Solubilization of Reactive Red 2 in the Mixed Micelles of Cetylpyridinium Chloride and TX-114
Abstract
1. Introduction
2. Results and Discussion
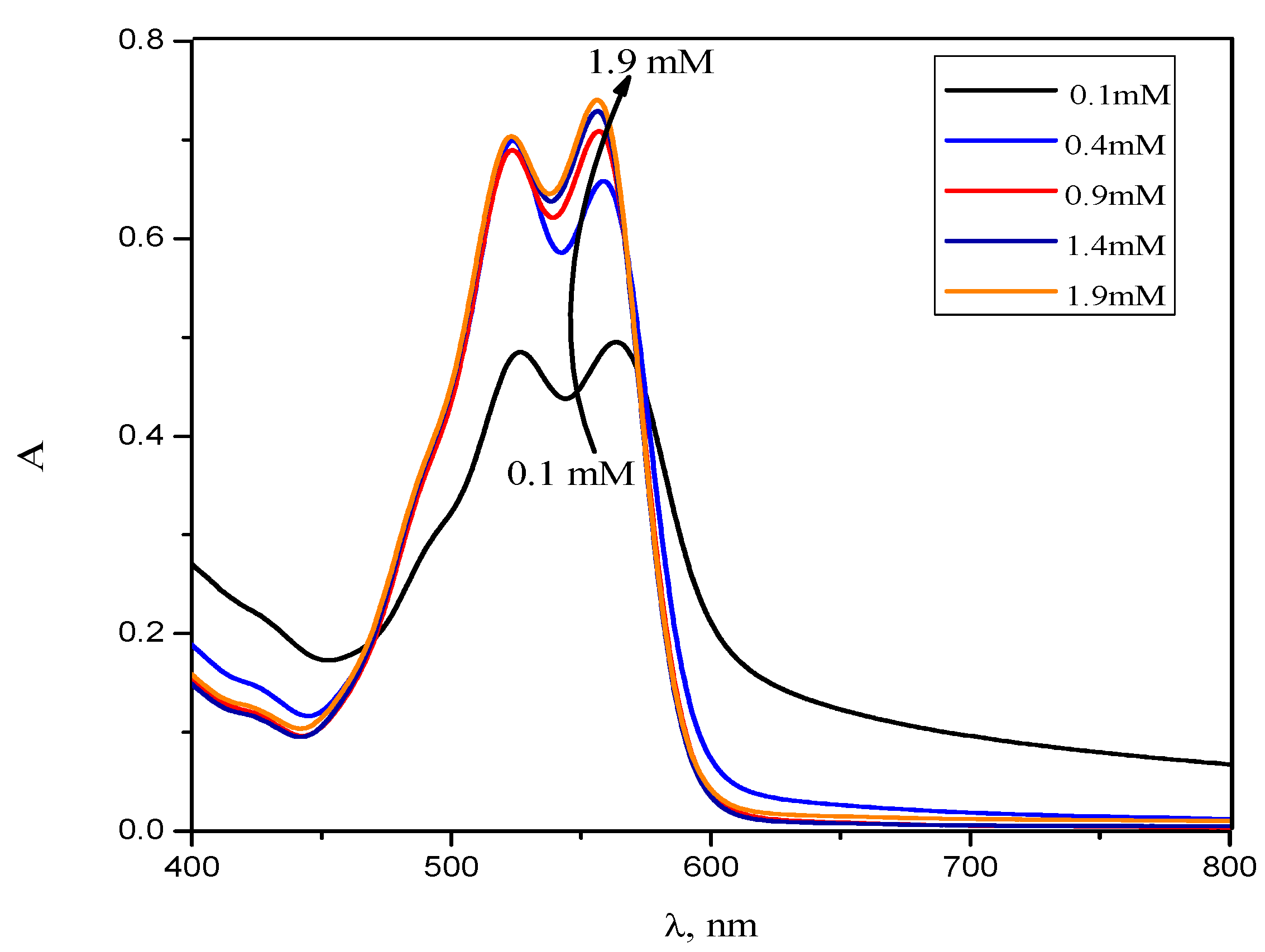
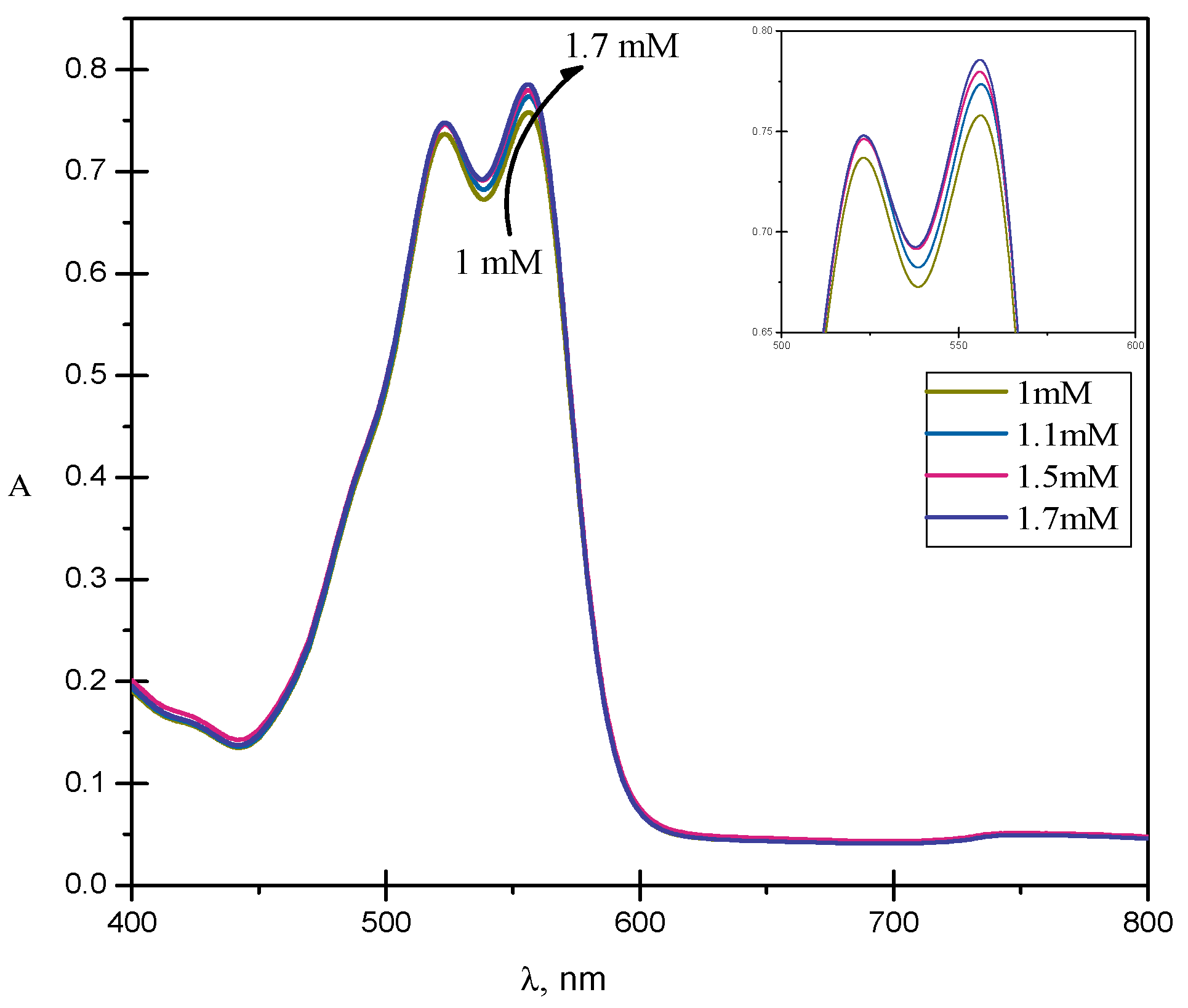
2.1. UV-Visible Spectroscopy
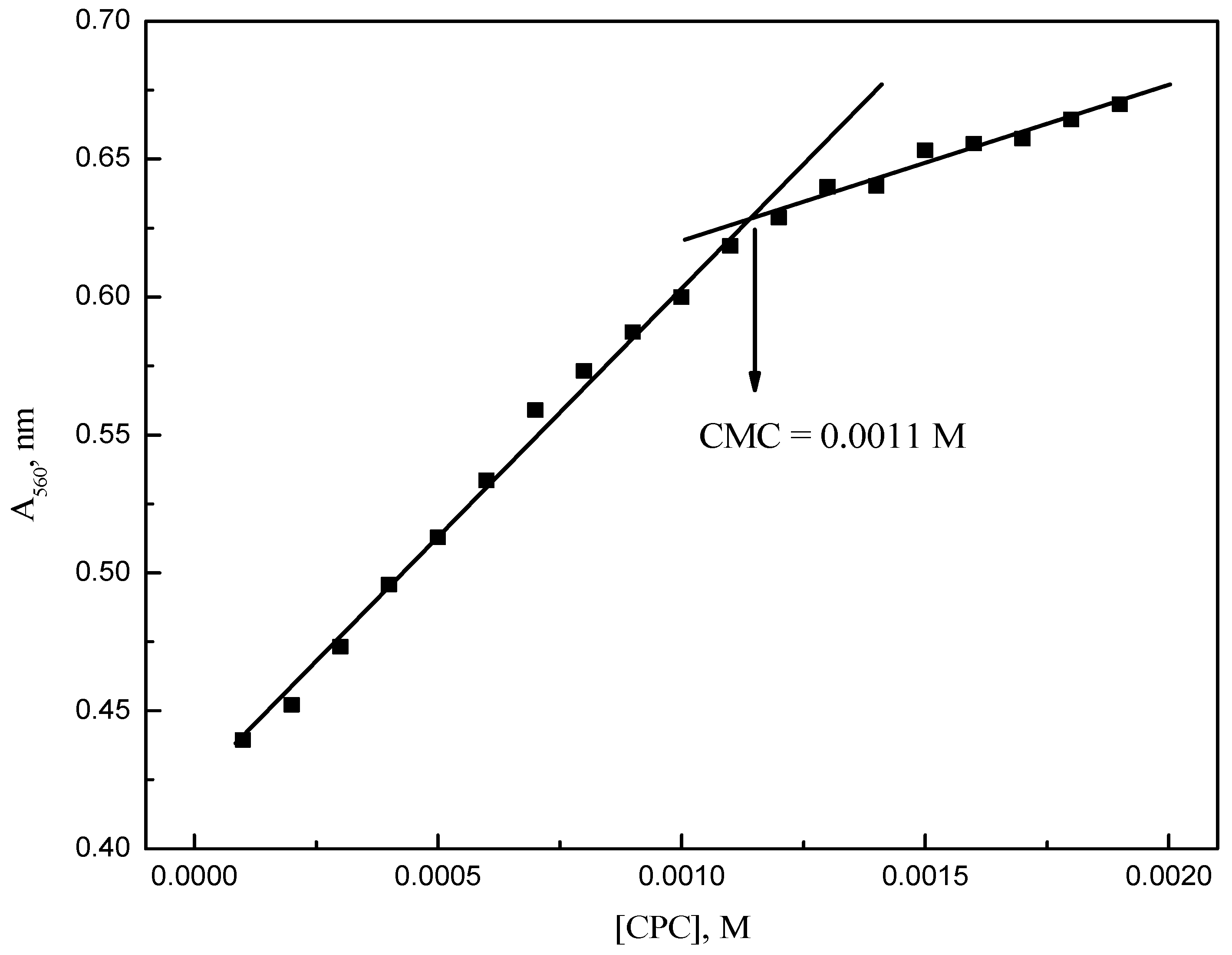
2.2. Electrical Conductivity Measurements
3. Material and Methods
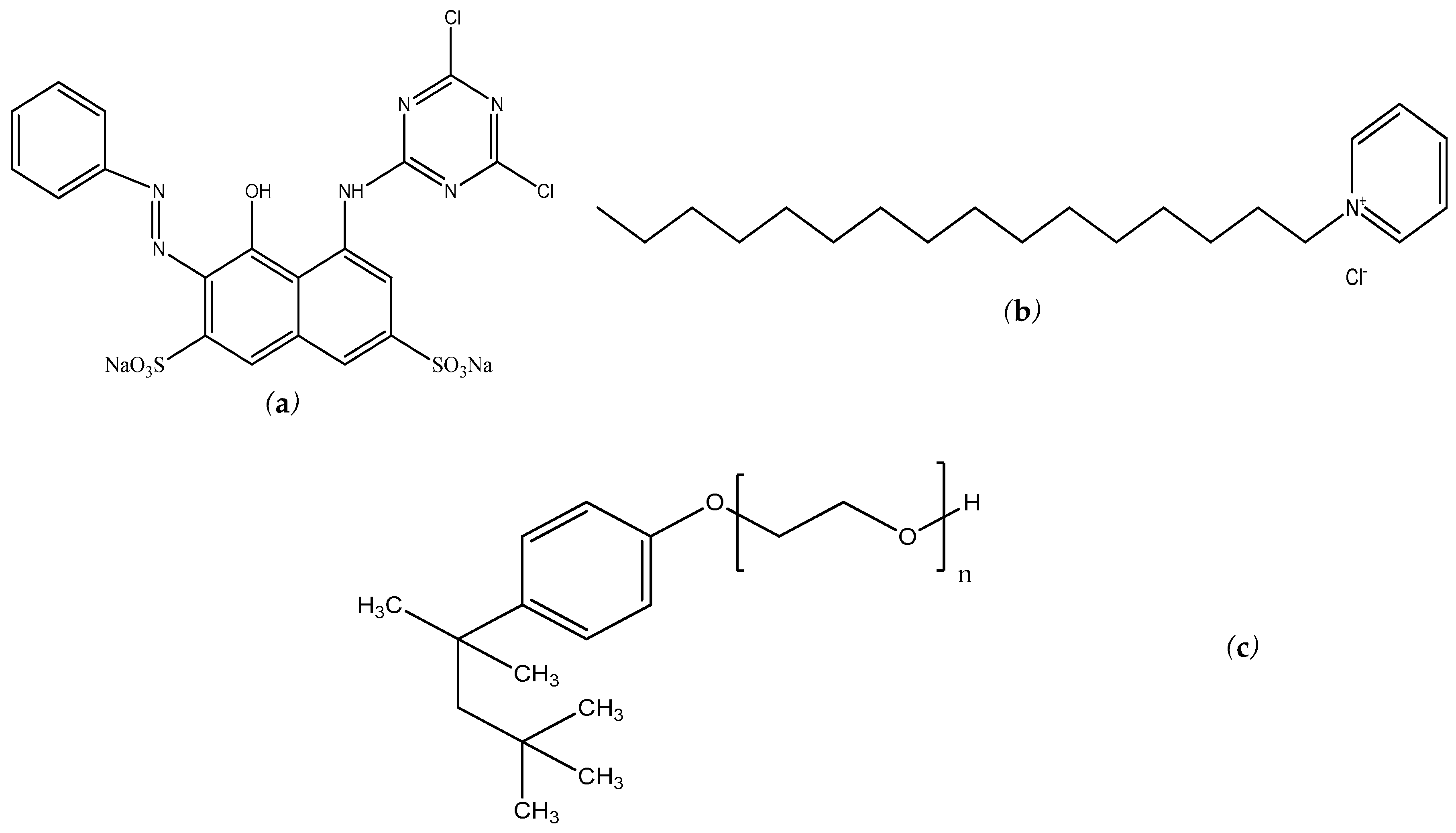
3.1. Reagents
3.2. Procedure
3.3. Conductometric Studies
3.4. UV-Visible Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laveda, F.; Núñez-Delicado, E.; García-Carmona, F.; Sánchez-Ferrer, A. Reversible Sodium Dodecyl Sulfate Activation of Latent Peach Polyphenol Oxidase by Cyclodextrins. Arch. Biochem. Biophys. 2000, 379, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sachin, K.M.; Karpe, S.A.; Singh, M.; Bhattarai, A. Self-assembly of sodium dodecylsulfate and dodecyltrimethylammonium bromide mixed surfactants with dyes in aqueous mixtures. R. Soc. Open Sci. 2019, 6, 181979. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Poddar, S. Studies on the Interaction of Surfactants with Cationic Dye by Absorption Spectroscopy. J. Colloid Interface Sci. 2000, 221, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Li, F.; Morrall, S.W.; Versteeg, D.J. The Relationship between the Interfacial Properties of Surfactants and Their Toxicity to Aquatic Organisms. Environ. Sci. Technol. 