Hz, CαH), 24.2 (CH2), and 12.5 (d, J = 15.3 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 23.2 ppm; IR (ATR) 2971, 1608, 1585, 1508, 1438, 1107, 1050, 1027, and 997 cm−1. HRMS (TOF-ESI) calcd. for C29H30O2P+ [M+] 441.1983 found 441.1982.
- 1-(2,4,6-trimethoxyphenyl)propyltriphenylphosphonium triflate (7b). Resin (533.7 mg, 86%). 1H NMR (400 MHz, CDCl3) 7.88–7.75 (m, 3H, Ph), 7.71–7.59 (m, 6H, Ph), 7.44–7.31 (m, 6H, Ph), 6.03 (s, 2H, aromatic), 5.00 (ddd, J = 17.5, 11.9, 3.2 Hz, 1H, CαH), 3.84 (s, 3H, OCH3), 3.46 (s, 3H, OCH3), 3.15 (s, 3H, OCH3), 2.77–2.62 (m, 1H, CHH), 2.13–2.00 (m, 1H, CHH), and 0.91 (td, J = 7.2, 1.1 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 162.7 (d, J = 2.8 Hz), 160.0 (d, J = 7.5 Hz), 135.0 (d, J = 3.0 Hz), 134.0 (d, J = 8.9 Hz), 130.1 (d, J = 12.0 Hz), 121.0 (q, J = 320.7 Hz, CF3), 118.8 (d, J = 82.4 Hz), 98.6 (d, J = 5.3 Hz), 91.0, 56.1 (OCH3), 55.7 (OCH3), 54.7 (OCH3), 36.4 (d, J = 45.5 Hz, CαH), and 21.6 (CH2), 13.0 (d, J = 15.1 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.7 ppm; IR (ATR) 2940, 1609, 1586, 1438, 1266, 1222, 1141, 1119, 1103, and 1030 cm−1. HRMS (TOF-ESI) calcd. for C30H32O3P+ [M+] 471.2089 found 471.2093.
- 1-(H-indol-3-yl)propyltriphenylphosphonium triflate (7c). White crystals (472.8 mg, 83%), mp 191–193 °C. 1H NMR (400 MHz, CDCl3) 10.22 (s, 1H, NH), 7.77–7.69 (m, 3H, Ph), 7.61–7.53 (m, 6H, Ph), 7.51–7.43 (m, 7H, aromatic), 7.07–6.99 (m, 1H, aromatic), 6.97–6.91 (m, 1H, aromatic), 6.81 (br t, J = 7.5 Hz, 1H, aromatic), 6.65 (br t, J = 3.0 Hz, 1H, aromatic), 4.73 (ddd, J = 14.4, 12.2, 2.4 Hz, 1H, CαH), 2.40–2.21 (m, 1H, CHH), 2.15–2.01 (m, 1H, CHH), and 0.91 (td, J = 7.1, 1.3 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 136.2, 135.2 (d, J = 2.0 Hz), 134.3 (d, J = 8.9 Hz), 130.3 (d, J = 12.0 Hz), 127.4 (d, J = 4.6 Hz), 126.3 (d, J = 7.8 Hz), 122.4, 121.0 (q, J = 318.8 Hz, CF3), 119.9, 117.8 (d, J = 80.9 Hz), 117.7, 112.9, 103.0 (d, J = 5.8 Hz), 36.8 (d, J = 45.2 Hz, CαH), 25.6 (CH2), and 12.4 (d, J = 14.9 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.9 ppm; IR (ATR) 3329, 1438, 1277, 1266, 1248, 1225, 1158, 1109, and 1030 cm−1. HRMS (TOF-ESI) calcd. for C29H27NP+ [M+] 420.1881 found 420.1882.
- 2-chloro-1-(2,4,6-trimethoxyphenyl)ethyltriphenylphosphonium tetrafluoroborate (7d). White crystals (457.3 mg, 79%), mp 159–160 °C. 1H NMR (400 MHz, CDCl3) 7.86–7.79 (m, 3H, Ph), 7.72–7.64 (m, 6H, Ph), 7.49–7.41 (m, 6H, Ph), 6.03 (s, 2H, aromatic), 5.50 (ddd, J = 16.4, 7.8, 7.1 Hz, 1H, CαH), 4.45–4.27 (m, 2H, CH2Cl), 3.83 (s, 3H, OCH3), and 3.42 (s, 6H, OCH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 163.5 (d, J = 2.7 Hz), 159.5 (br s), 135.4 (d, J = 3.1 Hz), 134.2 (d, J = 9.4 Hz), 130.3 (d, J = 12.4 Hz), 117.8 (d, J = 83.1 Hz), 98.3 (d, J = 5.1 Hz), 91.2, 55.9 (OCH3), 55.5 (OCH3), 41.7 (d, J = 5.0 Hz, CH2Cl), and 38.5 (d, J = 45.6 Hz, CαH) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 22.1 ppm; IR (ATR) 3354, 2843, 1608, 1590, 1438, 1340, 1209, 1157, 1142, 1118, 1103, 1032, and 997 cm−1. HRMS (TOF-ESI) calcd. for C29H29ClO3P+ [M+] 491.1543 found 491.1546.
