Novel Hyperspectral Analysis of Thin-Layer Chromatographic Plates—An Application to Fingerprinting of 70 Polish Grasses
Abstract
1. Introduction
2. Results and Discussion
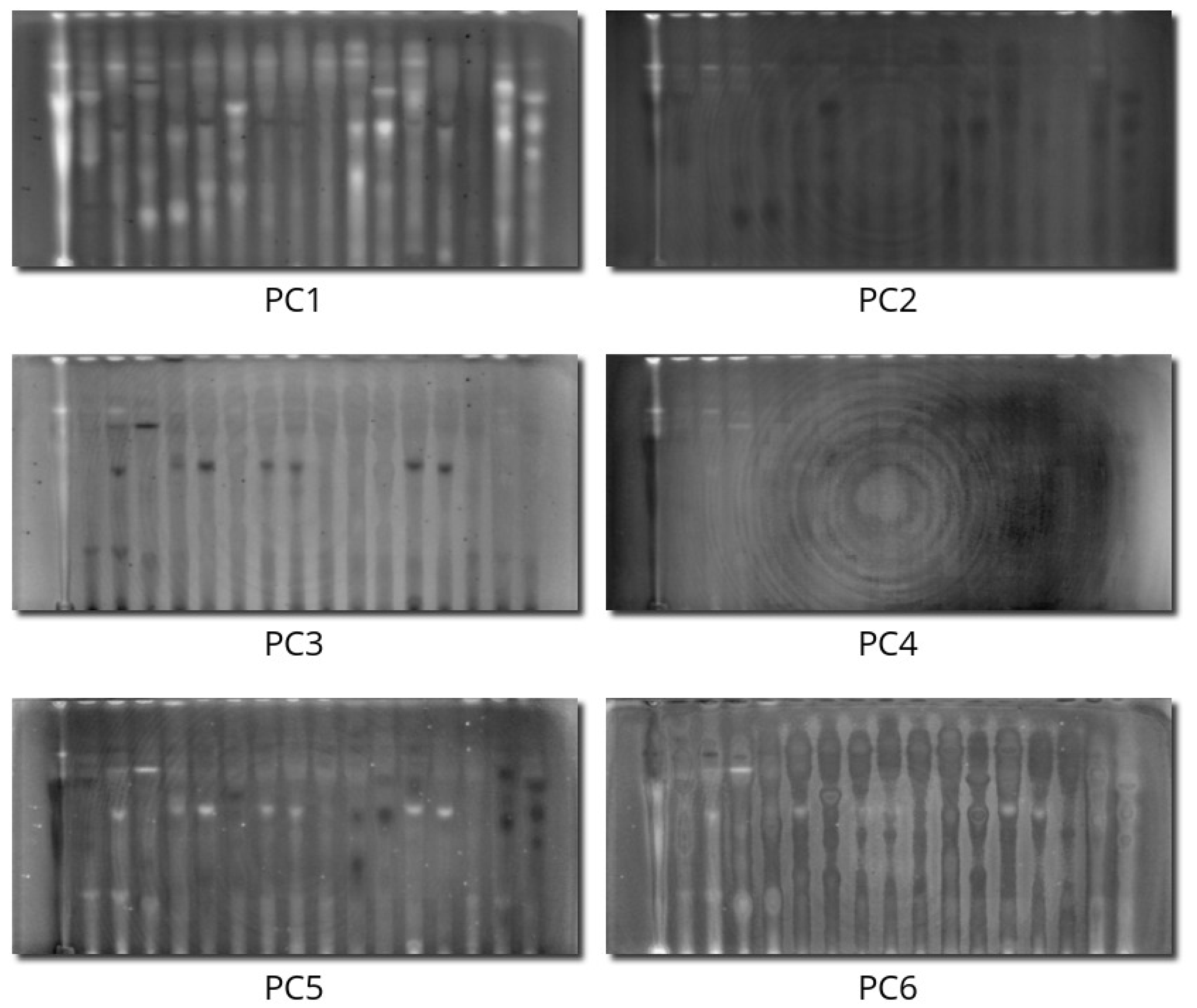
2.1. Principal Component Analysis
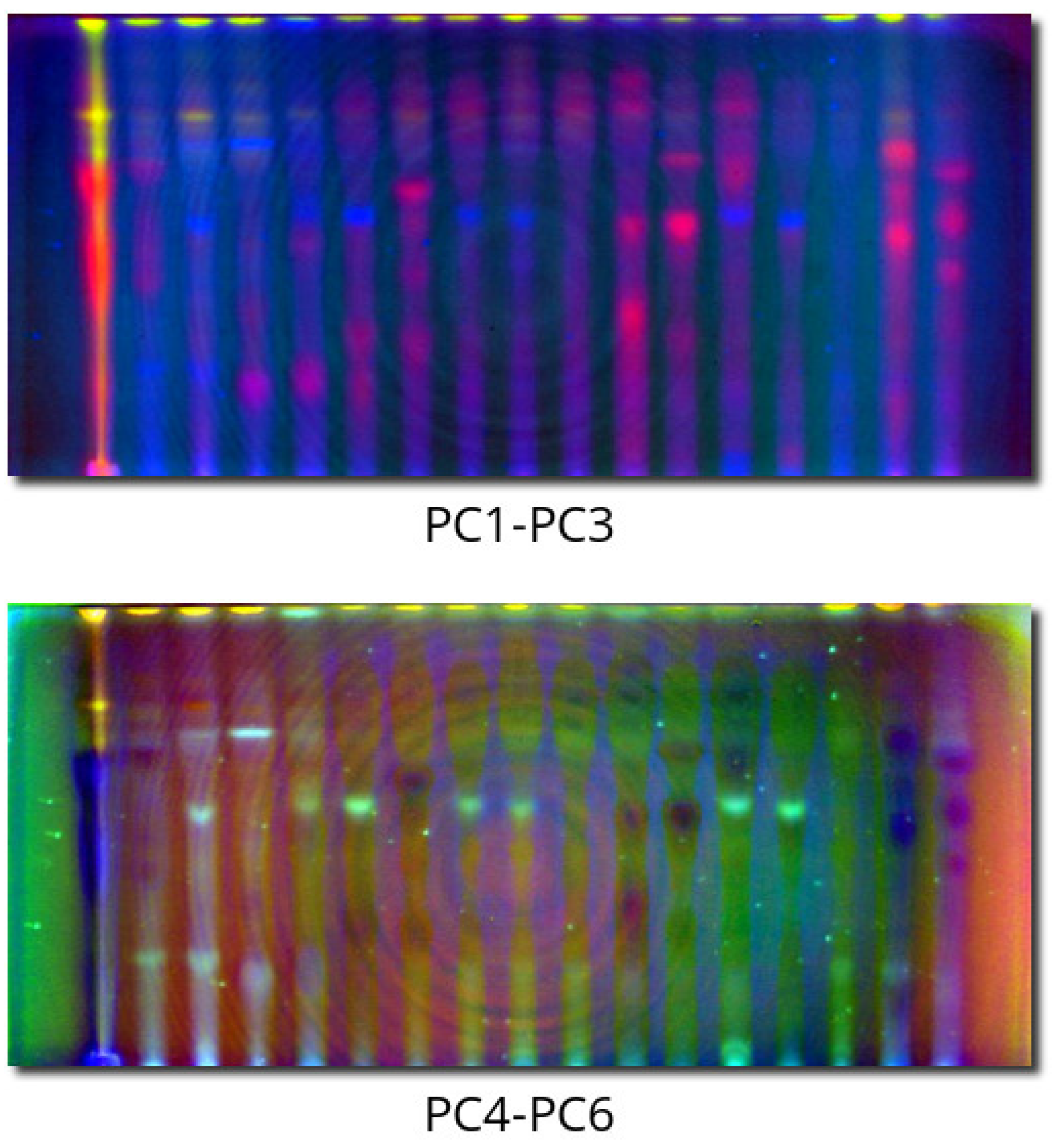
