A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps cicadae and Its Immunomodulatory Activity In Vitro and In Vivo
Abstract
1. Introduction
2. Results and Discussion
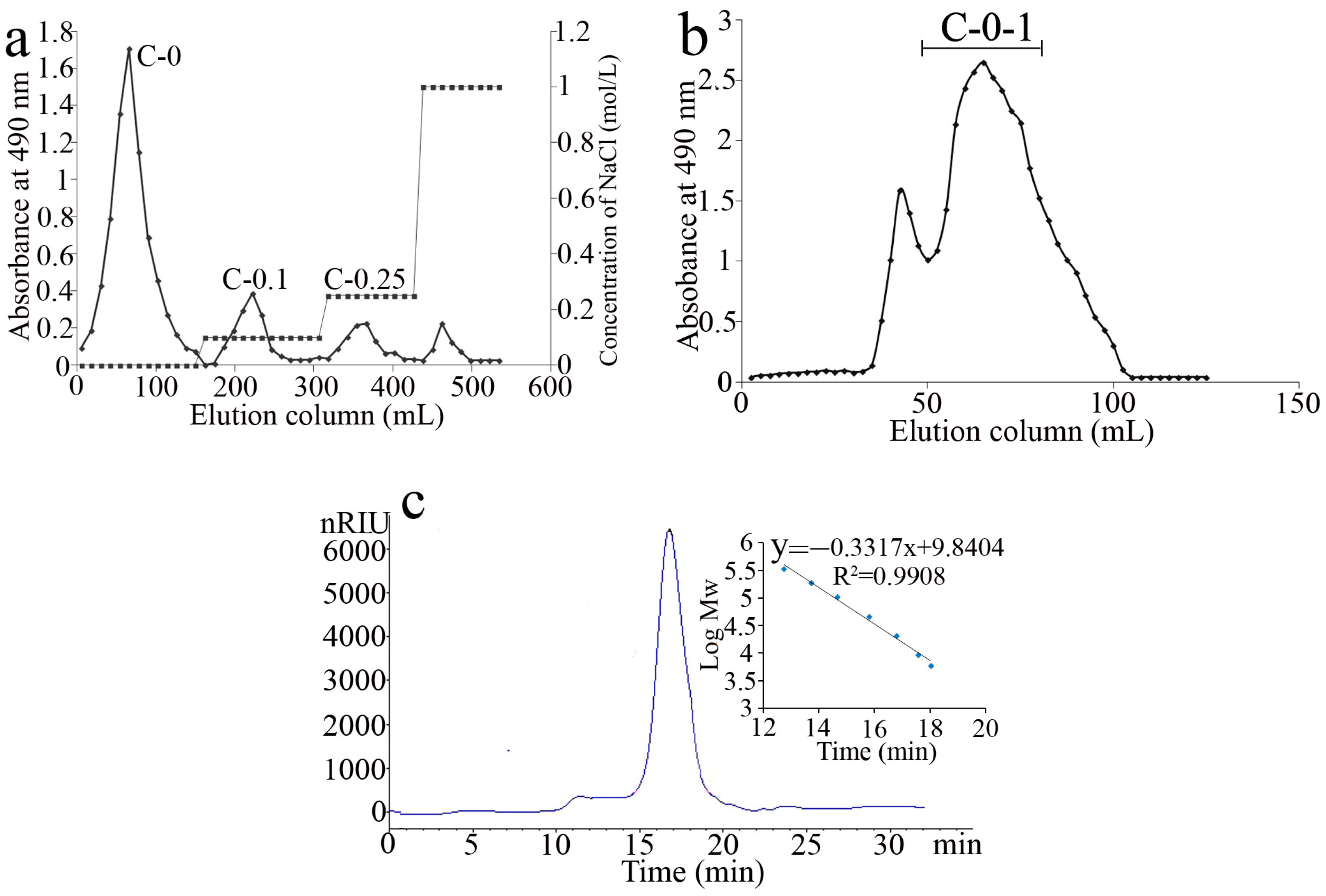
2.1. Extraction and Purification of Polysaccharides
2.2. Purity and Chemical Composition Analysis of C-0-1
2.3. Controlled Acid Hydrolysis and Methylation Analysis
2.4. NMR Analysis
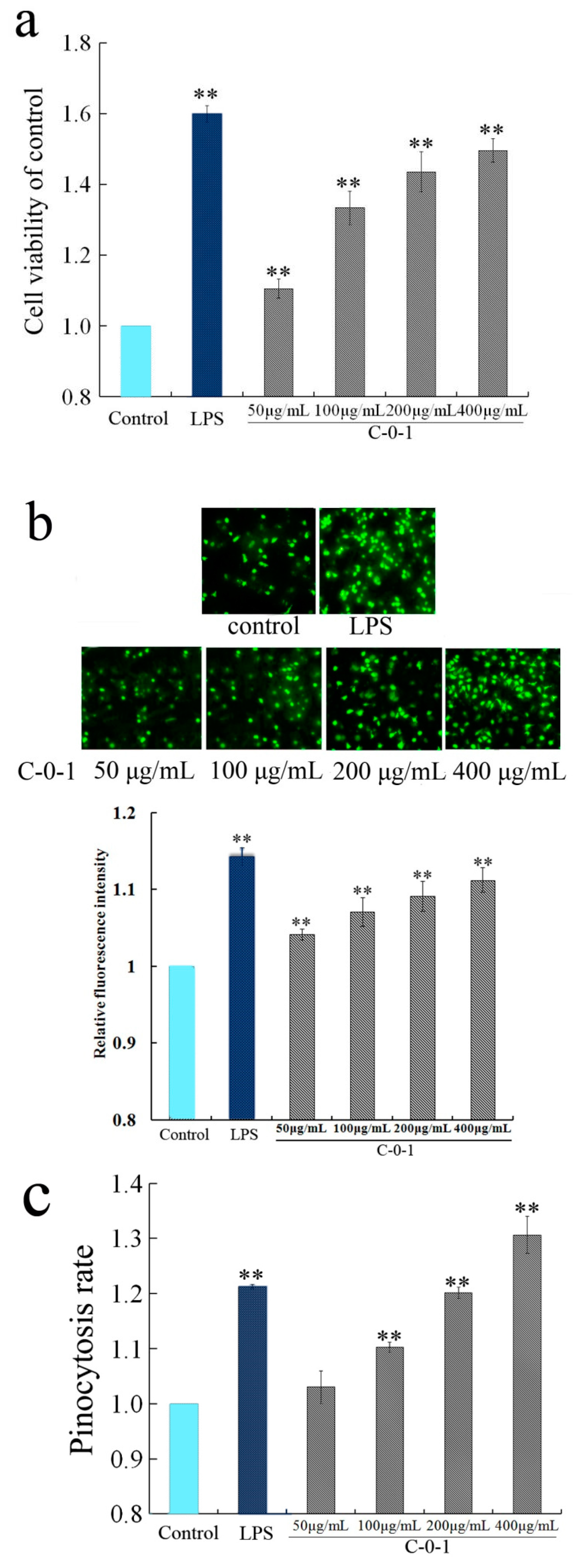
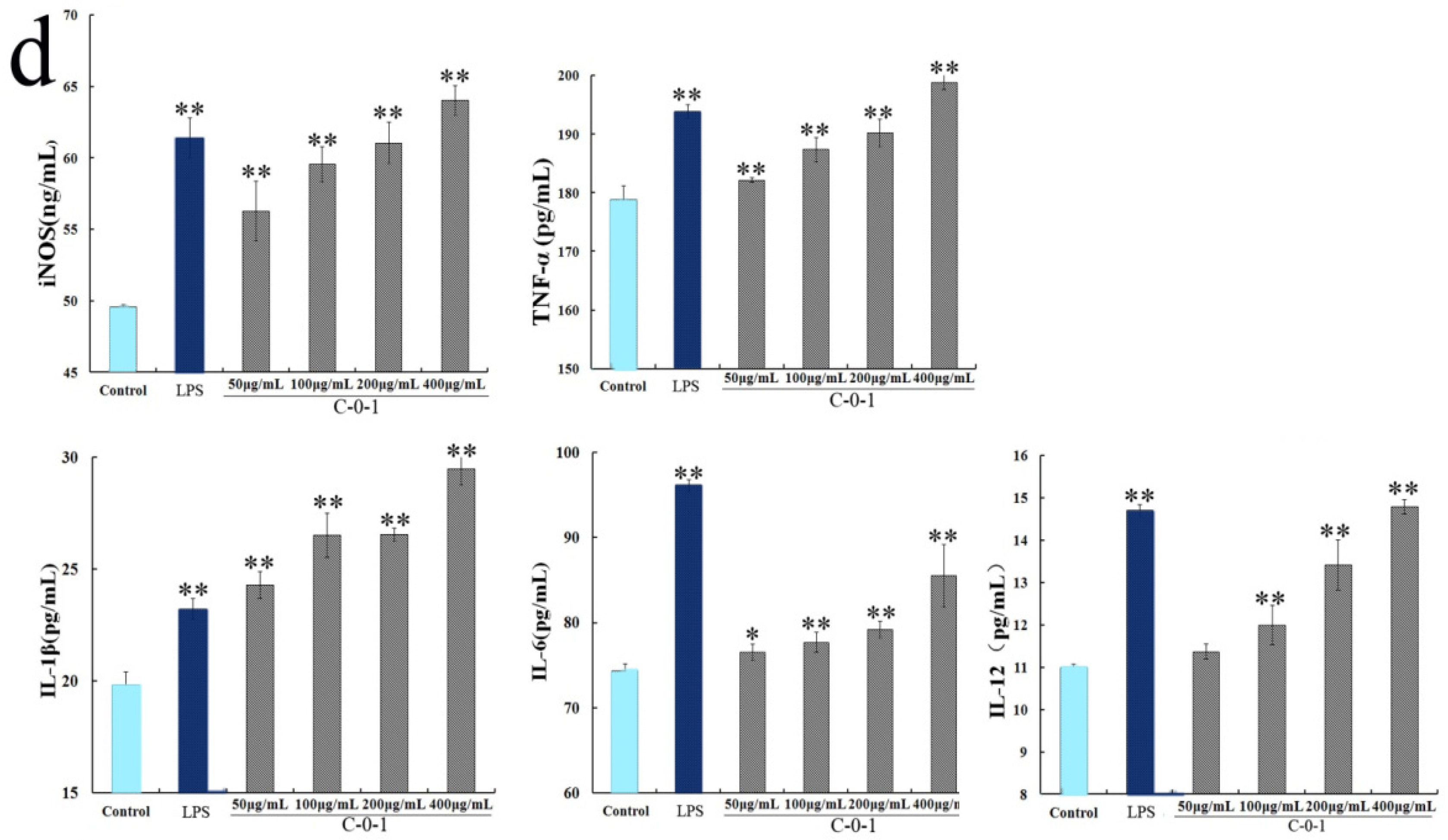
2.5. Determination of Macrophage Activation Activity
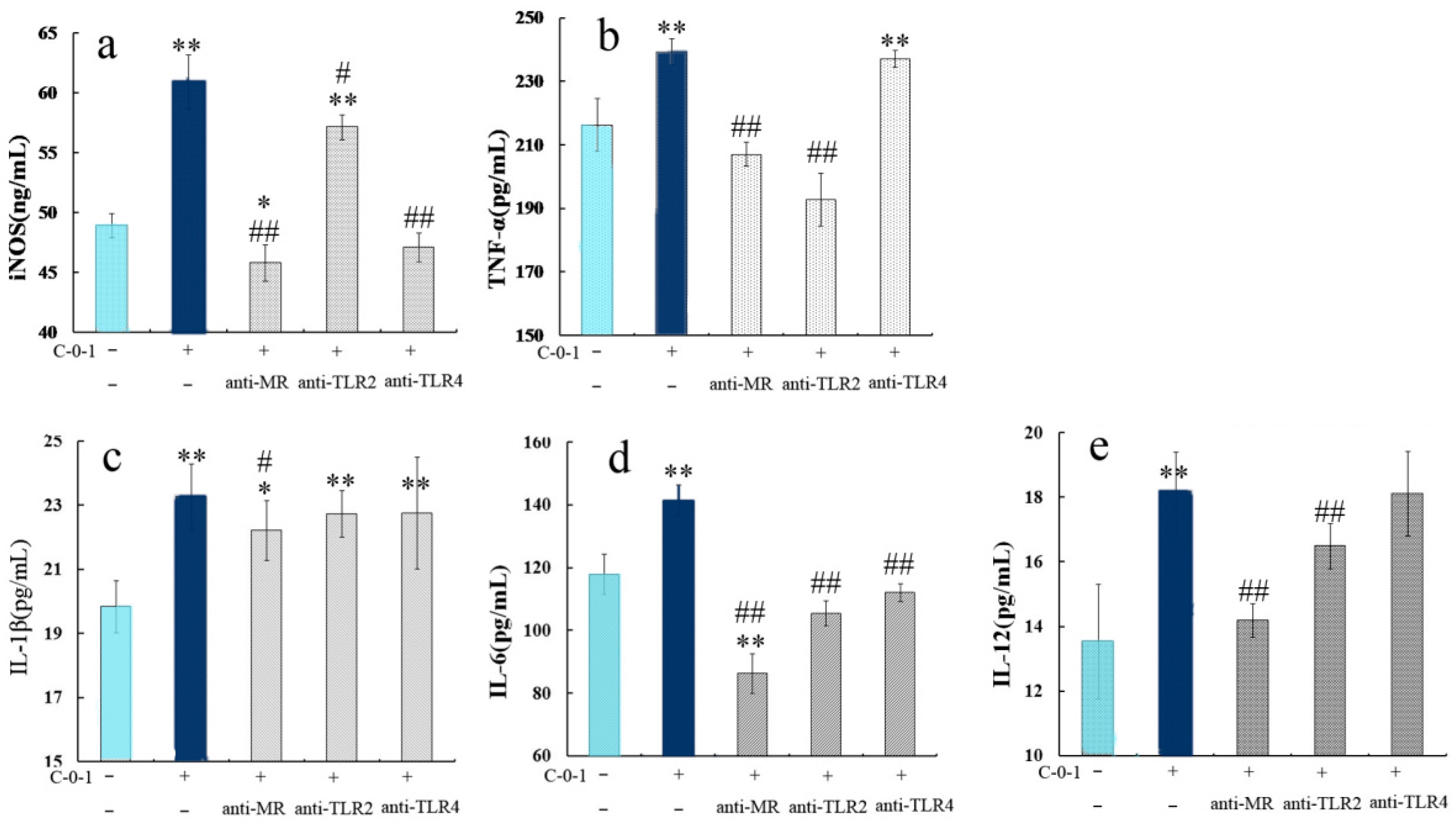
2.6. Inhibition of Cytokine Production Using Anti-PRR Antibodies
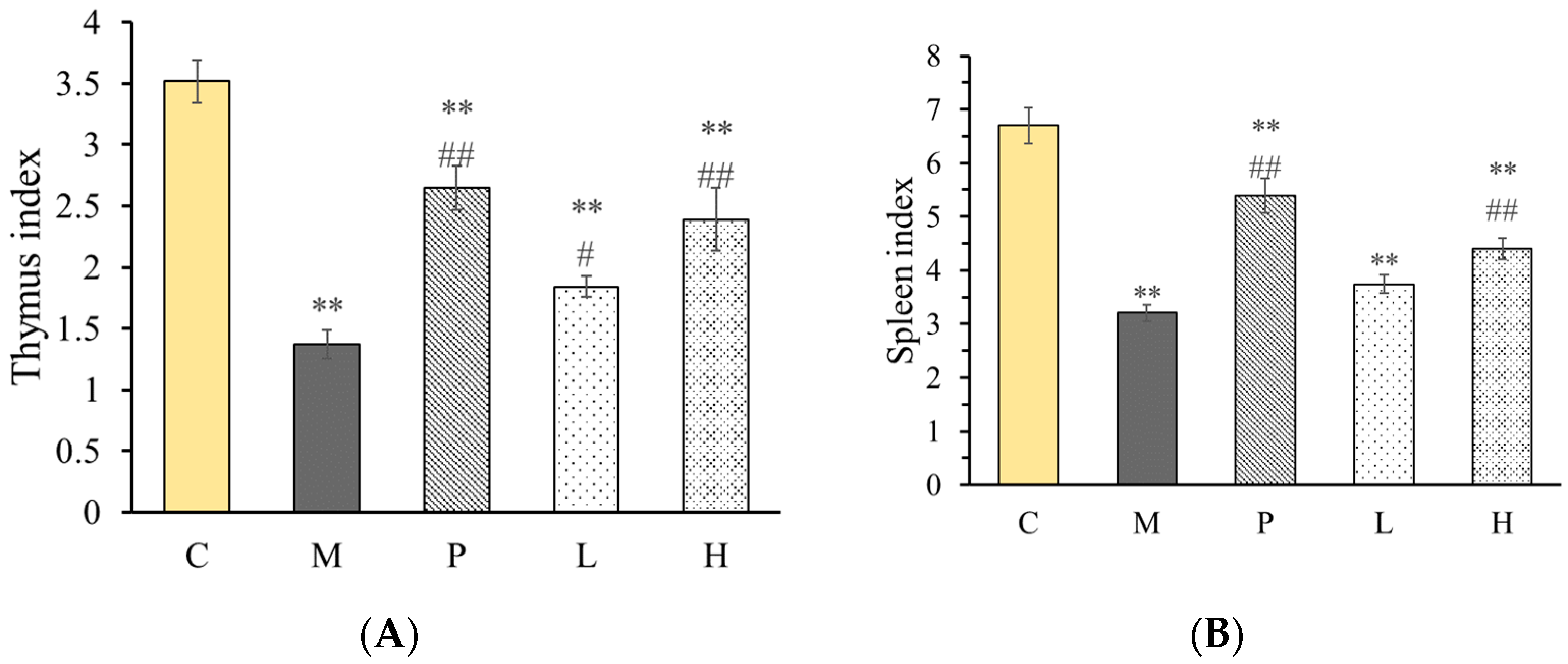
2.7. Effect of Polysaccharide on Immunomodulatory Activity in Mice
3. Materials and Methods
3.1. Materials
3.2. Extraction and Purification of Polysaccharides
3.3. Purity, and Chemical Composition Analysis
3.4. Controlled Acid Hydrolysis and Methylation Analysis
3.5. NMR Spectroscopy
3.6. Determination of Macrophage Activation
3.6.1. Effects of C-0-1 on RAW 264.7 Cells Viability
3.6.2. Determination of ROS Levels in RAW 264.7 Cells
3.6.3. Determination of Phagocytic Uptake
3.6.4. Measurement of Inducible Nitric Oxide Synthase (iNOS) Production
3.6.5. Cytokine Assays
3.6.6. Cytokine Production Inhibition Using Anti-PRR Antibodies
3.6.7. Statistical Analysis
3.7. Immunomodulatory Activity In Vivo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Olatunji, O.J.; Tang, J.; Tola, A.; Auberon, F.; Oluwaniyi, O.; Ouyang, Z. The genus Cordyceps: An extensive review of its traditional uses, phytochemistry and pharmacology. Fitoterapia 2018, 129, 293–316. [Google Scholar] [CrossRef]
