The Influence of the Side Chain Structure on the Photostability of Low Band Gap Polymers
Abstract
1. Introduction
2. Results and Discussion
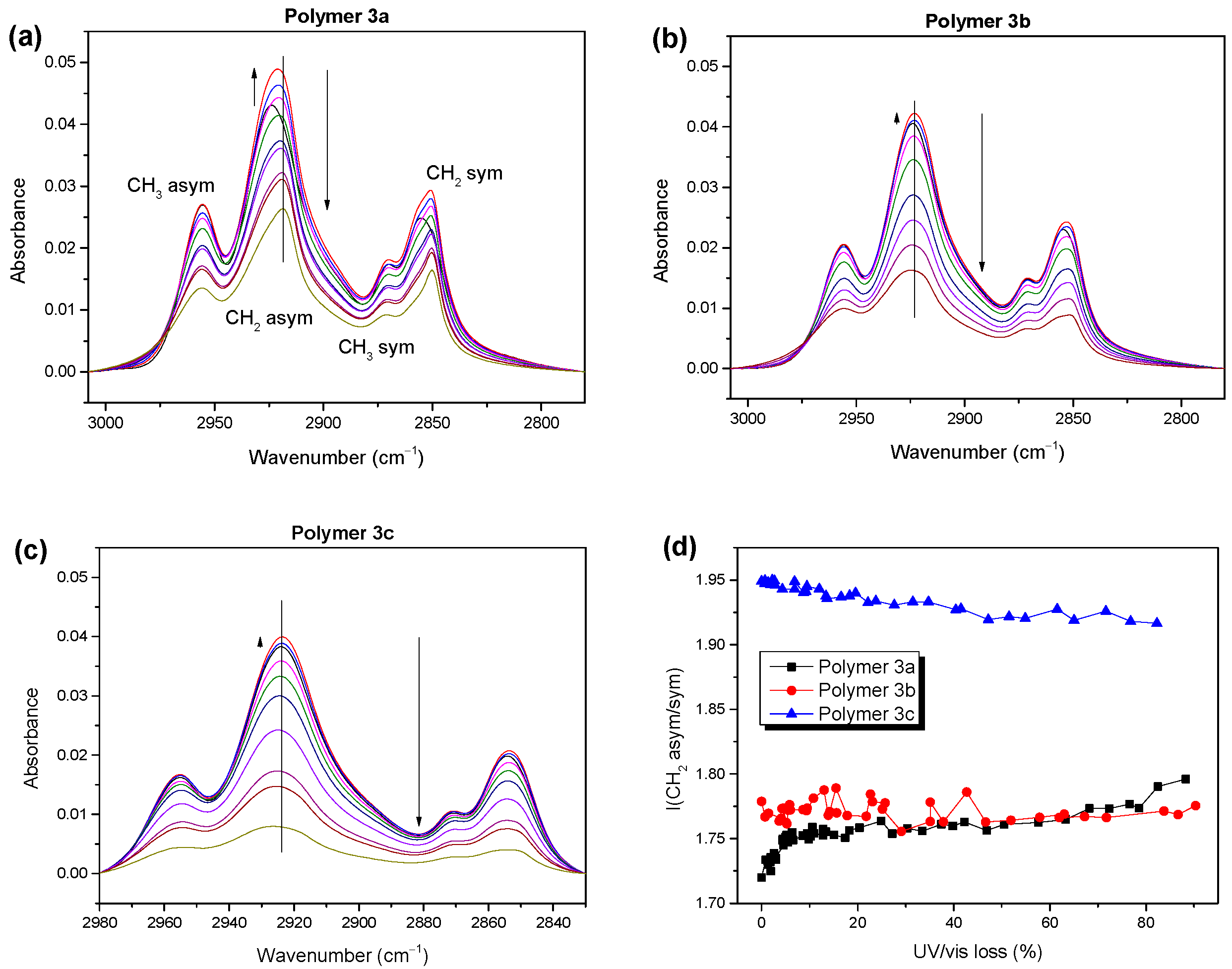
2.1. Electronic Structure and Molecular Orientation
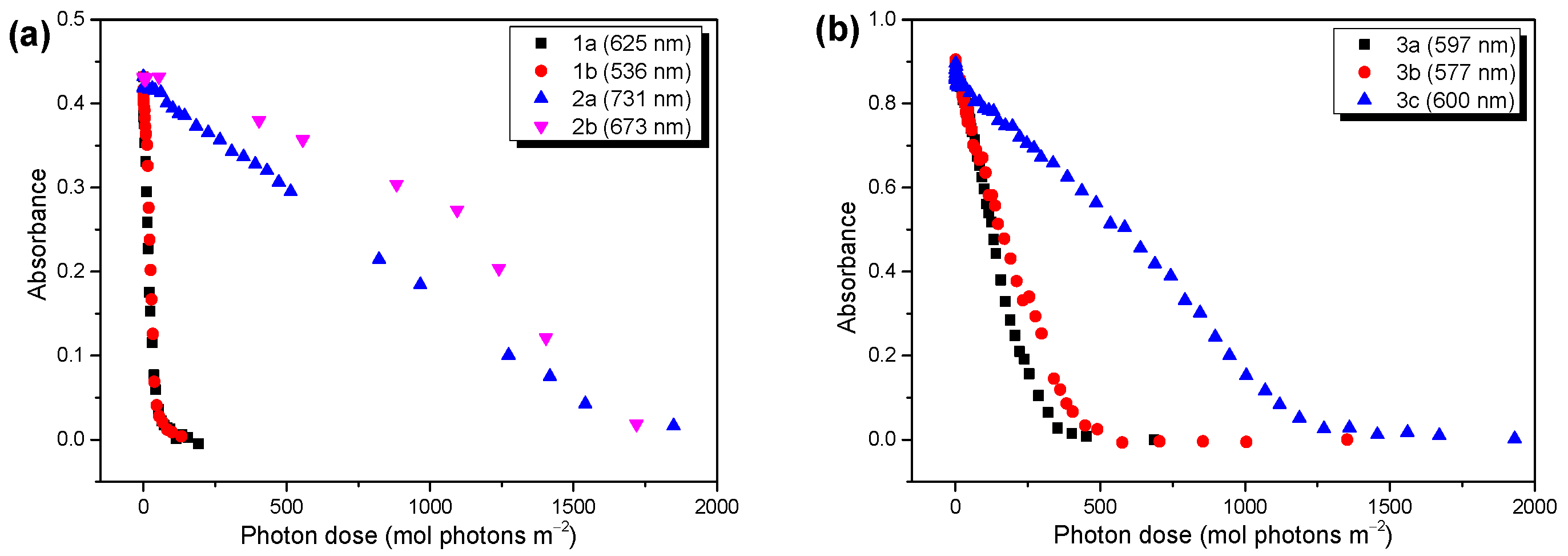
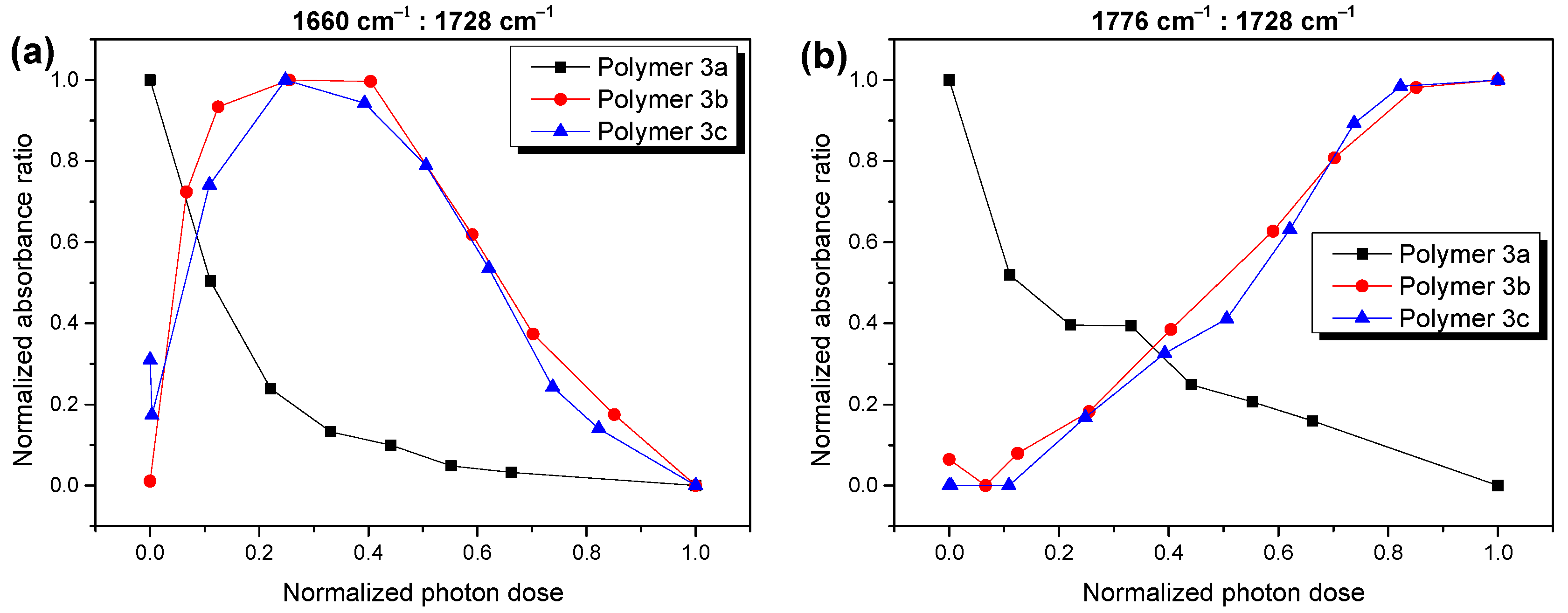
2.2. Photostability of the Investigated Low Band Gap Polymers
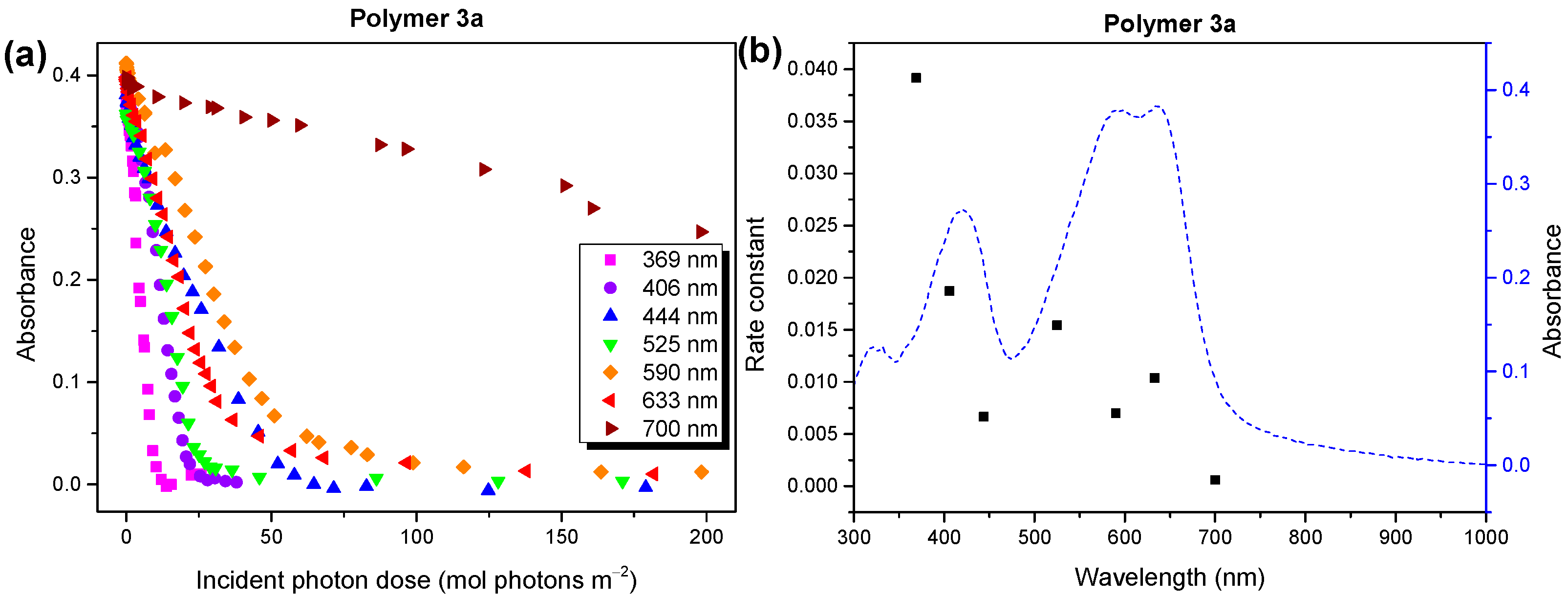
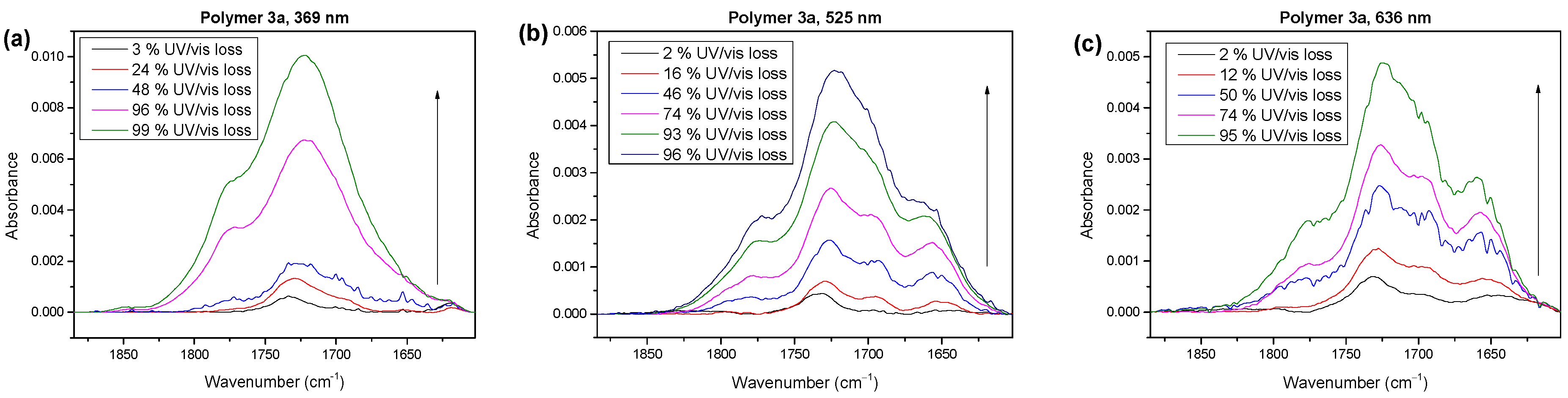
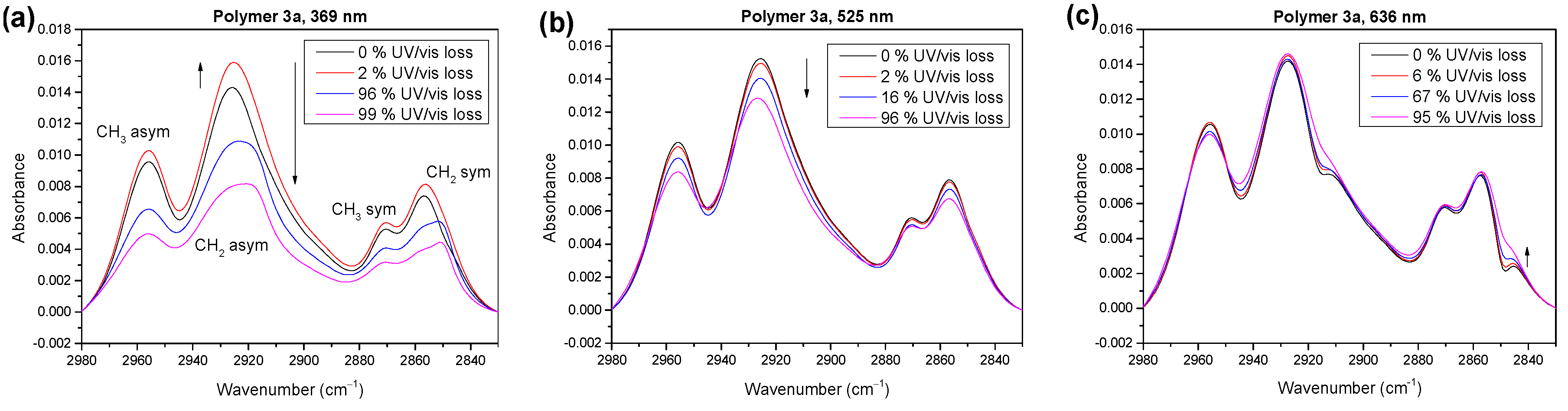
2.3. Wavelength Dependence of Degradation Mechanism of Polymer 3a
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.H.; Xiao, C.Y.; Xie, C.C.; Li, W.W. Flexible organic solar cells: Materials, large-area fabrication techniques and potential applications. Nano Energy 2021, 89, 26. [Google Scholar] [CrossRef]
- Bonnassieux, Y.; Brabec, C.J.; Cao, Y.; Carmichael, T.B.; Chabinyc, M.L.; Cheng, K.T.; Cho, G.; Chung, A.; Cobb, C.L.; Distler, A.; et al. The 2021 flexible and printed electronics roadmap. Flex. Print. Electron. 2022, 6, 023001. [Google Scholar] [CrossRef]
- Armin, A.; Li, W.; Sandberg, O.J.; Xiao, Z.; Ding, L.M.; Nelson, J.; Neher, D.; Vandewal, K.; Shoaee, S.; Wang, T.; et al. A History and Perspective of Non-Fullerene Electron Acceptors for Organic Solar Cells. Adv. Energy Mater. 2021, 11, 42. [Google Scholar] [CrossRef]
- Held, M.; Zakharko, Y.; Wang, M.; Jakubka, F.; Gannott, F.; Rumer, J.W.; Ashraf, R.S.; McCulloch, I.; Zaumseil, J. Photo- and electroluminescence of ambipolar, high-mobility, donor-acceptor polymers. Org. Electron. 2016, 32, 220–227. [Google Scholar] [CrossRef]
- Bürgi, L.; Turbiez, M.; Pfeiffer, R.; Bienewald, F.; Kirner, H.-J.; Winnewisser, C. High-Mobility Ambipolar Near-Infrared Light-Emitting Polymer Field-Effect Transistors. Adv. Mater. 2008, 20, 2217–2224. [Google Scholar] [CrossRef]
- Cheng, P.; Zhan, X.W. Stability of organic solar cells: Challenges and strategies. Chem. Soc. Rev. 2016, 45, 2544–2582. [Google Scholar] [CrossRef]