2001, 35, 954–959. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Asiri, A.M. Interaction of antipsychotic drug with novel surfactants: Micellization and binding studies. Chin. J. Chem. Eng. 2018, 26, 566–573. [Google Scholar] [CrossRef]
- Kumar, D.; Azum, N.; Rub, M.A.; Asiri, A.M. Aggregation behavior of sodium salt of ibuprofen with conventional and gemini surfactant. J. Mol. Liq. 2018, 262, 86–96. [Google Scholar] [CrossRef]
- Bhattarai, A.; Rub, M.A.; Posa, M.; Saha, B.; Kumar, D. Catalytic impacts of cationic twin headed and tailed gemini surfactants toward study of glycine and ninhydrin in sodium acetate-acetic acid buffer system. J. Mol. Liq. 2022, 360, 119442. [Google Scholar] [CrossRef]
- Bhattarai, A.; Rub, M.A.; Posa, M.; Saha, B.; Asiri, A.M.; Kumar, D. Studies of ninhydrin and phenylalanine in cationic dimeric Gemini micellar system: Spectrophotometric and conductometric measurements. Collids Surf. A Physicochem. Eng. Asp. 2022, 655, 130334. [Google Scholar] [CrossRef]
- Kumar, D.; Rub, M.A.; Bhattarai, A. Catalytic impact of twin headed geminis in study of ninhydrin with aspartic acid in an acetate buffer system. J. Mol. Liq. 2022, 359, 119324. [Google Scholar] [CrossRef]