- Pal, M.; Misra, K. Cordyceps sp.: The Precious Mushroom for High-Altitude Maladies. In Management of High Altitude Pathophysiology; Academic Press: Cambridge, MA, USA, 2018; pp. 93–114. [Google Scholar]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps sinensis: A review. J. Funct. Foods. 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Zhang, H.W.; Lin, Z.X.; Tung, Y.S.; Kwan, T.H.; Mok, C.K.; Leung, C.; Chan, L.S. Cordyceps sinensis (a traditional Chinese medicine) for treating chronic kidney disease. Cochrane Database Syst. Rev. 2014, 12, Cd008353. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.H.; Wang, W.; Zhang, H.Y.; Zhang, X.L.; Han, C.C. The Chemical Constituents and Pharmacological Actions of Cordyceps sinensis. Evid. Based Complement. Alternat. Med. 2015, 2015, 575063. [Google Scholar]
- Xiao, Y.; Huang, Q.; Zheng, Z.; Ma, H. Selenium release kinetics and mechanism from Cordyceps sinensis exopolysaccharide-selenium composite nanoparticles in simulated gastrointestinal conditions. Food Chem. 2021, 350, 129223. [Google Scholar] [PubMed]
- Yuan, Q.; Xie, F.; Tan, J.; Yuan, Y.; Mei, H.; Zheng, Y.; Sheng, R. Extraction, structure and pharmacological effects of the polysaccharides from Cordyceps sinensis: A review. J. Funct. Foods 2022, 89, 104909. [Google Scholar] [CrossRef]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Foods 2014, 6, 33–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Q.; Zhou, W.; Li, P.; Alolga, R.N.; Qi, L.-W.; Yin, X. A comparative proteomic characterization and nutritional assessment of naturally- and artificially-cultivated Cordyceps sinensis. J. Proteom. 2018, 181, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.F.; Kmonickova, E.; Han, R.L.; Zhou, W.; Yang, K.B.; Lu, H.F.; Wang, Z.Q.; Zhao, H.; Wang, H. Comprehensive evaluation of wild Cordyceps cicadae from different geographical origins by TOPSIS method based on the macroscopic infrared spectroscopy (IR) fingerprint. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wink, M.; Wang, P.; Lu, H.; Zhao, H.; Liu, H.; Wang, S.; Sun, Y.; Liang, Z. Biological characteristics, bioactive components and antineoplastic properties of sporoderm-broken spores from wild Cordyceps cicadae. Phytomedicine 2017, 36, 217–228. