Synthesis, Quality Control and Preliminary Activity Evaluation of a New Compound HM475
Abstract
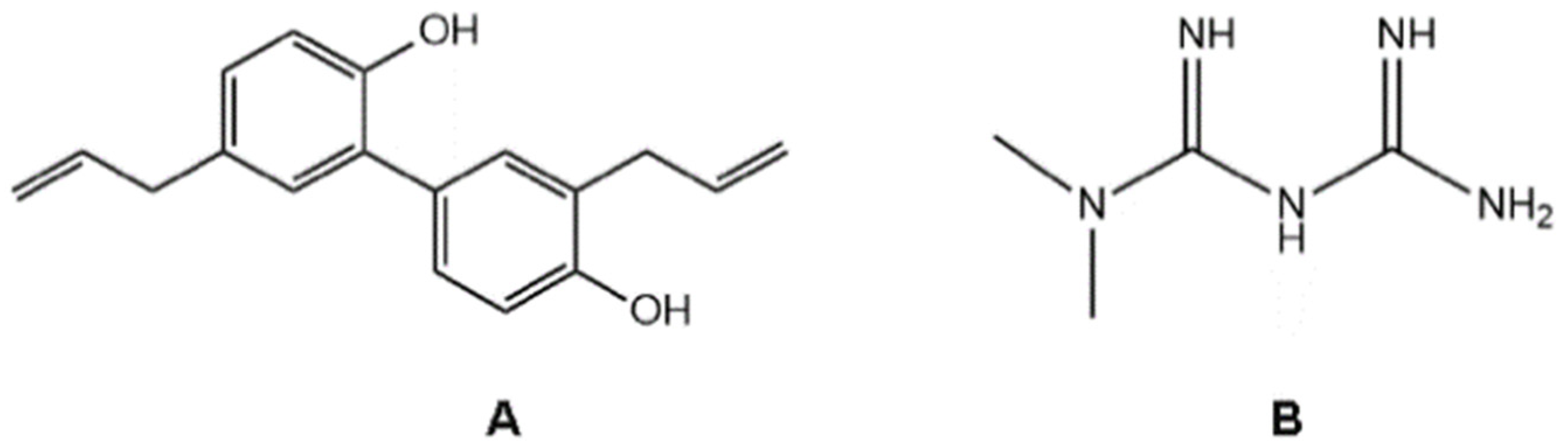
1. Introduction
2. Results and Discussion
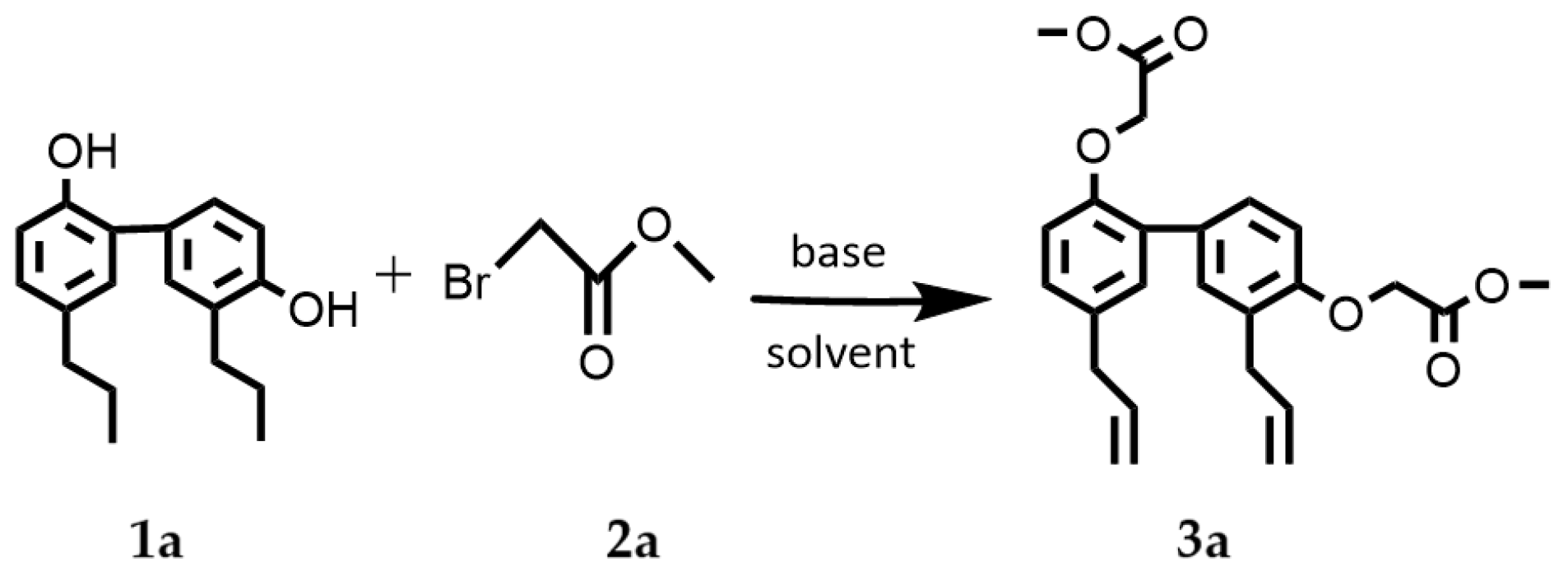
2.1. Synthesis of 3a
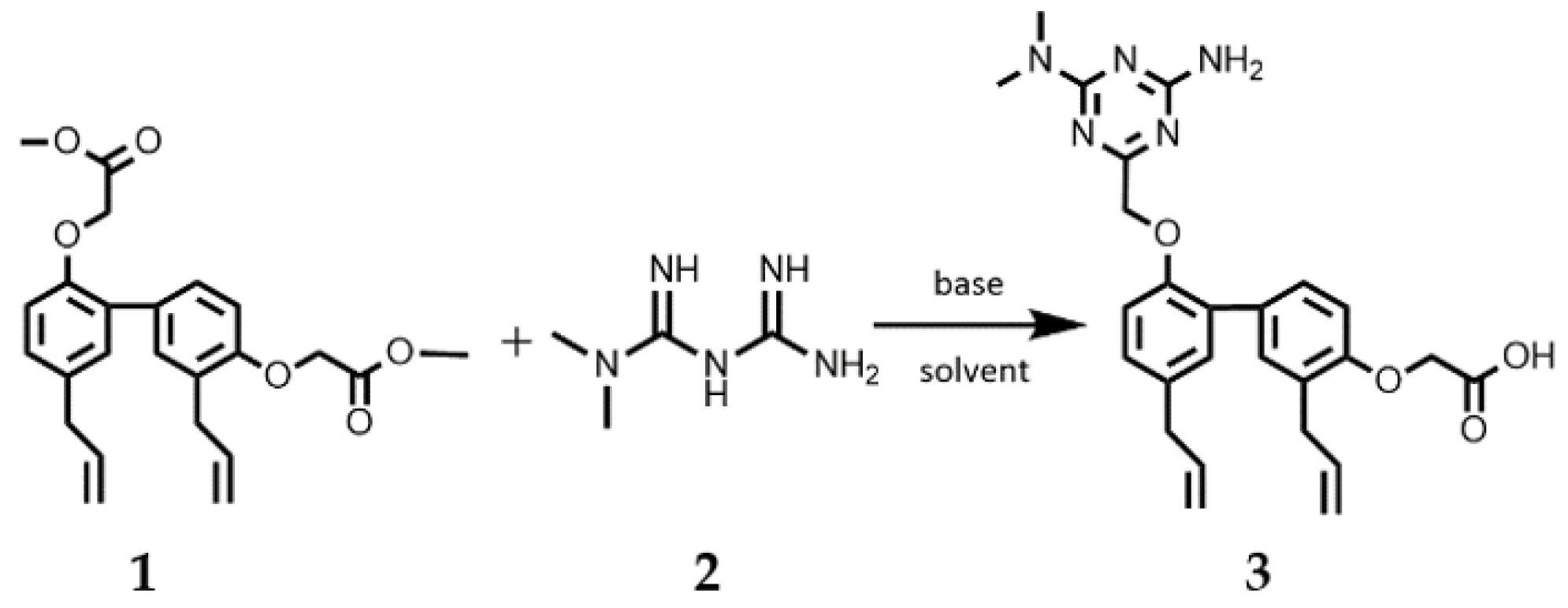
2.2. Synthesis of Compound 3 (HM475)
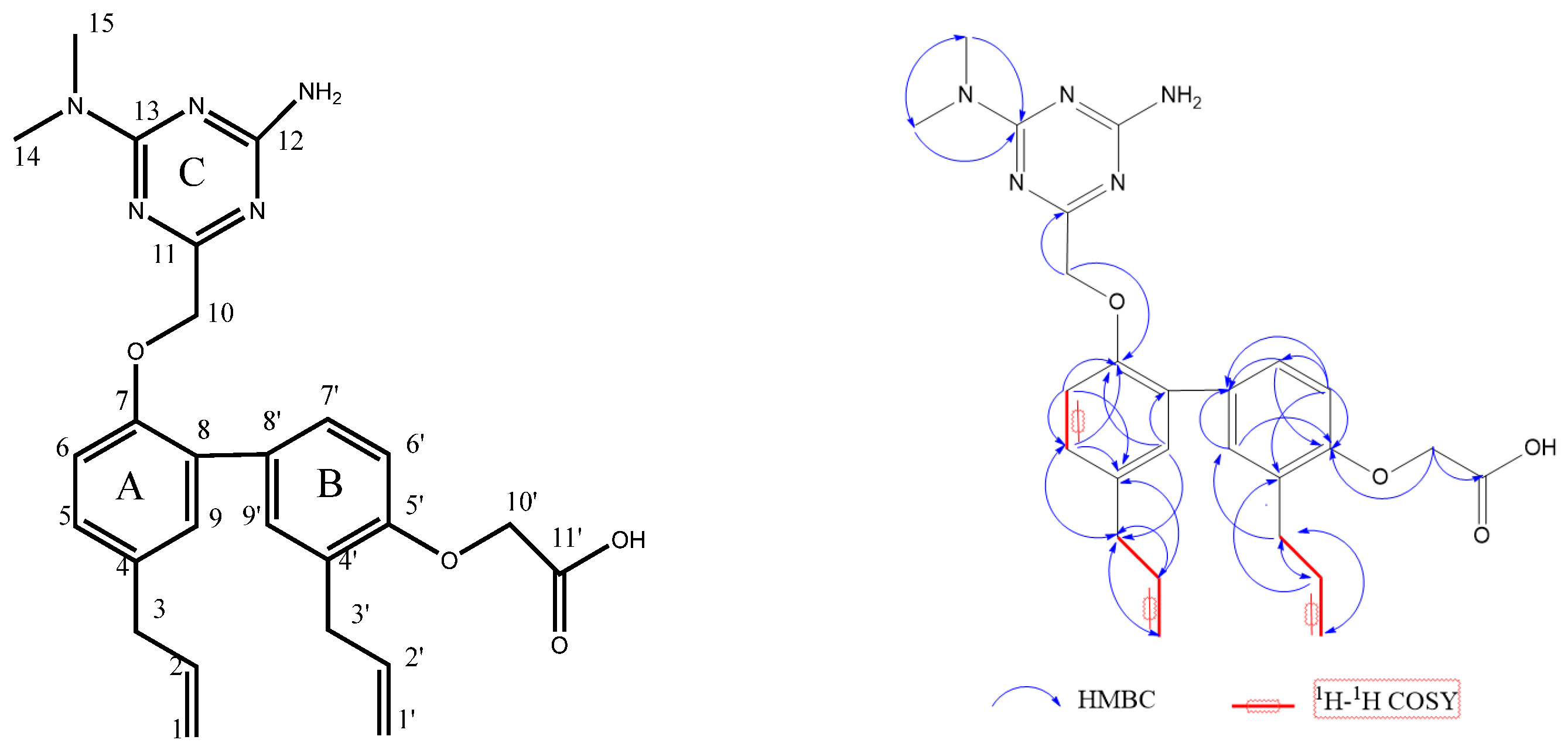
2.3. Characterization of Compound HM475
2.4. Studies of Quality Control
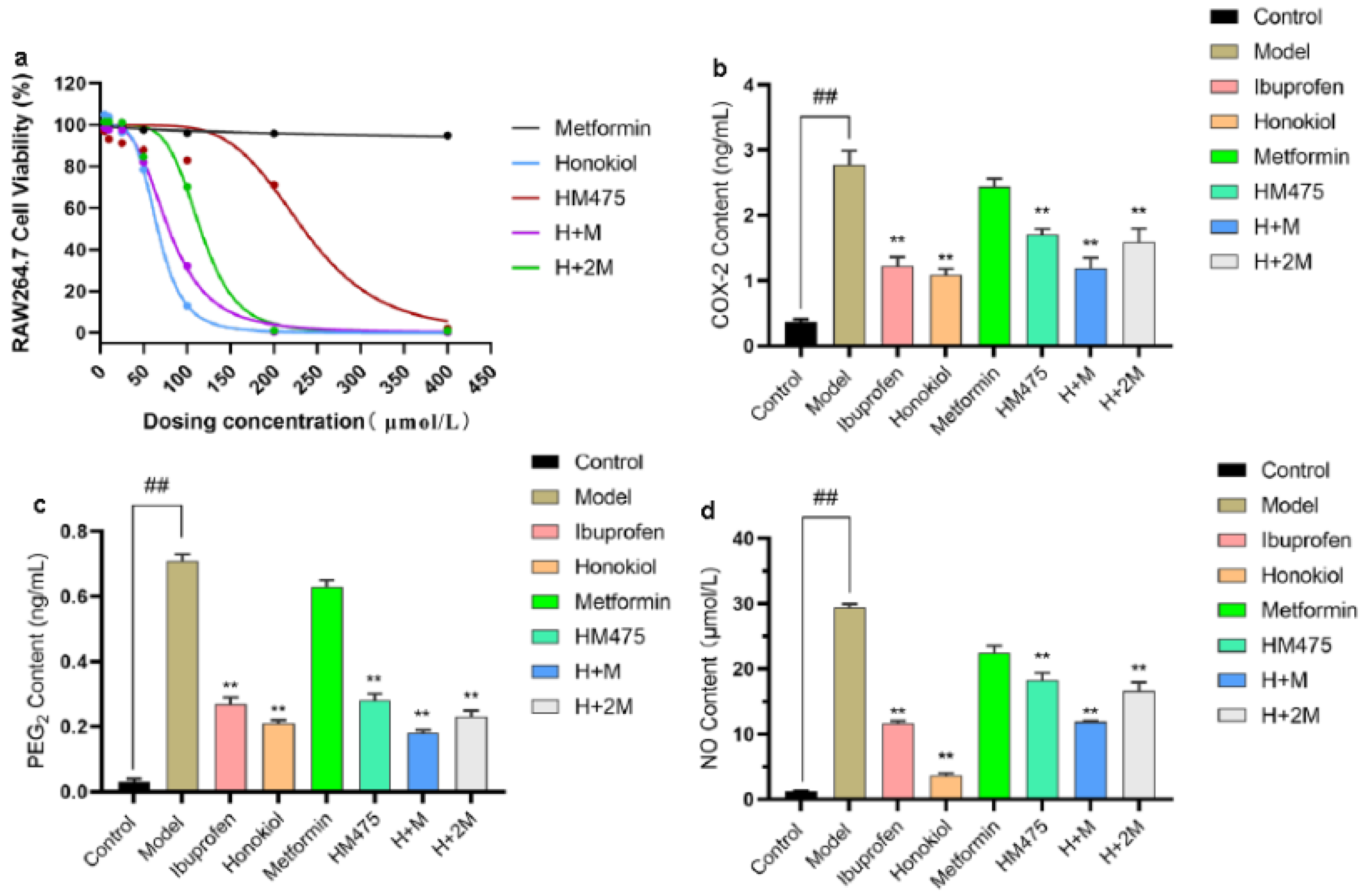
2.5. Evaluation of Anti-Inflammatory Activity of HM475 In Vitro
2.6. Study on the Activity of HM475 on PC-12 and BV-2 Cells
2.7. Evaluation of Anti-Insulin Resistance and Anti-Cancer Activity of HM475 In Vitro
3. Experimental Section
3.1. Synthetic Procedure for HM475
3.2. Crystal Structure Determination and Refinement of HM475
3.3. Determination of Physical and Chemical Properties, Impurity Inspection and Content Determination
3.4. Cell Culture and Determination of Cell Viability
3.5. Evaluation of Anti-Inflammatory, Insulin Resistance (IR), Neuroprotective and Anti-Breast Cancer Activities
3.6. General Apparatus and Chemicals
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability Statement
References
- Chen, C.; Zhang, Q.; Ye, Y. Honokiol: A naturally occurring lignan with pleiotropic bioactivities. Chin. J. Nat. Med. 2021, 19, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Chen, T.; Tseng, Y. Honokiol induces autophagic cell death in malignant glioma through reactive oxygen species-mediated regulation of the p53/PI3K/Akt/mTOR signaling pathway. Toxicol. Appl. Pharmacol. 2016, 304, 59–69. [Google Scholar] [CrossRef]
