Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemometrics
| Compound Annotation | Monoisotopic Mass (Da) Experimental_tR (min) | Molecular Formula | Italy | Greece | Egypt | Compound Class | Adduct Ion | MS/MS Fragment Ions (m/z) |
|---|---|---|---|---|---|---|---|---|
| 4-Hydroxycinnamyl aldehyde | 148.0514_9.06 | C9H8O2 | +++ | d a | d | Organic acid | [M-H]− | 119.05/101.04 |
| 3-Phenyllactid acid | 166.0621_10.12 | C9H10O3 | +++ | d | ++(Gr) | Organic acid | [M-H]− | 147.04/119.05 |
| Ferulic acid | 194.0572_9.30 | C10H10O4 | +++ | d | d | Organic acid | [M-H]− | 134.03/178.02 ** |
| Lumichrome | 242.0802_10.86 | C12H10N4O2 | +++ | d | d | Riboflavin metabolite | [M-H]− | 198.07/106.02 |
| Formononetin | 268.0737_14.15 | C16H12O4 | +++ | d | d | Isoflavone | [M-H]− | 252.04 |
| Glycitein | 284.0686_16.43 | C16H12O5 | +++ | d | d | Isoflavone | [M-H]− | 268.03 |
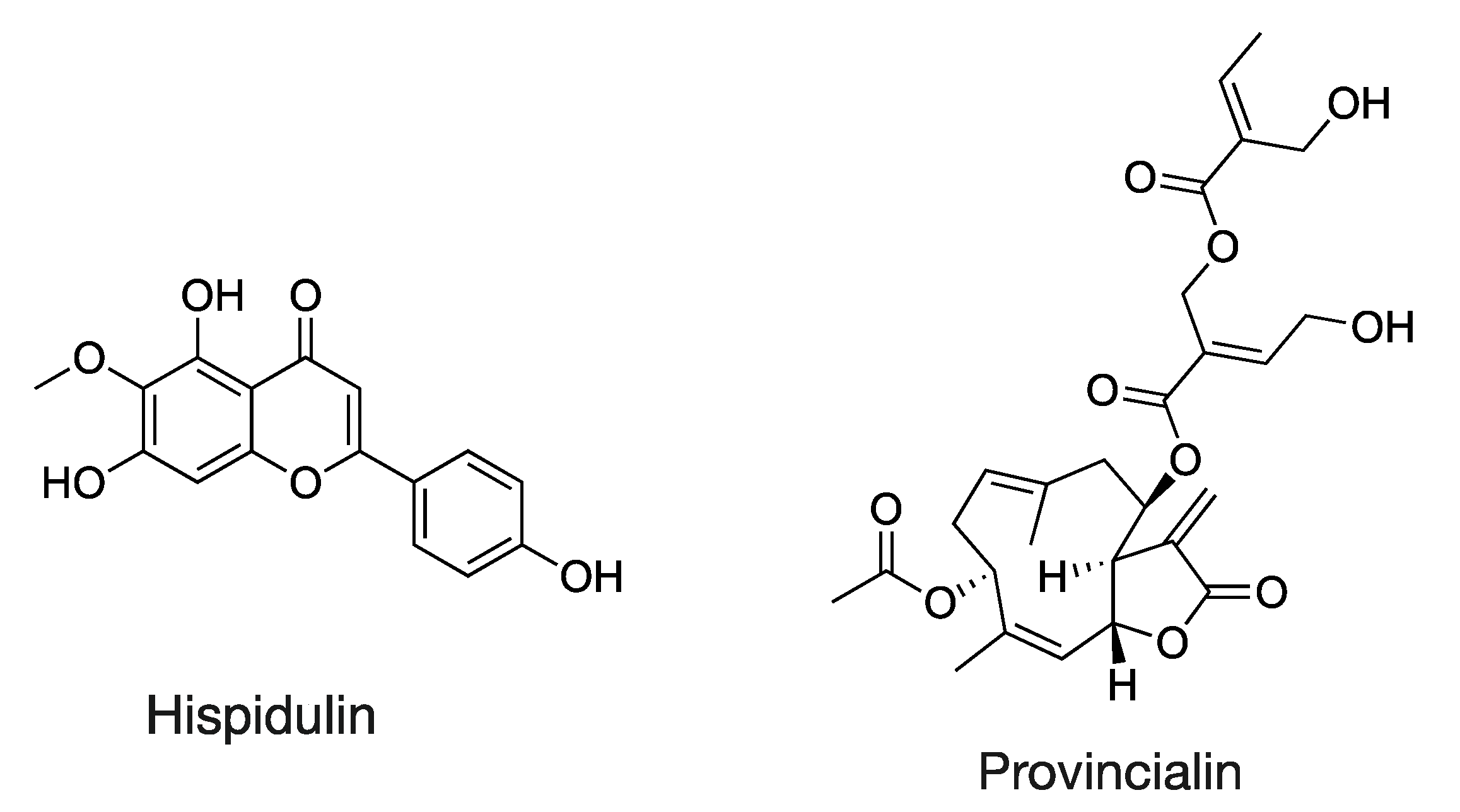
| Hispidulin | 300.0635_13.54 | C16H12O6 | +++ | d | d | Flavone | [M-H]− | 284.03, 136.98 ** |
| 3,7-Di-O-methyl quercetin | 330.0742_13.92 | C17H14O7 | +++ | d | d | Flavanol | [M-H]− | 271.02/299.02/314.04 |
| Naringenin | 272.0685_12.49 | C15H12O5 | +++ | d | d | Flavanone | [M-H]− | 151.00/119.05 ** |
| Sakuranetin | 286.0842_14.79 | C16H14O5 | +++ | d | d | Flavanone | [M-H]− | 119.04/165.01 ** |
| Dihydrokaempferol (Aromadendrin) | 288.0634_9.73 | C15H12O6 | +++ | d | d | Flavanonol | [M-H]− | 125.02/259.06 |
| Chrysin | 254.0578_15.57 | C15H10O4 | +++ | d | d | Flavone | [M-H]− | 253.05/143.05 ** |
| N-Feruloyltyramine | 313.1315_14.40 | C18H19NO4 | +++ | d | d | Amide | [M+H]+ | 120.08/103.05 |
| Secologanate | 374.1214_6.59 | C16H22O10 | +++ | d | d | Terpenoid | [M-H]− | 165.05/147.04 |
| Cinncassiol B | 422.1919_12.33 | C20H32O8 | +++ | d | d | Terpenoid | [M+Na-2H]− | 153.09 |
| Abscisic acid | 264.1362_11.07 | C15H20O4 | ++(Eg) | d | d | Sesquiterpene | [M-H]− | 204.11/219.13 ** |
| Corchorifatty acid F | 328.2253_14.51 | C18H32O5 | +++ | d | d | Fatty acid | [M-H]− | 211.13/229.14 |
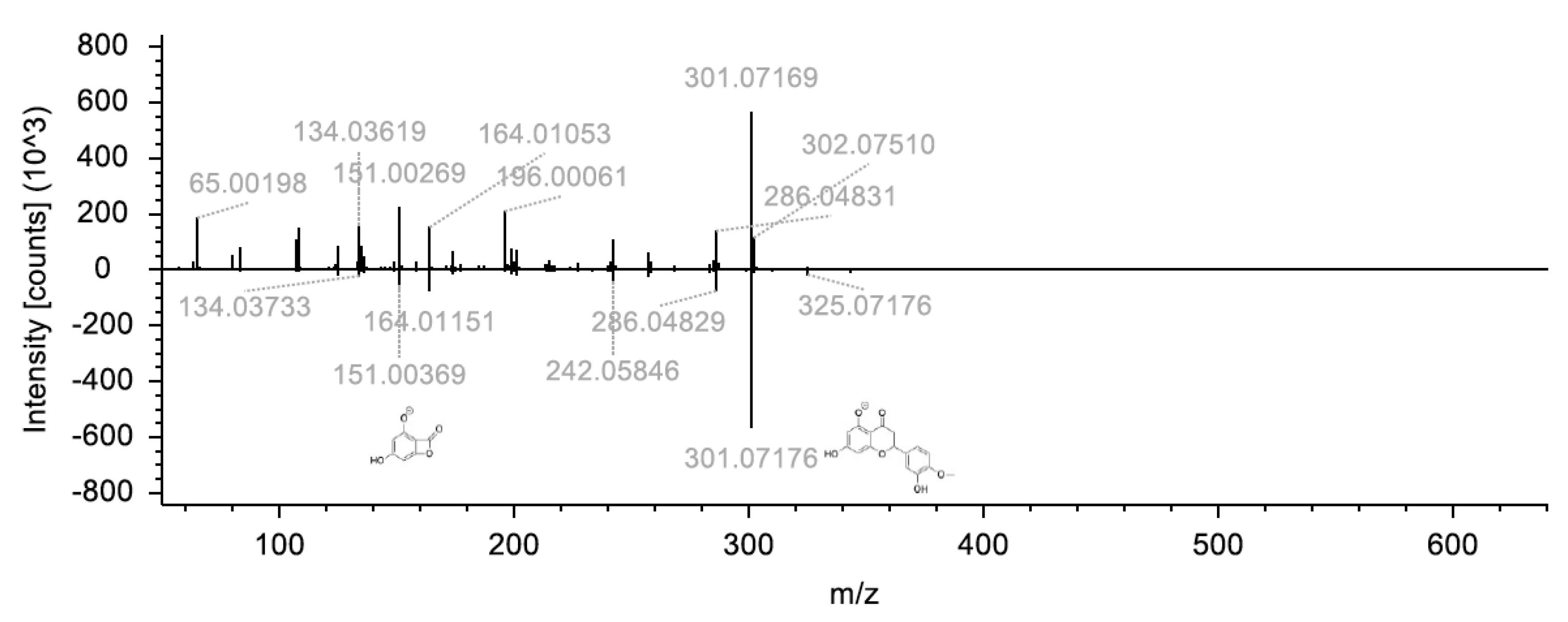
| Isorhamnetin 3-rutinoside 4”-rhamnoside | 770.2267_10.55 | C34H42O20 | +++ | d | d | Flavonoid glycoside | [M+H]+ | 301.07/286.04 |
| Pinocembrin | 256.0735_14.90 | C15H12O4 | +++ | ++(Eg) | d | Flavanone | [M+H]+ | 153.01/131.04 ** |
| Hesperetin | 302.0790_12.71 | C16H14O6 | +++ | d | d | Flavanone | [M+H]+ | 153.01/134.03 ** |
| Rutin | 610.1537_9.23 | C27H30O16 | +++ | d | d | Flavone | [M+H]+ | 287.05/317.06 ** |
| Sebacic acid | 202.1206_11.41 | C10H18O4 | ++(GR) | d | d | Carboxylic acid | [M+H]+ | 121.10/97.06 |
| Scopoletin | 192.0424_8.21 | C10H8O4 | ++(GR) | d | d | Coumarin | [M+H]+ | 193.04/133.02 ** |
| L-phenylalanine | 165.0790_2.93 | C9H11NO2 | ++(GR) | d | d | Amino acid | [M+H]+ | 120.08/103.05 |
| Acetophenone | 120.0576_10.81 | C8H8O | ++(GR) | d | d | Aromatic ketone | [M+H]+ | 121.06/103.05 ** |
| 4-Hydroxyquinoline | 145.0527_4.78 | C9H7NO | ++(GR) | d | d | Quinoline | [M+H]+ | 128.04 |
| 3Z-Hexenyl 2R-hydroxy-3-methylbutyrate | 200.1414_13.07 | C11H20O3 | d | ++(It) | d | Fatty acyls/Fatty esters | [M+H]+ | 67.05/55.05 |
| (3S, 7R)-iso-jasmonic acid | 210.1251_13.11 | C12H18O3 | d | ++(It) | d | Fatty acid | [M-H]− | 93.06/81.06 |
| 14-hydroxy-12-tetradecenoic acid | 242.1882_30.19 | C14H26O3 | d | ++(It) | d | Fatty acid | [M+H]+ | 95.08/81.06 |
| 9Z-Hexadecenamide | 253.2405_25.59 | C16H31NO | d | ++(It) | d | Fatty amide | [M+H]+ | 69.06/83.08 |
| Hesperidin | 610.1903_10.14 | C28H34O15 | d | ++(It) | d | Flavanone glycoside | [M-H]− | 301.07/151.00 ** |
| 10-Hydroxy-2-decenoic acid | 186.1248_13.04 | C10H18O3 | d | ++(It) | d | Fatty acid | [M-H]− | 185.11/139.11 ** |
| Hallactone B | 440.1160_9.69 | C20H24O9S | d | +++ | d | Terpenoid | [M-H]− | 393.10 |
| Provincialin | 518.2153_12.68 | C27H34O10 | d | +++ | d | Terpenoid | [M-H]− | 111.04 |
| Nepetaside | 346.1629_11.87 | C16H26O8 | d | ++(It) | d | Iridoid glucoside | [M-H]− | 183.10/185.11 |
| Patrinoside | 508.2155_10.37 | C21H34O11 | d | ++(It) | d | Iridoid glucoside | [M+FA-H]− | 183.10/139.11 |
| (4E, 6E, d14:2) sphingosine | 241.2041_30.19 | C14H27NO2 | d | ++(It) | d | Amino alcohol | [M+H]+ | 109.10/95.08 |
| Quercetin 3-O-sophoroside | 626.1491_8.15 | C27H30O17 | d | ++(It) | d | Flavonoid glycoside | [M-H]− | 300.02/271.02 ** |
| 11Z, 13E, 15-Hexadecatrienyl acetate | 278.2245_27.67 | C18H30O2 | d | d | +++ | Fatty acid ester | [M-H]− | 59.01 |
| Suberic acid | 174.0884_9.46 | C8H14O4 | d | d | ++(Gr) | Dicarboxylic acid | [M-H]− | 111.08/173.08 ** |
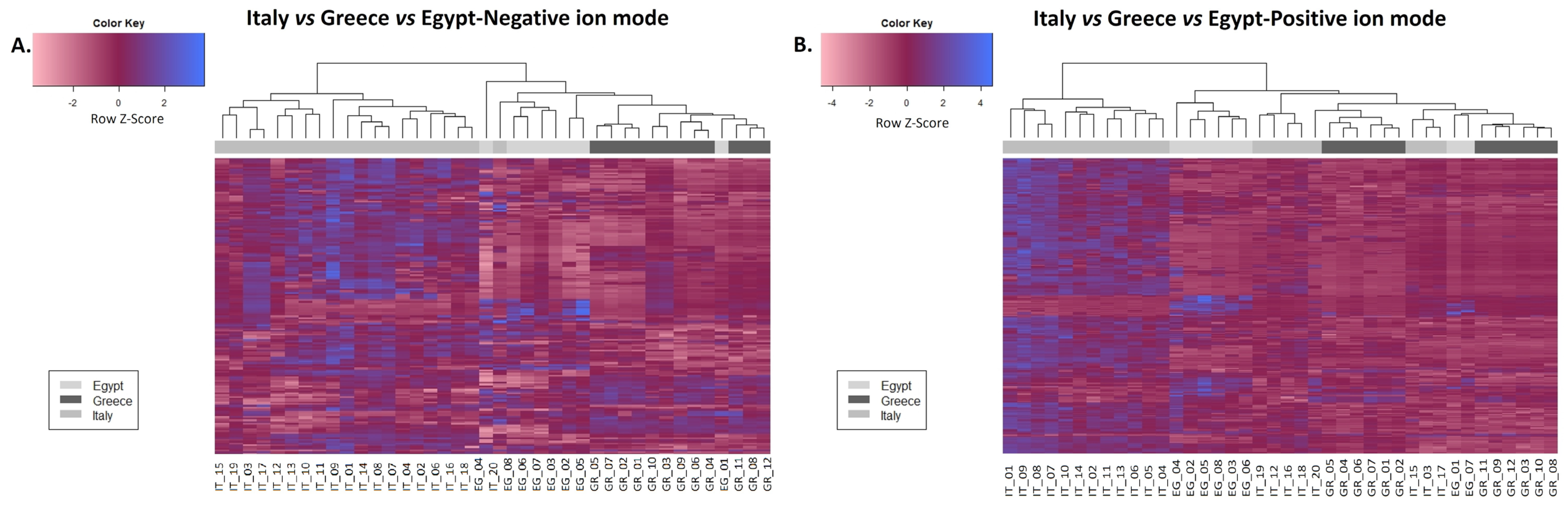
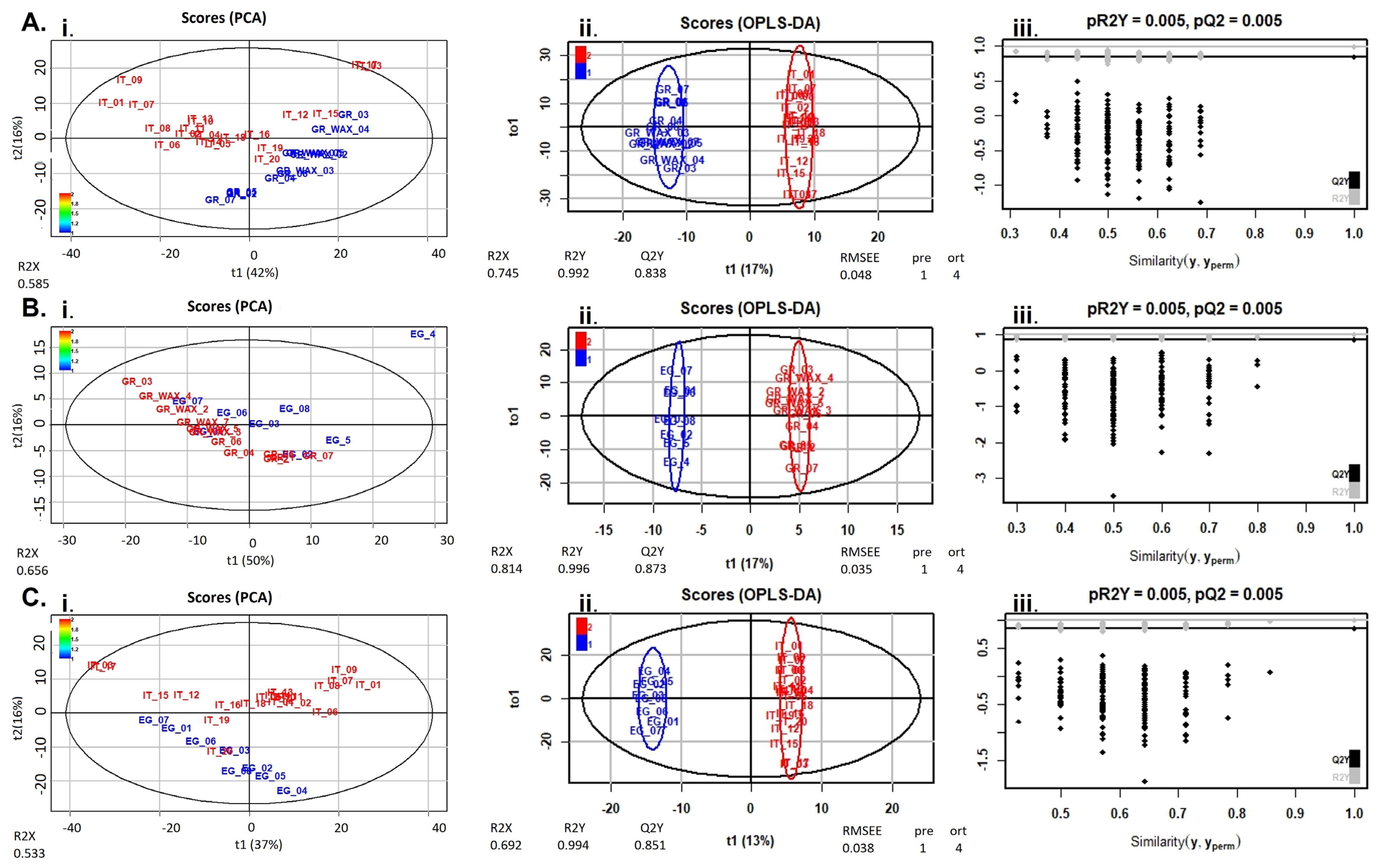

2.1.1. Flavonoids
2.1.2. Fatty and Organic Acids
2.1.3. Terpenes
2.1.4. Other Chemicals
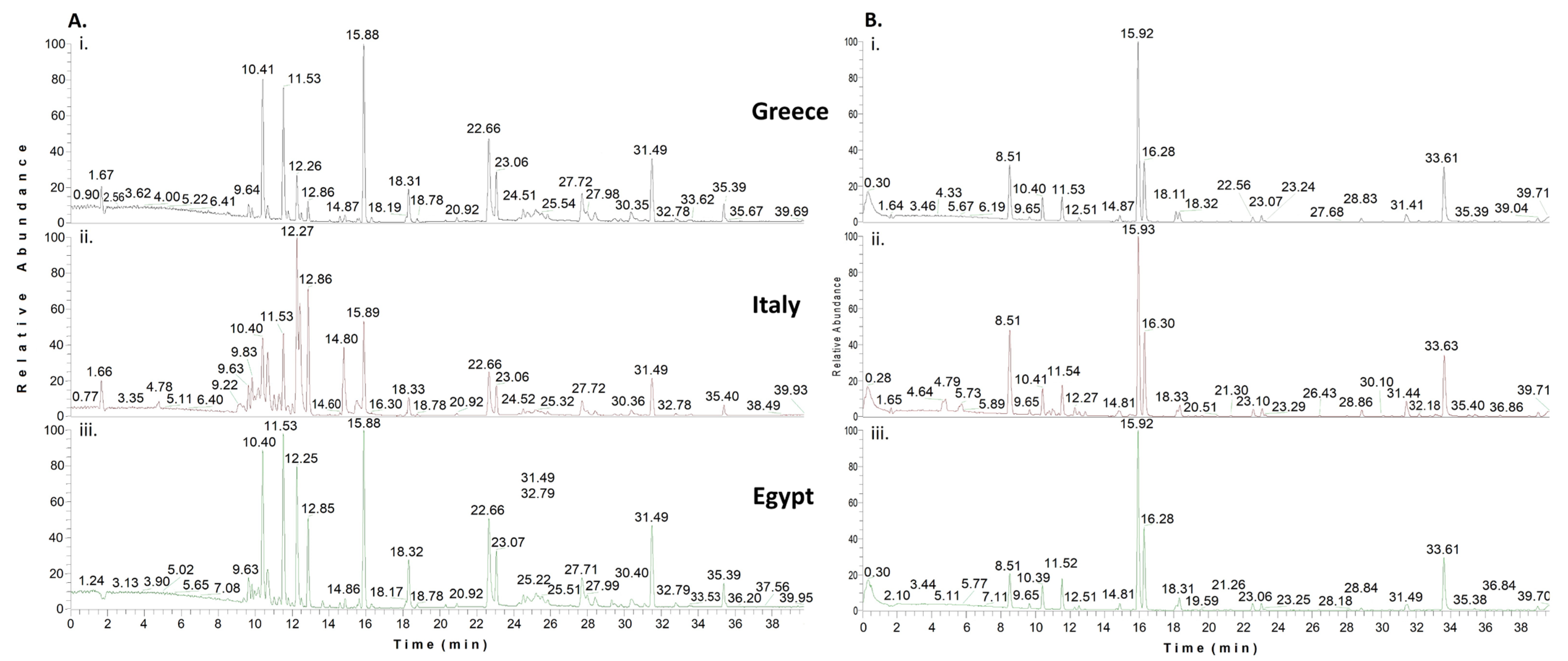
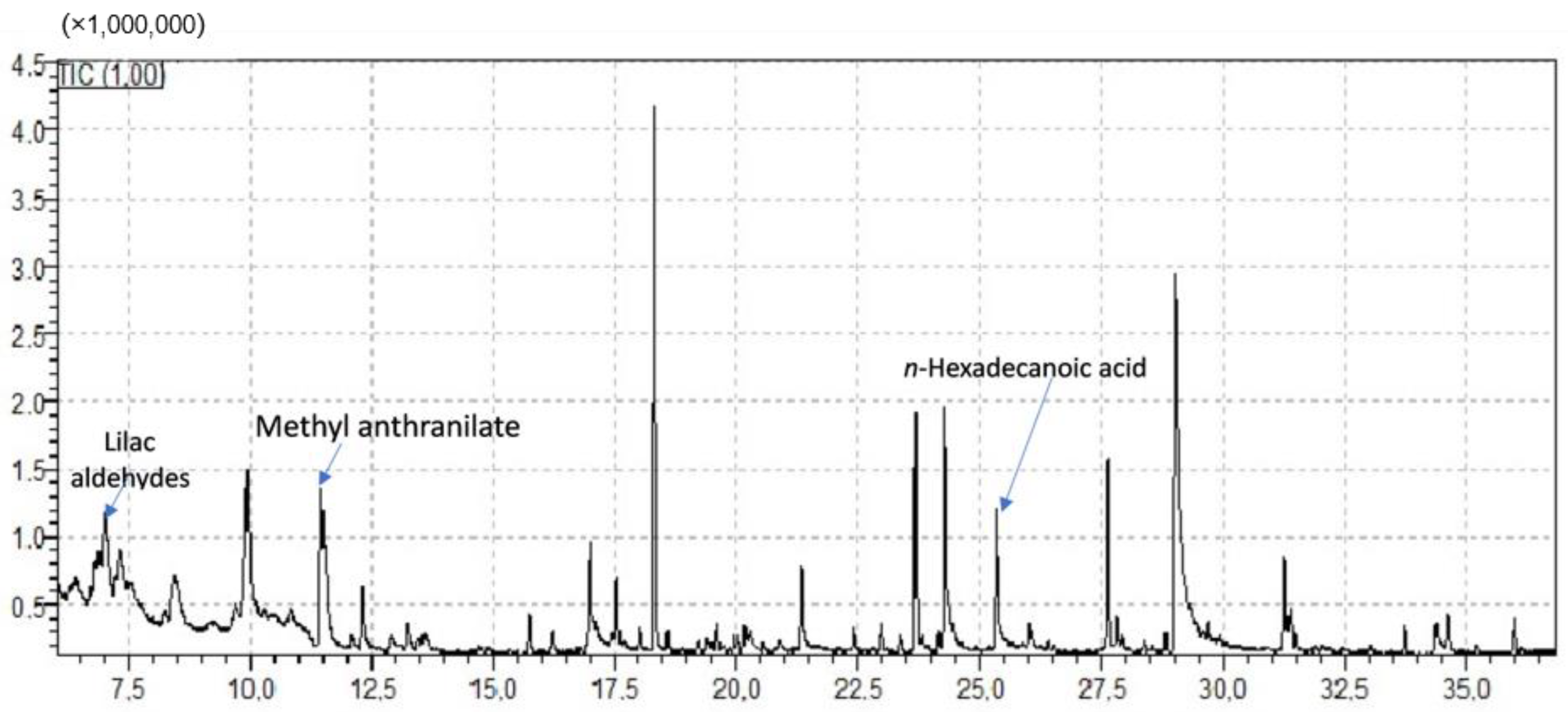
2.2. GC-MS Findings
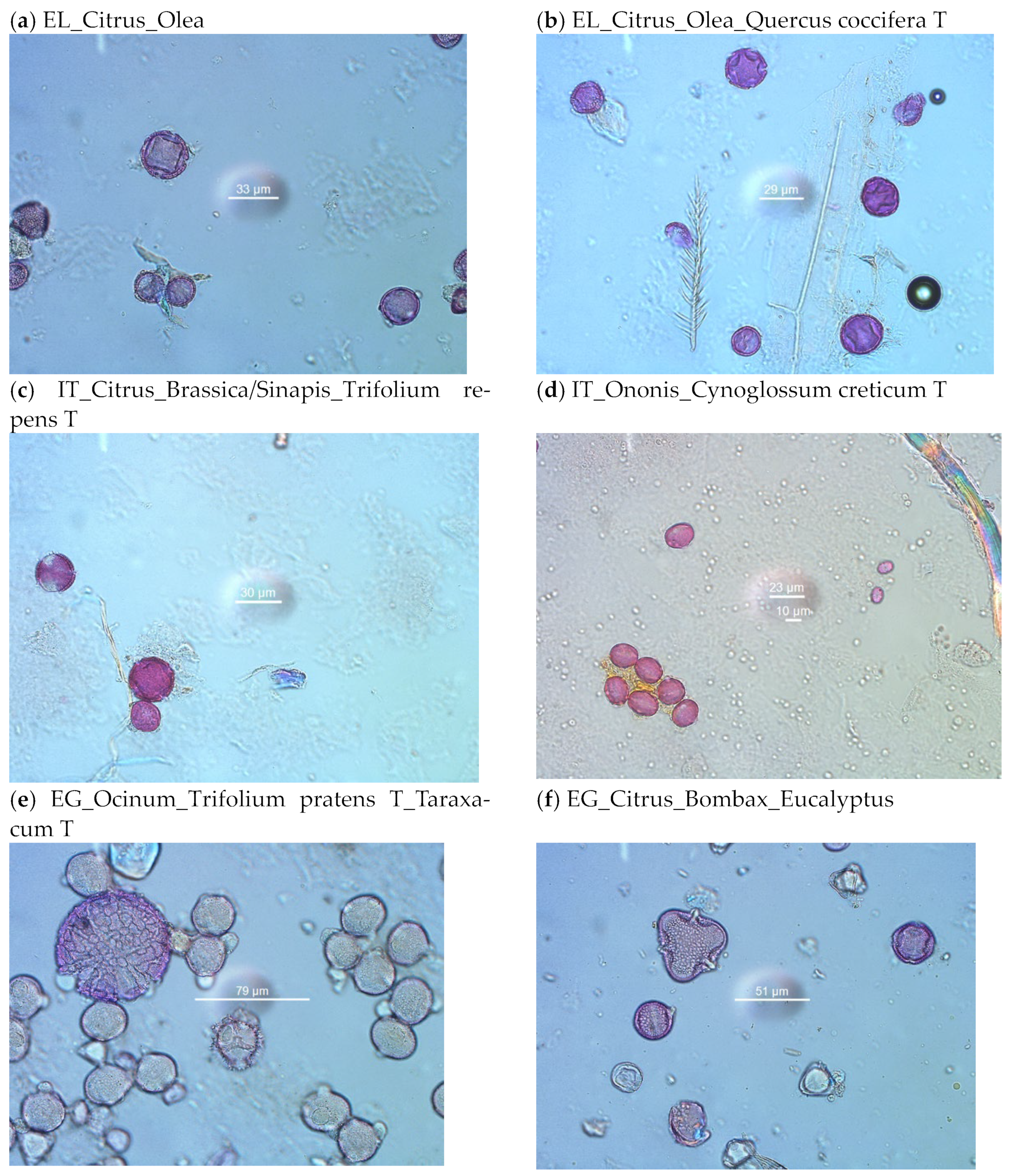
2.3. Melissopalynological Analysis