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhang, Z.L.; Wang, Y.Q.; Yang, M.; Wang, C.H.; Li, X.W.; Guo, Y.W. Chemical Constituents from Mycelia and Spores of Fungus Cordyceps cicadae. Chin. Herb. Med. 2017, 9, 188–192. [Google Scholar] [CrossRef]
- Yang, N.N.; Jiang, N.; Ma, Q.Y.; Kong, F.D.; Xie, Q.Y.; Zhou, L.M.; Yu, Z.F.; Zhao, Y.X. Chemical study of the strain Cordyceps spp. from cell fusion between Cordyceps militaris and Cordyceps cicadae. J. Asian Nat. Prod. Res. 2019, 21, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?–A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, C.; Wang, X.; Rui, X.; Zhang, Q.; Chen, X.; Dong, M.; Li, W. Structural characterization and immunomodulatory activity of intracellular polysaccharide from the mycelium of Paecilomyces cicadae TJJ1213. Food Res. Int. 2021, 147, 110515. [Google Scholar] [CrossRef]
- Xu, J.; Tan, Z.C.; Shen, Z.Y.; Shen, X.J.; Tang, S.M. Cordyceps cicadae polysaccharides inhibit human cervical cancer hela cells proliferation via apoptosis and cell cycle arrest. Food Chem. Toxicol. 2021, 148, 111971. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, T.; Zhang, X.; Zhang, F.; Linhardt, R.J. Structural and immunological studies on the polysaccharide from spores of a medicinal entomogenous fungus Paecilomyces cicadae. Carbohydr. Polym. 2021, 254, 117462. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Chen, S.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M.; Cui, S.W. Structural characterization of an α-1, 6-linked galactomannan from natural Cordyceps sinensis. Food Hydrocoll. 2018, 78, 77–91. [Google Scholar] [CrossRef]