- Azum, N.; Rub, M.A.; Khan, A.; Alotaibi, M.M.; Asiri, A.M.; Rahman, M.M. Mixed Micellization, Thermodynamic and Adsorption Behavior of Tetracaine Hydrochloride in the Presence of Cationic Gemini/Conventional Surfactants. Gels 2022, 8, 128. [Google Scholar] [CrossRef]
- Tehrani-Bagha, A.R.; Holmberg, K. Solubilization of Hydrophobic Dyes in Surfactant Solutions. Materials 2013, 6, 580–608. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Usman, M.; Bokhari, T.H.; Haq, A.U.; Saeed, M.; Rahman, H.M.A.U.; Siddiq, M.; Rasheed, A.; Nisa, M.U. The application of cationic-nonionic mixed micellar media for enhanced solubilization of Direct brown 2 dye. J. Mol. Liq. 2020, 301, 112408. [Google Scholar] [CrossRef]
- Tajalli, H.; Gilani, A.G.; Zakerhamidi, M.; Moghadam, M. Effects of surfactants on the molecular aggregation of rhodamine dyes in aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, N.; Sun, G. Intermolecular Interactions between Surfactants and Cationic Dyes and Effect on Antimicrobial Properties. Ind. Eng. Chem. Res. 2010, 49, 8347–8352. [Google Scholar] [CrossRef]
- Ali, A.; Uzair, S.; Malik, N.A.; Ali, M. Study of interaction between cationic surfactants and cresol red dye by electrical conductivity and spectroscopy methods. J. Mol. Liq. 2014, 196, 395–403. [Google Scholar] [CrossRef]
- Tabak, A.; Baltas, N.; Afsin, B.; Emirik, M.; Caglar, B.; Eren, E. Adsorption of Reactive Red 120 from aqueous solutions by cetylpyridinium-bentonite. J. Chem. Technol. Biotechnol. 2010, 85, 1199–1207. [Google Scholar] [CrossRef]
