Seven New Phenylhexanoids with Antioxidant Activity from Saxifraga umbellulata var. pectinata
Abstract
1. Introduction
2. Results and Discussion
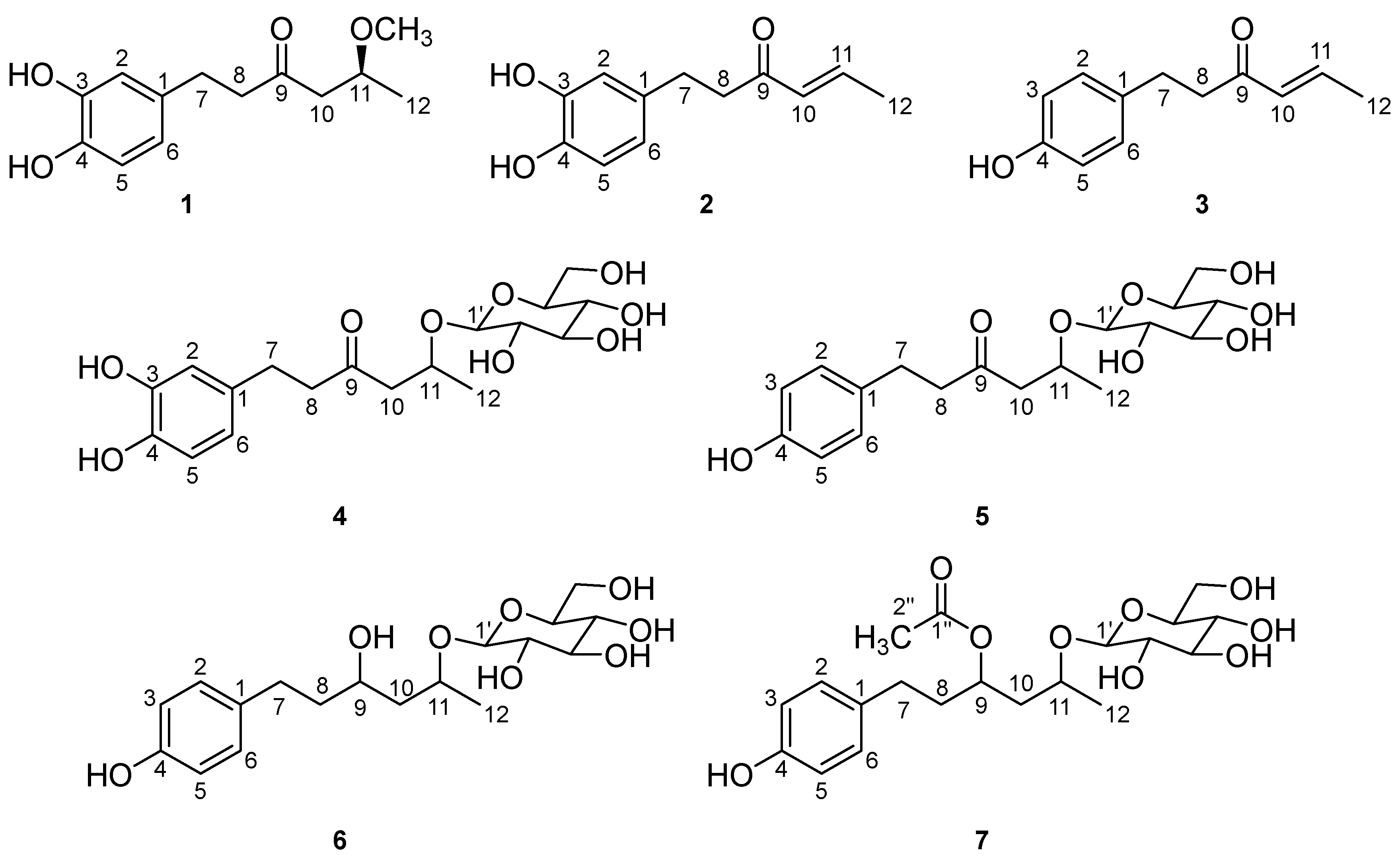
2.1. Identification of Compounds 1–7
2.2. The Antioxidant Activities of Compounds 1–7
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Compound 4
3.5. Determination of Antioxidant Activity
3.5.1. DPPH Radical Scavenging Assay
3.5.2. ABTS Radical Scavenging Assay
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| 1H-1H COSY | 1H-1H homonuclear chemical shift correlation spectroscopy |
| ABTS | 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic acid) ammonium salt |
| ANOVA | one-way analysis of variance |
| CD3OD | methanol-d4 |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EtOH | ethanol |
| HSQC | heteronuclear single quantum coherence spectroscopy |
| HMBC | heteronuclear multiple bond coherence spectroscopy |
| HR-ESI-MS | high-resolution electrospray ionization mass spectroscopy |
| IC50 | half inhibitory concentration |
| IR | infrared absorption spectrum |
| MeOH | methanol |
| NMR | nuclear magnetic resonance |
| IR | infrared absorption spectrum |
| SD | standard deviation |
| TLC | thin-layer chromatography |
References
- The Editorial Committee of Flora Reipublicae Popularis Sinicae (Zhongguo Zhiwu Zhi); Science Press: Beijing, China, 1992; Volume 34, p. 165.