- Aygül, U.; Egelhaaf, H.J.; Nagel, P.; Merz, M.; Schuppler, S.; Eichele, K.; Peisert, H.; Chassé, T. Photodegradation of C-PCPDTBT and Si-PCPDTBT: Influence of the Bridging Atom on the Stability of a Low-Band-Gap Polymer for Solar Cell Application. ChemPhysChem 2015, 16, 428–435. [Google Scholar] [CrossRef]
- Tournebize, A.; Bussiere, P.O.; Rivaton, A.; Gardette, J.L.; Medlej, H.; Hiorns, R.C.; Dagron-Lartigau, C.; Krebs, F.C.; Norrman, K. New Insights into the Mechanisms of Photodegradation/Stabilization of P3HT:PCBM Active Layers Using Poly(3-hexyl-d(13)-Thiophene). Chem. Mater. 2013, 25, 4522–4528. [Google Scholar] [CrossRef]
- Manceau, M.; Bundgaard, E.; Carlé, J.E.; Hagemann, O.; Helgesen, M.; Søndergaard, R.; Jørgensen, M.; Krebs, F.C. Photochemical stability of π-conjugated polymers for polymer solar cells: A rule of thumb. J. Mater. Chem. 2011, 21, 4132–4141. [Google Scholar] [CrossRef]
- Hintz, H.; Egelhaaf, H.J.; Peisert, H.; Chassé, T. Photo-oxidation and ozonization of poly(3-hexylthiophene) thin films as studied by UV/VIS and photoelectron spectroscopy. Polym. Degrad. Stabil. 2010, 95, 818–825. [Google Scholar] [CrossRef]
- Rivaton, A.; Chambon, S.; Manceau, M.; Gardette, J.-L.; Lamaitre, N.; Guillerez, S. Light-induced degradation of the active layer of polymer-based solar cells. Polym. Degrad. Stabil. 2010, 95, 278–284. [Google Scholar] [CrossRef]
- Manceau, M.; Rivaton, A.; Gardette, J.-L.; Guillerez, S.; Lemaitre, N. The mechanism of photo- and thermooxidation of poly(3-hexylthiophene) (P3HT) reconsidered. Polym. Degrad. Stabil. 2009, 94, 898–907. [Google Scholar] [CrossRef]
- Martynov, I.V.; Inasaridze, L.N.; Troshin, P.A. Resist or Oxidize: Identifying Molecular Structure-Photostability Relationships for Conjugated Polymers Used in Organic Solar Cells. Chemsuschem 2022, 15, e202101336. [Google Scholar] [CrossRef] [PubMed]
