Abstract
This work describes an ab initio principle computational examination of the optical, structural, elastic, electronic and mechanical characteristics of aluminum-based compounds AlRF3 (R = N, P) halide-perovskites. For optimization purposes, we used the Birch–Murnaghan equation of state and discovered that the compounds AlNF3 and AlPF3 are both structurally stable. The IRelast software was used to compute elastic constants (ECs) of the elastic properties. The aforementioned compounds are stable mechanically. They exhibit strong resistance to plastic strain, possess ductile nature and anisotropic behavior and are scratch-resistant. The modified Becke–Johnson (Tb-mBJ) approximation was adopted to compute various physical properties, revealing that AlNF3 and AlPF3 are both metals in nature. From the density of states, the support of various electronic states in the band structures are explained. Other various optical characteristics have been calculated from the investigations of the band gap energy of the aforementioned compounds. These compounds absorb a significant amount of energy at high levels. At low energy levels, the compound AlNF3 is transparent to incoming photons, whereas the compound AlPF3 is somewhat opaque. The examination of the visual details led us to the deduction that the compounds AlNF3 and AlPF3 may be used in making ultraviolet devices based on high frequency. This computational effort is being made for the first time in order to investigate the aforementioned properties of these chemicals, which have yet to be confirmed experimentally.
1. Introduction
As the world’s population is growing day by day, it is causing energy consumption to increase across the world. This results in a decrease in energy. For this purpose, new sources of energy, which are free from environmental problems, are necessary. Solar energy is renewable, does not cause environmental pollution, is available on a large scale and can be used as an excellent replacement for non-renewable energy sources. In recent years, numerous approaches for capturing solar energy have been developed, including solar architecture, solar heating, artificial photosynthesis, photovoltaics and photocatalytic water splitting. Among others, photovoltaics, which harness solar energy and utilize photovoltaic effects to turn sunlight into electricity, have drawn a significant amount of interest. A photovoltaic device known as a solar cell uses the sun’s energy to generate electricity. The solar chambers are typically divided into three age groups. The first age group of solar cells is wafer-based, the second compeers are based on thin film and the third group uses carbon-based assemblies. The first and second group of solar booths have been recycled for a long time, but their use has been constrained by their high cost, difficult manufacturing processes and negative environmental impact. Thus, researchers are searching for novel, inexpensive and pollution-free materials for solar cells. A variety of solar cell types, including multi-crystal silicon (mc-Si cells) and mono-crystal silicon solar chambers (c-Si cells), CIGS solar chambers, CdTe-based solar cells, quantum-dot-sensitized solar cells, organic photovoltaics and perovskite solar chambers, have all been described so far. To be exact, the cost of the materials and the efficiency of power renovation are important factors in the commercialization of solar cells. With considerable power conversion efficiency (PCE) of 25–26%, the third group of silicon-based solar cubicles had dominated up until this point. Perovskite solar chambers, a novel class of third-generation solar booths that demonstrate a PCE of 22.1%, are an alternative to silicon solar cells. Since the discovery of perovskites, they has remained a great source of interest for material scientists. Perovskites exhibit fascinating qualities, which is why they have tremendous use in nanotechnology, particularly in the field of nano-structured solar cells. A class of substances with a peculiar crystal structure, made up of cubic and diamond forms, is referred to as “perovskite”. Its superconducting, electronic and ferroelectric characteristics are very interesting and attractive, catching the attention of researchers.
Research interest in perovskite solar cells has increased dramatically since it was revealed that they are extraordinarily effective at absorbing light that may be turned into electricity. A tremendous amount of work has been conducted in this field for the creation of new perovskite compounds with outstanding characteristics. Perovskites have revealed a number of interesting features, including superconductivity, enormous magnetoresistance, spin-dependent-transport (spintronics) and catalytic abilities, explaining how the particles or combination of particles are active in the assembly. As an outcome, perovskites provide an interesting research environment for scientists. Perovskites are mostly present in nature as oxides formed with silicates (such as bridgmanite rocks). They can also be found in nature as fluoride, chloride, hydroxide, arsenide and intermetallic complexes. Although there are not many naturally occurring perovskite minerals, synthetic perovskites have an elemental makeup that covers the entire periodic table and a wide range of complex formulae, including metallic perovskites, hybrid organic–inorganic perovskites, metal-free perovskites and even noble gas-based perovskites.
Compounds with the chemical formula ABF3 have a fluoro-perovskite structure. In such halide perovskite structures, the cation A has 12 halide atoms bound to it, and the other cation, represented by B, has 6 fluorine atoms connected to it. Fluoro-perovskite materials are a distinct class of compounds which have solid crystalline characteristics and exceptional electronic properties, ranging from insulators to semiconductors. In the past few years, these compounds received significant attention due to their numerous applications in the field of radiation dosimeters, material scintillation, the semiconductor industry and as a lens material in photonic lithography [1,2,3]. The results of a large number of studies concluded that these complexes are usually stable mechanically and elastically anisotropic [4,5,6]. Uses of the ABF3 composite include high-efficiency photovoltaic and energy storage systems used in the automobile and electronics industries [7,8]. To generate stable fluoro-perovskites, F (fluorine) is normally coupled with other compounds, such as transition metals and organic and or inorganic compounds. Fluoro-perovskites with large band gaps are the best options. These components may be used to produce complicated lattice-matched compounds with large band gaps, permitting lattice-matching and group space engineering [1]. The huge energy band gap is a property shared by several substances. Because of their great potential and low absorption edges, these compounds may be employed as glass in vacuum ultraviolet and ultraviolet wavelengths [9,10,11]. Recently, some research work on such compounds has been published, which may be referred to in the references section [12,13,14]. Harmel et al. [15] utilized DFT to investigate various properties of BaCsF3 fluoro-perovskites, finding that CsBaF3 may be useful for optoelectronic devices because of its wide direct bandwidth and groups of the unreal component of the shielding properties in the ultraviolet field. Daniel et al. [16] investigated the important properties of LiBaF3 and discovered that these chemicals are more suitable for use in preparing devices for storing energy.