- Noor, S.; Taj, M.B.; Ashar, A. Solubilization of cationic dye in single and mixed micellar media. J. Mol. Liq. 2021, 330, 115613. [Google Scholar] [CrossRef]
- Shapovalov, S.; Ponomariov, V. Interaction of Dyes with Cationic Surfactants in Solutions: Determination of Critical Micelle Concentration. Int. Lett. Chem. Phys. Astron. 2019, 81, 27–34. [Google Scholar] [CrossRef]
- Amjad, S.; Shaukat, S.; Rahman, H.M.A.; Usman, M.; Farooqi, Z.H.; Nazar, M.F. Application of anionic-nonionic mixed micellar system for solubilization of methylene blue dye. J. Mol. Liq. 2022, 369, 120958. [Google Scholar] [CrossRef]
- Yusaf, A.; Usman, M.; Siddiq, M.; Bakhtiar, M.; Mansha, A.; Shaukat, S.; Rehman, H.F. Mixed Micellar Solubilization of Naphthol Green B Followed by Its Removal from Synthetic Effluent by Micellar-Enhanced Ultrafiltration under Optimized Conditions. Molecules 2022, 27, 6436. [Google Scholar] [CrossRef]
- Petcu, A.R.; Rogozea, E.A.; Lazar, C.A.; Olteanu, N.L.; Meghea, A.; Mihaly, M. Specific interactions within micelle microenvironment in different charged dye/surfactant systems. Arab. J. Chem. 2016, 9, 9–17. [Google Scholar] [CrossRef]