2.2. PCACI Results
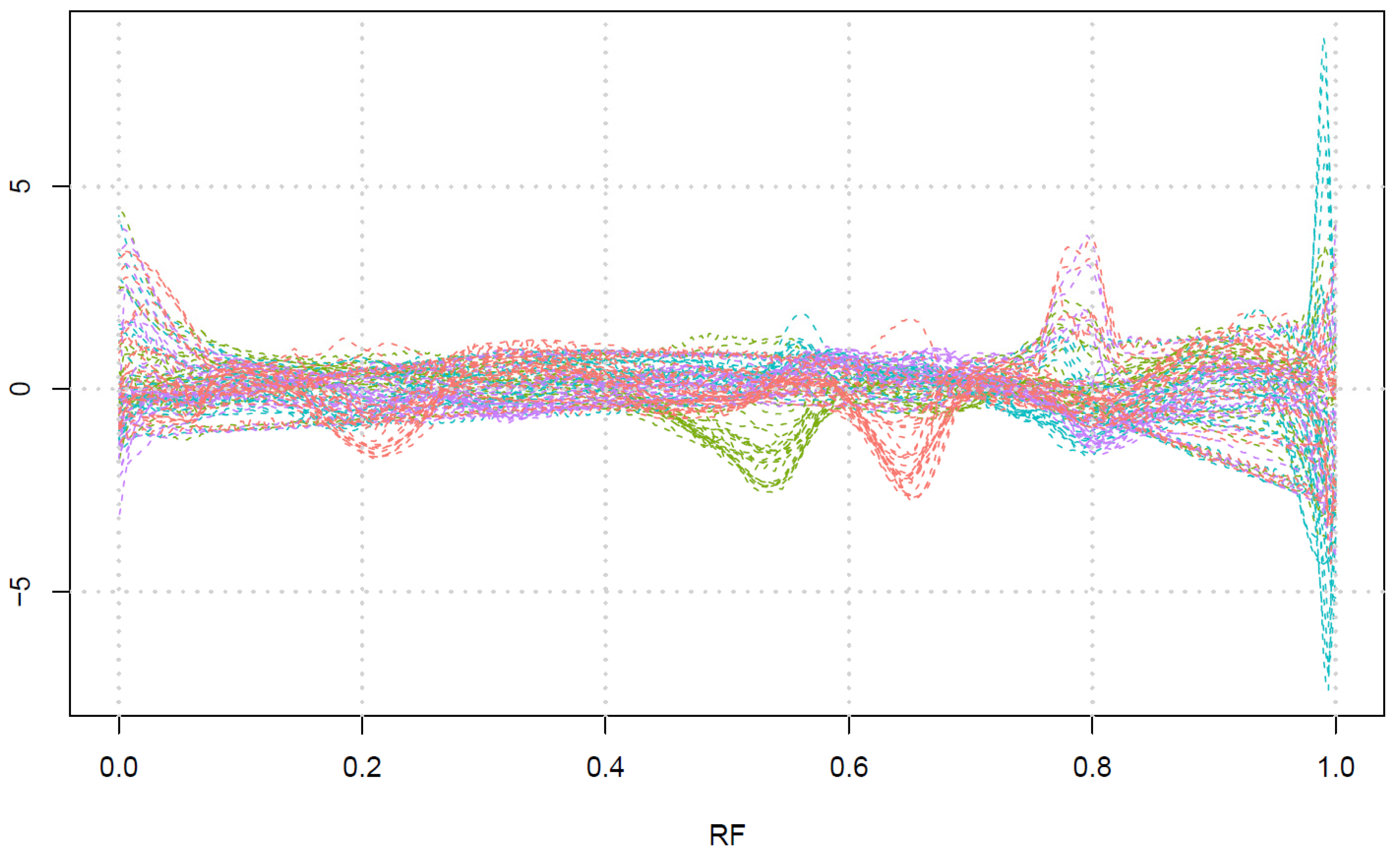
2.3. HCA Results
- The red class corresponds to the presence of a band around RF 0.2 and around RF 0.65, with or without a band around RF 0.8.
- The green class corresponds to the presence of a band around RF 0.55.
- The cyan class corresponds to the presence of strong bands glowing red or infrared at the end of the plate (RF close to 1), with very weak bands in other parts of the plate.
- The magenta class corresponds to the bands mainly around RF 0.8.
