(-)-Epigallocatechin-3-Gallate Attenuates the Adverse Reactions Triggered by Selenium Nanoparticles without Compromising Their Suppressing Effect on Peritoneal Carcinomatosis in Mice Bearing Hepatocarcinoma 22 Cells
Abstract
1. Introduction
2. Results and Discussion
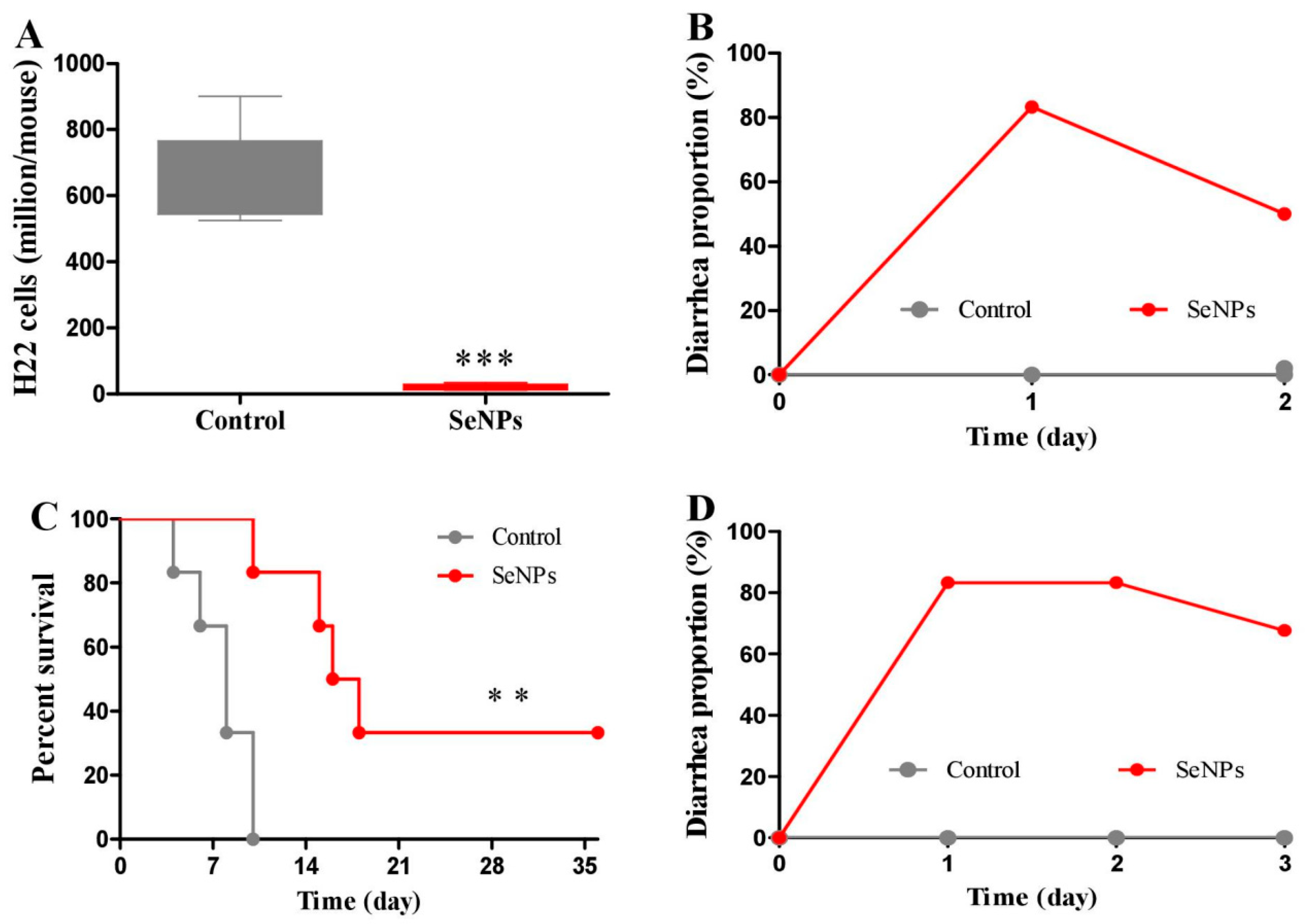
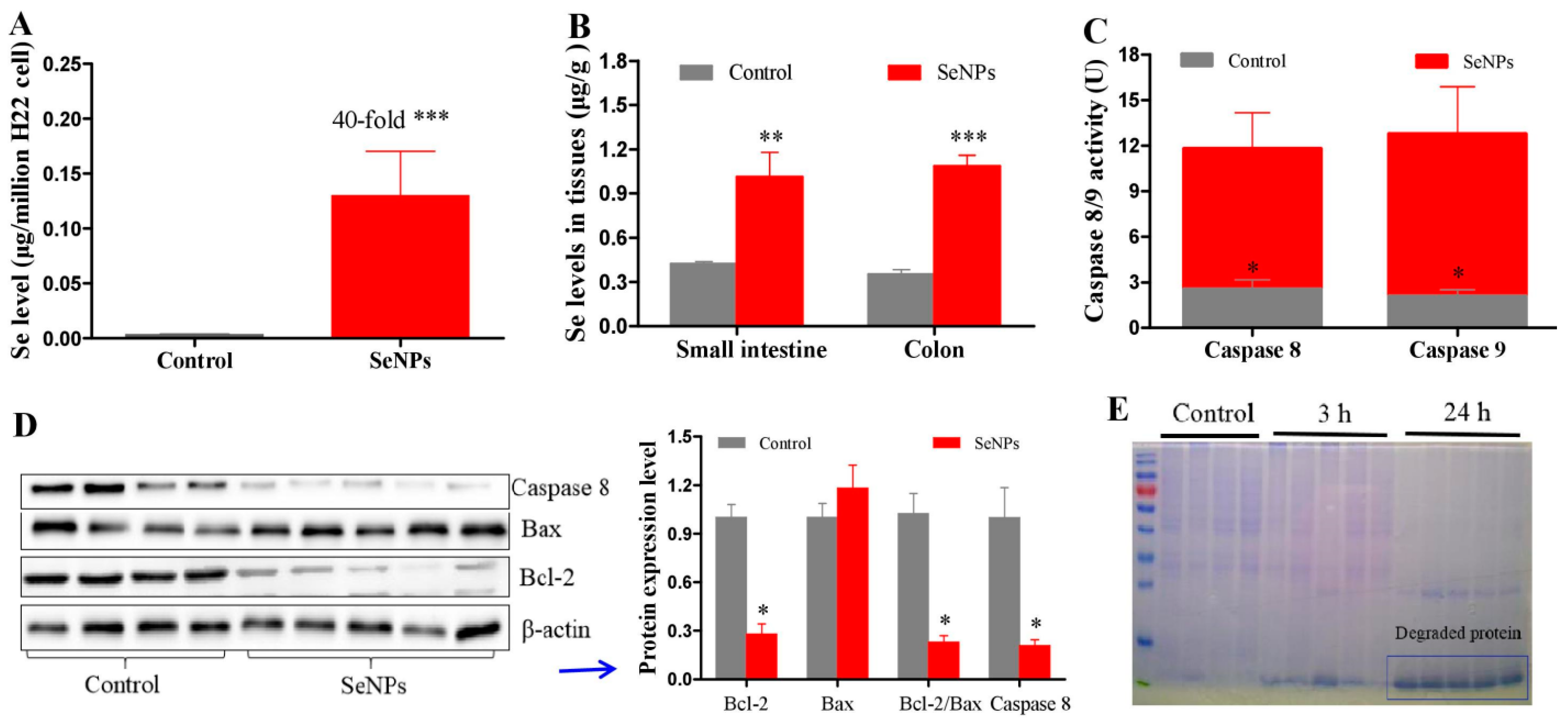
2.1. Effects of SeNPs on Peritoneal Carcinomatosis in Mice Bearing H22 Cells
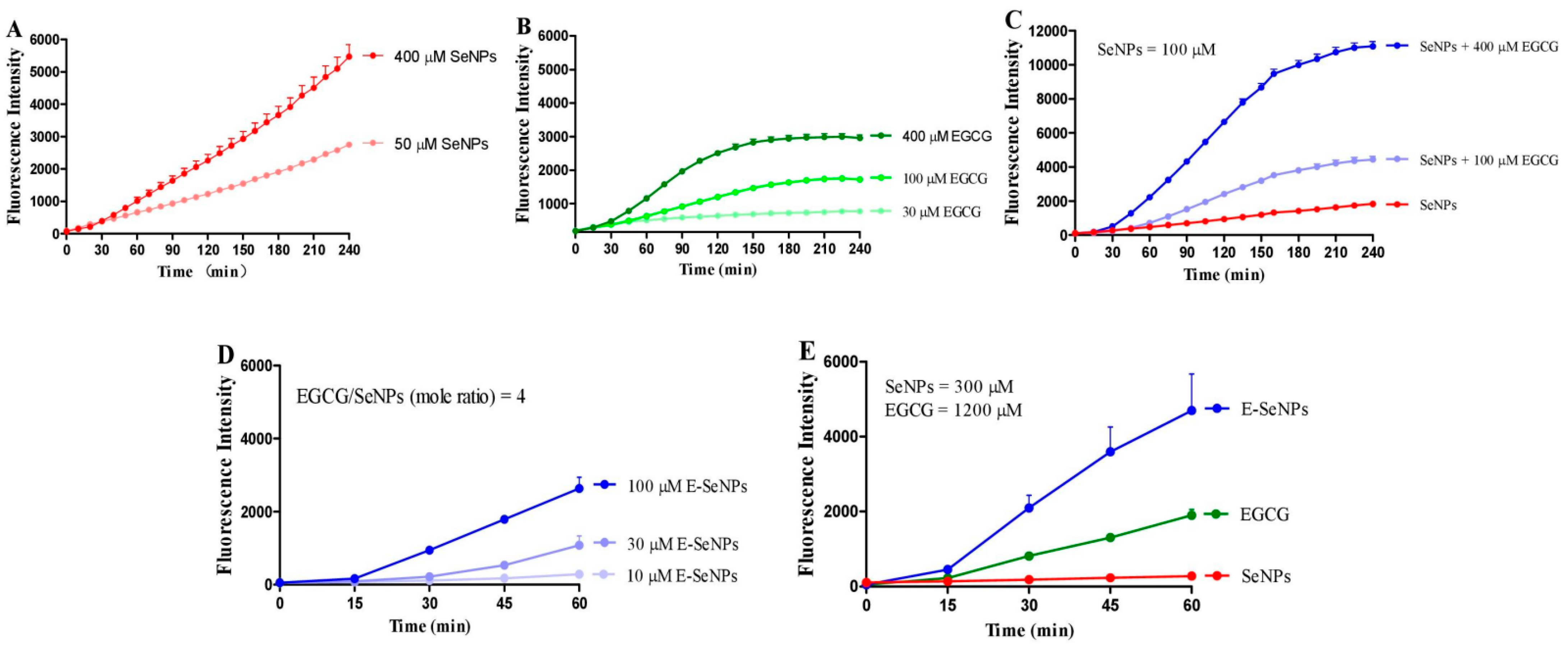
2.2. EGCG Enhances the Production of SeNP-Induced ROS in H22 Cell Suspension
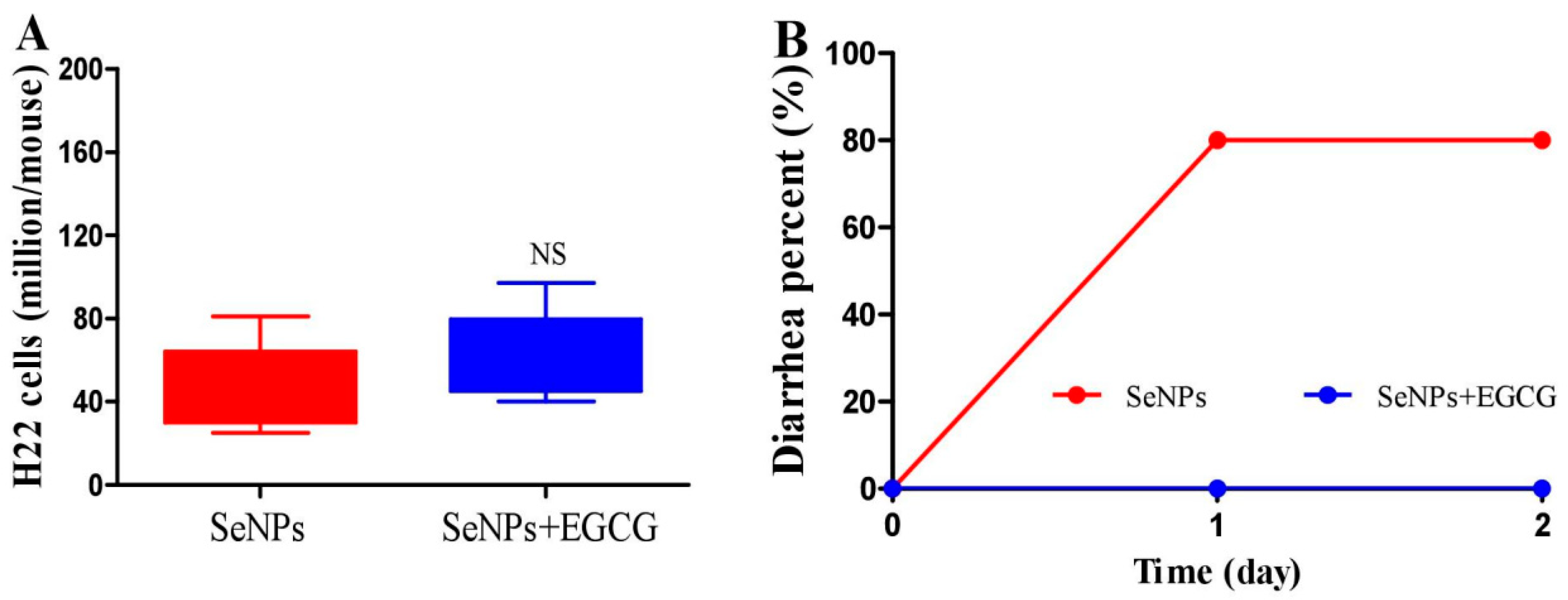
2.3. EGCG Reduces the Diarrhea Proportion Caused by SeNPs without Compromising Their Suppressing Efficacy on Peritoneal Carcinomatosis in Mice
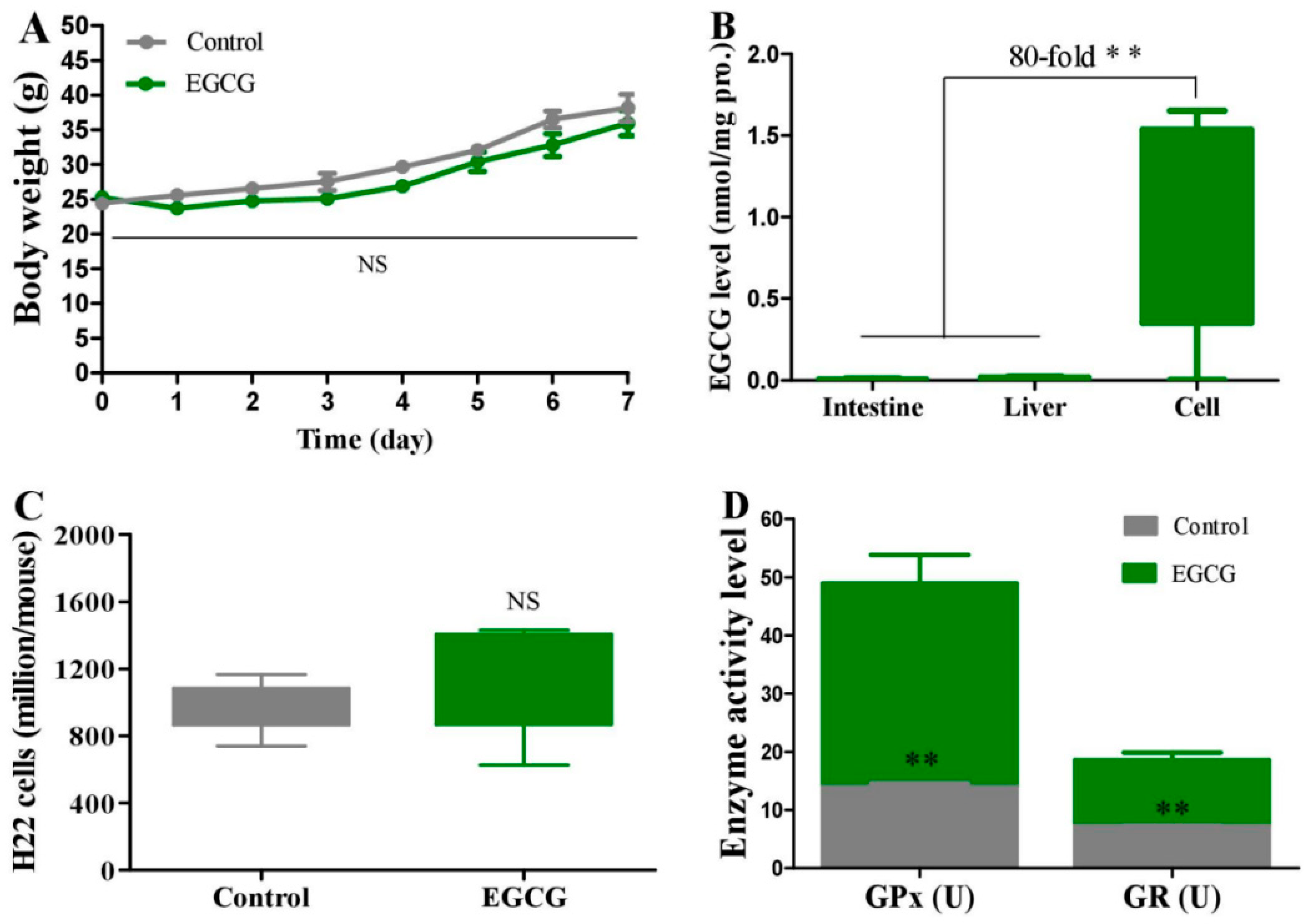
2.4. Effect of EGCG on Peritoneal Carcinomatosis in Mice Bearing H22 Cells
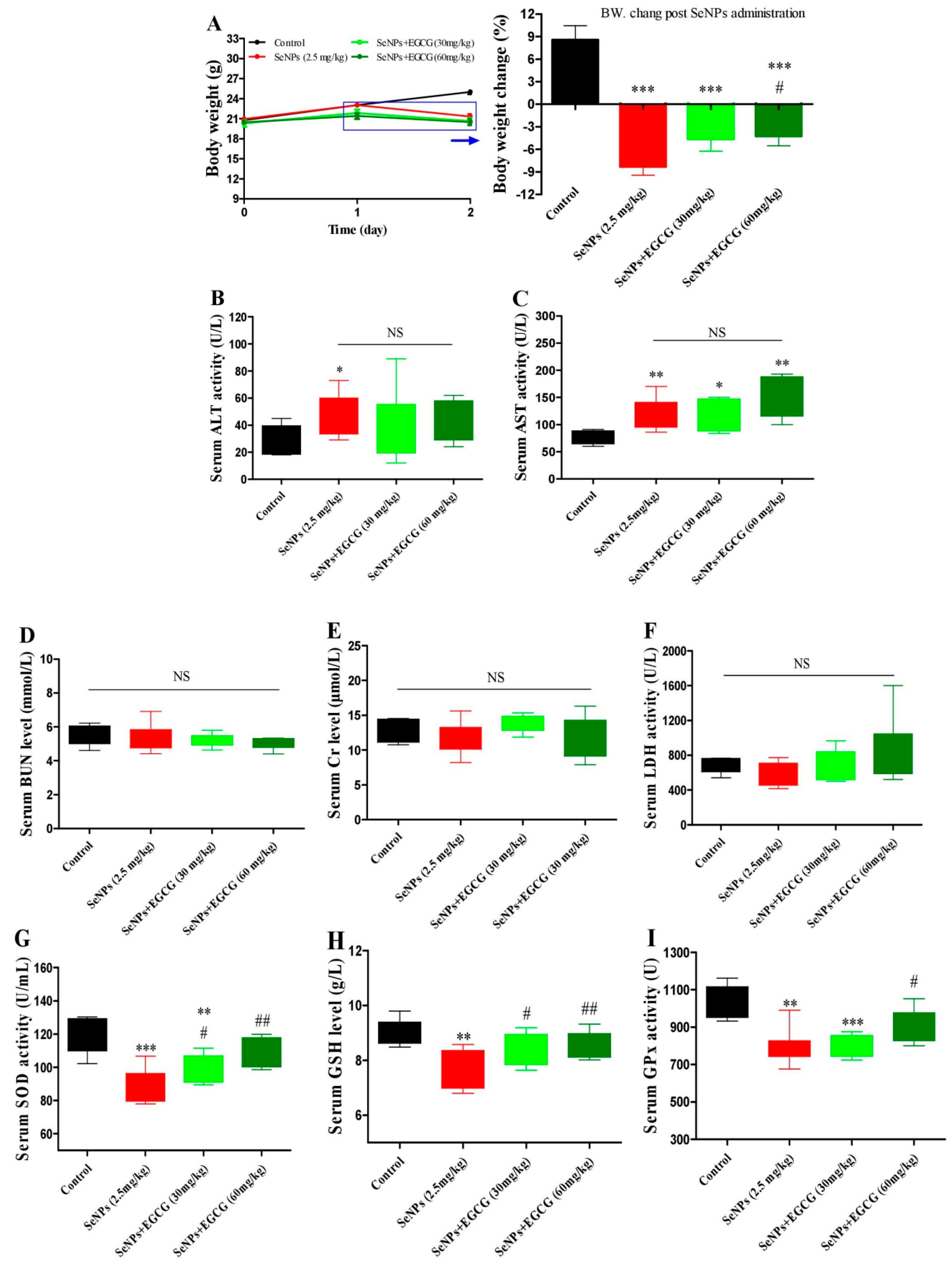
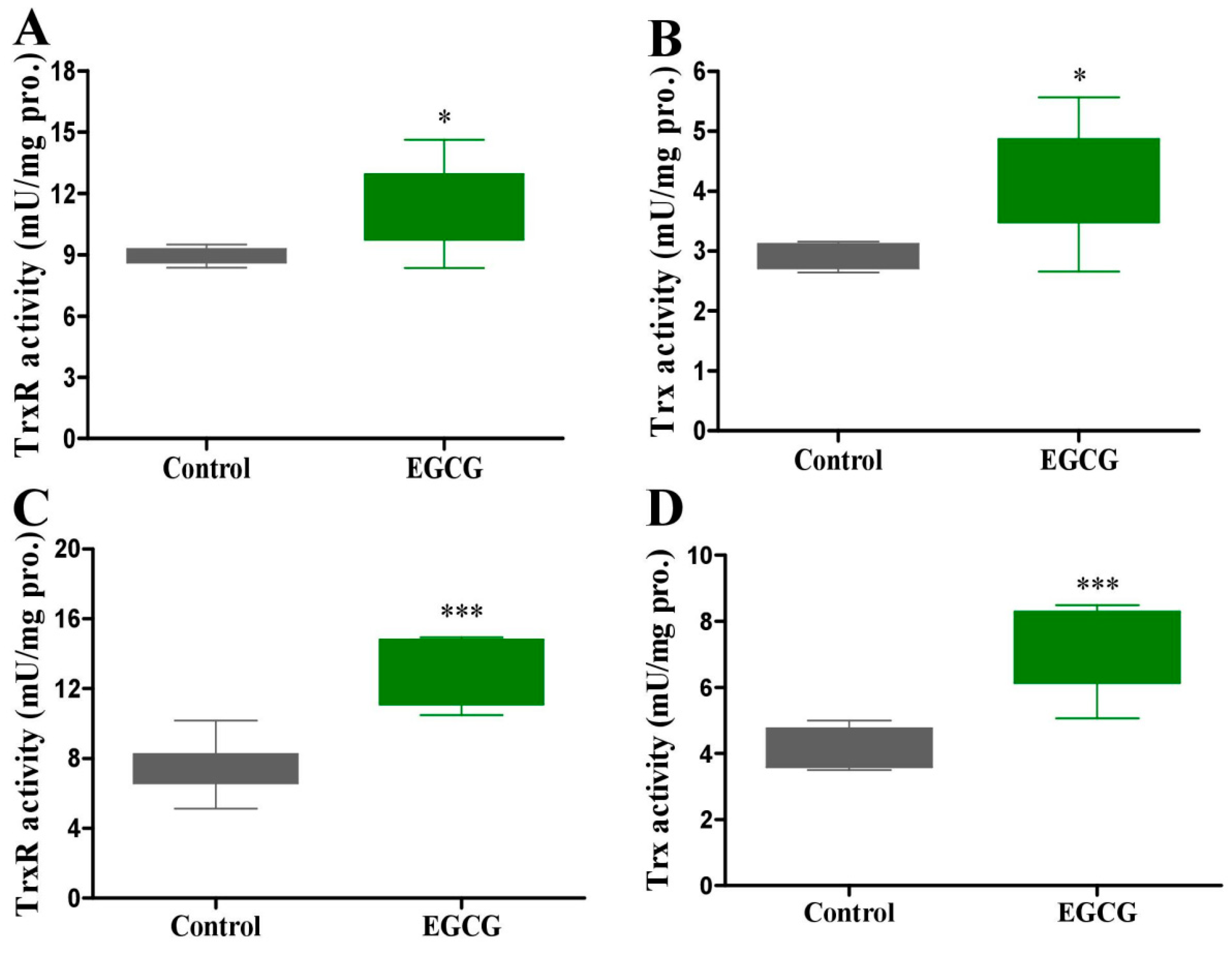
2.5. EGCG Protects against SeNP-Triggered Systemic Toxicity via Restoring Antioxidant Defense in Healthy Mice
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation and Characterization of SeNPs
3.3. Animals and H22 Model Mice
3.4. H22 Cell Collection and Count
3.5. ROS Measurement
3.6. Determination of Enzyme Activities
3.7. Determination of Selenium Level in Tissues
3.8. EGCG Measurement in H22 Cells and Tissues
3.9. Western Blot Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buyel, J.F. Plants as sources of natural and recombinant anti-cancer agents. Biotechnol. Adv. 2018, 36, 506–520. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Ji, X.L.; Hou, C.Y.; Gao, Y.G.; Xue, Y.Q.; Yan, Y.Z.; Guo, X.D. Metagenomic analysis of gut microbiota modulatory effects of jujube (Ziziphus jujuba Mill.) polysaccharides in a colorectal cancer mouse model. Food Funct. 2020, 11, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Hong, J. Prevention of chronic diseases by tea: Possible mechanisms and human relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.F.; Ryu, J.H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (-)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Yang, C.S.; Sang, S.D.; Lambert, J.D.; Lee, M.J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008, 52, S139–S151. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liao, J.; Kim, K.; Yurkow, E.J.; Yang, C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [Google Scholar] [CrossRef]