- Wang, W.; Huang, G.; An, C.; Xin, X.; Zhang, Y.; Liu, X. Transport behaviors of anionic azo dyes at interface between surfactant-modified flax shives and aqueous solution: Synchrotron infrared and adsorption studies. Appl. Surf. Sci. 2017, 405, 119–128. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A Spectrophotometric Investigation of the Interaction of Iodine with Aromatic Hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Kawamura, H.; Manabe, M.; Miyamoto, Y.; Fujita, Y.; Tokunaga, S. Partition coefficients of homologous ω-phenylalkanols between water and sodium dodecyl sulfate micelles. J. Phys. Chem. 1989, 93, 5536–5540. [Google Scholar] [CrossRef]
- Irshad, S.; Sultana, H.; Usman, M.; Saeed, M.; Akram, N.; Yusaf, A.; Rehman, A. Solubilization of direct dyes in single and mixed surfactant system: A comparative study. J. Mol. Liq. 2021, 321, 114201. [Google Scholar] [CrossRef]
- Sohail, M.; Rahman, H.M.A.; Asghar, M.N. Thermo-acoustic, spectroscopic, and electrochemical investigation of sparfloxacin–ionic surfactant interactions. J. Mol. Liq. 2021, 340, 117186. [Google Scholar] [CrossRef]
- Zaghbani, N.; Dhahbi, M.; Hafiane, A. Spectral study of Eriochrome Blue Black R in different cationic surfactant solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 1528–1531. [Google Scholar] [CrossRef]
- Pramanick, D.; Mukherjee, D. Molecular interaction of methylene blue with triton X-100 in reverse micellar media. J Colloid Interface Sci. 1993, 157, 131–134. [Google Scholar] [CrossRef]
