Co-Doping Effect of Mn2+ and Eu3+ on Luminescence in Strontiowhitlockite Phosphors
Abstract
:1. Introduction
2. Results and Discussion
2.1. PXRD and SHG Study
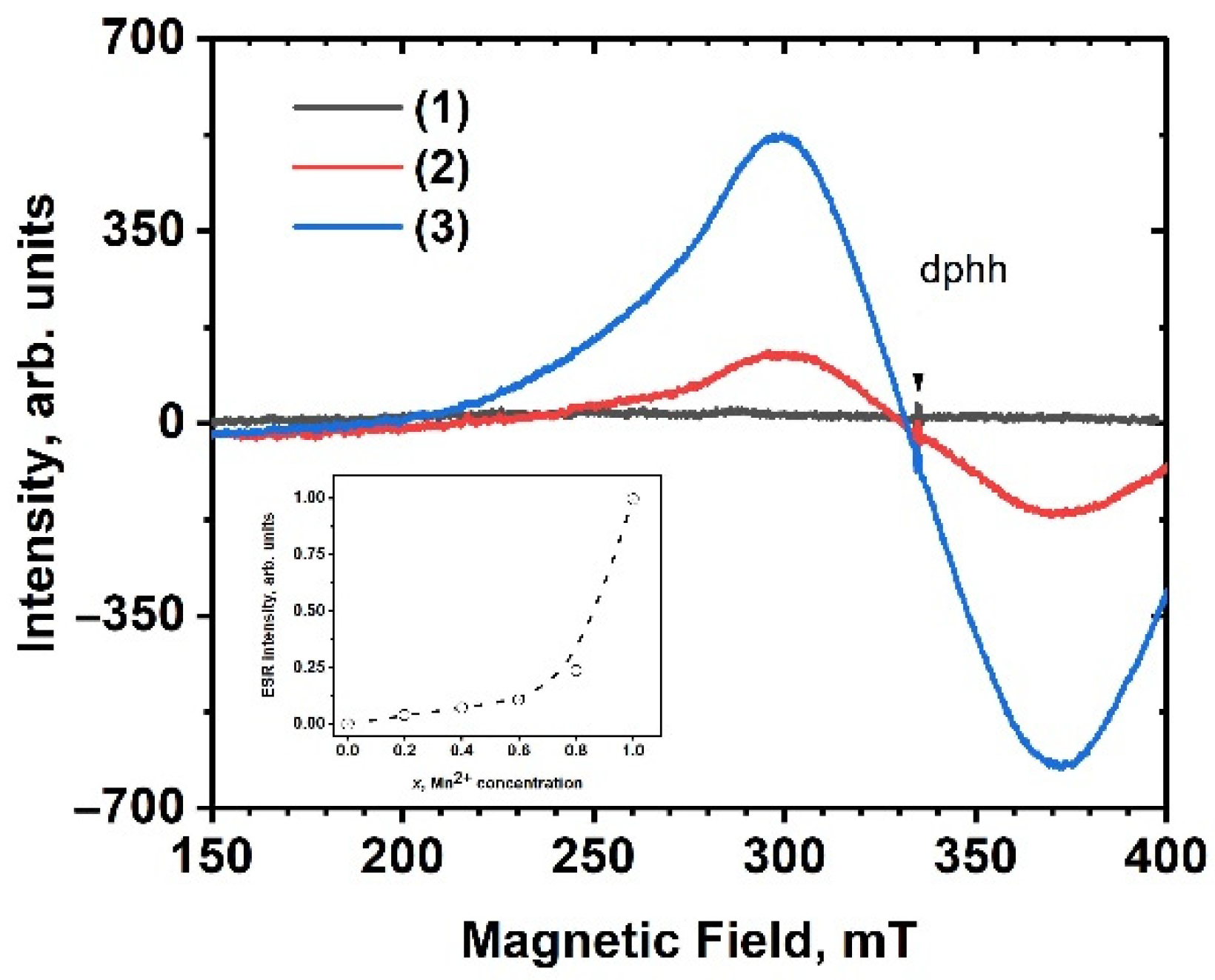
2.2. ESR Analyze
2.3. Diffuse Absorption