- Cordeiro, L.M.; Beilke, F.; Bettim, F.L.; Reinhardt Vde, F.; Rattmann, Y.D.; Iacomini, M. (1→2) and (1→6)-linked β-D-galactofuranan of microalga Myrmecia biatorellae, symbiotic partner of Lobaria linita. Carbohydr. Polym. 2012, 90, 1779–1785. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.J.; Yan, M.X.; Liu, X.; Wang, S.Y.; Xia, Z.; Xiao, B.; Cao, S.J.; Yang, B.Q.; Li, J. Purification, Chemical Characterization, and Bioactivity of an Extracellular Polysaccharide Produced by the Marine Sponge Endogenous Fungus Alternaria sp. SP-32. Mar. Biotechnol. 2016, 18, 301–313. [Google Scholar] [CrossRef]
- Kiho, T.; Tabata, H.; Ukai, S.; Hara, C. A minor, protein-containing galactomannan from a sodium carbonate extract of Cordyceps sinensis. Carbohydr. Res. 1986, 156, 189–197. [Google Scholar] [CrossRef]
- Nie, S.; Cui, S.W.; Xie, M.; Phillips, A.O.; Phillips, G.O. Bioactive polysaccharides from Cordyceps sinensis: Isolation, structure features and bioactivities. Bioact. Carbohydr. Diet. Fibre 2013, 1, 38–52. [Google Scholar] [CrossRef]
- Miyazaki, T.; Oikawa, N.; Yamada, H. Studies on Fungal Polysaccharides. XX. Galactomannan of Cordyceps sinensis. Chem. Pharm. Bull. 1977, 25, 3324–3328. [Google Scholar] [CrossRef]
- Kiho, T.; Ookubo, K.; Usui, S.; Ukai, S.; Hirano, K. Structural features and hypoglycemic activity of a polysaccharide (CS-F10) from the cultured mycelium of Cordyceps sinensis. Biol. Pharm. Bull. 1999, 22, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Yin, P.F.; Huang, D.F.; Li, W.J.; Xie, M.Y. Toll-like receptor 4-mediated ROS signaling pathway involved in Ganoderma atrum polysaccharide-induced tumor necrosis factor-alpha secretion during macrophage activation. Food Chem. Toxicol. 2014, 66, 14–22. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Grabowska, A.; Chain, B.M. Nitric oxide up regulates the release of inflammatory mediators by mouse macrophages. Eur. J. Immunol. 1995, 25, 947–951. [Google Scholar] [CrossRef]