- 1-(2,4-dimethoxyphenyl)ethyltriphenylphosphonium tetrafluoroborate (7e) [30]. Resin (385.7 mg, 75%). 1H NMR (400 MHz, CDCl3) δ 7.93–7.80 (m, 3H, Ph), 7.73–7.60 (m, 6H, Ph), 7.53–7.42 (m, 6H, Ph), 6.69–6.58 (m, 1H, aromatic), 6.42–6.32 (m, 2H, aromatic), 5.27–5.14 (m, 1H, CαH), 3.81 (s, 3H, OCH3), 3.41 (s, 3H, OCH3), and 1.82 (dd, J = 18.5, 7.4 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 161.8 (d, J = 3.0 Hz), 158.1 (d, J = 5.6 Hz), 135.2 (d, J = 3.0 Hz), 134.2 (d, J = 8.9 Hz), 130.3 (d, J = 12.1 Hz), 130.1 (d, J = 5.0 Hz), 117.3 (d, J = 82.4 Hz), 112.6 (d, J = 5.4 Hz), 105.5 (d, J = 2.6 Hz), 98.7 (d, J = 2.5 Hz), 55.6 (OCH3), 55.2 (OCH3), 29.7 (d, J = 45.7 Hz, CαH), and 16.5 (CH3) ppm; δ 31P{1H} NMR (161.9 MHz, CDCl3) δ 24.2 ppm; IR (ATR) 2936, 1608, 1506, 1486, 1438, 1301, 1209, 1107, 1049, 1023, and 996 cm−1. The spectra reported here are in agreement with previously published data [30].
- 1-(2,4,6-trimethoxyphenyl)decyltriphenylphosphonium tetrafluoroborate (7f). White crystals (564.6 mg, 86%), mp 110–112 °C. 1H NMR (400 MHz, CDCl3) 7.88–7.76 (m, 3H, Ph), 7.70–7.60 (m, 6H, Ph), 7.44–7.31 (m, 6H, Ph), 6.06 (s, 1H, aromatic), 6.03 (s, 1H, aromatic), 5.08 (ddd, J = 17.7, 12.0, 3.0 Hz, 1H, CαH), 3.85 (s, 3H, OCH3), 3.45 (s, 3H, OCH3), 3.16 (s, 3H, OCH3), and aliphatics (8xCH2): 2.81–2.64 (m, 1H), 1.99–1.81 (m, 1H), 1.32–1.09 (m, 14H), and 0.85 (t, J = 7.0 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 162.7 (d, J = 2.9 Hz), 160.1 (d, J = 3.6 Hz), 159.8 (d, J = 6.5 Hz), 135.0 (d, J = 3.0 Hz), 134.1 (d, J = 8.9 Hz), 130.2 (d, J = 12.1 Hz), 118.8 (d, J = 82.3 Hz), 98.8 (d, J = 5.4 Hz), 91.1 (br s), 56.1 (OCH3), 55.8 (OCH3), 54.7 (OCH3), 34.8 (d, J = 45.3 Hz, CαH), 31.9 (CH2), 29.5 (CH2), 29.3 (CH2), 29.3 (CH2), 28.8 (CH2), 28.1 (d, J = 13.6 Hz), 27.7 (CH2), 22.7 (CH2), and 14.2 (CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.7 ppm; IR (ATR) 2925, 2853, 1607, 1589, 1456, 1437, 1206, 1147, 1127, 1101, 1046, 1033, and 996 cm−1. HRMS (TOF-ESI) calcd. for C37H46O3P+ [M+] 569.3185 found 569.3185.