- Chiang, J.; Shen, Y.C.; Wang, Y.H.; Hou, Y.C.; Chen, C.C.; Liao, J.F.; Yu, M.C.; Juan, C.W.; Liou, K.T. Honokiol protects rats against eccentric exercise-induced skeletal muscle damage by inhibiting NF-κB induced oxidative stress and inflammation. Eur. J. Pharmacol. 2009, 610, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Chen, H.H.; Ko, C.H.; Chan, M.H. Neuroprotective activity of honokiol and magnolol in cerebellar granule cell damage. Eur. J. Pharmacol. 2006, 537, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Lin, H.; Liu, H.; Lu, Z.; Wang, H.; Fan, S.; Li, N. Honokiol Attenuates Sepsis-Associated Acute Kidney Injury via the Inhibition of Oxidative Stress and Inflammation. Inflammation 2019, 42, 826–834. [Google Scholar] [CrossRef]
- Tsai, S.; Huang, C.; Huang, S. Antiarrhythmic Effect of Magnolol and Honokiol during Acute Phase of Coronary Occlusion in Anesthetized Rats: Influence of L-NAME and Aspirin. Pharmacology 1999, 59, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preusse-Prange, A.; Wilms, H.; Lucius, R. Anti-inflammatory properties of Honokiol in activated primary microglia and astrocytes. J. Neuroimmunol. 2018, 323, 78–86. [Google Scholar] [CrossRef]
- Wang, D.; Dong, X.; Wang, C. Honokiol Ameliorates Amyloidosis and Neuroinflammation and Improves Cognitive Impairment in Alzheimer’s Disease Transgenic Mice. J. Pharmacol. Exp. Ther. 2018, 366, 470–478. [Google Scholar] [CrossRef]
- Chen, H.-H.; Chang, P.-C.; Chen, C.; Chan, M.-H. Protective and therapeutic activity of honokiol in reversing motor deficits and neuronal degeneration in the mouse model of Parkinson’s disease. Pharmacol. Rep. 2018, 70, 668–676. [Google Scholar] [CrossRef]
