Polyethylenimine Grafted onto Nano-NiFe2O4@SiO2 for the Removal of CrO42−, Ni2+, and Pb2+ Ions from Aqueous Solutions
Abstract
:1. Introduction
2. Results and Discussion
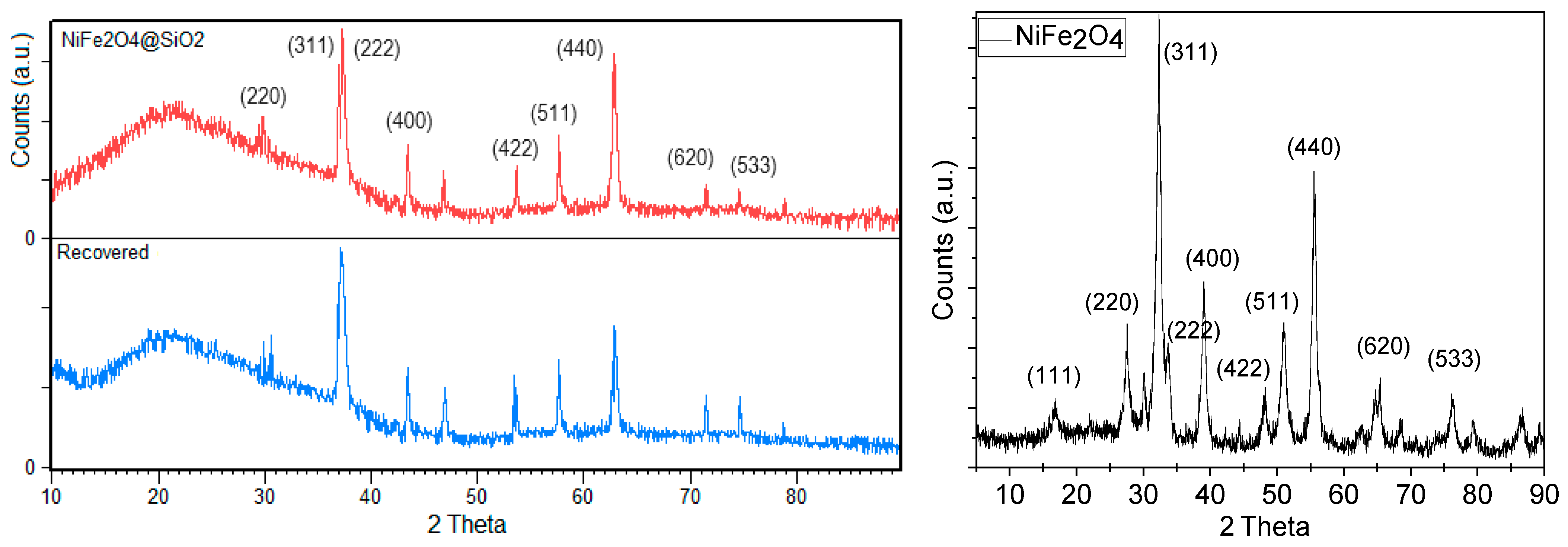
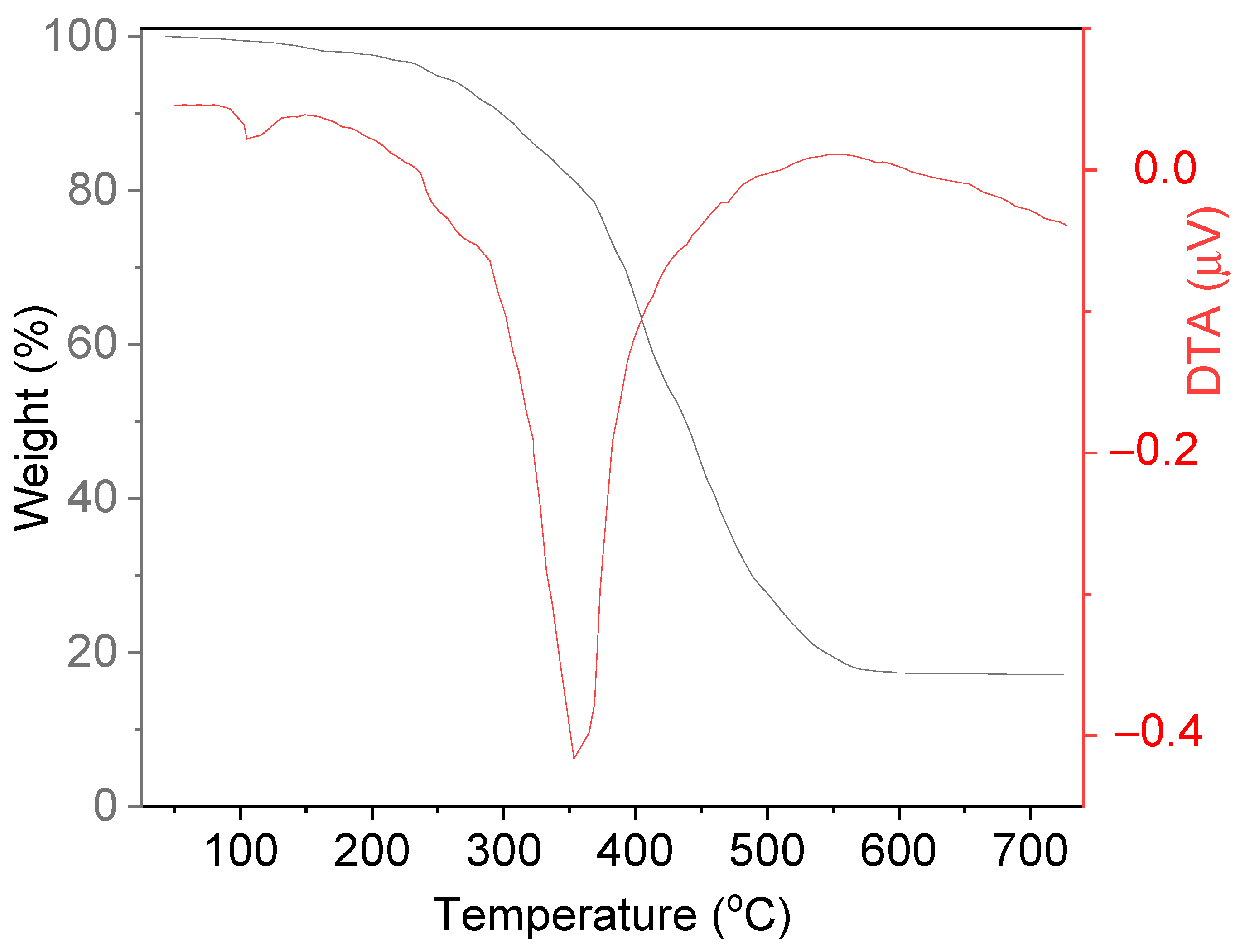
2.1. Characterization of the Adsorbent
2.2. Adsorption Studies for CrO42−, Ni2+, and Pb2+ Ions
2.2.1. Effect of pH
2.2.2. Effect of Contact Time
2.3. Adsorption Kinetics and Mechanism
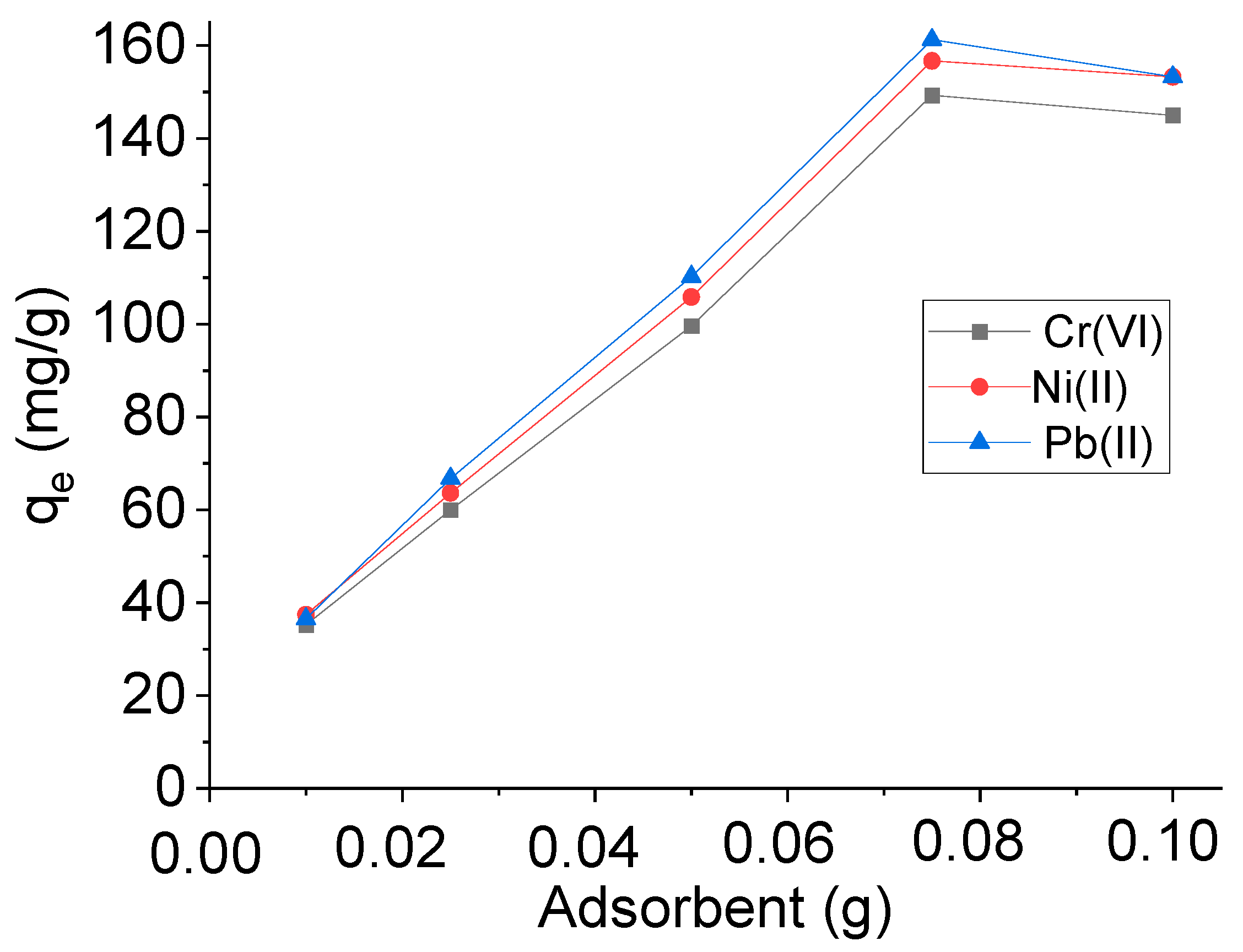
2.3.1. Effect of the Amount of Adsorbent
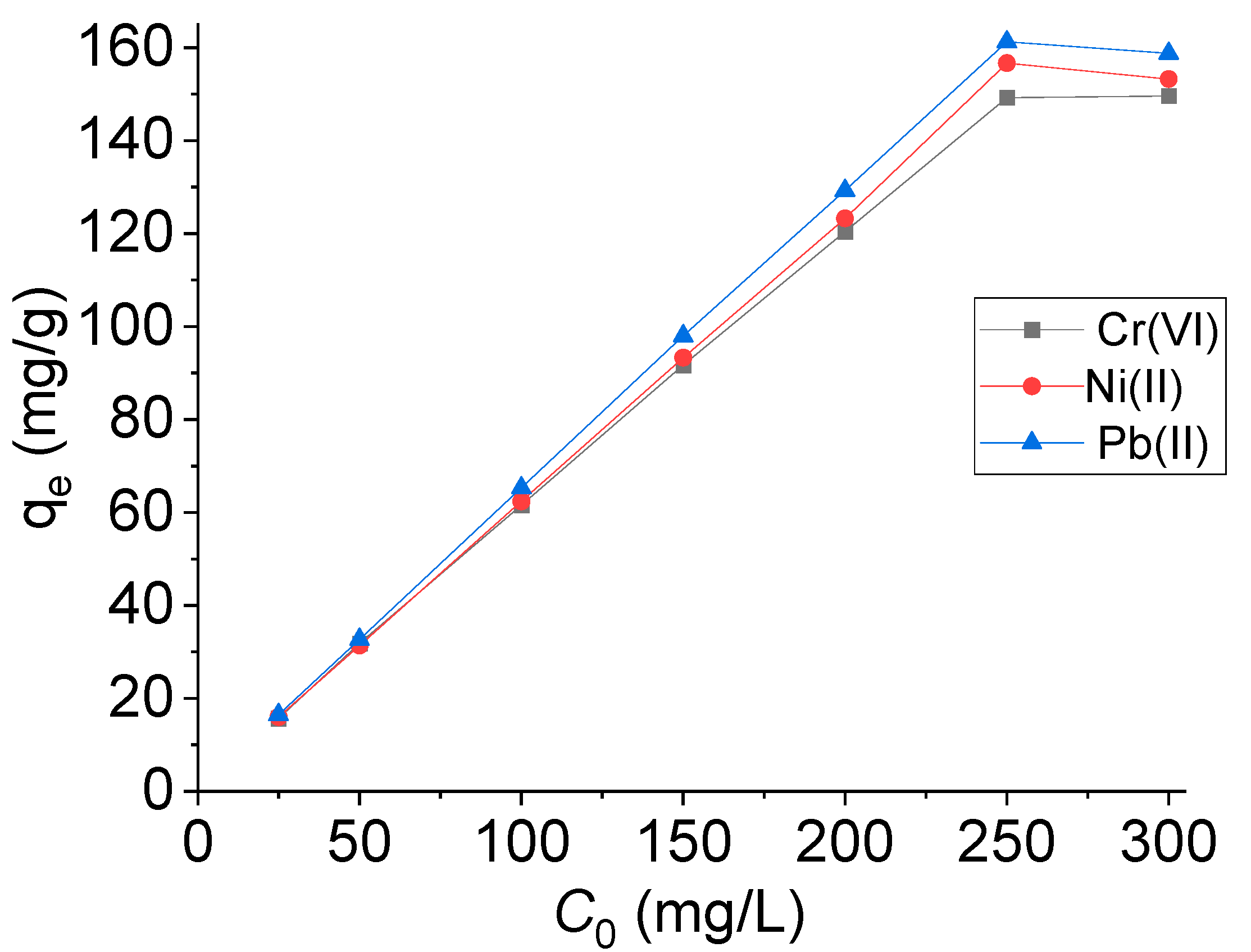
2.3.2. Effect of the Metal Ion Concentration
2.4. Adsorption Isotherms
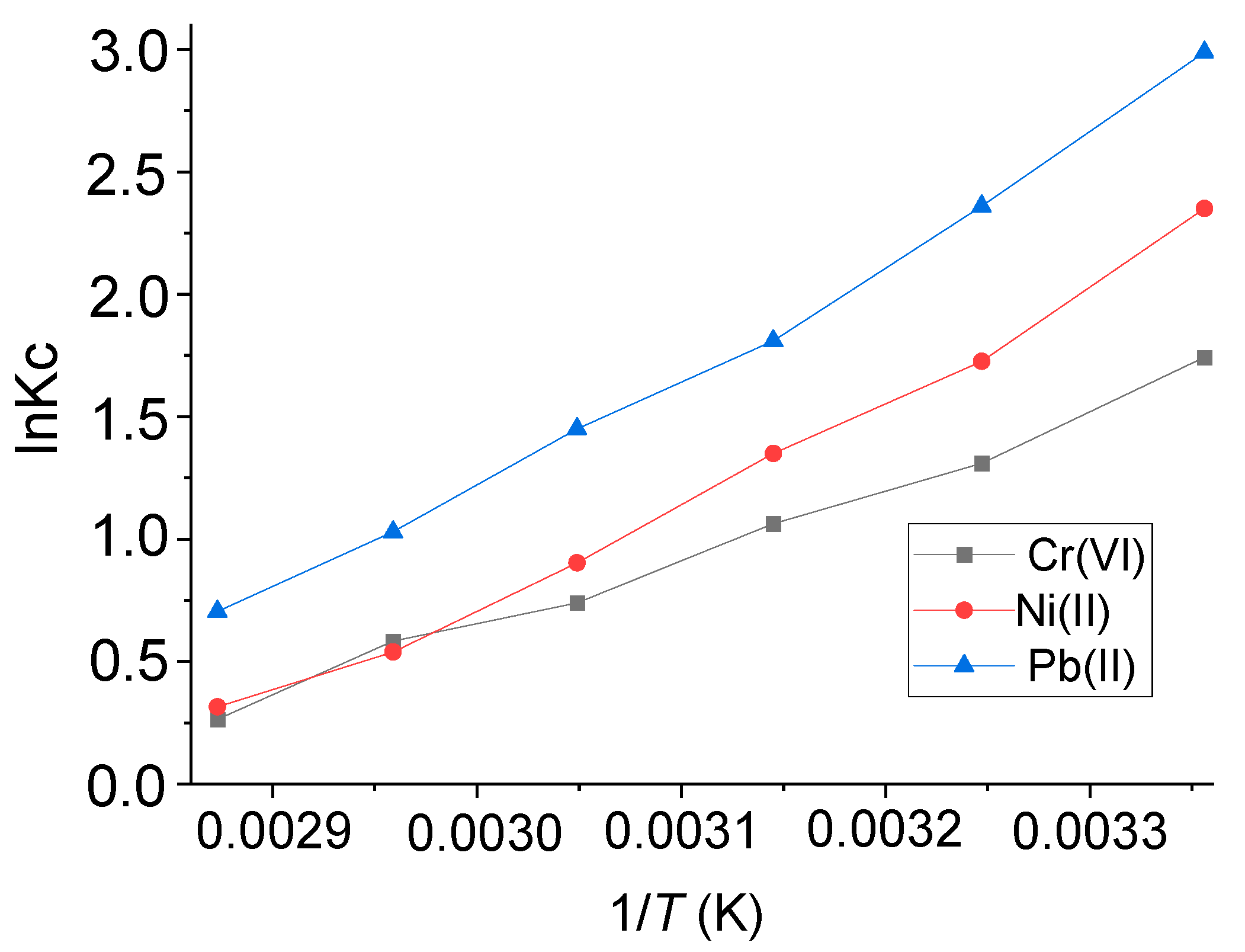
2.5. Adsorption Thermodynamics
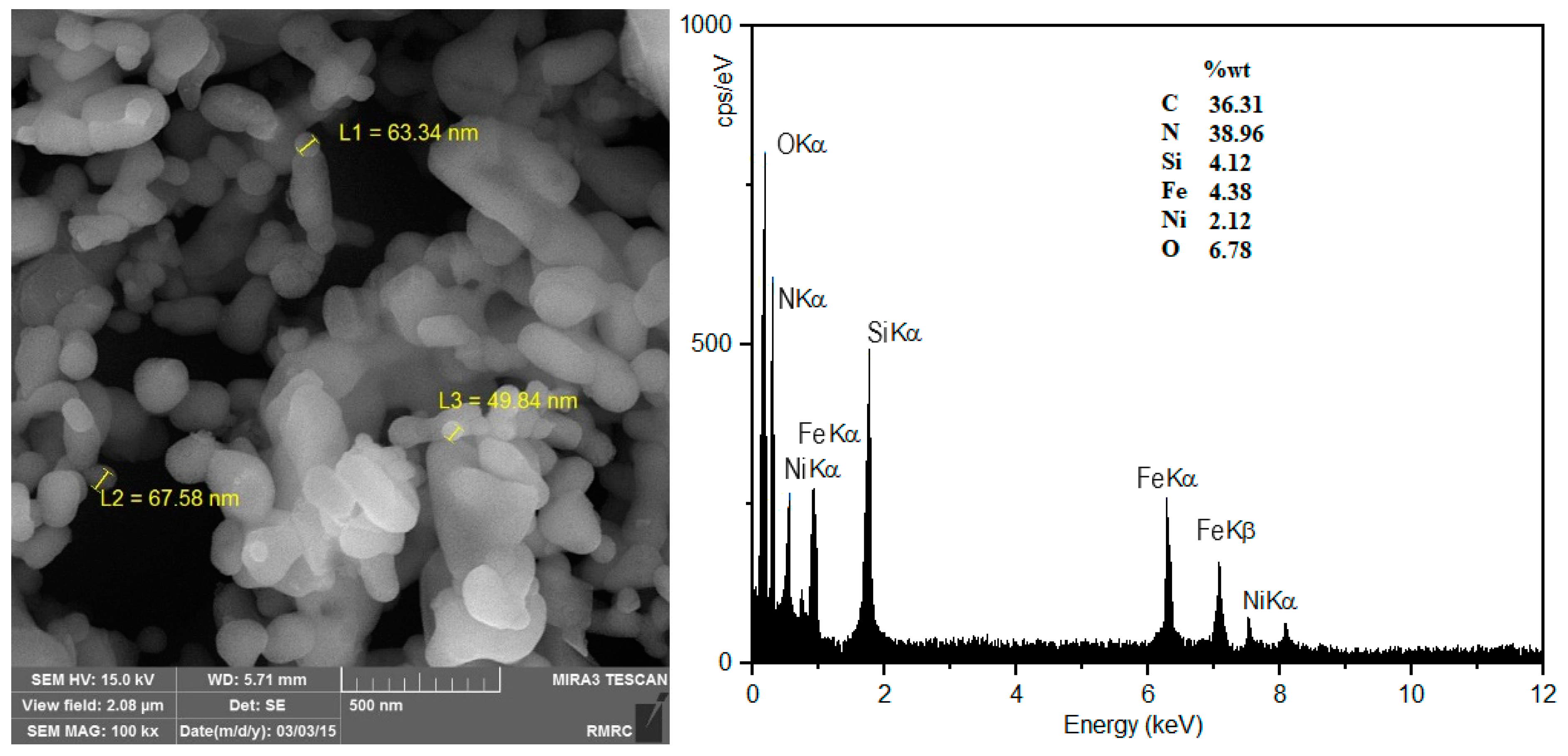
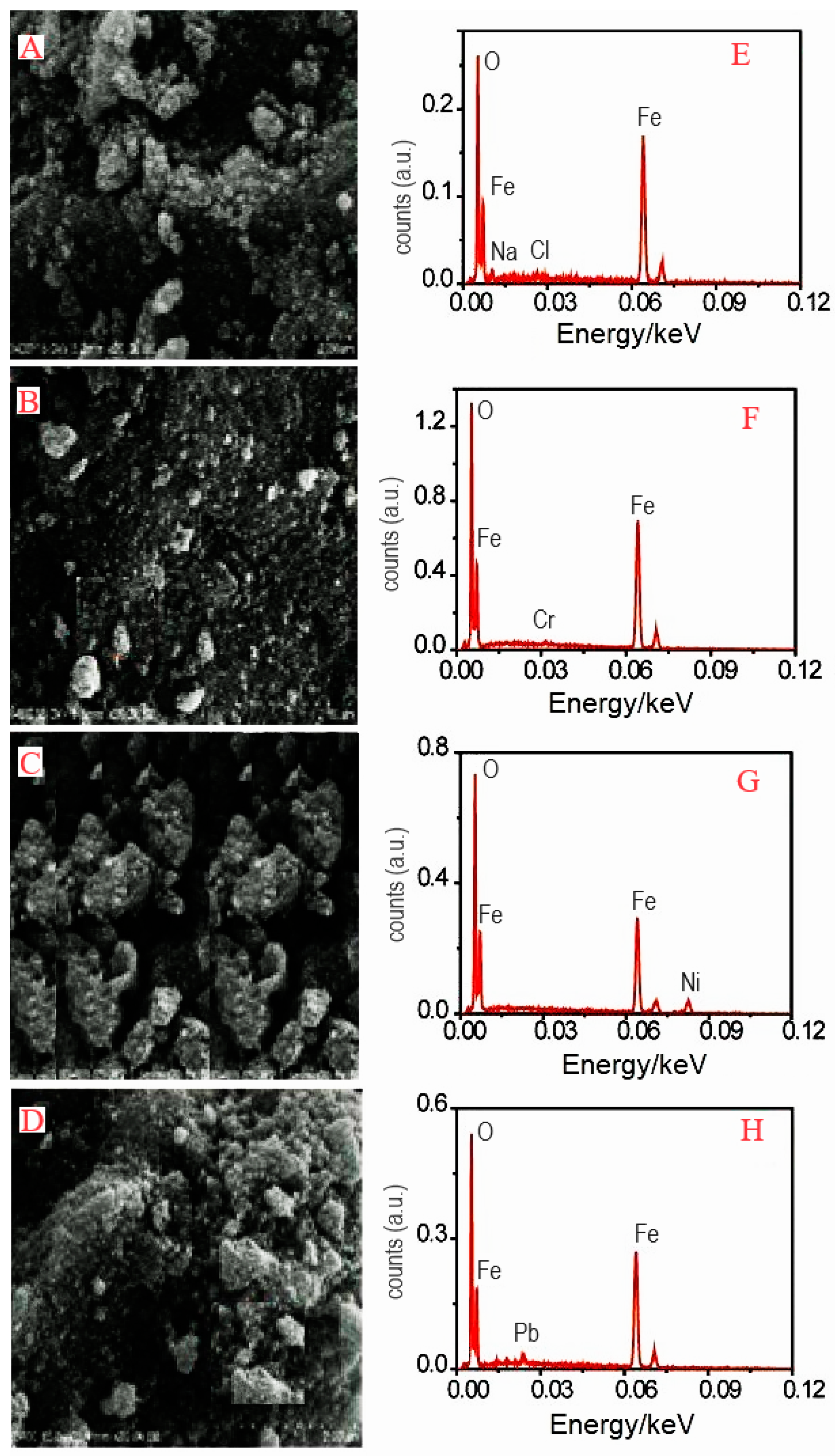
2.6. Scanning Electron Microscope (SEM)/Energy-Dispersive X-Ray (EDX) Analysis
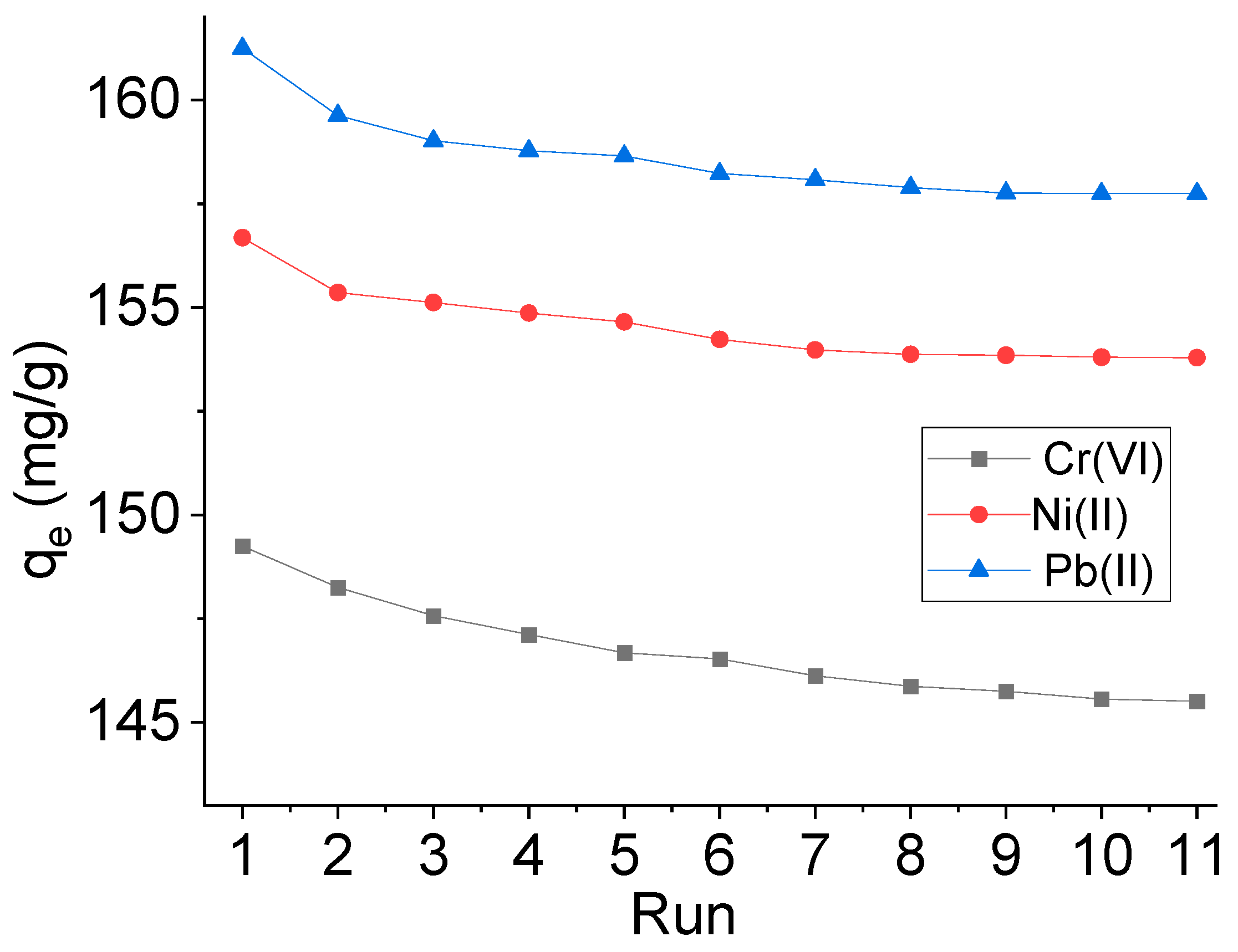
2.7. Adsorbent Recovery
3. Materials and Methods
3.1. Instrumentation
3.2. Reagents
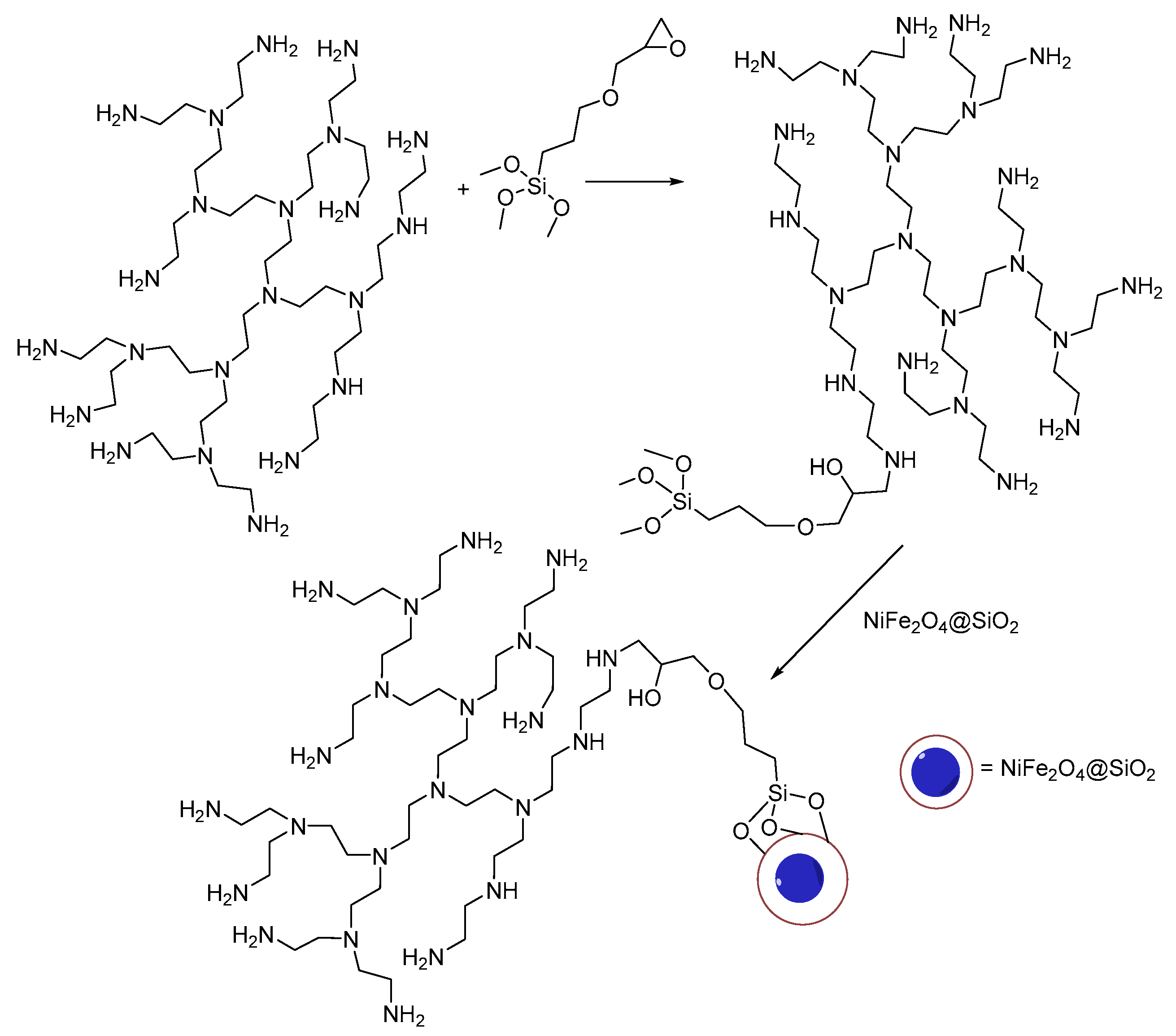
3.3. Synthesis of the NiFe2O4@SiO2–PEI Adsorbent
3.4. Adsorption Experiments
3.5. Adsorbent Recovery
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaim, W.; Schwederski, B.; Klein, A. Bioinorganic Chemistry: Inorganic Elements in the Chemistry of Life, 2nd ed.; Wiley Chichester: Hoboken, NJ, USA, 2013; ISBN 978-1-118-65926-7. [Google Scholar]
- Duffus, J.H. “Heavy Metals”—A Meaningless Term? (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 793–807. [Google Scholar] [CrossRef]
- Ethaib, S.; Al-Qutaifia, S.; Al-Ansari, N.; Zubaidi, S.L. Function of Nanomaterials in Removing Heavy Metals for Water and Wastewater Remediation: A Review. Environments 2022, 9, 123. [Google Scholar] [CrossRef]