3. Materials and Methods
3.1. Plant Material
3.2. Sample Preparation
3.3. Chromatographic Conditions
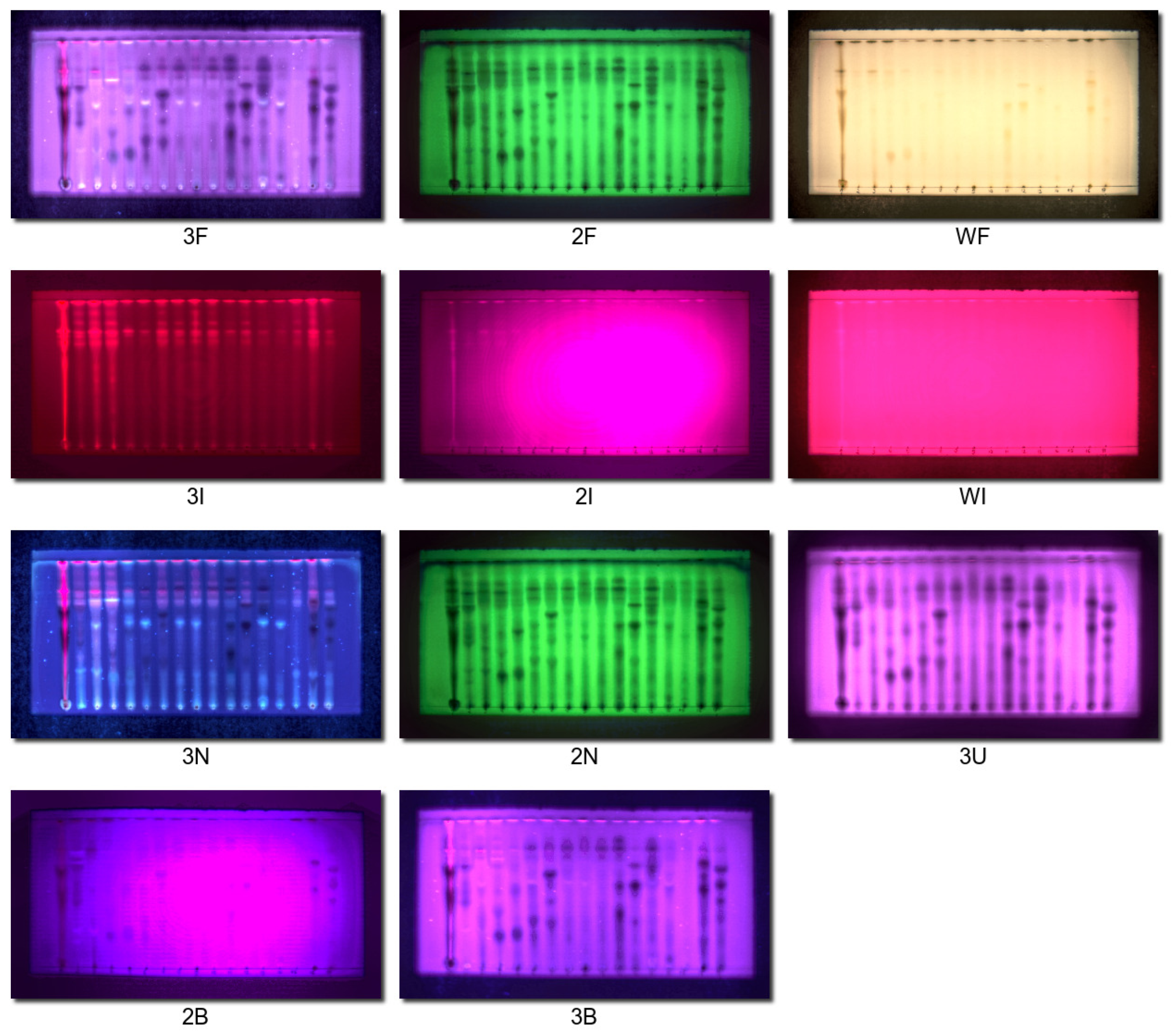
3.4. Image Acquisition Procedure
3.5. Principal Component Analysis
3.6. Principal Component Artificial Colouring of Images (PCACI)
- Each channel of an acquired image (for example, red color under UV illumination, green color under visible light, red color with an infrared filter) is unfolded from the matrix into a large long column vector.
- All column vectors are combined into one matrix with many rows (number of pixels) and a column number equivalent to the number of all processing channels.
- Scaled PCA is done on such a matrix, resulting in matrix scores of the same dimension (as the matrix is tall, the number of principal components is exactly the same as the original dimensionality).
