Improving Theaflavin-3,3′-digallate Production Efficiency Optimization by Transition State Conformation of Polyphenol Oxidase
Abstract
1. Introduction
2. Results
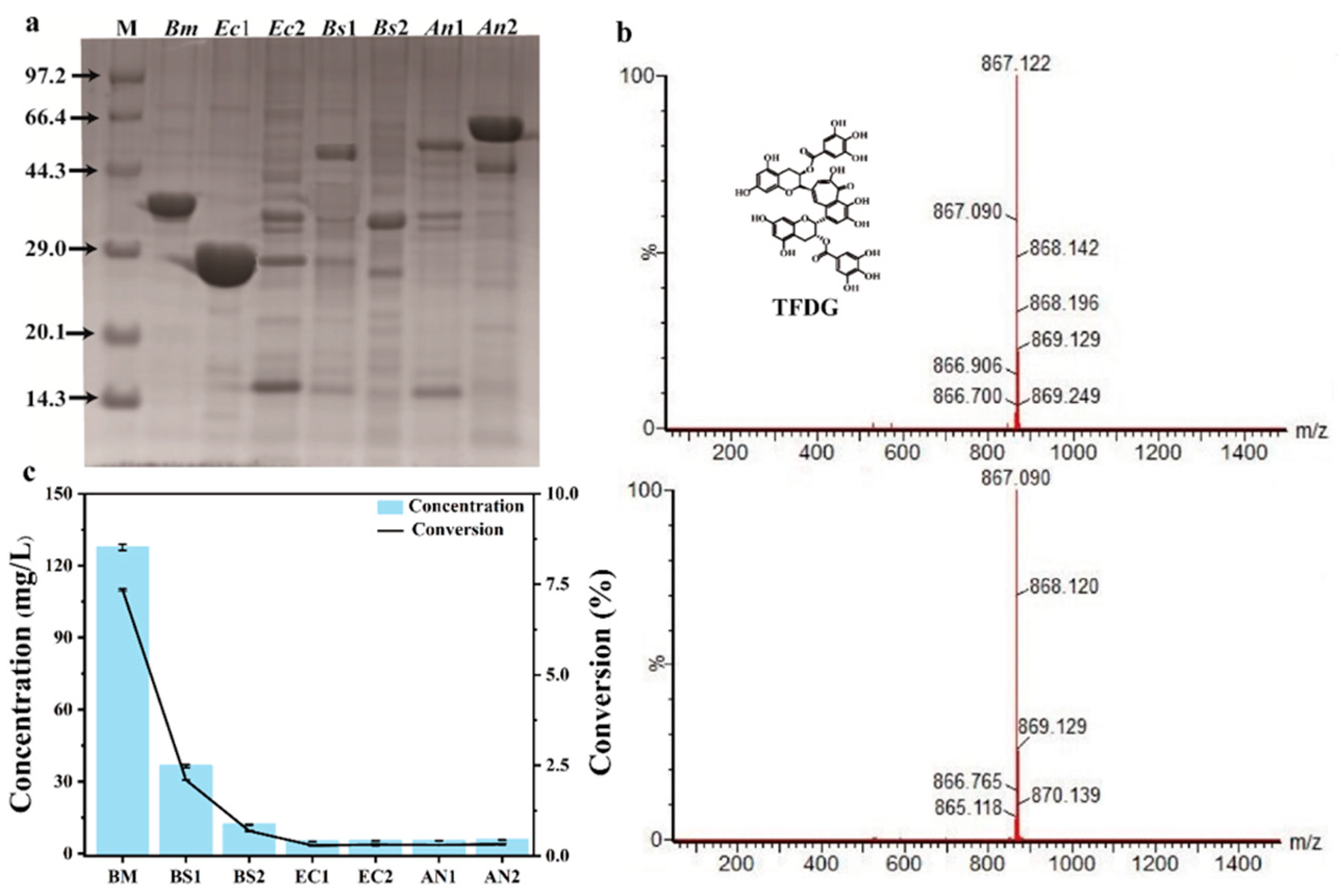
2.1. Screening Enzymes for TFDG Synthesis
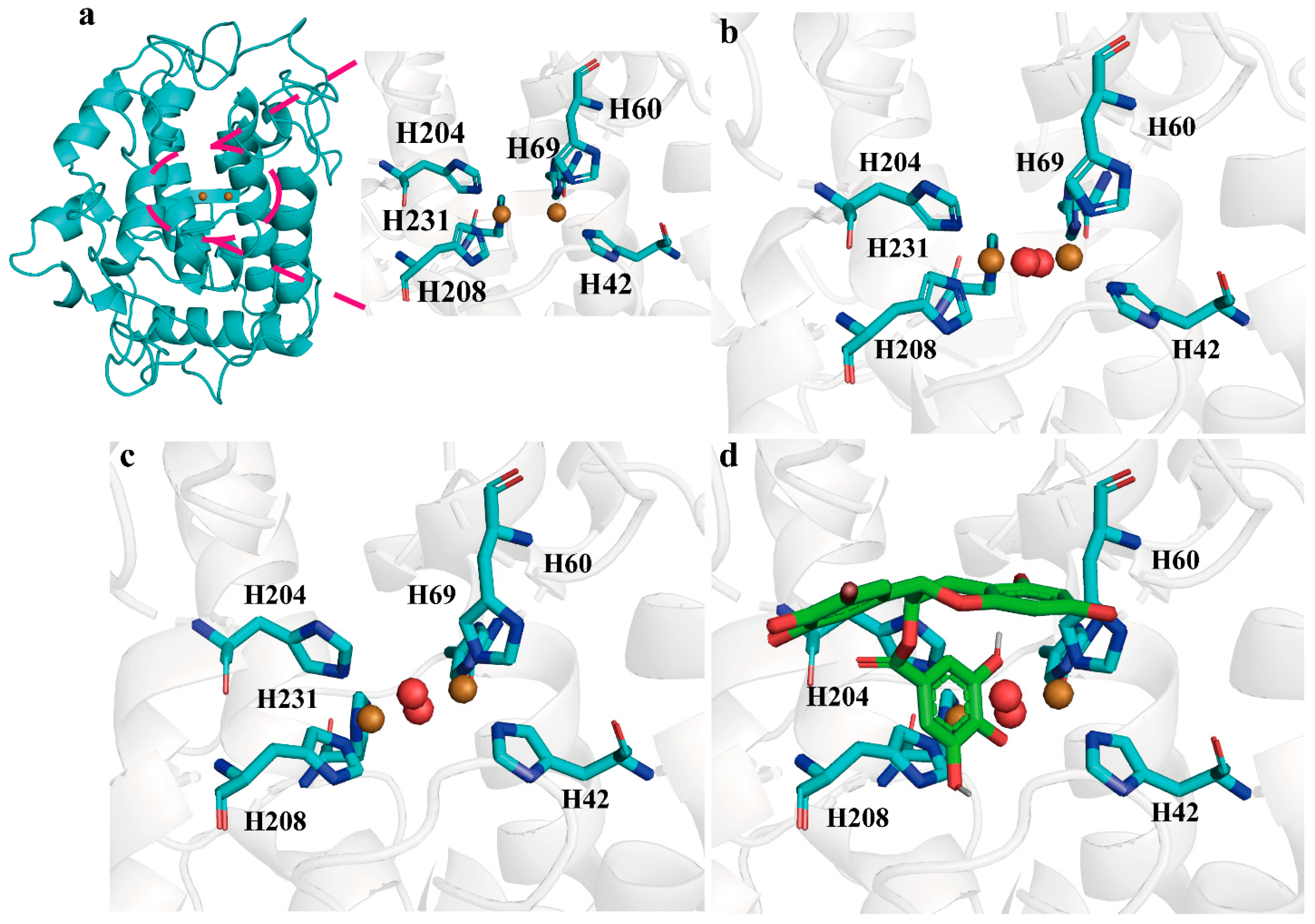
2.2. Structure and Catalytic Mechanism of BmTyr
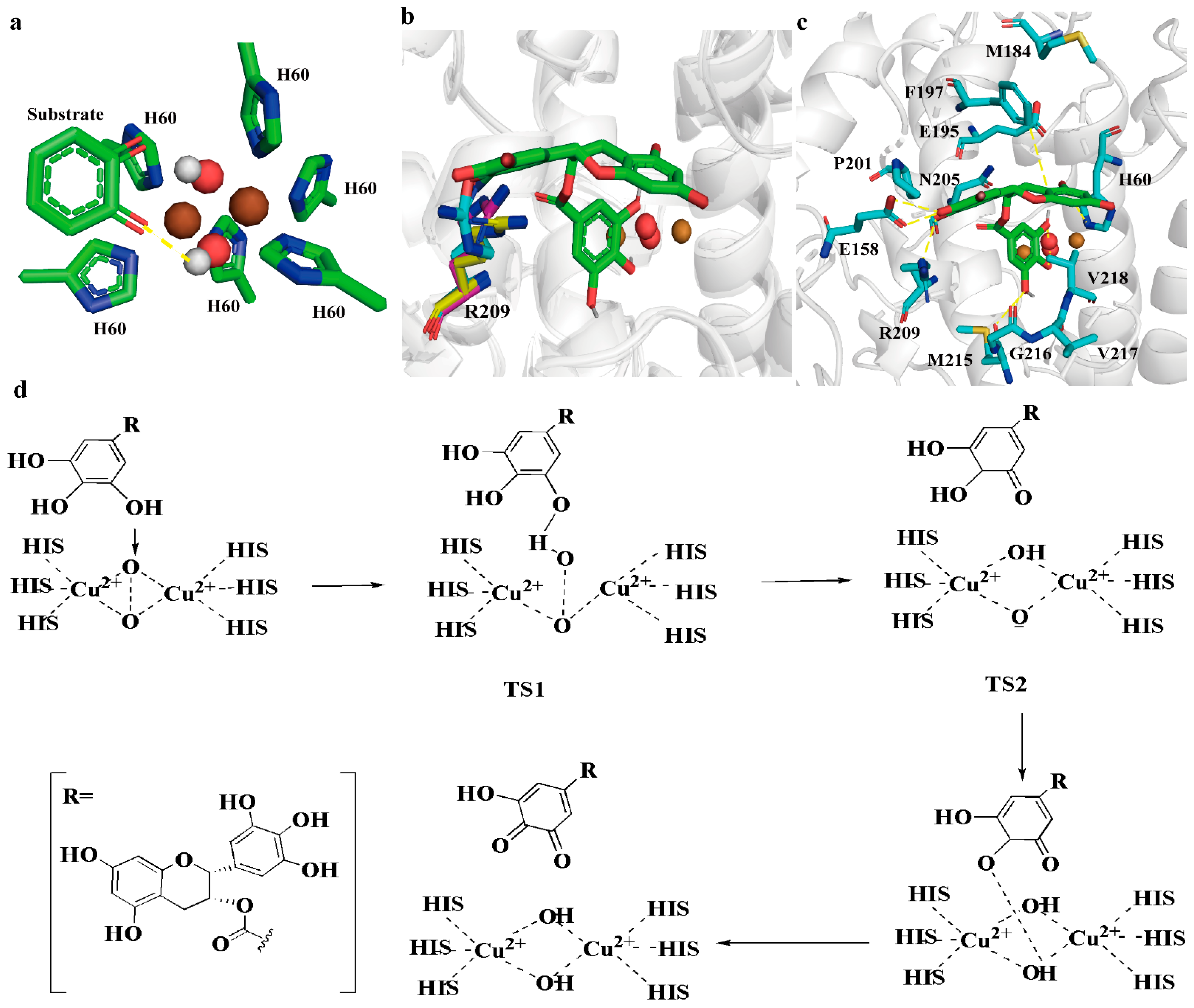
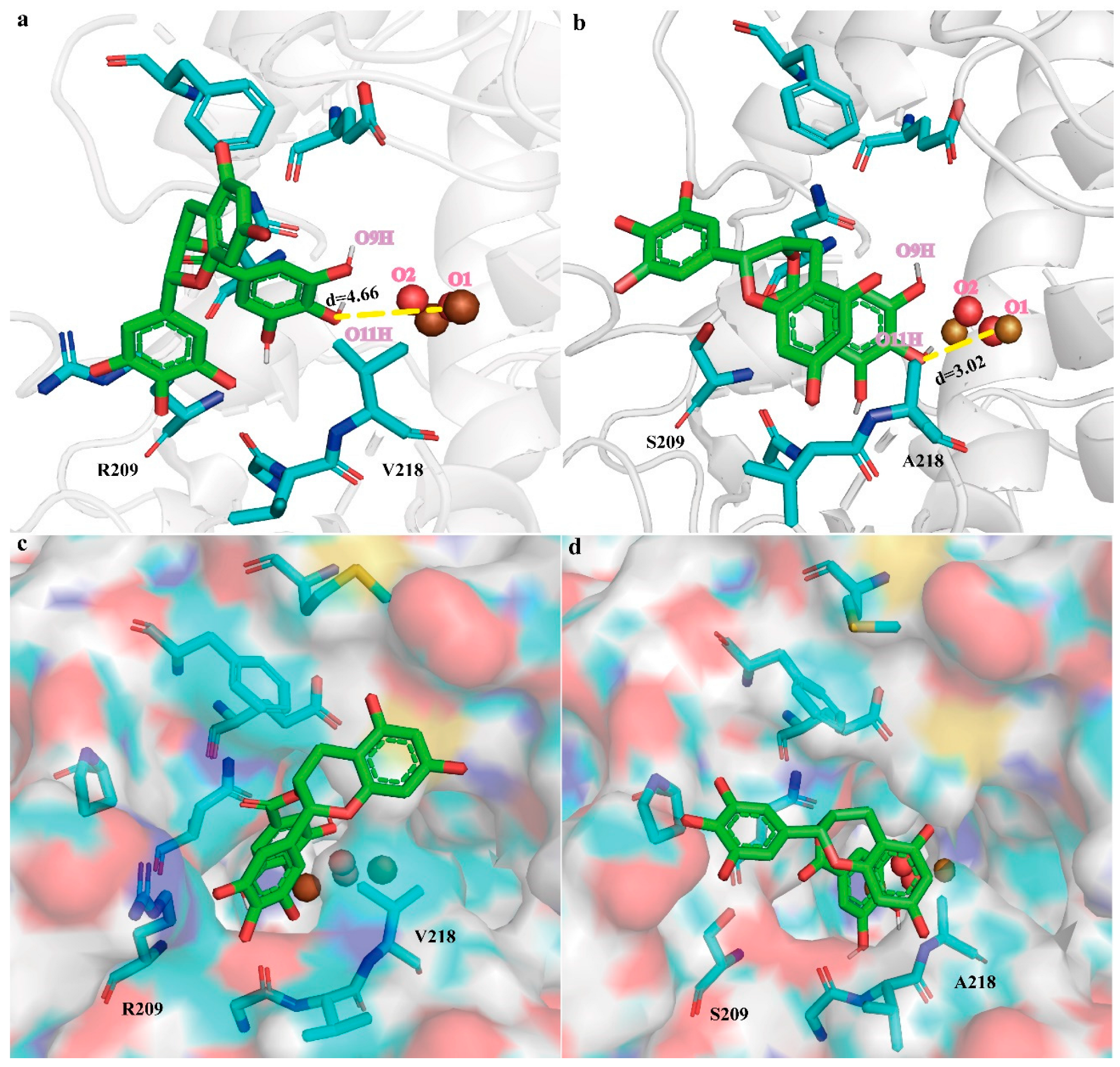
2.3. Improving BmTyr Catalytic Efficiency via TS2 Conformation Optimization
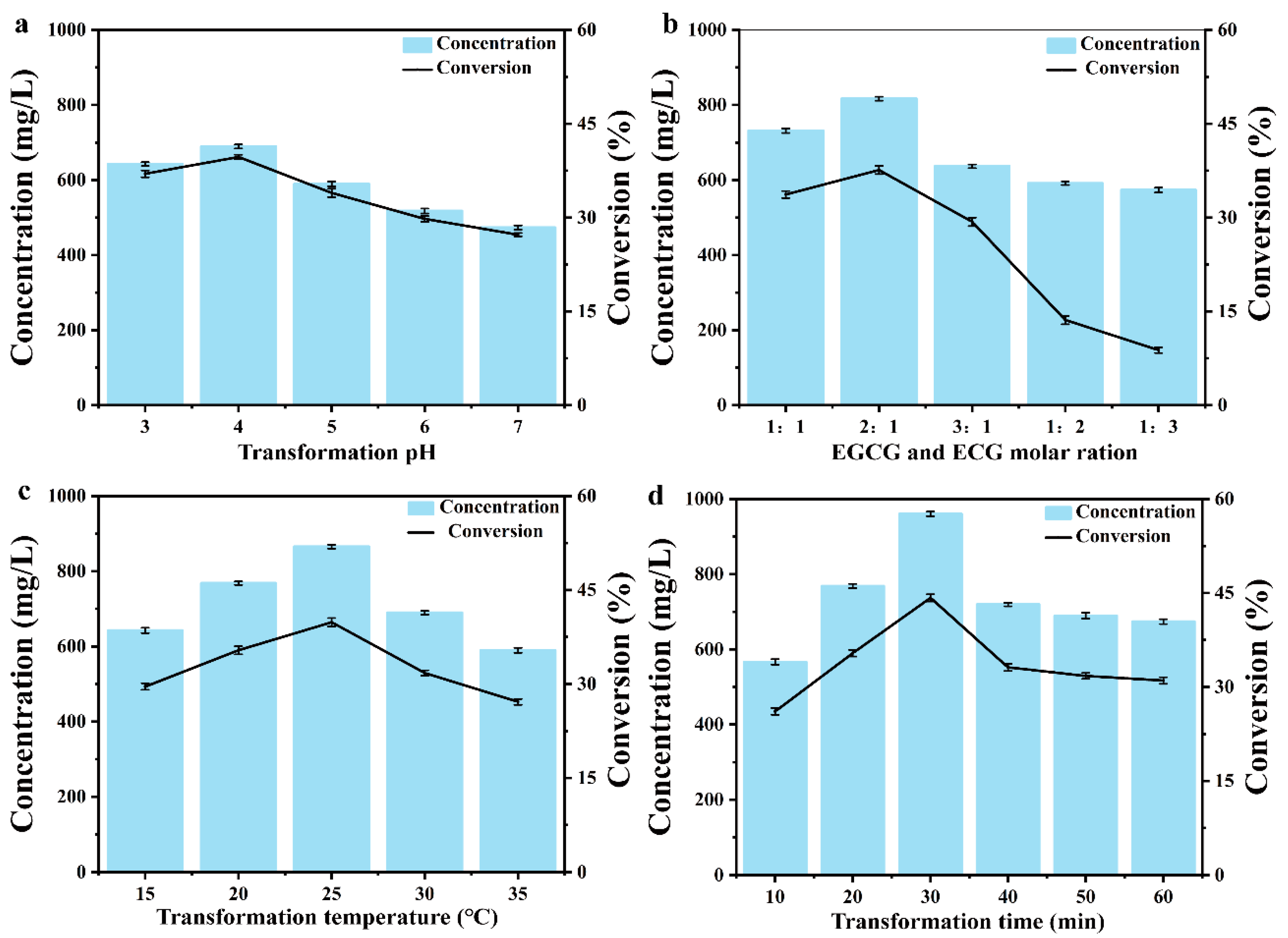
2.4. Optimize the Transformation System to Produce TFDG
3. Discussion and Conclusions
4. Materials and Methods
4.1. Strains, Plasmids, and Chemicals
4.2. Access Codes
4.3. Culture and Purification
4.4. Construction and Screening
4.5. HPLC Analysis
4.6. Protein Crystallization and Structure Determination
4.7. Initial Structural Preparation
4.8. Molecular Docking
4.9. MD Simulations
4.10. Rosetta Design
4.11. Directed Evolution Experiments
4.12. Activity Assay
4.13. Kinetic Assay
4.14. Optimum Reaction Conditions for TFDG Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shi, J.; Yang, G.; You, Q.; Sun, S.; Chen, R.; Lin, Z.; Simal-Gandara, J.; Lv, H. Updates on the chemistry, processing characteristics, and utilization of tea flavonoids in last two decades (2001–2021). Crit. Rev. Food Sci. Nutr. 2021, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Rankin, G.O.; Tu, Y.; Chen, Y.C. Inhibitory effects of the four main theaflavin derivatives found in black tea on ovarian cancer cells. Anticancer Res. 2016, 36, 643–651. [Google Scholar] [PubMed]