- Damiri, F.; Andra, S.; Kommineni, N.; Balu, S.K.; Bulusu, R.; Boseila, A.A.; Akamo, D.O.; Ahmad, Z.; Khan, F.S.; Rahman, M.d.H.; et al. Recent Advances in Adsorptive Nanocomposite Membranes for Heavy Metals Ion Removal from Contaminated Water: A Comprehensive Review. Materials 2022, 15, 5392. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, H.-X. Modified-biochar adsorbents (MBAs) for heavy-metal ions adsorption: A critical review. J. Environ. Chem. Eng. 2022, 10, 107393. [Google Scholar] [CrossRef]
- Mo, Z.; Tai, D.Z.; Zhang, H.; Shahab, A. A comprehensive review on the adsorption of heavy metals by zeolite imidazole framework (ZIF-8) based nanocomposite in water. Chem. Eng. J. 2022, 443, 136320. [Google Scholar] [CrossRef]
- Li, Y.; Yu, H.; Liu, L.; Yu, H. Application of co-pyrolysis biochar for the adsorption and immobilization of heavy metals in contaminated environmental substrates. J. Hazard. Mater. 2021, 420, 126655. [Google Scholar] [CrossRef]
- Farouz, M.; El-Dek, S.I.; ElFaham, M.M.; Eldemerdash, U. Ecofriendly sustainable synthetized nano-composite for removal of heavy metals from aquatic environment. Appl. Nanosci. 2022, 12, 1585–1600. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.-M.; Zhang, B.-G.; Dai, Y.-R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, X.; Li, T.; Zhang, Y.; Xu, H.; Sun, Y.; Gu, X.; Gu, C.; Luo, J.; Gao, B. MIL series of metal organic frameworks (MOFs) as novel adsorbents for heavy metals in water: A review. J. Hazard. Mater. 2022, 429, 128271. [Google Scholar] [CrossRef]
- Soliman, N.K.; Moustafa, A.F. Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Stylios, G.K.; Hassan, M.M.; Song, H.; Naddeo, V.; Figoli, A. Ag nanoparticles immobilized sulfonated polyethersulfone/polyethersulfone electrospun nanofiber membrane for the removal of heavy metals. Sci. Rep. 2022, 12, 5814. [Google Scholar] [CrossRef]
