Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study
Abstract
1. Introduction
1.1. Role of the Pellicle at the Tooth Surface
1.2. Olive Oil and Its Perspectives in Preventive Dentistry
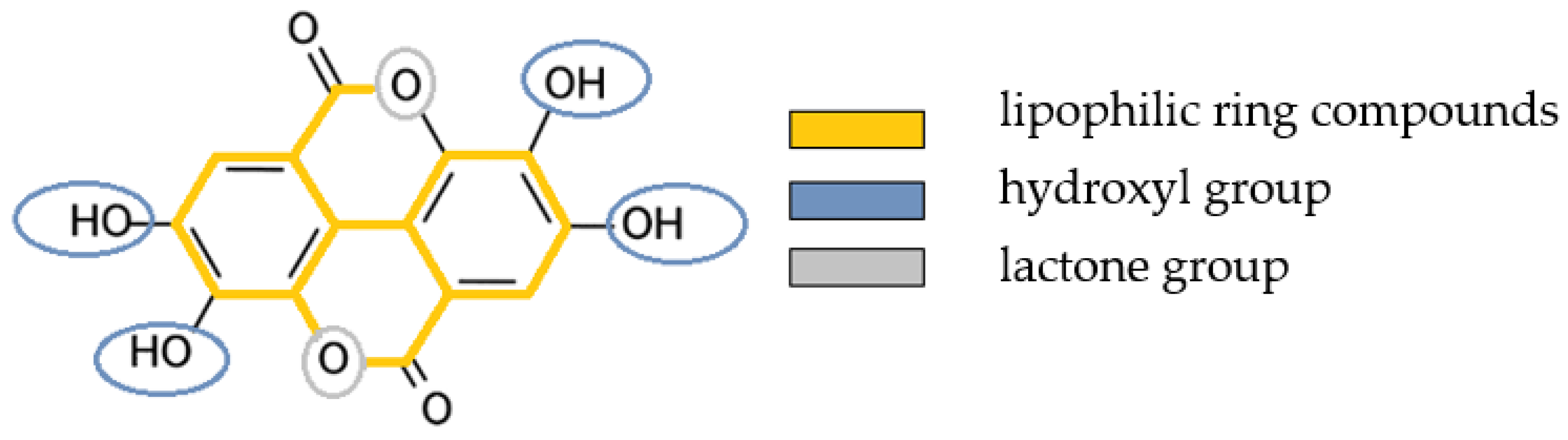