- Shi, M.; Lu, Y.; Wu, J.; Zheng, Z.; Lv, C.; Ye, J.; Qin, S.; Zeng, C. Beneficial effects of theaflavins on metabolic syndrome: From molecular evidence to gut microbiome. Int. J. Mol. Sci. 2022, 23, 7595. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ping, Z.; Gan, M.; Tao, Y.; Wang, L.; Shi, J.; Wu, X.; Zhang, W.; Yang, H.; Xu, Y. Theaflavin-3,3′-digallate represses osteoclastogenesis and prevents wear debris-induced osteolysis via suppression of ERK pathway. Acta Biomater. 2017, 48, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhou, R.; Huang, C.; Zhang, R.; Wang, J.; Zhang, Y.; Ding, J.; Li, X.; Zhou, J.; Cen, S. Identification of theaflavin-3,3′-digallate as a novel Zika virus protease inhibitor. Front. Pharmacol. 2020, 11, 514313. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Park, Y.I.; Cha, Y.E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid. Based Complement. Altern. Med. 2020, 2020, 5630838. [Google Scholar] [CrossRef] [PubMed]
- Novak, A.J.; Trauner, D. Complex natural products derived from pyrogallols. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2022; pp. 1–46. [Google Scholar]
- Leung, L.K.; Su, Y.; Chen, R.; Zhang, Z.; Huang, Y.; Chen, Z.-Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J. Nutr. 2001, 131, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Wang, H.; Jiang, Y.; Li, J.; Wang, J.; Yuan, H. Influence of enzyme source and catechins on theaflavins formation during in vitro liquid-state fermentation. LWT Food Sci. Technol. 2021, 139, 110291. [Google Scholar] [CrossRef]