The new ternary family compound AlRF3 (R = N, P) has the capacity to be used as a visual material in current electronic technologies. The composites that have forbidden a region width wider than 3.10 eV show better results in the ultraviolet spectrum. Because they are electrical conductors with great transparency across a small range of energies, AlNF3 and AlPF3 compounds exhibit metallic characteristics and are ideal candidates for electrical applications. The basic goal of the current research effort is to practice the DFT and the FP-LAPW approach for investigating the fundamental characteristics, such as the electronic, elastic, optical and structural properties of AlRF3 (R = N, P) fluoro-perovskites, which may be used as core data for upcoming experimental research efforts on the materials listed above.
2. Computational Methodology
The full potential linearized augmented plane wave (FP-LAPW) technique was recycled to calculate the data for these substances using the coding computer application WIEN2K [17,18,19,20,21,22]. The electronic and other parameters, such as optical, density of state and so on, were calculated using the Tran–Blaha modified Becke–Johnson exchange potential approximation (TB-mBJ) technique. GGA approximation was used to calculate the exchange–correlation potential, but this method resulted in an incorrect band gap value and needed to be adjusted using either the TB-mBJ method or GGA plus the multi-orbital mean-field Hubbard potential (GGA + U). The optical characteristics and band gap calculated using these methods corresponded well with the experimental data [23,24]. In this research, we looked into the particular FP-LAPW technique with the smallest radii in muffin-tin spheres (RMT), which was equal to eight, and, in our case, Kmax, which was the norm of the most extreme K in the plane wave expansion. R = (N, P) elements and F had RMTs of 1.64, 1.77 and 2.06 au. The Fourier extended charge density was reduced to Gmax = 13.0 au in the muffin-tin region, while the spherical harmonics were extended to lmax = 11.0. The self-consistent field values were observed to have been satisfied when the total energy was within the range of 0.001 Ry. Birch–Murnaghan’s equation of state [25] allowed us to draw a graph of energy vs. volume, from which we extracted the structural variables. The elastic constants (ECs) of our chosen crystals were obtained using the computer program IRelast, and the compounds’ elastic properties were then calculated [26]. The dielectric permeability was also recycled to assess the visual characteristics [27,28].
3. Results and Discussion
This segment of the study includes a full technical explanation of the results obtained utilizing the Tb-mBJ potential approaches.
3.1. Structural Properties
The unit cell of AlRF3 (R = N and P) crystals with space group Pm3m (# 221) had a cubic perovskite structure with a single molecule. The F atoms appeared at (½, ½, ½); the R atoms (R = N, P) at (0, ½, ½), (½, 0, ½) and (½, ½, 0); and the Al atoms at (0, 0, 0), respectively. Figure 1 depicts the arrangement of Al-based fluoro-perovskites cubic crystals. We calculated the total energy of the cell against volume to be around V0 (the volume of the building block in stable conditions). Utilizing Birch–Murnaghan’s equation of state [25], the volume optimization technique was used to obtain structural properties. We performed analytical calculations of our acquired locations using Birch–Murnaghan’s equation of state to fit the obtained information and achieve the lowest energy state features, such as an equilibrium lattice length ao, a bulk modulus B and its pressure derivative B’. As illustrated in Figure 2, the bottommost energy unit cell could be obtained by reducing the total energy for the appropriate volume of the unit block. The lowest state energy Eo was defined as the total minimum energy against volume. The volume was referred to as Vo, which stands for the optimal least volume. The compounds constructed with the peak optimal energy were expected to be more stable. The ideal structural parameters which we calculated are included in Table 1, including ao (optimized lattice constant), Eo (optimized lowest energy state), B (bulk modulus), Vo (optimized volume) and B’ (bulk modulus pressure derivative). Since the bulk modulus dropped with an increasing lattice constant, these consequences were consistent with the general trend of this approximation, implying that the estimated conclusions are very precise and genuine.
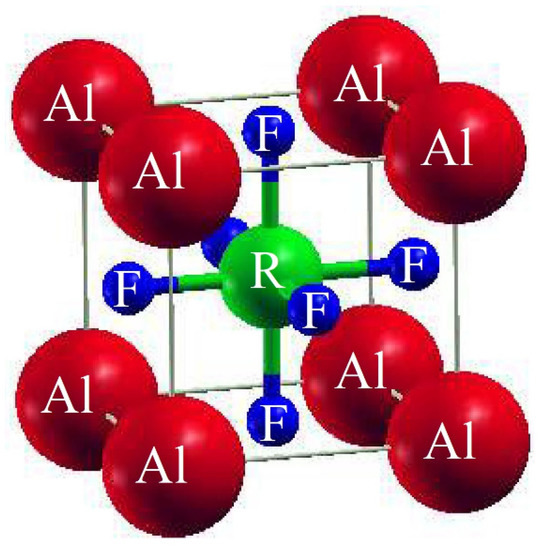
Figure 1.