- Li, G.-X.; Chen, Y.-K.; Hou, Z.; Xiao, H.; Jin, H.Y.; Lu, G.; Lee, M.-J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: A comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef]
- Baba, Y.; Sonoda, J.-I.; Hayashi, S.; Tosuji, N.; Sonoda, S.; Makisumi, K.; Nakajo, M. Reduction of oxidative stress in liver cancer patients by oral green tea polyphenol tablets during hepatic arterial infusion chemotherapy. Exp. Ther. Med. 2012, 4, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Staples, J.; Wataha, J.; Lewis, J.; Lockwood, P.; Schoenlein, P.; Rao, S.; Osaki, T.; Dickinson, D.; Kamatani, T.; et al. Protective effects of EGCG on salivary gland cells treated with gamma-radiation or cis-platinum(II)diammine dichloride. Anticancer Res. 2004, 24, 3065–3073. [Google Scholar]
- Enkhbat, T.; Nishi, M.; Yoshikawa, K.; Jun, H.; Tokunaga, T.; Takasu, C.; Kashihara, H.; Ishikawa, D.; Tominaga, M.; Shimada, M. Epigallocatechin-3-gallate enhances radiation sensitivity in colorectal cancer cells through Nrf2 activation and autophagy. Anticancer Res. 2018, 38, 6247–6252. [Google Scholar] [CrossRef]
- Arnér, E.S.J. Focus on mammalian thioredoxin reductases--important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef] [PubMed]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox. Signal 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Spallholz, J.E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 1994, 17, 45–64. [Google Scholar] [CrossRef] [PubMed]
- Seko, Y.; Imura, N. Active oxygen generation as a possible mechanism of selenium toxicity. Biomed. Environ. Sci. 1997, 10, 333–339. [Google Scholar]
- Wu, X.M.; Zhao, G.S.; He, Y.F.; Wang, W.P.; Yang, C.S.; Zhang, J.S. Pharmacological mechanisms of the anticancer action of sodium selenite against peritoneal cancer in mice. Pharmacol. Res. 2019, 147, 104360. [Google Scholar] [CrossRef]