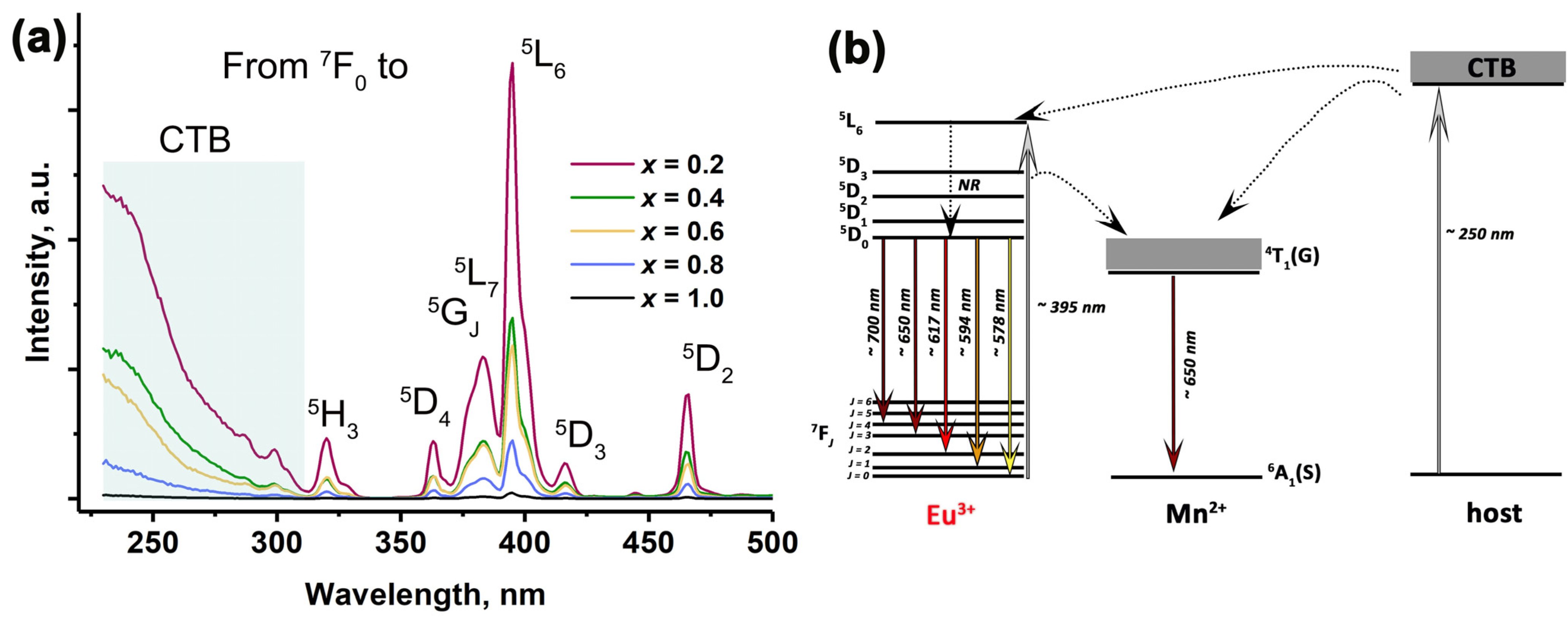
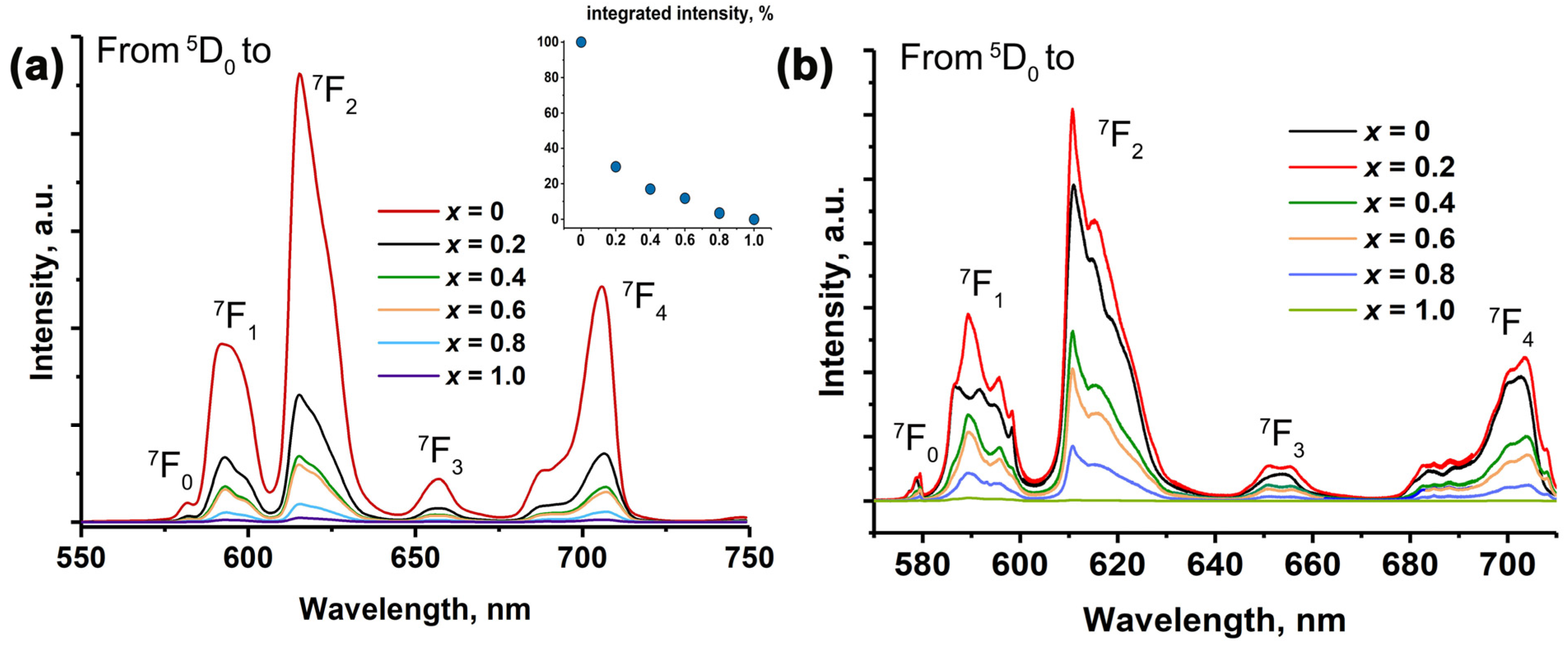
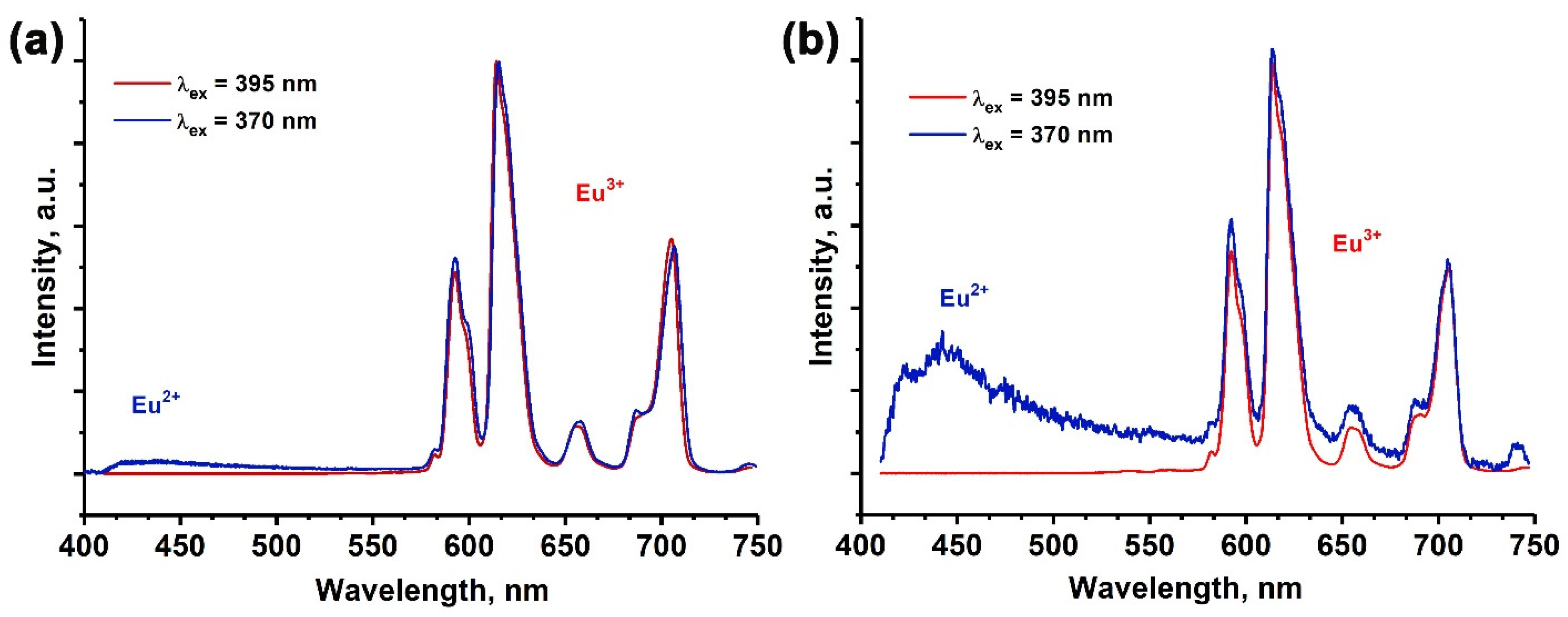
2.4. Photoluminescence Properties
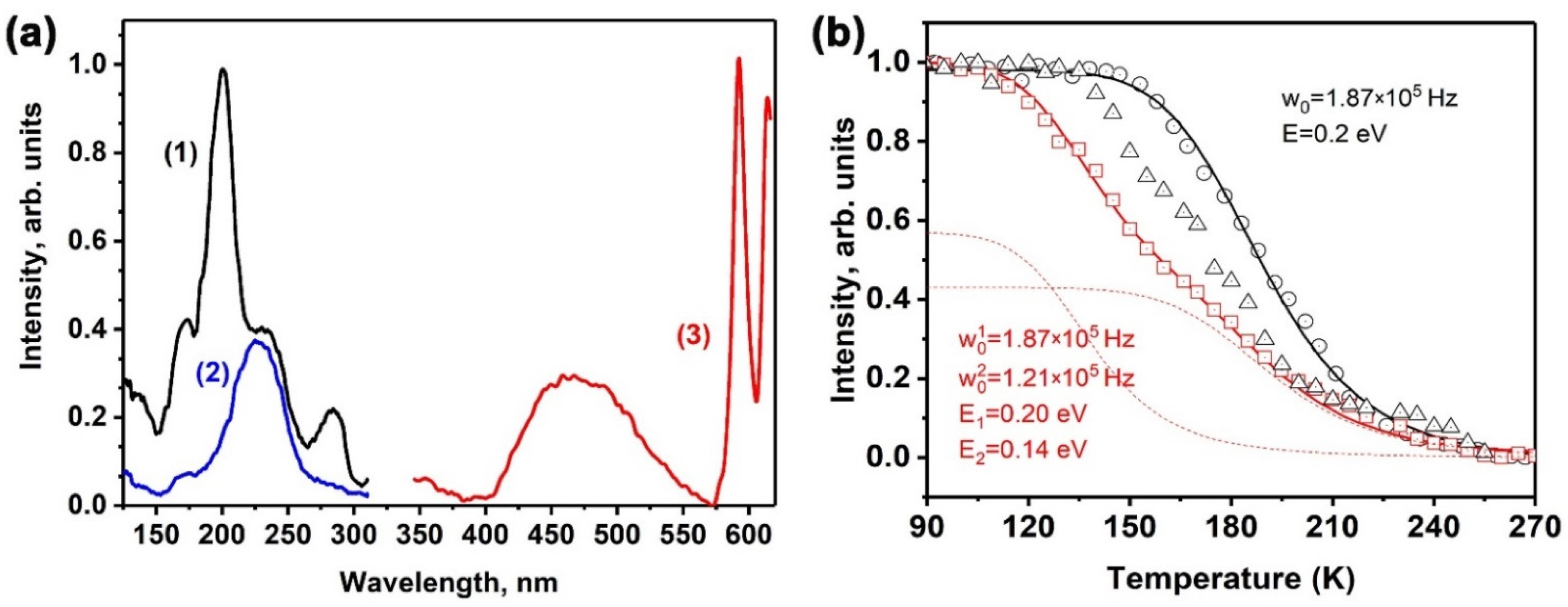
2.5. Temperature Dependence of Photoluminescence
2.6. The Decay Curves
2.7. The Abnormal Reduction Process
2.8. Color Characteristics
3. Materials and Methods
3.1. Synthesis
- Slow heating up to 200 °C for 8 h, followed by annealing for 8 h in air.
- Heating to 1100 °C for 12 h, followed by annealing for 24 h in air.