- Wu, H.; Lin, G.; Liu, C.; Chu, S.; Mo, C.; Liu, X. Progress and challenges in molecularly imprinted polymers for adsorption of heavy metal ions from wastewater. Trends Environ. Anal. Chem. 2022, 36, e00178. [Google Scholar] [CrossRef]
- Bao, S.; Yang, W.; Wang, Y.; Yu, Y.; Sun, Y.; Li, K. PEI grafted amino-functionalized graphene oxide nanosheets for ultrafast and high selectivity removal of Cr(VI) from aqueous solutions by adsorption combined with reduction: Behaviors and mechanisms. Chem. Eng. J. 2020, 399, 125762. [Google Scholar] [CrossRef]
- Godiya, C.B.; Liang, M.; Sayed, S.M.; Li, D.; Lu, X. Novel alginate/polyethyleneimine hydrogel adsorbent for cascaded removal and utilization of Cu2+ and Pb2+ ions. J. Environ. Manag. 2019, 232, 829–841. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhao, J.; Lei, L.; Kang, X.; Lu, R.; Chen, C.; Li, S.; Zhao, Y.; Yang, Q.; Chen, Z. Novel magnetically separable anhydride-functionalized Fe3O4@SiO2@PEI-NTDA nanoparticles as effective adsorbents: Synthesis, stability and recyclable adsorption performance for heavy metal ions. RSC Adv. 2019, 9, 9533–9545. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, J.; Wu, Z.; Deng, Q.; Tu, W.; Dai, G.; Zeng, Z.; Deng, S. Enhanced Cr(VI) removal by polyethylenimine- and phosphorus-codoped hierarchical porous carbons. J. Coll. Interfac. Sci. 2018, 523, 110–120. [Google Scholar] [CrossRef]
- Jin, X.; Xiang, Z.; Liu, Q.; Chen, Y.; Lu, F. Polyethyleneimine-Bacterial Cellulose Bioadsorbent for Effective Removal of Copper and Lead Ions from Aqueous Solution. Biores. Technol. 2017, 244, 844–849. [Google Scholar] [CrossRef]
- Tomonaga, H.; Tanigaki, Y.; Hayashi, K.; Matsuyama, T.; Ida, J. Adsorption properties of poly(NIPAM-co-AA) immobilized on silica-coated magnetite nanoparticles prepared with different acrylic acid content for various heavy metal ions. Chem. Eng. Res. Des. 2021, 171, 213–224. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Huang, X.; Wang, W.; Sun, P.; Li, Y.; Han, J. Functionalized biochar-supported magnetic MnFe2O4 nanocomposite for the removal of Pb(II) and Cd(II). RSC Adv. 2019, 9, 365–376. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, S.; Shao, Y.; Liu, J.; Xu, Z.; Zhu, D. Amino-functionalized Fe3O4@SiO2 core-shell magnetic nanomaterial as a novel adsorbent for aqueous heavy metals removal. J. Colloid Interface Sci. 2010, 349, 293–299. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Y.; Ding, Z. Network–Polymer–Modified Superparamagnetic Magnetic Silica Nanoparticles for the Adsorption and Regeneration of Heavy Metal Ions. Molecules 2023, 28, 7385. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Zhang, G.; Zhu, J.; Wu, Z. Adsorption of heavy metal ions in water by surface functionalized magnetic composites: A review. Environ. Sci. Water Res. Technol. 2022, 8, 907–925. [Google Scholar] [CrossRef]
- Thomas, B.; Alexander, L.K. Enhanced synergetic effect of Cr(VI) ion removal and anionic dye degradation with superparamagnetic cobalt ferrite meso–macroporous nanospheres. Appl. Nanosci. 2018, 8, 125–135. [Google Scholar] [CrossRef]
- Sureshkumar, V.; Daniel, S.C.G.K.; Ruckmani, K.; Sivakumar, M. Fabrication of chitosan–magnetite nanocomposite strip for chromium removal. Appl. Nanosci. 2016, 6, 277–285. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, H.; Fan, H.; Zhou, S.; Huang, J. One-Step Fabrication of Recyclable Konjac Glucomannan-Based Magnetic Nanoparticles for Highly Efficient Cr(VI) Adsorption. Molecules 2023, 28, 7100. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, M.; Taherkhani, M.; Kalhor, M. Preparation of some chromeno [4,3-d]pyrido[1,2-a]pyrimidine derivatives by ultrasonic irradiation using NiFe2O4@SiO2 grafted di(3-propylsulfonic acid) nanoparticles. New J. Chem. 2021, 45, 10718–10724. [Google Scholar] [CrossRef]