2.3.1. Melissopalynological Analysis of Citrus Honey from Sicily, Italy
| Family | Genus/ Species (*) | HONEY | HONEY | HONEYCOMB HONEY | HONEYCOMB HONEY | HONEYCOMB HONEY | HONEYCOMB HONEY | HONEYCOMB HONEY | HONEYCOMB HONEY | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Citrus-market | Citrus-market | Citrus-market | Citrus-market | Citrus-market | Citrus-market | Chiaramonte Gulfi (RG) | Chiaramonte Gulfi (RG) | Ispica (RG) | Ispica (RG) | Ispica (RG) | Ispica (RG) | Ispica (RG) | Chiaramonte Gulfi (RG) | Citrus-market | Citrus-market | Citrus-market | Citrus-market | Citrus-market | ||||||
| CH_IT SI_1 | CH_IT SI_2 | CH_IT SI_3 | CH_IT SI_4 | CH_IT SI_5 | CH_IT SI_6 | CH_IT SI_7 | CH_IT SI_8 | CH_IT SI_9 | CH_IT SI_10 | CH_IT SI_11 | CH_IT SI_12 | CH_IT SI_13 | CH_IT SI_14 | CH_IT SI_15 | CH_IT SI_16 | CH_IT SI_17 | CH_IT SI_18 | CH_IT SI_19 | Count | Mean | Min | Max | ||
| Fabaceae | (F1) | 67 | 32 | 25 | 41 | 47 | 15 | 69 | 68 | 32 | 35 | 82 | 59 | 36 | 66 | 23 | 52 | 59 | 34 | 92 | 19 | 49 | 15 | 92 |
| Brassicaceae | (B1) | 9 | 6 | 24 | 6 | 33 | 3 | 14 | 16 | 5 | 21 | 2 | 9 | 22 | 14 | 18 | 4 | 2 | 12 | 3 | 19 | 12 | 2 | 33 |
| Rutaceae | Citrus | 9 | 10 | 31 | 4 | 3 | 18 | 4 | 2 | 3 | 2 | 2 | <1 | 5 | 3 | 12 | 1 | 5 | 1 | 18 | 6 | <1 | 31 | |
| Rosaceae | (R1) | 1 | 2 | 1 | 1 | 1 | <1 | 6 | 4 | 3 | 2 | 2 | 4 | 8 | 2 | 4 | 4 | 1 | 1 | 18 | 3 | <1 | 8 | |
| Asteraceae | (A1) | 7 | 9 | 2 | 1 | 4 | 19 | 2 | 4 | 10 | 5 | 15 | 29 | 3 | 8 | 11 | 1 | 6 | 17 | 8 | 1 | 29 | ||
| Boraginaceae | (B2) | 3 | 33 | 12 | 44 | 9 | 25 | 2 | 26 | 5 | <1 | 6 | 5 | 3 | 8 | 1 | 15 | 12 | <1 | 44 | ||||
| Lamiaceae | (L1) | 4 | 2 | 1 | 8 | 3 | 3 | 2 | 2 | <1 | 3 | 10 | 3 | <1 | 8 | |||||||||
| Myrtaceae | Myrtus, Eucalyptus | 1 | 1 | 3 | 7 | 1 | 1 | 24 | 6 | 19 | 9 | 7 | 1 | 24 | ||||||||||
| Apiaceae | (A2) | 2 | 5 | 11 | <1 | 7 | 6 | 17 | 8 | 2 | 9 | 7 | <1 | 17 | ||||||||||
| Ranunculaceae | Clematis | 3 | 3 | 11 | 1 | 1 | 1 | 1 | 7 | 3 | 1 | 11 | ||||||||||||
| Fagaceae | Castanea | 10 | 1 | 2 | 6 | 1 | 10 | |||||||||||||||||
| Lythraceae | Lythrum | 8 | 2 | 2 | 5 | 2 | 8 | |||||||||||||||||
| SUM | 96 | 98 | 97 | 98 | 100 | 95 | 98 | 94 | 98 | 97 | 98 | 94 | 98 | 90 | 97 | 92 | 93 | 97 | 100 | |||||
2.3.2. Melissopalynological Analysis of Citrus Honey from Argos, Greece
| Family | Genus/Species (*) | Honey-Wax | Honey-Wax | Honey-Wax | Honey-Wax | Honey-Wax | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FIELD_1_001 | FIELD_2_001 | FIELD_2_002 | FIELD_3_001 | FIELD_3_002 | FIELD_4_001 | FIELD_4_002 | FIELD_5_001 | FIELD_5_002 | FIELD_6_001 | FIELD_7_001 | FIELD_7_002 | Count | Mean | Min | Max | ||
| Rutaceae | Citrus *1 | 17 | 66 | 35 | 79 | 63 | 66 | 75 | 36 | 67 | 24 | 33 | 73 | 12 | 53 | 17 | 79 |
| Brassicaceae | (B1) | 40 | 20 | 7 | 1 | 1 | 9 | 8 | 8 | 13 | 18 | 24 | 9 | 12 | 13 | 1 | 40 |
| Asteraceae | (A1) | 5 | 5 | 4 | 13 | 16 | 4 | 6 | 3 | 1 | 13 | 14 | 11 | 8 | 1 | 16 | |
| Boraginaceae | (B2) | 7 | 4 | 3 | 33 | 2 | 2 | 46 | 24 | 4 | 9 | 14 | 2 | 46 | |||
| Apiaceae | (A2) | <1 | 7 | 47 | <1 | 2 | <1 | <1 | 1 | 2 | 9 | 7 | <1 | 47 | |||
| Lamiaceae | (L1) | <1 | <1 | 1 | 1 | 1 | 4 | 3 | 7 | 8 | 2 | <1 | 7 | ||||
| Fabaceae | (F1) | 7 | 1 | 1 | 8 | 3 | 2 | 6 | 4 | 1 | 8 | ||||||
| Ericaceae | Erica, Arbutus | 2 | 1 | 4 | 3 | 3 | 5 | 3 | 1 | 4 | |||||||
| Rosaceae | (R1) | 17 | <1 | 2 | 1 | 4 | 5 | <1 | 17 | ||||||||
| Alliaceae | Allium | 1 | 2 | <1 | 6 | 3 | 3 | 1 | 6 | ||||||||
| Liliaceae | 2 | <1 | <1 | 1 | 4 | 1 | <1 | 2 | |||||||||
| Fagaceae | Castanea | 2 | 3 | 7 | 3 | 4 | 2 | 7 | |||||||||
| Myrtaceae | Eucalyptus | 2 | 1 | 6 | 3 | 3 | 1 | 6 | |||||||||
| Ranunculaceae | Clematis | <1 | <1 | 6 | 3 | 2 | <1 | 6 | |||||||||
| Araliaceae | Hedera | 6 | 3 | 2 | 5 | 3 | 6 | ||||||||||
| Asparagaceae | Asparagus | 1 | 7 | 2 | 4 | 1 | 7 | ||||||||||
| SUM | 96 | 99 | 100 | 99 | 100 | 99 | 100 | 99 | 97 | 99 | 98 | 100 |
2.3.3. Melissopalynological Analysis of Citrus Honey from Al Shaeir Island, Egypt
| Family | Genus/Species (*) | HBc-Control Citrus | HB1-CF Citrus | HB2-CF | HBc-AMPs Control | HB1-AMP + Citrus | HB2-AMP | HB1-Clover Control | HB1-Basil | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EG1-Citrus | EG2-Citrus | EG3-Clover | EG4-AMPs | EG5-Basil + Citrus | EG6-Basil + Borago+ Coriandrum+ Anethum + Carum | EG7-Clover | EG8-Basil | Count | Mean | Min | Max | ||
| Fabaceae | (F1) | 3 | 1 | 25 | 2 | <1 | 66 | 83 | 2 | 8 | 23 | <1 | 83 |
| Myrtaceae | Eucalyptus *1, Myrtus/Psidium | 3 | 62 | 50 | 57 | 26 | 16 | 46 | 7 | 37 | 3 | 62 | |
| Rutaceae | Citrus *1 | 79 | 29 | 18 | 1 | 6 | <1 | <1 | 7 | 19 | <1 | 79 | |
| Brassicaceae | (B1) | 3 | 4 | 1 | 91 | 24 | 3 | 6 | 21 | 1 | 91 | ||
| Apiaceae | (A2) | 2 | 2 | 7 | 6 | 1 | <1 | 6 | 3 | <1 | 7 | ||
| Malvaceae | (M1) | <1 | <1 | <1 | 2 | <1 | <1 | 6 | <1 | <1 | 2 | ||
| Lamiaceae | Ocinum | <1 | <1 | <1 | <1 | <1 | 5 | <1 | <1 | <1 | |||
| Asteraceae | (A1) | 7 | 1 | 5 | 3 | 4 | 1 | 7 | |||||
