Hydrogels Based on Natural Polymers Loaded with Bentonite and/or Halloysite: Composition Impact on Spectroscopic, Thermal, and Swelling Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. GGLA–ST, GGLA–ST–BET, and GGLA–ST–HAL Hydrogels
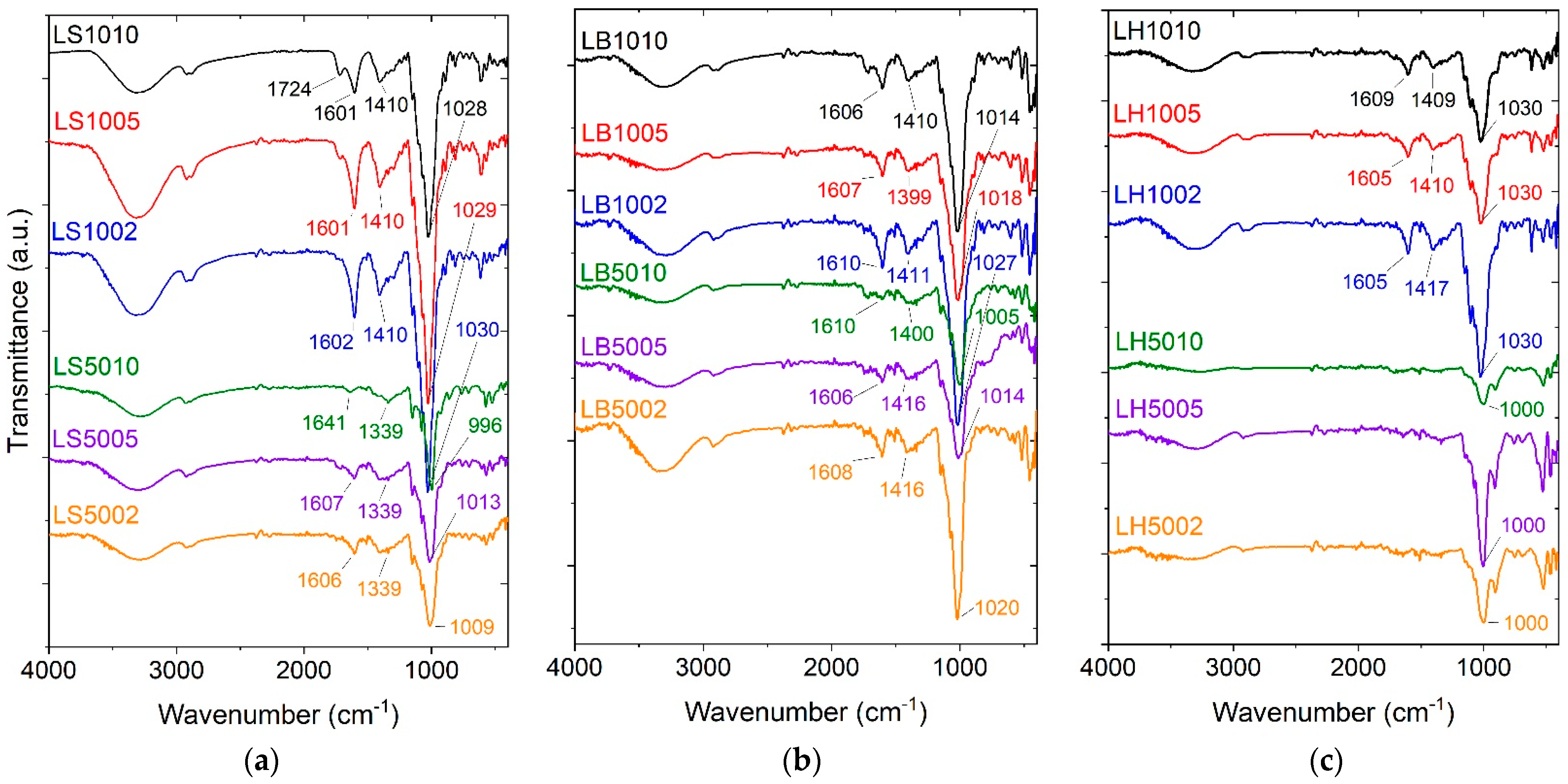
2.2. Fourier-Transform Infrared Spectroscopy (FT-IR)
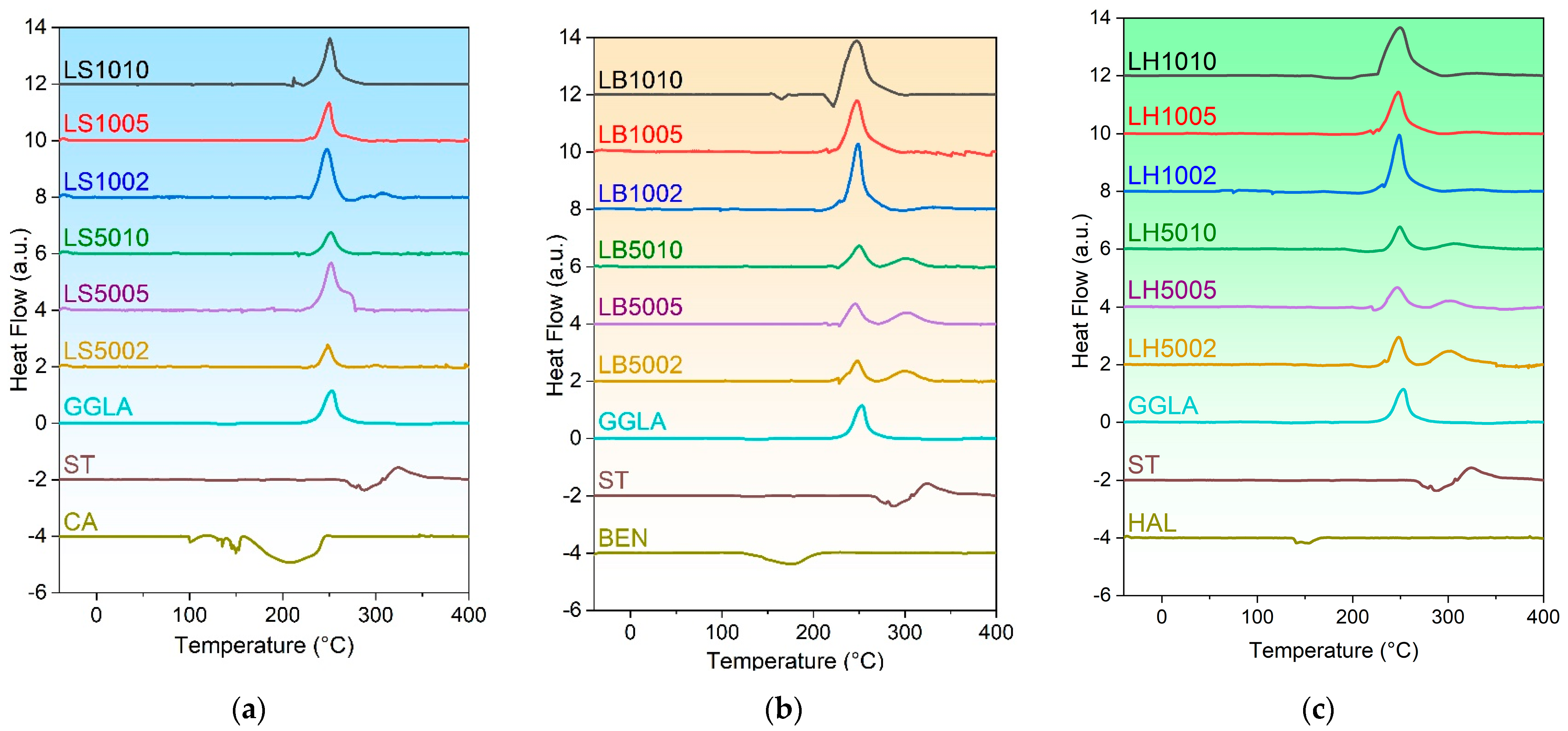
2.3. Differential Scanning Calorimetry (DSC)
2.4. Swelling Degree
2.5. State of Bound Water
3. Materials and Methods
3.1. Materials
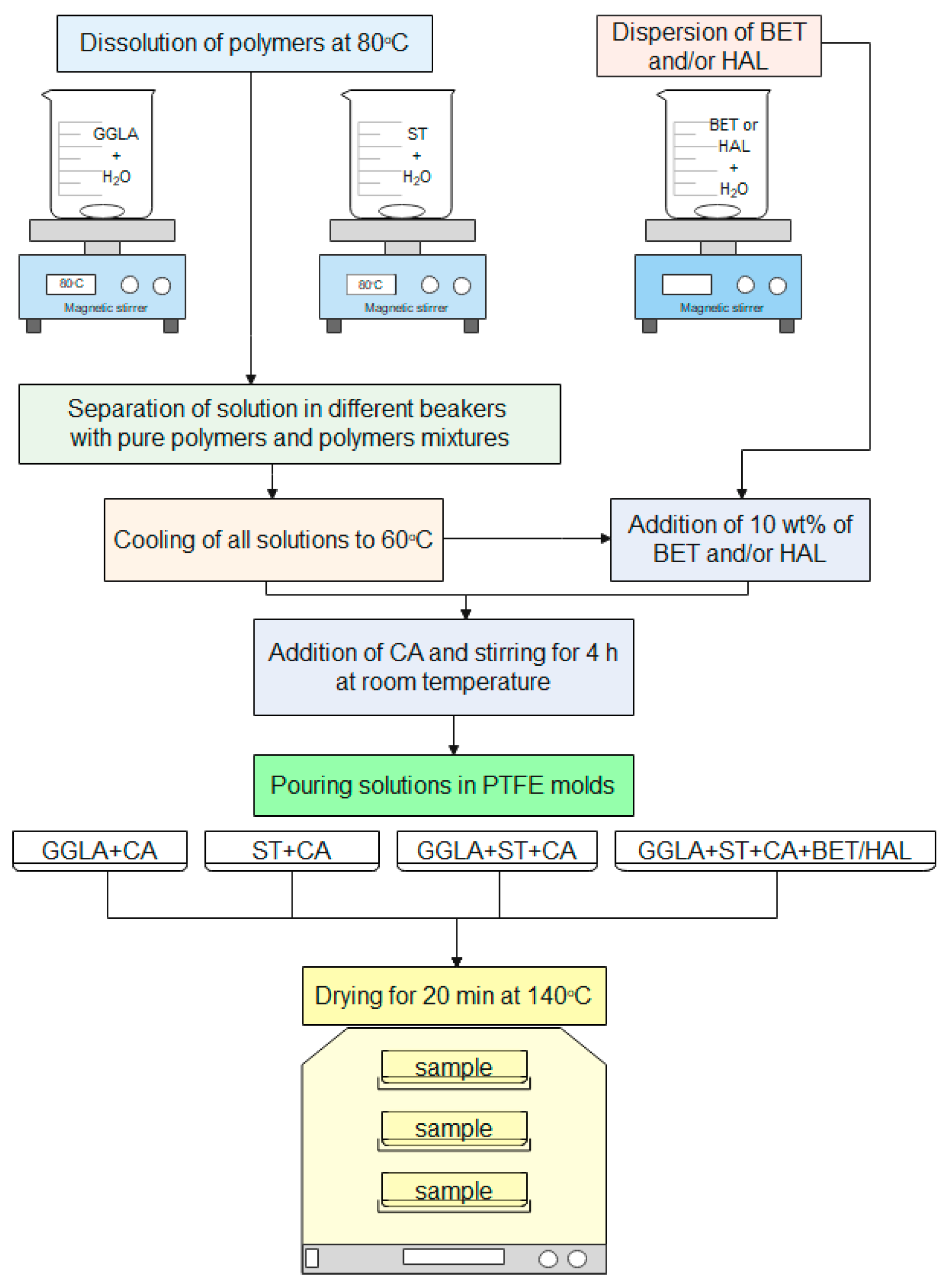
3.2. Hydrogel Synthesis
3.3. Nanocomposite Hydrogels Characterization
3.3.1. Infrared Spectroscopy
3.3.2. Thermal Analyses
3.3.3. Swelling Degree
3.3.4. State of Bound Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Altieri, M.A.; Toledo, V.M. The agroecological revolution in Latin America: Rescuing nature, ensuring food sovereignty and empowering peasants. J. Peasant. Stud. 2011, 38, 587–612. [Google Scholar] [CrossRef]
- Ni, B.; Liu, M.; Lu, S.; Xie, L.; Wang, Y. Environmentally friendly slow-release nitrogen fertilizer. J. Agric. Food Chem. 2011, 59, 10169–10175. [Google Scholar] [CrossRef] [PubMed]
- Davidson, D.; Gu, F.X. Materials for sustained and controlled release of nutrients and molecules to support plant growth. J. Agric. Food Chem. 2012, 60, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Pourjalili, N.; Bagheri Marandi, G.; Kurdtabar, M.; Rezanejade Bardajee, G. Synthesis and characterization of double network hydrogel based on gellan-gum for drug delivery. J. Macromol. Sci. Part A 2022, 59, 537–549. [Google Scholar] [CrossRef]
- An, Y.; Zhai, R.; Chen, J.; Xie, P. Preparation and application of a novel pH-responsive linalool carboxymethyl chitosan hydrogel. J. Macromol. Sci. Part A 2023, 60, 336–345. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, J.; Cai, W.; Li, J.; Kong, Y.; Zhou, M. Synthesis of oxidized carboxymethyl cellulose/chitosan hydrogels doped with graphene oxide for pH-and NIR-responsive drug delivery. Eur. Polym. J. 2023, 199, 112437. [Google Scholar] [CrossRef]