Traditional crystalline structure of compounds AlRF3 (R = N, P).
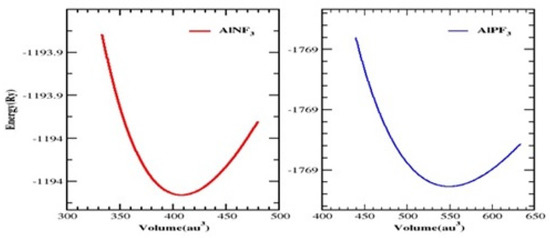
Figure 2.
Cubic perovskites AlRF3 (R = N, P) and their total energy against volume are plotted.

Table 1.
Optimized structural parameters of AlRF3 (R = N, P) obtained using Birch–Murnaghan’s energy versus volume equation.
3.2. Electronic Properties
In this section, we determined the real diagrams of energy band structures and density of states (DOS) to explore the electronic characteristics of AlRF3 (R = N, P) crystals. The basic forbidden region of semiconductors and insulating materials is widely known to be underestimated by local density approximations and GGA simulations [29,30]. The bulk of this is composite because its basic characteristics fail to produce both the exchange-correlation energy and its charge derivatives steadily. To solve band gap underestimation, the TB-mBJ was utilized, as it has been used effectively in recently published works [14,31,32]. Figure 3 illustrates the energy states obtained for AlMF3 (M = N and P). The Fermi level energy was selected as the null-energy level at the topmost of the valence band (VB). Since the VB maximum and conduction band (CB) minimum values overlap for AlNF3 and AlPF3, both are metals. Figure 4 depicts the total density of states (TDOS) and partial density of states (PDOS) for AlRF3 (R = N, P) crystals, providing a detailed understanding of the electronic structure. DOS shows how numerous electronic positions contribute to both bands. The perpendicular dashed lines at zero electron volts represent the Fermi energy level EF, whereas the DOS ranges from −12.5 to 10 eV. The conduction band is the group of states, which is on the right-side of EF line, while the valance group is to the left. The F-tot, Al-tot, F-p and Al-s states in the VB have prominent contributions.
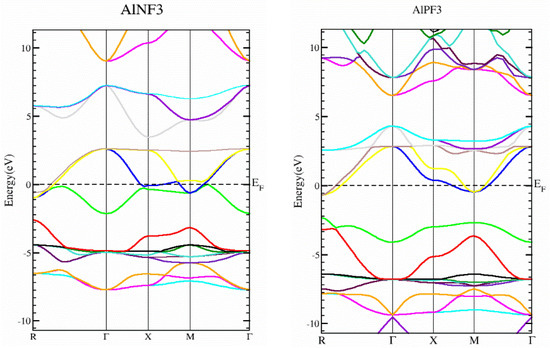
Figure 3.
The band structures of AlRF3 (R = N, P) compounds, achieved using the Tb-Mbj technique.
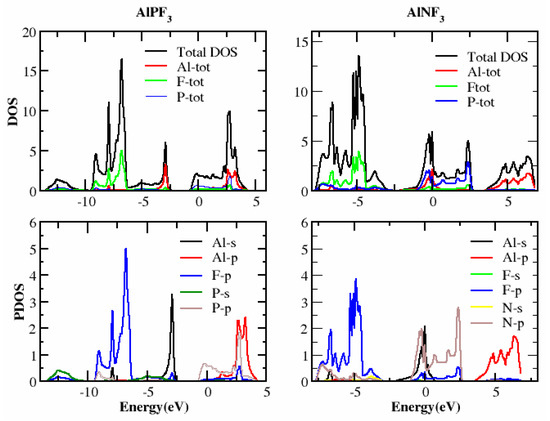
Figure 4.
TDOS and PDOS of the compound AlRF3 (R = N, P) (Tb-Mbj technique).
The energy ranged from −12.0 to −2.5 eV, −4 to −2.5 eV, −12.0 to −2.5 eV and −7.5 to −2.5 eV, for AlPF3. In the conduction band, the Al-tot, F-tot, Al-p and P-p states contributed the most in the energy ranges from 2.0 to 4 eV, −1.0 to 4.0 eV, 2.0 to 4.0 eV and −1.0 to 4.0 eV, respectively. In AlNF3, the biggest contributions were from F-tot, P-tot, F-p and N-p in the valance band from −8.0 to −2.5 eV, −8.0 to −2.5 eV, −10.0 to 0.0 eV and 10.0 to 0.0 eV, respectively. In the conduction band of the same compound, the highest contributions came from the Al-tot and Al-p states in the energy ranges of 2.5 to 10.0 eV and 2.5 to 8.0 eV, respectively, as illustrated in Figure 4.