- Munroe, M.E.; Arbiser, J.L.; Bishop, G.A. Honokiol, a natural plant product, inhibits inflammatory signals and alleviates inflammatory arthritis. J. Immunol. 2007, 179, 753–763. [Google Scholar] [CrossRef]
- Chao, L.K.; Liao, P.-C.; Ho, C.-L.; Wang, E.I.-C.; Chuang, C.-C.; Chiu, H.-W.; Hung, L.-B.; Hua, K.-F. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase C, mitogen-activated protein kinase, and the NF-κB pathway to reduce LPS-induced TNFα and NO expression. J. Agric. Food Chem. 2010, 58, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Dasari, R.; Bonsack, F.; Sukumari-Ramesh, S. Brain injury and repair after intracerebral hemorrhage: The role of microglia and brain-infiltrating macrophages. Neurochem. Int. 2021, 142, 104923. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, P.P.; Hu, K.L.; Li, L.N.; Yu, X.; Lu, Y.; Chang, H.S. Antidepressant-Like Effect and Mechanism of Action of Honokiol on the Mouse Lipopolysaccharide (LPS) Depression Model. Molecules 2019, 24, 2035. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-G.; Qiu, W.-Q.; Yu, L.; Pan, R.; Teng, J.-F.; Sang, Z.-P.; Law, B.Y.-K.; Zhao, Y.; Zhang, L.; Yan, L.; et al. Targeting microglial autophagic degradation of the NLRP3 inflammasome for identification of thonningianin A in Alzheimer’s disease. Inflamm. Regen. 2022, 42, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wu, T.; Li, H.; Li, X.; Li, Q.; Zhu, X.; Yu, M.; Kuo, S.-H.; Huang, F.; Wu, Y.-C. Dl-3-n-butylphthalide exerts dopaminergic neuroprotection through inhibition of neuroinflammation. Front. Aging Neurosci. 