- Brabec, C.J. Organic photovoltaics: Technology and market. Sol. Energy Mater. Sol. Cells 2004, 83, 273–292. [Google Scholar] [CrossRef]
- Yang, W.; Wang, W.; Wang, Y.; Sun, R.; Guo, J.; Li, H.; Shi, M.; Guo, J.; Wu, Y.; Wang, T.; et al. Balancing the efficiency, stability, and cost potential for organic solar cells via a new figure of merit. Joule 2021, 5, 1209–1230. [Google Scholar] [CrossRef]
- Hintz, H.; Egelhaaf, H.J.; Luer, L.; Hauch, J.; Peisert, H.; Chassé, T. Photodegradation of P3HT-A Systematic Study of Environmental Factors. Chem. Mater. 2011, 23, 145–154. [Google Scholar] [CrossRef]
- Manceau, M.; Rivaton, A.; Gardette, J.-L. Involvement of Singlet Oxygen in the Solid-State Photochemistry of P3HT. Macromol. Rapid Commun. 2008, 29, 1823–1827. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of Polymer Solar Cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef]
- Rabek, J.F. Polymer Photodegradation; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Abdou, M.S.A.; Holdcroft, S. Mechanisms of photodegradation of poly(3-alkylthiophenes) in solution. Macromolecules 1993, 26, 2954–2962. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Zhang, X.; Zhou, G.; Zhang, S. Microstructures and interaction analyses of phosphonium-based ionic liquids: A simulation study. J. Phys. Chem. B 2012, 116, 4934–4942. [Google Scholar] [CrossRef]
- Chen, L.; Mizukado, J.; Suzuki, Y.; Kutsuna, S.; Aoyama, Y.; Yoshida, Y.; Suda, H. An ESR study on superoxide radical anion generation and its involvement in the photooxidative degradation of poly-3-hexylthiophene in chlorobenzene solution. Chem. Phys. Lett. 2014, 605–606, 98–102. [Google Scholar] [CrossRef]
- Morse, G.E.; Tournebize, A.; Rivaton, A.; Chassé, T.; Taviot-Gueho, C.; Blouin, N.; Lozman, O.R.; Tierney, S. The effect of polymer solubilizing side-chains on solar cell stability. Phys. Chem. Chem. Phys. 2015, 17, 11884–11897. [Google Scholar] [CrossRef] [PubMed]