- Fan, L.; Tan, C.; Wang, L.; Pan, X.; Cao, M.; Wen, F.; Xie, W.; Nie, M. Preparation, characterization and the effect of carboxymethylated chitosan–cellulose derivatives hydrogels on wound healing. J. Appl. Polym. Sci. 2013, 128, 2789–2796. [Google Scholar] [CrossRef]
- Pal, K.; Banthia, A.; Majumdar, D.K. Polymeric hydrogels: Characterization and biomedical applications. Des. Monomers Polym. 2009, 12, 197–220. [Google Scholar] [CrossRef]
- Sabadini, R.C.; Martins, V.C.A.; Pawlicka, A. Synthesis and characterization of gellan gum: Chitosan biohydrogels for soil humidity control and fertilizer release. Cellulose 2015, 22, 2045–2054. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.-H.; Lin, C.-X.; Tong, D.-S.; Yu, W.-H. Synthesis of clay minerals. Appl. Clay Sci. 2010, 50, 1–11. [Google Scholar] [CrossRef]
- Lagaly, G.; Ogawa, M.; Dékány, I. Clay mineral organic interactions. Dev. Clay Sci. 2006, 1, 309–377. [Google Scholar] [CrossRef]
- Coelho, A.C.V.; Santos, P.D.S.; Santos, H.D.S. Argilas especiais: Argilas quimicamente modificadas-uma revisão. Química Nova 2007, 30, 1282–1294. [Google Scholar] [CrossRef]
- Wang, C.; He, Z.; Liu, Y.; Zhou, C.; Jiao, J.; Li, P.; Sun, D.; Lin, L.; Yang, Z. Chitosan-modified halloysite nanotubes as a controlled-release nanocarrier for nitrogen delivery. Appl. Clay Sci. 2020, 198, 105802. [Google Scholar] [CrossRef]
- Wei, H.; Wang, H.; Chu, H.; Li, J. Preparation and characterization of slow-release and water-retention fertilizer based on starch and halloysite. Int. J. Biol. Macromol. 