- Xu, P.; Hua, D.; Ma, C. Microbial transformation of propenylbenzenes for natural flavour production. Trends Biotechnol. 2007, 25, 571–576. [Google Scholar] [CrossRef]
- Lei, S.; Xie, M.; Hu, B.; Zhou, L.; Sun, Y.; Saeeduddin, M.; Zhang, H.; Zeng, X. Effective synthesis of theaflavin-3,3′-digallate with epigallocatechin-3-O-gallate and epicatechin gallate as substrates by using immobilized pear polyphenol oxidase. Int. J. Biol. Macromol. 2017, 94, 709–718. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, J.; Cui, D.; Zhao, D.; Li, Y.; Wan, X.; Zhao, J. Transcriptome and Metabolic Profiling Unveiled Roles of Peroxidases in Theaflavin Production in Black Tea Processing and Determination of Tea Processing Suitability. J. Agric. Food Chem. 2020, 68, 3528–3538. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Gong, Z.; Deng, Y.; Chen, L.; Li, Q.; Shao, Y.; Lin, L.; Xiao, W. Purification, characterization and enzymatic synthesis of theaflavins of polyphenol oxidase isozymes from tea leaf (Camellia sinensis). LWT Food Sci. Technol. 2017, 84, 263–270. [Google Scholar] [CrossRef]
- Sharma, A.; Gupta, G.; Ahmad, T.; Mansoor, S.; Kaur, B. Enzyme engineering: Current trends and future perspectives. Food Rev. Int. 2021, 37, 121–154. [Google Scholar] [CrossRef]