- Sai, N.; Leung, K.; Zadord, J.; Henkelman, G. First principles study of photo-oxidation degradation mechanisms in P3HT for organic solar cells. Phys. Chem. Chem. Phys. 2014, 16, 8092–8099. [Google Scholar] [CrossRef]
- Manceau, M.; Rivaton, A.; Gardette, J.-L.; Guillerez, S.; Lemaitre, N. Light-induced degradation of the P3HT-based solar cells active layer. Sol. Energy Mater. Sol. Cells 2011, 95, 1315–1325. [Google Scholar] [CrossRef]
- Silva, H.S.; Tournebize, A.; Begue, D.; Peisert, H.; Chasse, T.; Gardette, J.L.; Therias, S.; Rivaton, A.; Hiorns, R.C. A universal route to improving conjugated macromolecule photostability. RSC Adv. 2014, 4, 54919–54923. [Google Scholar] [CrossRef]
- Tournebize, A.; Dominguez, I.F.; Morse, G.E.; Taviot-Gueho, C.; Rivaton, A.; Peisert, H.; Chasse, T. Side chain structure and dispersity impact the photostability of low band gap polymers. Polym. Degrad. Stabil. 2017, 146, 155–160. [Google Scholar] [CrossRef]
- Shen, D.E.; Lang, A.W.; Collier, G.S.; Osterholm, A.M.; Smith, E.M.; Tomlinson, A.L.; Reynolds, J.R. Enhancement of Photostability through Side Chain Tuning in Dioxythiophene-Based Conjugated Polymers. Chem. Mater. 2022, 34, 1041–1051. [Google Scholar] [CrossRef]
- Mateker, W.R.; Heumueller, T.; Cheacharoen, R.; Sachs-Quintana, I.T.; McGehee, M.D.; Warnan, J.; Beaujuge, P.M.; Liu, X.; Bazan, G.C. Molecular Packing and Arrangement Govern the Photo-Oxidative Stability of Organic Photovoltaic Materials. Chem. Mater. 2015, 27, 6345–6353. [Google Scholar] [CrossRef]
- Dupuis, A.; Wong-Wah-Chung, P.; Rivaton, A.; Gardette, J.L. Influence of the microstructure on the photooxidative degradation of poly(3-hexylthiophene). Polym. Degrad. Stabil. 2012, 97, 366–374. [Google Scholar] [CrossRef]
- Bölke, S.; Batchelor, D.; Früh, A.; Lassalle-Kaiser, B.; Keller, T.; Trilling, F.; Forster, M.; Scherf, U.; Chassé, T.; Peisert, H. Influence of the Side Chain Structure on the Electronic Structure and Self-Organization Properties of Low Band Gap Polymers. ACS Appl. Energy Mater. 2022, 5, 15290–15301. [Google Scholar] [CrossRef]