1.3. Olive Oil as a Transport Medium of Bioactive Plant Compounds
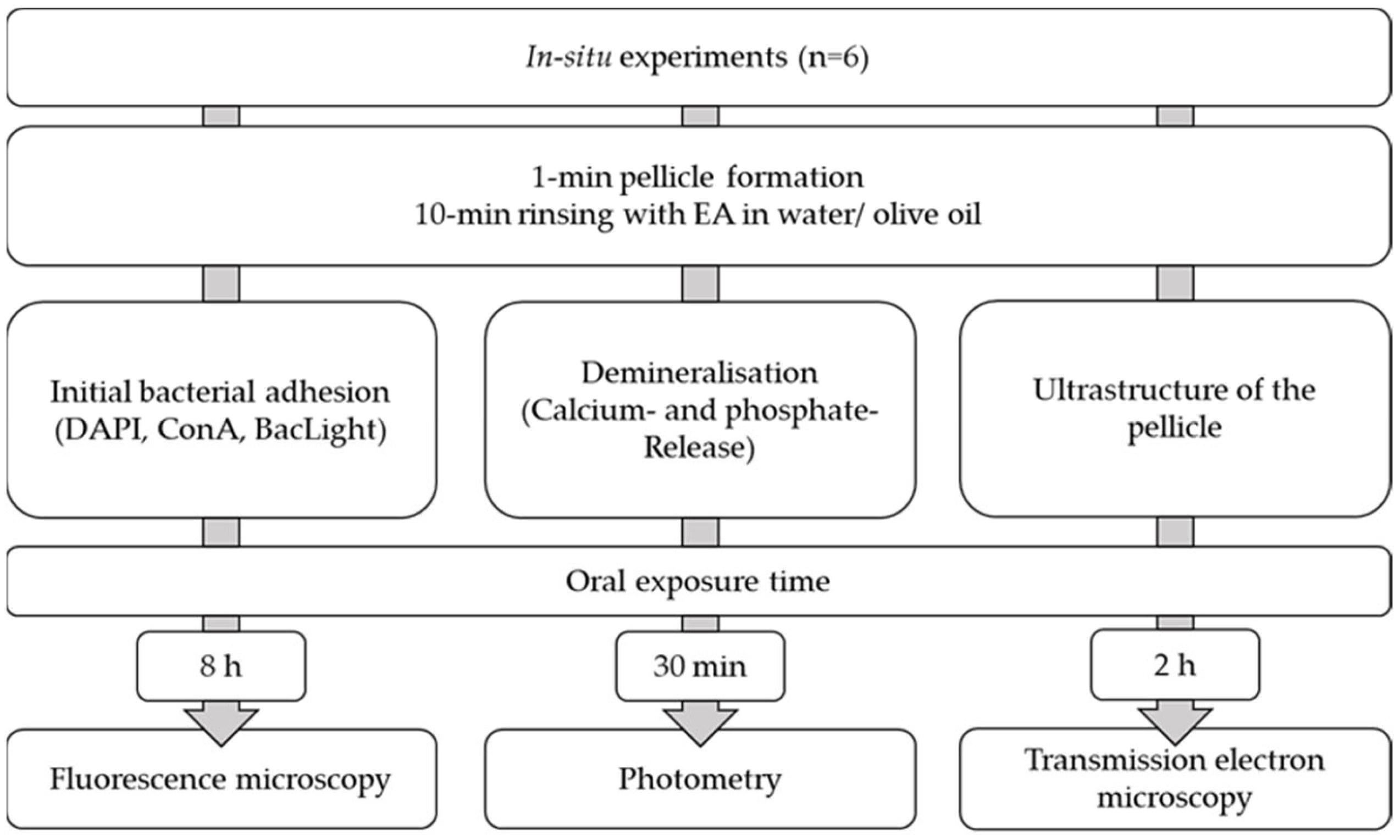
2. Results
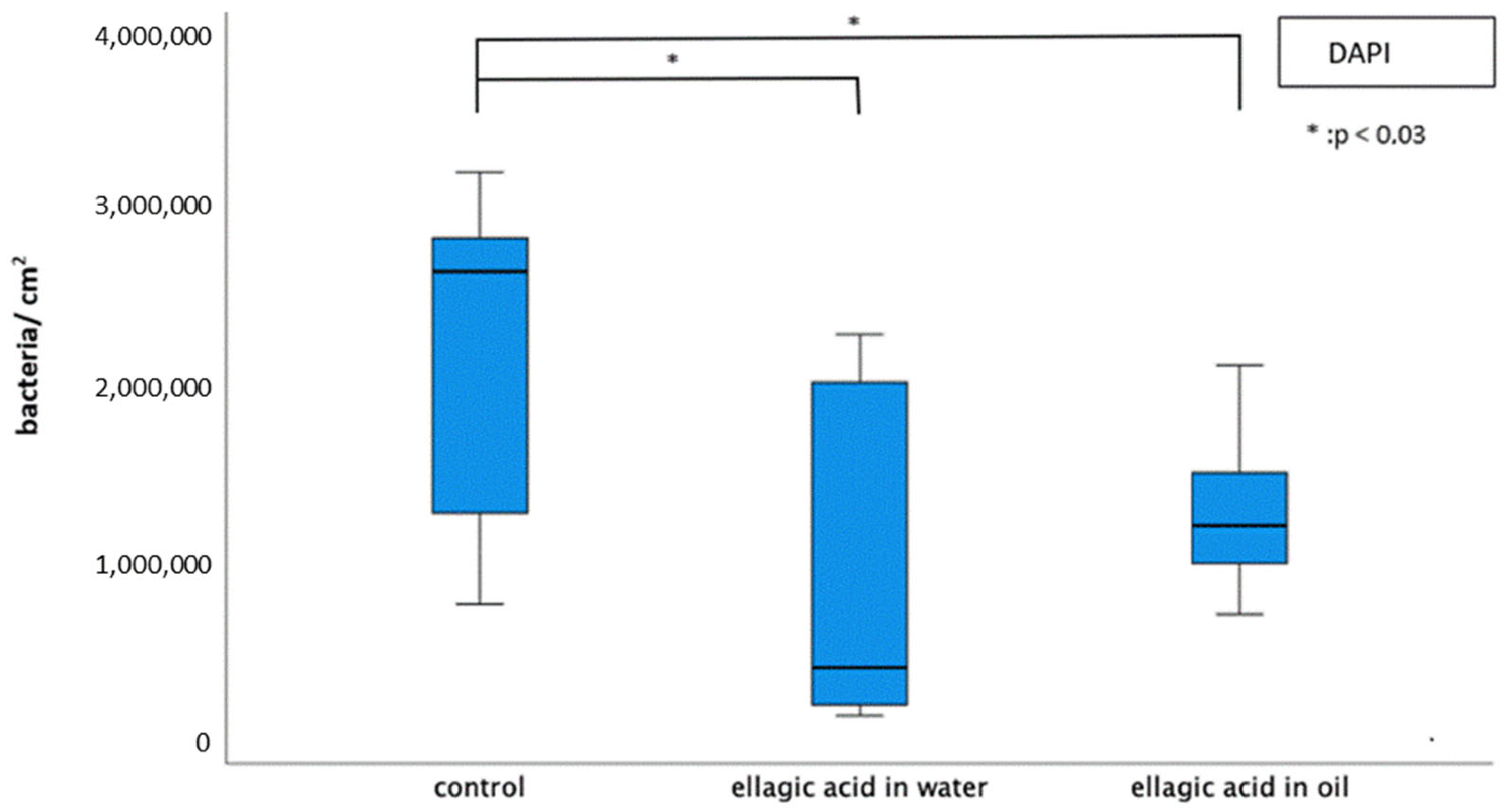
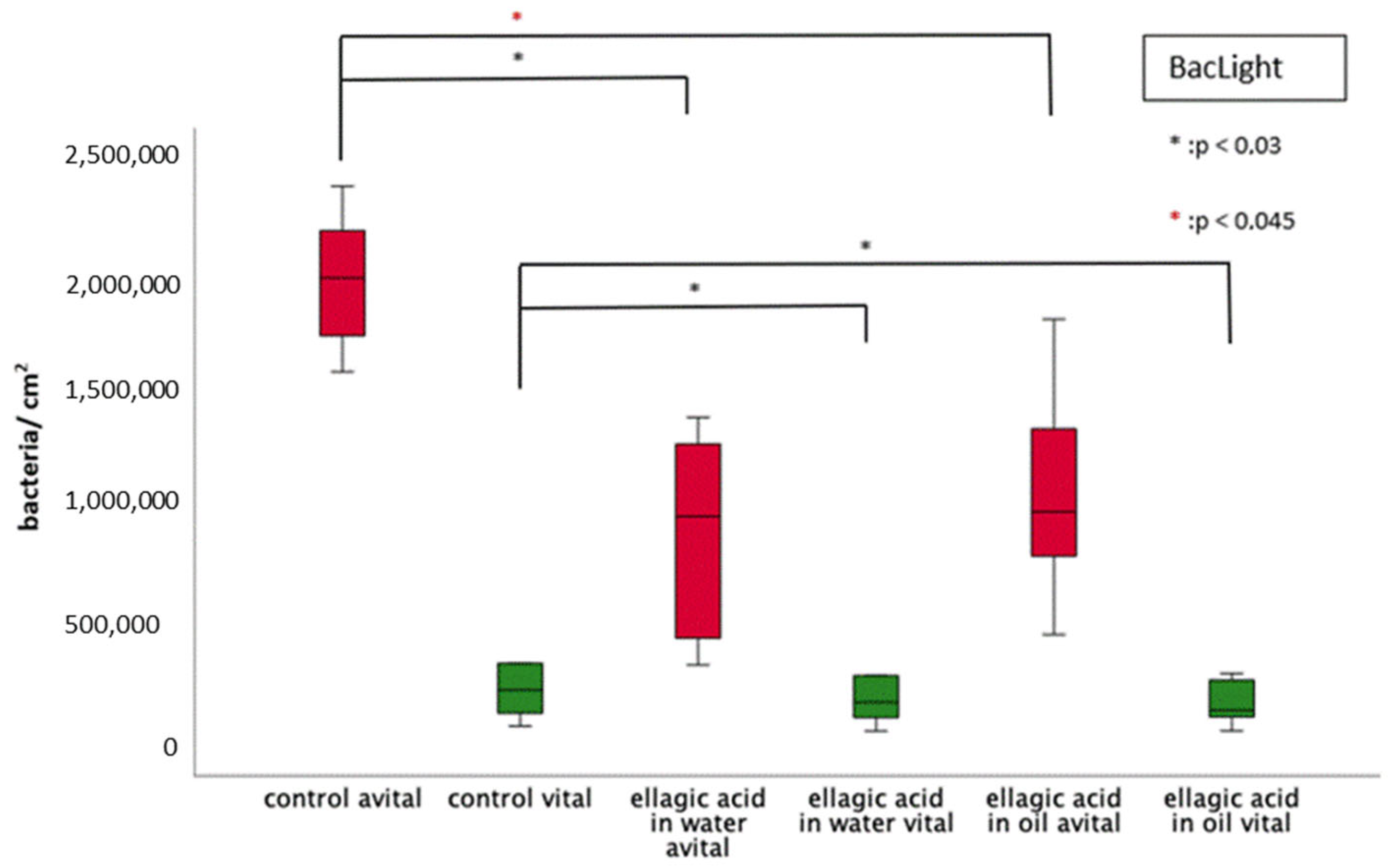
2.1. Initial Bacterial Adhesion
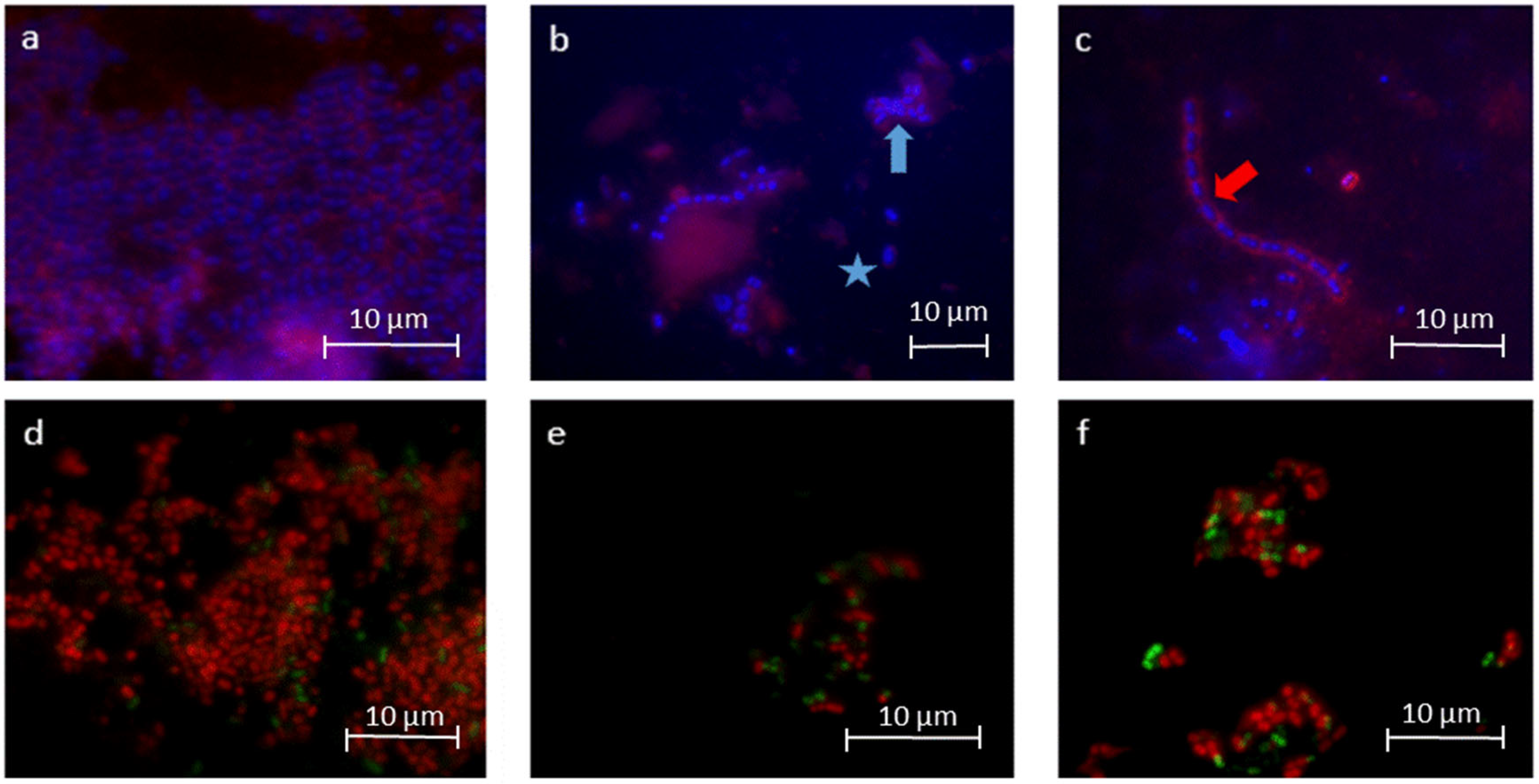
