Abstract
Platinum nanoparticles (PtNPs) are classical peroxidase-like nanozyme; self-agglomeration of nanoparticles leads to the undesirable reduction in stability and catalytic activity. Herein, a hybrid peroxidase-like nanocatalyst consisting of PtNPs in situ growing on g–C3N4 nanosheets with enhanced peroxidase-mimic catalytic activity (PtNP@g–C3N4 nanosheets) was prepared for H2O2 and oxidase-based colorimetric assay. g–C3N4 nanosheets can be used as carriers to solve the problem of poor stability of PtNPs. We observed that the catalytic ability could be maintained for more than 90 days. PtNP@g–C3N4 nanosheets could quickly catalyze the oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB), and the absorbance of blue color oxidized TMB (oxTMB) showed a robust linear relationship with the concentration of H2O2 (the detection limit (LOD): 3.33 μM). By utilizing H2O2 as a mediator, this strategy can be applied to oxidase-based biomolecules (glucose, organophosphorus, and so on, that generate or consume hydrogen peroxide) sensing. As a proof of concept, a sensitive assay of cholesterol that combined PtNP@g–C3N4 nanosheets with cholesterol oxidase (ChOx) cascade catalytic reaction was constructed with an LOD of 9.35 μM in a widespread range from 10 to 800 μM (R2 = 0.9981). In addition, we also verified its ability to detect cholesterol in fetal bovine serum. These results showed application prospect of PtNP@g–C3N4 nanosheets-based colorimetry in sensing and clinical medical detection.
1. Introduction
Since the first evidence of triazacyclonane-modified gold nanoparticles as substitutes for phosphodiesterase by Scrimin et al. in 2004 [1], especially due to the report of Fe3O4 nanoparticles with intrinsic peroxidase-like activity by Yan group in 2007 [2], nanomaterial-based artificial enzymes mimic, known as nanozymes, have received great research attention. Compared with natural enzymes, they possess the advantages of low cost, simple preparation, and tunable catalytic activity [3,4]. In recent years, various nanozymes including inorganic nanoparticles, carbon nanomaterials, metal-organic frameworks etc. have been used to mimic peroxidase, oxidase, and nuclease, et al. [5,6,7,8,9,10]. Particularly, ever-growing research interests have focused on peroxidase-mimic nanozymes owing to their application in sensing, catalysis, environmental protection, and other fields [11,12,13,14].
Among these nanozymes, platinum nanoparticles (PtNPs) are classical peroxidase-like nanozymes by virtue of their high chemistry stability and catalytic activity [15,16]. However, nanoparticles often self-aggregate during the catalytic reaction, thereby the active surface area is reduced, resulting in low stability and catalytic activity, which seriously limits the practical application of nanozymes [17,18]. In order to meet these challenges, we packaged platinum nanoparticles into a hyaluronidase-activated capsule and verified its high catalytic stability even under physiological conditions and antibacterial effect on diabetic wounds infected by bacteria [19]. A variety of nanosheets such as graphene, graphitic carbon nitride, and manganese dioxide were also used as support for inorganic nanoparticles decoration in order to hold preferable peroxidase-like performance, forming hierarchical nanohybrids [20]. Nanosheets could either offer synergistic effects on the intrinsic properties of the inorganic nanoparticles or prevent their aggregation, making the nanohybrids much more stable and attractive in catalytic sensing in comparison to the nanoparticles alone [21,22]. Graphite carbon nitride (g–C3N4) nanosheets have been intensely studied by virtue of their intrinsic peroxidase-like activity, excellent chemical stability, and appealing electronic structure [23]. Yang group [20] proved that the nanohybrid, with Au nanoparticles loaded on g–C3N4 nanosheets, possessed increased catalytic capability, and further constructed a colorimetric biosensing method based on its peroxidase-like activity.
In this work, a peroxidase-like hybrid nanocatalyst has been prepared that consists of PtNPs in situ growth on g–C3N4 nanosheets for H2O2 and oxidase-based colorimetric assay. Upon combining the excellent catalytic activity of PtNPs with the stability of g–C3N4 nanosheets, the catalytic performance of the hybrid catalyst such as stability and activity improved remarkably. PtNP@g–C3N4 nanosheets could quickly catalyze H2O2 to oxidize TMB, and the absorbance of blue color oxidized TMB (oxTMB) showed a robust linear relationship with the concentration of H2O2. As proof of the concept for oxidase-based sensing, a colorimetric assay of cholesterol that combined PtNP@g–C3N4 nanosheets with cholesterol oxidase (ChOx) cascade catalytic reaction was constructed. Cholesterol participates in the formation of cell membrane and can be used as a precursor for vitamin D, steroid hormone, etc. [24,25]. Cholesterol levels are associated with various diseases like cerebral thrombosis, lipid metabolic disorders, etc. [26]. As illustrated in Figure 1, H2O2 could be generated through the oxidation of cholesterol by cholesterol oxidase, following the PtNP@g–C3N4 nanosheets could quickly catalyze the oxidation reaction of TMB by the obtained H2O2, with a proportional production of blue color oxidized TMB (oxTMB). The absorbance of oxTMB showed a robust linear relationship with the concentration of cholesterol. Therefore, a highly sensitive platform for H2O2 and oxidase-based sensing has been developed which possesses great potential utility in the field of biosensing and medical disease detection.
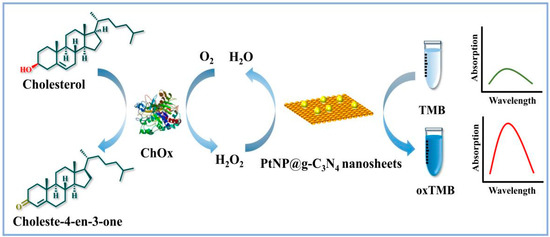
Figure 1.