- Braun, S.; Salaneck, W.R.; Fahlman, M. Energy-Level Alignment at Organic/Metal and Organic/Organic Interfaces. Adv. Mater. 2009, 21, 1450–1472. [Google Scholar] [CrossRef]
- Debe, M.K. Extracting physical structure information from thin organic films with reflection absorption infrared spectroscopy. J. Appl. Phys. 1984, 55, 3354–3366. [Google Scholar] [CrossRef]
- Pearce, H.A.; Sheppard, N. Possible importance of a metal-surface selection rule in interpretation of infrared-spectra of molecules adsorbed on particulate metals—Infrared-spectra from ethylene chemisorbed on silica-supported metal-catalysts. Surf. Sci. 1976, 59, 205–217. [Google Scholar] [CrossRef]
- Greenler, R.G. Infrared Study of Adsorbed Molecules on Metal Surfaces by Reflection Techniques. J. Chem. Phys. 1966, 44, 310–315. [Google Scholar] [CrossRef]
- Umemura, J. Reflection–Absorption Spectroscopy of Thin Films on Metallic Substrates. In Handbook of Vibrational Spectroscopy; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Früh, A.; Rutkowski, S.; Akimchenko, I.O.; Tverdokhlebov, S.I.; Frueh, J. Orientation analysis of polymer thin films on metal surfaces via IR absorbance of the relative transition dipole moments. Appl. Surf. Sci. 2022, 594, 153476. [Google Scholar] [CrossRef]
- Scharber, M.C.; Koppe, M.; Gao, J.; Cordella, F.; Loi, M.A.; Denk, P.; Morana, M.; Egelhaaf, H.-J.; Forberich, K.; Dennler, G.; et al. Influence of the Bridging Atom on the Performance of a Low-Bandgap Bulk Heterojunction Solar Cell. Adv. Mater. 2010, 22, 367. [Google Scholar] [CrossRef]
- Adams, J.H. Analysis of the nonvolatile oxidation products of polypropylene I. Thermal oxidation. J. Polym. Sci. Part A-1 Polym. Chem. 1970, 8, 1077–1090. [Google Scholar] [CrossRef]
- Ljungqvist, N.; Hjertberg, T. Oxidative degradation of poly (3-octylthiophene). Macromolecules 1995, 28, 5993–5999. [Google Scholar] [CrossRef]