- Jia, X.; Li, N.; Chen, J. A subchronic toxicity study of elemental nano-Se in sprague-dawley rats. Life Sci. 2005, 76, 1989–2003. [Google Scholar] [CrossRef]
- Zhao, G.S.; Wu, X.M.; Chen, P.P.; Zhang, L.Y.; Yang, C.S.; Zhang, J.S. Selenium nanoparticles are more efficient than sodium selenite in producing reactive oxygen species and hyper-accumulation of selenium nanoparticles in cancer cells generates potent therapeutic effects. Free Radic. Biol. Med. 2018, 126, 55–66. [Google Scholar] [CrossRef]
- Yan, L.; Spallholz, J.E. Generation of reactive oxygen species from the reaction of selenium compounds with thiols and mammary tumor cells. Biochem. Pharmacol. 1993, 45, 429–437. [Google Scholar] [PubMed]
- Fernandes, A.P.; Wallenberg, M.; Gandin, V.; Misra, S.; Tisato, F.; Marzano, C.; Rigobello, M.P.; Kumar, S.; Björnstedt, M. Mikael Björnstedt, Methylselenol formed by spontaneous methylation of selenide is a superior selenium substrate to the thioredoxin and glutaredoxin systems. PLoS ONE 2012, 7, e50727. [Google Scholar] [CrossRef]
- Wang, X.; Sun, K.; Tan, Y.P.; Wu, S.S.; Zhang, J.S. Efficacy and safety of selenium nanoparticles administered intraperitoneally for the prevention of growth of cancer cells in the peritoneal cavity. Free Radic. Biol. Med. 2014, 72, 1–10. [Google Scholar] [CrossRef]
- Jaffe, N.; Paed, D.; Traggis, D.; Salian, S.; Cassady, J.R. Improved outlook for ewing’s sarcoma with combination chemotherapy (vincristine, actinomycin D and cyclophosphamide) and radiation therapy. Cancer 1976, 38, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.D.; Bentzen, S.M.; Harari, P.M. Biologic basis for combining drugs with radiation. Semin. Radiat. Oncol. 2006, 16, 2–9. [Google Scholar] [CrossRef]
- Brunner, T.B. The rationale of combined radiotherapy and chemotherapy—Joint action of castor and pollux. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, G.; Singh, C.K.; Ndiaye, M.A.; Fedorowicz, S.; Molot, A.; Ahmad, N. Prostate cancer chemoprevention by natural agents: Clinical evidence and potential implications. Cancer Lett. 2018, 422, 9–18. [Google Scholar] [CrossRef]