- Xiang, B.; Ling, D.; Lou, H.; Gu, H. 3D hierarchical flower-like nickel ferrite/manganese dioxide toward lead(II) removal from aqueous water. J. Hazard. Mater. 2017, 325, 178–188. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, L.; Ma, P.-C.; Shao, Y.; Zhang, H.; Gao, W.; Li, Y. Development of carbon nanotubes/CoFe2O4 magnetic hybrid material for removal of tetrabromobisphenol A and Pb(II). J. Hazard. Mater. 2014, 265, 104–114. [Google Scholar] [CrossRef]
- Xu, W.; Song, Y.; Dai, K.; Sun, S.; Liu, G.; Yao, J. Novel ternary nanohybrids of tetraethylenepentamine and graphene oxide decorated with MnFe2O4 magnetic nanoparticles for the adsorption of Pb(II). J. Hazard. Mater. 2018, 358, 337–345. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; Amira, M.F.; Seleim, S.M.; Mohamed, A.K. Amino-decorated magnetic metal-organic framework as a potential novel platform for selective removal of chromium(VI), cadmium(II) and lead(II). J. Hazard. Mater. 2020, 381, 120979. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zheng, H.; Hu, X.; Wu, Y.; Tang, X.; He, Q.; Peng, S. Enhanced selective adsorption of lead(II) from complex wastewater by DTPA functionalized chitosan-coated magnetic silica nanoparticles based on anion-synergism. J. Hazard. Mater. 2022, 422, 126856. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Du, B.; Wei, Q.; Yang, J.; Hu, L.; Yan, L.; Xu, W. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J. Hazard. Mater. 2014, 278, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, B.; Lv, J.; He, Y.; Zhang, H.; Li, W.; Li, Y.; Wågberg, T.; Hu, G. Facile synthesis of sodium lignosulfonate/polyethyleneimine/sodium alginate beads with ultra-high adsorption capacity for Cr(VI) removal from water. J. Hazard. Mater. 2022, 436, 129270. [Google Scholar] [CrossRef] [PubMed]