- Alem, S.; Wakim, S.; Lu, J.; Robertson, G.; Ding, J.; Tao, Y. Degradation Mechanism of Benzodithiophene-Based Conjugated Polymers when Exposed to Light in Air. ACS Appl. Mater. Interfaces 2012, 4, 2993–2998. [Google Scholar] [CrossRef]
- Holdcroft, S. Photochain scission of the soluble electronically conducting polymer: Poly (3-hexylthiophene). Macromolecules 1991, 24, 2119–2121. [Google Scholar] [CrossRef]
- Wexler, A.S. Infrared determination of structural units in organic compounds by integrated intensity measurements: Alkanes, alkenes and monosubstituted alkyl benzenes. Spectrochim. Acta 1965, 21, 1725–1742. [Google Scholar] [CrossRef]
- Dettinger, U.; Egelhaaf, H.-J.; Brabec, C.J.; Latteyer, F.; Peisert, H.; Chasse, T. FTIR Study of the Impact of PC[60]BM on the Photodegradation of the Low Band Gap Polymer PCPDTBT under O2 Environment. Chem. Mater. 2015, 27, 2299–2308. [Google Scholar] [CrossRef]
- Gaber, B.P.; Peticolas, W.L. On the quantitative interpretation of biomembrane structure by Raman spectroscopy. Biochim. Et Biophys. Acta (BBA)-Biomembr. 1977, 465, 260–274. [Google Scholar] [CrossRef]
- Snyder, R.G. Group Moment Interpretation of the Infrared Intensities of Crystalline n-Paraffins. J. Chem. Phys. 1965, 42, 1744–1763. [Google Scholar] [CrossRef]
- Snyder, R.G.; Hsu, S.L.; Krimm, S. Vibrational spectra in the C-H stretching region and the structure of the polymethylene chain. Spectrochim. Acta Part A Mol. Spectrosc. 1978, 34, 395–406. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, S. ESR Study on Peroxide Modification of Polypropylene. Ind. Eng. Chem. Res. 1997, 36, 1130–1135. [Google Scholar] [CrossRef]
- Cnudde, P.; De Wispelaere, K.; Vanduyfhuys, L.; Demuynck, R.; Van der Mynsbrugge, J.; Waroquier, M.; Van Speybroeck, V. How Chain Length and Branching Influence the Alkene Cracking Reactivity on H-ZSM-5. ACS Catal. 2018, 8, 9579–9595. [Google Scholar] [CrossRef]