- Abdulah, R.; Miyazaki, K.; Nakazawa, M.; Koyama, H. Chemical forms of selenium for cancer prevention. J. Trace Elem. Med. Biol. 2005, 19, 141–150. [Google Scholar] [CrossRef]
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385. [Google Scholar] [CrossRef]
- Nissan, A.; Stojadinovic, A.; Garofalo, A.; Esquivel, J.; Piso, P. Evidence-based medicine in the treatment of peritoneal carcinomatosis: Past, present, and future. J. Surg. Oncol. 2009, 100, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Xiang, N.; Domann, F.E.; Zhong, W.X. Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells. Cancer Res. 2006, 66, 2296–2304. [Google Scholar] [CrossRef]
- Hu, H.B.; Jiang, C.; Schuster, T.; Li, G.X.; Daniel, P.T.; Lü, J.X. Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway. Mol. Cancer Ther. 2006, 5, 1873–1882. [Google Scholar] [CrossRef]
- Wu, S.S.; Sun, K.; Wang, X.; Wang, D.X.; Wan, X.C.; Zhang, J.S. Protonation of epigallocatechin-3-gallate (EGCG) results in massive aggregation and reduced oral bioavailability of EGCG-dispersed selenium nanoparticles. J. Agric. Food Chem. 2013, 61, 7268–7275. [Google Scholar] [CrossRef]
- Stein, A.; Voigt, W.; Jordan, K. Chemotherapy-induced diarrhea: Pathophysiology, frequency and guideline-based management. Ther. Adv. Med. Oncol. 2010, 2, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Andreyev, J.; Ross, P.; Donnellan, C.; Lennan, E.; Leonard, P.; Waters, C.; Wedlake, L.; Bridgewater, J.; Glynne-Jones, R.; Allum, W.; et al. Guidance on the Management of Diarrhoea During Cancer Chemotherapy. Lancet Oncol. 2014, 15, e447–e460. [Google Scholar] [CrossRef]
- McQuade, R.M.; Stojanovska, V.; Abalo, R.; Bornstein, J.C.; Nurgali, K. Chemotherapy-induced constipation and diarrhea: Pathophysiology, current and emerging treatments. Front. Pharmacol. 2016, 7, 414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Wang, X.F.; Xu, T.W. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with Se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Zhang, J.S.; Yu, H.Q. Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on selenoenzymes: Comparison with selenomethionine in mice. Free Radic. Biol. Med. 2007, 42, 1524–1533. [Google Scholar] [CrossRef]
