Design and Synthesis of Cofacially-Arrayed Polyfluorene Wires for Electron and Energy Transfer Studies
Abstract
1. Introduction
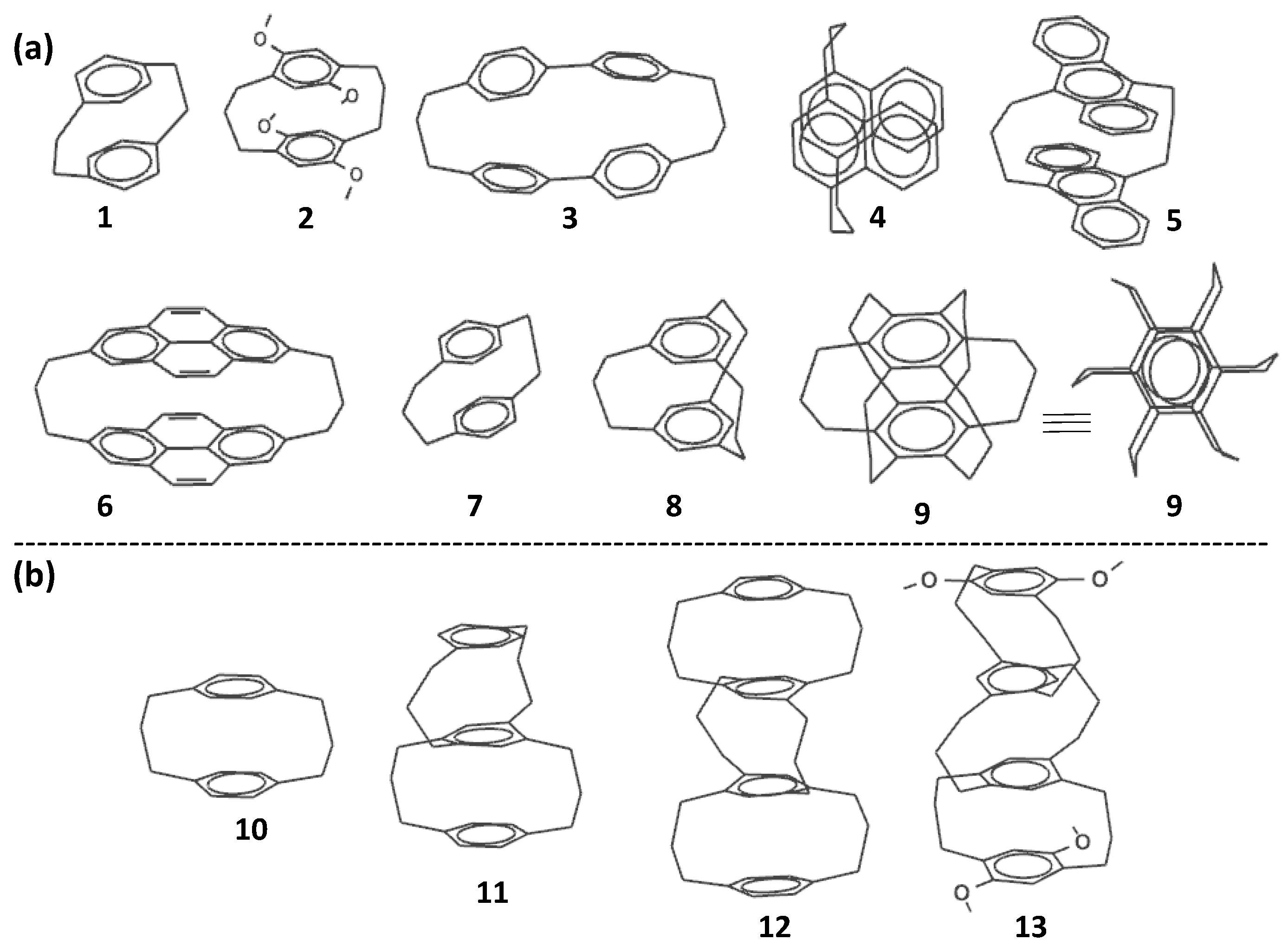
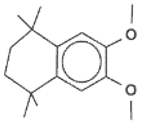
2. Cyclophanes as