- Sanchez-Lengeling, B.; Aspuru-Guzik, A. Inverse molecular design using machine learning: Generative models for matter engineering. Science 2018, 361, 360–365. [Google Scholar] [CrossRef]
- Jiang, L.; Althoff, E.A.; Clemente, F.R.; Doyle, L.; Rothlisberger, D.; Zanghellini, A.; Gallaher, J.L.; Betker, J.L.; Tanaka, F.; Barbas III, C. De novo computational design of retro-aldol enzymes. Science 2008, 319, 1387–1391. [Google Scholar] [CrossRef]
- Polatoğlu, İ.; Karataş, D. Modeling of molecular interaction between catechol and tyrosinase by DFT. J. Mol. Struct. 2020, 1202, 127192. [Google Scholar] [CrossRef]
- Rolff, M.; Schottenheim, J.; Decker, H.; Tuczek, F. Copper–O2 reactivity of tyrosinase models towards external monophenolic substrates: Molecular mechanism and comparison with the enzyme. Chem. Soc. Rev. 2011, 40, 4077–4098. [Google Scholar] [CrossRef]
- Hamann, J.N.; Herzigkeit, B.; Jurgeleit, R.; Tuczek, F. Small-molecule models of tyrosinase: From ligand hydroxylation to catalytic monooxygenation of external substrates. Coord. Chem. Rev. 2017, 334, 54–66. [Google Scholar] [CrossRef]
- Jiang, H.; Lai, W. Monophenolase and catecholase activity of Aspergillus oryzae catechol oxidase: Insights from hybrid QM/MM calculations. Org. Biomol. Chem. 2020, 18, 5192–5202. [Google Scholar] [CrossRef]
- Inoue, T.; Shiota, Y.; Yoshizawa, K. Quantum chemical approach to the mechanism for the biological conversion of tyrosine to dopaquinone. J. Am. Chem. Soc. 2008, 130, 16890–16897. [Google Scholar] [CrossRef]