2019, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Rui, W.; Li, S.; Xiao, H.; Xiao, M.; Shi, J. Baicalein attenuates neuroinflammation by inhibiting NLRP3/caspase-1/GSDMD pathway in MPTP-induced mice model of Parkinson’s disease. Int. J. Neuropsychopharmacol. 2020, 23, 762–773. [Google Scholar] [CrossRef]
- Sarrica, A.; Kirika, N.; Romeo, M.; Salmona, M.; Diomede, L.J. Safety and toxicology of magnolol and honokiol. Planta Med. 2018, 84, 1151–1164. [Google Scholar] [CrossRef]
- Bonnet, F.; Scheen, A. Understanding and overcoming metformin gastrointestinal intolerance. Diabetes Obes. Metab. 2017, 19, 473–481. [Google Scholar] [CrossRef]
- Kancherla, V.; Elliott, J.L., Jr.; Patel, B.B.; Holland, N.W.; Johnson, T.M., 2nd; Khakharia, A.; Phillips, L.S.; Oakley, G.P., Jr.; Vaughan, C.P. Long-term Metformin Therapy and Monitoring for Vitamin B12 Deficiency Among Older Veterans. J. Am. Geriatr. Soc. 2017, 65, 1061–1066. [Google Scholar] [CrossRef]
- Ma, L.; Chen, J.; Wang, X. Structural modification of honokiol, a biphenyl occurring in Magnolia officinalis: The evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship. J. Med. Chem. 2011, 54, 6469–6481. [Google Scholar] [CrossRef]
- De Iuliis, A.; Montinaro, E.; Fatati, G.; Plebani, M.; Colosimo, C. Diabetes mellitus and Parkinson’s disease: Dangerous liaisons between insulin and dopamine. Neural Regen. Res. 2022, 17, 523. [Google Scholar] [PubMed]
- Juan, H.; Xin, X.; Jian, W. Research progress on the pharmacological mechanism of traditional Chinese medicinal pair on prevention and treatment of diabetes. World Clin. Drugs 2020, 41, 405–409. [Google Scholar] [CrossRef]