Illustration of constructing PtNP/g–C3N4 as peroxidase mimic for cholesterol and H2O2 detection.
2. Results and Discussion
2.1. Characterization of PtNP@g–C3N4 Nanosheets
It could be observed in the TEM image (Figure 2A) that the g–C3N4 nanosheets were composed of thin layers with nanometer thickness. Meanwhile, the black dots (which represented platinum particles, about 4.3 nm diameter) were evenly loaded on g–C3N4 nanosheets (Figure 2B). These obtained images indicated the successful synthesis of PtNP@g–C3N4 nanosheets.
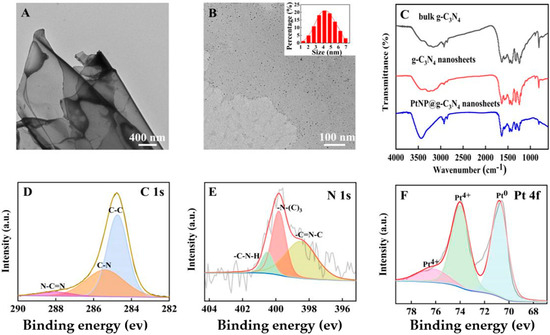
Figure 2.
(A) The TEM image of prepared g–C3N4 nanosheets; (B) The TEM image of PtNP@g–C3N4 nanosheets; (C) FT–IR spectra of three g–C3N4 products; (D) C 1s spectra of PtNP@g–C3N4 nanosheets through high–resolution XPS; (E) N 1s spectra of PtNP@g–C3N4 nanosheets through high-resolution XPS; (F) Pt 4f spectra of PtNP@g–C3N4 nanosheets through high-resolution XPS.
Further, the FT-IR spectra were scanned. The similarity (Figure 2C) of three g–C3N4 products indicated that nitric acid etching and PtNPs doping did not change the chemical structure of g–C3N4 materials. The 810 cm−1 sharp peak was caused by the out-of-plane bending vibration of the tri-s-triazine ring, and the series of peaks at 1200–1700 cm−1 were due to the stretching vibration of the aromatic CN heterocyclic unit. The peak at 3000~3600 cm−1 was caused by the vibration of the amine group or OH group [27].
At last, the chemical composition and elemental state of PtNP@g–C3N4 nanosheets were studied through high-resolution XPS. The C 1s spectrum was fitted into three peaks (Figure 2D): the 288.0 eV peak was attributed to N-C=N, 285.4 eV to C-N, and 284.6 eV to C-C, respectively. The N 1s spectrum (Figure 2E) could also be divided into three peaks, a peak at 400.5 eV corresponded to C-N-H, 399.8 eV to N-(C)3 and 398.5 eV to C=N-C, respectively. The divided peak of Pt 4f (Figure 2F) at 70.8 eV was attributed to Pt0 formed by NaBH4 reduction of Pt4+. Other peaks at 74.0 eV and 76.2 eV indicated the valence between divalent and tetravalent.
2.2. Catalytic Performance of PtNP@g–C3N4 Nanosheets
Peroxidase-mimic activity in our prepared PtNP@g–C3N4 nanosheets was explored through their catalytic capability to TMB by H2O2. The absorption peak at 662 nm originated from oxTMB. The existence of PtNP@g–C3N4 nanosheets could make the 662 nm absorption peak much higher (more than 0.5) than the system without PtNP@g–C3N4 nanosheets (only about 0.1, Figure 3A). While in the case of only PtNP@g–C3N4 nanosheets (without TMB and H2O2), the absorption of the system was nearly zero. In addition, the absorption of the system with PtNP@g–C3N4 nanosheets as catalyst (more than 0.5) was higher than in the case of g–C3N4 nanosheets (about 0.25, Figure 3A), which proved that the loading of PtNPs efficiently increased the peroxidase-mimic catalytic activity of g–C3N4 nanosheets. More importantly, the catalytic performance of PtNP@g–C3N4 nanosheets was stable. As shown in Figure 3B, it can still maintain good catalytic performance for more than 90 days.
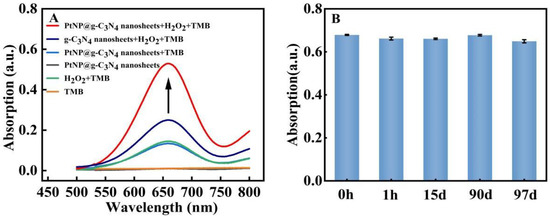
Figure 3.
Catalytic activity and stability exploration of PtNP@g–C3N4 nanosheets (A) The absorption spectra of various system; (B) The peak absorption of the system with PtNP@g–C3N4 nanosheets as catalysts in various times.
2.3. Kinetic Parameters of PtNP@g–C3N4 Nanosheets as Peroxidase Mimics
The steady-state kinetics of PtNP@g–C3N4 nanosheets was explored to investigate their catalytic activity. By changing the concentration of one substrate and keeping the alternative constant, the kinetic data was obtained and in good agreement with the Michaelis-Menten equation (Figure 4A,C). Furthermore, a typical Lineweaver-Burk diagram (Figure 4B,D) was constructed through the double-reciprocal method, which obtained the Michaelis constant (Km) and maximum initial velocity (Vmax) (Table 1). It turned out that the Km of PtNP@g–C3N4 nanosheets to TMB (0.446 mM) and H2O2 (0.105 mM) were lower than that of other typical peroxidase mimic nanozymes (Table 1), manifesting that PtNP@g–C3N4 nanosheets owed a higher affinity for the substrate.
