Analysis of Dibenzyltoluene Mixtures: From Fast Analysis to In-Depth Characterization of the Compounds
Abstract
1. Introduction
2. Results and Discussion
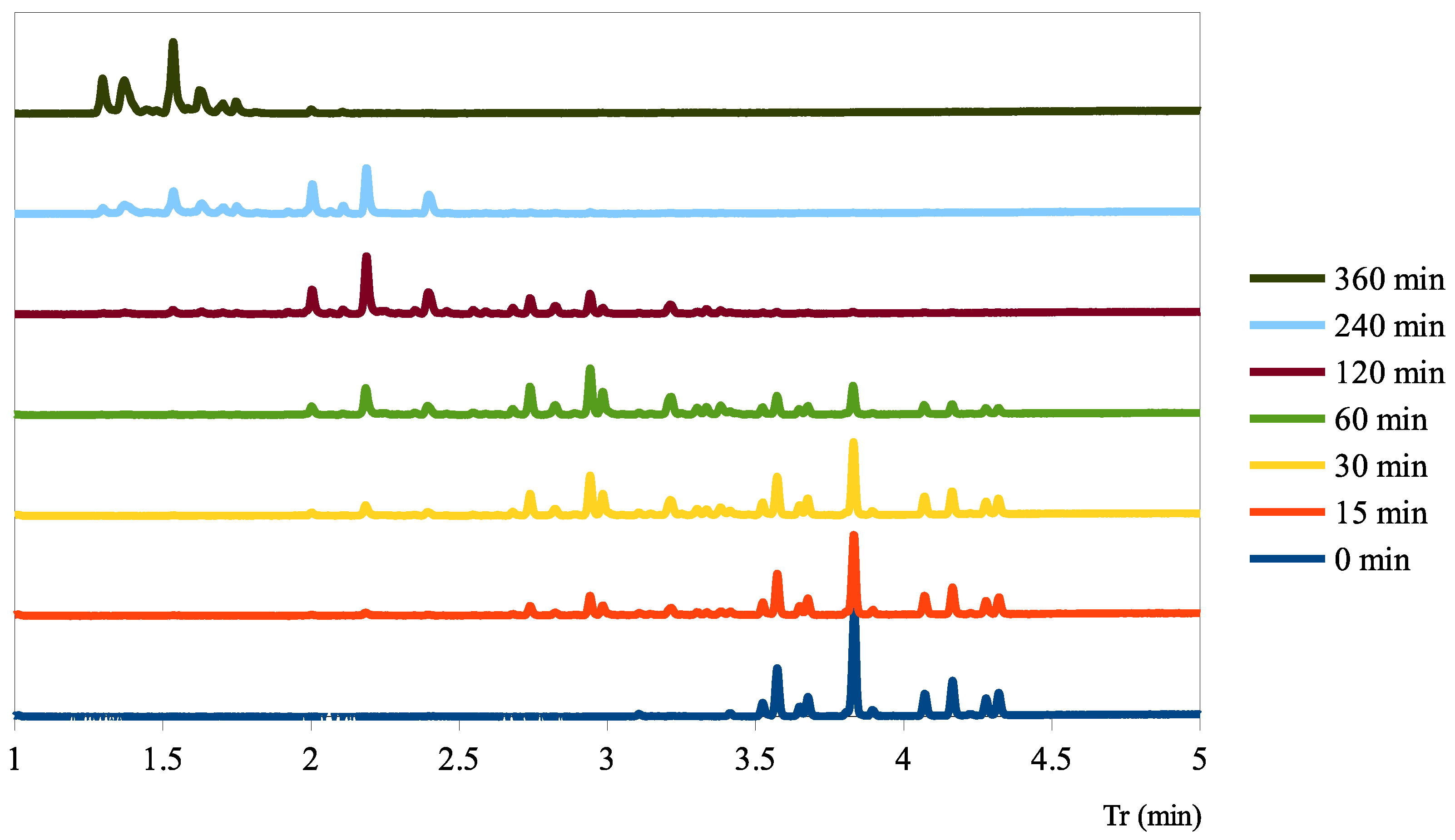
2.1. Evaluation of DOH Using Fast GC
2.2. Isomer Identification of the H0-DBT Mixture
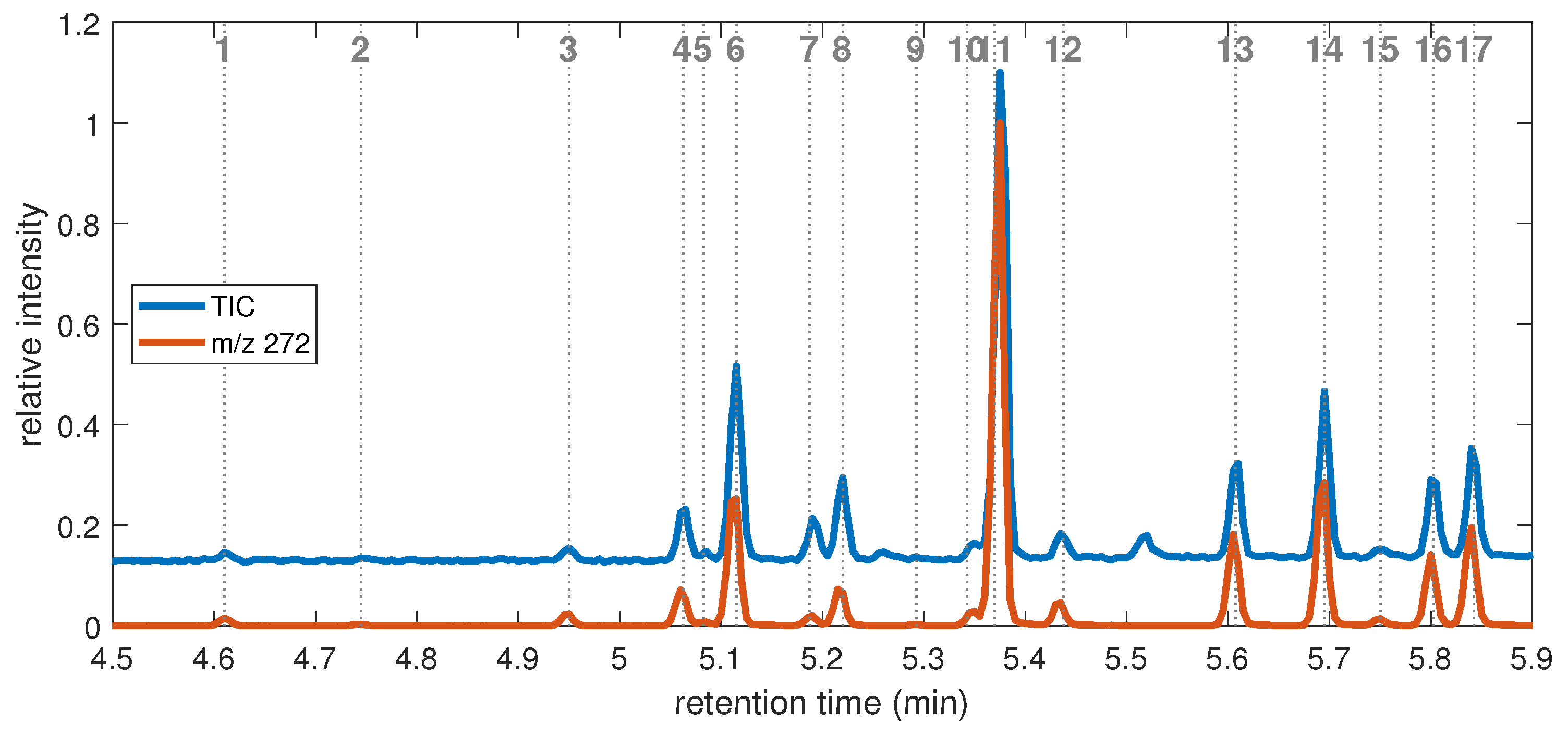
2.2.1. GC–MS Chromatogram
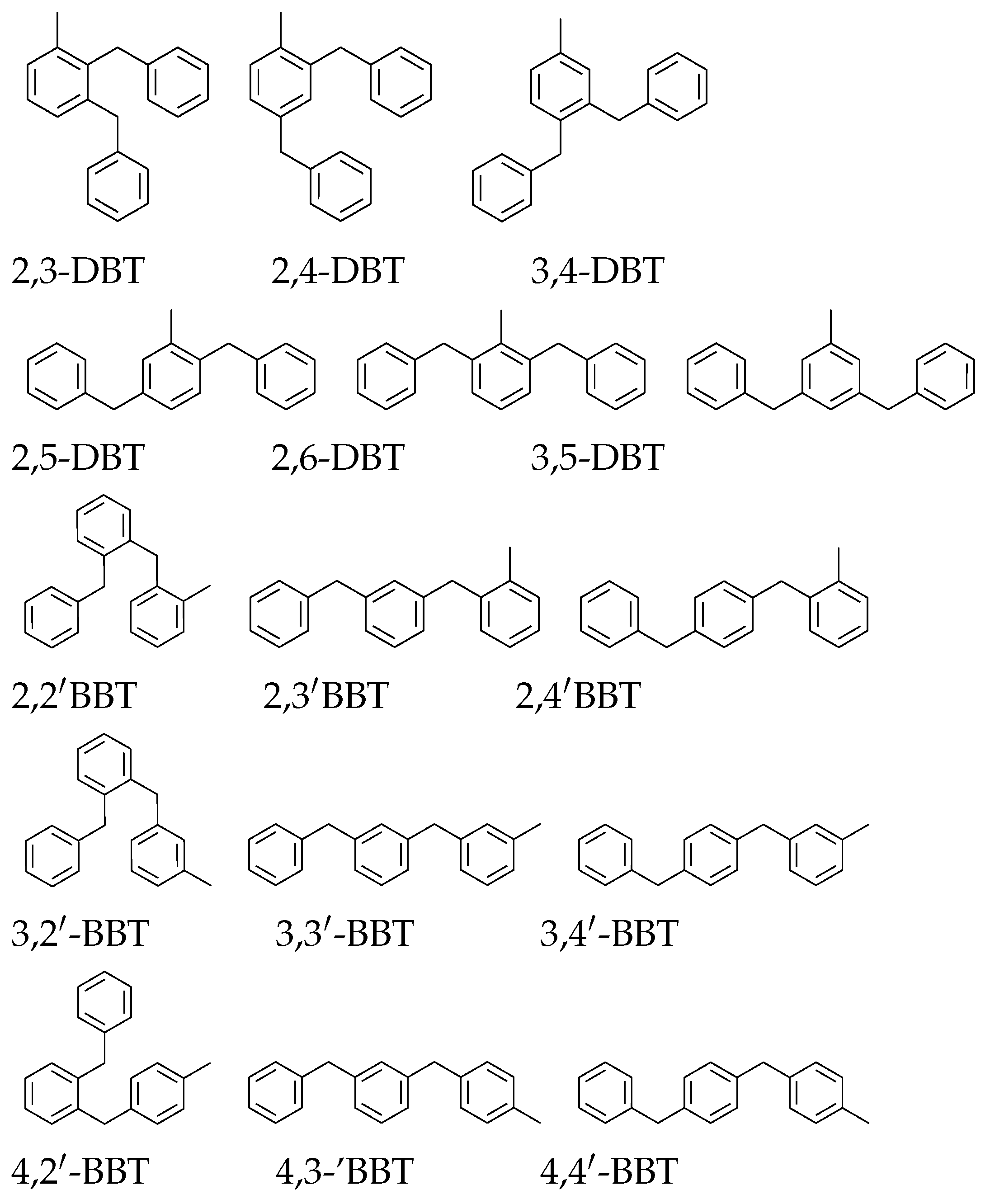
2.2.2. Friedel–Crafts Reactivity Hypothesis
2.2.3. Fractionation Results
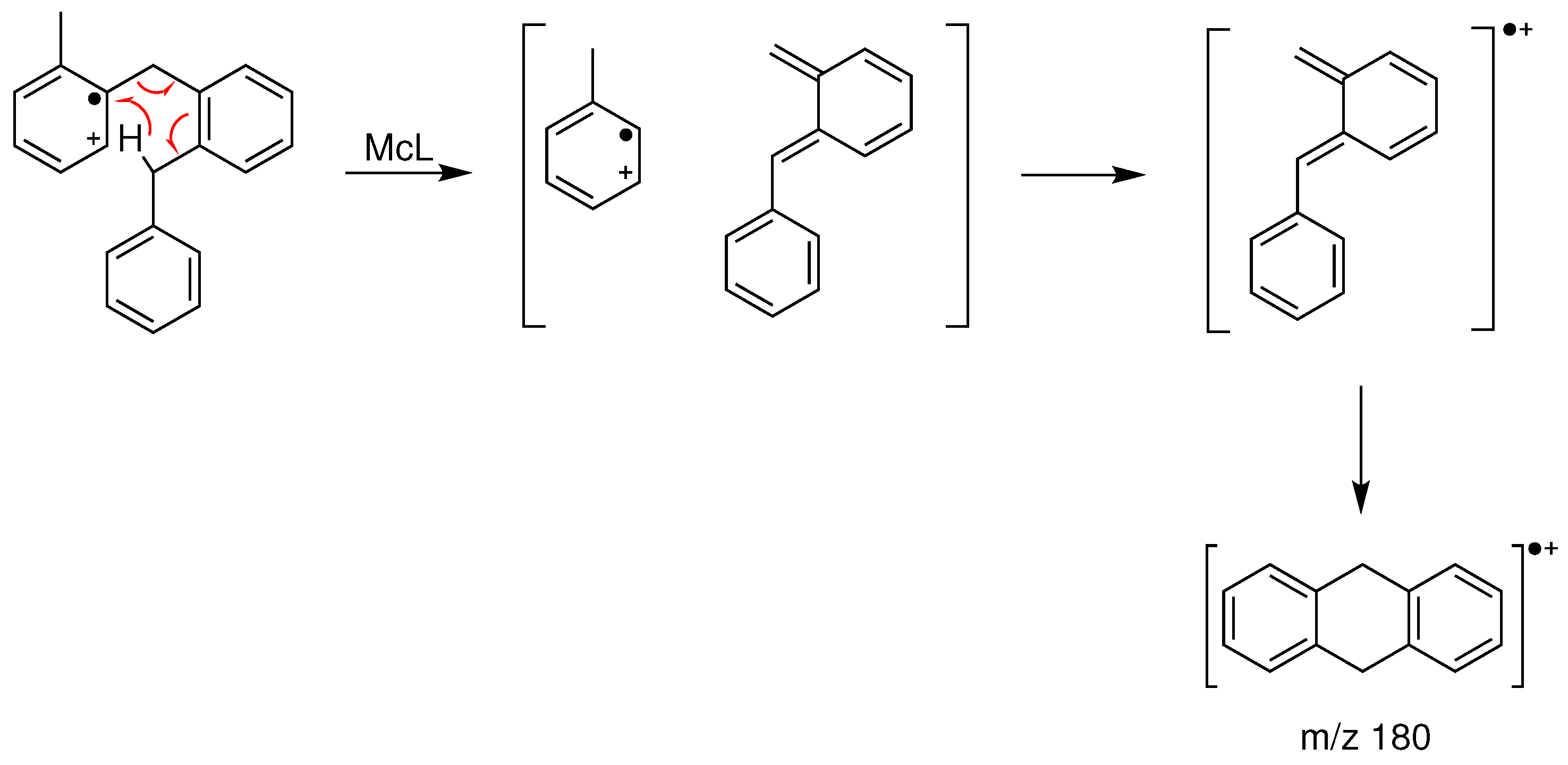
2.2.4. EI Mass Spectra
| pn | Attrib. | TIC | Ion Mass over Charge m/z | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 272 | 257 | 195 | 194 | 181 | 180 | 179 | 167 | 166 | 165 | 105 | 104 | 91 | ||||
| 4 | 2,3 DBT | 5.063 | 82,042 | 41 | 2 | 4 | 16 | 31 | 17 | 100 | 4 | 21 | 28 | 17 | ||
| 5 | 3,2 BBT | 5.083 | 30,024 | 60 | 12 | 47 | 83 | 100 | 23 | 25 | 58 | 27 | ||||
| 6 | 3,4 DBT | 5.115 | 259,092 | 51 | 4 | 21 | 50 | 15 | 100 | 5 | 27 | 33 | 17 | |||
| 7 | 2,2 BBT | 5.188 | 70,612 | 12 | 19 | 100 | 85 | 7 | 13 | 36 | 4 | 6 | 14 | |||
| 8 | 4,2 BBT | 5.220 | 121,124 | 31 | 4 | 21 | 84 | 100 | 9 | 15 | 38 | 6 | 11 | |||
| 9 | 3,5 DBT | 5.293 | <LOQ | 62 | 100 | |||||||||||
| 10 | 2,3 BBT | 5.343 | 40,338 | 94 | 10 | 14 | 21 | 23 | 80 | 26 | 56 | 33 | 100 | 46 | ||
| 11 | 2,4 DBT | 5.370 | 621,877 | 51 | 4 | 1 | 4 | 100 | 3 | 15 | 4 | 29 | 30 | 31 | ||
| 12 | 3,4 BBT | 5.438 | 52,165 | 73 | 7 | 5 | 100 | 8 | 23 | 44 | 35 | 45 | 19 | 47 | ||
| 13 | 2,5 DBT | 5.608 | 138,433 | 56 | 4 | 4 | 100 | 5 | 30 | 5 | 32 | 33 | 42 | |||