- 1-(H-indol-3-yl)decyltriphenylphosphonium tetrafluoroborate (7g). Creamy crystals (551.0 mg, 91%), mp 195–197 °C. 1H NMR (400 MHz, CDCl3) 9.70 (s, 1H, NH), 7.82–7.70 (m, 3H, Ph), 7.65–7.53 (m, 6H, Ph), 7.52–7.40 (m, 7H, aromatic), 7.12–7.01 (m, 1H, aromatic), 6.91–6.78 (m, 2H, aromatic), 6.77–6.68 (m, 1H, aromatic), 4.81–4.66 (m, 1H, CαH), and aliphatics (8xCH2): 2.27–2.01 (m, 2H), 1.42–0.98 (m, 14H), and 0.82 (t, J = 7.1 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 136.2, 135.3 (d, J = 2.9 Hz), 134.3 (d, J = 8.9 Hz), 130.4 (d, J = 12.0 Hz), 127.2 (d, J = 4.4 Hz), 126.6 (d, J = 6.9 Hz), 122.6, 120.1, 117.9 (d, J = 82.0 Hz), 117.5, 113.0, 103.4 (d, J = 5.9 Hz), 35.4 (d, J = 44.8 Hz, CαH), 32.0 (br s, CH2), 31.9 (CH2), 29.5 (CH2), 29.3 (CH2), 29.2 (CH2), 29.1 (CH2), 27.6 (d, J = 13.6 Hz), 22.7 (CH2), and 14.2 (CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.9 ppm; IR (ATR) 3357, 2927, 2854, 1484, 1459, 1436, 1340, 1106, 1055, 1020, 995, 744, 720, and 691 cm−1. HRMS (TOF-ESI) calcd. for C36H41NP+ [M+] 518.2977 found 518.2982.
- 1-(2,4-dimethoxyphenyl)phenylmethyltriphenylphosphonium tetrafluoroborate (7h) [30]. White crystals (357.4 mg, 62%), mp 209–211 °C (lit.: mp 199.5–200.5 °C [30]). 1H NMR (400 MHz, CDCl3) δ 7.84–7.75 (m, 3H), 7.67–7.58 (m, 8H), 7.51–7.40 (m, 6H), 7.32–7.19 (m, 1H), 7.14–7.07 (m, 2H), 7.02–6.95 (m, 1H), 6.52 (d, J = 18.2 Hz, 1H, CαH), 6.44–6.40 (m, 2H), 3.80 (s, 3H, OCH3), and 3.62 (s, 3H, OCH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 161.8 (d, J = 2.1 Hz), 157.7 (d, J = 6.5 Hz), 135.1 (d, J = 3.0 Hz), 134.5 (d, J = 9.0 Hz), 132.5 (d, J = 3.1 Hz), 131.6 (d, J = 6.1 Hz), 130.4 (d, J = 6.2 Hz), 130.1 (d, J = 12.2 Hz), 129.2 (d, J = 1.9 Hz), 129.0 (d, J = 2.8 Hz), 118.3 (d, J = 82.4 Hz), 112.8 (d, J = 3.5 Hz), 105.5 (d, J = 1.6 Hz), 99.1 (d, J = 1.6 Hz), 55.6 (OCH3), 55.5 (OCH3), and 41.8 (d, J = 45.5 Hz, CαH) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 22.0 ppm; IR (ATR) 3058, 1614, 1582, 1505, 1438, 1215, and 1035 cm−1. The spectra reported here are in agreement with previously published data [30].
- 1-(naphthalen-1-yl)-1-(2,4,6-trimethoxyphenyl)methyltriphenylphosphonium tetrafluoroborate (7i). Resin (485.8 mg, 74%). 1H NMR (400 MHz, CDCl3) 7.90–7.76 (m, 3H, aromatic), 7.75–7.62 (m, 4H, aromatic), 7.60–7.39 (m, 13H, aromatic), 7.40–7.27 (m, 2H, aromatic), 7.19 (d, J = 19.4 Hz, 1H, CαH), 6.08 (s, 2H, aromatic), 3.81 (s, 3H, OCH3), and 3.44 (s, 6H, OCH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 162.8 (d, J = 1.8 Hz), 158.6 (d, J = 5.5 Hz), 134.7 (d, J = 3.1 Hz), 134.5 (d, J = 8.9 Hz), 134.0, 131.4 (d, J = 8.1 Hz), 130.8 (d, J = 6.4 Hz), 129.9 (d, J = 12.1 Hz), 129.8, 129.3 (d, J = 1.9 Hz), 129.3, 127.1, 126.1, 124.8 (d, J = 2.1 Hz), 122.7, 120.3 (d, J = 82.5 Hz), 102.1 (d, J = 3.6 Hz), 91.5 (d, J = 1.3 Hz), 55.8 (OCH3), 55.4 (OCH3), and 37.7 (d, J = 49.6 Hz, CαH) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 22.4 ppm; IR (ATR) 2940, 1604, 1587, 1458, 1207, 1149, 1097, 1049, 996, and 690 cm−1. HRMS (TOF-ESI) calcd. for C38H34O3P+ [M+] 569.2246 found 569.2252.