| Portulacaceae | Portulaca | 5 | <1 | <1 | 3 | 5 | 5 | 5 | |||||
| Asparagaceae | Asparagus/Other *1 | 47 | 1 | 47 | 47 | 47 | |||||||
| SUM | 98 | 99 | 100 | 99 | 95 | 96 | 99 | 98 |

2.4. Environmental Factors
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Honey Samples
3.3. Sample Preparation
3.4. Ultra-High-Performance Liquid Chromatography Coupled to Orbitrap High-Resolution Mass Spectrometry Analysis of Honey Extracts
3.5. High-Performance Liquid Chromatography Photo Diode Array Electrospray Mass Spectrometry (HPLC-PDA-ESI/MS)
3.6. GC-MS Analysis
SPME Holder and Fibers
3.7. Data Post-Processing and Chemometrics Analysis
3.8. Melissopalynological Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cucu, A.-A.; Baci, G.-M.; Moise, A.R.; Dezsi, S.; Marc, B.D.; Stângaciu, S.; Dezmirean, D.S. Towards a Better Understanding of Nutritional and Therapeutic Effects of Honey and Their Applications in Apitherapy. Appl. Sci. 2021, 11, 4190. [Google Scholar] [CrossRef]
- Viteri, R.; Zacconi, F.; Montenegro, G.; Giordano, A. Bioactive compounds in Apis mellifera monofloral honeys. J. Food Sci. 2021, 86, 1552–1582. [Google Scholar] [CrossRef] [PubMed]
- Feknous, N.; Boumendjel, M. Natural bioactive compounds of honey and their antimicrobial activity. Czech J. Food Sci. 2022, 40, 163–178. [Google Scholar] [CrossRef]
- Mohammed, M.E.A. Factors Affecting the Physicochemical Properties and Chemical Composition of Bee’s Honey. Food Rev. Int. 2020, 38, 1330–1341. [Google Scholar] [CrossRef]
- Oddo, L.P.; Piazza, M.G.; Sabatini, A.G.; Accorti, M. Characterization of unifloral honeys. Apidologie 1995, 26, 453–465. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality, composition and health-protective properties of citrus honey: A review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef]
- Citriculture. Available online: https://swfrec.ifas.ufl.edu/hlb/database/pdf/22_DOnghia_09.pdf (accessed on 20 March 2022).
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef]
- EC-Food-Fraud-Quality. European Commission, Knowledge Centre for Food Fraud and Quality. Available online: https://knowledge4policy.ec.europa.eu/food-fraud-quality/topic/food-fraud_en (accessed on 3 March 2023).
- European Union. Council-Directive. COUNCIL DIRECTIVE 2001/110/EC of 20 December 2001 Relating to Honey; European Union: Maastricht, The Netherlands, 2001. [Google Scholar]
- European Union. Council-Directive. Directive 2014/63/EU of the European Parliament and of the Council of 15 May 2014 amending Council Directive 2001/110/EC Relating to Honey; European Union: Maastricht, The Netherlands, 2014. [Google Scholar]
- Oddo, L.P.; Piro, R.; Bruneau, É.; Guyot-Declerck, C.; Ivanov, T.; Piskulová, J.; Flamini, C.; Lheritier, J.; Morlot, M.; Russmann, H.; et al. Main European unifloral honeys: Descriptive sheets. Apidologie 2004, 35 (Suppl. S1), S38–S81. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Daferera, D.; Tarantilis, P.A.; Polissiou, M.; Harizanis, P.C. Ultrasound-assisted extraction of volatile compounds from citrus flowers and citrus honey. Food Chem. 2003, 82, 575–582. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Nikolaou, C.; Karabagias, V.K. Volatile fingerprints of common and rare honeys produced in Greece: In search of PHVMs with implementation of the honey code. Eur. Food Res. Technol. 2018, 245, 23–39. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.; González-Viñas, M.; Pérez-Coello, M. Differentiation of monofloral citrus, rosemary, eucalyptus, lavender, thyme and heather honeys based on volatile composition and sensory descriptive analysis. Food Chem. 2009, 112, 1022–1030. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Evaluation of four isolation techniques for honey aroma compounds. J. Sci. Food Agric. 2005, 85, 91–97. [Google Scholar] [CrossRef]
- Bonvehi, J.S.; Coll, F.V. Characterization of Citrus Honey (Citrus spp.) Produced in Spain. J. Agric. Food Chem. 1995, 43, 2053–2057. [Google Scholar] [CrossRef]
- Castro-Vázquez, L.; Díaz-Maroto, M.; Pérez-Coello, M. Aroma composition and new chemical markers of Spanish citrus honeys. Food Chem. 2007, 103, 601–606. [Google Scholar] [CrossRef]
- Gattuso, G.; Barreca, D.; Gargiulli, C.; Leuzzi, U.; Caristi, C. Flavonoid Composition of Citrus Juices. Molecules 2007, 12, 1641–1673. [Google Scholar] [CrossRef] [PubMed]
- Danieli, P.P.; Lazzari, F. Honey Traceability and Authenticity. Review of Current Methods Most Used to Face this Problem. J. Apic. Sci. 2022, 66, 101–119. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Louppis, A.P.; Karabournioti, S.; Kontakos, S.; Papastephanou, C.; Kontominas, M.G. Characterization and geographical discrimination of commercial Citrus spp. honeys produced in different Mediterranean countries based on minerals, volatile compounds and physicochemical parameters, using chemometrics. Food Chem. 2017, 217, 445–455. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Baira, E.; Iosifidou, S.; Bergele, K.; Manea-Karga, E.; Theologidis, I.; Barmpouni, T.; Tsipi, D.; Machera, K. Characterization of Ikaria Heather Honey by Untargeted Ultrahigh-Performance Liquid Chromatography-High Resolution Mass Spectrometry Metabolomics and Melissopalynological Analysis. Front. Chem. 2022, 10, 663. [Google Scholar] [CrossRef]