2019, 133, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Mynar, J.L.; Yoshida, M.; Lee, E.; Lee, M.; Okuro, K.; Kinbara, K.; Aida, T. High-water-content mouldable hydrogels by mixing clay and a dendritic molecular binder. Nature 2010, 463, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A.; Salari, D.; Reyhanitabar, A. On the preparation and swelling properties of hydrogel nanocomposite based on Sodium alginate-g-Poly (acrylic acid-co-acrylamide)/Clinoptilolite and its application as slow release fertilizer. J. Polym. Res. 2014, 21, 344. [Google Scholar] [CrossRef]
- Wang, Q.; Gao, Z. A constitutive model of nanocomposite hydrogels with nanoparticle crosslinkers. J. Mech. Phys. Solids 2016, 94, 127–147. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mat. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, X.; Ke, L.; Zhang, S.; Zhao, D.; Chen, H.; Xiao, X. Polysaccharides/mesoporous silica nanoparticles hybrid composite hydrogel beads for sustained drug delivery. J. Mater. Sci. 2017, 52, 3095–3109. [Google Scholar] [CrossRef]
- Barabadi, Z.; Azami, M.; Sharifi, E.; Karimi, R.; Lotfibakhshaiesh, N.; Roozafzoon, R.; Joghataei, M.T.; Ai, J. Fabrication of hydrogel based nanocomposite scaffold containing bioactive glass nanoparticles for myocardial tissue engineering. Mater. Sci. Eng. C 2016, 69, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Moatary, A.; Teimouri, A.; Bagherzadeh, M.; Chermahini, A.N.; Razavizadeh, R. Design and fabrication of novel chitin hydrogel/chitosan/nano diopside composite scaffolds for tissue engineering. Ceram. Int. 2017, 43, 1657–1668. [Google Scholar] [CrossRef]
- Mittal, H.; Kumar, V.; Ray, S.S. Adsorption of methyl violet from aqueous solution using gum xanthan/Fe3O4 based nanocomposite hydrogel. Int. J. Biol. Macromol. 2016, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rokicińska, A.; Natkański, P.; Dudek, B.; Drozdek, M.; Lityńska-Dobrzyńska, L.; Kuśtrowski, P. Co3O4-pillared montmorillonite catalysts synthesized by hydrogel-assisted route for total oxidation of toluene. Appl. Catal. B Environ. 2016, 195, 59–68. [Google Scholar] [CrossRef]