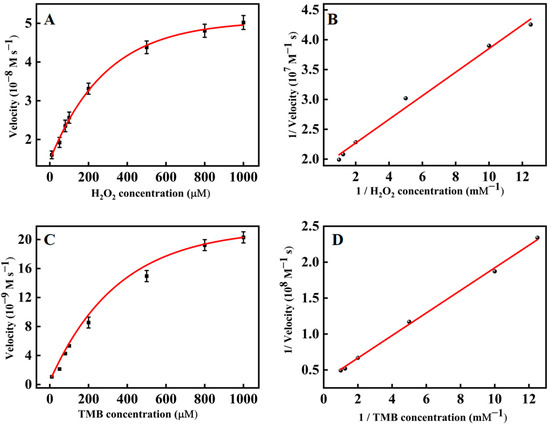
Figure 4.
The steady–state kinetics assay (A) Michaelis-Menten curve of H2O2 (TMB concentration was 1 mM); (B) Lineweaver-Burk plots with H2O2 as substrate; (C) Michaelis–Menten curve of TMB (H2O2 concentration was 0.1 mM); (D) Lineweaver-Burk plots with TMB as substrate.

Table 1.
Comparison of the Km and Vmax of Pt/GCNS nanocomplex, HRP, and other typical peroxidase mimic nanozymes.
2.4. Catalytic Mechanism of PtNP@g–C3N4 Nanosheets
Furthermore, the catalytic mechanism of PtNP@g–C3N4 nanosheets was explored. Peroxidase mimics usually have two catalytic pathways: the production of hydroxyl radicals (OH•) or the promotion of electron transfer. RhB could react with OH•, resulting in the reduction of its absorption at 550 nm [32]. Firstly, RhB was employed as a high-selective probe to capture and track OH•, which was generated in situ (Figure 5A). The absorbance of RhB almost stayed unchanged for 12 h starting from the addition of H2O2 and PtNP@g–C3N4 nanosheets, which proved that OH• was not produced. These results were further supported by UV absorption measurements by using methanol and isopropanol as OH• radical trap (Figure 5B). The addition of methanol or isopropanol had little effect on the absorbance of oxTMB. Later, the electrochemical method was used to investigate the electrocatalytic reduction behavior of our obtained catalyst to H2O2 (1 mM) in acetate buffer (Figure 5C). The reduction current of PtNP@g–C3N4 nanosheets modified glassy carbon electrode (GCE) was significantly increased compared with that of bare GCE. These results proved that PtNP@g–C3N4 nanosheets could increase the electron transfer between GCE and H2O2 with prominent catalytic ability. Therefore, the peroxidase-mimic activity of our obtained catalyst was probably attributed to the increasing electron transfer effects (the electron that transferred from TMB to H2O2 was increased by PtNP@g–C3N4 nanosheets). Moreover, PtNP@g–C3N4 nanosheets were negatively charged (the potential was −10.2 mV, Figure 5D) so they could adsorb positively charged TMB on their surface through electrostatic interaction, and thus the reaction rate was further promoted.
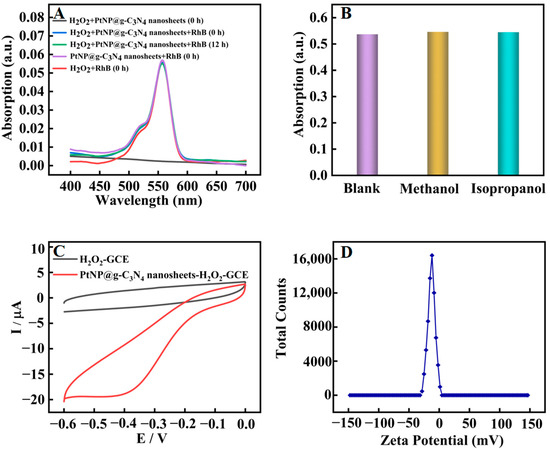
Figure 5.
Catalytic mechanism exploration of PtNP@g–C3N4 nanosheets; (A) UV absorption spectra of various system; (B) The absorbance value (662 nm) of oxTMB after the addition of methanol or isopropanol; (C) Cyclic voltammogram of bare GCE (black line) and PtNP@g–C3N4 nanosheets modified GCE (Red line) in acetate buffer containing 1 mM H2O2. Scan rate: 100 mv·s−1, platinum electrode as auxiliary, Ag/AgCl electrode as reference electrodes, and the geometric area of glassy carbon electrode used in the electrochemical experiments is 0.07 cm2; (D) Zeta potential diagram of PtNP@g–C3N4 nanosheets.
2.5. Optimization of Experiment Conditions for Detection of H2O2
In order to obtain an ideal analytical performance, the experiment conditions in the detection of H2O2 and cholesterol were optimized. In this study, A-A0 was used as a standard to select the optimum condition, A0 and A represented the absorbance responses value of the system with H2O2 at 0 and 600 μM, respectively, as for cholesterol at 0 and 2 mM.
The pH, reaction temperature and reaction time of the system in the detection of H2O2 were optimized. As shown in Figure 6A, A-A0 reached the maximum at pH = 3.6. As shown in Figure 6B, A-A0 of the system increased with time until it reached a balance within 30 min. Consequently, pH = 3.6, 45 °C and a reaction time of 30 min was chosen for subsequent experiments. Figure 6C showed that as the reaction temperature increased from 25 °C to 65 °C, A-A0 reached the maximum at 45 °C and then decreased gradually, perhaps because PtNP/g–C3N4 were easily inactivated at a high temperature.
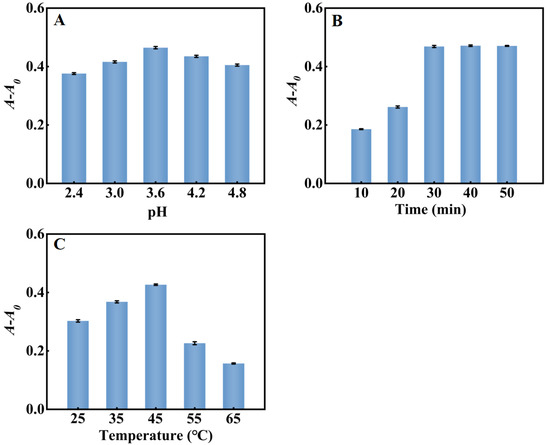
Figure 6.
