Highly Efficient Synthesis of Chlorogenic Acid Oleyl Alcohol Ester under Non-Catalytic and Solvent-Free Conditions
Abstract
1. Introduction
2. Results and Discussion
2.1. Influence of Reaction Parameters
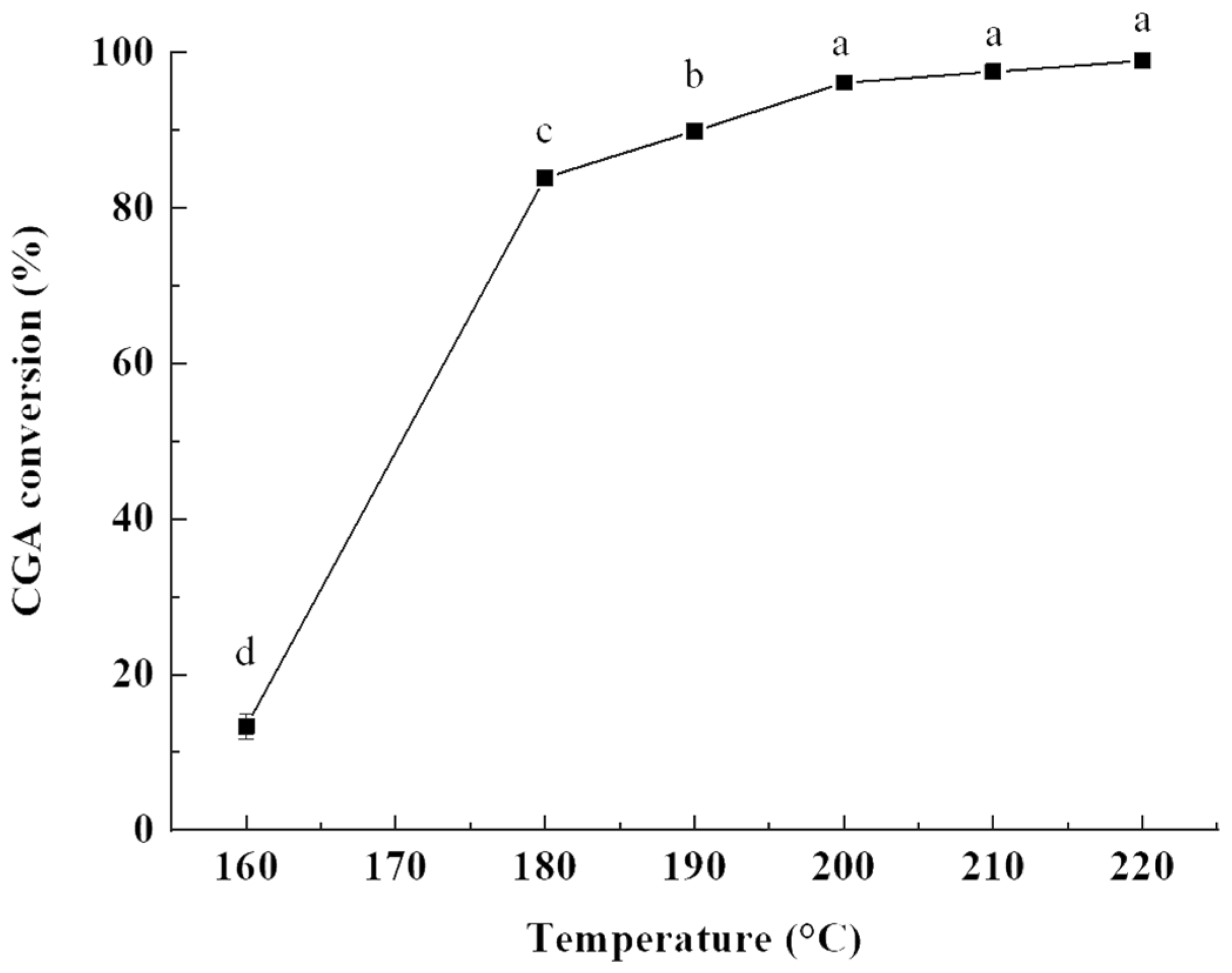
2.1.1. Influence of Reaction Temperature
2.1.2. Influence of Reaction Time
2.1.3. Influence of Molar Ratio of CGA to Oleyl Alcohol
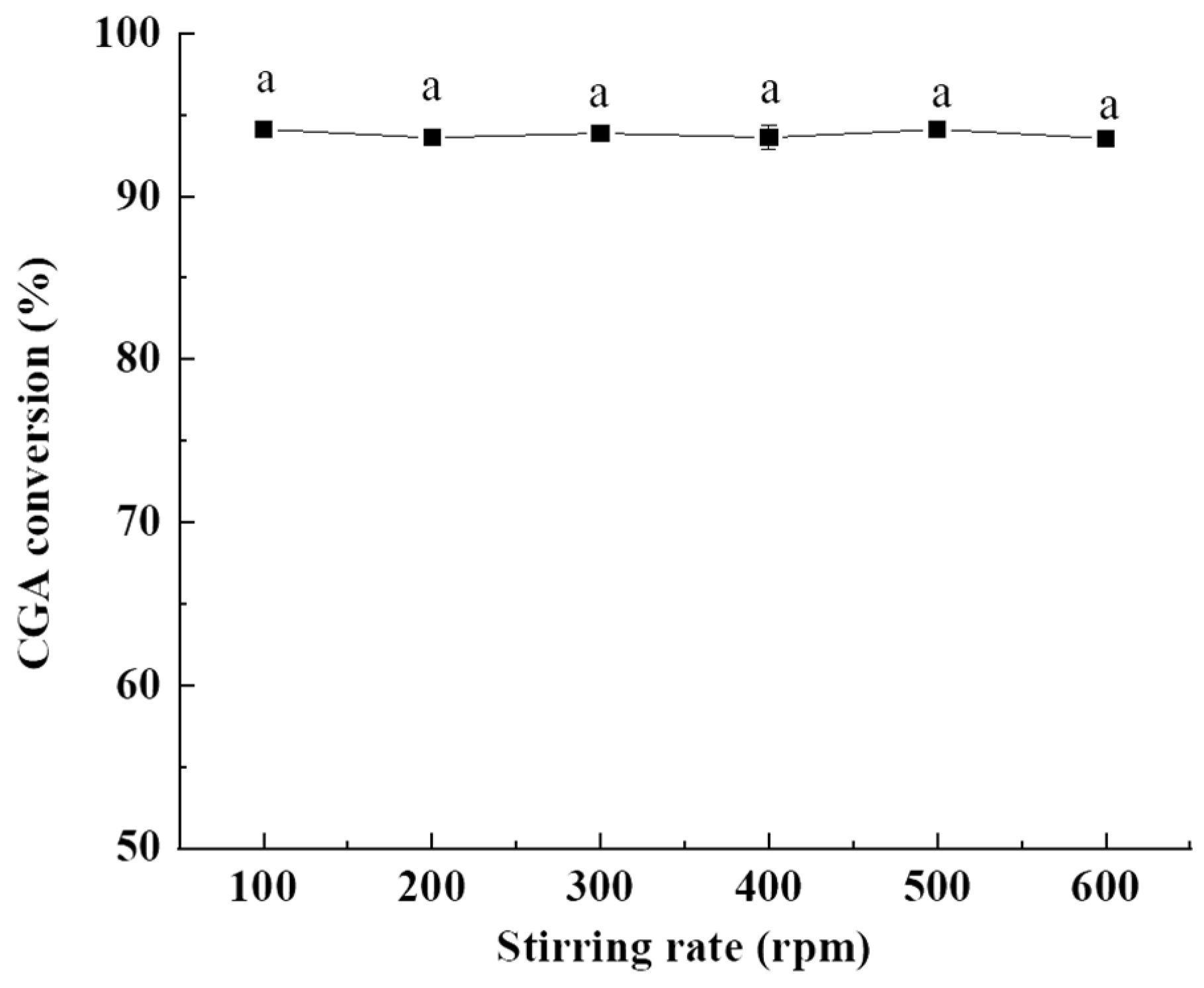
2.1.4. Influence of Stirring Rate
2.2. Purification of CGOA
2.3. Identification of CGOA
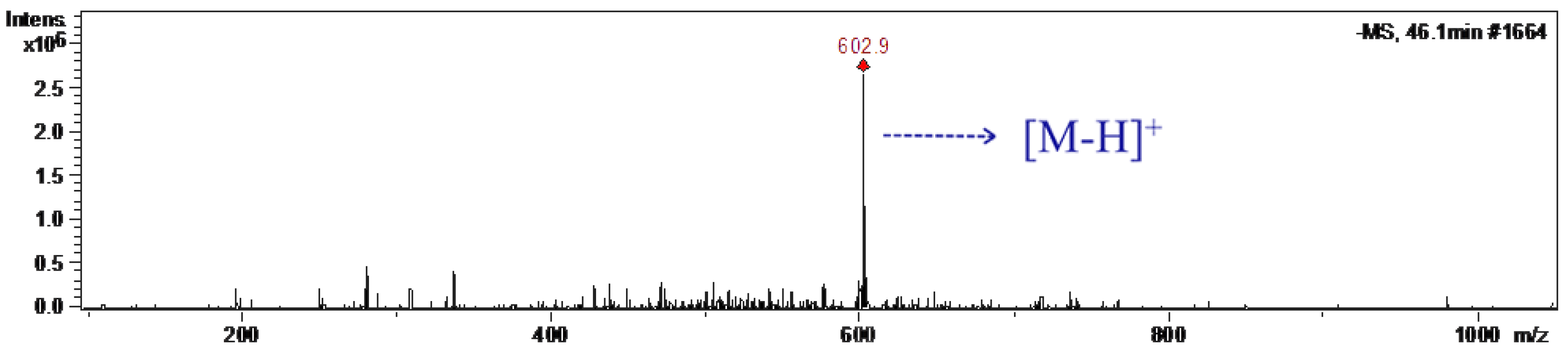
2.3.1. HPLC-MS Analysis of CGOA
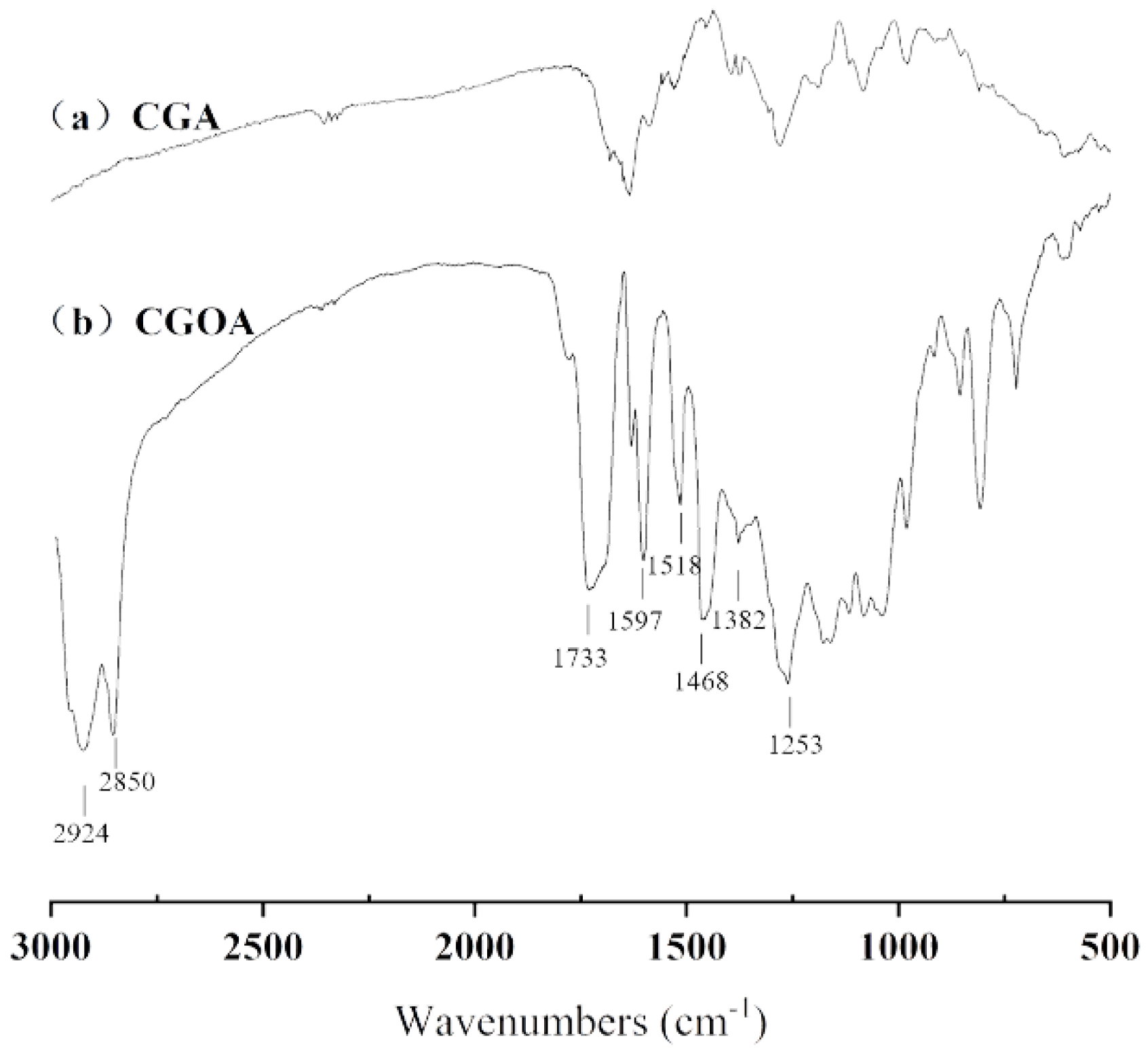
2.3.2. FT-IR Analysis of CGOA
2.3.3. NMR Analysis of CGOA
3. Materials and Methods
3.1. Materials
3.2. Preparation of CGOA
3.3. HPLC Analysis
3.4. Isolation and Purification of CGOA
3.5. HPLC-MS Analysis
3.6. FT-IR Analysis
3.7. NMR Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Iverson, F. Phenolic antioxidants: Health protection branch studies on butylated hydroxyanisole. Cancer Lett. 1995, 93, 49–54. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Villarino, M.; Sandin-Espana, P.; Melgarejo, P.; De Cal, A. High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agric. Food Chem. 2011, 59, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Alves Filho, E.G.; Sousa, V.M.; Rodrigues, S.; de Brito, E.S.; Fernandes, F.A.N. Green ultrasound-assisted extraction of chlorogenic acids from sweet potato peels and sonochemical hydrolysis of caffeoylquinic acids derivatives. Ultrason. Sonochem. 2020, 63, 104911. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Do, H.K.; Kim, D.Y.; Kim, W. Impact of chlorogenic acid on modulation of significant genes in dermal fibroblasts and epidermal keratinocytes. Biochem. Biophys. Res. Commun. 2021, 583, 22–28. [Google Scholar] [CrossRef]