- Three principal components are then chosen. Most frequently, the best result is achieved with the first three, but one should not be afraid of trying other combinations. The order of the chosen components (assignment to red, green, and blue, respectively) can also be set according to color preferences or a need to emphasize something with a desired color.
- The score values are scaled to be integer numbers in the range of 0–255 (the pixel with the minimal score becomes zero, the maximal-value pixel becomes 255), representing the intensity of one of the RGB colors in the image.
- To provide the consistency of the dark background idea, the median of the intensities is computed and checked. If it is larger than 128, a negative is taken to darken the image.
- The created artificial image is then stored in a common graphical format.
3.7. Hierarchical Cluster Analysis (HCA)
4. Conclusions
- The addition of infrared channels to video scanning is not redundant and can help to register which spots can glow in the infrared region when excited by UV or visible light. It can record phenomena that cannot be caught by visible-light photography or by densitometric scanning of the plate, as in our previous study.
- When PCA is performed on the hyperspectral data, the information in the infrared channels is encoded in one particular PC. It proves that this information is orthogonal to the other channels and increases the information in the whole dataset.
- Principal component analysis can reduce many channels to several (six in our case) orthogonal “pseudochannels”, which are uncorrelated and not redundant. Presenting them as artificial colors (PCACI) allows easy visualization and classification of spots in TLC fingerprints of complex mixtures.
- Hyperspectral photography is as effective as multi-mode densitometry; moreover, it contains infrared channels, which cannot be registered by a densitometer.
- There is a need for further research on the topic and hyperspectral photography seems to be an approach deserving of routine use in TLC fingerprinting.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Capitán-Vallvey, L.F.; López-Ruiz, N.; Martínez-Olmos, A.; Erenas, M.M.; Palma, A.J. Recent Developments in Computer Vision-Based Analytical Chemistry: A Tutorial Review. Anal. Chim. Acta 2015, 899, 23–56. [Google Scholar] [CrossRef] [PubMed]
- Rezazadeh, M.; Seidi, S.; Lid, M.; Pedersen-Bjergaard, S.; Yamini, Y. The Modern Role of Smartphones in Analytical Chemistry. TrAC Trends Anal. Chem. 2019, 118, 548–555. [Google Scholar] [CrossRef]
- Komsta, Ł. Chemometrics in Fingerprinting by Means of Thin Layer Chromatography. Chromatogr. Res. Int. 2012, 2012, 893246. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Andrić, F.; Milojković-Opsenica, D. Recent Trends in Image Evaluation of HPTLC Chromatograms. J. Liq. Chromatogr. Relat. Technol. 2020, 43, 291–299. [Google Scholar] [CrossRef]
- Bacsa, G.; Jávor, J.; Kiss, L. Determination of Neomycin Components by Thin Layer Chromatography with Videodensitometry. J. Liq. Chromatogr. 1984, 7, 2803–2811. [Google Scholar] [CrossRef]
- Simonovska, B.; Prošek, M.; Vovk, I.; Jelen-Žmitek, A. High-Performance Thin-Layer Chromatographic Separation of Ranitidine Hydrochloride and Two Related Compounds. J. Chromatogr. B Biomed. Sci. Appl. 1998, 715, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Milz, B.; Spangenberg, B. A Validated Quantification of Benzocaine in Lozenges Using TLC and a Flatbed Scanner. Chromatographia 2013, 76, 1307–1313. [Google Scholar] [CrossRef]