- 1-(H-indol-3-yl)-1-(naphthalen-1-yl)methyltriphenylphosphonium tetrafluoroborate (7j). Pink crystals (454.1 mg, 75%), mp 115–117 °C. 1H NMR (400 MHz, CDCl3) 9.81 (s, 1H, NH), 8.07–7.99 (m, 1H, aromatic), 7.87–7.75 (m, 2H, aromatic), 7.73–7.58 (m, 3H, aromatic), 7.54–7.35 (m, 15H, aromatic), 7.33–7.19 (m, 2H, aromatic), 7.07–6.95 (m, 3H, aromatic), 6.96 (d, J = 17.2 Hz, 1H, CαH), and 6.87–6.73 (m, 1H, aromatic) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 136.1, 135.3 (d, J = 3.0 Hz), 134.8 (d, J = 8.9 Hz), 134.2, 131.2 (d, J = 8.1 Hz), 130.3 (d, J = 12.0 Hz), 130.1 (d, J = 2.1 Hz), 130.0 (d, J = 1.8 Hz), 129.5, 128.5 (d, J = 7.1 Hz), 128.4 (d, J = 5.5 Hz), 127.8, 126.7, 126.0 (d, J = 6.4 Hz), 125.1 (d, J = 1.9 Hz), 122.6, 122.2, 120.2, 118.5 (d, J = 80.9 Hz), 117.5, 113.0, 104.9 (d, J = 4.8 Hz), and 38.4 (d, J = 47.1 Hz, CαH) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 19.6 ppm; IR (ATR) 3356, 3059, 1461, 1023, 997, 738, 719, and 690 cm−1. HRMS (TOF-ESI) calcd. for C37H29NP+ [M+] 518.2038 found 518.2036.
- 1-(tiophen-2-yl)-1-(2,4,6-trimethoxyphenyl)methyltriphenylphosphonium (7k). White crystals (575.7 mg, 94%), mp 195–197 °C (decomposition). 1H NMR (400 MHz, CDCl3) 7.83–7.76 (m, 3H, Ph), 7.66–7.58 (m, 6H, Ph), 7.39–7.29 (m, 6H, Ph), 7.24–7.19 (m, 1H, aromatic), 6.90–6.84 (m, 1H, aromatic), 6.84 (d, J = 18.7 Hz, 1H, CαH), 6.70 (br t, J = 3.5 Hz, 1H), 6.13 (s, 2H, aromatic), 3.86 (s, 3H, OCH3), and 3.48 (br s, 6H, OCH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 163.2 (d, J = 2.1 Hz), 158.9 (br s), 135.1 (d, J = 3.0 Hz), 134.3 (d, J = 8.8 Hz), 134.1 (d, J = 8.8 Hz), 130.2 (d, J = 12.2 Hz), 129.8 (d, J = 6.7 Hz), 127.4 (d, J = 4.1 Hz), 127.1 (d, J = 3.2 Hz), 119.0 (d, J = 82.5 Hz), 101.8 (d, J = 3.7 Hz), 91.2, 55.9 (OCH3), 55.5 (OCH3), and 37.8 (d, J = 49.2 Hz, CαH) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 21.9 ppm; IR (ATR) 3111, 2947, 1608, 1596, 1435, 1421, 1220, 1158, 1100, 1049, and 997 cm−1. HRMS (TOF-ESI) calcd. for C32H30O3PS+ [M+] 525.1653 found 525.1655.
- 1-(H-indol-3-yl)-1-(tiophen-2-yl)methyltriphenylphosphonium tetrafluoroborate (7l). Pink crystals (477.2 mg, 85%), mp 185–187 °C. 1H NMR (400 MHz, CDCl3) 9.87 (s, 1H, NH), 7.81–7.71 (m, 3H, Ph), 7.60–7.51 (m, 7H, aromatic), 7.40–7.30 (m, 6H, Ph), 7.21–7.15 (m, 2H, aromatic), 7.15–7.08 (m, 1H, aromatic), 6.95–6.87 (m, 2H, aromatic), 6.86–6.82 (m, 1H, aromatic), 6.78–6.72 (m, 1H, aromatic), and 6.45 (d, J = 16.4 Hz, 1H, CαH) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ aromatic carbons: 135.9, 135.6 (d, J = 2.9 Hz), 134.8 (d, J = 8.9 Hz), 130.4 (d, J = 12.1 Hz), 127.8 (d, J = 4.0 Hz), 127.7 (d, J = 3.2 Hz), 127.5 (d, J = 2.9 Hz), 127.4 (d, J = 2.7 Hz), 126.4 (d, J = 6.4 Hz), 123.1, 120.5, 117.5 (d, J = 82.0 Hz), 117.5, 113.2, 104.4 (d, J = 0.9 Hz), 104.4 (d, J = 1.7 Hz), and 38.4 (d, J = 47.5 Hz, CαH) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 19.9 ppm; IR (ATR) 3362, 3060, 1437, 1343, 1107, 1055, 996, and 688 cm−1. HRMS (TOF-ESI) calcd. for C31H25NPS+ [M+] 474.1445 found 474.1437.