- Sudhakar, M.; Sasikumar, S.J.; Silambanan, S.; Natarajan, D.; Ramakrishnan, R.; Nair, A.J.; Kiran, M.S. Chlorogenic acid promotes development of brown adipocyte-like phenotype in 3T3-L1 adipocytes. J. Funct. Foods 2020, 74, 104161. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.T.; Ye, Y.; Shan, J.H.; Yin, Z.M.; Luo, L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. INJ 2010, 41, 746–752. [Google Scholar] [CrossRef]
- Chen, Z.L.; Yang, Y.H.; Mi, S.M.; Fan, Q.S.; Sun, X.M.; Deng, B.C.; Wu, G.Y.; Li, Y.F.; Zhou, Q.C.; Ruan, Z. Hepatoprotective effect of chlorogenic acid against chronic liver injury in inflammatory rats. J. Funct. Foods 2019, 62, 103540. [Google Scholar] [CrossRef]
- Xue, Y.W.; Huang, F.; Tang, R.X.; Fan, Q.S.; Zhang, B.; Xu, Z.J.; Sun, X.M.; Ruan, Z. Chlorogenic acid attenuates cadmium-induced intestinal injury in Sprague-Dawley rats. Food Chem. Toxicol. 2019, 133, 110751. [Google Scholar] [CrossRef]
- Bao, L.P.; Li, J.S.; Zha, D.Q.; Zhang, L.; Gao, P.; Yao, T.; Wu, X.Y. Chlorogenic acid prevents diabetic nephropathy by inhibiting oxidative stress and inflammation through modulation of the Nrf2/HO-1 and NF-kB pathways. Int. Immunopharmacol. 2018, 54, 245–253. [Google Scholar] [CrossRef]
- Cho, A.S.; Jeon, S.M.; Kim, M.J.; Yeo, J.; Seo, K.I.; Choi, M.S.; Lee, M.K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.Y.; Cosma, G.; Gardner, H.; Castranova, V.; Vallyathan, V. Effect of chlorogenic acid on hydroxyl radical. Mol. Cell. Biochem. 2003, 247, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.E.; Chen, H.-H.; Chang, C.-I.; Huang, T.-C. Direct lipase-catalyzed lipophilization of chlorogenic acid from coffee pulp in supercritical carbon dioxide. Ind. Crops Prod. 2009, 30, 359–365. [Google Scholar] [CrossRef]