- Lee, J.S.; Kwon, D.S.; Lee, K.R.; Park, J.M.; Ha, S.-J.; Hong, E.K. Mechanism of macrophage activation induced by polysaccharide from Cordyceps militaris culture broth. Carbohydr. Polym. 2015, 120, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wen, J.; Huang, X.; Nie, Q.; Wu, X.; Ma, W.; Nie, S.; Xie, M. Interaction between polysaccharides and toll-like receptor 4: Primary structural role, immune balance perspective, and 3D interaction model hypothesis. Food Chem. 2022, 374, 131586. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, Y.J.; Lee, H.K.; Ryu, H.S.; Kim, J.S.; Yoon, M.J.; Kang, J.S.; Hong, J.T.; Kim, Y.; Han, S.B. Activation of macrophages by polysaccharide isolated from Paecilomyces cicadae through toll-like receptor 4. Food Chem. Toxicol. 2012, 50, 3190–3197. [Google Scholar] [CrossRef] [PubMed]
- Hagert, C.; Sareila, O.; Kelkka, T.; Jalkanen, S.; Holmdahl, R. The Macrophage Mannose Receptor Regulate Mannan-Induced Psoriasis, Psoriatic Arthritis, and Rheumatoid Arthritis-Like Disease Models. Front. Immunol. 2018, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Peng, X.; Lee, K.L.D.; Tang, J.C.O.; Cheung, P.C.K.; Wu, J.Y. Structural characterisation and immunomodulatory property of an acidic polysaccharide from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2011, 125, 637–643. [Google Scholar] [CrossRef]
- Liu, J.Y.; Feng, C.P.; Li, X.; Chang, M.C.; Meng, J.L.; Xu, L.J. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int. J. Biol. Macromol. 2016, 86, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 Cell’s Function in Immune System. In T Helper Cell Differentiation and Their Function; Sun, B., Ed.; Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2014; Volume 841, pp. 45–65. [Google Scholar]
- Lin, Y.; Pi, J.; Jin, P.; Liu, Y.; Mai, X.; Li, P.; Fan, H. Enzyme and microwave co-assisted extraction, structural characterization and antioxidant activity of polysaccharides from Purple-heart Radish. Food Chem. 2022, 372, 131274. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Dong, Z.; Wang, T.; Sun, K.; Zhao, Y.; Wang, B.; Chen, Y. Structure and immunoregulatory activity of β-d-galactofuranose-containing polysaccharides from the medicinal fungus Shiraia bambusicola. Int. J. Biol. Macromol. 2019, 129, 530–537. [Google Scholar] [CrossRef]