2.1.1. DAPI/Glucan
2.1.2. BacLight
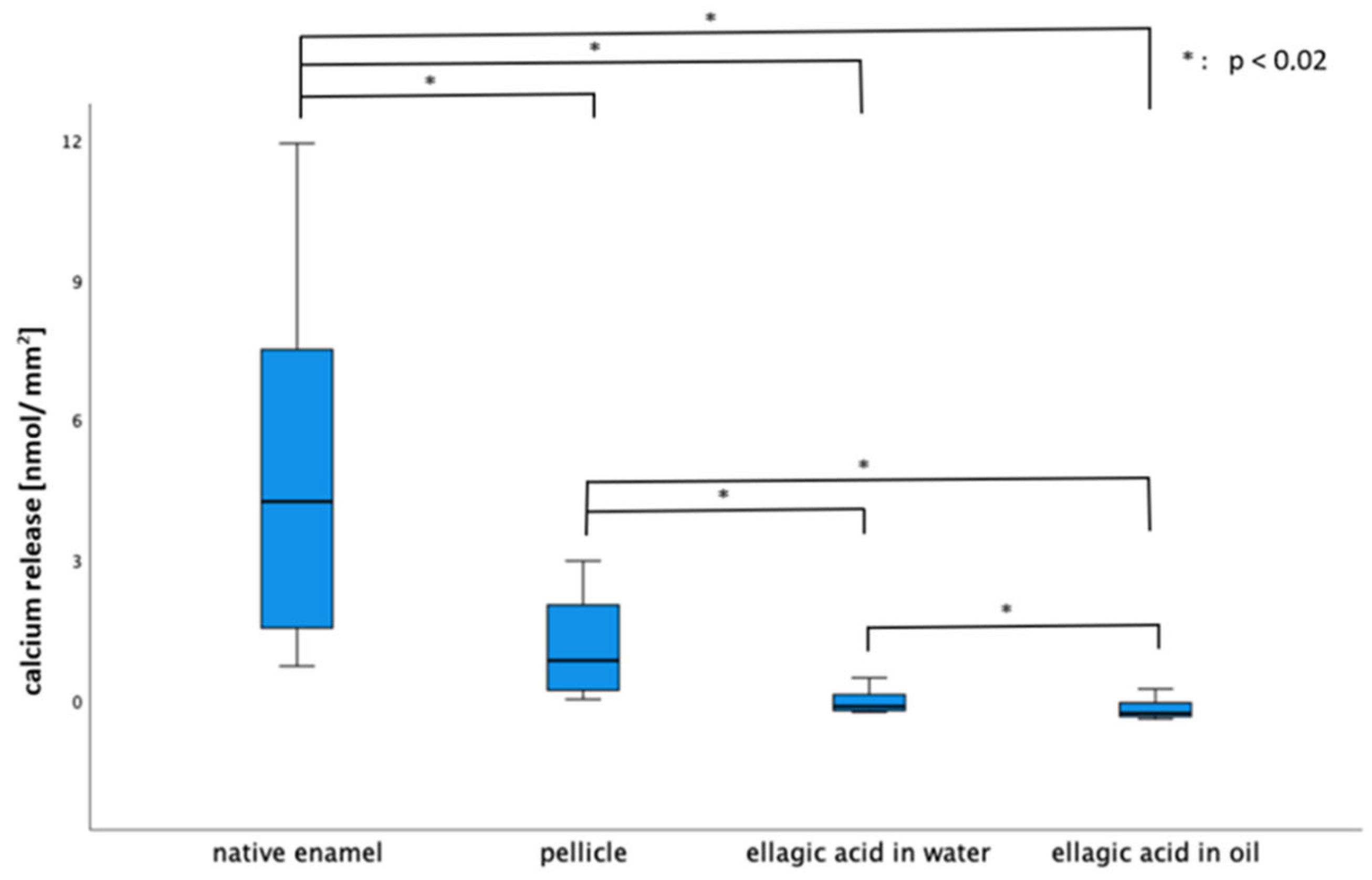
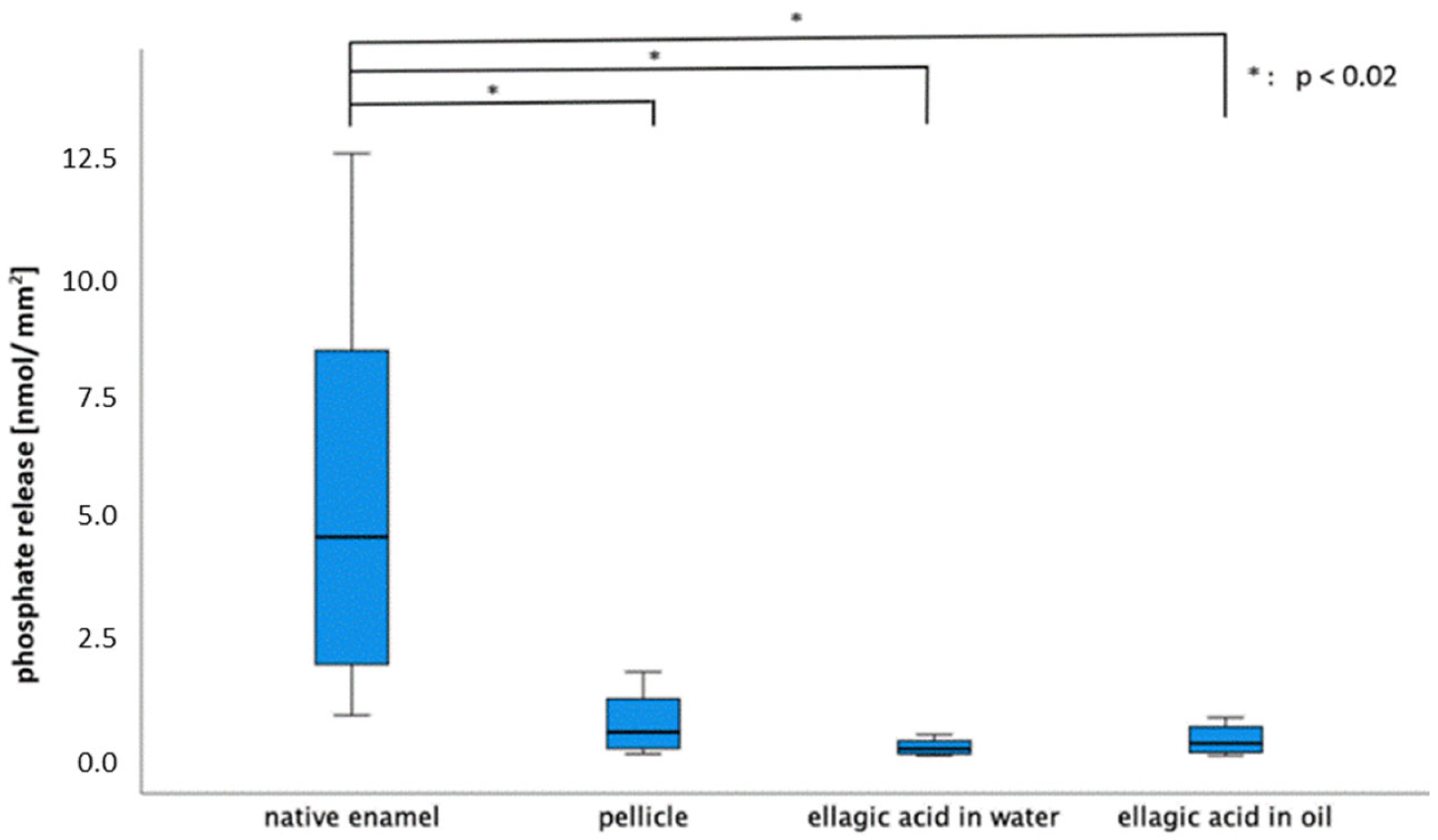
2.2. Calcium and Phosphate Release
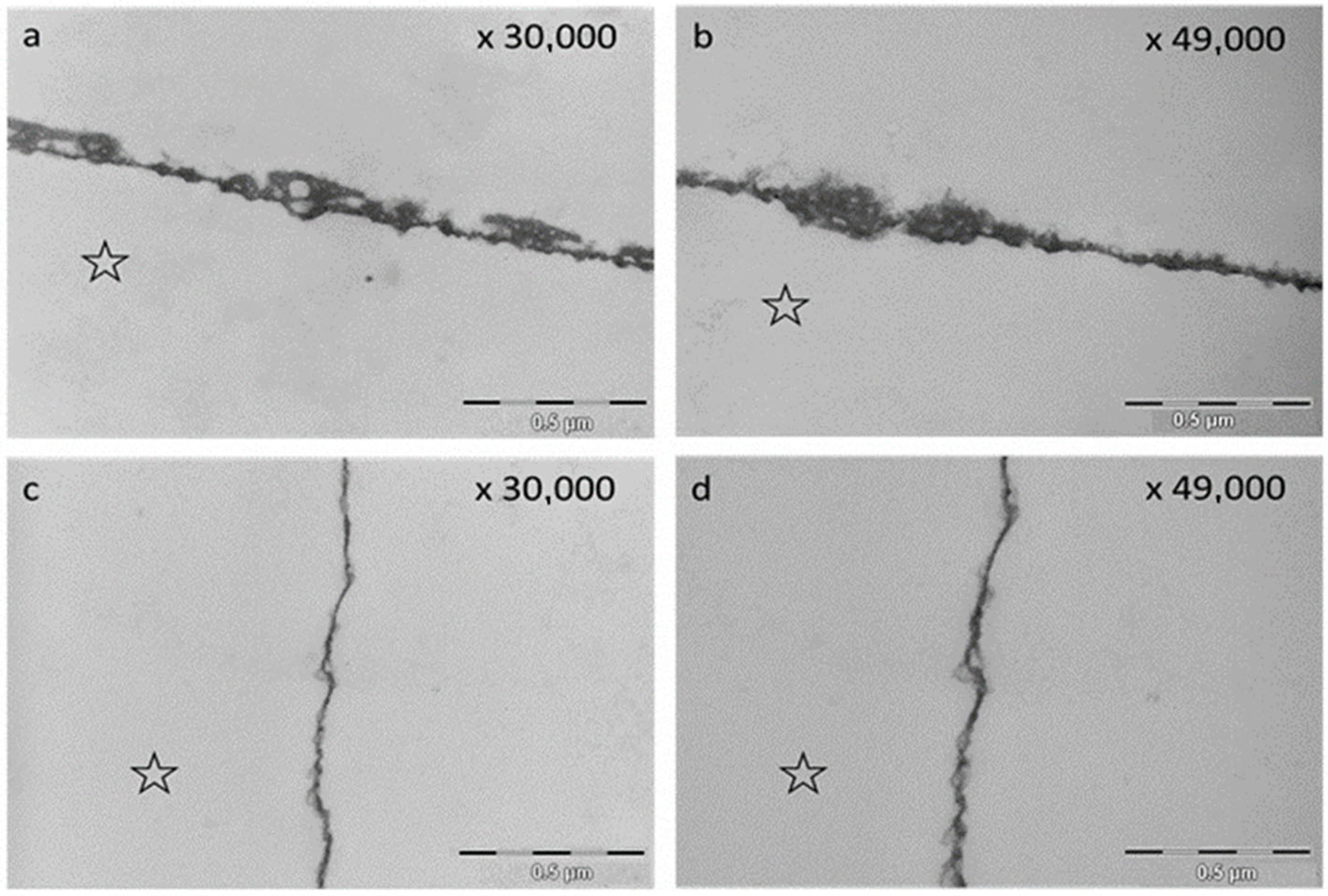
2.3. Transmission Electron Microscopy
3. Discussion
4. Material and Methods
4.1. Subjects
4.2. Specimens
4.3. Preparation of EA in Water/in Oil
4.4. Pellicle Formation
4.5. Initial Bacterial Colonization and Glucan Formation
4.5.1. DAPI/Glucan
- Score 0: no glucans detectable;
- Score 1: single glucan structures exhibiting cloudy formations;
- Score 2: distinct glucan structures exhibiting cloudy structures surrounding the bacteria;
- Score 3: distinct glucan structures surrounding at least 50% of the bacteria;
- Score 4: distinct glucan structures surrounding almost all bacteria.