- Milz, B.; Schnurr, P.; Grafmüller, J.; Oehler, K.; Spangenberg, B. A Validated Quantification of Sudan Red Dyes in Spicery Using TLC and a 16-Bit Flatbed Scanner. J. AOAC Int. 2018, 101, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Anders, B.; Doll, S.; Spangenberg, B. A Validated Quantification of Triclosan in Toothpaste Using High-Performance Thin-Layer Chromatography and a 48-Bit Flatbed Scanner. JPC—J. Planar Chromatogr.—Mod. TLC 2021, 34, 203–209. [Google Scholar] [CrossRef]
- Misztal, G.; Komsta, L. Determination of Bezafibrate and Ciprofibrate in Pharmaceutical Formulations by Densitometric and Videodensitometric TLC. J. Planar Chromatogr.—Mod. TLC 2005, 18, 188–193. [Google Scholar] [CrossRef]
- Skibiński, R.; Misztal, G.; Kudrzycki, M. Determination of Fluoxetine and Paroxetine in Pharmaceutical Formulations by Densitometric and Videodensitometric TLC. J. Planar Chromatogr.—Mod. TLC 2003, 16, 19–22. [Google Scholar] [CrossRef]
- Hopkała, H.; Pomykalski, A. TLC Analysis of Inhibitors of Cyclooxygenase and Videodensitometric Determination of Meloxicam and Tiaprofenic Acid. J. Planar Chromatogr.—Mod. TLC 2003, 16, 107–111. [Google Scholar] [CrossRef]
- Petrović, M.; Kastelan-Macan, M.; Ivanković, D.; Matecić, S. Video-Densitometric Quantitation of Fluorescence Quenching on Totally Irradiated Thin-Layer Chromatographic Plates. J. AOAC Int. 2000, 83, 1457–1462. [Google Scholar] [PubMed]
- Vovk, I.; Prosek, M. Reproducibility of Densitometric and Image Analysing Quantitative Evaluation of Thin-Layer Chromatograms. J. Chromatogr. A 1997, 779, 329–336. [Google Scholar] [CrossRef]
- Vovk, I.; Prošek, M. Quantitative Evaluation of Chromatograms from Totally Illuminated Thin-Layer Chromatographic Plates. J. Chromatogr. A 1997, 768, 329–333. [Google Scholar] [CrossRef]
- Fichou, D.; Morlock, G.E. Powerful Artificial Neural Network for Planar Chromatographic Image Evaluation, Shown for Denoising and Feature Extraction. Anal. Chem. 2018, 90, 6984–6991. [Google Scholar] [CrossRef] [PubMed]
- Komsta, Ł.; Cieśla, Ł.; Bogucka-Kocka, A.; Józefczyk, A.; Kryszeń, J.; Waksmundzka-Hajnos, M. The Start-to-End Chemometric Image Processing of 2D Thin-Layer Videoscans. J. Chromatogr. A 2011, 1218, 2820–2825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, X. Quantitative Evaluation of Thin-Layer Chromatography with Image Background Estimation Based on Charge-Coupled Device Imaging. J. Chromatogr. A 2006, 1109, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Ristivojević, P.; Dimkić, I.; Trifković, J.; Berić, T.; Vovk, I.; Milojković-Opsenica, D.; Stanković, S. Antimicrobial Activity of Serbian Propolis Evaluated by Means of MIC, HPTLC, Bioautography and Chemometrics. PLoS ONE 2016, 11, e0157097. [Google Scholar] [CrossRef] [PubMed]
- Fichou, D.; Morlock, G.E. Office Chromatography: Miniaturized All-in-One Open-Source System for Planar Chromatography. Anal. Chem. 2018, 90, 12647–12654. [Google Scholar] [CrossRef] [PubMed]
- Sowers, M.E.; Ambrose, R.; Bethea, E.; Harmon, C.; Jenkins, D. Quantitative Thin Layer Chromatography for the Determination of Medroxyprogesterone Acetate Using a Smartphone and Open-Source Image Analysis. J. Chromatogr. A 2022, 1669, 462942. [Google Scholar] [CrossRef] [PubMed]
- Milojković-Opsenica, D.M.; Trifković, J.Ð.; Ristivojević, P.M.; Andrić, F.L. Thin-Layer Chromatography in the Authenticity Testing of Bee-Products. J. Chromatogr. B 2022, 1188, 123068. [Google Scholar] [CrossRef]
- Simion, I.M.; Casoni, D.; Sârbu, C. Multivariate Color Scale Image Analysis—Thin Layer Chromatography for Comprehensive Evaluation of Complex Samples Fingerprint. J. Chromatogr. B 2021, 1170, 122590. [Google Scholar] [CrossRef] [PubMed]