- Dhibar, S.; Kar, P.; Khatua, B. Preparation of highly exfoliated and transparent polycarbonate/clay nanocomposites by melt blending of polycarbonate and poly (methyl methacrylate)/clay nanocomposites. J. Appl. Polym. Sci. 2012, 125, E601–E609. [Google Scholar] [CrossRef]
- Merino, S.; Martín, C.; Kostarelos, K.; Prato, M.; Vázquez, E. Nanocomposite hydrogels: 3D polymer–nanoparticle synergies for on-demand drug delivery. ACS Nano 2015, 9, 4686–4697. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Zohuriaan-Mehr, M.; Doroudiani, S. Superabsorbent hydrogel composites and nanocomposites: A review. Polym. Compos. 2011, 32, 277–289. [Google Scholar] [CrossRef]
- Zeng, Q.; Yu, A.; Lu, G.; Paul, D. Clay-based polymer nanocomposites: Research and commercial development. J. Nanosci. Nanotechnol. 2005, 5, 1574–1592. [Google Scholar] [CrossRef]
- Reddy, N.; Yang, Y. Citric acid cross-linking of starch films. Food Chem. 2010, 118, 702–711. [Google Scholar] [CrossRef]
- Widsten, P.; Dooley, N.; Parr, R.; Capricho, J.; Suckling, I. Citric acid crosslinking of paper products for improved high-humidity performance. Carbohydr. Polym. 2014, 101, 998–1004. [Google Scholar] [CrossRef]
- Zhou, J.; Tong, J.; Su, X.; Ren, L. Hydrophobic starch nanocrystals preparations through crosslinking modification using citric acid. Int. J. Biol. Macromol. 2016, 91, 1186–1193. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Ye, Z.; Ye, Z.; Deng, J.; Lin, J.; Wu, C.; Zhu, H. Citric acid crosslinked sphingan WL gum hydrogel films supported ciprofloxacin for potential wound dressing application. Carbohydr. Polym. 2022, 291, 119520. [Google Scholar] [CrossRef] [PubMed]
- Sabadini, R.C.; Silva, M.M.; Pawlicka, A.; Kanicki, J. Gellan gum–O,O′-bis(2-aminopropyl)-polyethylene glycol hydrogel for controlled fertilizer release. J. Appl. Polym. Sci. 2018, 135, 45636. [Google Scholar] [CrossRef]