3.2. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Wang, Y.; Wen, Y.; Zhang, F.; Liu, B. Luminescence properties of Ca10K(PO4)7:RE3+ (RE=Ce, Tb, Dy, Tm and Sm) under vacuum ultraviolet excitation. J. Alloys Compd. 2011, 509, 4649–4652. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, J.; Zhang, X.; Hao, Z.; Luo, Y. New orange–red phosphor Sr9Sc(PO4)7:Eu3+ for NUV-LEDs application. J. Alloys Compd. 2014, 587, 493–496. [Google Scholar] [CrossRef]
- Kim, K.-B.; Kim, Y.-I.; Chun, H.-G.; Cho, T.-Y.; Jung, J.-S.; Kang, J.-G. Structural and Optical Properties of BaMgAl10O17:Eu2+ Phosphor. Chem. Mater. 2002, 14, 5045–5052. [Google Scholar] [CrossRef]
- Chen, J.; Yang, C.; Chen, Y.; He, J.; Liu, Z.-Q.; Wang, J.; Zhang, J. Local Structure Modulation Induced Highly Efficient Far-Red Luminescence of La1−xLuxAlO3:Mn4+ for Plant Cultivation. Inorg. Chem. 2019, 58, 8379–8387. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhang, H.; Wei, R.; Zheng, M.; Zhang, L. Preparation, structural and luminescent properties of Ba2Gd2Si4O13:Eu3+ for white LEDs. Opt. Express 2011, 19, A201–A206. [Google Scholar] [CrossRef]
- Stefańska, D.; Dereń, P.J. Luminescence investigation and thermal stability of blue-greenish emission generated from Ca3MgSi2O8: Eu2+ phosphor. Opt. Mater. 2018, 80, 62–64. [Google Scholar] [CrossRef]
- Thulasiramudu, A.; Buddhudu, S. Optical characterization of Eu3+ and Tb3+ ions doped zinc lead borate glasses. Spectrochim. Acta Part A 2007, 66, 323–328. [Google Scholar] [CrossRef]
- Bungala Chinna, J.; Jakka, S.K.; Mohan Babu, A.; Sasikala, T.; Rama Moorthy, L. Green fluorescence of Tb3+- doped LBTAF glasses. Phys. B 2009, 404, 2020. [Google Scholar]
- Lü, W.; Jiao, M.; Shao, B.; Zhao, L.; You, H. Enhancing Photoluminescence Performance of SrSi2O2N2:Eu2+ Phosphors by Re (Re = La, Gd, Y, Dy, Lu, Sc) Substitution and Its Thermal Quenching Behavior Investigation. Inorg. Chem. 2015, 54, 9060–9065. [Google Scholar] [CrossRef]
- Osborne, R.A.; Cherepy, N.J.; Seeley, Z.M.; Payne, S.A.; Drobshoff, A.D.; Srivastava, A.M.; Beers, W.W.; Cohen, W.W.; Schlagel, D.L. New red phosphor ceramic K2SiF6:Mn4+. Opt. Mater. 2020, 107, 110140. [Google Scholar] [CrossRef]
- Xu, S.; Li, P.; Wang, Z.; Li, T.; Bai, Q.; Sun, J.; Yang, Z. Luminescence and energy transfer of Eu2+/Tb3+/Eu3+ in LiBaBO3 phosphors with tunable-color emission. J. Mater. Chem. C 2015, 3, 9112–9121. [Google Scholar] [CrossRef]
- Yuan, Y.; Lin, H.; Cao, J.; Guo, Q.; Xu, F.; Liao, L.; Mei, L. A novel blue-purple Ce3+ doped whitlockite phosphor: Synthesis, crystal structure, and photoluminescence properties. J. Rare Earths 2020, 39, 621–626. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.V.; Spassky, D.A.; Berdonosov, P.S.; Dzhevakov, P.B.; Lazoryak, B.I. Sr8MSm1−xEux(PO4)7 phosphors derived by different synthesis routes: Solid state, sol-gel and hydrothermal, the comparison of properties. J. Alloys Compd. 2021, 887, 161340. [Google Scholar] [CrossRef]
- Shuang, D.; Wanjun, T.; Guangyong, X.; Yuhong, Y.; Zhengxi, H. Synthesis and photoluminescence tuning of (Sr,Ln)9Mg1.5(PO4)7 phosphors by Ln (Ln = Y, La, Gd, Lu) substitution. J. Lumin. 2020, 224, 117331. [Google Scholar] [CrossRef]
- Britvin, S.N.; Pakhomovskii, Y.A.; Bogdanova, A.N.; Skiba, V.I. Strontiowhitlockite, Sr9Mg(PO3OH)(PO4)6, a new mineral species from the Kovdor Deposit, Kola Peninsula, U.S.S.R. Can. Mineral. 1991, 29, 87–93. [Google Scholar]
- Han, J.; Pan, F.; Zhou, W.; Qiu, Z.; Tang, M.; Wang, J.; Lian, S. Dual energy transfer controlled photoluminescence evolution in Eu and Mn co-activated β-Ca2.7Sr0.3(PO4)2 phosphors for solid-state lighting. RSC Adv. 2015, 5, 98026–98032. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, Z. Realization of color tuning via solid-solution and energy transfer in Ca3−xSrx(PO4)2:Eu2+,Mn2+ phosphors. J. Mater. Chem. C 2015, 3, 5339–5346. [Google Scholar] [CrossRef]