4.5.2. BacLight
4.6. Calcium and Phosphate Release
4.7. Transmission Electron Microscopy
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride Revolution and Dental Caries: Evolution of Policies for Global Use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Marinho, V.C.C.; Higgins, J.P.T.; Logan, S.; Sheiham, A. Systematic Review of Controlled Trials on the Effectiveness of Fluoride Gels for the Prevention of Dental Caries in Children. J. Dent. Educ. 2003, 67, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Walsh, T.; Worthington, H.V.; Glenny, A.M.; Appelbe, P.; Marinho, V.C.C.; Shi, X. Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst. Rev. 2010, 1, CD007868. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Burke, F.; Allen, P.F. Incidence, Prevalence and Global Distribution of Root Caries. Monogr. Oral Sci. 2017, 26, 1–8. [Google Scholar] [CrossRef]
- Frencken, J. Caries Epidemiology and Its Challenges. Monogr. Oral Sci. 2018, 27, 11–23. [Google Scholar] [CrossRef]
- Wen, P.Y.F.; Chen, M.X.; Zhong, Y.J.; Dong, Q.Q.; Wong, H.M. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2021, 101, 392–399. [Google Scholar] [CrossRef]
- Moore, C.; McLister, C.; Cardwell, C.; O’Neill, C.; Donnelly, M.; McKenna, G. Dental caries following radiotherapy for head and neck cancer: A systematic review. Oral Oncol. 2020, 100, 104484. [Google Scholar] [CrossRef]
- Hannig, M.; Joiner, A. The structure, function and properties of the acquired pellicle. Monogr. Oral Sci. 2006, 19, 29–64. [Google Scholar] [CrossRef]
- Dawes, C.; Pedersen, A.M.; Villa, A.; Ekström, J.; Proctor, G.B.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Kensche, A.; Carpenter, G. The mucosal pellicle—An underestimated factor in oral physiology. Arch. Oral Biol. 2017, 80, 144–152. [Google Scholar] [CrossRef]
- Siqueira, W.L.; Custodio, W.; McDonald, E.E. New Insights into the Composition and Functions of the Acquired Enamel Pellicle. J. Dent. Res. 2012, 91, 1110–1118. [Google Scholar] [CrossRef]
- Flemming, J.; Hannig, C.; Hannig, M. Caries Management—The Role of Surface Interactions in De- and Remineralization-Processes. J. Clin. Med. 2022, 11, 7044. [Google Scholar] [CrossRef]
- Hannig, M.; Hannig, C. The Pellicle and Erosion. In Erosive Tooth Wear: From Diagnosis to Therapy, 2nd ed.; Monogrphs in Oral Science; Karger: Basel, Switzerland, 2014; Volume 25, pp. 206–214. [Google Scholar] [CrossRef]
- Vukosavljevic, D.; Custodio, W.; Buzalaf, M.A.; Hara, A.T.; Siqueira, W.L. Acquired pellicle as a modulator for dental erosion. Arch. Oral Biol. 2014, 59, 631–638. [Google Scholar] [CrossRef]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef]
- Marin, L.M.; Xiao, Y.; Cury, J.A.; Siqueira, W.L. Modulation of Streptococcus mutans Adherence to Hydroxyapatite by Engineered Salivary Peptides. Microorganisms 2022, 10, 223. [Google Scholar] [CrossRef]
- Kirsch, J.; Hannig, M.; Winkel, P.; Basche, S.; Leis, B.; Putz, N.; Kensche, A.; Hannig, C. Influence of pure fluorides and stannous ions on the initial bacterial colonization in situ. Sci. Rep. 2019, 9, 18499. [Google Scholar] [CrossRef]
- Kensche, A.; Buschbeck, E.; König, B.; Koch, M.; Kirsch, J.; Hannig, C.; Hannig, M. Effect of fluoride mouthrinses and stannous ions on the erosion protective properties of the in situ pellicle. Sci. Rep. 2019, 9, 5336. [Google Scholar] [CrossRef]
- Kensche, A.; Kirsch, J.; Mintert, S.; Enders, F.; Pötschke, S.; Basche, S.; König, B.; Hannig, C.; Hannig, M. Impact of customary fluoride rinsing solutions on the pellicle’s protective properties and bioadhesion in situ. Sci. Rep. 2017, 7, 16584. [Google Scholar] [CrossRef]
- Hannig, C.; Gaeding, A.; Basche, S.; Richter, G.; Helbig, R.; Hannig, M. Effect of conventional mouthrinses on initial bioadhesion to enamel and dentin in situ. Caries Res. 2012, 47, 150–161. [Google Scholar] [CrossRef]
- Hertel, S.; Graffy, L.; Pötschke, S.; Basche, S.; Al-Ahmad, A.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Inula viscosa on the pellicle’s protective properties and initial bioadhesion in-situ. Arch. Oral Biol. 2016, 71, 87–96. [Google Scholar] [CrossRef]
- Kirsch, J.; Jung, A.; Hille, K.; König, B.; Hannig, C.; Kölling-Speer, I.; Speer, K.; Hannig, M. Effect of fragaria vesca, hamamelis and tormentil on the initial bacterial colonization in situ. Arch. Oral Biol. 2020, 118, 104853. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.-T.; Hannig, M.; Pötschke, S.; Höhne, F.; Hannig, C. Application of Plant Extracts for the Prevention of Dental Erosion: An In situ/In vitro Study. Caries Res. 2015, 49, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Hannig, C.; Sorg, J.; Spitzmüller, B.; Hannig, M.; Al-Ahmad, A. Polyphenolic beverages reduce initial bacterial adherence to enamel in situ. J. Dent. 2009, 37, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Hertel, S.; Pötschke, S.; Basche, S.; Delius, J.; Hoth-Hannig, W.; Hannig, M.; Hannig, C. Effect of Tannic Acid on the Protective Properties of the in situ Formed Pellicle. Caries Res. 2016, 51, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Cotter, D.; Sud, M.; Subramaniam, S. Lipid classification, structures and tools. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2011, 1811, 637–647. [Google Scholar] [CrossRef]
- Rawat, M.; Singh, D.; Saraf, S. Lipid carriers: A versatile delivery vehicle for proteins and peptides. Yakugaku Zasshi 2008, 128, 269–280. [Google Scholar] [CrossRef]
- Pauwels, E.K.J. The Protective Effect of the Mediterranean Diet: Focus on Cancer and Cardiovascular Risk. Med. Princ. Pr. 2011, 20, 103–111. [Google Scholar] [CrossRef]
- Kabara, J.J.; Swieczkowski, D.M.; Conley, A.J.; Truant, J.P. Fatty Acids and Derivatives as Antimicrobial Agents. Antimicrob. Agents Chemother. 1972, 2, 23–28. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Extra Virgin Olive Oil Polyphenols: Modulation of Cellular Pathways Related to Oxidant Species and Inflammation in Aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef]