- Fernández-Pérez, M.; Villafranca-Sanchez, M.; Gonzalez-Pradas, E.; Martinez-Lopez, F.; Flores-Cespedes, F. Controlled release of carbofuran from an alginate− bentonite formulation: Water release kinetics and soil mobility. J. Agric. Food Chem. 2000, 48, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Mohawesh, O.; Durner, W. Effects of bentonite, hydrogel and biochar amendments on soil hydraulic properties from saturation to oven dryness. Pedosphere 2019, 29, 598–607. [Google Scholar] [CrossRef]
- Vergaro, V.; Abdullayev, E.; Lvov, Y.M.; Zeitoun, A.; Cingolani, R.; Rinaldi, R.; Leporatti, S. Cytocompatibility and uptake of halloysite clay nanotubes. Biomacromolecules 2010, 11, 820–826. [Google Scholar] [CrossRef]
- Romainor, A.N.B.; Chin, S.F.; Pang, S.C.; Bilung, L.M. Preparation and characterization of chitosan nanoparticles-doped cellulose films with antimicrobial property. J. Nanomater. 2014, 2014, 710459. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, C.-W.; Wang, R.-Z.; Yang, L.; Du, S.-S.; Wang, F.-P.; Ruan, H.; He, G.-Q. Lipase-coupling esterification of starch with octenyl succinic anhydride. Carbohydr. Polym. 2012, 87, 2137–2144. [Google Scholar] [CrossRef]
- Castro-Cabado, M.; Parra-Ruiz, F.J.; Casado, A.; Román, J.S. Thermal crosslinking of maltodextrin and citric acid. Methodology to control the polycondensation reaction under processing conditions. Polym. Polym. Compos. 2016, 24, 643–654. [Google Scholar] [CrossRef]
- Cui, Z.; Beach, E.S.; Anastas, P.T. Modification of chitosan films with environmentally benign reagents for increased water resistance. Green Chem. Lett. Rev. 2011, 4, 35–40. [Google Scholar] [CrossRef]
- Noor, I.S.M.; Majid, S.R.; Arof, A.K.; Djurado, D.; Pawlicka, A. Characteristics of gellan gum—LiCF3SO3 polymer electrolyte. Solid. State Ion. 2012, 225, 649–653. [Google Scholar] [CrossRef]