- Koulis, G.A.; Tsagkaris, A.S.; Katsianou, P.A.; Gialouris, P.-L.P.; Martakos, I.; Stergiou, F.; Fiore, A.; Panagopoulou, E.I.; Karabournioti, S.; Baessmann, C.; et al. Thorough Investigation of the Phenolic Profile of Reputable Greek Honey Varieties: Varietal Discrimination and Floral Markers Identification Using Liquid Chromatography–High-Resolution Mass Spectrometry. Molecules 2022, 27, 4444. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Yang, S.; Zhang, W.; Zhang, J.; Zhao, W.; Chen, L.; Wen, Y.; Zhang, Y.; Lu, K.; et al. Strategy for comparative untargeted metabolomics reveals honey markers of different floral and geographic origins using ultrahigh-performance liquid chromatography-hybrid quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2017, 1499, 78–89. [Google Scholar] [CrossRef]
- Stavropoulou, M.-I.; Termentzi, A.; Kasiotis, K.M.; Cheilari, A.; Stathopoulou, K.; Machera, K.; Aligiannis, N. Untargeted Ultrahigh-Performance Liquid Chromatography-Hybrid Quadrupole-Orbitrap Mass Spectrometry (UHPLC-HRMS) Metabolomics Reveals Propolis Markers of Greek and Chinese Origin. Molecules 2021, 26, 456. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Brennan, L.; Fiehn, O.; Hankemeier, T.; Kristal, B.S.; van Ommen, B.; Pujos-Guillot, E.; Verheij, E.; Wishart, D.; Wopereis, S. Mass-spectrometry-based metabolomics: Limitations and recommendations for future progress with particular focus on nutrition research. Metabolomics 2009, 5, 435–458. [Google Scholar] [CrossRef]
- Zhang, W.; Lei, Z.; Huhman, D.; Sumner, L.W.; Zhao, P.X. MET-XAlign: A Metabolite Cross-Alignment Tool for LC/MS-Based Comparative Metabolomics. Anal. Chem. 2015, 87, 9114–9119. [Google Scholar] [CrossRef] [PubMed]
- De Vos, R.C.H.; Moco, S.; Lommen, A.; Keurentjes, J.; Bino, R.J.; Hall, R. Untargeted large-scale plant metabolomics using liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2007, 2, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, L.; Armada, D.; Celeiro, M.; Dagnac, T.; Llompart, M. Evaluating the Presence and Contents of Phytochemicals in Honey Samples: Phenolic Compounds as Indicators to Identify Their Botanical Origin. Foods 2021, 10, 2616. [Google Scholar] [CrossRef]
- Kanakis, P.; Termentzi, A.; Michel, T.; Gikas, E.; Halabalaki, M.; Skaltsounis, A.-L. From Olive Drupes to Olive Oil. An HPLC-Orbitrap-based Qualitative and Quantitative Exploration of Olive Key Metabolites. Planta Medica 2013, 79, 1576–1587. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Karabournioti, S. Discrimination of Clover and Citrus Honeys from Egypt According to Floral Type Using Easily Assessable Physicochemical Parameters and Discriminant Analysis: An External Validation of the Chemometric Approach. Foods 2018, 7, 70. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Nayik, G.A. Machine Learning Algorithms Applied to Semi-Quantitative Data of the Volatilome of Citrus and Other Nectar Honeys with the Use of HS-SPME/GC–MS Analysis, Lead to a New Index of Geographical Origin Authentication. Foods 2023, 12, 509. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- He, W.; Laaksonen, O.; Tian, Y.; Haikonen, T.; Yang, B. Chemical Composition of Juices Made from Cultivars and Breeding Selections of European Pear (Pyrus communis L.). J. Agric. Food Chem. 2022, 70, 5137–5150. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Martos, I.; Ferreres, F.; Radovic, B.S.; Anklam, E. HPLC flavonoid profiles as markers for the botanical origin of European unifloral honeys. J. Sci. Food Agric. 2001, 81, 485–496. [Google Scholar] [CrossRef]
- Martos, I.; Ferreres, F.; Tomás-Barberán, F.A. Identification of Flavonoid Markers for the Botanical Origin of Eucalyptus Honey. J. Agric. Food Chem. 2000, 48, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Tomas-Barberan, F.; Tomás-Lorente, F.; Ferreres, F.; Garcia-Viguera, C. Flavonoids as biochemical markers of the plant origin of bee pollen. J. Sci. Food Agric. 1989, 47, 337–340. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Truchado, P.; Ferreres, F.; Tomas-Barberan, F.A. Liquid chromatography–tandem mass spectrometry reveals the widespread occurrence of flavonoid glycosides in honey, and their potential as floral origin markers. J. Chromatogr. A 2009, 1216, 7241–7248. [Google Scholar] [CrossRef] [PubMed]
- Biluca, F.C.; de Gois, J.S.; Schulz, M.; Braghini, F.; Gonzaga, L.V.; Maltez, H.F.; Rodrigues, E.; Vitali, L.; Micke, G.A.; Borges, D.L.; et al. Phenolic compounds, antioxidant capacity and bioaccessibility of minerals of stingless bee honey (Meliponinae). J. Food Compos. Anal. 2017, 63, 89–97. [Google Scholar] [CrossRef]