- Zhong, G.-Y.; Wang, C.-H.; Liu, X. The resources and usage status of the commonly used Tibetan Medicinal Crop Dida. Chin. World Sci. Tech.-Mod. TCM Mater. Med. 2010, 12, 122–128. [Google Scholar]
- Luo, S.-Y.; Wang, W.-X.; Zhong, L.; Zhang, Y. Improvement of Quality Standard for Tibetan Medicine “Songdi”. Chin. World Sci. Tech.-Mod. TCM Mater. Med. 2022, 24, 3767–3773. [Google Scholar]
- Wu, R. Studies on the Chemical Constituents and Antibacterial Activity of Saxifrage umbellulata. Master’s Thesis, Lanzhou University of Technology, Lanzhou, China, 2014. [Google Scholar]
- Fei, Y.; Duan, H.; Niu, Y.-Z. The effect of flavonoids from Tibetan medicine ‘Songti’ on the oxidative damage of L02 hepatocytes. Tradit. Chin. Drug Res. Pharmacol. 2021, 32, 1260–1267. [Google Scholar]
- Jiang, W.; Zhong, G.-Y. A Kind of Diphenylnonane Compound and Its Composition and Its Preparation Method and Application. Chinese Patent 114149398 A, 8 March 2022. [Google Scholar]
- Fei, Y.; Jiang, W.; Zhong, G.-Y. Determination of total flavonoids content and chlorogenic ccid, rutin, quercetin in Tibetan medicinal herb ‘Songdi’ (Saxifraga umbellulata var. pectinata). Tradit. Chin. Drug Res. Pharmacol. 2013, 24, 411–415. [Google Scholar]
- Wu, R.; Ma, S.-Y. Flavonoios of Saxifraga umbellulata. Chem. Nat. Compd. 2017, 3, 467. [Google Scholar]
- Jiang, W.; Fei, Y.; Du, X.-L. Saxifraganoids A and B, two novel cucurbitane triterpenoid glycosides from Saxifraga umbellulata var. pectinate. Tetrahedron Lett. 2017, 58, 3541–3544. [Google Scholar] [CrossRef]
- Wu, R.; Yang, A.-M. Chemical constituents of Saxifraga umbellulata. Chem. Nat. Compd. 2015, 2015, 289–290. [Google Scholar]
- Yang, A.-M.; Wu, R.; Li, J.-Y. Chemical constituents of Saxifraga umbellulata. Adv. Mater. Res. 2013, 781–784, 2289–2291. [Google Scholar] [CrossRef]
- Kou, R.-W.; DU, S.-T.; Xia, B. Phenolic and steroidal metabolites from the cultivated edible Inonotus hispidus mushroom and their bioactivities. J. Agric. Food Chem. 2021, 69, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, W.; Hironori, T.; Hiroyuki, F.; Nobutoshi, T. Three new diarylheptanoid glycosides from Alnus japonica. Chem. Pharm. Bull. 1998, 46, 1054–1055. [Google Scholar]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Verma, K.; Nala, S. Free radicals as a double-edged sword: The cancer preventive and therapeutic roles of curcumin. Molecules 2020, 25, 5390. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, H. A review on free radicals and antioxidants. Infect. Disord. Drug Targets 2020, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q. Bridging free radical chemistry with drug discovery: A promising way for finding novel drugs efficiently. Eur. J. Med. Chem. 2020, 189, 112020. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-H.; Huang, J.; Zuo, H.-J.; Zhou, Z.-B.; Yang, C.-Y.; Huang, Z.-L. Monoterpenoid glycosides from the leaves of Ligustrum robustum and their bioactivities. Molecules 2022, 27, 3709. [Google Scholar] [CrossRef] [PubMed]
- Goupy, P.; Dufour, C.; Loonis, M. Quantitative kinetic analysis of hydrogen transfer reactions from dietary polyphenols to the DPPH radical. J. Agric. Food Chem. 2003, 51, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Silva, A. The antioxidant activity of prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Jansen, R.; Pilgrim, H. Hispolon, a yellow pigment from Inonotus hispidus. Phytochemistry 1996, 41, 927–929. [Google Scholar] [CrossRef]

| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 134.0 | 134.1 | 133.2 | |||
| 2 | 116.3 | 6.63 d (2.1) | 116.3 | 6.65 br.s | 130.3 | 7.00 d (8.5) |
| 3 | 146.2 | 146.2 | 116.1 | 6.68 d (8.5) | ||
| 4 | 144.5 | 144.5 | 156.6 | |||
| 5 | 116.5 | 6.67 d (8.0) | 116.5 | 6.68 d (8.0) | 116.1 | 6.68 d (8.5) |
| 6 | 120.6 | 6.50 dd (8.0, 2.1) | 120.6 | 6.53 br.d (8.0) | 130.3 | 7.00 d (8.5) |
| 7 | 30.0 | 2.72 t (4.0) | 30.8 | 2.76 t (7.5) | 30.4 | 2.79 m |
| 8 | 46.3 | 2.72 t (4.0) | 42.6 | 2.85 t (7.5) | 42.6 | 2.79 m |
| 9 | 211.3 | 202.6 | 202.5 | |||
| 10 | 50.6 | 2.67 dd (15.9, 7.5) 2.43 dd (15.9, 5.2) | 132.8 | 6.16 d (15.7) | 132.8 | 6.11 dq (15.8, 1.6) |
| 11 | 74.6 | 3.78 dqd (7.5, 6.2, 5.2) | 145.2 | 6.94 dq (14.1, 6.8) | 145.2 | 6.89 dq (15.8, 6.8) |
| 12 | 19.4 | 1.13 d (6.2) | 18.4 | 1.19 d (7.0) | 18.4 | 1.86 dd (6.8, 1.7) |
| -OCO3 | 56.8 | 3.27 s | ||||
| Position | 4 | 5 | 6 | 7 | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 134.1 | 133.3 | 134.5 | 133.8 | ||||
| 2 | 116.3 | 6.64 d (2.0) | 130.3 | 7.02 d (8.0) | 130.3 | 7.03 d (8.5) | 130.3 | 7.01 d (8.0) |
| 3 | 146.1 | 116.1 | 6.70 d (8.0) | 116.7 | 6.70 d (8.5) | 116.1 | 6.69 d (8.0) | |
| 4 | 144.4 | 156.5 | 156.3 | 156.4 | ||||
| 5 | 116.5 | 6.67 d (8.0) | 116.1 | 6.70 d (8.0) | 116.7 | 6.70 d (8.5) | 116.1 | 6.69 d (8.0) |
| 6 | 120.5 | 6.52 dd (8.0, 2.0) | 130.3 | 7.02 d (8.0) | 130.3 | 7.03 d (8.5) | 130.3 | 7.01 d (8.0) |
| 7 | 30.0 | 2.72 m | 29.7 | 2.77 m | 32.0 | 2.62 m | 31.6 | 2.55 m |