Models for the Study of π-Stacking
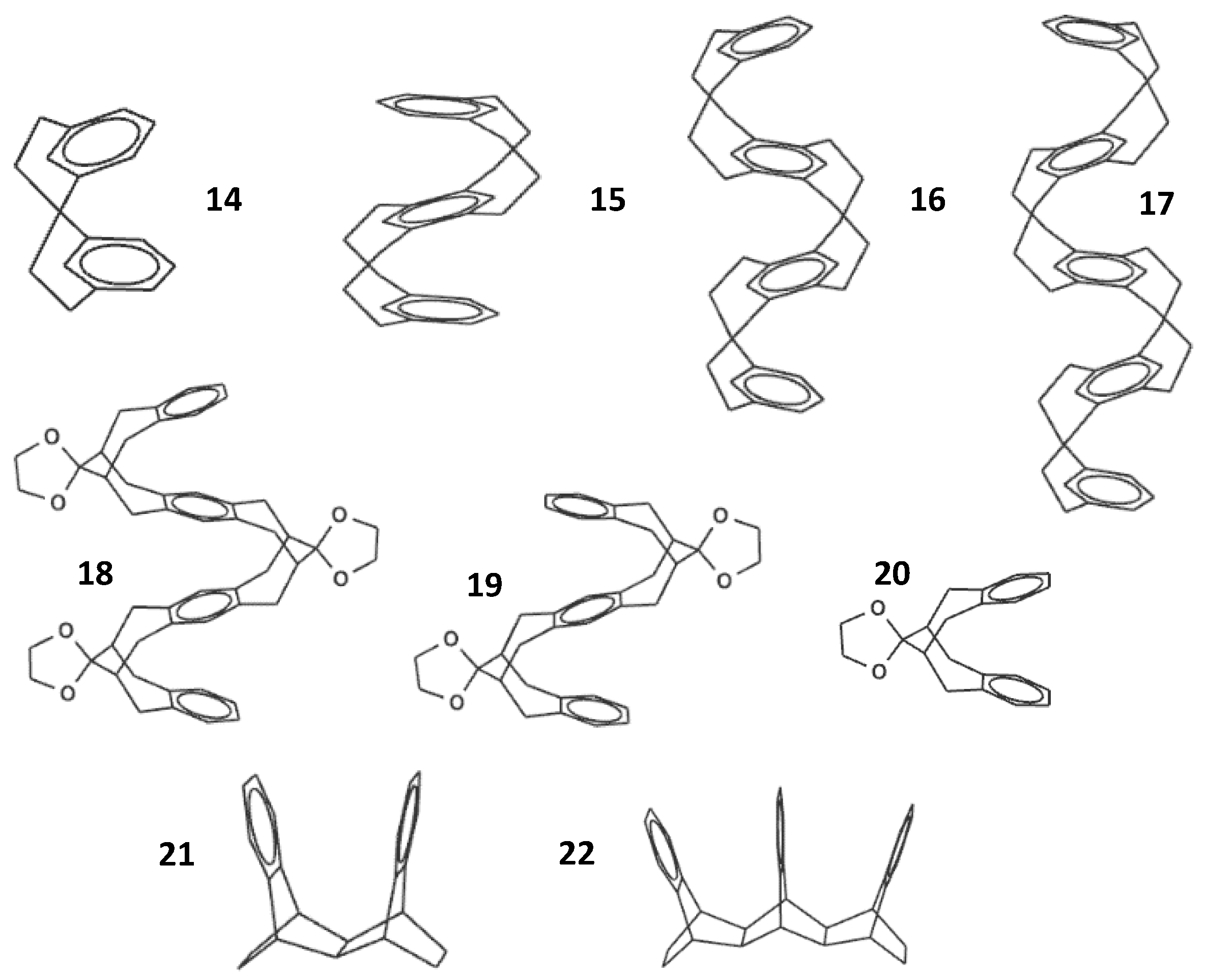
3. Multi-Decker Cyclophanes