- Hamed, A.R.; El-Hawary, S.S.; Ibrahim, R.M.; Abdelmohsen, U.R.; El-Halawany, A.M. Identification of Chemopreventive Components from Halophytes Belonging to Aizoaceae and Cactaceae through LC/MS—Bioassay Guided Approach. J. Chromatogr. Sci. 2020, 59, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Tourn, E.; Gallez, L.M.; Moreno, D.A.; Ferreres, F.; Tomás-Barberán, F.A. Identification of Botanical Biomarkers in Argentinean Diplotaxis Honeys: Flavonoids and Glucosinolates. J. Agric. Food Chem. 2010, 58, 12678–12685. [Google Scholar] [CrossRef]
- Čeksteryté, V.; Kurtinaitienė, B.O.; Venskutonis, P.R.; Pukalskas, A.; Kazernavičiūtė, R.I.; Balžekas, J.O. Evaluation of antioxidant activity and flavonoid composition in differently preserved bee products. Czech J. Food Sci. 2016, 34, 133–142. [Google Scholar] [CrossRef]
- Marzouk, M.S.; El-Toumy, S.; Merfort, I.; Nawwar, M.A. Polyphenolic metabolites of Rhamnus disperma. Phytochemistry 1999, 52, 943–946. [Google Scholar] [CrossRef]
- Kundo, N.K.; Manik, I.N.; Biswas, K.; Khatun, R.; Al-Amin, Y.; Alam, A.H.M.K.; Tanaka, T.; Sadik, G. Identification of Polyphenolics from Loranthus globosus as Potential Inhibitors of Cholinesterase and Oxidative Stress for Alzheimer’s Disease Treatment. BioMed Res. Int. 2021, 2021, 9154406. [Google Scholar] [CrossRef] [PubMed]
- Itokawa, H.; Oshida, Y.; Ikuta, A.; Inatomi, H.; Ikegami, S. Flavonol glycosides from the flowers of Cucurbita pepo. Phytochemistry 1981, 20, 2421–2422. [Google Scholar] [CrossRef]
- Lobo, J.A.; Méndez, Y.B. Diversity and foraging patterns of bees on flowers of Cucurbita pepo (Cucurbitaceae) in Costa Rica. Rev. De Biol. Trop. 2021, 69, 494–506. [Google Scholar] [CrossRef]
- Vidal, M.D.G.; De Jong, D.; Wien, H.C.; Morse, R.A. Pollination and fruit set in pumpkin (Cucurbita pepo) by honey bees. Braz. J. Bot. 2010, 33, 106–113. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Tradit. Complement. Med. 2016, 7, 360–366. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; García-Viguera, C.; Vit-Olivier, P.; Ferreres, F.; Tomás-Lorente, F. Phytochemical evidence for the botanical origin of tropical propolis from Venezuela. Phytochemistry 1993, 34, 191–196. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly and its unique fatty acid, 10-hydroxy-trans-2-decenoic acid, promote neurogenesis by neural stem/progenitor cells in vitro. Biomed. Res. 2007, 28, 261–266. [Google Scholar] [CrossRef]
- Brown, W.H.; Felauer, E.E.; Smith, M.V. Biosynthesis of Royal Jelly Acid from Sucrose. Nature 1962, 195, 75–76. [Google Scholar] [CrossRef]
- Plettner, E.; Slessor, K.N.; Winston, M.L. Biosynthesis of Mandibular Acids in Honey Bees (Apis mellifera): De novo Synthesis, Route of Fatty Acid Hydroxylation and Caste Selective β-Oxidation. Insect Biochem. Mol. Biol. 1998, 28, 31–42. [Google Scholar] [CrossRef]
- Blechert, S.; Brodschelm, W.; Hölder, S.; Kammerer, L.; Kutchan, T.M.; Mueller, M.J.; Xia, Z.Q.; Zenk, M.H. The octadecanoic pathway: Signal molecules for the regulation of secondary pathways. Proc. Natl. Acad. Sci. USA 1995, 92, 4099–4105. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cai, W.-J.; Yu, L.; Ding, J.; Feng, Y.-Q. Comprehensive Profiling of Phytohormones in Honey by Sequential Liquid–Liquid Extraction Coupled with Liquid Chromatography–Mass Spectrometry. J. Agric. Food Chem. 2017, 65, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Raviv, Z.; Cohen, S.; Reischer-Pelech, D. The anti-cancer activities of jasmonates. Cancer Chemother. Pharmacol. 2012, 71, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Radhika, V.; Kost, C.; Boland, W.; Heil, M. The Role of Jasmonates in Floral Nectar Secretion. PLoS ONE 2010, 5, e9265. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Shimada, H.; Yoshizumi, S.; Saka, M.; Yamahara, J.; Matsuda, H. Medicinal Foodstuffs. XIV. On the Bioactive Constituents of Moroheiya. (2): New Fatty Acids, Corchorifatty Acids A, B, C, D, E, and F, from the Leaves of Corchorus olitorius L. (Tiliaceae): Structures and Inhibitory Effect on NO Production in Mouse Peritoneal Macrophages. Chem. Pharm. Bull. 1998, 46, 1008–1014. [Google Scholar] [CrossRef]
- Cabañas-García, E.; Areche, C.; Jáuregui-Rincón, J.; Cruz-Sosa, F.; Balch, E.P.-M. Phytochemical Profiling of Coryphantha macromeris (Cactaceae) Growing in Greenhouse Conditions Using Ultra-High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2019, 24, 705. [Google Scholar] [CrossRef]
- Silva, E.L.; Almeida-Lafetá, R.C.; Borges, R.M.; Staerk, D. Dual high-resolution inhibition profiling and HPLC-HRMS-SPE-NMR analysis for identification of α-glucosidase and radical scavenging inhibitors in Solanum americanum Mill. Fitoterapia 2017, 118, 42–48. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Zhuo, Q.; Chen, X.; Liu, T.; Zhang, S.-Q. Quantitative and Discriminative Evaluation of Contents of Phenolic and Flavonoid and Antioxidant Competence for Chinese Honeys from Different Botanical Origins. Molecules 2018, 23, 1110. [Google Scholar] [CrossRef]
