Novel Indoline Spiropyrans Based on Human Hormones β-Estradiol and Estrone: Synthesis, Structure, Chromogenic and Cytotoxic Properties
Abstract
1. Introduction
2. Results and Discussion
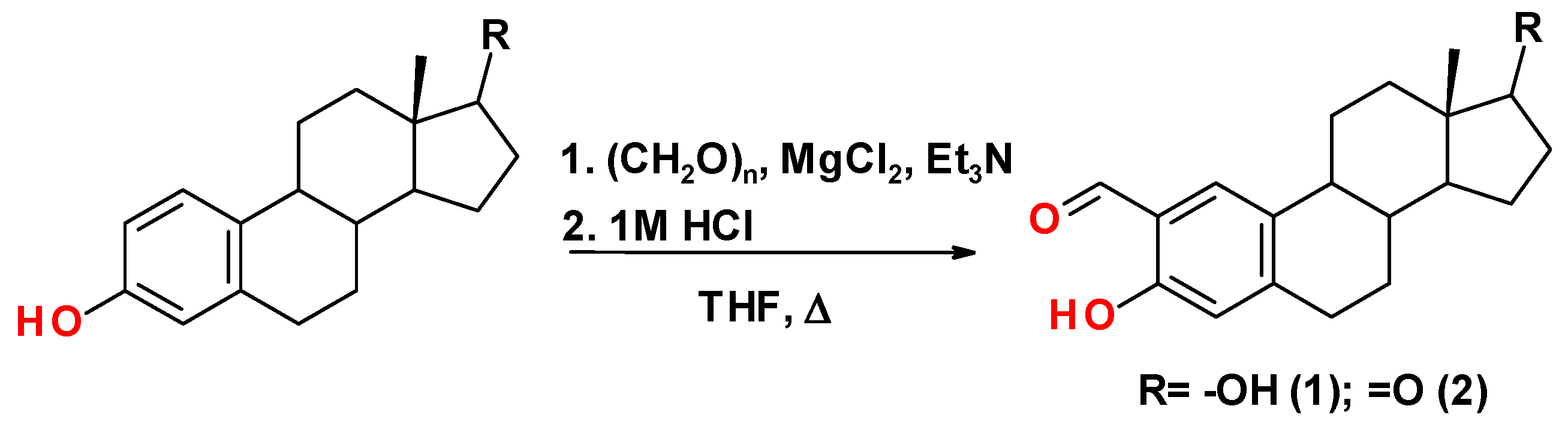
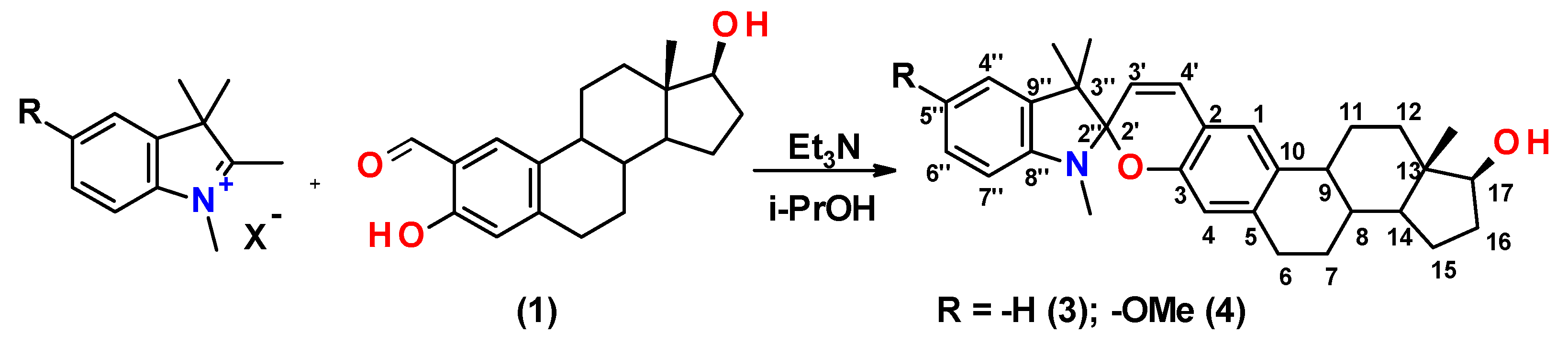
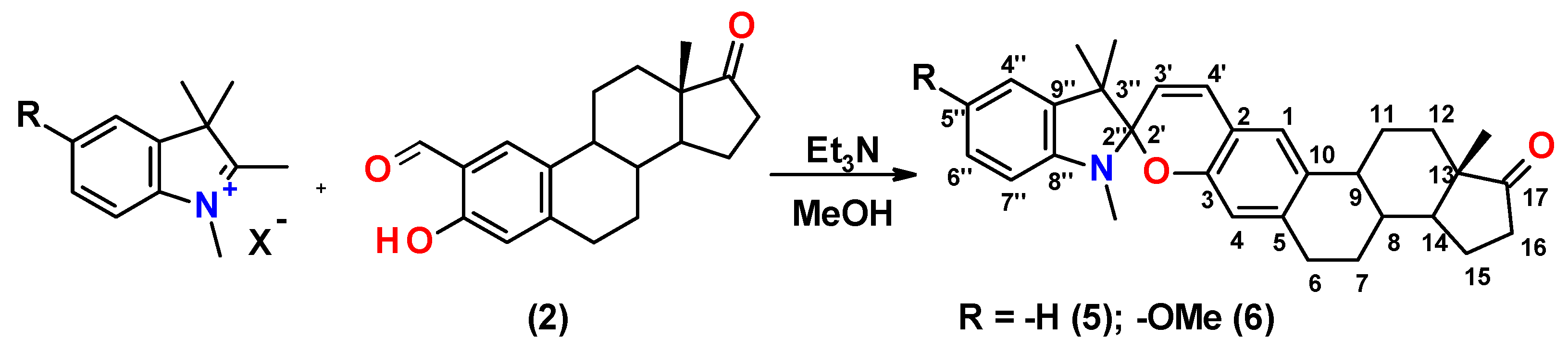
2.1. Synthesis
2.2. Structure Determination of Estrogen Derivatives
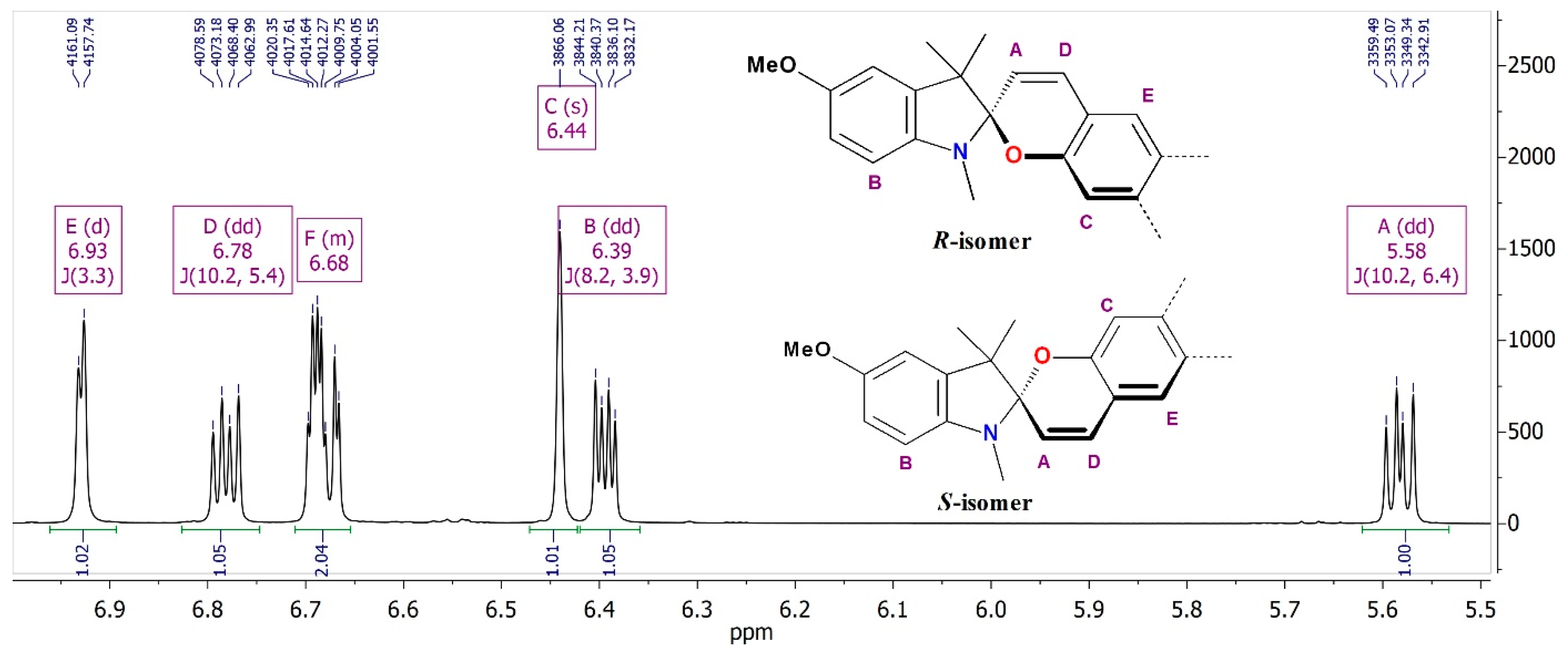
2.2.1. IR and NMR Spectroscopy
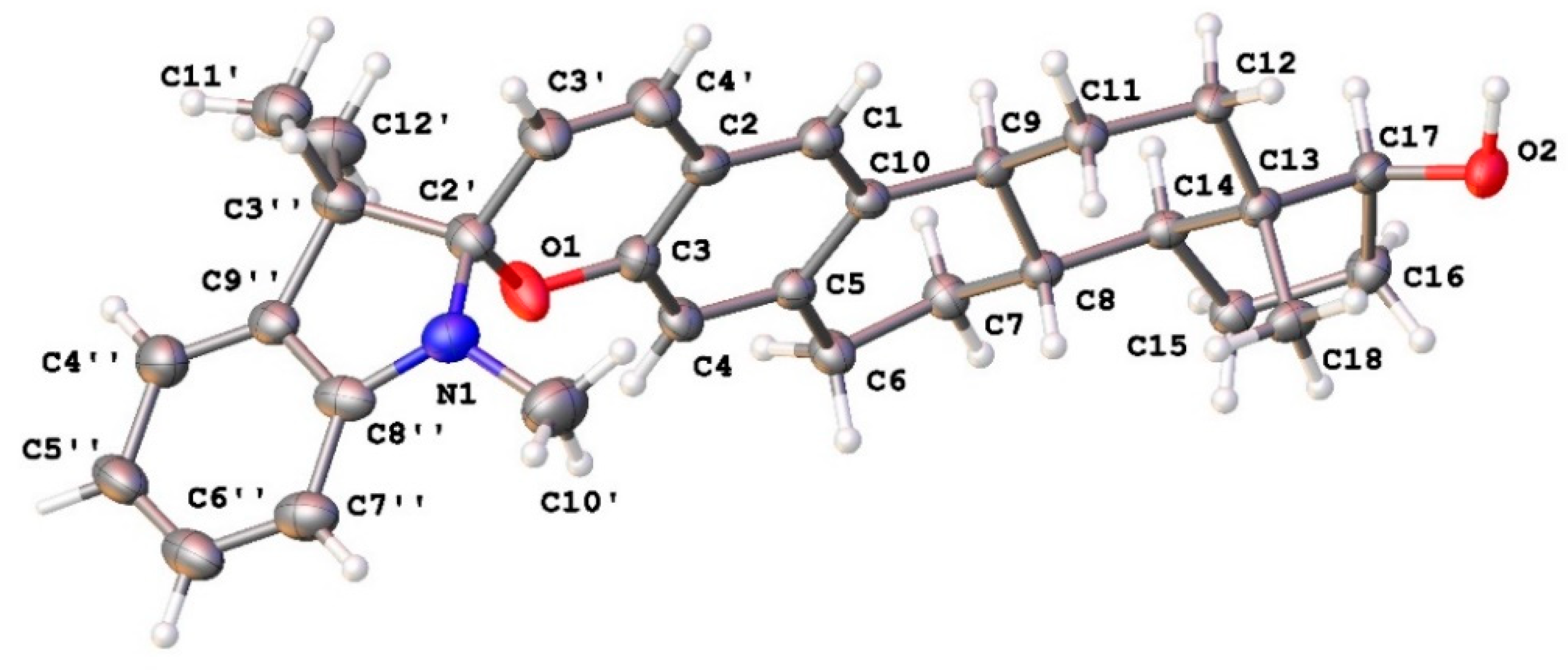
2.2.2. Single-Crystal X-ray Analysis
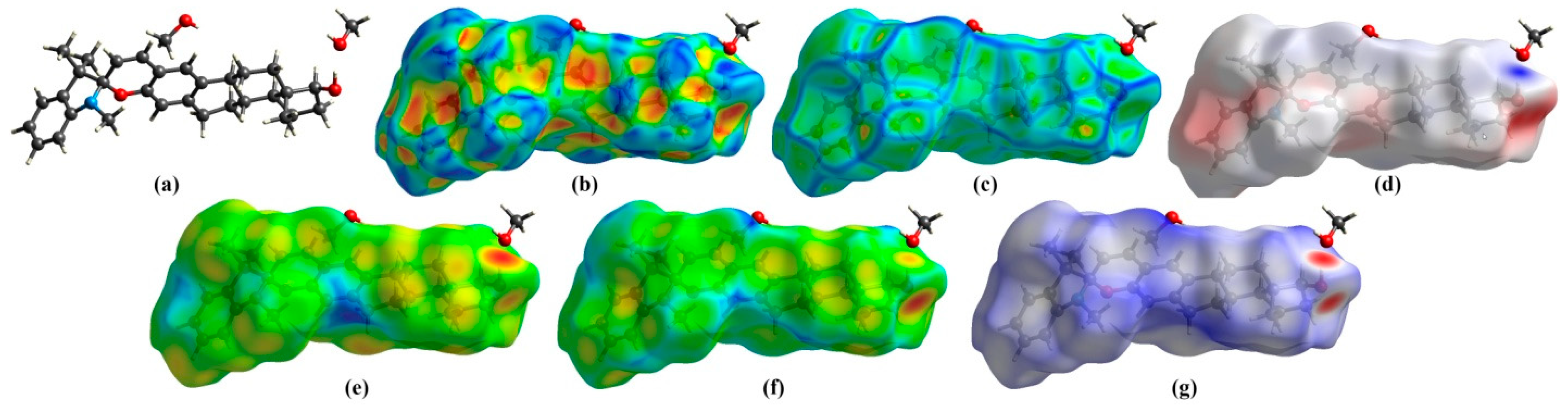
2.2.3. Hirshfeld Surface and 2D Fingerprint Plot Analysis
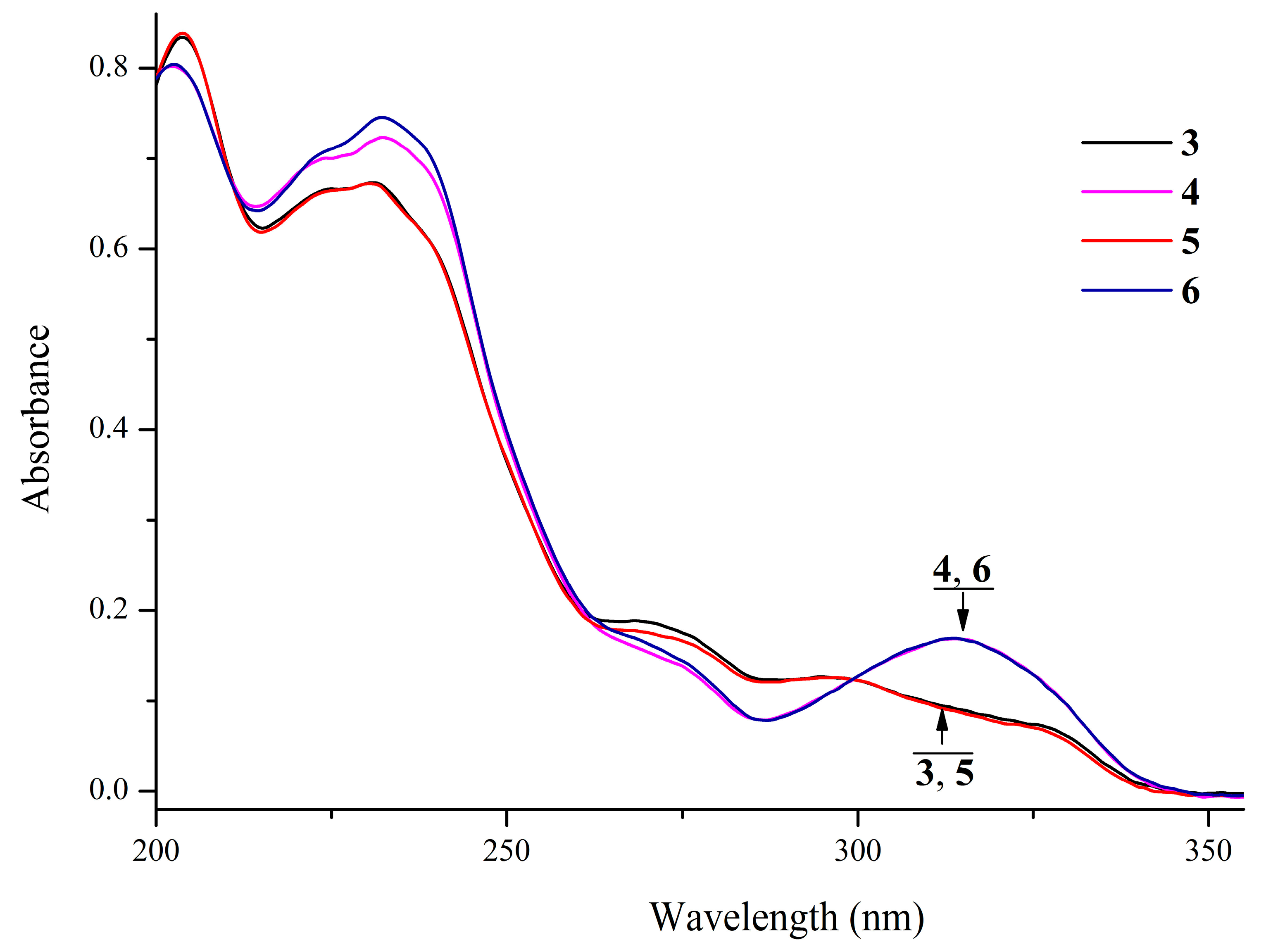
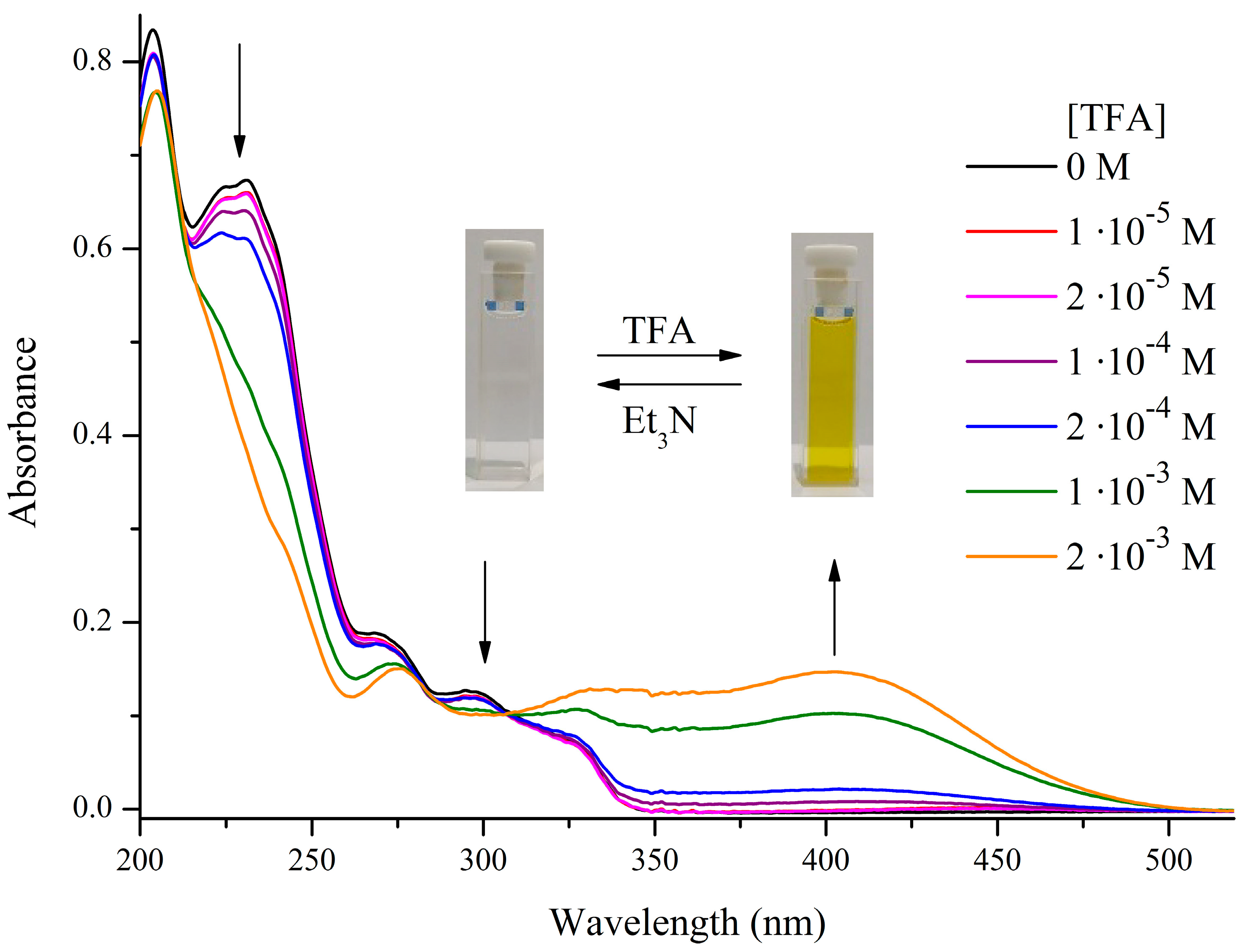
2.3. UV-Vis Spectral Studies
2.4. Quantum Chemical Investigations
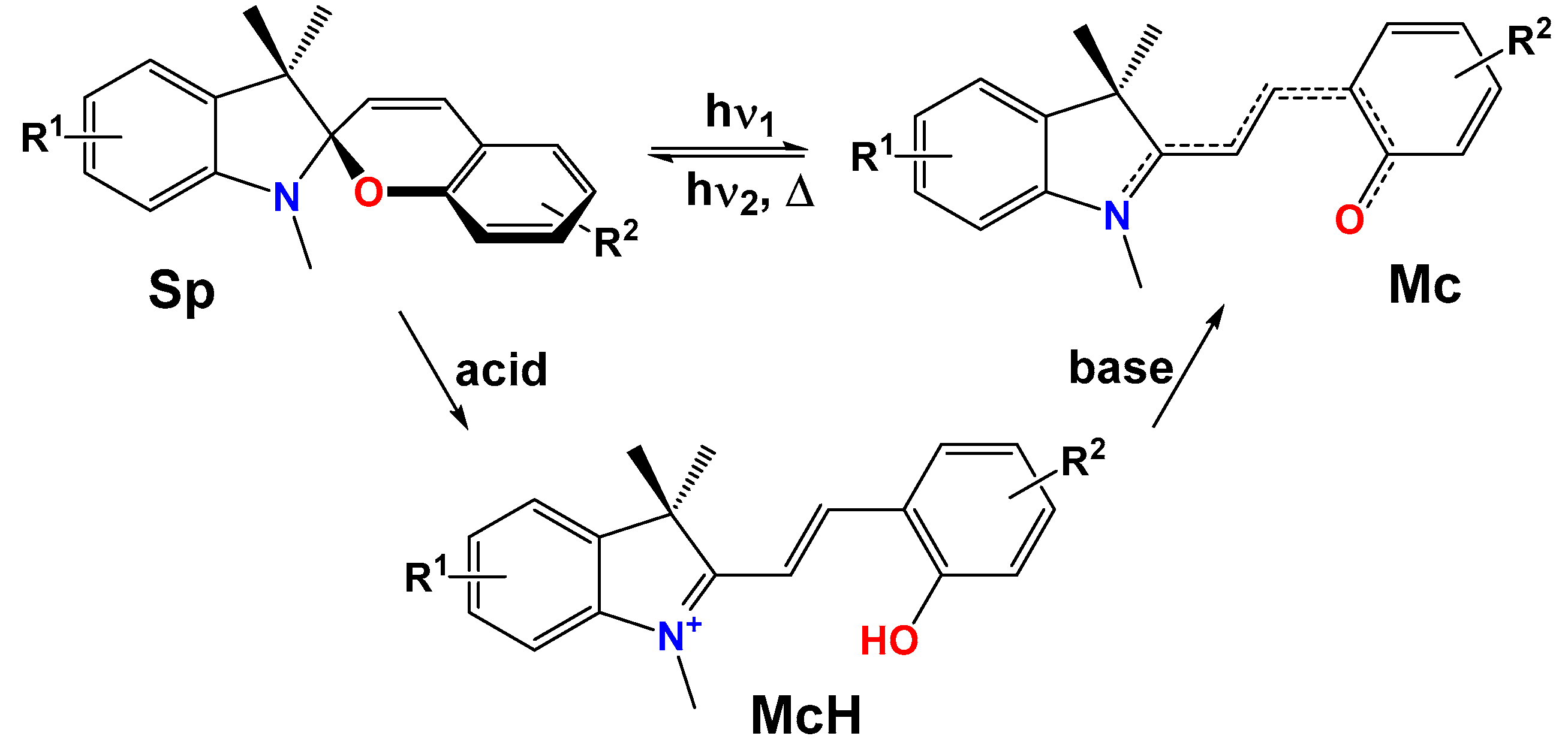
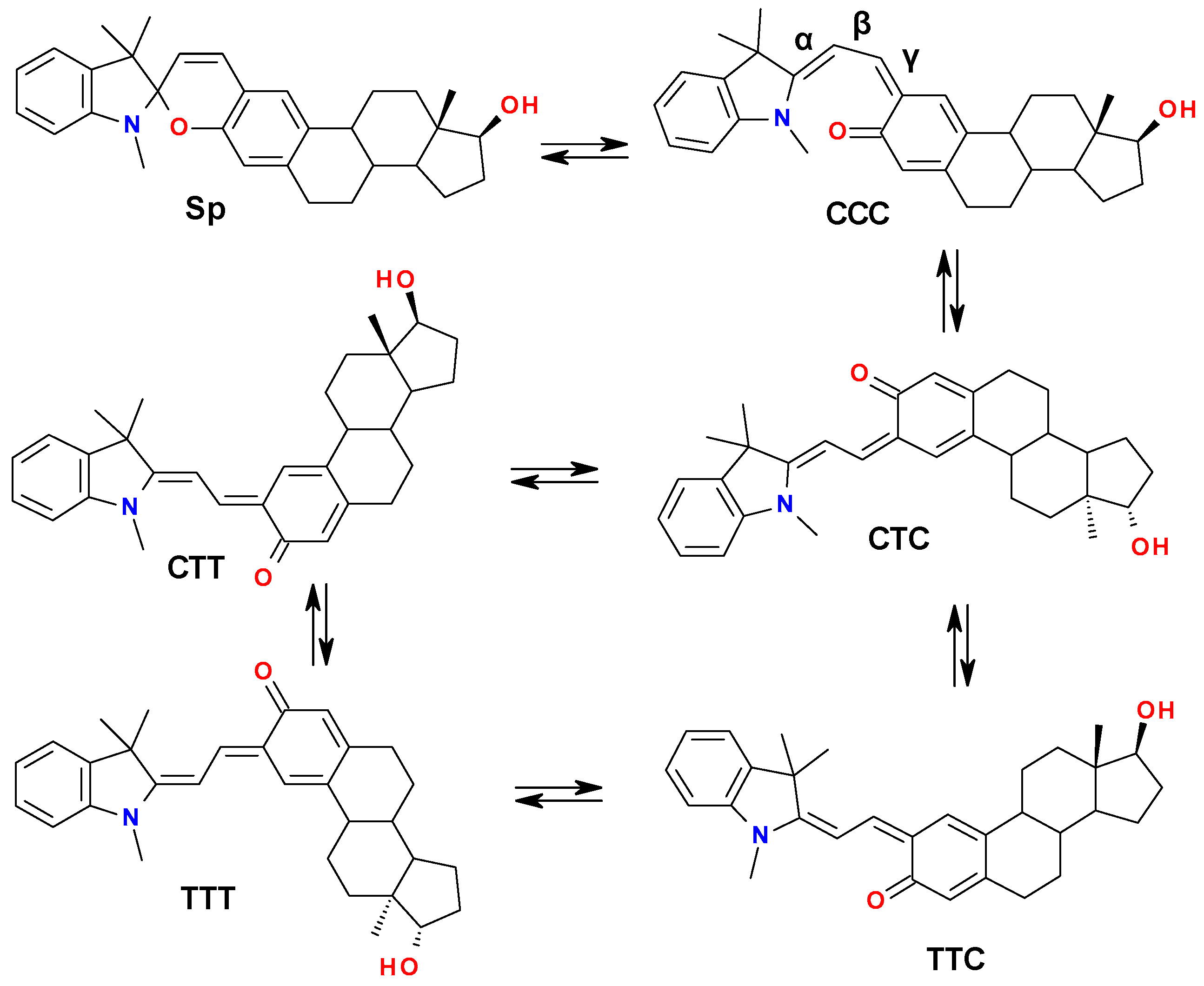
2.4.1. Isomerization of SPPs in the Ground State
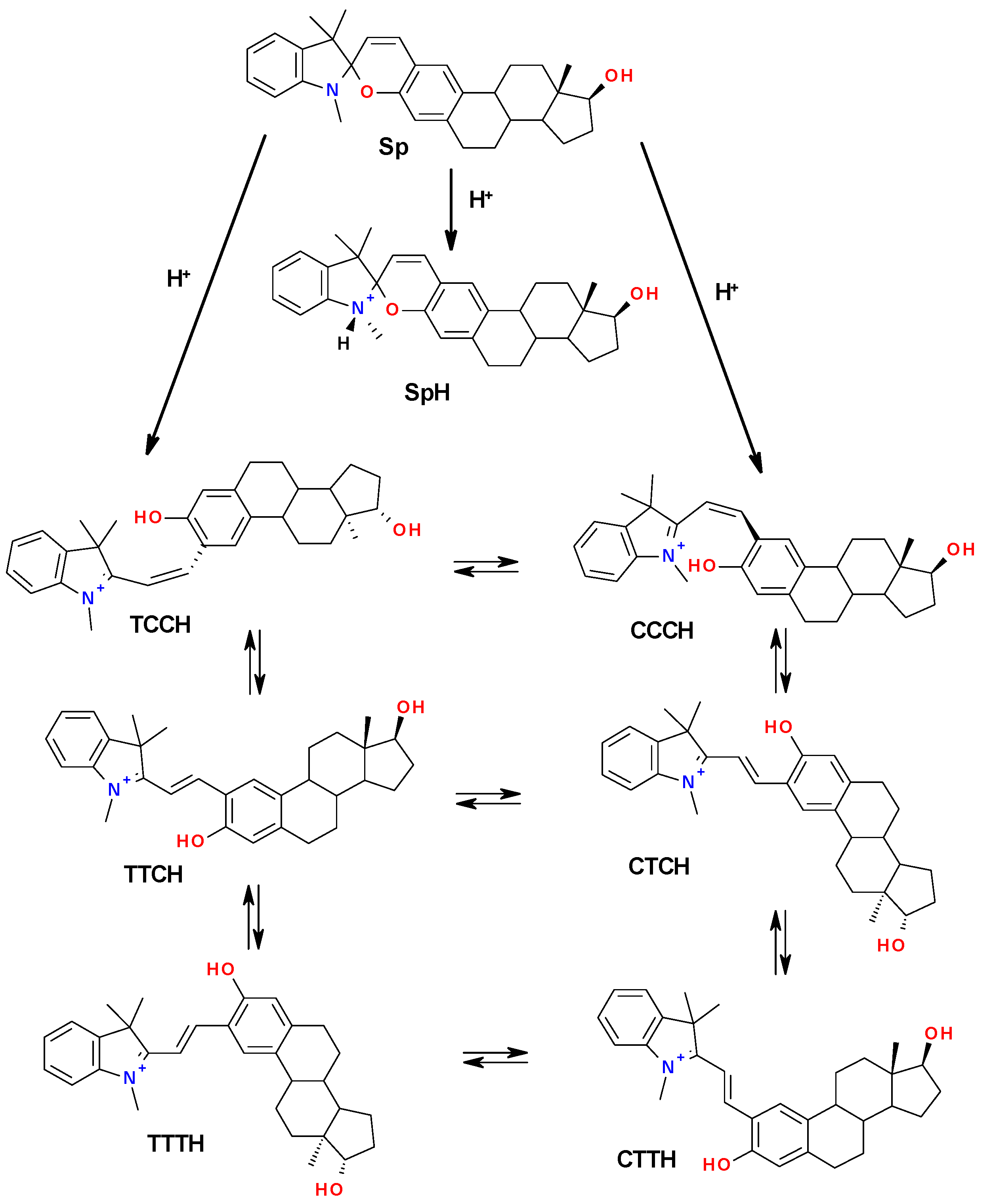
2.4.2. Mechanism of Acidochromic Transformations
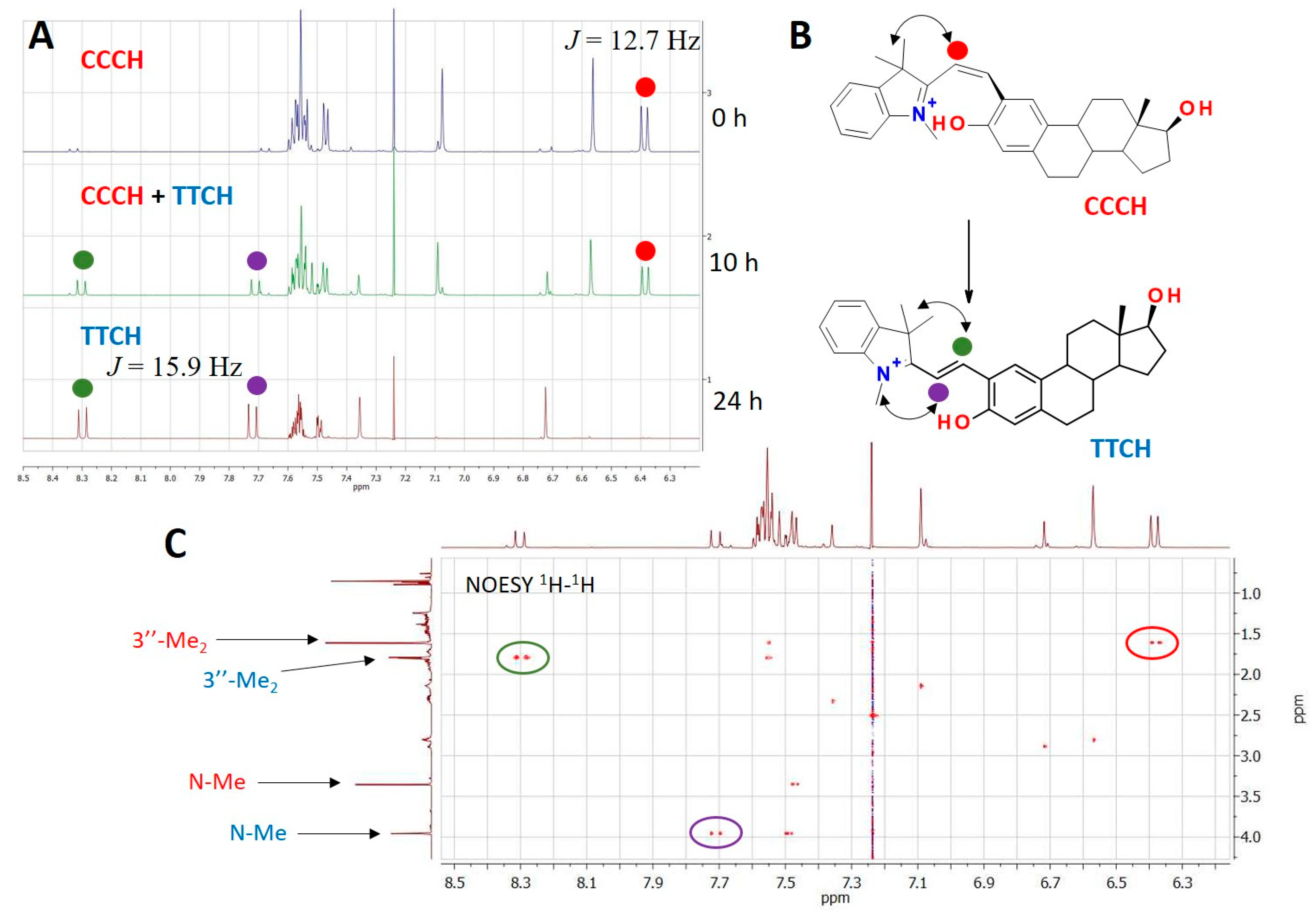
2.5. Determination of the Protonated McH Forms’ Structure
2.6. Cytotoxicity Study of the Spirocyclic and 2-Formyl Derivatives of β-Estradiol and Estrone
3. Materials and Methods
3.1. Synthetic Procedures and Spectral Data
3.2. Single-Crystal X-ray Analysis Methods
3.3. Hirshfeld Surface Analysis
3.4. Computational Methods
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hajializadeh, Z.; Khaksari, M. The Protective Effects of 17-β Estradiol and SIRT1 against Cardiac Hypertrophy: A Review. Heart Fail. Rev. 2022, 27, 725–738. [Google Scholar] [CrossRef]
- Meng, Q.; Li, Y.; Ji, T.; Chao, Y.; Li, J.; Fu, Y.; Wang, S.; Chen, Q.; Chen, W.; Huang, F.; et al. Estrogen Prevent Atherosclerosis by Attenuating Endothelial Cell Pyroptosis via Activation of Estrogen Receptor α-Mediated Autophagy. J. Adv. Res. 2021, 28, 149–164. [Google Scholar] [CrossRef]
- Dubey, R.K.; Jackson, E.K. Invited Review: Cardiovascular Protective Effects of 17β-Estradiol Metabolites. J. Appl. Physiol. 2001, 91, 1868–1883. [Google Scholar] [CrossRef]
- Schaufelberger, S.A.; Rosselli, M.; Barchiesi, F.; Gillespie, D.G.; Jackson, E.K.; Dubey, R.K. 2-Methoxyestradiol, an Endogenous 17β-Estradiol Metabolite, Inhibits Microglial Proliferation and Activation via an Estrogen Receptor-Independent Mechanism. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E313–E322. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Kim, J.H.; Cho, H.T.; Kim, Y.J. The Role of Estrogen in Adipose Tissue Metabolism: Insights into Glucose Homeostasis Regulation. Endocr. J. 2014, 61, 1055–1067. [Google Scholar] [CrossRef]
- Costeira, R.; Lee, K.A.; Murray, B.; Christiansen, C.; Castillo-Fernandez, J.; Ni Lochlainn, M.; Capdevila Pujol, J.; Macfarlane, H.; Kenny, L.C.; Buchan, I.; et al. Estrogen and COVID-19 Symptoms: Associations in Women from the COVID Symptom Study. PLoS ONE 2021, 16, e0257051. [Google Scholar] [CrossRef] [PubMed]
- Al-kuraishy, H.M.; Al-Gareeb, A.I.; Faidah, H.; Al-Maiahy, T.J.; Cruz-Martins, N.; Batiha, G.E.-S. The Looming Effects of Estrogen in Covid-19: A Rocky Rollout. Front. Nutr. 2021, 8, 649128. [Google Scholar] [CrossRef]