| 14 | 2,6 DBT | 5.695 | 228,073 | 50 | 1 | 1 | 2 | 100 | 3 | 14 | 5 | 32 | 31 | 31 | ||
| 15 | 4,3 DBT | 5.750 | 33,111 | 74 | 98 | 100 | 56 | 51 | 35 | |||||||
| 16 | 2,4 BBT | 5.803 | 118,206 | 100 | 15 | 98 | 8 | 20 | 89 | 43 | 65 | 24 | 95 | 54 | ||
| 17 | 4,4 BBT | 5.843 | 157,482 | 75 | 13 | 2 | 100 | 6 | 14 | 61 | 33 | 46 | 16 | 5 | 34 | |
2.2.5. Synthesis and Final Attribution
3. Materials and Methods
3.1. Experimental Instrumentation
3.2. Fractionation Conditions
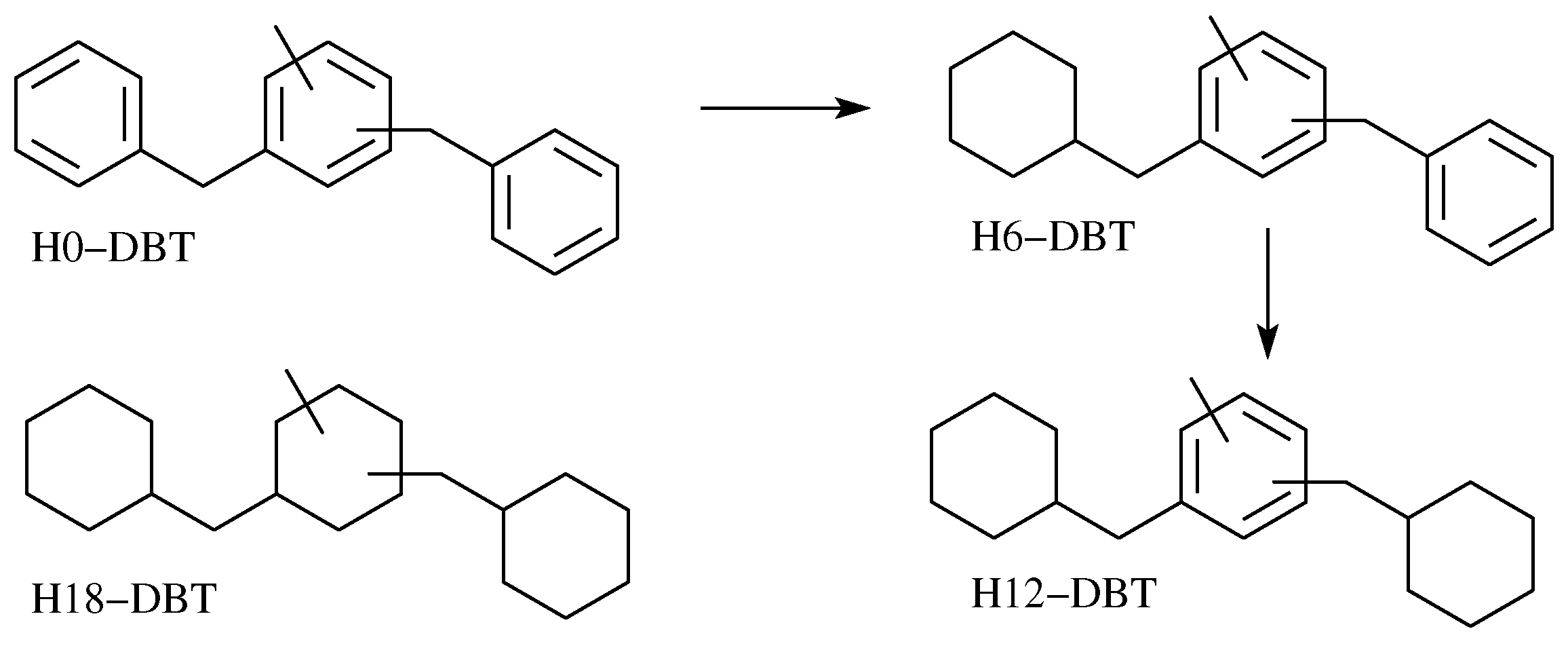
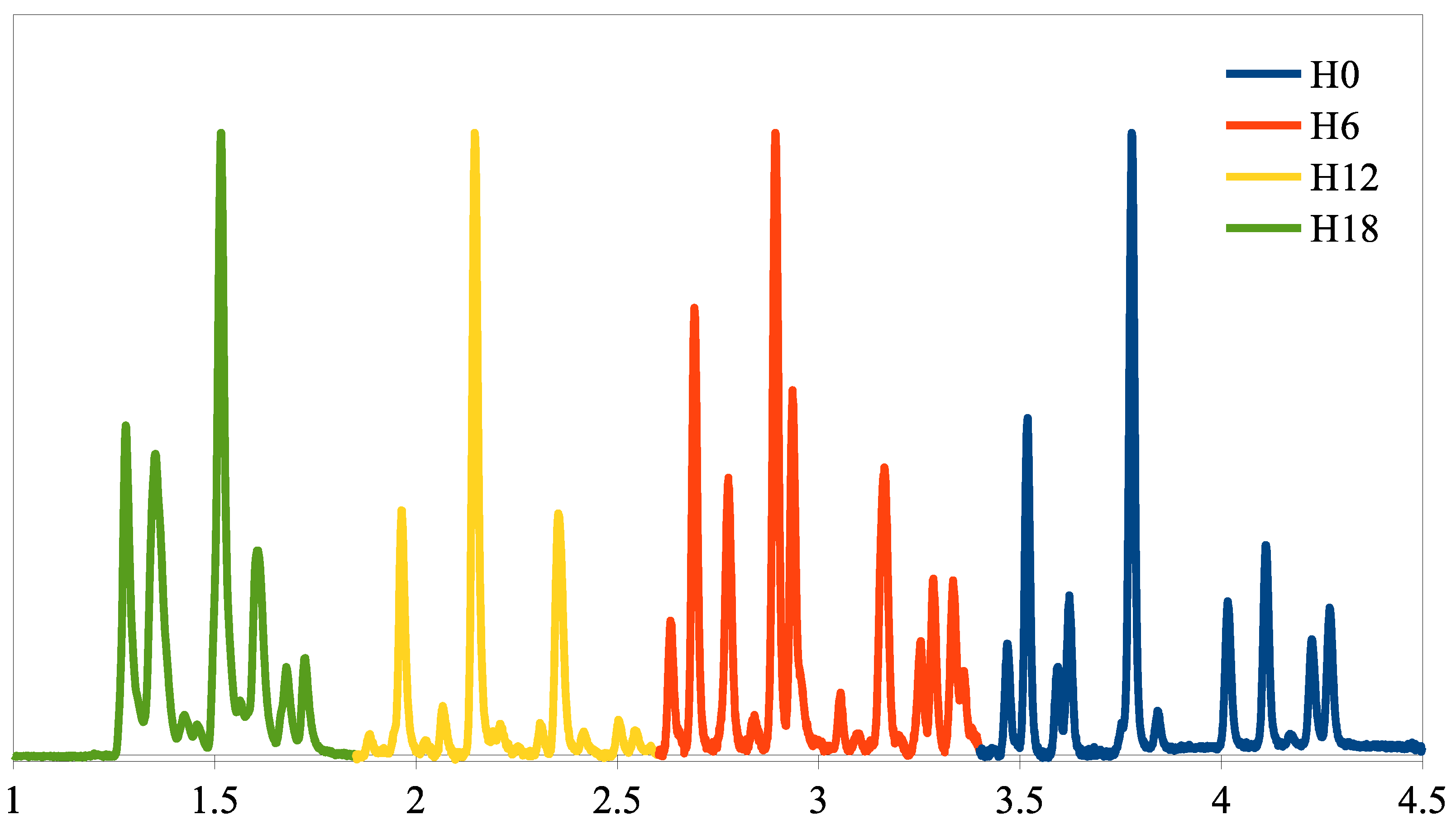
3.3. Synthesis of H6, H12, and H18-Dbt by H0-Dbt Hydrogenation
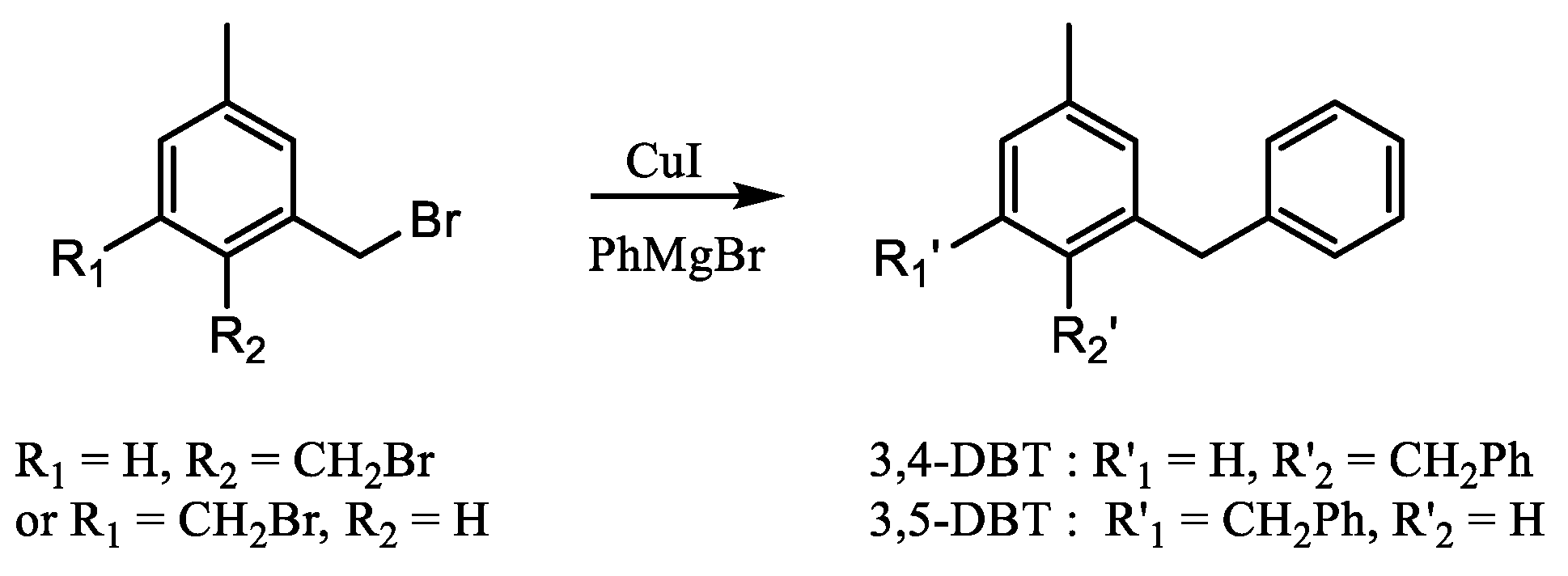
3.4. Synthesis of 3,4- and 3,5-Di(benzyl)toluene
- 3,4-Di(benzyl)toluene
- 3,5-Di(benzyl)toluene
3.5. Synthesis of 2,4- and 2,5-Di(benzyl)toluene
- 2,4-Di(benzyl)toluene (oil)
- 2,5-Di(benzyl)toluene (oil)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and Alternatives for International Transport of Renewable Hydrogen. Renew. Sustain. Energy Rev. 2021, 135, 110171. [Google Scholar] [CrossRef]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid Organic Hydrogen Carriers for Transportation and Storing of Renewable Energy—Review and Discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Valentini, F.; Marrocchi, A.; Vaccaro, L. Liquid Organic Hydrogen Carriers (LOHCs) as H-Source for Bio-Derived Fuels and Additives Production. Adv. Energy Mater. 2022, 12, 2103362. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems. ChemSusChem 2014, 7, 229–235. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Dürr, S.; Seidel, A.; Müller, K.; Bösmann, A.; Wasserscheid, P. Hydrogen Storage Using a Hot Pressure Swing Reactor. Energy Environ. Sci. 2017, 10, 1652–1659. [Google Scholar] [CrossRef]