- Jia, S.; Xiaoxia, G.; Junsheng, T. Modern research on compatibility mechanism of Chinese materia medica pair. Chin. Tradit. Herb. Drugs 2017, 48, 4367–4374. [Google Scholar] [CrossRef]
- Huttunen, K.; Leppänen, J.; Kemppainen, E.; Palonen, P.; Rautio, J.; Järvinen, T.; Vepsäläinen, J. Towards Metformin Prodrugs. Synthesis 2008, 2008, 3619–3624. [Google Scholar] [CrossRef]
- Chen, Z.; Bo, H.; Aiqing, B.; Xiao, L. Copper-catalyzed arylation of biguanide derivatives via C-N cross-coupling reactions. Org. Biomol. Chem. 2015, 13, 11432–11437. [Google Scholar] [CrossRef]
- Brzozowski, Z.; Saczewski, F.; Gdaniec, M. Synthesis, structural characterization and antitumor activity of novel 2,4-diamino-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2000, 35, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Shili, L.; Wenjing, Z. Synthesis, Characterization, and Biological Evaluations of 1,3,5-Triazine Derivatives of Metformin Cyclization with Berberine and Magnolol in the Presence of Sodium Methylate. Molecules 2017, 22, 1752. [Google Scholar] [CrossRef]
- Cui, R.; Juanxia, W.; Youzhen, T. Synthesis, Characterization and Biological Evaluation of Magnolol and Honokiol Derivatives with 1,3,5-Triazine of Metformin Cyclization. Molecules 2020, 25, 5779. [Google Scholar] [CrossRef]
- Hao, M.; Li, Y.; Liu, L.; Yuan, X.; Gao, Y.; Guan, Z.; Li, W. The design and synthesis of a novel compound of berberine and baicalein that inhibits the efficacy of lipid accumulation in 3T3-L1 adipocytes. Bioorg. Med. Chem. 2017, 25, 5506–5512. [Google Scholar] [CrossRef]