- Koval, I.A.; Gamez, P.; Belle, C.; Selmeczi, K.; Reedijk, J. Synthetic models of the active site of catechol oxidase: Mechanistic studies. Chem. Soc. Rev. 2006, 35, 814–840. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gertz, E.M.; Agarwala, R.; Schäffer, A.A.; Yu, Y.-K. PSI-BLAST pseudocounts and the minimum description length principle. Nucleic Acids Res. 2009, 37, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Mauracher, S.G.; Molitor, C.; Michael, C.; Kragl, M.; Rizzi, A.; Rompel, A. High level protein-purification allows the unambiguous polypeptide determination of latent isoform PPO4 of mushroom tyrosinase. Phytochemistry 2014, 99, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, C.; Zhao, S.; Liu, Y.; Zhang, S.; Zhao, Q.; Wang, F.; Xu, G.; Huang, J.; Liu, Z. Improved yield of theaflavin-3,3′-digallate from Bacillus megaterium tyrosinase via directed evolution. Food Chem. 2022, 375, 131848. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.P.; Wang, L.P.; Yu, J.; Yue, P.X.; Jiang, X.; Feng, W.Y.; Chenzhou, Y.Q.; Li, X.H. In Studies on optimum conditions of synthesizing theaflavins by using bio-enzyme method. Appl. Mech. Mater. 2012, 138, 929–932. [Google Scholar] [CrossRef]
- Ngure, F.M.; Wanyoko, J.K.; Mahungu, S.M.; Shitandi, A.A. Catechins depletion patterns in relation to theaflavin and thearubigins formation. Food Chem. 2009, 115, 8–14. [Google Scholar] [CrossRef]