- Ruiz-Caro, R.; Veiga-Ochoa, M.D. Characterization and dissolution study of chitosan freeze-dried systems for drug controlled release. Molecules 2009, 14, 4370–4386. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska-Czubenko, J.; Gierszewska-Druzynska, M. Effect of ionic crosslinking on the water state in hydrogel chitosan membranes. Carbohydr. Polym. 2009, 77, 590–598. [Google Scholar] [CrossRef]
- Don, T.-M.; King, C.-F.; Chiu, W.-Y.; Peng, C.-A. Preparation and characterization of chitosan-g-poly (vinyl alcohol)/poly (vinyl alcohol) blends used for the evaluation of blood-contacting compatibility. Carbohydr. Polym. 2006, 63, 331–339. [Google Scholar] [CrossRef]
- Menzel, C.; Olsson, E.; Plivelic, T.S.; Andersson, R.; Johansson, C.; Kuktaite, R.; Järnström, L.; Koch, K. Molecular structure of citric acid cross-linked starch films. Carbohydr. Polym. 2013, 96, 270–276. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Y.; Lin, J.; Lin, S. Study on starch-graft-acrylamide/mineral powder superabsorbent composite. Polymer 2003, 44, 6513–6520. [Google Scholar] [CrossRef]
- Sabadini, R.C.; Fernandes, M.; de Zea Bermudez, V.; Pawlicka, A.; Silva, M.M. Eco-friendly superabsorbent hydrogels based on starch, gellan gum, citric acid, and nanoclays for soil humidity control. J. Appl. Polym. Sci. 2022, 139, e52998. [Google Scholar] [CrossRef]
- Sabadini, R.C.; Raphael, E.; Marques, S.T.; Berci Filho, P.; Pawlicka, A. Alginate-Jeffamine covalently crosslinked hydrogel. Mol. Cryst. Liq. Cryst. 2014, 604, 240–247. [Google Scholar] [CrossRef]
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 112–117. [Google Scholar]
- Zohuriaan-Mehr, M.; Kabiri, K. Superabsorbent polymer materials: A review. Iran. Polym. J. 2008, 17, 451–477. [Google Scholar]
- Yoshida, H.; Hatakeyama, T.; Hatakeyama, H. Characterization of water in polysaccharide hydrogels by DSC. J. Therm. Anal. Calorim. 1993, 40, 483–489. [Google Scholar] [CrossRef]