- Amr, A.; Elsayed, E.; Al-Omar, M.; Badr Eldin, H.; Nossier, E.; Abdallah, M. Design, Synthesis, Anticancer Evaluation and Molecular Modeling of Novel Estrogen Derivatives. Molecules 2019, 24, 416. [Google Scholar] [CrossRef]
- Carmona-Negrón, J.A.; Santana, A.; Rheingold, A.L.; Meléndez, E. Synthesis, Structure, Docking and Cytotoxic Studies of Ferrocene–Hormone Conjugates for Hormone-Dependent Breast Cancer Application. Dalton Trans. 2019, 48, 5952–5964. [Google Scholar] [CrossRef]
- Theron, A.; Prudent, R.; Nolte, E.; Van Den Bout, I.; Punchoo, R.; Marais, S.; Du Toit, P.; Hlophe, Y.; Van Papendorp, D.; Lafanechère, L.; et al. Novel in Silico-Designed Estradiol Analogues Are Cytotoxic to a Multidrug-Resistant Cell Line at Nanomolar Concentrations. Cancer Chemother. Pharmacol. 2015, 75, 431–437. [Google Scholar] [CrossRef]
- Leese, M.P.; Leblond, B.; Smith, A.; Newman, S.P.; Di Fiore, A.; De Simone, G.; Supuran, C.T.; Purohit, A.; Reed, M.J.; Potter, B.V.L. 2-Substituted Estradiol Bis-Sulfamates, Multitargeted Antitumor Agents: Synthesis, In Vitro SAR, Protein Crystallography, and In Vivo Activity. J. Med. Chem. 2006, 49, 7683–7696. [Google Scholar] [CrossRef] [PubMed]
- Rzheznikov, V.M.; Golubovskaya, L.E.; Mayatskaya, E.E.; Keda, B.I.; Sushinina, L.P.; Titova, T.A.; Tolkachev, V.N.; Osetrova, I.P.; Smirnova, Z.S. Antitumor Steroids. 3. Synthesis and Biological Activity of 11β-Hydroxyestra-1,3,5(10)-Triene Derivatives with Bis-(2-Chloroethyl)Amino-Containing Substituents in the 3-Position. Pharm. Chem. J. 2008, 42, 111–114. [Google Scholar] [CrossRef]
- Seeger, H.; Huober, J.; Wallwiener, D.; Mueck, A.O. Inhibition of Human Breast Cancer Cell Proliferation with Estradiol Metabolites is as Effective as with Tamoxifen. Horm. Metab. Res. 2004, 36, 277–280. [Google Scholar] [CrossRef]
- Kumar, B.S.; Raghuvanshi, D.S.; Hasanain, M.; Alam, S.; Sarkar, J.; Mitra, K.; Khan, F.; Negi, A.S. Recent Advances in Chemistry and Pharmacology of 2-Methoxyestradiol: An Anticancer Investigational Drug. Steroids 2016, 110, 9–34. [Google Scholar] [CrossRef]
- Verenich, S.; Gerk, P.M. Therapeutic Promises of 2-Methoxyestradiol and Its Drug Disposition Challenges. Mol. Pharm. 2010, 7, 2030–2039. [Google Scholar] [CrossRef] [PubMed]
- Parks, M.; Tillhon, M.; Donà, F.; Prosperi, E.; Scovassi, A.I. 2-Methoxyestradiol: New Perspectives in Colon Carcinoma Treatment. Mol. Cell. Endocrinol. 2011, 331, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M.; Mohanakrishnan, A.K.; Hollingshead, M.; Hamel, E. The Effect of Exchanging Various Substituents at the 2-Position of 2-Methoxyestradiol on Cytotoxicity in Human Cancer Cell Cultures and Inhibition of Tubulin Polymerization. J. Med. Chem. 2002, 45, 4748–4754. [Google Scholar] [CrossRef] [PubMed]
- Solum, E.J.; Cheng, J.-J.; Sylte, I.; Vik, A.; Hansen, T.V. Synthesis, Biological Evaluation and Molecular Modeling of New Analogs of the Anti-Cancer Agent 2-Methoxyestradiol: Potent Inhibitors of Angiogenesis. RSC Adv. 2015, 5, 32497–32504. [Google Scholar] [CrossRef]