- Ishurd, O.; Kennedy, J. The anti-cancer activity of polysaccharide prepared from Libyan dates (Phoenix dactylifera L.). Carbohydr. Polym. 2005, 59, 531–535. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, J.; Li, D.; Song, S.; Song, L.; Fu, Y.; Zhang, L. Structural investigation of a uronic acid-containing polysaccharide from abalone by graded acid hydrolysis followed by PMP-HPLC–MSn and NMR analysis. Carbohydr. Res. 2015, 402, 95–101. [Google Scholar] [CrossRef]
- Ballance, S.; Børsheim, K.Y.; Inngjerdingen, K.; Paulsen, B.S.; Christensen, B.E. A re-examination and partial characterisation of polysaccharides released by mild acid hydrolysis from the chlorite-treated leaves of Sphagnum papillosum. Carbohydr. Polym. 2007, 67, 104–115. [Google Scholar] [CrossRef]
- Geng, L.H.; Hu, W.C.; Liu, Y.J.; Wang, J.; Zhang, Q.B. A heteropolysaccharide from Saccharina japonica with immunomodulatory effect on RAW 264.7 cells. Carbohydr. Polym. 2018, 201, 557–565. [Google Scholar] [CrossRef]
- Jeong, J.W.; Hwang, S.J.; Han, M.H. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Zhang, X. Structure and Immuno-Stimulating Activities of a New Heteropolysaccharide from Lentinula edodes. J. Agric. Food Chem. 2012, 60, 11560–11566. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, C.; Zhou, D.; Ou, S.; Liu, Z.; Huang, H. Immune-enhancing activities of chondroitin sulfate in murine macrophage RAW 264.7 cells. Carbohydr. Polym. 2018, 198, 611–619. [Google Scholar] [CrossRef]
- Wang, J.; Nie, S.; Cui, S.W.; Wang, Z.; Phillips, A.O.; Phillips, G.O.; Li, Y.; Xie, M. Structural characterization and immunostimulatory activity of a glucan from natural Cordyceps sinensis. Food Hydrocoll. 2017, 67, 139–147. [Google Scholar] [CrossRef]