- Yanovska, E.; Savchenko, I.; Petrenko, O.; Davydov, V. Adsorption of some toxic metal ions on pine sawdust in situ immobilized by polyaniline. Appl. Nanosci. 2022, 12, 861–868. [Google Scholar] [CrossRef]
- Zhao, Y.-G.; Shen, H.-Y.; Pan, S.-D.; Hu, M.-Q. Synthesis, characterization and properties of ethylenediamine-functionalized Fe3O4 magnetic polymers for removal of Cr(VI) in wastewater. J. Hazard. Mater. 2010, 182, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-M.; An, Q.-D.; Xiao, Z.-Y.; Zhai, S.-R.; Yang, D.-J. Efficient removal of Pb(II), Cr(VI) and organic dyes by polydopamine modified chitosan aerogels. Carbohydr. Polym. 2018, 202, 306–314. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Peng, L.; Han, S.; Hao, C.; Jiang, C.; Wang, H.; Fan, X. Effective removal of heavy metals from water using porous lignin-based adsorbents. Chemosphere 2021, 279, 130504. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Liu, J.; Liu, J.; Xia, F.; Wang, C.; Dahlgren, R.A.; Liu, W. Mechanism of Cr(VI) removal by magnetic greigite/biochar composites. Sci. Total Environ. 2020, 700, 134414. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Q.; Chen, J.; Yang, J.; Zhang, Y.; Chen, Y.; Li, X.; Du, W.; Liang, A.; Ho, S.-H.; et al. Adsorption behavior of Cr(VI) by magnetically modified Enteromorpha prolifera based biochar and the toxicity analysis. J. Hazard. Mater. 2020, 395, 122658. [Google Scholar] [CrossRef]
- Cai, W.; Dionysiou, D.D.; Fu, F.; Tang, B. CTAB–intercalated molybdenum disulfide nanosheets for enhanced simultaneous removal of Cr(VI) and Ni(II) from aqueous solutions. J. Hazard. Mater. 2020, 396, 122728. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lee, X.; Macazo, F.C.; Grattieri, M.; Cai, R.; Minteer, S.D. Fast and Efficient Removal of Chromium(VI) Anionic Species by a Reusable Chitosan-Modified Multi-Walled Carbon Nanotube Composite. Chem. Engin. J. 2018, 339, 259–267. [Google Scholar] [CrossRef]