3.3. Elastic Properties
The elastic property is the reaction to external pressure by a crystal. This property is used to calculate the crystal’s stability. The IRelast package, which was included in Wien2k and is specially built for cubic systems, was used in the calculation of ECs [33]. The three distinct ECs are C11, C12 and C44, which are recycled to achieve ductility and mechanical stability in any particular crystal. Table 2 summarizes these independent constants. For a cubic crystal construction to be mechanically stable, the following ECs criteria should be satisfied: C11 − C12 > 0, C11 > 0, C44 > 0, C11 − C44 > 0, C11 + 2C12 > 0 and B > 0 [34]. The ECs and Cij values determined here reflect the compound’s elastic stability. AlPF3 has a C11 value of 82.03 GPa, which is lower than AlNF3’s value of 117.78 GPa. As a result, AlPF3 is somewhat harder than AlNF3. The tendency of materials to create microscopic cracks is thoroughly connected to the crystal’s elastic anisotropy, represented by A, which may be used specifically in the study of engineering. The value of A was obtained for the aforementioned compounds with the help of the obtained values of C11, C12 and C44, according to Equation (1).
A = 2C44/(C11 − C12)

Table 2.
Computed values of ECs, A, G, v and B/G for the AlRF3 compounds (R = N, P).
A = 1 for an isotropic material, while anything less than 1 implies anisotropy.
Because the values of A for both the compounds were not equal to one, we were able to claim that our compounds possessed an anisotropic nature, and that the degree of anisotropy was indicated by the amount of variance. According to Table 2, this was 0.28 for AlPF3 and 0.48 for AlNF3, which means that AlNF3 is more anisotropic in nature. “Shear modulus G, Young’s modulus E and Poisson ratio v” were calculated using ECs, with the help of the following formulas [35,36,37]:
Table 2 shows the E, A, v and G values obtained from the equations above. A variety of factors can be recycled to assess the material’s ductility or brittleness. (C11 − C44) is the compound’s ductility, while Cauchy’s pressure is the difference between C11 and C44 [38]. If C11 − C44 has a positive value, the compounds will be ductile, and if it is negative, the compounds will be brittle. Both materials have a positive Cauchy’s pressure value, which is 0.49 GPa for AlPF3 and 0.45 Gpa for AlNF3, showing that they are ductile. The Pugh ratio, or B/G, is the second methodology used for determining whether it is brittle or ductile. The greater value of “B/G” from the critical value of 1.75, the greater the compound’s ductility will be and vice versa [39]. Our compounds were ductile because their values were 44.84 and 9.91 for AlPF3 and AlNF3, respectively. This means that AlNF3 is somewhat less ductile than AlPF3. In order to differentiate between the ductile and brittle nature of any compound, T. Frantsevich et al. [40,41], using v, obtained 0.26 as the critical value. Therefore, if a material’s “v” value is less than 0.26, it will be brittle, and vice versa. Table 2 shows that both ternary AlRF3 (R = N, P) compounds had a value greater than 0.26, namely, 0.49 for AlPF3 and 0.460 for AlNF3, signifying its ductile nature. Finally, we discovered that the AlRF3 (R = N, P) compounds are mechanically ductile, anisotropic, tough and fracture-resistant. Based on these results, we can definitely see the elastic properties being used in a range of current electrical devices.
4. Optical Properties
We subjected our materials to packets of light with energies ranging from 0 to 14.5 eV, and all the visual properties of our compounds were calculated using the predicted equilibrium lattice constant. The dielectric function ε(ω) was used to determine all optical characteristics.
4.1. The Refractive Index
Using ε1(ω) and ε2(ω), we determined several physical characteristics, such as the refractive index η(ω), optical conductivity σ(ω), absorption coefficient i(ω) and reflectivity r(ω) of our compounds. Figure 5 depicts the computed value of the refractive index 4.3 as 6, and the values of refractive index η(0) = 4.6 at 0 eV for the crystals AlPF3 and AlNF3, as seen in the refraction spectrum. The curves of Figure 6 clarify that the refractive indexes of the aforementioned compounds slightly deviated from each other. Figure 5 shows that AlPF3 had the highest refractive index value of 2.4 and 2.4 at photon energies of 5 and 10 eV, respectively, whereas the peak refractive index of AlNF3 was 2.1 at 6.5 eV. We determined the refraction of light from a particular composite based on its refractive index, which was extremely useful for photoelectric purposes. The refractive index was more than 1 (η(ω) > 1) because when a photon enters a compound, it feels hindrance due to the photon–electron interaction, as revealed in the same Figure. The greater the ε(ω) of a substance when it passes through, the more photons are refracted. Each process that raises the electron density of a substance also enhances its refractive index.
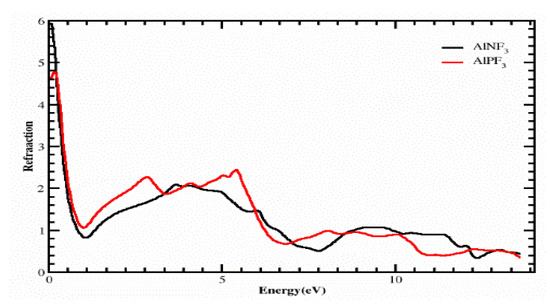
Figure 5.
Calculated refractive index of compounds AlRF3 (R = N, P).
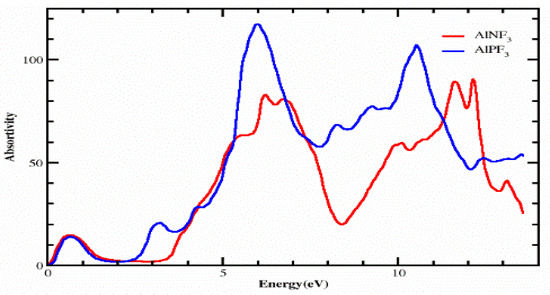
Figure 6.