- Ali, A.; Rohini, A.K.; Noh, Y.S.; Moon, D.J.; Lee, H.J. Hydrogenation of Dibenzyltoluene and the Catalytic Performance of Pt/Al2O3 with Various Pt Loadings for Hydrogen Production from Perhydro-Dibenzyltoluene. Int. J. Energy Res. 2022, 46, 6672–6688. [Google Scholar] [CrossRef]
- Park, S.; Naseem, M.; Lee, S. Experimental Assessment of Perhydro-Dibenzyltoluene Dehydrogenation Reaction Kinetics in a Continuous Flow System for Stable Hydrogen Supply. Materials 2021, 14, 7613. [Google Scholar] [CrossRef]
- Ouma, C.N.M.; Modisha, P.; Bessarabov, D. Insight into the adsorption of a liquid organic hydrogen carrier, perhydro-i-dibenzyltoluene (i = m, o, p), on Pt, Pd and PtPd planar surfaces. RSC Adv. 2018, 8, 31895–31904. [Google Scholar] [CrossRef]
- Sisáková, K.; Podrojková, N.; Oriňaková, R.; Oriňak, A. Novel Catalysts for Dibenzyltoluene as a Potential Liquid Organic Hydrogen Carrier Use—A Mini-review. Energy Fuels 2021, 35, 7608–7623. [Google Scholar] [CrossRef]
- Do, G.; Preuster, P.; Aslam, R.; Bösmann, A.; Müller, K.; Arlt, W.; Wasserscheid, P. Hydrogenation of the Liquid Organic Hydrogen Carrier Compound Dibenzyltoluene—Reaction Pathway Determination by 1H NMR Spectroscopy. React. Chem. Eng. 2016, 1, 313–320. [Google Scholar] [CrossRef]