- Liang, S.H.; Zhao, Q.; Wei, X.Z.; Sun, C. Highly efficient synthesis of chlorogenic oleate using acyl chloride method. LWT—Food Sci. Technol. 2022, 154, 112817. [Google Scholar] [CrossRef]
- Zhu, S.; Meng, N.; Chen, S.W.; Li, Y. Study of acetylated EGCG synthesis by enzymatic transesterification in organic media. Arab. J. Chem. 2020, 13, 8824–8834. [Google Scholar] [CrossRef]
- Ménard, R.; Caillol, S.; Allais, F. Ferulic acid-based renewable esters and amides-containing epoxy thermosets from wheat bran and beetroot pulp: Chemo-enzymatic synthesis and thermo-mechanical properties characterization. Ind. Crops Prod. 2017, 95, 83–95. [Google Scholar] [CrossRef]
- Xiang, Z.N.; Ning, Z.X. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT—Food Sci. Technol. 2008, 41, 1189–1203. [Google Scholar] [CrossRef]
- Figueroa-Espinoza, M.C.; Villeneuve, P. Phenolic acids enzymatic lipophilization. J. Agric. Food Chem. 2005, 53, 2779–2787. [Google Scholar] [CrossRef]
- He, B.Q.; Deng, T.; Li, J.X.; Yan, F.; Wang, H.; Huang, Y.; Peng, C. An innovative auto-catalytic esterification for the production of phytosterol esters: Experiment and kinetics. RSC Adv. 2014, 4, 64319–64327. [Google Scholar] [CrossRef]
- Pinnarat, T.; Savage, P.E. Assessment of noncatalytic biodiesel synthesis using supercritical reaction conditions. Ind. Eng. Chem. Res. 2008, 47, 6801–6808. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Huang, K.-C.; Su, C.-H. Green process for the preparation of phytosterol esters: Microwave-mediated noncatalytic synthesis. Chem. Eng. J. 2020, 382, 122796. [Google Scholar] [CrossRef]