Computed absorption coefficient of complexes AlRF3 (R = N, P).
4.2. The Absorption Coefficient
The absorption coefficient I(ω) was calculated using the dielectric function, as shown in Figure 6. From Figure 6, it is clear that our crystals have appreciable absorption coefficients at energies varying from 0 eV to 14 eV for AlPF3 and AlNF3. Both of these compounds have essentially identical critical values, which are 0.0 eV for AlPF3 and AlNF3, respectively. At energy levels of 0.50, 3.30, 4.30, 6.0 and 11.0 eV, AlPF3 exhibited absorption peaks of 18.0, 23.0, 30.0, 120.0 and 100.0, respectively. At 0.50, 7.20, 7.0, 10.0, 12.0 and 12.50 eV energy, AlNF3 had absorption peaks of 18.0, 85.0, 80.0, 60.0, 85.0, and 86.0, respectively.
4.3. The Reflectivity
Figure 7 explains the reflectivity R(ω), which was estimated from the dielectric permittivity exposed through the variation of energy from 0.0 eV to 14.0 eV. For AlPF3 and AlNF3, the reflectivity values at zero-frequency R(0) were 0.42 and 0.54, correspondingly. As the incident photon energy was enhanced, the reflectivity of both complexes first increased up to approximately 0.4 eV and then declined to 0.09 at 1.0 eV, and after 1.0 eV, it once again rose. Afterward, the values separated, and AlNF3 had values of 0.20, 0.35 and 0.4 at 5.2, 6.6 and 10.6 eV, respectively. Similarly, further peaks of 0.19, 0.45 and 0.4 were detected for AlPF3 at 2.2, 60 and 10.5–0 eV, respectively. In comparison to AlNF3, AlPF3 had an extremely low reflectance of 0 eV. After 1.0 eV, it stayed low for AlNF3 compared to AlPF3 at higher energies, indicating that AlNF3 was more transparent than AlPF3 in this energy range. The transparency of the material suggested that these crystals may be used to produce lenses.
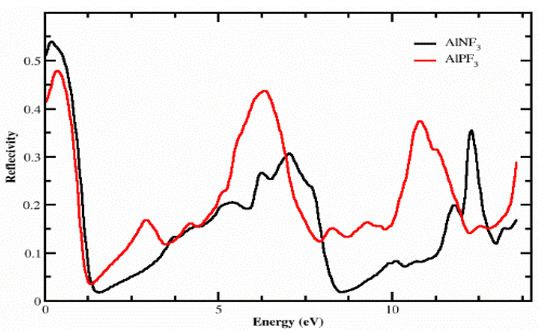
Figure 7.
The computed R(ω) of light for the compounds AlRF3 (R = N, P).
4.4. Optical Conductivity
Photon conduction is mathematically represented as σ(ω), which describes the electrons’ movement in a substance produced by the application of an electromagnetic field. We may study the conductivity σ(ω) using the dielectric function, as revealed in Figure 8.
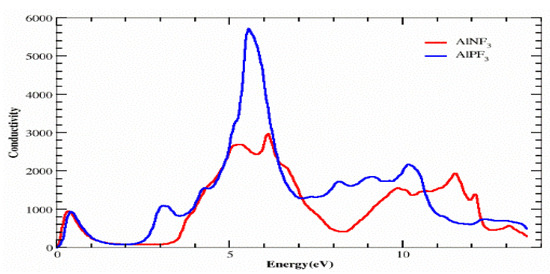
Figure 8.
Calculated optical conductivity σ(ω) of AlRF3 (R = N, P) compounds.
For both substances, photon conductivity began at 0 eV. Other peaks for AlPF3 were 1000, 0, 2200, 5800 and 2800 at 0.4, 2.0, 3.2, 5.9 and 10.3 eV, respectively. Figure 8 depicts the peaks for AlNF3 as 1000, 0, 3000, 3100, 1900 and 2000 at 0.4, 2.0, 5.0, 6.3, 9.9 and 11.5 eV, respectively. As a consequence, it was discovered that the AlPF3 crystal is optically more conductive at low energy than AlNF3.
5. Conclusions
In the presented study, we effectively studied the structural, elastic, electronic and optical characteristics of ternary fluoro-perovskite AlRF3 (R = N, P) crystals. The most accurate and recent results are as follows. Based on improved structural parameters, we determined that AlRF3 (R = N, P) is cubic and structurally stable. The IRelast program was applied to guess flexible constraints, such as fundamental ECs, “anisotropy factor, Poison ratio, ductility, Cauchy’s pressure, shear modulus, Pugh ratio and Young modulus”. Based on these basic elastic properties, it was determined that the aforementioned crystals were elastically stable, anisotropic, scratch-resistant and ductile. As a result of these discoveries, we are confident in the use of these materials in a wide range of current electrical technologies. To investigate the basic electrical properties of our crystals, the TB-mBJ approximation was adopted. The chemicals AlPF3 and AlNF3 were revealed to be conductors. F-tot, Al-tot, F-p and Al-s states in the VB contributed the most to the DOS, with energies ranging from −12.0 to −2.50 eV, −4 to −2.50 eV, −12.0 to −2.50 eV and −7.5 to −2.5 eV for AlPF3. For AlPF3, the biggest contribution in the conduction band came from the Al-tot, F-tot, Al-p and P-p states, with energies ranging from 2.0 to 4.0 eV, −1.0 to 4.0 eV, 2.0 to 4.0 eV and −1.0 to 4.0 eV, respectively. In contrast, the greatest contributions to AlNF3 were from F-tot, P-tot, F-p and N-p, from −8.0 to −2.50 eV, −8.0 to −2.50 eV, −10.0 to 0 eV and 10.0 to 0 eV, respectively. In the same compound’s conduction band, the greatest contributions came from the Al-tot and Al-p states, with energies ranging from 2.50 eV to 10.0 eV and 2.5 to 8.0 eV, respectively.