- Nolte, E.; Joubert, A.; Lakier, R.; Van Rensburg, A.; Mercier, A. Exposure of Breast and Lung Cancer Cells to a Novel Estrone Analog Prior to Radiation Enhances Bcl-2-Mediated Cell Death. Int. J. Mol. Sci. 2018, 19, 2887. [Google Scholar] [CrossRef]
- Kozlenko, A.S.; Ozhogin, I.V.; Pugachev, A.D.; Lukyanova, M.B.; El-Sewify, I.M.; Lukyanov, B.S. A Modern Look at Spiropyrans: From Single Molecules to Smart Materials. Top Curr. Chem. 2023, 381, 8. [Google Scholar] [CrossRef] [PubMed]
- Keyvan Rad, J.; Balzade, Z.; Mahdavian, A.R. Spiropyran-Based Advanced Photoswitchable Materials: A Fascinating Pathway to the Future Stimuli-Responsive Devices. J. Photochem. Photobiol. C 2022, 51, 100487. [Google Scholar] [CrossRef]
- Towns, A. Spiropyran Dyes. Phys. Sci. Rev. 2021, 6, 341–368. [Google Scholar] [CrossRef]
- Kortekaas, L.; Browne, W.R. The Evolution of Spiropyran: Fundamentals and Progress of an Extraordinarily Versatile Photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Mukhanov, E.L.; Ozhogin, I.V.; Kozlenko, A.S.; Metelitsa, A.V.; Lukyanov, B.S. Isomerization and Changes of the Properties of Spiropyrans by Mechanical Stress: Advances and Outlook. Chem. Heterocycl. Compd. 2021, 57, 122–130. [Google Scholar] [CrossRef]
- Lerch, M.M.; Hansen, M.J.; van Dam, G.M.; Szymanski, W.; Feringa, B.L. Emerging Targets in Photopharmacology. Angew. Chem. Int. Ed. 2016, 55, 10978–10999. [Google Scholar] [CrossRef] [PubMed]
- Klajn, R. Spiropyran-Based Dynamic Materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef]
- Minkin, V.I. Light-Controlled Molecular Switches Based on Bistable Spirocyclic Organic and Coordination Compounds. Russ. Chem. Rev. 2013, 82, 1–26. [Google Scholar] [CrossRef]
- Fagan, A.; Bartkowski, M.; Giordani, S. Spiropyran-Based Drug Delivery Systems. Front. Chem. 2021, 9, 720087. [Google Scholar] [CrossRef]
- Ghani, M.; Heiskanen, A.; Kajtez, J.; Rezaei, B.; Larsen, N.B.; Thomsen, P.; Kristensen, A.; Žukauskas, A.; Alm, M.; Emnéus, J. On-Demand Reversible UV-Triggered Interpenetrating Polymer Network-Based Drug Delivery System Using the Spiropyran–Merocyanine Hydrophobicity Switch. ACS Appl. Mater. Interfaces 2021, 13, 3591–3604. [Google Scholar] [CrossRef]
- Cardano, F.; Del Canto, E.; Giordani, S. Spiropyrans for Light-Controlled Drug Delivery. Dalton Trans. 2019, 48, 15537–15544. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, J.; Carling, C.-J.; Almutairi, A. Photocontrolled Release Using One-Photon Absorption of Visible or NIR Light. J. Control. Release 2015, 219, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Das, J.; Biswas, S.; Maiti, T.K.; Pradeep Singh, N.D. A Spiropyran–Coumarin Platform: An Environment Sensitive Photoresponsive Drug Delivery System for Efficient Cancer Therapy. J. Mater. Chem. B 2017, 5, 3940–3944. [Google Scholar] [CrossRef]
- Seiler, V.K.; Callebaut, K.; Robeyns, K.; Tumanov, N.; Wouters, J.; Champagne, B.; Leyssens, T. Acidochromic Spiropyran–Merocyanine Stabilisation in the Solid State. CrystEngComm 2018, 20, 3318–3327. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, H.; Zhang, G.; Zhou, Y.; Fan, L.; Shi, L.; Zhang, C.; Shuang, S.; Dong, C. Substituent Effect on the Acid-Induced Isomerization of Spiropyran Compounds. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 202, 13–17. [Google Scholar] [CrossRef]