- Zhou, N.; Sun, Y.-P.; Zheng, X.-K.; Wang, Q.-H.; Yang, Y.-Y.; Bai, Z.-Y.; Kuang, H.-X.; Feng, W.-S. A Metabolomics-Based Strategy for the Mechanism Exploration of Traditional Chinese Medicine: Descurainia Sophia Seeds Extract and Fractions as a Case Study. Evid.-Based Complement. Altern. Med. 2017, 2017, 2845173. [Google Scholar] [CrossRef]
- Li, W.; Liu, C.; He, M.; Li, J.; Cai, Y.; Ma, Y.; Xu, J. Largely different contents of terpenoids in beef red-flesh tangerine and its wild type. BMC Plant Biol. 2017, 17, 36. [Google Scholar] [CrossRef]
- Niogret, J.; Epsky, N.D.; Schnell, R.J.; Boza, E.J.; Kendra, P.E.; Heath, R.R. Terpenoid Variations within and among Half-Sibling Avocado Trees, Persea americana Mill. (Lauraceae). PLoS ONE 2013, 8, e73601. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Jerković, I.; Bifulco, E.; Marijanovic, Z.; Congiu, F.; Bubalo, D. Riboflavin and lumichrome in Dalmatian sage honey and other unifloral honeys determined by LC–DAD technique. Food Chem. 2012, 135, 1985–1990. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Bifulco, E.; Caboni, P.; Sarais, G.; Cottiglia, F.; Floris, I. Lumichrome and Phenyllactic Acid as Chemical Markers of Thistle (Galactites tomentosa Moench) Honey. J. Agric. Food Chem. 2010, 59, 364–369. [Google Scholar] [CrossRef]
- Cavendish, R.L.; Santos, J.D.S.; Neto, R.B.; Paixão, A.O.; Oliveira, J.V.; de Araujo, E.D.; e Silva, A.A.B.; Thomazzi, S.M.; Cardoso, J.C.; Gomes, M.Z. Antinociceptive and anti-inflammatory effects of Brazilian red propolis extract and formononetin in rodents. J. Ethnopharmacol. 2015, 173, 127–133. [Google Scholar] [CrossRef]
- Karlander, S.-G.; Karlsson, K.-A.; Leffler, H.; Lilja, A.; Samuelsson, B.; Steen, G. The structure of sphingomyelin of the honey bee (Apis mellifera). Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1972, 270, 117–131. [Google Scholar] [CrossRef]
- Morikawa, T.; Ando, S.; Matsuda, H.; Kataoka, S.; Muraoka, O.; Yoshikawa, M. Inhibitors of Nitric Oxide Production from the Rhizomes of Alpinia galanga: Structures of New 8-9’ Linked Neolignans and Sesquineolignan. Chem. Pharm. Bull. 2005, 53, 625–630. [Google Scholar] [CrossRef]
- Seiji, O.; Hirohide, H. Polyphenols in Salix species II. Polyphenols from the bark of Salix pet-susu Kimura. Mokuzai Gakkaishi/J. Jpn. Wood Res. Soc. 1996, 42, 618–623. [Google Scholar]
- Ratana, B.; Pimpinum, P.; Aree, N.; Wilart, P.; Prachya, K. 4′-hydroxycinnamaldehyde from Alpinia galanga (Linn.) induces human leukemic cell apoptosis via mitochondrial and endoplasmic reticulum stress pathways. Asian Pac. J. Cancer Prev. 2011, 12, 593–598. [Google Scholar]
- Alissandrakis, E.; Kibaris, A.C.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Flavour compounds of Greek cotton honey. J. Sci. Food Agric. 2005, 85, 1444–1452. [Google Scholar] [CrossRef]
- Antika, L.D.; Tasfiyati, A.N.; Hikmat, H.; Septama, A.W. Scopoletin: A review of its source, biosynthesis, methods of extraction, and pharmacological activities. Z. Nat. C 2022, 77, 303–316. [Google Scholar] [CrossRef]
- Kesnerova, L.; Mars, R.A.T.; Ellegaard, K.M.; Troilo, M.; Sauer, U.; Engel, P. Disentangling metabolic functions of bacteria in the honey bee gut. PLoS Biol. 2017, 15, e2003467. [Google Scholar] [CrossRef]
- Machado, A.M.; Miguel, M.G.; Vilas-Boas, M.; Figueiredo, A.C. Honey Volatiles as a Fingerprint for Botanical Origin—A Review on their Occurrence on Monofloral Honeys. Molecules 2020, 25, 374. [Google Scholar] [CrossRef]
- Alissandrakis, E.; Tarantilis, P.A.; Harizanis, P.C.; Polissiou, M. Comparison of the Volatile Composition in Thyme Honeys from Several Origins in Greece. J. Agric. Food Chem. 2007, 55, 8152–8157. [Google Scholar] [CrossRef]
- Seisonen, S.; Kivima, E.; Vene, K. Characterisation of the aroma profiles of different honeys and corresponding flowers using solid-phase microextraction and gas chromatography–mass spectrometry/olfactometry. Food Chem. 2015, 169, 34–40. [Google Scholar] [CrossRef]
- Ferreres, F.; Andrade, P.; Tomás-Barberán, F.A. Natural Occurrence of Abscisic Acid in Heather Honey and Floral Nectar. J. Agric. Food Chem. 1996, 44, 2053–2056. [Google Scholar] [CrossRef]
- Makowicz, E.; Kafarski, P.; Jasicka-Misiak, I. Chromatographic fingerprint of the volatile fraction of rare Hedera helix honey and biomarkers identification. Eur. Food Res. Technol. 2018, 244, 2169–2179. [Google Scholar] [CrossRef]
- Khallouki, F.; Akdad, M.; Bouddine, T.; Hajji, L.; Owen, R.W. HPLC-ESI-MS and GC-EI-MS Identification and Quantitation of Polyphenolics and Alkaloids in Moroccan Jujube Honeys. J. Apic. Sci. 2020, 64, 287–299. [Google Scholar] [CrossRef]
- Yuan, H.-Y.; Kwaku, O.R.; Pan, H.; Han, J.-X.; Yang, C.-R.; Xu, M. Iridoid glycosides from the Genus Gentiana (Gentianaceae) and their Chemotaxonomic Sense. Nat. Prod. Commun. 2017, 12, 1663–1670. [Google Scholar] [CrossRef]