- Van der Westhuizen, M.; Steenkamp, L.; Steenkamp, P.; Apostolides, Z. Alternative pathway implicated as an influencing factor in the synthesis of theaflavin. Biocatal. Biotransform 2015, 33, 298–309. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post refinement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef]
- Sendovski, M.; Kanteev, M.; Ben-Yosef, V.S.; Adir, N.; Fishman, A. First structures of an active bacterial tyrosinase reveal copper plasticity. J. Mol. Biol. 2011, 405, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.C.; Myers, J.B.; Folta, T.; Shoja, V.; Heath, L.S.; Onufriev, A. H++: A server for estimating pKas and adding missing hydrogens to macromolecules. Nucleic Acids Res. 2005, 33 (Suppl. S2), W368–W371. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef]
- Jakalian, A.; Bush, B.L.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Jakalian, A.; Jack, D.B.; Bayly, C.I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: II. Parameterization and validation. J. Comput. Chem. 2002, 23, 1623–1641. [Google Scholar] [CrossRef]
- Case, D.A.; Betz, R.M.; Cerutti, D.S.; Cheatham, T.E., III; Darden, T.A.; Duke, R.E.; Giese, T.J.; Gohlke, H.; Götz, A.W.; Homeyer, N.; et al. Amber 16; University of California: San Francisco, CA, USA, 2016. [Google Scholar]
- Berendsen, H.J.; Postma, J.V.; Van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh Ewald: An N⋅log (N) method for Ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Ryckaert, J.-P.; Ciccotti, G.; Berendsen, H.J. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Garcia-Molina, F.; Muñoz, J.L.; Varón, R.; Rodriguez-Lopez, J.N.; García-Cánovas, F.; Tudela, J. A review on spectrophotometric methods for measuring the monophenolase and diphenolase activities of tyrosinase. J. Sci. Food Agric. 2007, 55, 9739–9749. [Google Scholar] [CrossRef] [PubMed]
- Narai-Kanayama, A.; Kawashima, A.; Uchida, Y.; Kawamura, M.; Nakayama, T. Specificity of tyrosinase-catalyzed synthesis of theaflavins. J. Mol. Catal. B Enzym. 2016, 133, S452–S458. [Google Scholar] [CrossRef]

| Enzyme | Substrate | Specific Activity (U mg−1) | Vmax (U min−1 mg−1 ) | Km (mM) | Vmax/Km (U min−1 mg−1 mM−1 ) | E0 (J/mol k) |
|---|---|---|---|---|---|---|
| WT | ECG | 10.93 ± 0.82 | 123.24 ± 1.19 | 59.62 ± 0.91 | 2.07 ± 0.05 | 1.28 × 10−5 |
| EGCG | 15.26 ± 0.78 | 35.26 ± 1.25 | 10.35 ± 0.36 | 3.41 ± 0.03 | 1.25 × 10−5 | |
| Mu1 (R209S) | ECG | 16.57 ± 0.96 | 146.26 ± 1.53 | 43.87 ± 0.12 | 3.33 ± 0.83 | 1.29 × 10−5 |
| EGCG | 28.63 ± 0.96 | 116.59 ± 0.95 | 18.62 ± 0.48 | 6.26 ± 0.65 | 1.29 × 10−5 | |
| Mu2 (V217L) | ECG | 31.23 ± 0.89 | 174.53 ± 1.69 | 28.51 ± 0.48 | 6.12 ± 0.51 | 1.29 × 10−5 |
| EGCG | 26.04 ± 0.55 | 145.89 ± 1.04 | 26.58 ± 0.37 | 5.48 ± 0.46 | 1.29 × 10−5 | |
| Mu3 (V218A) | ECG | 27.66 ± 0.78 | 154.69 ± 1.68 | 36.75 ± 1.27 | 4.20 ± 0.41 | 1.29 × 10−5 |
| EGCG | 33.78 ± 0.78 | 165.62 ± 0.98 | 28.51 ± 0.19 | 6.51 ± 0.63 | 1.29 × 10−5 | |
| Mu4 (V218A/R209S) | ECG | 51.14 ± 1.05 | 474.53 ± 10.56 | 32.51 ± 4.54 | 14.59 ± 0.64 | 1.31 × 10−5 |