- Mandal, M.; Banik, D.; Karak, A.; Manna, S.K.; Mahapatra, A.K. Spiropyran–Merocyanine Based Photochromic Fluorescent Probes: Design, Synthesis, and Applications. ACS Omega 2022, 7, 36988–37007. [Google Scholar] [CrossRef] [PubMed]
- Miguez, F.B.; Moreira, O.B.O.; De Oliveira, M.A.L.; Denadai, Â.M.L.; De Oliveira, L.F.C.; De Sousa, F.B. Reversible Electrospun Fibers Containing Spiropyran for Acid and Base Vapor Sensing. J. Mat. Res. 2023, 38, 547–556. [Google Scholar] [CrossRef]
- Keyvan Rad, J.; Ghomi, A.R.; Karimipour, K.; Mahdavian, A.R. Progressive Readout Platform Based on Photoswitchable Polyacrylic Nanofibers Containing Spiropyran in Photopatterning with Instant Responsivity to Acid–Base Vapors. Macromolecules 2020, 53, 1613–1622. [Google Scholar] [CrossRef]
- Babazadeh-Mamaqani, M.; Roghani-Mamaqani, H.; Abdollahi, A.; Salami-Kalajahi, M. Optical Chemosensors Based on Spiropyran-Doped Polymer Nanoparticles for Sensing PH of Aqueous Media. Langmuir 2022, 38, 9410–9420. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, S.; Mikesell, L.; Whisman, N.; Fang, M.; Steenwinkel, T.E.; Chen, K.; Luck, R.L.; Werner, T.; Liu, H. Near-Infrared Hybrid Rhodol Dyes with Spiropyran Switches for Sensitive Ratiometric Sensing of PH Changes in Mitochondria and Drosophila Melanogaster First-Instar Larvae. ACS Appl. Bio Mater. 2019, 2, 4986–4997. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Jia, J.; Chen, X.; Lv, Y.; Guo, Y.; Li, J. A Ratiometric Near-Infrared Fluorescence Strategy Based on Spiropyran in Situ Switching for Tracking Dynamic Changes of Live-Cell Lysosomal PH. Dyes Pigm. 2019, 166, 433–442. [Google Scholar] [CrossRef]
- Chen, L.; Wu, J.; Schmuck, C.; Tian, H. A Switchable Peptide Sensor for Real-Time Lysosomal Tracking. Chem. Commun. 2014, 50, 6443–6446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gao, Q.; Tong, Q.; Wu, G. Fluorescent Probes Based on Benzothiazole-Spiropyran Derivatives for PH Monitoring in Vitro and in Vivo. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 225, 117506. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Zheng, Y.; Shen, J.; Yang, W.; Yin, M. “On–off–on” Switchable Sensor: A Fluorescent Spiropyran Responds to Extreme PH Conditions and Its Bioimaging Applications. ACS Appl. Mater. Interfaces 2014, 6, 19515–19519. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.K.; Zhao, Z.; Gildner, M.B.; Shoulders, B.A.; Velasquez, T.L.; Blumenthal, M.O.; Wang, L.; Li, X.; Hudnall, T.W.; Betancourt, T.; et al. Synthesis, Optical Properties and in Vitro Cell Viability of Novel Spiropyrans and Their Photostationary States. Tetrahedron 2021, 80, 131854. [Google Scholar] [CrossRef]