| 8 | 46.2 | 2.80 m | 46.2 | 2.80 m | 40.8 | 1.71 m | 37.0 | 1.95 m 1.84 m |
| 9 | 211.9 | 211.9 | 70.1 | 3.74 tt (8.5, 4.3) | 73.3 | 5.08 m | ||
| 10 | 51.6 | 2.53 dd (15.9, 5.4) 2.83 dd (15.9, 7.4) | 51.5 | 2.53 dd (15.9, 5.4) 2.84 dd (15.9, 7.3) | 45.5 | 1.84 m, 1.58 m | 42.5 | 1.97 m 1.68 m |
| 11 | 72.4 | 4.34 m | 72.3 | 4.34 m | 74.3 | 4.10 m | 72.7 | 3.97 dq (7.8, 6.0) |
| 12 | 20.3 | 1.20 d (6.2) | 20.3 | 1.20 d (6.2 ) | 20.1 | 1.21 d (6.0) | 20.0 | 1.19 d (6.0) |
| Glc-1′ | 102.4 | 4.34 d (7.8) | 102.3 | 4.34 d (7.7) | 102.3 | 4.36 d (7.8) | 101.8 | 4.32 d (7.7) |
| Glc-2′ | 75.0 | 3.12 m | 75.0 | 3.12 dd (9.2, 7.8) | 75.1 | 3.15 dd (9.1, 7.8) | 75.1 | 3.14 dd (8.8, 7.7) |
| Glc-3′ | 77.8 | 3.25 m | 77.8 | 3.25 m | 77.9 | 3.28 m | 77.8 | 3.25 m |
| Glc-4′ | 71.7 | 3.26 m | 71.7 | 3.26 m | 71.7 | 3.28 m | 71.7 | 3.30 m |
| Glc-5′ | 78.0 | 3.35 m | 78.0 | 3.36 m | 78.0 | 3.37 m | 78.0 | 3.34 m |
| Glc-6′ | 62.9 | 3.65 dd (11.9, 5.3) 3.84 dd (11.9, 1.9) | 62.9 | 3.64 dd (11.9, 5.3) 3.84 dd (11.9, 1.9) | 62.9 | 3.67 dd (11.8, 5.5) 3.87 dd (11.8, 1.7) | 62.9 | 3.68 dd (11.8, 5.4) 3.79 dd (11.8, 2.3) |
| C=O | 173.0 | |||||||
| CH3 | 21.3 | 2.01 s | ||||||
| Compound | IC50 (μM) | |
|---|---|---|
| DPPH | ABTS | |
| 1 | 48.66 ± 0.94 | 13.99 ± 2.53 b |
| 2 | 53.85 ± 1.17 | 13.11 ± 0.94 b |
| 3 | >100 | 28.85 ± 0.18 |
| 4 | 43.95 ± 1.91 | 28.44 ± 3.86 c |
| 5 | >100 | 33.04 ± 1.43 |
| 6 | >100 | 38.10 ± 3.94 |
| 7 | >100 | 27.03 ± 0.55 c |
| L-ascorbic acid a | 30.41 ± 1.40 | 23.51 ± 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Chen, D.; Liu, M.; Yu, Y.; Zhang, Y.; Huang, J. Seven New Phenylhexanoids with Antioxidant Activity from Saxifraga umbellulata var. pectinata. Molecules 2023, 28, 3928. https://doi.org/10.3390/molecules28093928
Huang J, Chen D, Liu M, Yu Y, Zhang Y, Huang J. Seven New Phenylhexanoids with Antioxidant Activity from Saxifraga umbellulata var. pectinata. Molecules. 2023; 28(9):3928. https://doi.org/10.3390/molecules28093928
Chicago/Turabian StyleHuang, Jiao, Donglin Chen, Mengying Liu, Yarui Yu, Yi Zhang, and Jing Huang. 2023. "Seven New Phenylhexanoids with Antioxidant Activity from Saxifraga umbellulata var. pectinata" Molecules 28, no. 9: 3928. https://doi.org/10.3390/molecules28093928
APA StyleHuang, J., Chen, D., Liu, M., Yu, Y., Zhang, Y., & Huang, J. (2023). Seven New Phenylhexanoids with Antioxidant Activity from Saxifraga umbellulata var. pectinata. Molecules, 28(9), 3928. https://doi.org/10.3390/molecules28093928