Author Contributions
A.A.P., H.K., M.S., R.K. and H.O.E.: investigation, conceptualization, writing—original draft preparation, visualization, data curation; H.K., M.S., R.K., H.O.E., A.Z.D., A.A., R.C., N.R. and A.K.: supervision, formal analysis, investigation, writing—original draft preparation; H.K., M.S., R.K., N.R., A.U., A.A.K. and A.Z.D.: visualization, data curation; H.K., M.S., R.K., A.A., R.C., N.R. and A.K.: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research, King Saud University through the Vice Deanship of Scientific Research Chairs: the Research Chair of Prince Sultan Bin Abdul Aziz International Prize for Water.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research, King Saud University for funding through the Vice Deanship of Scientific Research Chairs: the Research Chair of Prince Sultan Bin Abdul Aziz International Prize for Water.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Nishimatsu, T.; Terakubo, N.; Mizuseki, H.; Kawazoe, Y.; Pawlak, D.A.; Shimamura, K.; Fukuda, T. Band structures of perovskite-like fluorides for vacuum-ultraviolet-transparent lens materials. Jpn. J. Appl. Phys. 2002, 41, 365. [Google Scholar] [CrossRef]
- Husain, M.; Rahman, N.; Reshak, A.H.; Zulfiqar; Habib, A.; Ali, S.; Laref, A.; Al Bakri, A.M.M.; Bila, J. Insight into the physical properties of the inter-metallic titanium-based binary compounds. Eur. Phys. J. Plus 2021, 136, 624. [Google Scholar] [CrossRef]
- Rahman, N.; Husain, M.; Yang, J.; Sajjad, M.; Murtaza, G.; Ul Haq, M.; Habib, A.; Rauf, A.; Karim, A.; Nisar, M.; et al. First principle study of structural, electronic, optical and mechanical properties of cubic fluoro-perovskites: (CdXF3, X = Y, Bi). Eur. Phys. J. Plus 2021, 136, 347. [Google Scholar] [CrossRef]
- Rahman, N.; Yang, J.; Sohail, M.; Khan, R.; Iqbal, A.; Maouche, C.; Khan, A.A.; Husain, M.; Khattak, S.A.; Khan, S.N.; et al. Insight into metallic oxide semiconductor (SnO2, ZnO, CuO, α-Fe2O3, WO3)-carbon nitride (g-C3N4) heterojunction for gas sensing application. Sens. Actuators A Phys. 2021, 332, 113–128. [Google Scholar] [CrossRef]
- Murtaza, G.; Sadique, G.; Aliabad, H.R.; Khalid, M.; Naeem, S.; Afaq, A.; Amin, B.; Ahmad, I. First principle study of cubic perovskites: AgTF3 (T = Mg, Zn). Phys. B Condens. Matter 2011, 406, 4584–4589. [Google Scholar] [CrossRef]
- Körbel, S.; Marques, M.A.L.; Botti, S. Stability and electronic properties of new inorganic perovskites from high-throughput ab initio calculations. J. Mater. Chem. C 2016, 4, 3157–3167. [Google Scholar] [CrossRef]
- Dubrovin, R.M.; Alyabyeva, L.N.; Siverin, N.V.; Gorshunov, B.P.; Novikova, N.N.; Boldyrev, K.N.; Pisarev, R.V. Incipient multiferroicity in P n m a fluoroperovskite NaMnF3. Phys. Rev. B 2020, 101, 180–403. [Google Scholar] [CrossRef]
- Kumar, Y.A.; Kumara, K.D.; Kim, H.-J. A novel electrode for supercapacitors: Efficient PVP-assisted synthesis of Ni3S2 nanostructures grown on Ni foam for energy storage. Dalton Trans. 2020, 49, 4050–4059. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; Kumar, Y.A.; Alshgari, R.A.; Joo, S.W. Facile fabrication of novel heterostructured tin disulfide (SnS2)/tin sulfide (SnS)/N-CNO composite with improved energy storage capacity for high-performance supercapacitors. J. Electroanal. Chem. 2021, 899, 115695. [Google Scholar] [CrossRef]
- Pallavolu, M.R.; AnilKumar, Y.; Mani, G.; Nallapureddy, R.R.; Parvathala, A.; Albaqami, M.D.; Karami, A.M.; Joo, S.W. A novel hybridized needle-like Co3O4/N-CNO composite for superior energy storage asymmetric supercapacitors. J. Alloys Compd. 2022, 56, 164–447. [Google Scholar] [CrossRef]
- Mubarak, A.A. Ab initio Study of Ag-Based Fluoroperovskite AgMF3 (M = Co and Ni) Compounds. J. Electron. Mater. 2018, 47, 887898. [Google Scholar] [CrossRef]
- Bouich, A.; Marí-Guaita, J.; Sahraoui, B.; Palacios, P.; Marí, B. Tetrabutylammonium (TBA)-Doped Methylammonium Lead Iodide: High Quality and Stable Perovskite Thin Films. Front. Energy Res. 2022, 10, 840817. [Google Scholar] [CrossRef]