- Shinohara, M.; Ashikaga, Y.; Xu, W.; Kim, S.; Fukaminato, T.; Niidome, T.; Kurihara, S. Photochemical OFF/ON Cytotoxicity Switching by Using a Photochromic Surfactant with Visible Light Irradiation. ACS Omega 2022, 7, 6093–6098. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Wang, C.; Peng, P.; Hossain, M.; Jiang, T.; Fu, W.; Liao, Y.; Su, M. Visible Light Mediated Killing of Multidrug-Resistant Bacteria Using Photoacids. J. Mater. Chem. B 2013, 1, 997–1001. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Zolotukhin, P.V.; Mukhanov, E.L.; Rostovtseva, I.A.; Makarova, N.I.; Tkachev, V.V.; Beseda, D.K.; Metelitsa, A.V.; Lukyanov, B.S. Novel Molecular Hybrids of Indoline Spiropyrans and α-Lipoic Acid as Potential Photopharmacological Agents: Synthesis, Structure, Photochromic and Biological Properties. Bioorg. Med. Chem. Lett. 2021, 31, 127709. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Zolotukhin, P.V.; Tkachev, V.V.; Pugachev, A.D.; Kozlenko, A.S.; Belanova, A.A.; Aldoshin, S.M.; Lukyanov, B.S. Synthesis and Study of New Indoline Spiropyran and Its Derivative with α-Lipoic Acid Exhibiting Low Cytotoxicity. Russ. Chem. Bull. 2021, 70, 1388–1393. [Google Scholar] [CrossRef]
- Hofsløkken, N.U.; Skattebøl, L. Convenient Method for the Ortho-Formylation of Phenols. Acta Chem. Scand. 1999, 53, 258–262. [Google Scholar] [CrossRef]
- Akselsen, Ø.W.; Hansen, T.V. Ortho-Formylation of Estrogens. Synthesis of the Anti-Cancer Agent 2-Methoxyestradiol. Tetrahedron 2011, 67, 7738–7742. [Google Scholar] [CrossRef]
- Ozhogin, I.V.; Mukhanov, E.L.; Chernyshev, A.V.; Pugachev, A.D.; Lukyanov, B.S.; Metelitsa, A.V. Synthesis and Study of New Photochromic Unsymmetrical Bis-Spiropyrans with Nonequivalent Heteroarene Fragments Conjugated through the Common 2H,8H-Pyrano[2,3-f]Chromene Moiety. J. Mol. Struct. 2020, 1221, 128808. [Google Scholar] [CrossRef]
- Mukhanov, E.L.; Dorogan, I.V.; Chernyshev, A.V.; Bezuglyi, S.O.; Ozhogin, I.V.; Lukyanova, M.B.; Lukyanov, B.S. Theoretical and Experimental Study of New Photochromic Bis-Spiropyrans with Hydroxyethyl and Carboxyethyl Substituents. Int. J. Photoenergy 2013, 2013, 752949. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Ashfaq, M.; Munawar, K.S.; Tahir, M.N.; Dege, N.; Yaman, M.; Muhammad, S.; Alarfaji, S.S.; Kargar, H.; Arshad, M.U. Synthesis, Crystal Structure, Hirshfeld Surface Analysis, and Computational Study of a Novel Organic Salt Obtained from Benzylamine and an Acidic Component. ACS Omega 2021, 6, 22357–22366. [Google Scholar] [CrossRef] [PubMed]
- Riwar, L.-J.; Trapp, N.; Kuhn, B.; Diederich, F. Substituent Effects in Parallel-Displaced π-π Stacking Interactions: Distance Matters. Angew. Chem. Int. Ed. 2017, 56, 11252–11257. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Ozhogin, I.V.; Kozlenko, A.S.; Tkachev, V.V.; Shilov, G.V.; Makarova, N.I.; Rostovtseva, I.A.; Borodkin, G.S.; El-Sewify, I.M.; Aldoshin, S.M.; et al. Comprehensive Study of Substituent Effects on Structure and Photochromic Properties of 1,3-Benzoxazine-4-One Spiropyrans. J. Mol. Struc. 2023, 1277, 134898. [Google Scholar] [CrossRef]