3.9. Reactions of 1-Hydroxyalkylphosphonium Salts with Amide-Type Substrates
To a solution of 1-hydroxyalkylphosphonium salt (1.0 mmol) in CH3CN (1 cm3) placed in a glass vial sealed with a screw-cap, an amide-type substrate (1 mmol) was added. The reaction was carried out under the conditions given in Scheme 4. Then, 1-(N-acylamino)alkylphosphonium salts 9 were precipitated using Et2O.
- 1-(N-acetylamino)propyltriphenylphosphonium tetrafluoroborate (9a) [24]. White crystals (386.3 mg, 86%), mp 199–201 °C (lit.: mp 185–186 °C [24]). 1H NMR (400 MHz, CDCl3) δ 7.87–7.76 (m, 4H, Ph + NH), 7.74–7.66 (m, 12H, Ph), 5.67 (dddd, J = 12.0, 9.2, 7.7, 2.7 Hz, 1H, CαH), 2.13–1.97 (m, 1H, CHH), 1.91 (d, J = 1.2 Hz, 3H, CH3C=O), 1.86–1.70 (m, 1H, CHH), and 1.12 (td, J = 7.2, 1.3 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.6 (d, J = 3.0 Hz, C=O), aromatic carbons: 135.4 (d, J = 3.0 Hz), 134.3 (d, J = 9.3 Hz), 130.6 (d, J = 12.3 Hz), 117.2 (d, J = 81.7 Hz), 49.6 (d, J = 53.4 Hz, CαH), 25.2 (d, J = 5.4 Hz, CH2), 22.3 (CH3C=O), and 11.5 (d, J = 14.1 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 26.3 ppm; IR (ATR) 3330, 1683, 1522, 1440, 1286, 1109, 1062, 1020, and 996 cm−1. The spectra reported here are in agreement with previously published data [24].
- 1-(N-benzoylamino)propyltriphenylphosphonium tetrafluoroborate (9b) [24]. White crystals (383.5 mg, 75%), mp 197–198 °C (lit.: mp 198–199 °C [24]). 1H NMR (400 MHz, CDCl3) δ 8.39 (dd, J = 8.2, 2.5 Hz, 1H, NH), 7.83–7.73 (m, 8H, Ph), 7.72–7.68 (m, 3H, Ph), 7.68–7.59 (m, 6H, Ph), 7.48–7.42 (m, 1H, Ph), 7.39–7.33 (m, 2H, Ph), 5.81 (dtd, J = 11.5, 8.3, 3.0 Hz, 1H, CαH), 2.54–2.37 (m, 1H, CHH), 1.89–1.77 (m, 1H, CHH), and 1.17 (td, J = 7.2, 1.2 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 168.5 (d, J = 2.3 Hz, C=O), aromatic carbons: 134.9 (d, J = 3.0 Hz), 134.4 (d, J = 9.4 Hz), 132.3, 131.7, 130.1 (d, J = 12.3 Hz), 128.6, 127.4, 117.9 (d, J = 81.7 Hz), 51.4 (d, J = 51.7 Hz, CαH), 24.9 (d, J = 5.2 Hz, CH2), and 11.7 (d, J = 13.9 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 27.0 ppm; IR (ATR) 3357, 1670, 1508, 1483, 1438, 1111, 1070, 1030, and 996 cm−1. The spectra reported here are in agreement with previously published data [24].
- 1-(N-benzyloxycarbonylamino)propyltriphenylphosphonium tetrafluoroborate (9c) [24]. White crystals (465.5 mg, 86%), mp 176–177 °C (lit.: mp 161–162 °C [24]). 1H NMR (400 MHz, CDCl3) δ 7.81–7.71 (m, 3H, Ph), 7.70–7.56 (m, 12H, Ph), 7.33–7.24 (m, 3H, Ph), 7.24–7.17 (m, 2H, Ph), 6.93 (d, J = 9.1 Hz, 1H, NH), 5.45–5.33 (m, 1H, CαH), 4.98, 4.89 (ABq, J = 12.5 Hz, 2H, CH2), 2.28–2.14 (m, 1H, CHH), 1.83–1.70 (m, 1H, CHH), and 1.15 (td, J = 7.2, 1.3 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 156.9 (d, J = 2.9 Hz, C=O), aromatic carbons: 136.1, 135.3 (d, J = 2.9 Hz), 134.3 (d, J = 9.3 Hz), 130.5 (d, J = 12.3 Hz), 128.6, 128.1, 128.0, 117.1 (d, J = 81.2 Hz), 67.4 (CH2Ph), 52.7 (d, J = 52.9 Hz, CαH), 25.0 (d, J = 6.2 Hz, CH2), and 11.5 (d, J = 13.9 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 25.6 ppm; IR (ATR) 3334, 1712, 1519, 1439, 1227, 1110, 1063, 1035, 1009, and 995 cm−1. The spectra reported here are in agreement with previously published data [24].