- Ji, H.; Huang, Z.; Xia, Z.; Molokeev, M.S.; Atuchin, V.V.; Huang, S. Cation Substitution Dependent Bimodal Photoluminescence in Whitlockite Structural Ca3−xSrx(PO4)2:Eu2+ (0 ≤ x ≤ 2) Solid Solution Phosphors. Inorg. Chem. 2014, 53, 11119–11124. [Google Scholar] [CrossRef]
- Szyszka, K.; Nowak, N.; Kowalski, R.M.; Zukrowski, J.; Wiglusz, R.J. Anomalous luminescence properties and cytotoxicity assessment of Sr3(PO4)2 co-doped with Eu2+/3+ ions for luminescence temperature sensing. J. Mater. Chem. C 2022, 10, 9092–9105. [Google Scholar] [CrossRef]
- Belik, A.A.; Izumi, F.; Stefanovich, S.Y.; Malakho, A.P.; Lazoryak, B.I.; Leonidov, I.A.; Leonidova, O.N.; Davydov, S.A. Polar and Centrosymmetric Phases in Solid Solutions Ca3−xSrx(PO4)2 (0 ≤ x ≤ 16/7). Chem. Mater. 2002, 14, 3197–3205. [Google Scholar] [CrossRef]
- Zhou, N.; Gao, P.; Yang, Y.; Zhong, Y.; Xia, M.; Zhang, Y.; Tian, Y.; Lu, X.; Zhou, Z. Novel orange–red emitting phosphor Sr8ZnY(PO4)7:Sm3+ with enhanced emission based on Mg2+ and Al3+ incorporation for plant growth LED lighting. J. Taiwan Inst. Chem. Engin. 2019, 104, 360–368. [Google Scholar] [CrossRef]
- Ding, X.; Li, Z.; Xia, D. New whitlockite-type structure material Sr9Y(PO4)7 and its Eu2+ doped green emission properties under NUV light. J. Lumin. 2020, 221, 117114. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Aksenov, S.M.; Nikiforov, I.V.; Stefanovich, S.Y.; Lazoryak, B.I. Symmetry Inhomogeneity of Ca9−xZnxEu(PO4)7 Phosphor Determined by Second-Harmonic Generation and Dielectric and Photoluminescence Spectroscopy. Cryst. Growth Des. 2020, 20, 6461–6468. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Nikiforov, I.V.; Spassky, D.A.; Dikhtyar, Y.Y.; Aksenov, S.M.; Stefanovich, S.Y.; Lazoryak, B.I. Luminescence of Eu3+ as a probe for the determination of the local site symmetry in β-Ca3(PO4)2-related structures. CrystEngComm 2019, 21, 5235–5242. [Google Scholar] [CrossRef]
- Sipina, E.V.; Spassky, D.A.; Krutyak, N.R.; Morozov, V.A.; Zhukovskaya, E.S.; Belik, A.A.; Manylov, M.S.; Lazoryak, B.I.; Deyneko, D.V. Abnormal Eu3+ → Eu2+ Reduction in Ca9−xMnxEu(PO4)7 Phosphors: Structure and Luminescent Properties. Materials 2023, 16, 1383. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hou, D.; Yan, J.; Zhou, L.; Kuang, X.; Liang, H.; Huang, Y.; Zhang, B.; Tao, Y. Energy Transfer and Tunable Luminescence of NaLa(PO3)4:Tb3+/Eu3+ under VUV and Low-Voltage Electron Beam Excitation. J. Phys. Chem. 2014, 118, 3220–3229. [Google Scholar] [CrossRef]
- Wang, Z.; Lou, S.; Li, P. Luminescent properties and energy transfer of Sr3La(PO4)3:Sm3+, Eu3+ for white LEDs. J. Alloys Compd. 2014, 586, 536–541. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Gong, M. Site-occupancy, luminescent properties and energy transfer of a violet-to-red color-tunable phosphor Ca10Li(PO4)7: Ce3+, Mn2+. J. Lumin. 2017, 183, 348–354. [Google Scholar] [CrossRef]
- Guo, H.; Devakumar, B.; Li, B.; Huang, X. Novel Na3Sc2(PO4)3:Ce3+,Tb3+ phosphors for white LEDs: Tunable blue-green color emission, high quantum efficiency and excellent thermal stability. Dyes Pigm. 2018, 151, 81–88. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Yang, Z.; Guo, Q. A novel, warm, white light-emitting phosphor Ca2PO4Cl:Eu2+, Mn2+ for white LEDs. J. Mater. Chem. 2014, 2, 7823–7829. [Google Scholar] [CrossRef]