- Jelsch, C.; Ejsmont, K.; Huder, L. The Enrichment Ratio of Atomic Contacts in Crystals, an Indicator Derived from the Hirshfeld Surface Analysis. IUCrJ 2014, 1, 119–128. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Q.; Li, P.; Fang, B.; Yin, M. Photochromism of Neutral Spiropyran in the Crystalline State at Room Temperature. J. Mater. Chem. C 2021, 9, 6290–6296. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Tkachev, V.V.; Kozlenko, A.S.; Ozhogin, I.V.; Lukyanova, M.B.; Dmitriev, V.S.; Aldoshin, S.M.; Lukyanov, B.S. Features of Crystallization of Indoline Spiropyran with a Cationic Substituent. Russ. J. Gen. Chem. 2022, 92, 1384–1391. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Tkachev, V.V.; Ozhogin, I.V.; Lukyanova, M.B.; Aldoshin, S.M.; Minkin, V.I.; Mukhanov, E.L.; Metelitsa, A.V.; Stankevich, N.V.; Lukyanov, B.S. Structures of Spiropyrans Exhibiting Photochromic Properties in the Solid State. Russ. Chem. Bull. 2021, 70, 2090–2099. [Google Scholar] [CrossRef]
- Funasako, Y.; Ason, M.; Takebayashi, J.; Inokuchi, M. Solid-State Photochromism of Salts of Cationic Spiropyran with Various Anions: A Correlation between Reaction Cavity Volumes and Reactivity. Cryst. Growth Des. 2019, 19, 7308–7314. [Google Scholar] [CrossRef]
- Futami, Y.; Chin, M.L.S.; Kudoh, S.; Takayanagi, M.; Nakata, M. Conformations of Nitro-Substituted Spiropyran and Merocyanine Studied by Low-Temperature Matrix-Isolation Infrared Spectroscopy and Density-Functional-Theory Calculation. Chem. Phys. Lett. 2003, 370, 460–468. [Google Scholar] [CrossRef]
- Hobley, J.; Malatesta, V. Energy Barrier to TTC–TTT Isomerisation for the Merocyanine of a Photochromic Spiropyran. Phys. Chem. Chem. Phys. 2000, 2, 57–59. [Google Scholar] [CrossRef]
- Dorogan, I.V.; Minkin, V.I. Theoretical Modeling of Electrocyclic 2H-Pyran and 2H-1,4-Oxazine Ring Opening Reactions in Photo- and Thermochromic Spiropyrans and Spirooxazines. Chem. Heterocycl. Compd. 2016, 52, 730–735. [Google Scholar] [CrossRef]
- Kortekaas, L.; Chen, J.; Jacquemin, D.; Browne, W.R. Proton-Stabilized Photochemically Reversible E/Z Isomerization of Spiropyrans. J. Phys. Chem. B 2018, 122, 6423–6430. [Google Scholar] [CrossRef]
- Sheng, Y.; Leszczynski, J.; Garcia, A.A.; Rosario, R.; Gust, D.; Springer, J. Comprehensive Theoretical Study of the Conversion Reactions of Spiropyrans: Substituent and Solvent Effects. J. Phys. Chem. B 2004, 108, 16233–16243. [Google Scholar] [CrossRef]
- Aldoshin, S.M.; Bozhenko, K.V.; Utenyshev, A.N. Quantum Chemical Study of the Cspiro-O Bond Dissociation in Spiropyran Molecules. Russ. Chem. Bull. 2013, 62, 1740–1743. [Google Scholar] [CrossRef]
- Cottone, G.; Noto, R.; La Manna, G. Density Functional Theory Study of the Trans-Trans-Cis (TTC)→Trans-Trans-Trans (TTT) Isomerization of a Photochromic Spiropyran Merocyanine. Molecules 2008, 13, 1246–1252. [Google Scholar] [CrossRef]
- Pugachev, A.D.; Ozhogin, I.V.; Makarova, N.I.; Rostovtseva, I.A.; Lukyanova, M.B.; Kozlenko, A.S.; Borodkin, G.S.; Tkachev, V.V.; El-Sewify, I.M.; Dorogan, I.V.; et al. Novel Polychromogenic Fluorine-Substituted Spiropyrans Demonstrating Either Uni- or Bidirectional Photochromism as Multipurpose Molecular Switches. Dyes Pigm. 2022, 199, 110043. [Google Scholar] [CrossRef]