- Rashid, S.; Usman, M.; Shahzad, T.; Saeed, M.; Haq, A.U.; Ibrahim, M.; Siddiq, M.; Iram, M. The Differential Spectroscopic Investigation of Partitioning of Reactive Dyes in Micellar Media of Cationic Surfactant, Cetyl Trimethylammonium Bromide (CTAB). Z. Phys. Chem. 2018, 233, 183–199. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, J.; Yin, X. Effect of anionic-nonionic-mixed surfactant micelles on solubilization of PAHs. J. Air Waste Manag. Assoc. 2013, 63, 694–701. [Google Scholar] [CrossRef]
- Choi, T.-S.; Shimizu, Y.; Shirai, H.; Hamada, K. Solubilization of disperse dyes in cationic gemini surfactant micelles. Dye. Pigment. 2000, 45, 145–152. [Google Scholar] [CrossRef]
- El-Aila, H.J.Y. Interaction of nonionic surfactant Triton-X-100 with ionic surfactants. J. Dispers. Sci. Technol. 2009, 30, 1277–1280. [Google Scholar] [CrossRef]
- Rehman, H.; Usman, M.; Bokhari, T.H.; Rahman, H.M.A.U.; Mansha, A.; Siddiq, M.; Rasheed, A.; Nisa, M.U. Effects of nonionic surfactant (TX-100) on solubilizing power of cationic surfactants (CTAB and CPC) for Direct Red 13. Colloids Surf. A Physicol. Chem. Eng. Asp. 2020, 586, 124241. [Google Scholar] [CrossRef]
- López-Díaz, D.; García-Mateos, I.; Velázquez, M.M. Synergism in mixtures of zwitterionic and ionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2005, 270–271, 153–162. [Google Scholar] [CrossRef]