- Panja, S.; Hanson, S.; Wang, C. EDTA–inspired polydentate hydrogels with exceptionally high heavy metal adsorption capacity as reusable adsorbents for wastewater purification. ACS Appl. Mater. Interfaces 2020, 12, 25276–25285. [Google Scholar] [CrossRef] [PubMed]
- Largitte, L.; Pasquier, R. A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem. Eng. Res. Des. 2016, 109, 495–504. [Google Scholar] [CrossRef]
- Ghashang, M.; Khosravian, P.; Ghayoor, H. Effective removal of penicillin from aqueous solution using Zinc oxide/natural-Zeolite composite nano-powders prepared via ball milling technique. Rec. Pat. Nanotechnol. 2017, 11, 154–164. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. On adsorptions in solution. Z. Phys. Chem. 1907, 57, 385–471. [Google Scholar] [CrossRef]
- Haghighatian, S.; Mazarei, E.; Doroodmand, M.M.; Klein, A.; Memarpoor-Yazdi, M. A new whole-cell biocatalyst for sulfur dioxide filtering and degradation. Biores. Technol. 2020, 314, 123755. [Google Scholar] [CrossRef]
- Siddiqui, S.H. The removal of Cu2+, Ni2+ and Methylene Blue (MB) from aqueous solution using Luffa Actangula Carbon: Kinetics, thermodynamic and isotherm and response methodology. Groundw. Sust. Develop. 2018, 6, 141–149. [Google Scholar] [CrossRef]




| Linear Equations a | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CrO42− | Ni2+ | Pb2+ | |||||||
| k | R2 | qe (mg/g) | k | R2 | qe (mg/g) | k | R2 | qe (mg/g) | |
| Pseudo-first order | 0.118 | 0.999 | 151.07 | 0.097 | 0.992 | 169.78 | 0.102 | 0.996 | 159.66 |
| Pseudo-second order | 0.0014 | 0.981 | 163.93 | 0.0008 | 0.968 | 175.43 | 0.0011 | 0.980 | 178.57 |
| Elovich equation | α | R2 | β | α | R2 | β | α | R2 | β |
| 135.57 | 0.928 | 0.026 | 10.85 | 0.983 | 0.022 | 79.89 | 0.963 | 0.023 | |
| Models | Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CrO42− | Ni2+ | Pb2+ | |||||||