- Dowds, M.; Stenspil, S.G.; De Souza, J.H.; Laursen, B.W.; Cacciarini, M.; Nielsen, M.B. Orthogonal- and Path-Dependent Photo/Acidoswitching in an Eight-State Dihydroazulene-Spiropyran Dyad. ChemPhotoChem 2022, 6, e202200152. [Google Scholar] [CrossRef]
- CrysAlis Pro (Version 171.41.93a), Rigaku Oxford Diffraction. 2015. Available online: https://www.rigaku.com/products/crystallography/crysalis (accessed on 1 April 2023).
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, 3814, 3814–3816. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward Reliable Density Functional Methods without Adjustable Parameters: The PBE0 Model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, Approximate and Parallel Hartree–Fock and Hybrid DFT Calculations. A ‘Chain-of-Spheres’ Algorithm for the Hartree–Fock Exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An Overlap Fitted Chain of Spheres Exchange Method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef]
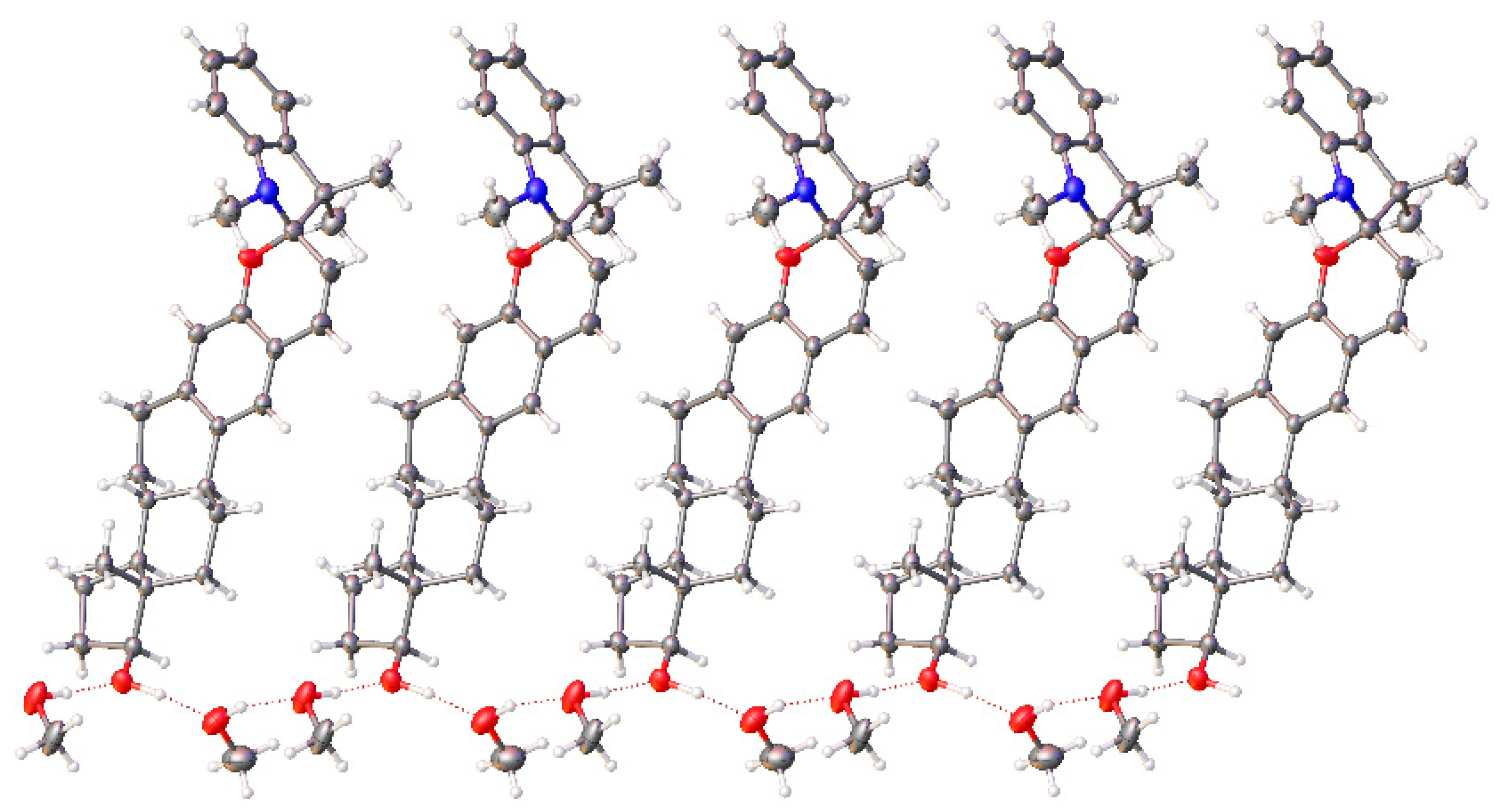


| Short Contacts | l, Å | l − VdW, Å |
|---|---|---|
| O(2)…O(1S) | 2.678 | −0.362 |
| O(2)…O(2S) | 2.675 | −0.365 |
| O(1S)…O(2S) | 2.664 | −0.376 |
| C(4′)…H(6B) | 2.857 | −0.043 |
| H(12D)…C(8″) | 2.814 | −0.086 |
| H(2)…O(1S) | 1.663 | 1.057 |
| H(2)…H(1S) | 2.307 | −0.093 |
| H(2)…C(1S) | 2.506 | −0.394 |
| H(2)…H(1SB) | 2.386 | −0.014 |
| H(7B)…O(2S) | 2.647 | −0.073 |
| O(2)…H(2S) | 1.707 | −1.013 |
| C(17)…H(2S) | 2.879 | −0.021 |
| H(2)…H(2S) | 2.356 | −0.044 |
| H(1S)…O(2S) | 1.852 | −0.868 |
| H(1S)…C(3S) | 2.865 | −0.035 |
| H(1S)…H(2S) | 2.347 | −0.053 |
| C(1S)…O(2S) | 3.195 | −0.025 |
| Contact | Cxy (Exy) |
|---|---|
| O…H | 0.058 (1.11) |
| N…H | 0.008 (1.11) |
| C…H | 0.127 (1.11) |
| H…H | 0.807 (0.99) |
| No. | Structure | Form | Absorption λmax, nm (ε · 10−3, M−1·cm−1) |
|---|---|---|---|
| (3) | 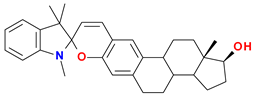 | Sp | 204 (41.8), 231 (33.7), 268 (9.5), 296 (6.4), 323 sh (3.8) |
| McH | 402 | ||
| (4) | 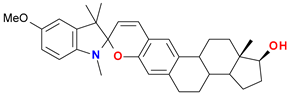 | Sp | 202 (40.1), 232 (36.2), 269 sh (7.9), 314 (8.5) |
| McH | 402 | ||
| (5) |  | Sp | 204 (42.0); 231 (33.6); 268 (8.9); 296 (6.3); 323 sh (3.7) |
| McH | 400 | ||
| (6) |  | Sp | 202 (40.2); 232 (37.3); 269 sh (8.4); 314 (8.4) |
| McH | 397 |
| Isomer | G, a.u. | Grel, kcal/mol | Energy Barrier, kcal/mol |
|---|---|---|---|
| Sp | −1407.19203162 | 0 | - |
| TSSp-CCC | −1407.17068088 | 13.4 | 13.4 |
| CCC | −1407.17508024 | 10.6 | - |
| TSCCC-CTC | −1407.15417442 | 23.8 | 13.2 |
| CTC | −1407.18312876 | 5.6 | - |
| TSCTC-CTT | −1407.15251755 | 24.8 | 19.2 |
| CTT | −1407.18443424 | 4.8 | - |
| TSCTC-TTC | −1407.16277247 | 18.4 | 12.8 |
| TTC | −1407.18757602 | 2.8 | - |
| TSCTT-TTT | −1407.16040986 | 19.8 | 15.0 |
| TSTTC-TTT | −1407.15498830 | 23.2 | 20.4 |
| TTT | −1407.18760296 | 2.8 | - |
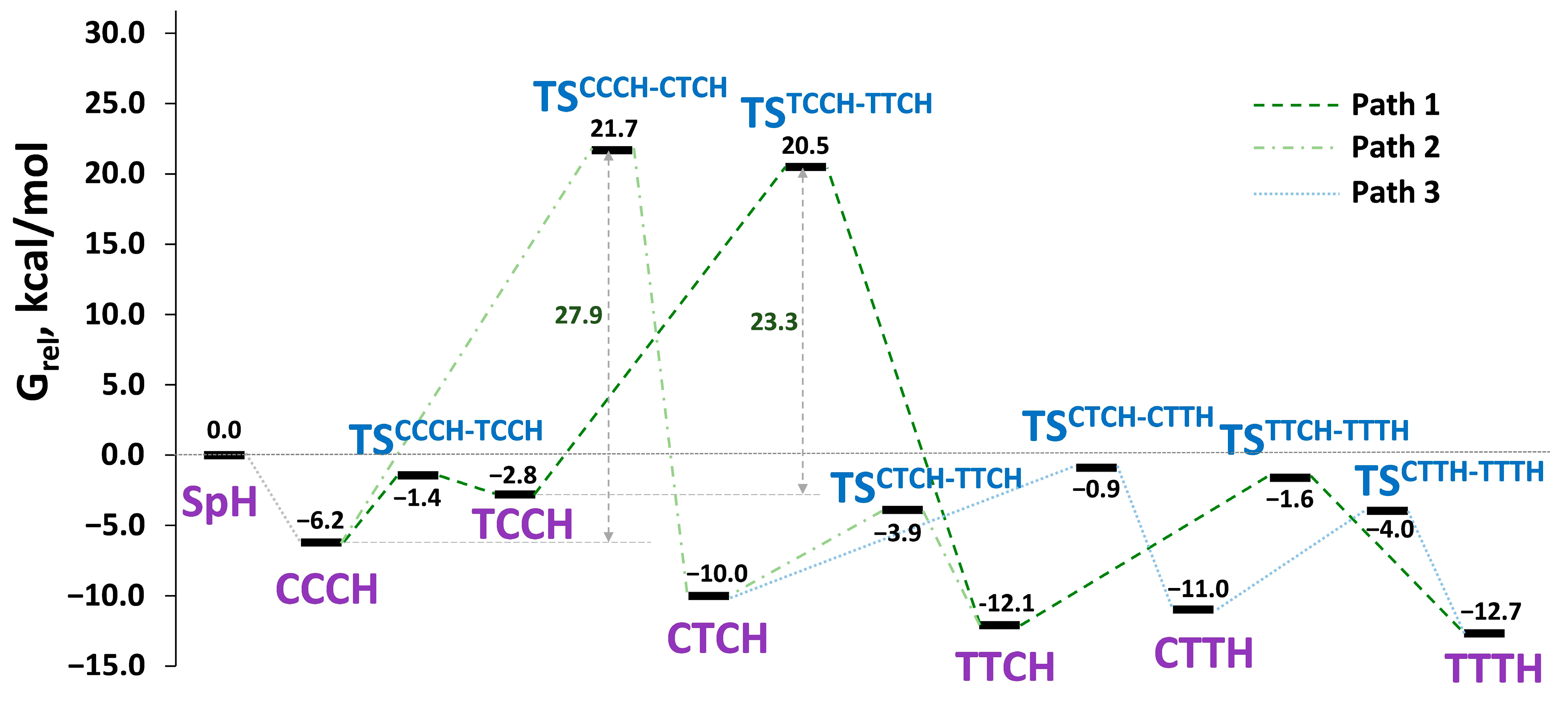
| Isomer | G, a.u. | Grel, kcal/mol | Energy Barrier, kcal/mol |
|---|---|---|---|
| SpH | −1407.61996533 | 0 | - |
| CCCH | −1407.62985427 | −6.2 | - |
| TSCCCH-TCCH | −1407.62225265 | −1.4 | 4.8 |
| TCCH | −1407.62441867 | −2.8 | - |
| TSCCCH-CTCH | −1407.58540033 | 21.7 | 27.9 |
| CTCH | −1407.63590909 | −10.0 | - |