- Srivastava, M.; Mukhopadhyay, P.; Chakraborty, R. Efficient monooleoyl glycerol synthesis employing hybrid ultrasonic-infrared-wave promoted reactor: Concurrent catalytic and noncatalytic esterification kinetics. Int. J. Chem. Kinet. 2019, 52, 61–73. [Google Scholar] [CrossRef]
- Rani, K.N.P.; Neeharika, T.S.V.R.; Kumar, T.P.; Satyavathi, B.; Sailu, C. Kinetics of non-catalytic esterification of free fatty acids present in Jatropha oil. J. Oleo Sci. 2016, 65, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, L.J.L.; Laguerre, M.; Lecomte, J.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Villeneuve, P. Lipase-catalyzed synthesis of chlorogenate fatty esters in solvent-free medium. Enzyme Microb. Technol. 2007, 41, 721–726. [Google Scholar] [CrossRef]
- Lorentz, C.; Dulac, A.; Pencreac’h, G.; Ergan, F.; Richomme, P.; Soultani-Vigneron, S. Lipase-catalyzed synthesis of two new antioxidants: 4-O- and 3-O-palmitoyl chlorogenic acids. Biotechnol. Lett. 2010, 32, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Guyot, B.; Gueule, D.; Pina, M.; Graille, J.; Farines, V.; Farines, M. Enzymatic synthesis of fatty esters in 5-caffeoyl quinic acid. Eur. J. Lipid Sci. Technol. 2000, 102, 93–95. [Google Scholar] [CrossRef]
- Adnani, A.; Basri, M.; Chaibakhsh, N.; Ahangar, H.A.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Rahman, M.B.A. Chemometric analysis of lipase-catalyzed synthesis of xylitol esters in a solvent-free system. Carbohydr. Res. 2011, 346, 472–479. [Google Scholar] [CrossRef]
- Hardhianti, M.P.W.; Rochmadi; Azis, M.M. Kinetic studies of esterification of rosin and pentaerythritol. Processes 2022, 10, 39. [Google Scholar] [CrossRef]