- Lassen, B.; Holmberg, K.; Brink, C.; Carlen, A.; Olsson, J. Binding of Salivary Proteins and Oral Bacteria to Hydrophobic and Hydrophilic Surfaces In-Vivo and In-Vitro. Colloid Polym. Sci. 1994, 272, 1143–1150. [Google Scholar] [CrossRef]
- Olsson, J.; van der Heijde, Y.; Holmberg, K. Plaque formation in vivo and bacterial attachment in vitro on permanently hydrophobic and hydrophilic surfaces. Caries Res. 1992, 26, 428–433. [Google Scholar] [CrossRef]
- Hannig, C.; Kirsch, J.; Al-Ahmad, A.; Kensche, A.; Hannig, M.; Kümmerer, K. Do edible oils reduce bacterial colonization of enamel in situ? Clin. Oral Investig. 2012, 17, 649–658. [Google Scholar] [CrossRef]
- Hannig, C.; Wagenschwanz, C.; Pötschke, S.; Kümmerer, K.; Kensche, A.; Hoth-Hannig, W.; Hannig, M. Effect of Safflower Oil on the Protective Properties of the in situ Formed Salivary Pellicle. Caries Res. 2012, 46, 496–506. [Google Scholar] [CrossRef]
- Peckys, D.; De Jonge, N.; Hannig, M. Oil droplet formation on pellicle covered tooth surfaces studied with environmental scanning electron microscopy. J. Microsc. 2019, 274, 158–167. [Google Scholar] [CrossRef]
- Saltveit, M.E. Synthesis and metabolism of phenolic compounds. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Wiley Blackwell: Oxford, UK, 2018; Volume 1–2, pp. 115–123. [Google Scholar]
- Eberhardt, M.V.; Lee, C.Y.; Liu, R.H. Nutrition—Antioxidant activity of fresh apples. Nature 2000, 405, 903–904. [Google Scholar] [CrossRef]
- Zhao, C.-N.; Tang, G.-Y.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Liu, Q.; Mao, Q.-Q.; Shang, A.; Li, H.-B. Phenolic Profiles and Antioxidant Activities of 30 Tea Infusions from Green, Black, Oolong, White, Yellow and Dark Teas. Antioxidants 2019, 8, 215. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 186864. [Google Scholar] [CrossRef]
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Patra, J.K.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2019, 151, 104584. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M. The oral cavity—A key system to understand substratum-dependent bioadhesion on solid surfaces in man. Clin. Oral Investig. 2009, 13, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Schestakow, A.; Hannig, M. Effects of Experimental Agents Containing Tannic Acid or Chitosan on the Bacterial Biofilm Formation in Situ. Biomolecules 2020, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Wittpahl, G.; Kölling-Speer, I.; Basche, S.; Herrmann, E.; Hannig, M.; Speer, K.; Hannig, C. The Polyphenolic Composition of Cistus incanus Herbal Tea and Its Antibacterial and Anti-adherent Activity against Streptococcus mutans. Planta Med. 2015, 81, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Hoth-Hannig, W.; Deng, S.; Jin, X.; Fu, B.; Hannig, M. The effect of polyphenol-containing solutions on in situ biofilm formation on enamel and dentin. J. Dent. 2020, 102, 103482. [Google Scholar] [CrossRef] [PubMed]
- Veeresham, C.; Kishan, B.; Chandra, R.; Karnati, M. Anti-arthritic activity of root bark of Oroxylum indicum (L.) vent against adjuvant-induced arthritis. Pharmacogn. Res. 2013, 5, 121–128. [Google Scholar] [CrossRef]
- Zaveri, M.; Khandhar, A.; Jain, S. Quantification of Baicalein, Chrysin, Biochanin-A and Ellagic Acid in Root Bark of Oroxylum indicum by RPHPLC with UV Detection. Eurasian J. Anal. Chem. 2008, 3, 245–257. [Google Scholar]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Amakura, Y.; Okada, M.; Tsuji, S.; Tonogai, Y. High-performance liquid chromatographic determination with photodiode array detection of ellagic acid in fresh and processed fruits. J. Chromatogr. A 2000, 896, 87–93. [Google Scholar] [CrossRef]
- Häkkinen, S.; Heinonen, M.; Kärenlampi, S.; Mykkänen, H.; Ruuskanen, J.; Törrönen, R. Screening of selected flavonoids and phenolic acids in 19 berries. Food Res. Int. 1999, 32, 345–353. [Google Scholar] [CrossRef]
- Williner, M.R.; Pirovani, M.E.; Güemes, D.R. Ellagic acid content in strawberries of different cultivars and ripening stages. J. Sci. Food Agric. 2003, 83, 842–845. [Google Scholar] [CrossRef]
- Fikry, E.M.; Gad, A.; Eid, A.H.; Arab, H.H. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharmacother. 2018, 110, 878–886. [Google Scholar] [CrossRef]
- Seo, C.S.; Jeong, S.J.; Yoo, S.R.; Lee, N.R.; Shin, H.K. Quantitative Analysis and In vitro Anti-Inflammatory Effects of Gallic Acid, Ellagic Acid, and Quercetin from Radix Sanguisorbae. Pharmacogn. Mag. 2016, 12, 104–108. [Google Scholar] [CrossRef]
- Baek, B.; Lee, S.H.; Kim, K.; Lim, H.-W.; Lim, C.-J. Ellagic acid plays a protective role against UV-B-induced oxidative stress by up-regulating antioxidant components in human dermal fibroblasts. Korean J. Physiol. Pharmacol. 2016, 20, 269–277. [Google Scholar] [CrossRef]
- Karimi, A.; Moradi, M.-T.; Rabiei, M.; Alidadi, S. In vitro anti-adenoviral activities of ethanol extract, fractions, and main phenolic compounds of pomegranate (Punica granatum L.) peel. Antivir. Chem. Chemother. 2020, 28, 1–6. [Google Scholar] [CrossRef]
- Park, S.W.; Kwon, M.J.; Yoo, J.Y.; Choi, H.-J.; Ahn, Y.-J. Antiviral activity and possible mode of action of ellagic acid identified in Lagerstroemia speciosa leaves toward human rhinoviruses. BMC Complement. Altern. Med. 2014, 14, 171. [Google Scholar] [CrossRef]
- Sampaio, A.D.G.; Gontijo, A.V.L.; Araujo, H.M.; Koga-Ito, C.Y. In Vivo Efficacy of Ellagic Acid against Candida albicans in a Drosophila melanogaster Infection Model. Antimicrob. Agents Chemother. 2018, 62, 16–18. [Google Scholar] [CrossRef]
- Loo, W.T.Y.; Jin, L.J.; Cheung, M.N.B.; Chow, L.W.C. Evaluation of Ellagic acid on the activities of oral bacteria with the use of adenosine triphosphate (ATP) bioluminescence assay. Afr. J. Biotechnol. 2010, 9, 3938–3943. [Google Scholar]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006, 40, 206–210. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Tegos, G.; Stermitz, F.R.; Lomovskaya, O.; Lewis, K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob. Agents Chemother. 2002, 46, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Hirt, H.M.; M’pia, B.; Lindsey, K. Natural Medicine in the Tropics; Anamed International e.V.: Winnenden, Germany, 2008. [Google Scholar]