- Aslam, R.; Khan, M.H.; Ishaq, M.; Müller, K. Thermophysical Studies of Dibenzyltoluene and Its Partially and Fully Hydrogenated Derivatives. J. Chem. Eng. Data 2018, 63, 4580–4587. [Google Scholar] [CrossRef]
- Chen, X.; Gierlich, C.H.; Schötz, S.; Blaumeiser, D.; Bauer, T.; Libuda, J.; Palkovits, R. Hydrogen Production Based on Liquid Organic Hydrogen Carriers through Sulfur Doped Platinum Catalysts Supported on TiO2. ACS Sustain. Chem. Eng. 2021, 9, 6561–6573. [Google Scholar] [CrossRef]
- Kim, T.W.; Kim, M.; Kim, S.K.; Choi, Y.N.; Jung, M.; Oh, H.; Suh, Y.W. Remarkably Fast Low-Temperature Hydrogen Storage into Aromatic Benzyltoluenes over MgO-Supported Ru Nanoparticles with Homolytic and Heterolytic H2 Adsorption. Appl. Catal. B Environ. 2021, 286, 119889. [Google Scholar] [CrossRef]
- Shi, L.; Qi, S.; Qu, J.; Che, T.; Yi, C.; Yang, B. Integration of Hydrogenation and Dehydrogenation Based on Dibenzyltoluene as Liquid Organic Hydrogen Energy Carrier. Int. J. Hydrogen Energy 2019, 44, 5345–5354. [Google Scholar] [CrossRef]
- Ali, A.; Rohini, A.K.; Lee, H.J. Dehydrogenation of Perhydro-Dibenzyltoluene for Hydrogen Production in a Microchannel Reactor. Int. J. Hydrogen Energy 2022, 47, 20905–20914. [Google Scholar] [CrossRef]
- Markiewicz, M.; Zhang, Y.Q.; Bösmann, A.; Brückner, N.; Thöming, J.; Wasserscheid, P.; Stolte, S. Environmental and Health Impact Assessment of Liquid Organic Hydrogen Carrier (LOHC) Systems—Challenges and Preliminary Results. Energy Environ. Sci. 2015, 8, 1035–1045. [Google Scholar] [CrossRef]