- Panchal, B.; Chang, T.; Qin, S.J.; Sun, Y.Z.; Wang, J.X.; Bian, K. Optimization and kinetics of tung nut oil transesterification with methanol using novel solid acidic ionic liquid polymer as catalyst for methyl ester synthesis. Renew. Energy 2020, 151, 796–804. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Varma, M.N.; Madras, G. Synthesis of isoamyl laurate and isoamyl stearate in supercritical carbon dioxide. Appl. Biochem. Biotechnol. 2007, 141, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Guyot, B.; Bosquette, B.; Pina, M.; Graille, J. Esterification of phenolic acids from green coffee with an immobilized lipase from Candida antarctica in solvent-free medium. Biotechnol. Lett. 1997, 19, 529–532. [Google Scholar] [CrossRef]
- Stamatis, H.; Sereti, V.; Kolisis, F.N. Studies on the enzymatic synthesis of lipophilic derivatives of natural antioxidants. J. Am. Oil Chem. Soc. 1999, 76, 1505–1510. [Google Scholar] [CrossRef]
- Twu, Y.K.; Shih, I.L.; Yen, Y.H.; Ling, Y.F.; Shieh, C.J. Optimization of lipase-catalyzed synthesis of octyl hydroxyphenylpropionate by response surface methodology. J. Agric. Food Chem. 2005, 53, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Endo, A.; Kitahara, N.; Yamagishi, T.; Aoyagi, S.; Hara, S. Factors determining the reaction temperature of the solvent-free enzymatic synthesis of trehalose esters. Carbohydr. Res. 2019, 482, 107739. [Google Scholar] [CrossRef] [PubMed]
- Adnani, A.; Basri, M.; Malek, E.A.; Salleh, A.B.; Rahman, M.B.A.; Chaibakhsh, N.; Rahman, R.N.Z.R.A. Optimization of lipase-catalyzed synthesis of xylitol ester by Taguchi robust design method. Ind. Crops Prod. 2010, 31, 350–356. [Google Scholar] [CrossRef]
- Farah, A.; de Paulis, T.; Trugo, L.C.; Martin, P.R. Effect of roasting on the formation of chlorogenic acid lactones in coffee. J. Agric. Food Chem. 2005, 53, 1505–1513. [Google Scholar] [CrossRef]