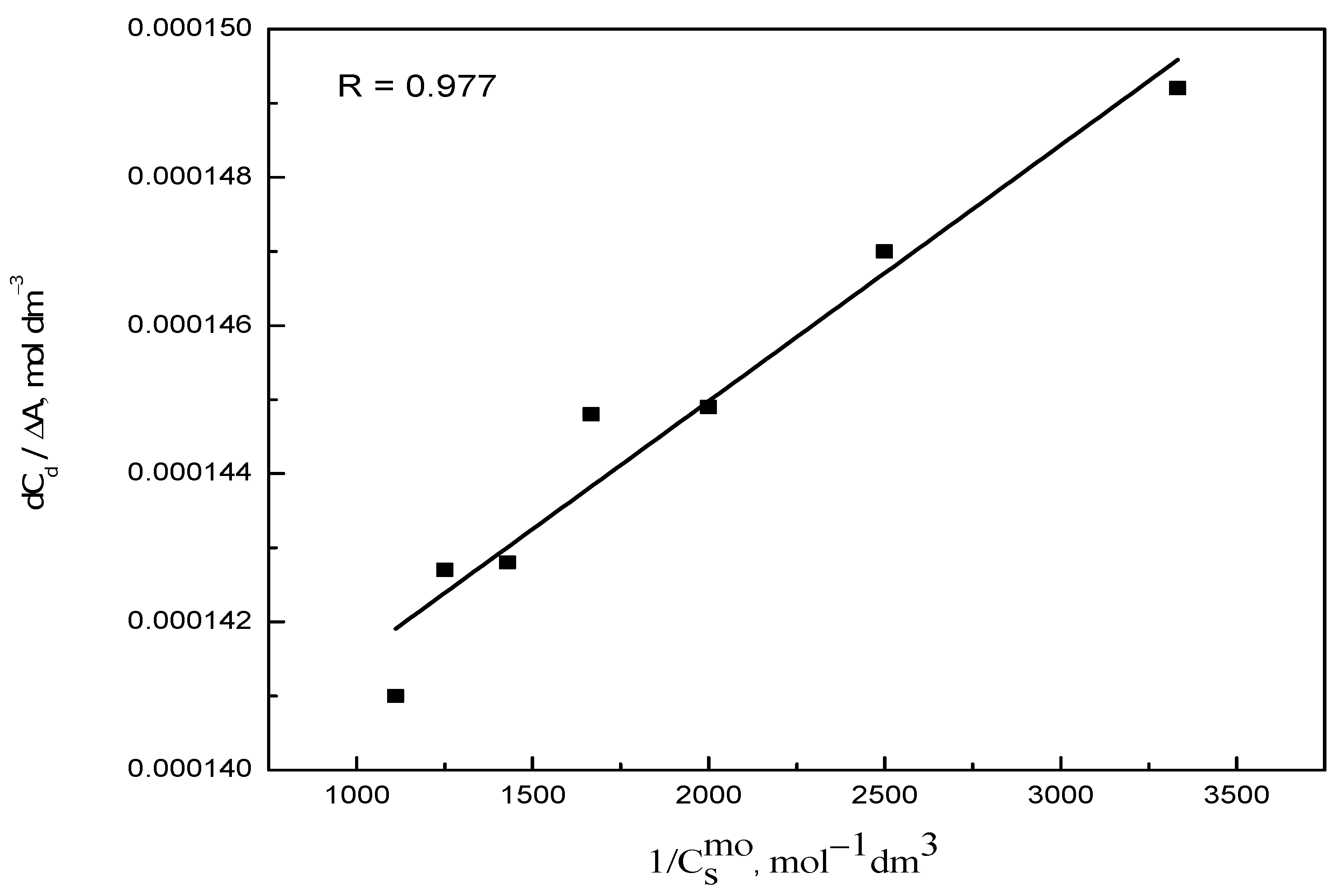

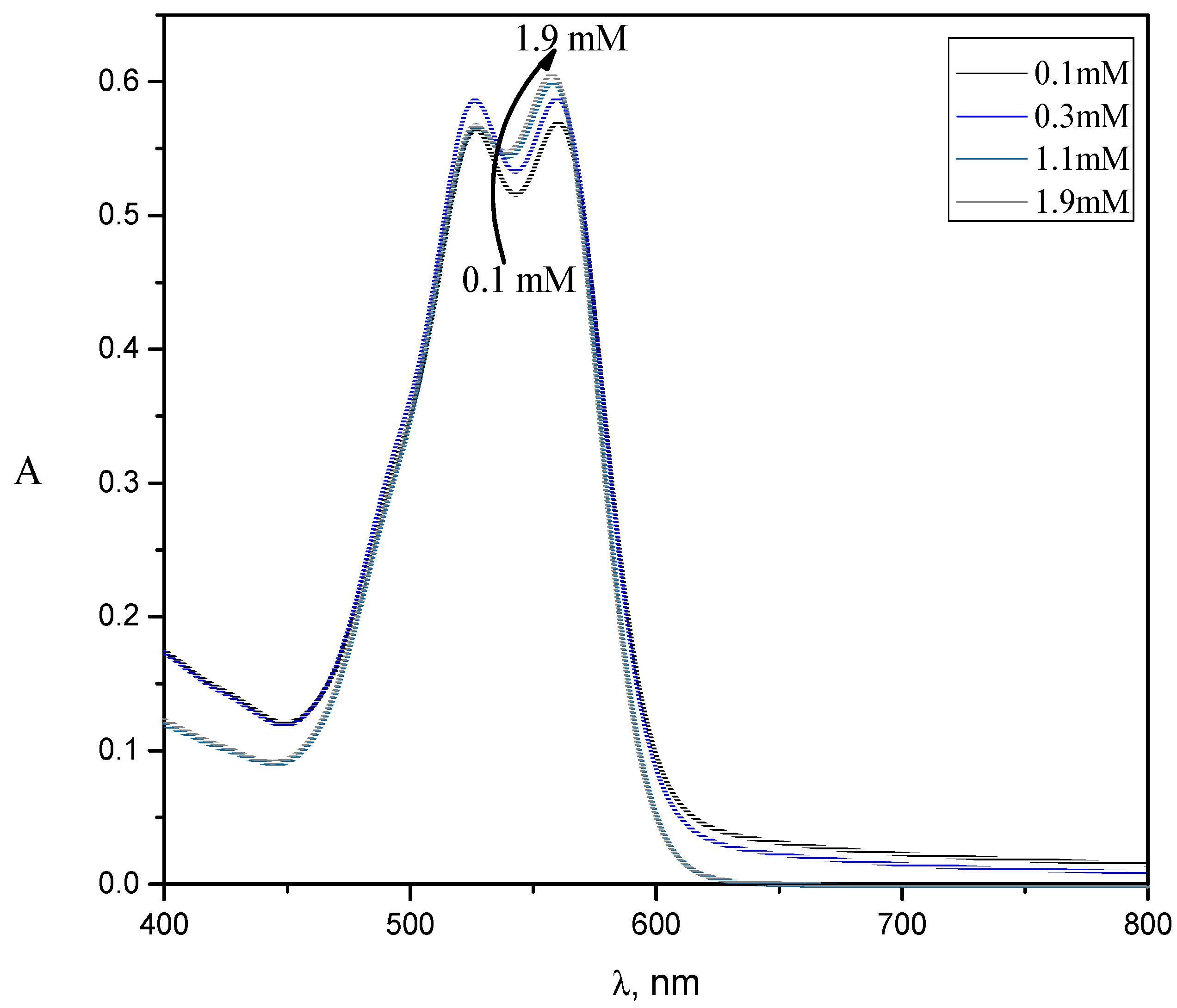
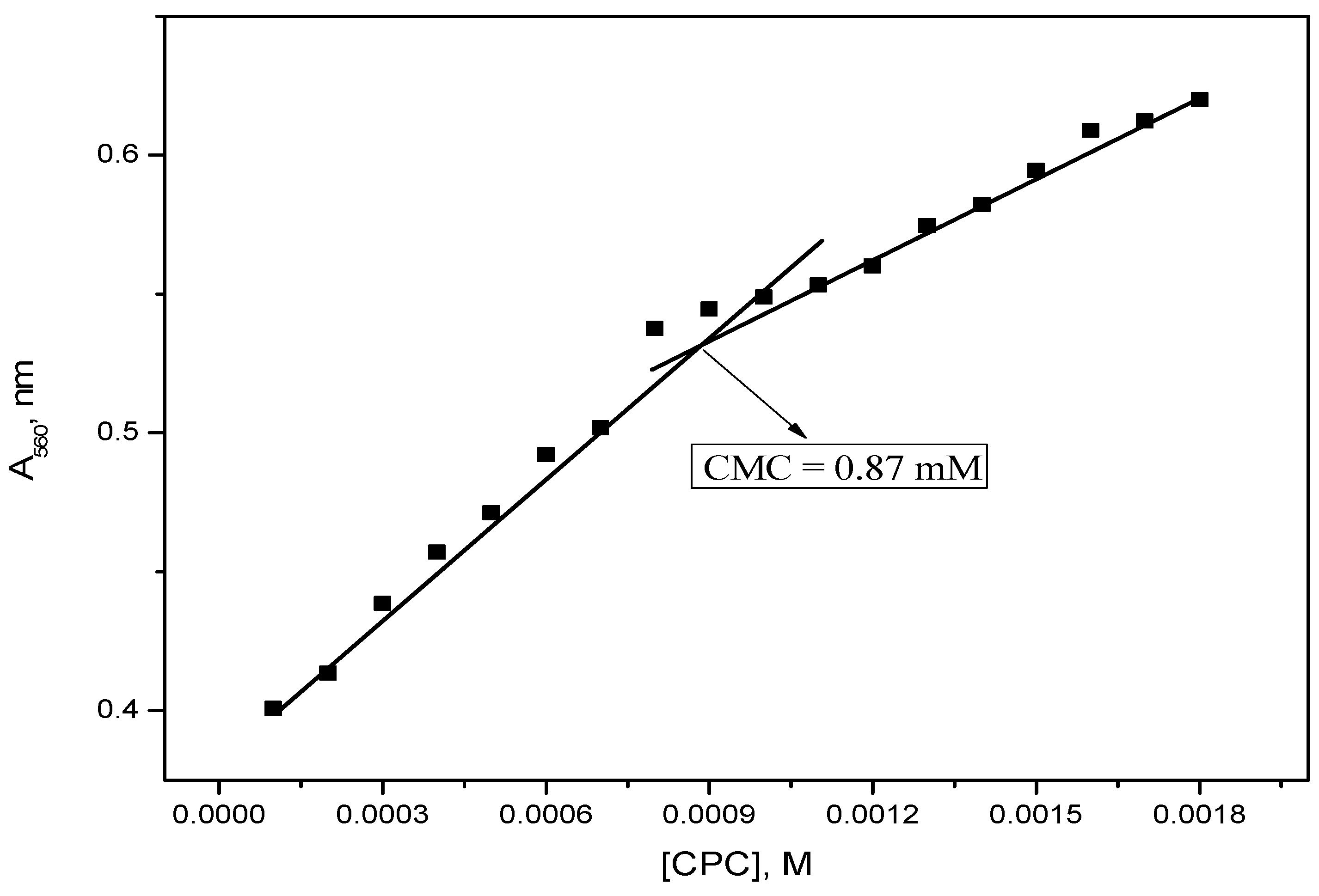
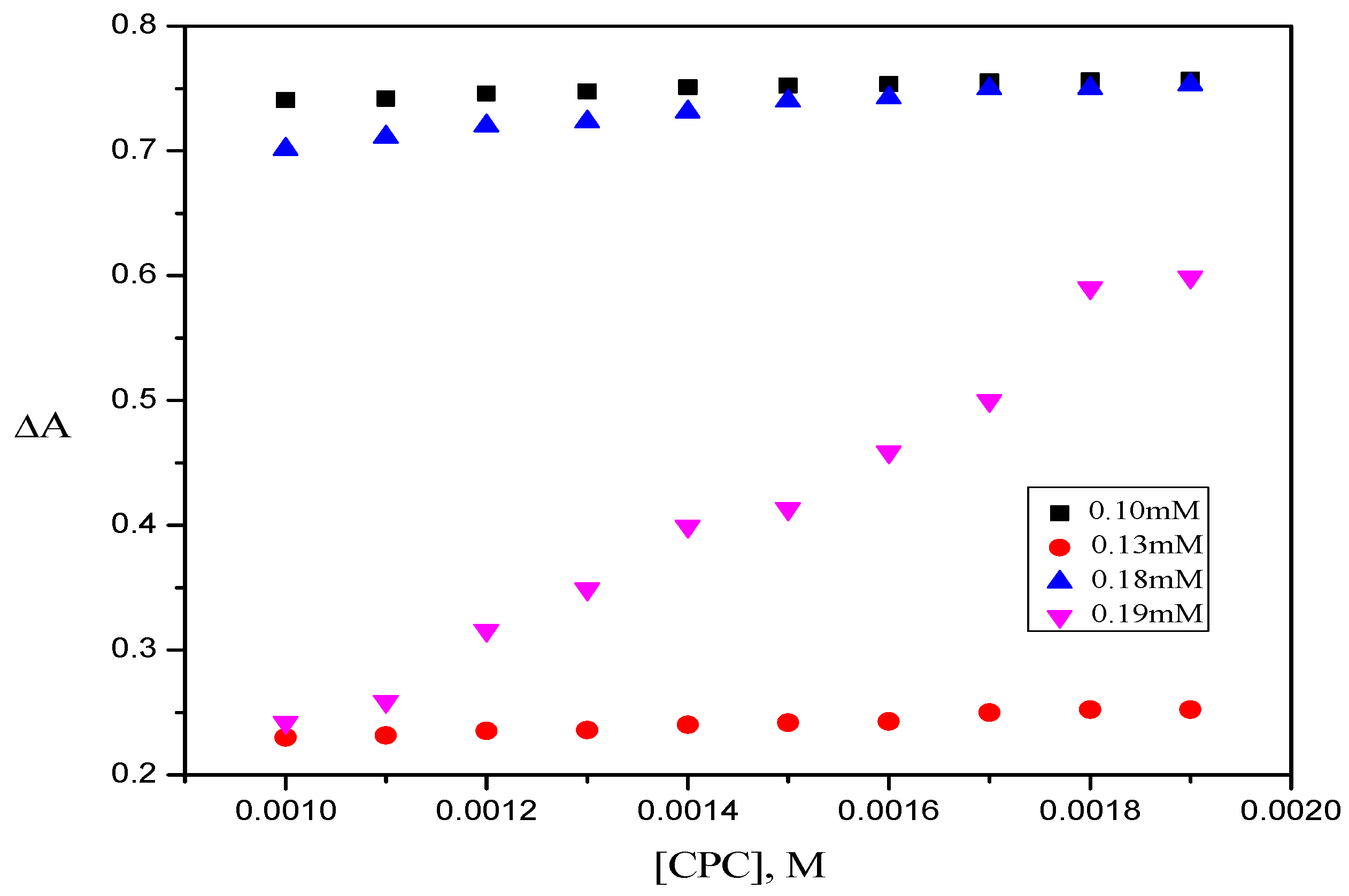
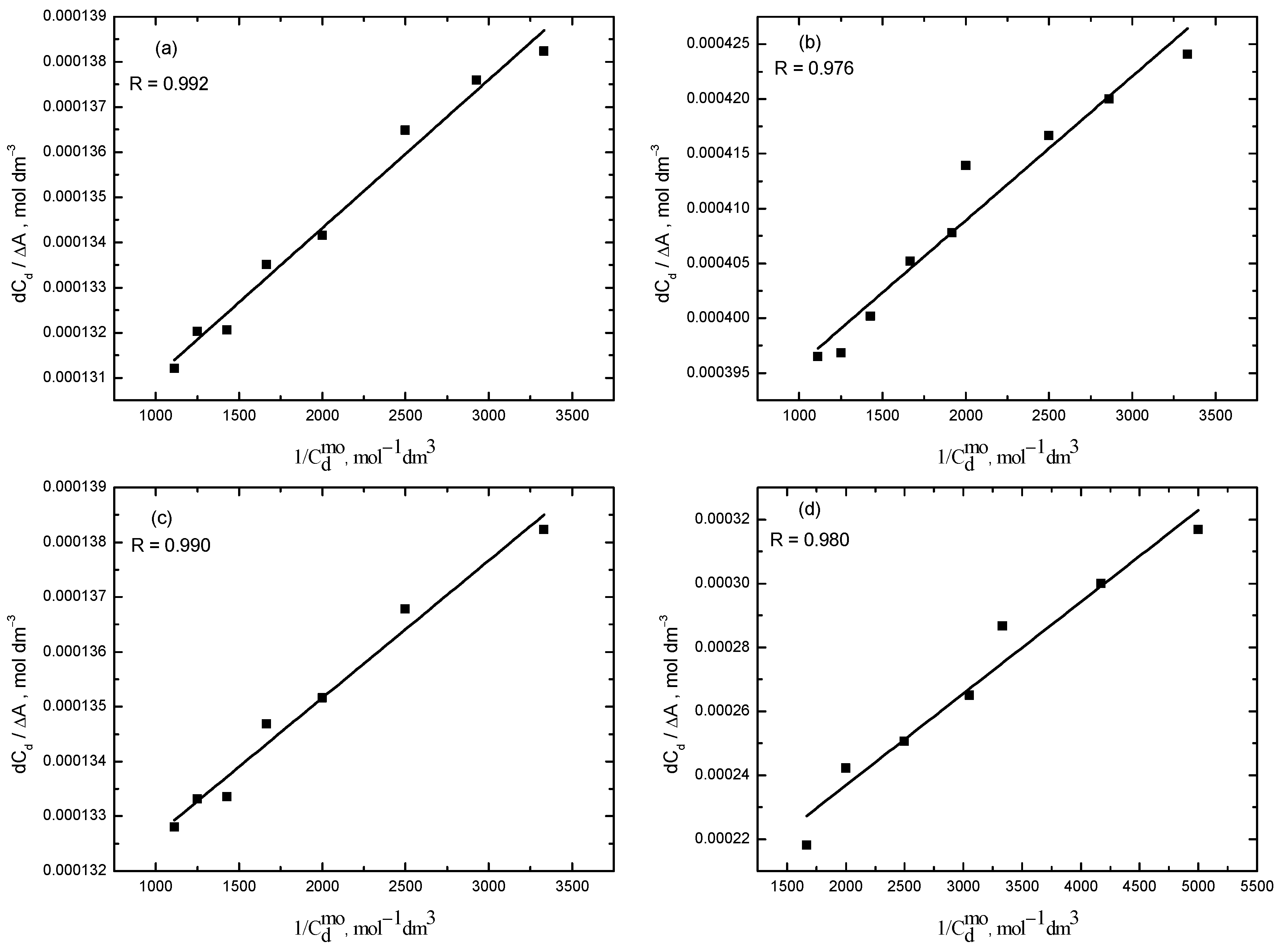



| Dye–Surfactant System | Kb × 10−3 (dm3/mol) | ΔGb (kJ/mol) | Kc × 10−3 (dm3/mol) | Kx × 10−4 | ΔGp (kJ/mol) |
|---|---|---|---|---|---|
| RR2–CPC | 40 ± 2 | −26.3 ± 0.3 | 26 ± 1 | 1500 ± 2 | −34.6 ± 0.3 |
| RR2–TX-114 | 23 ± 1 | −24.8 ± 0.2 | 2.4 ± 0.5 | 14 ± 1 | −29.3 ± 0.3 |
| Conc. of TX-114 (mM) | Kb × 10−3 (dm3/mol) | ΔGb (kJ/mol) | Kc × 10−3 (dm3/mol) | Kx × 10−4 | ΔGp (kJ/mol) |
|---|---|---|---|---|---|
| 0.10 | 39 ± 3 | −26.2 ± 0.2 | 29 ± 1 | 163 ± 1 | −35.5 ± 0.2 |
| 0.13 | 29 ± 2 | −25.5 ± 0.2 | 19 ± 1 | 107 ± 1 | −34.4 ± 0.2 |
| 0.18 | 52 ± 2 | −26.9 ± 0.2 | 35 ± 1 | 192 ± 1 | −35.9 ± 0.2 |
| 0.19 | 6 ± 1 | −21.7 ± 0.2 | 2 ± 1 | 8 ± 1 | −28.0 ± 0.2 |
| Temperature (K) | CMC (mM) | ∆G°m (kJ/mol) | ΔH°m (kJ/mol) | ΔS°m (kJ/Kmol) | |
|---|---|---|---|---|---|
| 293.15 | 1.03 | −36.1 | −1.9 | 0.116 | 0.640 |
| 303.15 | 1.07 | −37.0 | −2.1 | 0.115 | 0.646 |
| 313.15 | 1.08 | −38.2 | −2.2 | 0.115 | 0.647 |
| 323.15 | 1.10 | −39.9 | −2.4 | 0.117 | 0.628 |
| Temperature (K) | CMC (mM) | ∆G°m (kJ/mol) | ΔH°m (kJ/mol) | ΔS°m (kJ/Kmol) | |
|---|---|---|---|---|---|