- Song, J.; Lei, X.; Luo, J.; Everaert, N.; Zhao, G.; Wen, J.; Yang, Y. The Effect of epigallocatechin-3-gallate on small intestinal morphology, antioxidant capacity and anti-inflammatory effect in heat-stressed broilers. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1030–1038. [Google Scholar] [CrossRef]
- Wang, D.X.; Wang, Y.J.; Wan, X.C.; Yang, C.S.; Zhang, J.S. Green tea polyphenol (-)-epigallocatechin-3-gallate triggered hepatotoxicity in mice: Responses of major antioxidant enzymes and the Nrf2 rescue pathway. Toxicol. Appl. Pharmacol. 2015, 283, 65–74. [Google Scholar] [CrossRef]
- Sahin, K.; Tuzcu, M.; Gencoglu, H.; Dogukan, A.; Timurkan, M.; Sahin, N.; Aslan, A.; Kucuk, O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010, 87, 240–245. [Google Scholar] [CrossRef]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer prevention with green tea and its principal constituent, EGCG: From early investigations to current focus on human cancer stem cells. Mol. Cells 2018, 41, 73–82. [Google Scholar]
- Chikara, S.; Nagaprashantha, L.D.; Singhal, J.; Horne, D.; Awasthi, S.; Singhal, S.S. Oxidative stress and dietary phytochemicals: Role in ancer chemoprevention and treatment. Cancer Lett. 2018, 413, 122–134. [Google Scholar] [CrossRef]
- Wang, D.; Wei, Y.; Wang, T.; Wan, X.; Yang, C.S.; Reiter, R.J.; Zhang, J. Melatonin attenuates (-)-epigallocatehin-3-gallate-triggered hepatotoxicity without compromising its downregulation of hepatic gluconeogenic and lipogenic genes in mice. J. Pineal Res. 2015, 59, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Gromer, S.; Urig, S.; Becker, K. The thioredoxin system--from science to clinic. Med. Res. Rev. 2004, 24, 40–89. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.; Awwad, R.T.; Smart, D.D.K.; Spitz, D.R.; Gius, D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006, 236, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Zhang, B.X.; Li, X.M.; Han, X.; Liu, R.J.; Fang, J.G. Small molecule inhibitors of mammalian thioredoxin reductase as potential anticancer agents: An update. Med. Res. Rev. 2019, 39, 5–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; Li, X.M.; Han, X.; Liu, R.J.; Fang, J.G. Targeting the thioredoxin system for cancer therapy. Trends Pharmacol. Sci. 2017, 38, 794–808. [Google Scholar] [CrossRef]