- Arar, R.; Ouahrani, T.; Varshney, D.; Khenata, R.; Murtaza, G.; Rached, D.; Bouhemadou, A.; Al-Douri, Y.; Bin Omran, S.; Reshak, A. Structural, mechanical and electronic properties of sodium based fluoroperovskites NaXF3 (X = Mg, Zn) from first-principle calculations. Mater. Sci. Semicon. Proc. 2015, 33, 127–135. [Google Scholar] [CrossRef]
- Abdullah, A.; Husain, M.; Rahman, N.; Khan, R.; Iqbal, Z.; Zulfiqar, S.; Sohail, M.; Umer, M.; Murtaza, G.; Khan, S.N.; et al. Computational investigation of structural, magnetic, elastic, and electronic properties of Half-Heusler ScVX (X = Si, Ge, Sn, and Pb) compounds. Eur. Phys. J. Plus 2021, 136, 1176. [Google Scholar] [CrossRef]
- Chouit, N.; Korba, S.A.; Slimani, M.; Meradji, H.; Ghemid, S.; Khenata, R. First-principles study of the structural, electronic and thermal properties of CaLiF3. Phys. Scr. 2013, 88, 35702. [Google Scholar] [CrossRef]
- Seddik, T.; Khenata, R.; Merabiha, O.; Bouhemadou, A.; Bin-Omran, S.; Rached, D. Elastic, electronic and thermodynamic properties of fluoro-perovskite KZnF3 via first-principles calculations. Appl. Phys. A 2012, 106, 645–653. [Google Scholar] [CrossRef]
- Harmel, M.; Khachai, H.; Haddou, A.; Khenata, R.; Murtaza, G.; Abbar, B.; Bin Omran, S.; Khalfa, M. Ab initio study of the mechanical, thermal and optoelectronic properties of the cubic CsBaF3. Acta Phys. Pol. 2015, 128, 34–42. [Google Scholar] [CrossRef]
- Koteras, K.; Gawraczyński, J.; Derzsi, M.; Mazej, Z.; Grochala, W. Lattice Dynamics of KAgF3 Perovskite, Unique 1D Antiferromagnet. Chemistry 2021, 3, 94–103. [Google Scholar] [CrossRef]
- Dubrovin, R.M.; Garcia-Castro, A.C.; Siverin, N.V.; Novikova, N.N.; Boldyrev, K.N.; Romero, A.H.; Pisarev, R.V. Incipient geometric lattice instability of cubic fluoroperovskites. Phys. Rev. B 2021, 104, 144304. [Google Scholar] [CrossRef]
- Dubrovin, R.M.; Siverin, N.; Syrnikov, P.P.; Novikova, N.N.; Boldyrev, K.N.; Pisarev, R. Lattice dynamics and microscopic mechanisms of the spontaneous magnetodielectric effect in the antiferromagnetic fluoroperovskites KCoF3 and RbCoF3. Phys. Rev. B 2019, 100, 24429. [Google Scholar] [CrossRef]
- Vaitheeswaran, G.; Kanchana, V.; Zhang, X.; Ma, Y.; Svane, A.; Christensen, N.E. Calculated high-pressure structural properties, lattice dynamics and quasi particle band structures of perovskite fluorides KZnF3, CsCaF3 and BaLiF3. J. Phys. Condens. Matter 2016, 28, 315403. [Google Scholar] [CrossRef]
- Vaitheeswaran, G.; Kanchana, V.; Kumar, R.S.; Cornelius, A.L.; Nicol, M.F.; Svane, A.; Christensen, N.E.; Eriksson, O. High-pressure structural study of fluoro-perovskite CsCdF 3 up to 60 GPa: A combined experimental and theoretical study. Phys. Rev. B 2010, 81, 75105. [Google Scholar] [CrossRef]
- Vaitheeswaran, G.; Kanchana, V.; Kumar, R.S.; Cornelius, A.L.; Nicol, M.F.; Svane, A.; Delin, A.; Johansson, B. High-pressure structural, elastic, and electronic properties of the scintillator host material KMgF3. Phys. Rev. B 2007, 76, 14107. [Google Scholar] [CrossRef]
- Korba, S.A.; Meradji, H.; Ghemid, S.; Bouhafs, B. First principles calculations of structural, electronic and optical properties of BaLiF3. Comput. Mater. Sci. 2009, 44, 1265–1271. [Google Scholar] [CrossRef]
- Furetta, C.; Santopietro, F.; Sanipoli, C.; Kitis, G. Thermoluminescent (TL) properties of the perovskite KMgF3 activated by Ce and Er impurities. Appl. Radiat. Isot. 2001, 55, 533–542. [Google Scholar] [CrossRef]
- Hamioud, F.; AlGhamdi, G.S.; Al-Omari, S.; Mubarak, A.A. Ab initio investigation of the structural, electronic, magnetic and optical properties of the perovskite TlMnX3 (X = F, Cl) compounds. Int. J. Mod. Phys. B 2016, 30, 1650031. [Google Scholar] [CrossRef]
- Cheriet, A.; Lagoun, B.; Halit, M.; Zaabat, M.; Abdelhakim, C.; Hamza, L. First-principles study of structural, electronic, optical and elastic properties of cadmium based Fluoro-Perovskite MCdF3 (M = Rb, Tl). Solid State Phenom. 2019, 297, 173–186. [Google Scholar] [CrossRef]