- Dong, S.; Liang, H.; Wang, Y.; Wang, J.; Guan, X.; Yao, Y.; Xu, Z. Immunological Enhancement of Crude Polysaccharides from Plantago asiatica L. on Immunosuppressed Mice Induced by Cyclophosphamide. Sci. Technol. Food Ind. 2018, 39, 289–293. [Google Scholar]

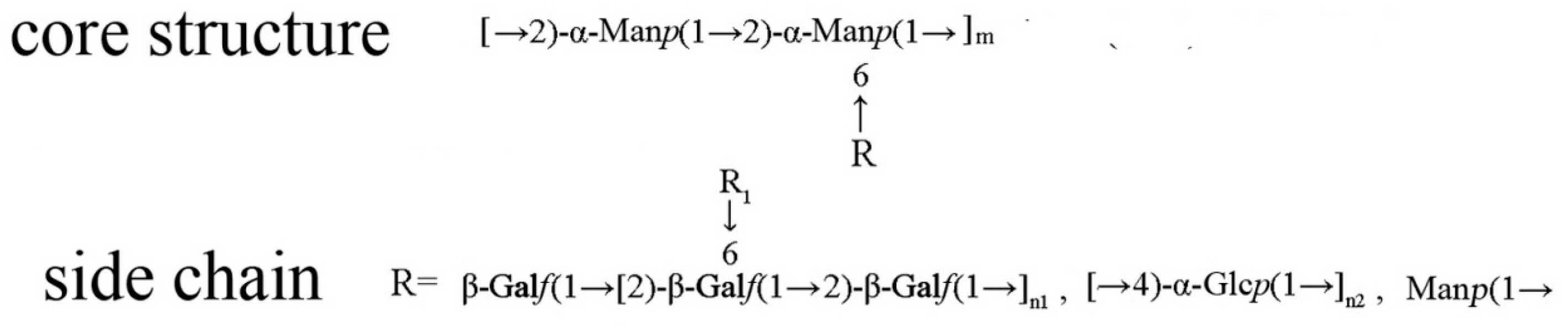

| Methylation Product | Linkage Type | Main MS (m/z) | Molar Ratio (%) | |
|---|---|---|---|---|
| C-0-1 | C-0-1P | |||
| 1,4-Ac2-2,3,5,6-Me4-D-Gal | Galf(1→ | 117,161,205,277 | 13.4 | — |
| 1,5-Ac2-2,3,4,6-Me4-D-Man | Manp(1→ | 117,129,145,161,205 | 5.2 | — |
| 1,2,5-Ac3-3,4,6-Me3-D-Man | →2)Manp(1→ | 129,161,189 | 14.8 | 74.5 |
| 1,4,5-Ac3-2,3,6-Me3-D-Glc | →4)Glcp-(1→ | 113,117,131,161,173,233 | 11.2 | 8.9 |
| 1,2,4-Ac3-3,5,6-Me3-D-Gal | →2)Galf(1→ | 117,129,143,161 | 8.8 | 3.9 |
| 1,5,6-Ac3-2,3,4-Me3-D-Man | →6)Manp(1→ | 117,129,161,189 | 4.6 | — |
| 1,2,5,6-Ac4-3,4-Me2-D-Man | →2,6)Manp(1→ | 129,189 | 18.9 | 12.7 |
| 1,2,4,6-Ac4-3,5-Me3-D-Gal | →2,6)Galf(1→ | 117,129,189 | 23.2 | — |
| Residue | H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 |
|---|---|---|---|---|---|---|
| A (1→4)-α-D-Glcp | 5.25 | 3.53 | 3.7 | 3.58 | — | 3.78, 3.63 |
| 99.0 | 71.7 | 74.0 | 77.5 | — | 60.6 | |
| B (1→2)-β-D-Galf | 5.1 | 4.04 | 4.1 | 3.89 | 3.75 | 3.55, 3.76 |
| 106.7 | 86 | 75.1 | 82.4 | 70 | 62.5 | |
| C (1→2,6)-β-D-Galf | 5.03 | 4.01 | 4.1 | 3.89 | 3.6 | 3.56, 3.8 |
| 106.9 | 86 | 75.1 | 82.4 | 70.8 | 69 | |
| D (1→2)-α-D-Manp | 5.01 | 3.88 | 3.76 | 3.35 | 3.6 | 3.65, 3.75 |
| 101.7 | 80 | 70.6 | 69 | 74 | 61 | |
| E (1→2,6)-α-D-Manp | 4.92 | 3.88 | 3.76 | 3.35 | 3.6 | 3.9, 3.6 |
| 98.0 | 80 | 70.6 | 69 | 74 | 67 | |
| F β-D-Galf(1→ | 4.9 | 4.01 | 4.07 | 3.94 | 3.75 | 3.63, — |
| 107.4 | 82 | 78.5 | 82.7 | 70.9 | 62.3 |
| Fungus | Polysaccharide Resource | Component | Molecular Weight | Linkages | Reference |
|---|---|---|---|---|---|