- Szyszka, K.; Watras, A.; Wiglusz, R.J. Strontium Phosphate Composite Designed to Red-Emission at Different Temperatures. Materials 2020, 13, 4468. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, K.; Wu, Z.-C.; Mei, Y.-M.; Kuang, S.-P.; Li, D.-X. The reduction of Eu3+ to Eu2+ in a new orange–red emission Sr3P4O13: Eu phosphor prepared in air and its photoluminescence properties. Ceram. Int. 2014, 40, 8827–8831. [Google Scholar] [CrossRef]
- Xie, G.; Wu, M.; Li, T.; You, Q.; Tang, W. Luminescence Enhancement of Red-Emitting Sr9MnK(PO4)7 Phosphor via Energy Transfer and Charge Compensation. Phys. Status Solidi B 2022, 259, 2200259. [Google Scholar] [CrossRef]
- Tang, W.; Ding, C. Luminescence Tuning of Sr8MgCe(PO4)7: Eu2+, Mn2+ Phosphors: Structure Refinement, Site Occupancy, and Energy Transfer. Z. Anorg. Allg. Chem. 2018, 644, 893–900. [Google Scholar] [CrossRef]
- Belik, A.A.; Izumi, F.; Ikeda, T.; Lazoryak, B.I.; Morozov, V.A.; Malakho, A.P.; Stefanovich, S.Y.; Grebenev, V.V.; Shelmenkova, O.V.; Kamiyama, T.; et al. Structural changes and phase transitions in whitlockite-like phosphates. Phosphorus Sulfur Silicon Relat. Elem. 2002, 177, 1899–1902. [Google Scholar] [CrossRef]
- Belik, A.A.; Izumi, F.; Ikeda, T.; Okui, M.; Malakho, A.P.; Morozov, V.A.; Lazoryak, B.I. Whitlockite-Related Phosphates Sr9A(PO4)7 (A=Sc, Cr, Fe, Ga, and In): Structure Refinement of Sr9In(PO4)7 with Synchrotron X-ray Powder Diffraction Data. J. Solid State Chem. 2002, 168, 237–244. [Google Scholar] [CrossRef]
- Bessière, A.; Benhamou, R.A.; Wallez, G.; Lecointre, A.; Viana, B. Site occupancy and mechanisms of thermally stimulated luminescence in Ca9Ln(PO4)7 (Ln=lanthanide). Acta Mater. 2012, 60, 6641–6649. [Google Scholar] [CrossRef]
- Luo, J.; Zhou, W.; Fan, J.; Sun, Z.; Zhang, X. Composition modification for tuning the luminescent property in Sr19(Mg,Mn)2(PO4)14: Eu2+ phosphors. J. Lumin. 2021, 239, 118369. [Google Scholar] [CrossRef]
- Miura, M.; Hasegawa, A.; Watanabe, M. The Electron Spin Resonance of Mn2+ Ion in Polyphosphate. Bull. Chem. Soc. Jpn. 1968, 41, 1035–1038. [Google Scholar] [CrossRef]
- Naga Bhaskararao, Y.; Satyavathi, K.; Subba Rao, M.; Cole, S. Synthesis and characterization of Mn2+ doped CdOZn3(PO4)2 nanocomposites. J. Mol. Struct. 2017, 1130, 585–591. [Google Scholar] [CrossRef]
- Jerroudi, M.; Bih, L.; Haily, E.; Bejjit, L.; Haddad, M.; Manoun, B.; Lazor, P. ESR, physical and structural studies on Mn2+ doped in mixed alkali phosphate glasses. Mater. Today Proc. 2021, 37, 3876–3881. [Google Scholar] [CrossRef]
- Kaneva, E.; Shendrik, R.; Mesto, E.; Bogdanov, A.; Vladykin, N. Spectroscopy and crystal chemical properties of NaCa2[Si4O10]F natural agrellite with tubular structure. Chem. Phys. Lett. 2020, 738, 136868. [Google Scholar] [CrossRef]
- Singh, V.; Gundu Rao, T.K.; Zhu, J.-J. Preparation, luminescence and defect studies of Eu2+-activated strontium hexa-aluminate phosphor prepared via combustion method. J. Solid State Chem. 2006, 179, 2589–2594. [Google Scholar] [CrossRef]
- Dhoble, S.J.; Moharil, S.V.; Gundu Rao, T.K. Correlated ESR, PL and TL studies on Sr5(PO4)3Cl:Eu thermoluminescence dosimetry phosphor. J. Lumin. 2007, 126, 383–386. [Google Scholar] [CrossRef]
- Choi, N.-S.; Park, K.-W.; Park, B.-W.; Zhang, X.-M.; Kim, J.-S.; Kung, P.; Margaret Kim, S. Eu2+–Mn2+ energy transfer in white-light-emitting T-phase (Ba,Ca)2SiO4:Eu2+, Mn2+ phosphor. J. Lumin. 2010, 130, 560–566. [Google Scholar] [CrossRef]
- Guo, N.; You, H.; Jia, C.; Ouyang, R.; Wu, D. A Eu2+ and Mn2+-coactivated fluoro-apatite-structure Ca6Y2Na2(PO4)6F2 as a standard white-emitting phosphor via energy transfer. Dalton Trans. 2014, 43, 12373–12379. [Google Scholar] [CrossRef] [PubMed]
- Kaneva, E.; Shendrik, R.; Pankrushina, E.; Dokuchits, E.; Radomskaya, T.; Pechurin, M.; Ushakov, A. Frankamenite: Relationship between the Crystal–Chemical and Vibrational Properties. Minerals 2023, 13, 1017. [Google Scholar] [CrossRef]