- Ratkiewicz, A.; Truong, T.N. Kinetics of the C–C bond beta scission reactions in alkyl radical reaction class. J. Phys. Chem. A 2012, 116, 6643–6654. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S.C.; Liu, S.; You, W. Development of Fluorinated Benzothiadiazole as a Structural Unit for a Polymer Solar Cell of 7% Efficiency. Angew. Chem. Int. Ed. 2011, 50, 2995–2998. [Google Scholar] [CrossRef]
- Carlé, J.E.; Helgesen, M.; Zawacka, N.K.; Madsen, M.V.; Bundgaard, E.; Krebs, F.C. A comparative study of fluorine substituents for enhanced stability of flexible and ITO-free high-performance polymer solar cells. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 893–899. [Google Scholar] [CrossRef]
- Bura, T.; Beaupre, S.; Legare, M.A.; Quinn, J.; Rochette, E.; Blaskovits, J.T.; Fontaine, F.G.; Pron, A.; Li, Y.N.; Leclerc, M. Direct heteroarylation polymerization: Guidelines for defect-free conjugated polymers. Chem. Sci. 2017, 8, 3913–3925. [Google Scholar] [CrossRef]
- Kim, J.; Yun, M.H.; Kim, G.H.; Lee, J.; Lee, S.M.; Ko, S.J.; Kim, Y.; Dutta, G.K.; Moon, M.; Park, S.Y.; et al. Synthesis of PCDTBT-Based Fluorinated Polymers for High Open-Circuit Voltage in Organic Photovoltaics: Towards an Understanding of Relationships between Polymer Energy Levels Engineering and Ideal Morphology Control. ACS Appl. Mater. Interfaces 2014, 6, 7523–7534. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.F.; Takita, R.; Kikuzaki, Y.; Ozawa, F. Palladium-Catalyzed Dehydrohalogenative Polycondensation of 2-Bromo-3-hexylthiophene: An Efficient Approach to Head-to-Tail Poly(3-hexylthiophene). J. Am. Chem. Soc. 2010, 132, 11420–11421. [Google Scholar] [CrossRef] [PubMed]
- Campeau, L.C.; Parisien, M.; Leblanc, M.; Fagnou, K. Biaryl synthesis via direct arylation: Establishment of an efficient catalyst for intramolecular processes. J. Am. Chem. Soc. 2004, 126, 9186–9187. [Google Scholar] [CrossRef]