- Avachat, A.M.; Patel, V.G. Self nanoemulsifying drug delivery system of stabilized ellagic acid–phospholipid complex with improved dissolution and permeability. Saudi Pharm. J. 2014, 23, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Kensche, A.; Reich, M.; Kümmerer, K.; Hannig, M.; Hannig, C. Lipids in preventive dentistry. Clin. Oral Investig. 2012, 17, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Kensche, A.; Dürasch, A.; König, B.; Henle, T.; Hannig, C.; Hannig, M. Characterization of the in situ pellicle ultrastructure formed under the influence of bovine milk and milk protein isolates. Arch. Oral Biol. 2019, 104, 133–140. [Google Scholar] [CrossRef]
- Kensche, A.; Reich, M.; Hannig, C.; Kümmerer, K.; Hannig, M. Modification of the Lipid Profile of the Initial Oral Biofilm In Situ Using Linseed Oil as Mouthwash. Nutrients 2021, 13, 989. [Google Scholar] [CrossRef]
- Hannig, M.; Hess, N.J.; Hoth-Hannig, W.; De Vrese, M. Influence of salivary pellicle formation time on enamel demineralization—An in situ pilot study. Clin. Oral Investig. 2003, 7, 158–161. [Google Scholar] [CrossRef]
- Flemming, J.; Meyer-Probst, C.T.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Preventive Applications of Polyphenols in Dentistry—A Review. Int. J. Mol. Sci. 2021, 22, 4892. [Google Scholar] [CrossRef]
- Nayak, A.; Carpenter, G.H. A physiological model of tea-induced astringency. Physiol. Behav. 2008, 95, 290–294. [Google Scholar] [CrossRef]
- Joiner, A.; Muller, D.; Elofsson, U.M.; Arnebrant, T. Ellipsometry analysis of the in vitro adsorption of tea polyphenols onto salivary pellicles. Eur. J. Oral Sci. 2004, 112, 510–515. [Google Scholar] [CrossRef]
- de Souza, E.S.C.M.; da Silva Ventura, T.M.; de Pau, L.; la Silva, C.; de Lima Leite, A.; Buzalaf, M.A.R. Effect of gels containing chlorhexidine or epigallocatechin-3-gallate on the protein composition of the acquired enamel pellicle. Arch. Oral Biol. 2017, 82, 92–98. [Google Scholar] [CrossRef]
- Kandra, L.; Gyémánt, G.; Zajácz, A.; Batta, G. Inhibitory effects of tannin on human salivary alpha-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef]
- Jean-Gilles, D.; Li, L.Y.; Vaidyanathan, V.G.; King, R.; Cho, B.; Worthen, D.R.; Chichester, C.O.; Seeram, N.P. Inhibitory effects of polyphenol punicalagin on type-II collagen degradation in vitro and inflammation in vivo. Chem. Interact. 2013, 205, 90–99. [Google Scholar] [CrossRef]
- Yanagida, A.; Kanda, T.; Tanabe, M.; Matsudaira, F.; Cordeiro, J.G.O. Inhibitory effects of apple polyphenols and related compounds on cariogenic factors of mutans streptococci. J. Agric. Food Chem. 2000, 48, 5666–5671. [Google Scholar] [CrossRef]
- Lei, M.; Jiang, F.C.; Cai, J.; Hu, S.; Zhou, R.; Liu, G.; Wang, Y.H.; Wang, H.B.; He, J.R.; Xiong, X.G. Facile microencapsulation of olive oil in porous starch granules: Fabrication, characterization, and oxidative stability. Int. J. Biol. Macromol. 2018, 111, 755–761. [Google Scholar] [CrossRef]
- Rehage, M.; Delius, J.; Hofmann, T.; Hannig, M. Oral astringent stimuli alter the enamel pellicle’s ultrastructure as revealed by electron microscopy. J. Dent. 2017, 63, 21–29. [Google Scholar] [CrossRef]
- Buchalla, W.; Attin, T.; Roth, P.; Hellwig, E. Influence of olive oil emulsions on dentin demineralization in vitro. Caries Res. 2003, 37, 100–107. [Google Scholar] [CrossRef]
- Wiegand, A.; Gutsche, M.; Attin, T. Effect of olive oil and an olive-oil-containing fluoridated mouthrinse on enamel and dentin erosion in vitro. Acta Odontol. Scand. 2007, 65, 357–361. [Google Scholar] [CrossRef]
- Hannig, C.; Hannig, M.; Rehmer, O.; Braun, G.; Hellwig, E.; Al-Ahmad, A. Fluorescence microscopic visualization and quantification of initial bacterial colonization on enamel in situ. Arch. Oral Biol. 2007, 52, 1048–1056. [Google Scholar] [CrossRef]
- Hannig, C.; Spitzmüller, B.; Hannig, M. Characterisation of lysozyme activity in the in situ pellicle using a fluorimetric assay. Clin. Oral Investig. 2008, 13, 15–21. [Google Scholar] [CrossRef]
- Kensche, A.; Basche, S.; Bowen, W.; Hannig, M.; Hannig, C. Fluorescence microscopic visualization of non cellular components during initial bioadhesion in situ. Arch. Oral Biol. 2013, 58, 1271–1281. [Google Scholar] [CrossRef]
- Attin, T.; Becker, K.; Hannig, C.; Buchalla, W.; Hilgers, R. Method to detect minimal amounts of calcium dissolved in acidic solutions. Caries Res. 2005, 39, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Becker, K.; Hannig, C.; Buchalla, W.; Wiegand, A. Suitability of a malachite green procedure to detect minimal amounts of phosphate dissolved in acidic solutions. Clin. Oral Investig. 2005, 9, 203–207. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flemming, J.; Meyer-Probst, C.T.; Hille, K.; Basche, S.; Speer, K.; Kölling-Speer, I.; Hannig, C.; Hannig, M. Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study. Molecules 2023, 28, 3803. https://doi.org/10.3390/molecules28093803
Flemming J, Meyer-Probst CT, Hille K, Basche S, Speer K, Kölling-Speer I, Hannig C, Hannig M. Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study. Molecules. 2023; 28(9):3803. https://doi.org/10.3390/molecules28093803
Chicago/Turabian StyleFlemming, Jasmin, Clara Theres Meyer-Probst, Kristin Hille, Sabine Basche, Karl Speer, Isabelle Kölling-Speer, Christian Hannig, and Matthias Hannig. 2023. "Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study" Molecules 28, no. 9: 3803. https://doi.org/10.3390/molecules28093803
APA StyleFlemming, J., Meyer-Probst, C. T., Hille, K., Basche, S., Speer, K., Kölling-Speer, I., Hannig, C., & Hannig, M. (2023). Olive Oil as a Transport Medium for Bioactive Molecules of Plants?—An In Situ Study. Molecules, 28(9), 3803. https://doi.org/10.3390/molecules28093803