- Fridrichová, J.; Bačík, P.; Ertl, A.; Wildner, M.; Dekan, J.; Miglierini, M. Jahn-Teller distortion of Mn3+-occupied octahedra in red beryl from Utah indicated by optical spectroscopy. J. Mol. Struct. 2018, 1152, 79–86. [Google Scholar] [CrossRef]
- Sabatini, J.F.; Salwin, A.E.; McClure, D.S. High-energy optical-absorption bands of transition-metal ions in fluoride host crystals. Phys. Rev. B 1975, 11, 3832–3841. [Google Scholar] [CrossRef]
- True, M.; Kirm, M.; Negodine, E.; Vielhauer, S.; Zimmerer, G. VUV spectroscopy of Tm3+ and Mn2+ doped LiSrAlF6. J. Alloys Compd. 2004, 374, 36–39. [Google Scholar] [CrossRef]
- Garcia, C.R.; Oliva, J.; Garcia-Lobato, M.A.; Martínez, A.I.; Ochoa-Valiente, R.; Hirata, G.A. Red-emitting SrGe4O9:Eu3+ phosphors obtained by combustion synthesis. Ceram. Int. 2017, 43, 12876–12881. [Google Scholar] [CrossRef]
- Liang, C.-H.; Chang, Y.-C.; Chang, Y.-S. Synthesis and Photoluminescence Characteristics of Color-Tunable BaY2ZnO5:Eu3+ Phosphors. Appl. Phys. Lett. 2008, 93, 211902. [Google Scholar] [CrossRef]
- Ferhi, M.; Horchani-Naifer, K.; Férid, M. Spectroscopic properties of Eu3+-doped KLa(PO3)4 and LiLa(PO3)4 powders. Opt. Mater. 2011, 34, 12–18. [Google Scholar] [CrossRef]
- Su, B.; Xie, H.; Tan, Y.; Zhao, Y.; Yang, Q.; Zhang, S. Luminescent properties, energy transfer, and thermal stability of double perovskites La2MgTiO6:Sm3+, Eu3+. J. Lumin. 2018, 204, 457–463. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, G.; Zhou, L.; Lu, D.; Li, Y.; Xiong, X.; Yang, K.; Chen, M.; Pan, Y.; Wu, P.; et al. Luminescent properties and energy transfer process of Sm3+-Eu3+ co-doped MY2(MoO4)4 (M=Ca, Sr and Ba) red-emitting phosphors. Solid State Sci. 2016, 59, 44–51. [Google Scholar] [CrossRef]
- Félix-Quintero, H.; Falcony, C.; Hernández, A.J.; Camarillo, G.E.; Flores, J.C.; Murrieta, S.H. Mn2+ to Eu3+ energy transfer in zinc phosphate glass. J. Lumin. 2020, 225, 117337. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Yu, Y.; Zheng, C. Influences of Mn2+/Eu3+ dopants on the microstructures and optical properties of glass-embedded CsPbBr3 quantum dots. Opt. Mater. Express 2023, 13, 1488–1496. [Google Scholar] [CrossRef]
- Zhang, H.; Lü, M.; Xiu, Z.; Wang, S.; Zhou, G.; Zhou, Y.; Wang, S.; Qiu, Z.; Zhang, A. Synthesis and photoluminescence properties of a new red emitting phosphor: Ca3(VO4)2:Eu3+; Mn2+. Mater. Res. Bull. 2007, 42, 1145–1152. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, G.; Wang, W.; Ma, L.; Wang, X.; Jin, Z. Tuning of Emission by Eu3+ Concentration in a Pyrophosphate: The Effect of Local Symmetry. Inorg. Chem. 2020, 59, 2241–2247. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Spassky, D.A.; Morozov, V.A.; Aksenov, S.M.; Kubrin, S.P.; Molokeev, M.S.; Lazoryak, B.I. Role of the Eu3+ Distribution on the Properties of β-Ca3(PO4)2 Phosphors: Structural, Luminescent, and 151Eu Mössbauer Spectroscopy Study of Ca9.5−1.5xMgEux(PO4)7. Inorg. Chem. 2021, 60, 3961–3971. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Q.; Jiang, L.; Liao, L.; Liu, H.; Mei, L. A novel apatite, Lu5(SiO4)3N:(Ce,Tb), phosphor material: Synthesis, structure and applications for NUV-LEDs. Phys. Chem. Chem. Phys. 2016, 18, 15545–15554. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, H.; Li, B.; Du, J.; Hao, J.; Hu, C.; Wang, Y.; Yi, X.; Pang, R.; Li, C. Energy transfer and luminescence properties of a green-to-red color tunable phosphor Sr8MgY(PO4)7:Tb3+,Eu3+. J. Mater. Sci.-Mater. Electron. 2019, 30, 9421–9428. [Google Scholar] [CrossRef]