- Cai, W.Q.; Zhang, L.W.; Song, Y.L.; Wang, B.L.; Zhang, B.X.; Cui, X.M.; Hu, G.; Liu, Y.; Wu, J.; Fang, J. Small molecule inhibitors of mammalian thioredoxin reductase. Free Radic. Biol. Med. 2012, 52, 257–265. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Peng, S.; Liu, R.; Li, X.; Hou, Y.; Han, X.; Fang, J. Thioredoxin reductase inhibitors: A patent review. Expert Opin. Ther. Pat. 2017, 27, 547–556. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Wang, D.; Li, S.; Sun, K.; Wan, X.; Taylor, E.W.; Zhang, J. Inhibition of glutathione synthesis eliminates the adaptive response of ascitic hepatoma 22 cells to nedaplatin that targets thioredoxin reductase. Toxicol. Appl. Pharmacol. 2012, 265, 342–350. [Google Scholar] [CrossRef]
- Singh, S.; Singh, D.K.; Meena, A.; Dubey, V.; Masood, N.; Luqman, S. Rutin protects tbutyl hydroperoxide-induced oxidative impairment via modulating the Nrf2 and iNOS activity. Phytomedicine 2019, 55, 92–104. [Google Scholar] [CrossRef]
- Chen, X.Y.; Cheng, C.; Zuo, X.Z.; Huang, W. Astragalin alleviates cerebral ischemia-reperfusion injury by improving anti-oxidant and anti-inflammatory activities and inhibiting apoptosis pathway in rats. BMC Complement. Med. Ther. 2020, 20, 120. [Google Scholar] [CrossRef]
- Tayemeh, M.B.; Kalbassi, M.R.; Paknejad, H.; Joo, H.S. Dietary nanoencapsulated quercetin homeostated transcription of redox-status orchestrating genes in zebrafish (Danio rerio) exposed to silver nanoparticles. Environ. Res. 2020, 185, 109477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, J.J.; Jing, Z.H.; Ordovas, J.M.; Wang, J.; Shen, L.R. Anti-fatigue and anti-oxidant effects of curcumin supplementation in exhaustive swimming mice via Nrf2/Keap1 signal pathway. Curr. Res. Food Sci. 2022, 5, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Ogborne, R.M.; Rushworth, S.A.; O’Connell, M.A. Epigallocatechin activates haem oxygenase-1 expression via protein kinase Cdelta and Nrf. Biochem. Biophys. Res. Commun. 