- 1-(2-Oxopyrrolidin-1-yl)propyltriphenylphosphonium tetrafluoroborate (9d) [24]. White crystals (366.0 mg, 77%), mp 189–191 °C (lit.: mp 186–188 °C [24]). 1H NMR (400 MHz, CDCl3) δ 7.91–7.80 (m, 3H, Ph), 7.79–7.65 (m, 12H, Ph), and 5.72 (ddd, J = 12.4, 10.5, 3.1 Hz, 1H, CαH), CH2 groups: 3.57–3.42 (m, 1H), 3.32–3.17 (m, 1H), 2.48–2.28 (m, 1H), 2.27–2.09 (m, 2H), 1.98–1.80 (m, 3H), and 1.07 (td, J = 7.2, 1.3 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 177.0 (d, J = 2.3 Hz, C=O), aromatic carbons: 135.6 (d, J = 3.1 Hz), 134.3 (d, J = 9.7 Hz), 130.8 (d, J = 12.4 Hz), 117.1 (d, J = 81.4 Hz), 53.3 (d, J = 51.4 Hz, CαH), 46.9 (CH2N), 30.3 (CH2), 22.8 (d, J = 5.0 Hz, CH2), 18.6 (CH2), and 11.5 (d, J = 14.1 Hz, CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 24.7 ppm; IR (ATR) 2880, 1692, 1509, 1438, 1405, 1271, 1109, 1047, 1035, and 997 cm−1. The spectra reported here are in agreement with previously published data [24].
- (N-Acetylamino)methyltriphenylphosphonium tetrafluoroborate (9e) [24,31]. White crystals (383.3 mg, 91%), mp 191–193 °C (lit.: mp 191–193 °C [24]). 1H NMR (400 MHz, CDCl3) δ 9.67 (t, J = 6.2 Hz, 1H, NH), 7.86–7.76 (m, 9H, Ph), 7.74–7.63 (m, 6H, Ph), 5.13 (dd, J = 6.3, 2.9 Hz, 2H, CH2), and 1.89 (d, J = 1.4 Hz, 3H, CH3) ppm; 13C{1H} NMR (100 MHz, CDCl3) δ 172.2 (d, J = 1.4 Hz, C=O), aromatic carbons: 135.3 (d, J = 3.1 Hz), 134.2 (d, J = 9.8 Hz), 130.3 (d, J = 12.6 Hz), 117.5 (d, J = 83.9 Hz), 37.6 (d, J = 56.8 Hz, CH2), and 22.6 (CH3) ppm; 31P{1H} NMR (161.9 MHz, CDCl3) δ 20.7 ppm; IR (ATR) 3356, 1671, 1508, 1483, 1437, 1111, 1071, 1030, and 996 cm−1. The spectra reported here are in agreement with previously published data [24,31].
- 2-Carbamoyletyhyltriphenylphosphonium tetrafluoroborate (10) [24]. White crystals (315.9 mg, 75%), mp 145–147 °C (lit.: mp 146–148 °C [24]). 1H NMR (400 MHz, CDCl3) δ 7.85–7.64 (m, 15H, Ph), 6.98 (br s, 1H, NH), 5.33 (br s, 1H, NH), 3.49–3.41 (m, 2H, CH2), and 2.85–2.78 (m, 2H); 13C{1H} NMR (100 MHz, CDCl3) δ 171.3 (d, J = 14.2 Hz), 135.4 (d, J = 3.0 Hz), 133.5 (d, J = 10.0 Hz), 130.7 (d, J = 12.7 Hz), 117.5 (d, J = 86.7 Hz), 27.3 (d, J = 2.9 Hz), and 19.1 (d, J = 56.0 Hz); 31P{1H} NMR (161,9 MHz, CDCl3) δ 24.6; IR (ATR) 3422, 3198, 1669, 1441, 1025, and 996 cm−1. The spectra reported here are in agreement with previously published data [24].
4. Conclusions
An efficient and convenient method for the preparation of 1-hydroxyalkylphosphonium salts was optimized and improved. This synthesis is based on the reaction of aldehydes with triarylphosphonium salts in a stoichiometric ratio under mild conditions: r.t., 30 min, without chromatography. The properties of the compounds obtained were determined on the basis of almost 50 examples, which also helped identify factors affecting their stability in solutions. The selected products were isolated and purified by crystallization from CH3CN/Et2O or CH2Cl2/Et2O and then reacted with aromatic systems (120 °C, 2 h) or amide-type substrates (amide/carbamate/lactam; 50 °C, 2 h). The two protocols, ‘step-by-step’ and ‘one-pot’, have been described and compared with each other. New products such as 1-arylalkylphosphonium salts and 1-(N-acylamino)alkylphosphonium salts were formed with good or very good yields.