| EGCG | 86.58 ± 0.95 | 1265.62 ± 13.32 | 46.58 ± 3.15 | 27.17 ± 0.88 | 1.34 × 10−5 | |
| Mu5 (V218A/V217L) | ECG | 57.58 ± 1.31 | 524.69 ± 9.32 | 33.75 ± 2.56 | 15.54 ± 0.93 | 1.32 × 10−5 |
| EGCG | 73.16 ± 1.45 | 865.62 ± 13.46 | 45.41 ± 4.23 | 19.06 ± 0.67 | 1.33 × 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Gao, C.; Song, W.; Wei, W.; Chen, X.; Gao, C.; Liu, J.; Wu, J.; Liu, L. Improving Theaflavin-3,3′-digallate Production Efficiency Optimization by Transition State Conformation of Polyphenol Oxidase. Molecules 2023, 28, 3831. https://doi.org/10.3390/molecules28093831
Huang Y, Gao C, Song W, Wei W, Chen X, Gao C, Liu J, Wu J, Liu L. Improving Theaflavin-3,3′-digallate Production Efficiency Optimization by Transition State Conformation of Polyphenol Oxidase. Molecules. 2023; 28(9):3831. https://doi.org/10.3390/molecules28093831
Chicago/Turabian StyleHuang, Ying, Changzheng Gao, Wei Song, Wanqing Wei, Xiulai Chen, Cong Gao, Jia Liu, Jing Wu, and Liming Liu. 2023. "Improving Theaflavin-3,3′-digallate Production Efficiency Optimization by Transition State Conformation of Polyphenol Oxidase" Molecules 28, no. 9: 3831. https://doi.org/10.3390/molecules28093831
APA StyleHuang, Y., Gao, C., Song, W., Wei, W., Chen, X., Gao, C., Liu, J., Wu, J., & Liu, L. (2023). Improving Theaflavin-3,3′-digallate Production Efficiency Optimization by Transition State Conformation of Polyphenol Oxidase. Molecules, 28(9), 3831. https://doi.org/10.3390/molecules28093831