- Modisha, P.M.; Jordaan, J.H.L.; Bösmann, A.; Wasserscheid, P.; Bessarabov, D. Analysis of reaction mixtures of perhydro-dibenzyltoluene using two-dimensional gas chromatography and single quadrupole gas chromatography. Int. J. Hydrogen Energy 2018, 43, 5620–5636. [Google Scholar] [CrossRef]
- Commandeur, R.; Missos, D.E. Process for the Synthesis of Benzyltoluene and Dibenzyltoluene with Low Chlorine Content. European Patent EP0435737B1, 19 July 1995. [Google Scholar]
- Olah, G.A.; Kobayashi, S.; Tashiro, M. Aromatic substitution. XXX. Friedel-Crafts benzylation of benzene and toluene with benzyl and substituted benzyl halides. J. Am. Chem. Soc. 1972, 94, 7448–7461. [Google Scholar] [CrossRef]
- Coq, B.; Gourves, V.; Figuéras, F. Benzylation of toluene by benzyl chloride over protonic zeolites. Appl. Catal. A Gen. 1993, 100, 69–75. [Google Scholar] [CrossRef]
- Kim, T.W.; Jo, Y.; Jeong, K.; Yook, H.; Han, J.W.; Jang, J.H.; Han, G.B.; Park, J.H.; Suh, Y.W. Tuning the isomer composition is a key to overcome the performance limits of commercial benzyltoluene as liquid organic hydrogen carrier. J. Energy Storage 2023, 60, 106676. [Google Scholar] [CrossRef]
- Zhou, J.; Kim, E.J.; Chung, J.S.; Kang, S.G. Using first-principles modeling to investigate dehydrogenation of perhydro-dibenzyltoluene on Pt/M surface alloys (M = Fe, Ni, and Cu). Fuel 2023, 331, 125779. [Google Scholar] [CrossRef]
- Aslam, R.; Minceva, M.; Müller, K.; Arlt, W. Development of a Liquid Chromatographic Method for the Separation of a Liquid Organic Hydrogen Carrier Mixture. Sep. Purif. Technol. 2016, 163, 140–144. [Google Scholar] [CrossRef]