This confirms the high reactivity of 1-hydroxyalkylphosphonium salts, which are not necessarily generated in situ.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29010018/s1, Supporting information includes apparatus for the synthesis; 1H-, 13C-, 31P-NMR, IR, and MS spectra of all new compounds; comparison of in-house results with available literature data [24]; Table S1. Characteristic signals (1H and 31P{1H} NMR) for the identification of compounds 1o-x; Table S2. Comparison of yields in one-pot and step-by-step methodology for the reactions of 1-hydroxyalkylphosphonium salts 1 with the amide-type substrates.
Author Contributions
Conceptualization, J.A.; Formal analysis, J.A., A.K., A.P.-H., M.G. and K.E.; Investigation, J.A., A.K., A.P.-H., M.G., D.K. and D.M.; Methodology, J.A.; Supervision, J.A.; Writing—original draft, J.A.; Writing—review & editing, J.A., A.K., A.P.-H. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Silesian University of Technology (Poland) BKM Grant No. 04/020/BKM23/1080. This research was also supported by the Rector’s Proquality Grant, Silesian University of Technology (Poland), No. 04/020/RGJ22/1041.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maryanoff, B.E.; Reitz, A.B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Johnson, A.W. Ylides and Imines of Phosphorus; A Wiley-Interscience Publication: New York, NY, USA, 1993; pp. 221–300. [Google Scholar]
- Kolodiazhnyi, O.I. Phosphorus Ylides; Wiley: Weinheim, Germany, 1999; pp. 359–539. [Google Scholar]
- Hilton, M.C.; Dolewski, R.D.; McNally, A. Selective Functionalization of Pyridines via Heterocyclic Phosphonium Salts. J. Am. Chem. Soc. 2016, 138, 13806–13809. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, J.-H.; Xiao, J.-C. Nucleophilic arylation with tetraarylphosphonium salts. Nat. Commun. 2016, 7, 10337. [Google Scholar] [CrossRef]
- Babu, K.N.; Massarwe, F.; Shioukhi, I.; Masarwa, A. Sequential Selective C-H and C(sp3)-+P Bond Functionalizations: An Entry to Bioactive Arylated Scaffolds. Angew. Chem. Int. Ed. 2021, 60, 26199–26209. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, M.; Wang, Y.; Man, X.; Lu, X.; Mou, Z.; Luo, Y.; Liang, H. Nickel-Catalyzed Reductive Csp2–Csp3 Cross Coupling Using Phosphonium Salts. Org. Lett. 2021, 23, 8183–8188. [Google Scholar] [CrossRef] [PubMed]
- Adamek, J.; Grymel, M.; Kuźnik, A.; Październiok-Holewa, A. 1-Aminoalkylphosphonium Derivatives: Smart Synthetic Equivalents of N-Acyliminium-Type Cations, and Maybe Something More: A Review. Molecules 2022, 27, 1562. [Google Scholar] [CrossRef] [PubMed]
- Selva, M.; Perosa, A.; Noè, M. Organophosphorous Chemistry; Tebby, J.C., Loakes, D., Allen, D.W., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2016; pp. 132–169. [Google Scholar]
- Werner, T. Phosphonium Salt Organocatalysis. Adv. Synth. Catal. 2009, 351, 1469–1481. [Google Scholar] [CrossRef]
- He, R.; Ding, C.; Marouka, K. Phosphonium salts as chiral phase-transfer catalysts: Asymmetric Michael and Mannich reactions of 3-aryloxindoles. Angew. Chem. 2009, 48, 4559–4561. [Google Scholar] [CrossRef]
- Bradaric, C.J.; Downard, A.; Kennedy, C.; Robertson, A.J.; Zhou, Y. Industrial preparation of phosphonium ionic liquids. Green Chem. 2003, 5, 143–152. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef]
- Zielonka, J.; Joseph, J.; Sikora, A.; Hardy, M.; Ouari, O.; Vasquez-Vivar, J.; Cheng, G.; Lopez, M.; Kalyanaraman, B. Mitochondria-Targeted Triphenylphosphonium-Based Compounds: Syntheses, Mechanisms of Action, and Therapeutic and Diagnostic Applications. Chem. Rev. 2017, 117, 10043–10120. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H. Umsetzung von Triphenylphosphin mit Aldehyden. Angew. Chem. 1960, 72, 77. [Google Scholar] [CrossRef]