2008, 373, 584–588. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Wu, C.H.; Ho, C.Y.; Yen, G.C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J. Nutr. Biochem. 2013, 24, 475–483. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Adhami, V.M.; Bharali, D.J.; Hafeez, B.B.; Asim, M.; Khwaja, S.I.; Ahmad, N.; Cui, H.; Mousa, S.A.; Mukhtar, H. Introducing nanochemoprevention as a novel approach for cancer control: Proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res. 2009, 69, 1712–1716. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Pinheiro, M.; Reis, S. Epigallocatechin Gallate Nanodelivery Systems for Cancer Therapy. Nutrients 2016, 8, 307. [Google Scholar] [CrossRef]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Wang, Y.J.; Chen, P.P.; Zhao, G.S.; Sun, K.; Li, D.X.; Wan, X.C.; Zhang, J.S. Inverse relationship between elemental selenium nanoparticle size and inhibition of cancer cell growth in vitro and in vivo. Food Chem. Toxicol. 2015, 85, 71–77. [Google Scholar] [CrossRef]
- Zhao, G.S.; Dong, R.X.; Teng, J.Y.; Yang, L.; Liu, T.; Wu, X.M.; He, Y.; Wang, Z.; Pu, H.; Wang, Y. N-Acetyl-l-cysteine enhances the effect of selenium nanoparticles on cancer cytotoxicity by increasing the production of selenium-induced reactive oxygen species. ACS Omega 2020, 5, 11710–11720. [Google Scholar] [CrossRef]
- Smith, A.D.; Levander, O.A. High-throughput 96-well microplate assays for determining specific activities of glutathione peroxidase and thioredoxin reductase. Methods Enzymol. 2002, 347, 113–121. [Google Scholar]
- Carlberg, I.; Mannervik, B. Glutathione reductase. Methods Enzymol. 1985, 113, 484–490. [Google Scholar]
- Zhang, L.; Ning, M.; Xu, Y.; Wang, C.; Zhao, G.; Cao, Q.; Zhang, J. Predicting the cytotoxic potency of cigarette smoke by assessing the thioredoxin reductase inhibitory capacity of cigarette smoke extract. Int. J. Environ. Res. Public Health 2016, 13, 348. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Landrum, L.M.; Gold, M.A.; Moore, K.N.; Myers, T.K.N.; McMeekin, D.S.; Walker, J.L. Intraperitoneal chemotherapy for patients with advanced epithelial ovarian cancer: A review of complications and completion rates. Gynecol Oncol. 2008, 108, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.X.; Wang, D.X.; Wang, X.X.; Zhang, K.; Chen, P.P.; Yang, C.S.; Zhang, J. Epigallocatechin-3-gallate enhances key enzymatic activities of hepatic thioredoxin and glutathione systems in selenium-optimal mice but activates hepatic Nrf2 responses in selenium-deficient mice. Redox Biol. 2016, 10, 221–232. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ban, Q.; Chi, W.; Wang, X.; Wang, S.; Hai, D.; Zhao, G.; Zhao, Q.; Granato, D.; Huang, X. (-)-Epigallocatechin-3-Gallate Attenuates the Adverse Reactions Triggered by Selenium Nanoparticles without Compromising Their Suppressing Effect on Peritoneal Carcinomatosis in Mice Bearing Hepatocarcinoma 22 Cells. Molecules 2023, 28, 3904. https://doi.org/10.3390/molecules28093904
Ban Q, Chi W, Wang X, Wang S, Hai D, Zhao G, Zhao Q, Granato D, Huang X. (-)-Epigallocatechin-3-Gallate Attenuates the Adverse Reactions Triggered by Selenium Nanoparticles without Compromising Their Suppressing Effect on Peritoneal Carcinomatosis in Mice Bearing Hepatocarcinoma 22 Cells. Molecules. 2023; 28(9):3904. https://doi.org/10.3390/molecules28093904
Chicago/Turabian StyleBan, Qiuyan, Wenjing Chi, Xiaoxiao Wang, Shiqiong Wang, Dan Hai, Guangshan Zhao, Qiuyan Zhao, Daniel Granato, and Xianqing Huang. 2023. "(-)-Epigallocatechin-3-Gallate Attenuates the Adverse Reactions Triggered by Selenium Nanoparticles without Compromising Their Suppressing Effect on Peritoneal Carcinomatosis in Mice Bearing Hepatocarcinoma 22 Cells" Molecules 28, no. 9: 3904. https://doi.org/10.3390/molecules28093904
APA StyleBan, Q., Chi, W., Wang, X., Wang, S., Hai, D., Zhao, G., Zhao, Q., Granato, D., & Huang, X. (2023). (-)-Epigallocatechin-3-Gallate Attenuates the Adverse Reactions Triggered by Selenium Nanoparticles without Compromising Their Suppressing Effect on Peritoneal Carcinomatosis in Mice Bearing Hepatocarcinoma 22 Cells. Molecules, 28(9), 3904. https://doi.org/10.3390/molecules28093904