Optimization of the H2O2 sensing system (A) pH; (B) reaction time; (C) reaction temperature. Error bars indicated the standard deviations of three experiments.
2.6. Optimization of Experiment Conditions for Detection of Cholesterol
The pH, reaction temperature, TMB concentration, and reaction time of the system in the detection of cholesterol were also optimized. As shown in Figure 7A, A-A0 reached the maximum at pH = 3. Under strong acid conditions, the decrease in the electron cloud density of nitrogen atoms in TMB would weaken the interaction between PtNP/g–C3N4 and TMB molecules, inhibiting the catalytic activity of PtNP/g–C3N4. Conversely, when the pH was higher than 3, unstable H2O2 tended to decompose into O2 and H2O, which greatly reduced the amount of H2O2. The results showed that, similar to natural peroxidase HRP, PtNP/g–C3N4 exhibited better catalytic activity in the acidic solution. As shown in Figure 7B, with the increase of TMB concentration, A-A0 gradually increased and then reached the maximum when 1000 μM TMB was used. As shown in Figure 7C, with the increasing of reaction temperature from 25 °C to 65 °C, A-A0 reached the maximum at 45 °C and then decreased gradually, owing to the fact that cholesterol oxidase and PtNP/g–C3N4 were easily inactivated at a high temperature. The reaction time also had an effect on the system. As shown in Figure 7D, A-A0 of the system increased with the reaction time until it reached equilibrium within 30 min. Thus, pH 3.0, 45 °C, 1000 μM TMB, and the reaction time of 30 min were chosen for subsequent cholesterol experiments.
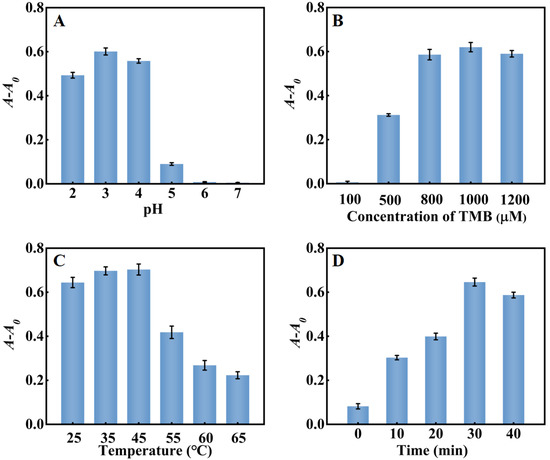
Figure 7.
Optimization of system for detection of cholesterol (A) pH; (B) TMB concentration; (C) reaction temperature; and (D) reaction time. Error bars indicated the standard deviations of three experiments.
2.7. Colorimetric Assay of H2O2
With their peroxidase-mimic catalysis performance, PtNP@g–C3N4 nanosheets could promote the colorimetric reaction and achieve high assay sensitivity. According to the principle in which H2O2 assay was first investigated. Figure 8A showed that under the best conditions (the optimization of experiment conditions was in Figure 6), the absorbance value of the system was increased with H2O2 concentration from 0 to 500 μM. Figure 8B also illustrated a fine linear relationship (R2 = 0.9959) between A-A0 and H2O2 concentration within the scope 5~200 μM with a low LOD of 3.33 μM (the LOD was estimated according to the equation: LOD = 3σ/S, S: the linear slope in Figure 8B, y = 0.0011x + 0.0027; σ: the standard deviation of blank sample with ten experiments), which was lower than many reports of H2O2 assays that based on peroxidase-mimic nanozymes (Table 2).
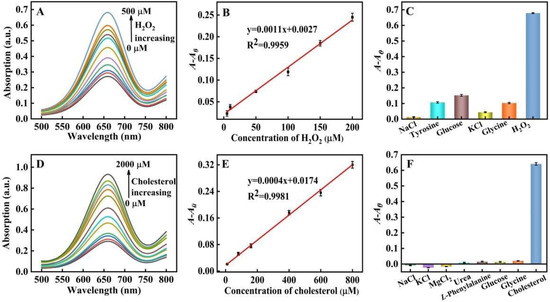
Figure 8.
(A) UV-visible absorption spectra of the system under various H2O2 concentrations (0, 5, 10, 50, 100, 150, 200, 250, 300, 400, and 500 μM, respectively); (B) The linear relationship between H2O2 concentration (the concentration from left to right was 5, 10, 50, 100, 150 and 200 μM) and A-A0; (C) The selectivity towards H2O2 and potential interferents (the concentration was all set at 600 ìM); (D) UV-visible absorption spectra under various cholesterol concentrations; (0, 10, 80, 160, 400, 600, 800, 1200, 1400, 1600, 1800, and 2000 μM, respectively); (E) The linear relationship between cholesterol concentration and A-A0. The cholesterol concentration from bottom to top was 10, 80, 160, 400, 600 and 800 μM; (F) The selectivity towards cholesterol and potential interferents (the concentrations were all set at 2 mM).

Table 2.
The detection limits and linear ranges of H2O2 detection methods in different catalyst systems.
Control experiments were also performed with coexistent NaCl, tyrosine, KCl, glucose, and glycine in blood as potential interfering substances for the evaluation of the selectivity in H2O2 assay (Figure 8C), H2O2 or interfering substances were added to the system with a final concentration of 600 μM. A-A0 of the system was more than 0.6 in the case of H2O2, as for interfering substances, which was below 0.15. These results proved the high selectively and application prospects of our proposed method of serum sample detection.
2.8. Colorimetric Assay of Cholesterol
On the basis of the fact that the catalytic activity of PtNP@g–C3N4 nanosheets showed a strong dependence on the concentration of H2O2, which could be produced during the oxidization of many biological substrates by their specific oxidase, our proposed strategy could be exploited as a platform for oxidases-based colorimetric assay. As a proof of concept, a method for cholesterol detection has been established based on PtNP@g–C3N4 nanosheets and cholesterol oxidase (ChOx) cascade catalytic reaction.