- Xie, S.; Uesato, S.; Inouye, H.; Fujita, T.; Murai, F.; Tagawa, M.; Shingu, T. Absolute structure of nepetaside, a new iridoid glucoside from Nepeta cataria. Phytochemistry 1988, 27, 469–472. [Google Scholar] [CrossRef]
- Valiakos, E.; Marselos, M.; Sakellaridis, N.; Constantinidis, T.; Skaltsa, H. Ethnopharmacological approach to the herbal medicines of the “Antidotes” in Nikolaos Myrepsos’ Dynameron. J. Ethnopharmacol. 2015, 163, 68–82. [Google Scholar] [CrossRef]
- Arshad, A.; Ahemad, S.; Saleem, H.; Saleem, M.; Zengin, G.; Abdallah, H.H.; Tousif, M.I.; Ahemad, N.; Mahomoodally, M.F. RP-UHPLC-MS Chemical Profiling, Biological and In Silico Docking Studies to Unravel the Therapeutic Potential of Heliotropium crispum Desf. as a Novel Source of Neuroprotective Bioactive Compounds. Biomolecules 2021, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Shin, H.-S.; Tundis, R.; Gonçalves, S.; Tantengco, O.A.G.; Campos, M.G.; Acquaviva, R.; Malfa, G.A.; Romano, A.; Robles, J.A.H.; et al. Plant Species of Sub-Family Valerianaceae—A Review on Its Effect on the Central Nervous System. Plants 2021, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Pieri, V.; Schwaiger, S.; Ellmerer, E.P.; Stuppner, H. Iridoid Glycosides from the Leaves of Sambucus ebulus. J. Nat. Prod. 2009, 72, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Tomassini, L.; Ventrone, A.; Frezza, C.; Cometa, M.F. A new iridoid diglycoside from Sambucus ebulus L. Nat. Prod. Res. 2019, 34, 2137–2143. [Google Scholar] [CrossRef]
- Demirezer, L.; Güvenalp, Z.; Schiewe, H.-J.; Strietzel, I.; Harmandar, M.; Zeeck, A. Iridoids from Centranthus longiflorus subsp. longiflorus. Phytochemistry 1999, 51, 909–912. [Google Scholar] [CrossRef]
- Tomassini, L.; Foddai, S.; Ventrone, A.; Nicoletti, M. Iridoid Glucosides from Viburnum Macrocephalum. Nat. Prod. Commun. 2008, 3, 845–846. [Google Scholar] [CrossRef]
- Taguchi, H.; Endo, T. Patrinoside, a new iridoid glycoside from Patrinia scabiosaefolia. Chem. Pharm. Bull. 1974, 22, 1935–1937. [Google Scholar] [CrossRef]
- Losito, I.; Abbattista, R.; De Ceglie, C.; Castellaneta, A.; Calvano, C.D.; Cataldi, T.R. Bioactive Secoiridoids in Italian Extra-Virgin Olive Oils: Impact of Olive Plant Cultivars, Cultivation Regions and Processing. Molecules 2021, 26, 743. [Google Scholar] [CrossRef]
- Kanaze, F.I.; Termentzi, A.; Gabrieli, C.; Niopas, I.; Georgarakis, M.; Kokkalou, E. The phytochemical analysis and antioxidant activity assessment of orange peel (Citrus sinensis) cultivated in Greece-Crete indicates a new commercial source of hesperidin. Biomed. Chromatogr. 2008, 23, 239–249. [Google Scholar] [CrossRef]
- Swaileh, K.M.; Abdulkhaliq, A. Analysis of aflatoxins, caffeine, nicotine and heavy metals in Palestinian multifloral honey from different geographic regions. J. Sci. Food Agric. 2013, 93, 2116–2120. [Google Scholar] [CrossRef]
- Tette, P.A.; Guidi, L.R.; Bastos, E.M.; Fernandes, C.; Gloria, M.B.A. Synephrine—A potential biomarker for orange honey authenticity. Food Chem. 2017, 229, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xue, A.; Li, X.; Huang, X.; Ning, F.; Zhang, X.; Liu, T.; Chen, H.; Luo, L. Analysis of chemical composition of nectars and honeys from Citrus by extractive electrospray ionization high resolution mass spectrometry. LWT Food Sci. Technol. 2020, 131, 109748. [Google Scholar] [CrossRef]
- Rowe, L.; Gibson, D.; Bahlai, C.A.; Gibbs, J.; Landis, D.A.; Isaacs, R. Flower traits associated with the visitation patterns of bees. Oecologia 2020, 193, 511–522. [Google Scholar] [CrossRef] [PubMed]
- De Ibarra, N.H.; Holtze, S.; Bäucker, C.; Sprau, P.; Vorobyev, M. The role of colour patterns for the recognition of flowers by bees. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20210284. [Google Scholar] [CrossRef] [PubMed]
- Von Der Ohe, W.; Oddo, L.P.; Piana, M.L.; Morlot, M.; Martin, P. Harmonized methods of melissopalynology. Apidologie 2004, 35, S18–S25. [Google Scholar] [CrossRef]
- Thrasyvoulou, A.; Tananaki, C.; Goras, G.; Karazafiris, E.; Dimou, M.; Liolios, V.; Kanelis, D.; Gounari, S. Legislation of honey criteria and standards. J. Apic. Res. 2018, 57, 88–96. [Google Scholar] [CrossRef]
- Tomaseli, V.; Ferrauto, G.; Longhitano, N.; Zizza, A. La flora Apistica dei Monti Iblei (Sicilia Sud-Orientale). Tec. Agric. 1999, 4, 89–120. [Google Scholar]
- Hatjina, F.; Dimou, M.; Haristos, L.; Kostarelou-Damianidou, M. Pollen Grain Characteristics. Of Plants from Peloponnese. Available online: https://www.apiservices.biz/documents/articles-en/pollen_grain_characteristics_of_plants_from_peloponnese.pdf (accessed on 10 December 2022).
- Vascular-Plants-Checklist-of-Greece. Available online: https://portal.cybertaxonomy.org/flora-greece/intro (accessed on 20 November 2022).