- Guo, M.-X.; Liao, S.-L.; Wu, X.; Wang, J.-X.; Chen, D.-Q.; Shi, K.-X.; Tan, Y.-Z.; Feng, Y.-F. Synthesis and characterization of magnolol and honokiol derivatives and evaluation of their anti-inflammatory and anti-tumor activities in vitro. Nat. Prod. Res. Dev. 2020, 32, 749. [Google Scholar]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medicine Science and Technology Press: Beijing, China, 2020; Volume 4. [Google Scholar]
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]


| Entry | Base (Equiv) | Solvent | Temp (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | Na2CO3 (2.0) | MeOH | 65 | 77.5 |
| 2 | Na2CO3 (2.0) | EtOH | 65 | 24.1 |
| 3 | Na2CO3 (2.0) | MeCN | 65 | 22.9 |
| 4 | K2CO3 (2.0) | MeOH | 65 | 18.9 |
| 5 | K2CO3 (2.0) | EtOH | 65 | 5.9 |
| 6 | K2CO3 (2.0) | MeCN | 65 | 5.6 |
| 7 | Na2CO3 (2.0) | MeOH | 25 | 37.5 |
| 8 | Na2CO3 (2.0) | MeOH | 45 | 47.9 |
| 9 | Na2CO3 (2.0) | MeOH | 67 | 69.3 |
| 10 | Na2CO3 (1.5) | MeOH | 65 | 63.4 |
| 11 | Na2CO3 (1.0) | MeOH | 65 | 42.1 |
| Entry | Base | Solvent | Temp (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | Na2CO3 | MeOH | 65 | 10.8 |
| 2 | Na2CO3 | EtOH | 65 | 7.4 |
| 3 | Na2CO3 | MeCN | 65 | 4.9 |
| 4 | K2CO3 | MeOH | 65 | 14.3 |
| 5 | K2CO3 | EtOH | 65 | 9.8 |
| 6 | K2CO3 | MeCN | 65 | 6.6 |
| 7 | NaOH | MeOH | 65 | 7.3 |
| 8 | NaOH | EtOH | 65 | 5.0 |
| 9 | NaOH | MeCN | 65 | 3.3 |
| 10 | MeONa | EtOH | 65 | 12.2 |
| 11 | MeONa | DMF | 65 | 1.2 |
| 12 | MeONa | MeCN | 65 | 8.2 |
| 13 | MeONa | MeOH | 25 | 11.1 |
| 14 | MeONa | MeOH | 45 | 11.5 |
| 15 | MeONa | MeOH | 65 | 17.8 |