As shown in Figure 8D,E, the absorbance of our proposed assay system increased with the concentration of cholesterol. The linear detection range for cholesterol was 10~800 μM (R2 = 0.9981) with a LOD of 9.35 μM (the LOD was estimated according to the equation: LOD = 3σ/S, S: the linear slope in Figure 8E, y = 0.0004x + 0.0174; σ: the standard deviation of blank sample with ten experiments), which reached or was lower than many reported cholesterol assays that also depended on peroxidase mimic nanozymes (Table 3).

Table 3.
The detection limits and linear ranges of cholesterol detection methods in different catalyst systems.
Control experiments were carried out with coexistent inorganic salts, urea, L-phenylalanine, glucose, and glycine in blood as potential interfering substances. As shown in Figure 8F, when the interfering substances or cholesterol were added to the system with a final concentration of 2 mM, A-A0 was more than 0.6 in case of cholesterol, nevertheless A-A0 of the system containing interfering substances was nearly zero. These results proved the high selectivity of our proposed method, which showed the application prospects for serum sample detection.
2.9. Assay of Cholesterol in Fetal Bovine Serum
Our proposed platform was further used in the cholesterol analysis of fetal bovine serum to testify for its application for biological samples. Different standard concentrations of cholesterol were added into the solution that contained 10% fetal calf serum. As shown in Figure 9, the absorbance value increased in proportion to the cholesterol concentration, and a good linear relationship has been found between the cholesterol concentration and A-A0 (R2 = 0.9917). These results showed that our established platform has a certain application prospect in clinical medical detection.
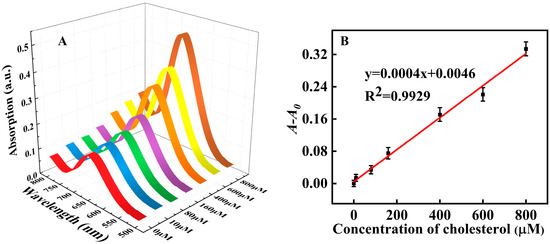
Figure 9.
Detection of cholesterol in fetal calf serum; (A) UV-visible absorption spectra with various cholesterol concentrations added to solution that contained 10% fetal calf serum; (B) The linear relationship between cholesterol concentration and A-A0.
3. Materials and Methods
3.1. Materials and Instruments
Cholesterol (≥99%) was purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Fetal bovine serum (FBS) was purchased from Zhejiang Tianhang Biotechnology Co., Ltd. (Huzhou, China). 3,3′,5,5′-tetramethylbenzidine (TMB, ≥99.7%), glycine (≥99), l-phenylalanine (≥99%), melamine (99%), urea (≥99.5%), dimethyl sulfoxide (DMSO, 95%), glucose (AR), and polyvinylpyrrolidone (PVP, 95%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). Cholesterol oxidase (ChOx, ≥95%), Rhodamine B (RhB, AR), and chloroplatinic acid hexahydrate (H2PtCl6.6H2O, AR) were obtained from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Polyethylene glycol mono-4-octylphenyl ether (Triton X-100) was provided by Saen Chemical Technology (Shanghai, China) Co., Ltd. All the aqueous solutions in this study were prepared with ultrapure water (>18.2 MΩ·cm, Millipore-D 24uv).
UV-vis absorption spectra were collected by TU-1901 UV-visible spectrophotometer (Beijing, China). The morphologies were performed on a Hitachi HT-7700 transmission electron microscope (TEM, Tokyo, Japan). Fourier transform infrared spectra (FT-IR) were recorded on a Thermo Scientific Nicolet iS10 (Waltham, MA, USA). X-ray photoelectron spectroscopy (XPS) measurement was measured by Thermo Scientific Escalab 250Xi (Waltham, MA, USA). Zeta-potential analysis was obtained from Anton Paar. Electrochemical data was conducted at CHI 660E electrochemical workstation (Shanghai, China).
3.2. Synthesis of PtNP@g–C3N4 Nanosheets
First, the bulk g–C3N4 was prepared according to our previous method [41]. After 2 h ultrasonic treatment in 100 mL 5 M nitric acid, 1 g bulk g–C3N4 was refluxed for 24 h under 120 °C. The product was then washed to neutrality with ultrapure water through 5 min centrifugation at 2680× g repeatedly. After thorough drying, 200 mg power was again dispersed in 40 mL ultrapure water and was treated with ultrasound for 2 h. Then the dispersive g–C3N4 nanosheets were obtained.
PtNP@g–C3N4 nanosheets (3 mg/mL g–C3N4 nanosheets included) were synthesized as follow: After 0.22 µm filter dialysis to remove the large size nanosheets, the 1 mL g–C3N4 nanosheets solution was mingled with 7 mL polyvinylpyrrolidone (PVP, 16 mg) solution, and 70 µL 0.1 M H2PtCl6.6H2O, followed with heating at 95 °C for 20 min. After that 500 µL freshly prepared 150 mM NaBH4 was dropped into the above solution continued with stirring at 95 °C for 30 min. Lastly, after centrifugation and washing with ultrapure water to neutrality, the obtained precipitate was ultrasonically treated in 1 mL ultrapure water and then kept at 4 °C in refrigerator, with ultrasonic treatment for 10 min before use.
3.3. Investigation of PtNP@g–C3N4 Nanosheets Peroxidase-Mimic Activity
We investigated the catalytic activity of PtNP@g–C3N4 nanosheets as below: 5 μL obtained (3 mg/mL g–C3N4 nanosheets included) PtNP@g–C3N4 nanosheets, 50 μL of 1 mM H2O2, 50 μL of 1 mM TMB, and 150 μL of 0.2 M pH 3.0 acetate buffer were mixed in a tube with a final volume of 500 μL. After incubation at 35 °C for 40 min, the absorbance of the mixture from 500 to 800 nm was detected through UV-visible spectroscopy.