- Tang, B.; Isacsson, U.; Edwards, Y. Chemical Characterization and Screening of Emission Profiles of Bituminous Sealants Using Solid-Phase Microextraction. Energy Fuels 2006, 20, 1528–1535. [Google Scholar] [CrossRef]
- Zoccali, M.; Tranchida, P.Q.; Mondello, L. Fast gas chromatography-mass spectrometry: A review of the last decade. TrAC Trends Anal. Chem. 2019, 118, 444–452. [Google Scholar] [CrossRef]
- Kuck, D. Mass spectrometry of alkylbenzenes and related compounds. Part I. Gas-phase ion chemistry of alkylbenzene radical cations. Mass Spectrom. Rev. 1990, 9, 187–233. [Google Scholar] [CrossRef]
- Miranda, R.; Delgado, F.; Velasco, L.; Pérez, J.; Salmón, M. Mass spectrometric detection and identification of ortho, para-benzyltoluenes and oligotoluenes. Rapid Commun. Mass Spectrom. 2000, 14, 188–193. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Balasubramanian, M.; Siskin, M. Aqueous high-temperature chemistry of carbo- and heterocycles. 2. Monosubstituted benzenes: Benzyl alcohol, benzaldehyde and benzoic acid. Energy Fuels 1990, 4, 499–505. [Google Scholar] [CrossRef]
- NIST Mass Spectrometry Data Center; Wallace, W.E. Mass Spectra. In NIST Chemical Webbook, Reference Standard NIST Database; Wallace, W.E., Lindstrom, P., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023; Volume 69, p. 20899. [Google Scholar]
- Meyerson, S.; Drews, H.; Fields, E.K. Mass Spectra of ortho-Substituted Diarylmethanes. J. Am. Chem. Soc. 1964, 86, 4964–4967. [Google Scholar] [CrossRef]