| 16 | MeONa | MeOH | 67 | 16.8 |
| Empirical Formula | C26H29N5O4 |
|---|---|
| Mr | 476.55 |
| T [K] | 100 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a [Å] | 4.2879(3) |
| b [Å] | 15.4405(9) |
| c [Å] | 19.29(12) |
| α [°] | 107.806(6) |
| β [°] | 96.102(6) |
| γ [°] | 95.991(6) |
| V [Å3] | 1196.61(14) |
| Z | 2 |
| Dc [gcm–3] | 1.323 |
| θ range for data collection [°] | 4.461–73.512 |
| Limiting indices, hkl | −5 ≤ h ≤ 3; −17 ≤ k ≤ 19; −23 ≤ l ≤ 23 |
| Reflections collected | 4656 |
| Independent reflections (Rint) | 3616 |
| Good-of-fit on F2 | 0.996 |
| R1/wR2 [I > 2σ(I)] [a] | 0.0875/0.0683 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Xie, L.; Zhang, J.; Cao, H.; Wang, J.; Wu, X.; Feng, Y. Synthesis, Quality Control and Preliminary Activity Evaluation of a New Compound HM475. Molecules 2023, 28, 3753. https://doi.org/10.3390/molecules28093753
Guo J, Xie L, Zhang J, Cao H, Wang J, Wu X, Feng Y. Synthesis, Quality Control and Preliminary Activity Evaluation of a New Compound HM475. Molecules. 2023; 28(9):3753. https://doi.org/10.3390/molecules28093753
Chicago/Turabian StyleGuo, Jieqing, Luming Xie, Jing Zhang, Han Cao, Juanxia Wang, Xia Wu, and Yifan Feng. 2023. "Synthesis, Quality Control and Preliminary Activity Evaluation of a New Compound HM475" Molecules 28, no. 9: 3753. https://doi.org/10.3390/molecules28093753
APA StyleGuo, J., Xie, L., Zhang, J., Cao, H., Wang, J., Wu, X., & Feng, Y. (2023). Synthesis, Quality Control and Preliminary Activity Evaluation of a New Compound HM475. Molecules, 28(9), 3753. https://doi.org/10.3390/molecules28093753