3.4. Steady-State Kinetic Assays
Steady-state kinetic analysis of PtNP/g–C3N4 peroxidase-like activity was carried out by time scanning spectrum. TMB with varied concentration was added into the tube with 50 μL 1 mM H2O2, 150 μL acetate buffer (0.2 M, pH 3.0), and 5 μL PtNP/g–C3N4 mixed, and the absorbance value of the mixture following the change of time (2 min) was measured at a maximum absorption wavelength. The reaction speed of the system with different TMB concentrations could be obtained by the Lambert-Beer law (A = εbc, A represents the measured absorbance value, the molar absorption coefficient ε = 39,000 mol−1cm−1, the thickness of absorption layer b = 0.2 cm, c is the concentration of the light-absorbing substance) and the Michaelis-Menten equation 1/V = (Km/Vmax) × 1/[S] + 1/Vmax (V, [S], Vmax and Km represents initial rate, substrate concentration, the maximum reaction rate, and the Michaelis constant, respectively) could be curved, with Km and Vmax being calculated through the equation. Subsequently, according to the same process, different concentrations of H2O2 were added to a mixture containing 5 μL PtNP/g–C3N4, 50 μL 10 mM TMB, and 150 μL acetate buffer, and Km and Vmax could also be calculated.
3.5. H2O2 Colorimetric Assay
In the colorimetric detection of H2O2, 100 μL H2O2 with various concentration, 10 μL PtNP@g–C3N4, 50 μL 10 mM TMB, 150 μL acetic acid buffer (0.2 mol/L, pH = 3.0), and 190 μL ultrapure water were mixed in a tube. After that the tube was incubated at 45 °C for 10 min and then the absorbance value of the mixture was detected.
3.6. Cholesterol Colorimetric Assay
As for the detection of cholesterol, 20 μL cholesterol with different concentration, 10 μL 100 U/mL ChOx and 265 μL ultrapure water were mixed in a tube, which was then incubated at 37 °C for 30 min. Later, 5 μL PtNP@g–C3N4, 50 μL 10 mM TMB, 150 μL acetic acid buffer (0.2 mol/L, pH = 3.0) was mixed with the solution, continued by a 30 min incubation at 45 °C. Finally, UV-visible spectroscopy was used to measure the absorbance value of the mixture.
4. Conclusions
In summary, we have fabricated a hybrid catalyst through PtNPs in situ growth on g–C3N4 nanosheets with increased peroxidase-mimic preformance. Based on their peroxidase-like capability to catalyze the oxidation of TMB, PtNP@g–C3N4 nanosheets have been used to develop a sensitive colorimetric strategy for H2O2 assay (LOD: 3.33 μM). More meaningfully, a colorimetric platform based on this strategy can be exploited for assay of many biological substrates where H2O2 can be generated or consumed based on specific oxidase-catalyzed oxidation. As a proof of concept, a quantitative colorimetric assay for cholesterol (LOD: 9.35 μM) was conducted based on PtNP@g–C3N4 nanosheets and cholesterol oxidase cascade catalytic reaction, which is more sensitive than many reported methods that depended on peroxidase-mimic nanozymes. The PtNP@g–C3N4 nanosheets based detection platform also exhibits high selectivity. Moreover, it works well in serum samples, indicating the promising potential applications in the clinical diagnosis of biomolecules related to H2O2. We plan to develop composite materials based on g–C3N4 nanosheets with excellent catalytic performance and apply it to biochemical analysis.
Author Contributions
Conceptualization, Y.C. and C.S.; methodology, G.Y.; validation, G.Y., Y.C., R.S. and S.G.; formal analysis, G.Y., Y.C., R.C. and S.G.; resources, C.S.; data curation, Y.C., S.G. and X.Z.; writing—original draft preparation, Y.C. and C.S.; writing—review and editing, C.S., Z.Q., Y.R. and Y.L.; project administration, C.S.; funding acquisition, C.S., Z.Q. and Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NSFC of China (21705002), Natural Science Foundation of Education Committee of Anhui Province (2022AH050890, KJ2021A0176), Open Foundation of Key Laboratory of Agricultural Sensors, Ministry of Agriculture, Anhui Agricultural University (KLAS2022KF004), the Nature Science Major Project for Anhui Provincial University (2022AH040125), Open Foundation of Hunan Provincial Key Laboratory of Cytochemistry, Changsha University of Science and Technology (2021xbhx01).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Abbreviations
PtNPs: Pt nanoparticles; PtNP/g–C3N4 nanosheets, Pt nanoparticles loaded graphitic carbon nitride nanosheets; TMB, 3,3′, 5,5′-tetramethylbenzidine; HRP, horseradish peroxidase; DMSO, dimethyl sulfoxide; PVP, polyvinylpyrrolidone; ChOx, cholesterol oxidase; RhB, Rhodamine B; H2PtCl6·6H2O, chloroplatinic acid hexahydrate; Triton X-100, polyethylene glycol mono-4-octylphenyl ether; TEM, transmission electron microscope; XPS, X-ray photoelectron spectroscopy; FT-IR, Fourier transform infrared spectra.