- Alimi, B.A.; Workneh, T.S. Structural and physicochemical properties of heat moisture treated and citric acid modified acha and iburu starches. Food Hydrocoll. 2018, 81, 449–455. [Google Scholar] [CrossRef]
- Coutinho, D.F.; Sant, S.V.; Shin, H.; Oliveira, J.T.; Gomes, M.E.; Neves, N.M.; Khademhosseini, A.; Reis, R.L. Modified gellan gum hydrogels with tunable physical and mechanical properties. Biomaterials 2010, 31, 7494–7502. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyema, T.; Yamauchi, A.; Hatakeyema, H. Studies on bound water in poly (vinyl alcohol). Hydrogel by DSC and FT-NMR. Eur. Polym. J. 1984, 20, 61–64. [Google Scholar] [CrossRef]
- Ostrowska-Czubenko, J.; Pieróg, M.; Gierszewska-Drużyńska, M. Water state in chemically and physically crosslinked chitosan membranes. J. Appl. Polym. Sci. 2013, 130, 1707–1715. [Google Scholar] [CrossRef]
- Androsits, B. Thermal Analysis Fundamentals and Applications and Polymer Science Editors: T. Hatakeyama and FX Quinn. J. Therm. Anal. Calorim. 1999, 58, 237. [Google Scholar] [CrossRef]

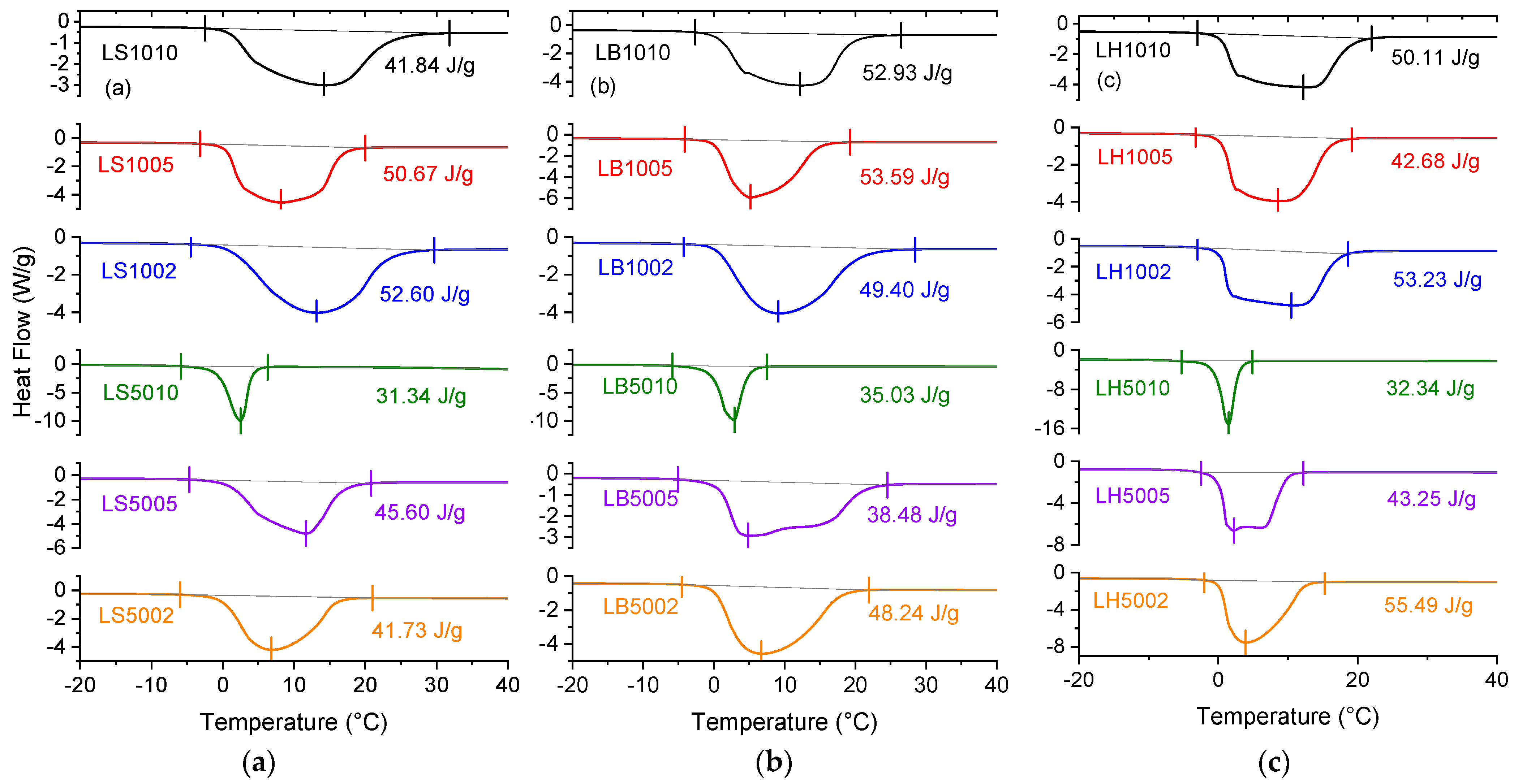
| Sample | S | ΔHm (J/g) | wf (g/g) | wnf (g/g) |
|---|---|---|---|---|
| LS1010 | 28 | 41.84 | 0.12 | 0.88 |
| LS1005 | 31 | 50.67 | 0.15 | 0.85 |
| LS1002 | 32 | 52.60 | 0.16 | 0.84 |
| LS5010 | 4 | 31.34 | 0.09 | 0.91 |
| LS5005 | 10 | 45.60 | 0.14 | 0.86 |
| LS5002 | 18 | 41.73 | 0.12 | 0.84 |
| LB1010 | 14 | 52.93 | 0.16 | 0.84 |
| LB1005 | 17 | 53.59 | 0.16 | 0.84 |
| LB1002 | 22 | 49.40 | 0.15 | 0.85 |
| LB5010 | 3 | 35.03 | 0.10 | 0.90 |
| LB5005 | 16 | 38.48 | 0.11 | 0.89 |
| LB5002 | 18 | 48.24 | 0.14 | 0.86 |
| LH1010 | 28 | 50.11 | 0.15 | 0.85 |
| LH1005 | 22 | 42.68 | 0.13 | 0.87 |
| LH1002 | 29 | 53.23 | 0.16 | 0.84 |
| LH5010 | 3 | 32.34 | 0.10 | 0.90 |
| LH5005 | 6 | 43.25 | 0.13 | 0.87 |
| LH5002 | 21 | 55.49 | 0.17 | 0.83 |
| Sample Name: LXYYZZ | |
|---|---|