4. Rationale for the Design of a New Class of Cofacially π-Stacked Polyfluorenes
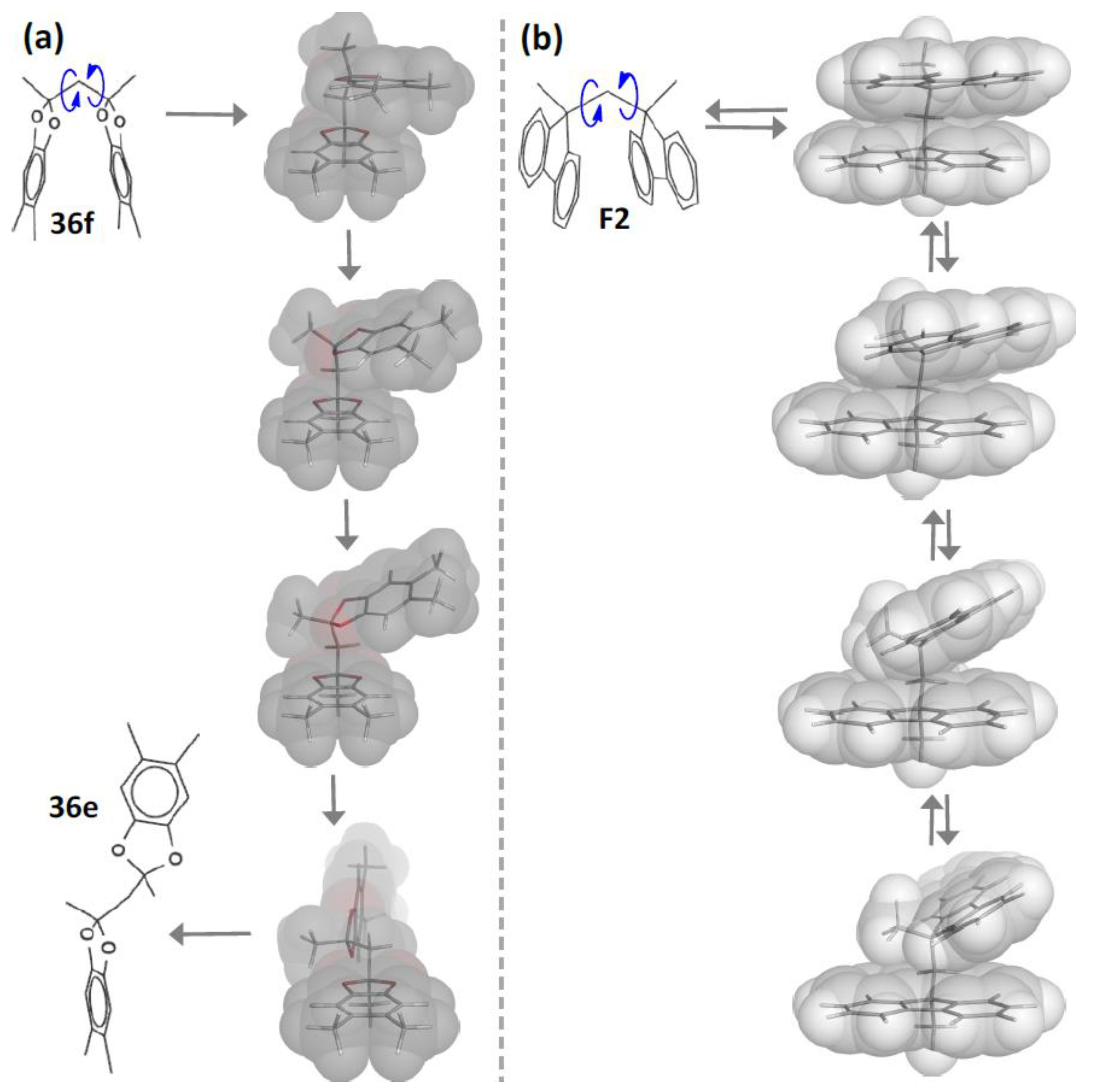