- Khan, H.; Sohail, M.; Rahman, N.; Khan, R.; Hussain, M.; Khan, A.U.A.; Abed, A.; Dewidar, A.Z.; Elansary, H.O.; Yessoufou, K. Theoretical Investigations into the Different Properties of Al-Based Fluoroperovskite AlMF3 (M = Cr, B) Compounds by the TB-MBJ Potential Method. Materials 2022, 15, 5942. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Althubeiti, K.; Algethami, M.; Rahman, N.; Sohail, M.; Mao, Q.; Zaman, Q.; Ilyash, A.U.N.; Mohammad, A.A.; Mianaki, A.K.; et al. Observation of Quantum Criticality in Antiferromagnetic based (Ce1−xYx)2Ir3Ge5 Kondo-Lattice System. J. Magn. Magn. Mater. 2022, 556, 169361P. [Google Scholar] [CrossRef]
- Saddique, J.; Husain, M.; Rahman, N.; Khan, R.; Zulfiqar; Iqbal, A.; Sohail, M.; Khattak, S.A.; Khan, S.N.; Khan, A.A.; et al. Modeling structural, elastic, electronic and optical properties of ternary cubic barium based fluoroperovskites MBaF3 (M = Ga and In) compounds based on DFT. Mater. Sci. Semicond. Process. 2022, 139, 106345. [Google Scholar] [CrossRef]
- Roy, A.; Mukherjee, S.; Sarkar, S.; Auluck, S.; Prasad, R.; Gupta, R.; Garg, A. Effects of site disorder, off-stoichiometry and epitaxial strain on the optical properties of magnetoelectric gallium ferrite. J. Phys. Condens. Matter 2012, 24, 435501. [Google Scholar] [CrossRef]
- Han, M.; Ozaki, T.; Yu, J. Rapid Communication Magnetic ordering and exchange interactions in multiferroic GaFeO3. Phys. Rev. B 2007, 75, 060404(R). [Google Scholar] [CrossRef]
- Khan, H.; Sohail, M.; Rahman, N.; Khan, R.; Ullah, A.; Hussain, M.; Khan, A.; Hegazy, H. Theoretical study of different aspects of Al-based Fluoroperovskite AlMF3 (M = Cu, Mn) compounds using TB-MBJ potential approximation method for generation of energy. Results Phys. 2022, 42, 105982. [Google Scholar] [CrossRef]
- Rahman, N.; Husain, M.; Khan, R.; Sohail, M.; Zaman, T.; Khan, A.A.; Murtaza, G.; Neffati, R.; Khan, A. First-principles calculations to investigate structural, elastic, optical, and thermoelectric properties of narrow band gap semiconducting cubic ternary fluoroperovskites barium based BaMF3 (M = Ag and Cu) compounds. J. Mater. Res. Technol. 2022, 21, 2168–2177. [Google Scholar] [CrossRef]
- Jamal, M.; Bilal, M.; Ahmad, I.; Jalali-Asadabadi, S. IRelast package. J. Alloys Compd. 2018, 735, 569–579. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The compressibility of media under extreme pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244. [Google Scholar] [CrossRef]
- Berger, J.; Hauret, G.; Rousseau, M. Brillouin scattering investigation of the structural phase transition of TlCdF3 and RbCaF3. Solid State Commun. 1978, 25, 569–571. [Google Scholar] [CrossRef]
- Azam, S.; Khan, S.A. A first principles study of electronic and optical properties of the polar quaternary chalcogenides β-A2Hg3Ge2S8 (A = K and Rb). Mater. Sci. Semicond. Process 2015, 34, 250–259. [Google Scholar] [CrossRef]
- Sohail, M.; Husain, M.; Rahman, N.; Althubeiti, K.; Algethami, M.; Khan, A.A.; Iqbal, A.; Ullah, A.; Khanf, A.; Khan, R. First-Principal Investigations of Electronic, Structural, Elastic, and Optical Properties of the Fluoroperovskite TlLF3 (L = Ca, Cd) Compounds using TB-mBJ Potential method. RSC Adv. 2022, 12, 7002–7008. [Google Scholar] [CrossRef]
- Shah, S.A.; Husain, M.; Rahman, N.; Sohail, M.; Khan, R.; Khan, A.A.; Ullah, A.; Abdelmohsen, S.A.M.; Khan, A.M.M.A. Insight into the exemplary structural, Elastic, Electronic and Optical natural of GaBeCl3 and LnBeCl3: A DFT study. RSC Adv. 2022, 12, 8172–8177. [Google Scholar] [CrossRef]
- Sulaman, M.; Song, Y.; Yang, S.; Saleem, M.I.; Li, M.; Veeramalai, C.P.; Zhi, R.; Jiang, Y.; Cui, Y.; Hao, Q.; et al. Interlayer of PMMA Doped with Au Nanoparticles for High-Performance Tandem Photodetectors: A Solution to Suppress Dark Current and Maintain High Photocurrent. ACS Appl. Mater. Interfaces 2020, 12, 23. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).