- Keller, T.; Gahlmann, T.; Riedl, T.; Scherf, U. Direct Arylation Polycondensation (DAP) Synthesis of Alternating Quaterthiophene-Benzothiadiazole Copolymers for Organic Solar Cell Applications. Chempluschem 2019, 84, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]



| IP | Φorg/Δ | ||||
|---|---|---|---|---|---|
| Polymer | PEI | Au | PEI | Au | |
| 3a | 5.2 | 5.2 | 2.01 | 3.7/+0.4 | 4.5/−0.7 |
| 3b | 5.1 | 5.1 | 2.01 | 3.7/+0.4 | 4.4/−0.8 |
| 3c | 4.9 | 4.9 | 1.90 | 3.7/+0.4 | 4.3/−0.9 |
| Polymer | 3a | 3b | 3c |
|---|---|---|---|
| room temperature | 15° | 14° | 22° |
| annealed (130 °C) | 15° | 16° | 24° |
| Polymer | Mn [kg/mol] | Mw [kg/mol] | Mw/Mn | DP |
|---|---|---|---|---|
| 1a * | 12.6 | 14.4 | 1.14 | 14 |
| 1b * | 9.7 | 15.4 | 1.59 | 11 |
| 2a * | 7.2 | 10.2 | 1.42 | 10 |
| 2b * | 8.0 | 10.0 | 1.25 | 12 |
| 3a # | 10.3 | 13.4 | 1.30 | 9 |
| 3b # | 17.1 | 23.9 | 1.40 | 14 |
| 3c # | 16.7 | 58.5 | 3.50 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bölke, S.; Keller, T.; Trilling, F.; Forster, M.; Scherf, U.; Chassé, T.; Peisert, H. The Influence of the Side Chain Structure on the Photostability of Low Band Gap Polymers. Molecules 2023, 28, 3858. https://doi.org/10.3390/molecules28093858
Bölke S, Keller T, Trilling F, Forster M, Scherf U, Chassé T, Peisert H. The Influence of the Side Chain Structure on the Photostability of Low Band Gap Polymers. Molecules. 2023; 28(9):3858. https://doi.org/10.3390/molecules28093858
Chicago/Turabian StyleBölke, Sven, Tina Keller, Florian Trilling, Michael Forster, Ullrich Scherf, Thomas Chassé, and Heiko Peisert. 2023. "The Influence of the Side Chain Structure on the Photostability of Low Band Gap Polymers" Molecules 28, no. 9: 3858. https://doi.org/10.3390/molecules28093858
APA StyleBölke, S., Keller, T., Trilling, F., Forster, M., Scherf, U., Chassé, T., & Peisert, H. (2023). The Influence of the Side Chain Structure on the Photostability of Low Band Gap Polymers. Molecules, 28(9), 3858. https://doi.org/10.3390/molecules28093858