| Cordyceps sinensis | Ascocarps | Man:Gal = 3:5 | 23 kDa | Backbone: (1→2) and (1→6) -α-D-Manp. Side chain: non-reducing terminal β-Galf, (1→5)-β-Galf, and non-reducing terminal α-D-Manp. Branch point: O-6/O-4. | [21] |
| Cordyceps sinensis | Nature Ascocarps | Man:Glc:Gal = 24:7:69 | 7.2 kDa | Backbone: (1→6)-α-D-Manp. Side chain: non-reducing terminal, (1→5), (1→6)-β-D-Galf, (1→2)-α-D-Manp, and non-reducing terminal α-D-Manp. Branch point: O-2/O-4. | [18] |
| Cordyceps sinensis | Ascocarps | Man:Gal = 1:1 | - | Backbone: (1→2)-α-D-Manp. Side chain: non-reducing terminal, (1→3), (1→5), (1→6)-β-Galf, and non-reducing terminal α-D-Manp. Branch point: O-6/O-4. | [23] |
| Cordyceps sinensis | Cultured mycelium | Man:Glc:Gal = 24:33:43 | 15 kDa | Backbone: (1→2)-α-D-Manp. Side chain: terminal, (1→5), (1→6)-β-Galf, and non-reducing terminal α-D-Glcp. | [24] |
| NC | MC | PC | C-L | C-H | |
|---|---|---|---|---|---|
| Th1/Th2 | 3.13 ± 0.11 | 3.42 ± 0.01 ** | 3.18 ± 0.14 ## | 3.36 ± 0.01 ** | 3.17 ± 0.10 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, F.; Luo, L.; Zhang, L. A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps cicadae and Its Immunomodulatory Activity In Vitro and In Vivo. Molecules 2023, 28, 3867. https://doi.org/10.3390/molecules28093867
Gao F, Luo L, Zhang L. A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps cicadae and Its Immunomodulatory Activity In Vitro and In Vivo. Molecules. 2023; 28(9):3867. https://doi.org/10.3390/molecules28093867
Chicago/Turabian StyleGao, Fei, Lingling Luo, and Leifang Zhang. 2023. "A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps cicadae and Its Immunomodulatory Activity In Vitro and In Vivo" Molecules 28, no. 9: 3867. https://doi.org/10.3390/molecules28093867
APA StyleGao, F., Luo, L., & Zhang, L. (2023). A New Galactoglucomannan from the Mycelium of the Medicinal Parasitic Fungus Cordyceps cicadae and Its Immunomodulatory Activity In Vitro and In Vivo. Molecules, 28(9), 3867. https://doi.org/10.3390/molecules28093867