| X | S—no BET and/or HAL, B—bentonite, and H—halloysite |
| YY | Gellan gum (%): 00 = 0%, 10 = 100%, 75 = 75%, 50 = 50%, and 25 = 25% |
| ZZ | CA (%): 02 = 2%, 05 = 5%, and 10 = 10% |
| Sample Name | GGLA (wt.%) | ST (wt.%) | CA (wt.%) |
|---|---|---|---|
| LX1010 | 100 | 0 | 10 |
| LX1005 | 100 | 0 | 5 |
| LX1002 | 100 | 0 | 2 |
| LX7510 | 75 | 25 | 10 |
| LX7505 | 75 | 25 | 5 |
| LX7502 | 75 | 25 | 2 |
| LX5010 | 50 | 50 | 10 |
| LX5005 | 50 | 50 | 5 |
| LX5002 | 50 | 50 | 2 |
| LX2510 | 25 | 75 | 10 |
| LX2505 | 25 | 75 | 5 |
| LX2502 | 25 | 75 | 2 |
| LX0010 | 0 | 100 | 10 |
| LX0005 | 0 | 100 | 5 |
| LX0002 | 0 | 100 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabadini, R.C.; Fernandes, M.; Bermudez, V.d.Z.; Pawlicka, A.; Silva, M.M. Hydrogels Based on Natural Polymers Loaded with Bentonite and/or Halloysite: Composition Impact on Spectroscopic, Thermal, and Swelling Properties. Molecules 2024, 29, 131. https://doi.org/10.3390/molecules29010131
Sabadini RC, Fernandes M, Bermudez VdZ, Pawlicka A, Silva MM. Hydrogels Based on Natural Polymers Loaded with Bentonite and/or Halloysite: Composition Impact on Spectroscopic, Thermal, and Swelling Properties. Molecules. 2024; 29(1):131. https://doi.org/10.3390/molecules29010131
Chicago/Turabian StyleSabadini, Rodrigo César, Mariana Fernandes, Verónica de Zea Bermudez, Agnieszka Pawlicka, and Maria Manuela Silva. 2024. "Hydrogels Based on Natural Polymers Loaded with Bentonite and/or Halloysite: Composition Impact on Spectroscopic, Thermal, and Swelling Properties" Molecules 29, no. 1: 131. https://doi.org/10.3390/molecules29010131
APA StyleSabadini, R. C., Fernandes, M., Bermudez, V. d. Z., Pawlicka, A., & Silva, M. M. (2024). Hydrogels Based on Natural Polymers Loaded with Bentonite and/or Halloysite: Composition Impact on Spectroscopic, Thermal, and Swelling Properties. Molecules, 29(1), 131. https://doi.org/10.3390/molecules29010131