- Cobzac, S.C.A.; Olah, N.K.; Casoni, D. Application of HPTLC Multiwavelength Imaging and Color Scale Fingerprinting Approach Combined with Multivariate Chemometric Methods for Medicinal Plant Clustering According to Their Species. Molecules 2021, 26, 7225. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, E.; Ward, G.; Pattanaik, S.; Debevec, P. 04—HDR Image Capture. In High Dynamic Range Imaging; The Morgan Kaufmann Series in Computer Graphics; Reinhard, E., Ward, G., Pattanaik, S., Debevec, P., Eds.; Morgan Kaufmann: San Francisco, CA, USA, 2006; pp. 115–165. ISBN 978-0-12-585263-0. [Google Scholar]
- Gadowski, S.; Tomiczak, K.; Komsta, L. High Dynamic Range in Videodensitometry—A Comparative Study to Classic Videoscanning on Gentiana Extracts. J. Planar Chromatogr.—Mod. TLC 2023, 36, 3–8. [Google Scholar] [CrossRef]
- Gagliano, J.; Anselmo-Moreira, F.; Sala-Carvalho, W.R.; Furlan, C.M. What Is Known about the Medicinal Potential of Bamboo? Adv. Tradit. Med. 2021, 22, 467–495. [Google Scholar] [CrossRef]
- Kumar, P.; Singh, S.; Sharma, A.; Kaur, G.; Kaur, R.; Singh, A.N. Arundo donax L.: An Overview on Its Traditional and Ethnomedicinal Importance, Phytochemistry, and Pharmacological Aspects. J. Herbmed Pharmacol. 2021, 10, 269–280. [Google Scholar] [CrossRef]
- Karami, S.; Yargholi, A.; Sadati Lamardi, S.N.; Soleymani, S.; Shirbeigi, L.; Rahimi, R. A Review of Ethnopharmacology, Phytochemistry and Pharmacology of Cymbopogon Species. Res. J. Pharmacogn. 2021, 8, 83–112. [Google Scholar] [CrossRef]
- Ekpenyong, C.E.; Akpan, E.; Nyoh, A. Ethnopharmacology, Phytochemistry, and Biological Activities of Cymbopogon Citratus (DC.) Stapf Extracts. Chin. J. Nat. Med. 2015, 13, 321–337. [Google Scholar] [CrossRef]
- Oladeji, O.S.; Adelowo, F.E.; Ayodele, D.T.; Odelade, K.A. Phytochemistry and Pharmacological Activities of Cymbopogon Citratus: A Review. Sci. Afr. 2019, 6, e00137. [Google Scholar] [CrossRef]
- Singh, A.; Lal, U.; Mukhtar, H.; Singh, P.; Shah, G.; Dhawan, R. Phytochemical Profile of Sugarcane and Its Potential Health Aspects. Pharmacogn. Rev. 2015, 9, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Gebashe, F.; Aremu, A.O.; Finnie, J.F.; Van Staden, J. Grasses in South African Traditional Medicine: A Review of Their Biological Activities and Phytochemical Content. S. Afr. J. Bot. 2019, 122, 301–329. [Google Scholar] [CrossRef]
- Wróbel-Szkolak, J.; Cwener, A.; Komsta, Ł. Data Fusion from Several Densitometric Modes in Fingerprinting of 70 Grass Species. JPC—J. Planar Chromatogr.—Mod. TLC 2022, 35, 287–297. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel-Szkolak, J.; Cwener, A.; Komsta, Ł. Novel Hyperspectral Analysis of Thin-Layer Chromatographic Plates—An Application to Fingerprinting of 70 Polish Grasses. Molecules 2023, 28, 3745. https://doi.org/10.3390/molecules28093745
Wróbel-Szkolak J, Cwener A, Komsta Ł. Novel Hyperspectral Analysis of Thin-Layer Chromatographic Plates—An Application to Fingerprinting of 70 Polish Grasses. Molecules. 2023; 28(9):3745. https://doi.org/10.3390/molecules28093745
Chicago/Turabian StyleWróbel-Szkolak, Joanna, Anna Cwener, and Łukasz Komsta. 2023. "Novel Hyperspectral Analysis of Thin-Layer Chromatographic Plates—An Application to Fingerprinting of 70 Polish Grasses" Molecules 28, no. 9: 3745. https://doi.org/10.3390/molecules28093745
APA StyleWróbel-Szkolak, J., Cwener, A., & Komsta, Ł. (2023). Novel Hyperspectral Analysis of Thin-Layer Chromatographic Plates—An Application to Fingerprinting of 70 Polish Grasses. Molecules, 28(9), 3745. https://doi.org/10.3390/molecules28093745