- Peng, M.; Pei, Z.; Hong, G.; Su, Q. The reduction of Eu3+ to Eu2+ in BaMgSiO4:Eu prepared in air and the luminescence of BaMgSiO4:Eu2+ phosphor. J. Mater. Chem. 2003, 13, 1202–1205. [Google Scholar] [CrossRef]
- Grandhe, B.K.; Bandi, V.R.; Jang, K.; Kim, S.-S.; Shin, D.-S.; Lee, Y.-I.; Lim, J.-M.; Song, T. Reduction of Eu3+ to Eu2+ in NaCaPO4:Eu phosphors prepared in a non-reducing atmosphere. J. Alloys Compd. 2011, 509, 7937–7942. [Google Scholar] [CrossRef]
- Chen, J.; Liang, Y.; Zhu, Y.; Liu, S.; Li, H.; Lei, W. Abnormal reduction of Eu3+ to Eu2+ in Sr5(PO4)3Cl:Eu phosphor and its enhanced red emission by the charge compensation. J. Lumin. 2019, 214, 116569. [Google Scholar] [CrossRef]
- Deyneko, D.V.; Spassky, D.A.; Antropov, A.V.; Ril’, A.I.; Baryshnikova, O.V.; Pavlova, E.T.; Lazoryak, B.I. Anomalous oxidation state of europium in the Sr3(PO4)2-type phosphors doped with alkaline cations. Mater. Res. Bull. 2023, 165, 112296. [Google Scholar] [CrossRef]
- Pei, Z.; Su, Q.; Zhang, J. The valence change from RE3+ to RE2+ (RE = Eu, Sm, Yb) in SrB4O7: RE prepared in air and the spectral properties of RE2+. J. Alloys Compd. 1993, 198, 51–53. [Google Scholar] [CrossRef]
- Le Bail, A.; Duroy, H.; Fourquet, J.L. Ab-initio structure determination of LiSbWO6 by X-ray powder diffraction. Mater. Res. Bull. 1988, 23, 447–452. [Google Scholar] [CrossRef]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
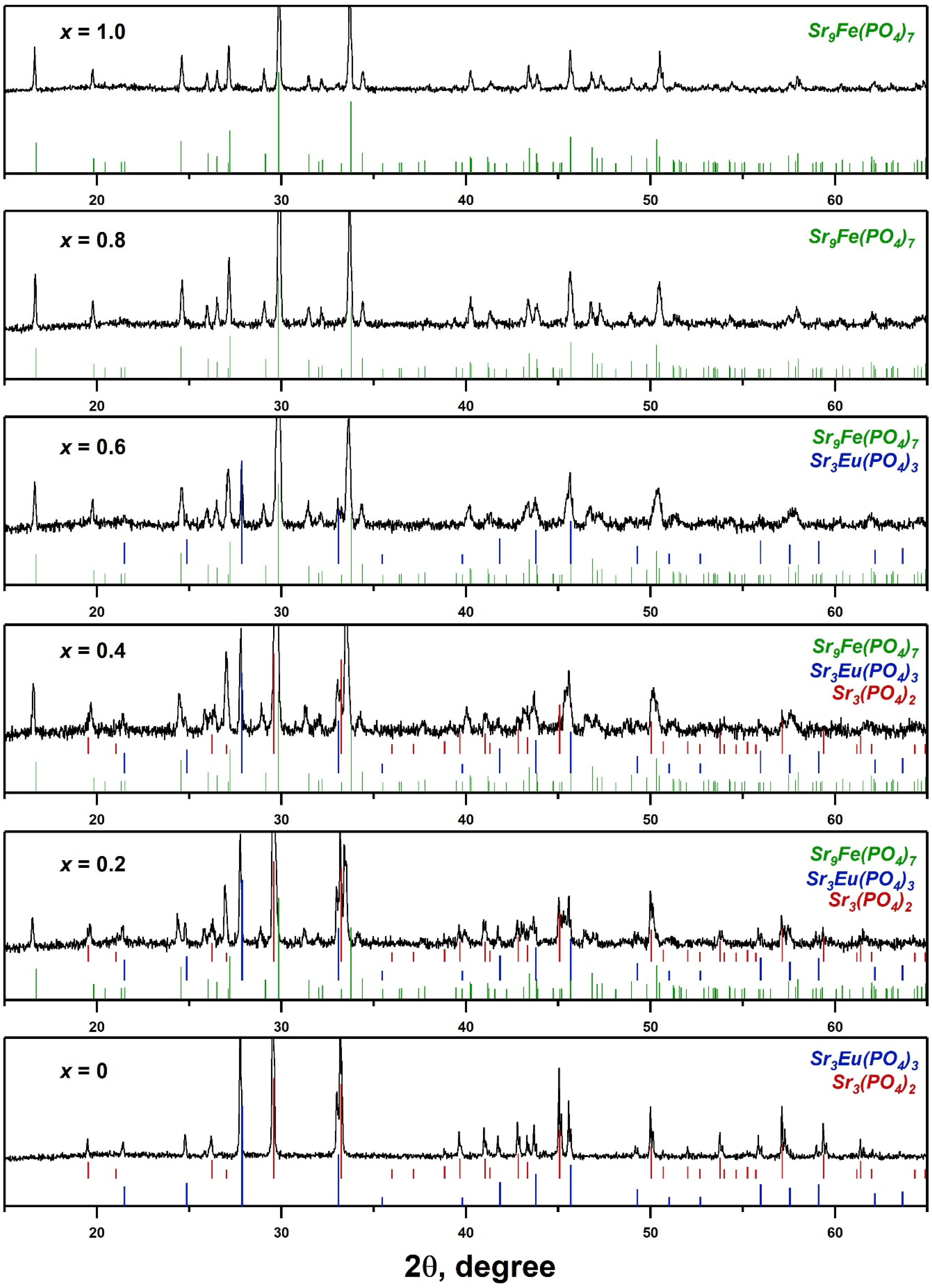






| Whitlockite-Type | Palmierite-Type | Eulytite-Type | SHG | |
|---|---|---|---|---|
| Sr9Fe1.5(PO4)7 | Sr3(PO4)2 | Sr3Eu(PO4)3 | ||
| sp.gr. m | sp.gr. m | sp.gr. 3d | ||
| Centrosymmetric | Centrosymmetric | Non-Centrosymmetric | ||
| x = 0 | 0 | 45% | 55% | 1.1 |
| x = 0.2 | 49% | 23% | 28% | 0.7 ± 0.1 |
| x = 0.4 | 67% | 13% | 20% | 0.5 ± 0.1 |
| x = 0.6 | 83% | 0 | 17% | 0.3 ± 0.1 |
| x = 0.8 | 100% | 0 | 0 | 0.1 ± 0.1 |
| x = 1.0 | 100% | 0 | 0 | 0.1 ± 0.1 |
| Conditions | Present Work | Remarks |
|---|---|---|
| (1) No oxidizing ions should be present in the host. | There were no oxidizing ions in the SrMnxEu host. | |
| (2) The dopant R3+ ions must replace host cations with a different oxidation state. | Eu3+ replaced Sr2+ ions in SrMnxEu host. | In the β-Ca3(PO4)2 structure, Eu3+ can also replace Ca2+ ions in the host. Eu2+ emission was found in some hosts. |
| (3) The host cations must have similar radii to the divalent R2+ ions. | rVIII(Eu2+) = 1.25 Å was close to rVIII(Sr2+) = 1.26 Å. | The similarity of the ionic radii explains the more common abnormal reduction in air in the Sr-based host compared to the Ca-based one. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikiforov, I.V.; Spassky, D.A.; Krutyak, N.R.; Shendrik, R.Y.; Zhukovskaya, E.S.; Aksenov, S.M.; Deyneko, D.V. Co-Doping Effect of Mn2+ and Eu3+ on Luminescence in Strontiowhitlockite Phosphors. Molecules 2024, 29, 124. https://doi.org/10.3390/molecules29010124
Nikiforov IV, Spassky DA, Krutyak NR, Shendrik RY, Zhukovskaya ES, Aksenov SM, Deyneko DV. Co-Doping Effect of Mn2+ and Eu3+ on Luminescence in Strontiowhitlockite Phosphors. Molecules. 2024; 29(1):124. https://doi.org/10.3390/molecules29010124
Chicago/Turabian StyleNikiforov, Ivan V., Dmitry A. Spassky, Nataliya R. Krutyak, Roman Yu. Shendrik, Evgenia S. Zhukovskaya, Sergey M. Aksenov, and Dina V. Deyneko. 2024. "Co-Doping Effect of Mn2+ and Eu3+ on Luminescence in Strontiowhitlockite Phosphors" Molecules 29, no. 1: 124. https://doi.org/10.3390/molecules29010124
APA StyleNikiforov, I. V., Spassky, D. A., Krutyak, N. R., Shendrik, R. Y., Zhukovskaya, E. S., Aksenov, S. M., & Deyneko, D. V. (2024). Co-Doping Effect of Mn2+ and Eu3+ on Luminescence in Strontiowhitlockite Phosphors. Molecules, 29(1), 124. https://doi.org/10.3390/molecules29010124