- Lee, W.Y.; Sim, W.; Choi, K.D. Biscyclophanes. Part 1: Synthesis of a common-nuclear bis[1.1.1 ]orthocyclophane. First member of a new family of cyclophanes. J. Chem. Soc. Perkin Trans. 1 1992, 7, 881–885. [Google Scholar] [CrossRef]
- Zhuang, L.; Wai, J.S.; Embrey, M.W.; Fisher, T.E.; Egbertson, M.S.; Payne, L.S.; Guare, J.P.; Vacca, J.P.; Hazuda, D.J.; Felock, P.J.; et al. Design and Synthesis of 8-Hydroxy-[1,6]Naphthyridines as Novel Inhibitors of HIV-1 Integrase in Vitro and in Infected Cells. J. Med. Chem. 2003, 46, 453–456. [Google Scholar] [CrossRef]
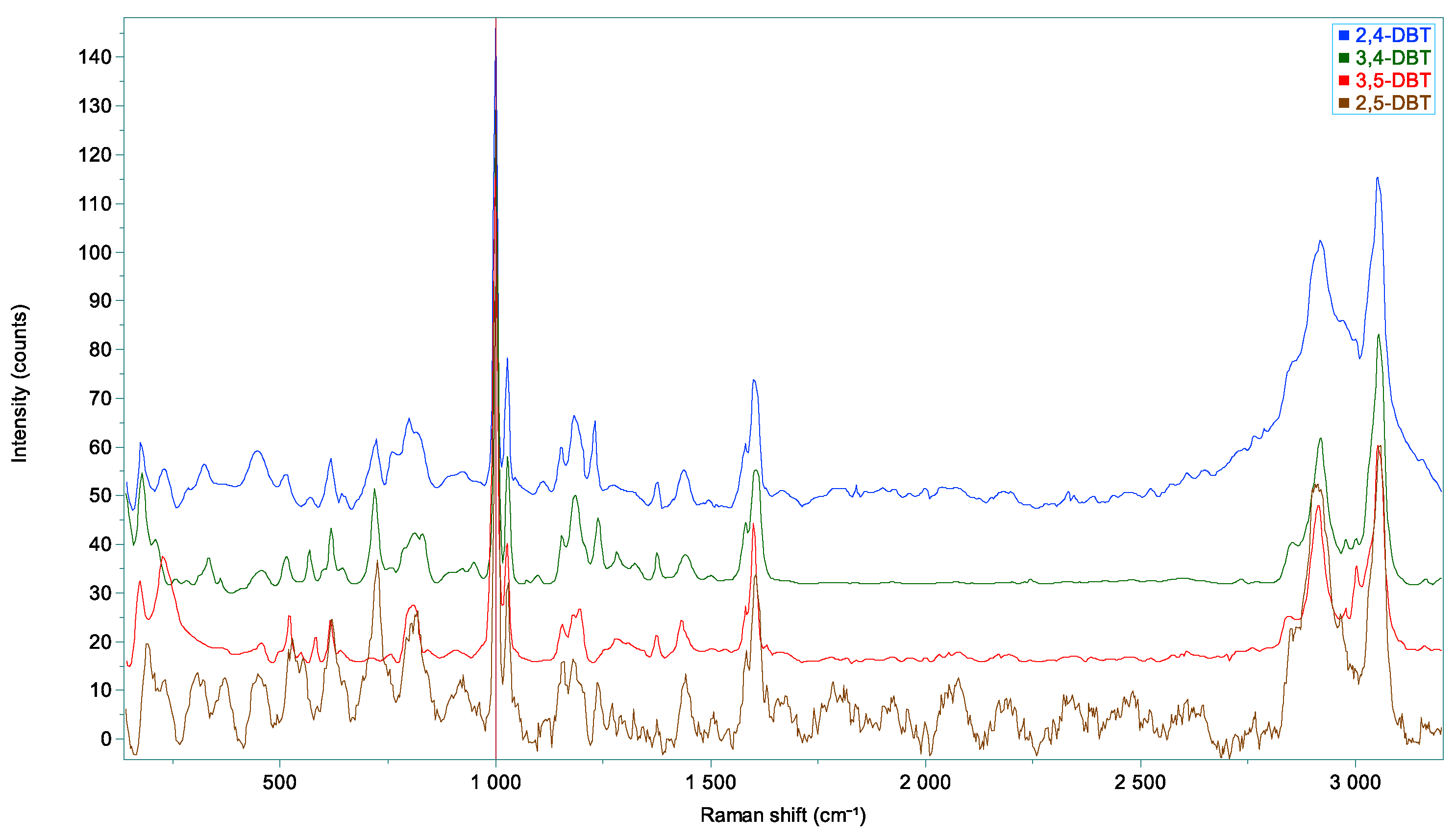

| Vibrations | Toluene | 2,4-DBT | 3,4-DBT | 3,5-DBT | 2,5-DBT |
|---|---|---|---|---|---|
| 173 (m) | 176 (m) | 173 (m) | |||
| 216 (m) | 229 (m) | 209 (w) | 225 (m) | ||
| 320 (m) | 333 (w) | ||||
| Aromatic ring deformation vibrations | 444 (m) | 450 (w) | 455 (w) | ||
| 517 (m) | 511 (m) | 513 (m) | 518 (m) | ||
| 568 (w) | 567 (m) | 577 (m) | |||
| Aromatic out-of-plane C-H deformation vibrations | 623 (w) | 617 (m) | 616 (m) | 617 (m) | |
| 722 (s) | 718 (s) | - | 724 | ||
| 783 (s) | 760 (sh), 798 (m), 816 (sh) | 785 (sh), 811 (m), 828 (sh) | 809 (m) | 818 | |
| Aromatic in-plane C-H deformation vibrations | 1002 (vs), 1027 (s) | 1000 (vs), 1027 (s) | 999 (vs), 1026 (s) | 1000 (vs), 1027 (s) | 1002 (vs), 1027 (s) |
| Alkane C-C vibrations: skeletal vibrations | 1156 (w), 1179 (w) | 1150 (m), 1180 (m) | 1152 (m), 1185 (m) | 1153 (m), 1181 (m) | 1179 |
| 1209 (m) | 1229 (m) | 1238 (m) | 1234 | ||
| Alkane C-H deform. vibrations | 1378 (m) | 1375 (w) | 1375 (w) | 1375 (w) | |
| 1437 (m) | 1439 (w) | 1431 (m) | 1442 | ||