References
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-Like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Dong, H.; Fan, Y.; Zhang, W.; Gu, N.; Zhang, Y. Catalytic Mechanisms of Nanozymes and Their Applications in Biomedicine. Bioconjugate Chem. 2019, 30, 1273–1296. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-Dimensional MnO2 Nanozyme-Mediated Homogeneous Electrochemical Detection of Organophosphate Pesticides without the Interference of H2O2 and Color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Zhang, S.; Chen, X.; Li, P.; Gao, Y.; Xie, S.; Zhang, A.; Wang, H. Plasma-Assisted Controllable Doping of Nitrogen into MoS2 Nanosheets as Efficient Nanozymes with Enhanced Peroxidase-Like Catalysis Activity. ACS Appl. Mater. Interfaces 2020, 12, 17547–17556. [Google Scholar] [CrossRef]
- Qin, N.; Pan, A.; Yuan, J.; Ke, F.; Wu, X.; Zhu, J.; Liu, J.; Zhu, J. One-Step Construction of a Hollow Au@Bimetal–Organic Framework Core–Shell Catalytic Nanoreactor for Selective Alcohol Oxidation Reaction. ACS Appl. Mater. Interfaces 2021, 13, 12463–12471. [Google Scholar] [CrossRef]
- Qin, N.; Wu, X.; Liu, X.; Xue, Z.; Muddassir, M.; Sakiyama, H.; Xia, C.; Zhang, C.; Zhu, L.; Ke, F. Well-Arranged Hollow Au@Zn/Ni-MOF-2-NH2 Core–Shell Nanocatalyst with Enhanced Catalytic Activity for Biomass-Derived d-Xylose Oxidation. ACS Sustain. Chem. Eng. 2022, 10, 5396–5403. [Google Scholar] [CrossRef]
- Wang, H.; Song, A.; Chen, H.; Zhang, W.; Xue, Z. Charge-Storage Nickel Substrate-Boosted CuP2 Nanosheet for the Electrochemical Oxygen Evolution Reaction. Inorg. Chem. 2022, 61, 12489–12493. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, D.; Zeng, G.; Chi, H.; Li, L.; He, Y.; Ke, F.; Xiao, J.; Ye, S. Dysprosium Doped CoFe2O4 with Enhanced Magnetic Property and Photodegradation Activity of Methyl Orange. Mater. Lett. 2021, 284, 128966. [Google Scholar] [CrossRef]
- Huang, Y.; Gu, Y.; Liu, X.; Deng, T.; Dai, S.; Qu, J.; Yang, G.; Qu, L. Reusable ring-like Fe3O4/Au Nanozymes with Enhanced Peroxidase-Like Activities for Colorimetric-SERS Dual-Mode Sensing of Biomolecules in Human Blood. Biosens. Bioelectron. 2022, 209, 114253. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Y.; Wang, Q.; Lin, A.; Wei, H. Nanozyme-Enabled Analytical Chemistry. Anal. Chem. 2022, 94, 312–323. [Google Scholar] [CrossRef]
- Navyatha, B.; Singh, S.; Nara, S. AuPeroxidase nanozymes: Promises and applications in biosensing. Biosens. Bioelectron. 2021, 175, 112882. [Google Scholar] [CrossRef] [PubMed]
- Romanholo, P.; Razzino, C.A.; Raymundo-Pereira, P.A.; Prado, T.M.; Sgobbi, L.F. Biomimetic Electrochemical Sensors: New Horizons and Challenges in Biosensing Applications. Biosens. Bioelectron. 2021, 8, 113242. [Google Scholar] [CrossRef]
- Paudyal, J.; Wang, P.; Zhou, F.; Liu, Y.; Cai, Y.; Xiao, Y. Platinum-Nanoparticle-Modified Single-Walled Carbon Nanotube-Laden Paper Electrodes for Electrocatalytic Oxidation of Methanol. ACS Appl. Nano Mater. 2021, 4, 13798–13806. [Google Scholar] [CrossRef]
- Draz, M.S.; Lakshminaraasimulu, N.K.; Krishnakumar, S.; Battalapalli, D.; Vasan, A.; Kanakasabapathy, M.K.; Sreeram, A.; Kallakuri, S.; Thirumalaraju, P.; Li, Y.; et al. Motion-Based Immunological Detection of Zika Virus Using Pt-Nanomotors and a Cellphone. ACS Nano 2018, 12, 5709–5718. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, T.; Kang, E. Hairy Hybrid Nanorattles of Platinum Nanoclusters with Dual-Responsive Polymer Shells for Confined Nanocatalysis. Macromolecules 2016, 49, 5649–5659. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Sun, X.; Chen, L.; Xu, Z. Boron Nitride Nanosheet/CuS Nanocomposites as Mimetic Peroxidase for Sensitive Colorimetric Detection of Cholesterol. Sens. Actuators B Chem. 2017, 246, 118–126. [Google Scholar] [CrossRef]
- Chen, L.; Xing, S.; Qing, Z.; Chen, Q.; Zou, Z.; Quan, K.; Lei, Y.; Liu, J.; Yang, R. A Glucose-Powered Activatable Nanozyme Breaking pH and H2O2 Limitations for Treating Diabetic Infections. Angew. Chem. Int. Ed. 2021, 60, 23534–23539. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.; Wang, X.; Guo, F.; Wen, H.; Yang, T.; Wang, J. Enhanced Peroxidase-Like Activity of AuNPs Loaded Graphitic Carbon Nitride Nanosheets for Colorimetric Biosensing. Anal. Chim. Acta 2019, 1091, 69–75. [Google Scholar] [CrossRef]
- Fu, L.; Chen, G.; Jiang, N.; Yu, J.; Lin, C.; Yu, A. In Situ Growth of Metal Nanoparticles on Boron Nitride Nanosheets as Highly Efficient Catalysts. J. Mater. Chem. A 2016, 4, 19107–19115. [Google Scholar] [CrossRef]
- Tang, S.; Wang, M.; Li, G.; Li, X.; Chen, W.; Zhang, L. Ultrasensitive Colorimetric Determination of Silver(I) Based on the Peroxidase Mimicking Activity of a Hybrid Material Composed of Graphitic Carbon Nitride and Platinum Nanoparticles. Microchim. Acta 2018, 185, 273. [Google Scholar] [CrossRef]