| TSTCCH-TTCH | −1407,58729335 | 20.5 | 23.3 |
| TSCTCH-TTCH | −1407.62617824 | −3.9 | 6.1 |
| TTCH | −1407.63924105 | −12.1 | - |
| TSCTCH-CTTH | −1407.62138572 | −0.9 | 9.1 |
| CTTH | −1407.63745967 | −11.0 | - |
| TSTTCH-TTTH | −1407.62253176 | −1.6 | 10.5 |
| TSCTTH-TTTH | −1407.62627058 | −4.0 | 7.0 |
| TTTH | −1407.64015436 | −12.7 | - |
| Group | Viability, % | pMW * | Percent Change * | |
|---|---|---|---|---|
| Med [25…75] * | M ± SD * | |||
| DMSO (control) | 99.5 [96.75…100] | 97.88 ± 3.24 | - | - |
| (1) | 97 [96…98.5] | 97.41 ± 1.78 | 0.296 | Non-significant |
| (2) | 97 [97…98.25] | 97.5 ± 1.679 | 0.272 | Non-significant |
| (3) | 72.5 [67.75…99] | 72 ± 7.568 | 0.001 | 27.1% decrease |
| (4) | 85 [83…87.25] | 85.08 ± 4.23 | 0.001 | 14.6% decrease |
| (5) | 96.5 [94.5…99] | 96.17 ± 3.51 | 0.139 | Non-significant |
| (6) | 97 [93.75…99] | 96.5 ± 3.06 | 0.193 | Non-significant |
| Group | Viability, % | pMW | Percent Change * | |
|---|---|---|---|---|
| Med [25…75] | M ± SD | |||
| DMSO (control) | 99.5 [96.75…100] | 97.88 ± 3.24 | - | - |
| (1) | 58 [50.5…70.75] | 59.75 ± 16.88 | 0.001 | 41.7% decrease |
| (2) | 50.5 [45.5…56] | 50.92 ± 9.99 | 0.001 | 49.3% decrease |
| (3) | 32 [29…39] | 32.33 ± 10.75 | 0.001 | 67.8% decrease |
| (4) | 60.5 [47…65] | 57.42 ± 10.99 | 0.001 | 39.2% decrease |
| (5) | 73.5 [43.75…98] | 69.83 ± 30.36 | 0.007 | 26.1% decrease |
| (6) | 98 [96.75…99] | 97.5 ± 2.15 | 0.215 | Non-significant |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozhogin, I.V.; Pugachev, A.D.; Makarova, N.I.; Belanova, A.A.; Kozlenko, A.S.; Rostovtseva, I.A.; Zolotukhin, P.V.; Demidov, O.P.; El-Sewify, I.M.; Borodkin, G.S.; et al. Novel Indoline Spiropyrans Based on Human Hormones β-Estradiol and Estrone: Synthesis, Structure, Chromogenic and Cytotoxic Properties. Molecules 2023, 28, 3866. https://doi.org/10.3390/molecules28093866
Ozhogin IV, Pugachev AD, Makarova NI, Belanova AA, Kozlenko AS, Rostovtseva IA, Zolotukhin PV, Demidov OP, El-Sewify IM, Borodkin GS, et al. Novel Indoline Spiropyrans Based on Human Hormones β-Estradiol and Estrone: Synthesis, Structure, Chromogenic and Cytotoxic Properties. Molecules. 2023; 28(9):3866. https://doi.org/10.3390/molecules28093866
Chicago/Turabian StyleOzhogin, Ilya V., Artem D. Pugachev, Nadezhda I. Makarova, Anna A. Belanova, Anastasia S. Kozlenko, Irina A. Rostovtseva, Peter V. Zolotukhin, Oleg P. Demidov, Islam M. El-Sewify, Gennady S. Borodkin, and et al. 2023. "Novel Indoline Spiropyrans Based on Human Hormones β-Estradiol and Estrone: Synthesis, Structure, Chromogenic and Cytotoxic Properties" Molecules 28, no. 9: 3866. https://doi.org/10.3390/molecules28093866
APA StyleOzhogin, I. V., Pugachev, A. D., Makarova, N. I., Belanova, A. A., Kozlenko, A. S., Rostovtseva, I. A., Zolotukhin, P. V., Demidov, O. P., El-Sewify, I. M., Borodkin, G. S., Metelitsa, A. V., & Lukyanov, B. S. (2023). Novel Indoline Spiropyrans Based on Human Hormones β-Estradiol and Estrone: Synthesis, Structure, Chromogenic and Cytotoxic Properties. Molecules, 28(9), 3866. https://doi.org/10.3390/molecules28093866