| Langmuir | b | R2 | qmax (mg/g) | b | R2 | qmax (mg/g) | b | R2 | qmax (mg/g) |
| 0.075 | 0.972 | 181.81 | 0.105 | 0.976 | 178.57 | 0.441 | 0.997 | 166.67 | |
| Freundlich | kf | R2 | n | kf | R2 | n | kf | R2 | n |
| 0.015 | 0.910 | 0.646 | 0.016 | 0.893 | 0.675 | 0.0014 | 0.814 | 0.553 | |
| Temkin | B | R2 | A | B | R2 | A | B | R2 | A |
| 0.0247 | 0.931 | 367.1 | 0.0226 | 0.885 | 298.3 | 0.0272 | 0.838 | 1.584 | |
| ΔG0 (kJ/mol) (T = 298 K) | ΔS0 (J/K·mol) | ΔH0 (kJ/mol) | ||||||
|---|---|---|---|---|---|---|---|---|
| CrO42− | Ni2+ | Pb2+ | CrO42− | Ni2+ | Pb2+ | CrO42− | Ni2+ | Pb2+ |
| −4.18 | −5.58 | −7.37 | −67.96 | −98.92 | −106.64 | −24.43 | −35.06 | −38.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalaj, M.; Khatami, S.-M.; Kalhor, M.; Zarandi, M.; Anthony, E.T.; Klein, A. Polyethylenimine Grafted onto Nano-NiFe2O4@SiO2 for the Removal of CrO42−, Ni2+, and Pb2+ Ions from Aqueous Solutions. Molecules 2024, 29, 125. https://doi.org/10.3390/molecules29010125
Khalaj M, Khatami S-M, Kalhor M, Zarandi M, Anthony ET, Klein A. Polyethylenimine Grafted onto Nano-NiFe2O4@SiO2 for the Removal of CrO42−, Ni2+, and Pb2+ Ions from Aqueous Solutions. Molecules. 2024; 29(1):125. https://doi.org/10.3390/molecules29010125
Chicago/Turabian StyleKhalaj, Mehdi, Seyed-Mola Khatami, Mehdi Kalhor, Maryam Zarandi, Eric Tobechukwu Anthony, and Axel Klein. 2024. "Polyethylenimine Grafted onto Nano-NiFe2O4@SiO2 for the Removal of CrO42−, Ni2+, and Pb2+ Ions from Aqueous Solutions" Molecules 29, no. 1: 125. https://doi.org/10.3390/molecules29010125
APA StyleKhalaj, M., Khatami, S.-M., Kalhor, M., Zarandi, M., Anthony, E. T., & Klein, A. (2024). Polyethylenimine Grafted onto Nano-NiFe2O4@SiO2 for the Removal of CrO42−, Ni2+, and Pb2+ Ions from Aqueous Solutions. Molecules, 29(1), 125. https://doi.org/10.3390/molecules29010125