- Wittig, G.; Schlosser, M. Über die Herstellung von Vinyläthern, Vinylthioäthern und Vinylhalogeniden auf der Phosphylen-Basis; IV. Mitteil. Über Phosphin-alkylene als olefinbildende Reagenzien. Chem. Ber. 1961, 94, 1373–1383. [Google Scholar] [CrossRef]
- Hellmann, H.; Bader, J.; Birkner, H.; Schumacher, O. Hydroxymethyl-phosphine, Hydroxymethyl-phosphoniumsalze und Chlormethyl-phosphoniumsalze. Liebigs Ann. Chem. 1962, 659, 49–63. [Google Scholar] [CrossRef]
- Henderson, W.; Olsen, G.M. Application of electrospray mass spectrometry to the characterization of hydroxymethylphosphonium salts, -phosphines, and their oxide, sulfide and selenide derivatives. Polyhedron 1996, 15, 2105–2115. [Google Scholar] [CrossRef]
- Allen, D.W.; Millar, I.T. Unusually low PCH coupling constants in the nuclear magnetic resonance spectra of phosphonium salts and phosphine oxides. Tetrahedron Lett. 1968, 9, 745–750. [Google Scholar] [CrossRef]
- Anders, E.; Gaβner, T.; Stankowiak, A. [1-(Aryl-bzw. Alkylcarbonyloxy)alkyl]phosphonium-Salze]—Herstellungsmethoden. Chem. Ber. 1985, 118, 124–131. [Google Scholar] [CrossRef]
- Burton, D.J.; Wiemers, D.M. A practical synthesis of fluoromethyltriphenylphosphonium salts. J. Fluor. Chem. 1985, 27, 85–89. [Google Scholar] [CrossRef]
- Davis, M.C.; Parrish, D.A. Synthesis of 4-(N,N-Dialkylamino)benzyltriphenylphosphonium Iodides from Hydroxymethyltriphenylphosphonium Iodide and N,N-Dialkylaniline. Synth. Commun. 2008, 38, 3909–3918. [Google Scholar] [CrossRef]
- Dal Canto, R.A.; Roskamp, E.J. Addition of triphenylphosphonium salts to aldehydes. Remarkable counterion effects on phosphorus proton couplings. J. Org. Chem. 1992, 57, 406–407. [Google Scholar] [CrossRef]
- Adamek, J.; Zielezny, P.; Erfurt, K. Synthesis of N-Protected 1-Aminoalkylphosphonium Salts from Amides, Carbamates, Lactams, or Imides. J. Org. Chem. 2021, 86, 5852–5862. [Google Scholar] [CrossRef] [PubMed]
- Hazra, G.; Masarwa, A. Synthesis and Functionalization of Thiophosphonium Salts: A Divergent Approach to Access Thioether, Thioester, and Dithioester Derivatives. Org. Lett. 2023, 25, 6396–6400. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, B.D.; Williams, C.M.; Anders, E.; Bernhardt, P.V. Exploiting the Anders–Gaßner variant on the Wittig reaction: New methodology for the synthesis of 3,3-dimethylacroyl enol esters. Tetrahedron 2008, 64, 6482–6487. [Google Scholar] [CrossRef]
- Huang, W.; Xu, J. In Situ Generation of Formaldehyde and Triphenylphosphine from (Hydroxymethyl)triphenylphosphonium and Its Application in Wittig Olefination. Synth. Commun. 2015, 45, 1777–1782. [Google Scholar] [CrossRef]
- Konrath, R.; Sekine, K.; Jevtovikj, I.; Paciello, R.A.; Hashmi, A.S.K.; Schaub, T. Performance enhancing additives for reusable ruthenium-triphos catalysts in the reduction of CO2 to dimethoxymethane. Green Chem. 2020, 22, 6464–6470. [Google Scholar] [CrossRef]
- Seyferth, D.; Heeren, J.K.; Singh, G.; Grim, S.O.; Hughes, W.B. Studies in phosphinemethylene chemistry: XIII. Routes to triphenylphosphine-halomethylenes and -dihalomethylenes. J. Organomet. Chem. 1966, 5, 267–274. [Google Scholar] [CrossRef]
- Adamek, J.; Węgrzyk, A.; Krawczyk, M.; Erfurt, K. Catalyst-free Friedel-Crafts reaction of 1-(N-acylamino)alkyltriarylphosphonium salts with electron-rich arenes. Tetrahedron 2018, 74, 2575–2583. [Google Scholar] [CrossRef]
- Adamek, J.; Mrowiec-Białoń, J.; Październiok-Holewa, A.; Mazurkiewicz, R. Thermogravimetrical investigations of the dealkoxycarbonylation of N-acyl-α-triphenylphosphonioglycinates. Thermochim. Acta 2011, 512, 22–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).