- Chen, J.N.; Liu, W. Lipophilization of phenolic acids through esterification using p-toluenesulfonic acid as catalyst. Grain Oil Sci. Technol. 2018, 1, 91–96. [Google Scholar]
- Panchal, B.M.; Deshmukh, S.A.; Sharma, M.R. Production and kinetic transesterification of biodiesel from yellow grease with dimethyl carbonate using methanesulfonic acid as a catalyst. Environ. Prog. Sustain. Energy 2017, 36, 802–807. [Google Scholar] [CrossRef]
- Refaat, A.A. Different techniques for the production of biodiesel from waste vegetable oil. Int. J. Environ. Sci. Technol. 2009, 7, 183–213. [Google Scholar] [CrossRef]
- Winkler, H.; Vorwerg, W.; Wetzel, H. Synthesis and properties of fatty acid starch esters. Carbohydr. Polym. 2013, 98, 208–216. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, C.; Liu, H.; Chen, Y.; Wei, X.; Liang, S. Highly Efficient Synthesis of Chlorogenic Acid Oleyl Alcohol Ester under Non-Catalytic and Solvent-Free Conditions. Molecules 2023, 28, 3948. https://doi.org/10.3390/molecules28093948
Sun C, Liu H, Chen Y, Wei X, Liang S. Highly Efficient Synthesis of Chlorogenic Acid Oleyl Alcohol Ester under Non-Catalytic and Solvent-Free Conditions. Molecules. 2023; 28(9):3948. https://doi.org/10.3390/molecules28093948
Chicago/Turabian StyleSun, Cong, Hui Liu, Yanran Chen, Xianzhi Wei, and Shaohua Liang. 2023. "Highly Efficient Synthesis of Chlorogenic Acid Oleyl Alcohol Ester under Non-Catalytic and Solvent-Free Conditions" Molecules 28, no. 9: 3948. https://doi.org/10.3390/molecules28093948
APA StyleSun, C., Liu, H., Chen, Y., Wei, X., & Liang, S. (2023). Highly Efficient Synthesis of Chlorogenic Acid Oleyl Alcohol Ester under Non-Catalytic and Solvent-Free Conditions. Molecules, 28(9), 3948. https://doi.org/10.3390/molecules28093948