- Qiu, H.; Pu, F.; Ran, X.; Liu, C.; Ren, J.; Qu, X. Nanozyme as Artificial Receptor with Multiple Readouts for Pattern Recognition. Anal. Chem. 2018, 90, 11775–11779. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Zhang, X.; Zhang, X.; Lin, Z.; Lin, Q.; Ql, A. 5,10,15,20-Tetrakis (4-Carboxylphenyl) Porphyrin Functionalized NiCo2S4 yolk-shell Nanospheres: Excellent Peroxidase-Like Activity, Catalytic Mechanism and Fast Cascade Colorimetric Biosensor for Cholesterol. Sens. Actuators B Chem. 2021, 326, 128850. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, Y. Designing Recognition Molecules and Tailoring Functional Surfaces for In Vivo Monitoring of Small Molecules in the Brain. Acc. Chem. Res. 2018, 51, 688–698. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Rawat, K. Au@Carbon Dot Nanoconjugates As a Dual Mode Enzyme-Free Sensing Platform for Cholesterol. J. Mater. Chem. B 2017, 5, 5425–5432. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Xu, T.; Li, Y.; Wang, C. Size Effect of Pt Co-Catalyst on Photocatalytic Efficiency of g–C3N4 for Hydrogen Evolution. Appl. Surf. Sci. 2019, 464, 36–42. [Google Scholar]
- Navadeepthy, D.; Rebekah, A.; Viswanathan, C.; Ponpandian, N. N-Doped Graphene/ZnFe2O4: A Novel Nanocomposite for Intrinsic Peroxidase Based Sensing of H2O2. Mater. Res. Bull. 2017, 95, 1–8. [Google Scholar]
- Muhammad, N.; Sajid, R.; Nawshad, M.; Mian, H.N.; Aqif, A.C.; Muhammad, H.M.; Shakir, A.S.; Akhtar, H. Biomimetic Nitrogen Doped Titania Nanoparticles as a Colorimetric Platform for Hydrogen Peroxide Detection. J. Colloid Interface Sci. 2017, 505, 1147–1157. [Google Scholar]
- Hu, L.; Yuan, Y.; Zhang, L.; Zhao, J.; Majeed, S.; Xu, G. Copper Nanoclusters as Peroxidase Mimetics and Their Applications to H2O2 and Glucose Detection. Anal. Chim. Acta 2013, 762, 83–86. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, Y.; Shen, J.; Qi, W.; Wang, H. Fabrication of Polyethyleneimine-Functionalized Reduced Graphene Oxide-Hemin-Bovine Serum Albumin (PEI-rGO-Hemin-BSA) Nanocomposites as Peroxidase Mimetics for the Detection of Multiple Metabolites. Anal. Chim. Acta 2019, 1070, 80–87. [Google Scholar] [CrossRef]
- Cai, S.; Fu, Z.; Xiao, W.; Xiong, Y.; Wang, C.; Yang, R. Zero-Dimensional/Two-Dimensional AuxPd100–x Nanocomposites with Enhanced Nanozyme Catalysis for Sensitive Glucose Detection. ACS Appl. Mater. Interfaces 2020, 12, 11616–11624. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sun, B.; Wang, X.; Qiao, F.; Ai, S. 2D Ultrathin Nanosheets of Co–Al Layered Double Hydroxides Prepared in l-Asparagine Solution: Enhanced Peroxidase-Like Activity and Colorimetric Detection of Glucose. J. Mater. Chem. B 2013, 1, 2268–2274. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.K.; Loh, P.Y.; Sow, C.H.; Chin, W.S. CoOOH Nanosheet Electrodes: Simple Fabrication for Sensitive Electrochemical Sensing of Hydrogen Peroxide and Hydrazine. Biosens. Bioelectron. 2013, 39, 255–260. [Google Scholar] [CrossRef]
- Chen, L.; Sun, K.; Li, P.; Fan, X.; Sun, J.; Ai, S. DNA-Enhanced Peroxidase-Like Activity of Layered Double Hydroxide Nanosheets and Applications in H2O2 and Glucose Sensing. Nanoscale 2013, 5, 10982–10988. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Qi, C.; Zheng, X.; Zheng, J. Facile Synthesis of Silver Nanoparticle-Decorated Graphene Oxide Nanocomposites and Their Application for Electrochemical Sensing. New J. Chem. 2015, 39, 9358–9362. [Google Scholar] [CrossRef]
- Kuo, C.; Lan, W.-J.; Chen, C. Redox Preparation of Mixed-valence Cobalt Manganese Oxide Nanostructured Materials: Highly Efficient Noble Metal-Free Electrocatalysts for Sensing Hydrogen Peroxide. Nanoscale 2014, 6, 334–341. [Google Scholar] [CrossRef]
- Umar, A.; Ahmad, R.; Hwang, S.W.; Kim, S.H.; Al-Hajry, A.; Hahn, Y.B. Development of Highly Sensitive and Selective Cholesterol Biosensor Based on Cholesterol Oxidase Co-Immobilized with α-Fe2O3 Micro-Pine Shaped Hierarchical Structures. Electrochim. Acta 2014, 135, 396–403. [Google Scholar] [CrossRef]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric Detection of Cholesterol Based on Highly Efficient Peroxidase Mimetic Activity of Graphene Quantum Dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Nantaphol, S.; Chailapakul, O. Sensitive and Selective Electrochemical Sensor Using Silver Nanoparticles Modified Glassy Carbon Electrode for Determination of Cholesterol in Bovine Serum. Sens. Actuators B Chem. 2015, 207, 193–198. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, G.; Gao, S.; Zhang, L.; Yu, M.; Song, C.; Lu, Y. Highly Rapid and Non-Enzymatic Detection of Cholesterol Based on Carbon Nitride Quantum Dots as Fluorescent Nanoprobes. RSC Adv. 2020, 10, 39596–39600. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).