| 293.15 | 0.87 | −32.4 | −5.0 | 0.009 | 0.484 |
| 303.15 | 0.89 | −41.5 | −5.2 | 0.120 | 0.505 |
| 313.15 | 0.92 | −47.6 | −6.2 | 0.132 | 0.332 |
| 323.15 | 0.98 | −51.9 | −7.1 | 0.139 | 0.230 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaqoob, T.; Shaukat, S.; Alonaizan, R.; Ullah, R.; Khan, I.; Nazar, M.F.; Abd Ur Rahman, H.M. Solubilization of Reactive Red 2 in the Mixed Micelles of Cetylpyridinium Chloride and TX-114. Molecules 2023, 28, 3952. https://doi.org/10.3390/molecules28093952
Yaqoob T, Shaukat S, Alonaizan R, Ullah R, Khan I, Nazar MF, Abd Ur Rahman HM. Solubilization of Reactive Red 2 in the Mixed Micelles of Cetylpyridinium Chloride and TX-114. Molecules. 2023; 28(9):3952. https://doi.org/10.3390/molecules28093952
Chicago/Turabian StyleYaqoob, Tayyba, Saadia Shaukat, Rasha Alonaizan, Ramzan Ullah, Imran Khan, Muhammad Faizan Nazar, and Hafiz Muhammad Abd Ur Rahman. 2023. "Solubilization of Reactive Red 2 in the Mixed Micelles of Cetylpyridinium Chloride and TX-114" Molecules 28, no. 9: 3952. https://doi.org/10.3390/molecules28093952
APA StyleYaqoob, T., Shaukat, S., Alonaizan, R., Ullah, R., Khan, I., Nazar, M. F., & Abd Ur Rahman, H. M. (2023). Solubilization of Reactive Red 2 in the Mixed Micelles of Cetylpyridinium Chloride and TX-114. Molecules, 28(9), 3952. https://doi.org/10.3390/molecules28093952