- Galarini, R.; Ricciardelli D’Albore, M.; Ricciardelli D’Albore, G.; Mediterranean-Melissopalynology. Mediterranean Melissopalynology. Available online: http://www.izsum.it/Melissopalynology/ (accessed on 8 September 2022).
- Esmael, M.E.M.; Salem, M.H.A.; Mahgoub, M.S.E.; El-Barbary, N.S.S. Photographer Guide of Pollen Grains Collected from Apiaries, Beheira Governorates—In Alexandria and El (West Nile Delta, Egypt). Alex. J. Agric. Sci. 2016, 61, 267–290. [Google Scholar]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, L.; Yu, M.; Li, Z.; Ke, Z.; Qian, X.; Ruan, X.; He, L.; Wei, F.; Zhao, Y.; et al. Seasonal variation influences flavonoid biosynthesis path and content, and antioxidant activity of metabolites in Tetrastigma hemsleyanum Diels & Gilg. PLoS ONE 2022, 17, e0265954. [Google Scholar] [CrossRef]
- Devkota, A.; Dall’Acqua, S.; Comai, S.; Innocenti, G.; Jha, P.K. Centella asiatica (L.) urban from Nepal: Quali-quantitative analysis of samples from several sites, and selection of high terpene containing populations for cultivation. Biochem. Syst. Ecol. 2010, 38, 12–22. [Google Scholar] [CrossRef]
- Sun, C.; Shang, X.; Ding, H.; Cao, Y.; Fang, S. Natural variations in flavonoids and triterpenoids of Cyclocarya paliurus leaves. J. For. Res. 2020, 32, 805–814. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Anastasiadou, P.; Papadopoulos, A.; Machera, K. Revisiting Greek Propolis: Chromatographic Analysis and Antioxidant Activity Study. PLoS ONE 2017, 12, e0170077. [Google Scholar] [CrossRef]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Wolf, S.; Schmidt, S.; Müller-Hannemann, M.; Neumann, S. In silico fragmentation for computer assisted identification of metabolite mass spectra. BMC Bioinform. 2010, 11, 148. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Behm, F.; von der Ohe, K.; Henrich, W. Zuverlässigkeit der Pollenanalyse von Honig. Bestimmung der Pollenhäufigkeit. Dtsch. Lebensm. 1996, 92, 183–187. [Google Scholar]
- PalDat. A Palynological Database. Available online: https://www.paldat.org/ (accessed on 20 December 2022).
- Palynological-Database. Beekeeping Plants. Available online: https://honeybeeroutes.gr/plants/ (accessed on 10 November 2022).
- Pollen-Wiki. Pollen Atlas. Stebler Th. Available online: https://pollen.tstebler.ch/MediaWiki/index.php?title=Pollenatlas (accessed on 20 September 2022).
- CREA-Pollen-Atlas. Available online: https://pollenatlas.net/ (accessed on 10 October 2022).
- Raimondo, F.M.; Domina, G.; Spadaro, V. Checklist of the vascular flora of Sicily. Quad. Bot. Amb. Appl. 2010, 21, 189–252. [Google Scholar]
- Taha, E.-K.A.; Taha, R.A.; Al-Kahtani, S. Nectar and pollen sources for honeybees in Kafrelsheikh province of northern Egypt. Saudi J. Biol. Sci. 2017, 26, 890–896. [Google Scholar] [CrossRef]
- Plants-of-the-World-Online. Available online: https://powo.science.kew.org/ (accessed on 10 November 2022).
- Flora-Italiana. Available online: http://luirig.altervista.org/flora/taxa/floraindice.php (accessed on 10 November 2022).
- African-Plant-Database. Available online: https://africanplantdatabase.ch/en (accessed on 10 November 2022).
- Gazala, N.; Nowar, E. Survey of different pollen sources gathering by honey bee at Qunatir Al-khiria, Qaluobia Governorate. J. Plant Prot. Pathol. 2014, 5, 755–771. [Google Scholar]
- Hassanien, M.M. Microscopic and Chemical Analysis of Honey and Bee Bread at Certain Apiaries in Qalyubia Governorate and Available Honey in Local Market, Egypt. Master’s Thesis, Ain Shams University, Cairo, Egypt, 2018. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasiotis, K.M.; Baira, E.; Iosifidou, S.; Manea-Karga, E.; Tsipi, D.; Gounari, S.; Theologidis, I.; Barmpouni, T.; Danieli, P.P.; Lazzari, F.; et al. Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS. Molecules 2023, 28, 3967. https://doi.org/10.3390/molecules28093967
Kasiotis KM, Baira E, Iosifidou S, Manea-Karga E, Tsipi D, Gounari S, Theologidis I, Barmpouni T, Danieli PP, Lazzari F, et al. Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS. Molecules. 2023; 28(9):3967. https://doi.org/10.3390/molecules28093967
Chicago/Turabian StyleKasiotis, Konstantinos M., Eirini Baira, Styliani Iosifidou, Electra Manea-Karga, Despina Tsipi, Sofia Gounari, Ioannis Theologidis, Theodora Barmpouni, Pier Paolo Danieli, Filippo Lazzari, and et al. 2023. "Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS" Molecules 28, no. 9: 3967. https://doi.org/10.3390/molecules28093967
APA StyleKasiotis, K. M., Baira, E., Iosifidou, S., Manea-Karga, E., Tsipi, D., Gounari, S., Theologidis, I., Barmpouni, T., Danieli, P. P., Lazzari, F., Dipasquale, D., Petrarca, S., Shairra, S., Ghazala, N. A., Abd El-Wahed, A. A., El-Gamal, S. M. A., & Machera, K. (2023). Fingerprinting Chemical Markers in the Mediterranean Orange Blossom Honey: UHPLC-HRMS Metabolomics Study Integrating Melissopalynological Analysis, GC-MS and HPLC-PDA-ESI/MS. Molecules, 28(9), 3967. https://doi.org/10.3390/molecules28093967