| Aromatic C=C stretching vibrations | 1586 (sh), 1601 (s) | 1579 (sh), 1599 (s) | 1579 (sh), 1604 (s) | 1579 (sh), 1599 (s) | 1604 (s) |
| Alkane C-H stretching Vibrations | 2870 (w), 2918 (s), 2982 (sh) | 2848 (sh), 2916 (s), 2975 (sh) | 2853 (sh), 2918 (s), 2976 (sh) | 2843 (sh), 2913 (s), 2975 (sh) | 2912 (s) |
| Aromatic =C-H stretching vibrations | 3004, 3056 (s) | 3000 (w), 3051 (s) | 3000 (w), 3053 (s) | 3001 (w), 3051 (s) | 3058 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Louarn, E.; Fache, F.; Vanoye, L.; Bonhommé, A.; Pitault, I.; Meille, V. Analysis of Dibenzyltoluene Mixtures: From Fast Analysis to In-Depth Characterization of the Compounds. Molecules 2023, 28, 3751. https://doi.org/10.3390/molecules28093751
Ji X, Louarn E, Fache F, Vanoye L, Bonhommé A, Pitault I, Meille V. Analysis of Dibenzyltoluene Mixtures: From Fast Analysis to In-Depth Characterization of the Compounds. Molecules. 2023; 28(9):3751. https://doi.org/10.3390/molecules28093751
Chicago/Turabian StyleJi, Xiaolong, Essyllt Louarn, Fabienne Fache, Laurent Vanoye, Anne Bonhommé, Isabelle Pitault, and Valérie Meille. 2023. "Analysis of Dibenzyltoluene Mixtures: From Fast Analysis to In-Depth Characterization of the Compounds" Molecules 28, no. 9: 3751. https://doi.org/10.3390/molecules28093751
APA StyleJi, X., Louarn, E., Fache, F., Vanoye, L., Bonhommé, A., Pitault, I., & Meille, V. (2023). Analysis of Dibenzyltoluene Mixtures: From Fast Analysis to In-Depth Characterization of the Compounds. Molecules, 28(9), 3751. https://doi.org/10.3390/molecules28093751