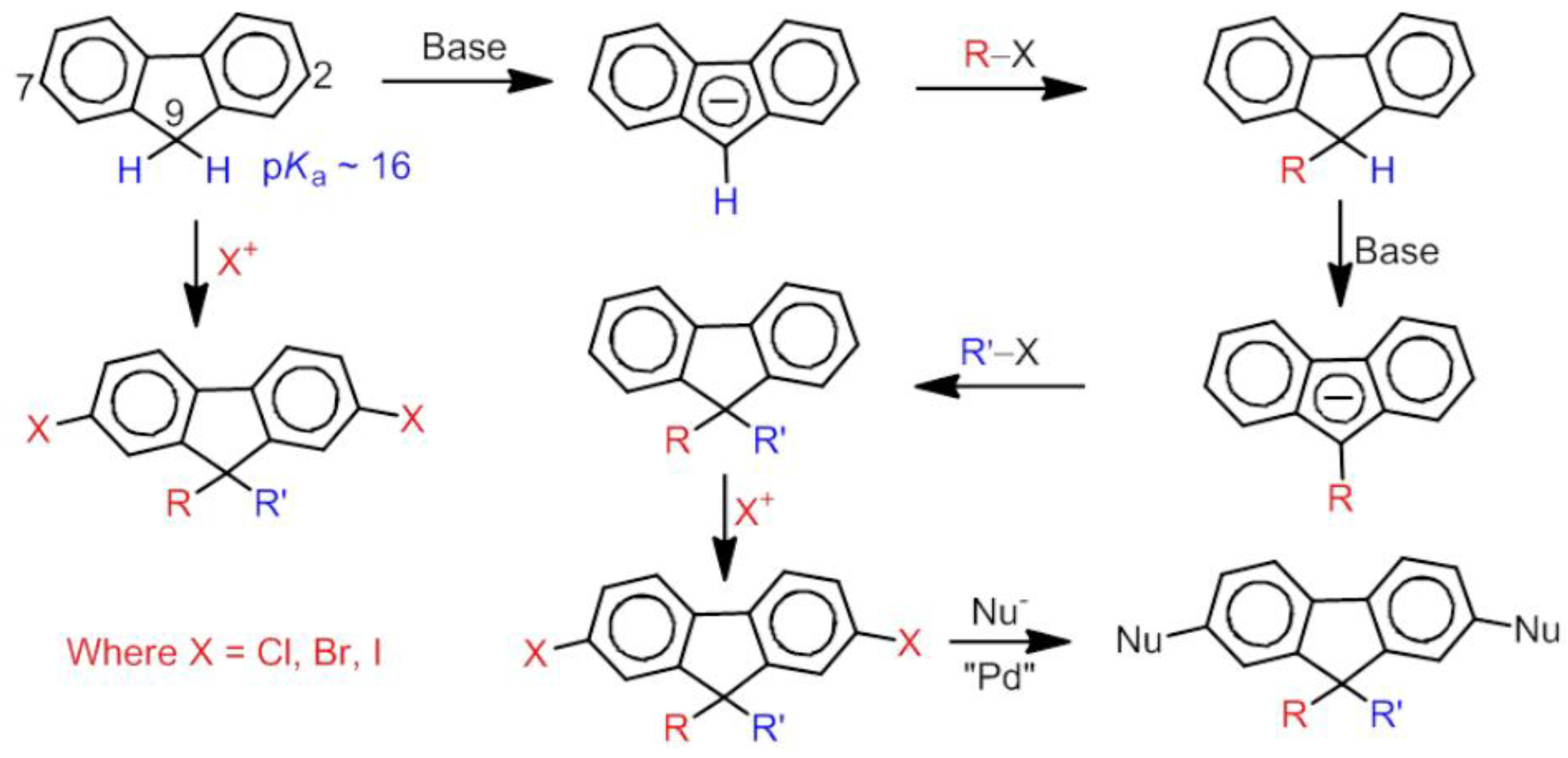
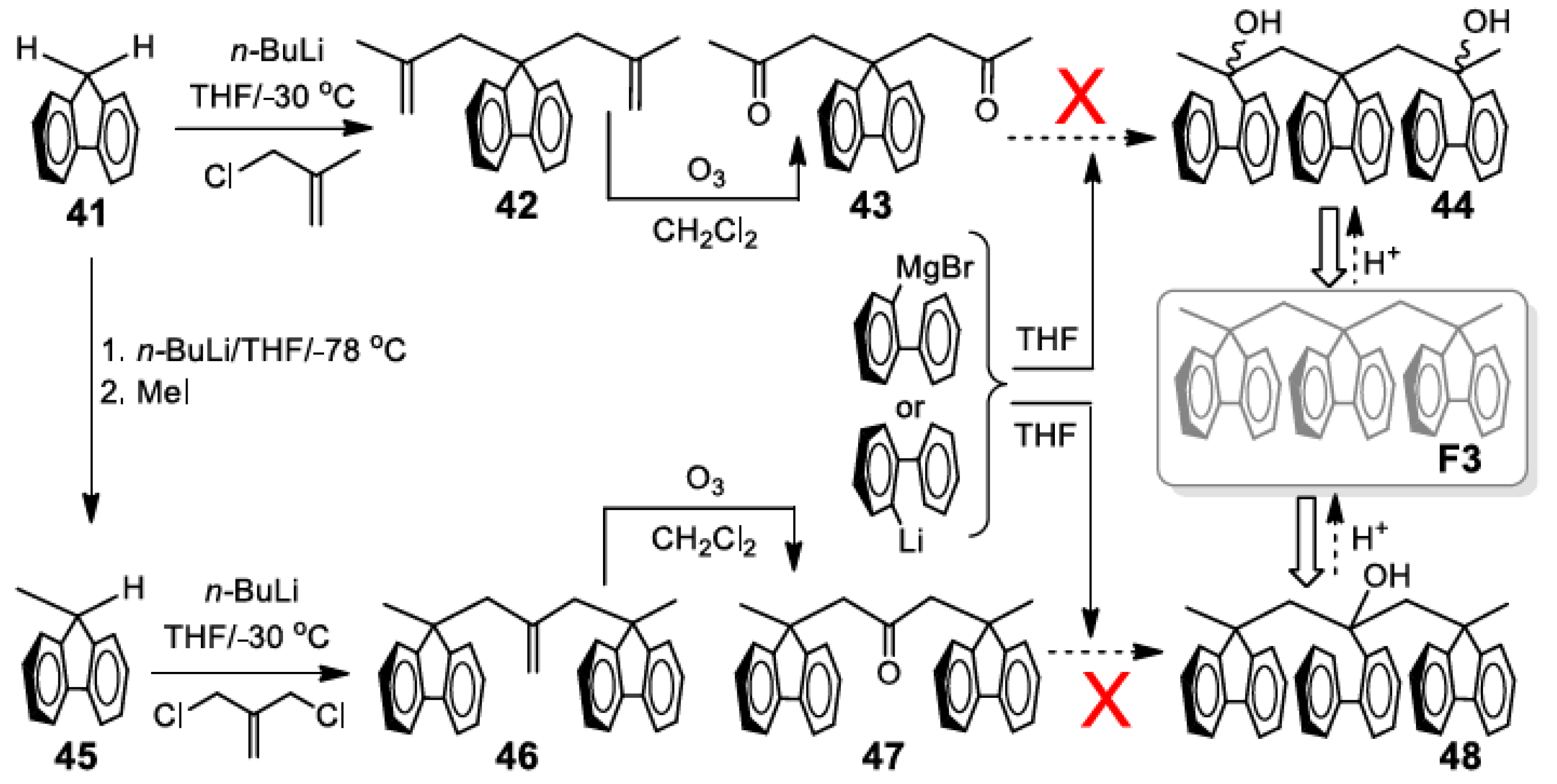
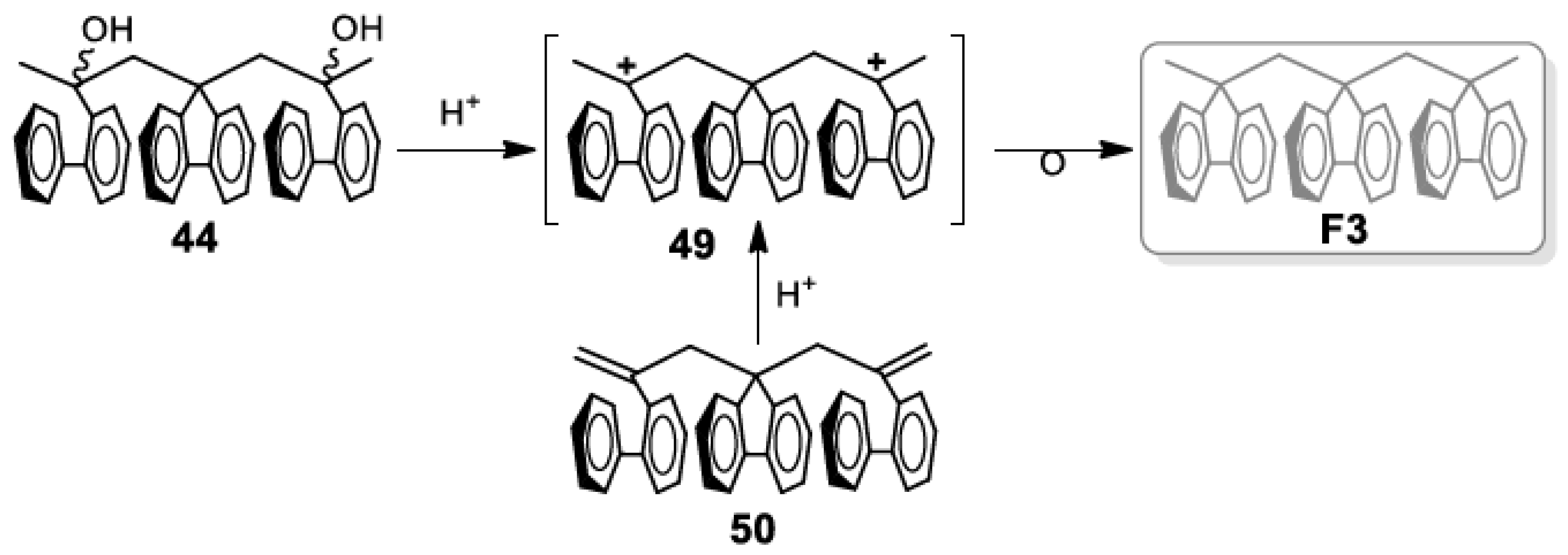
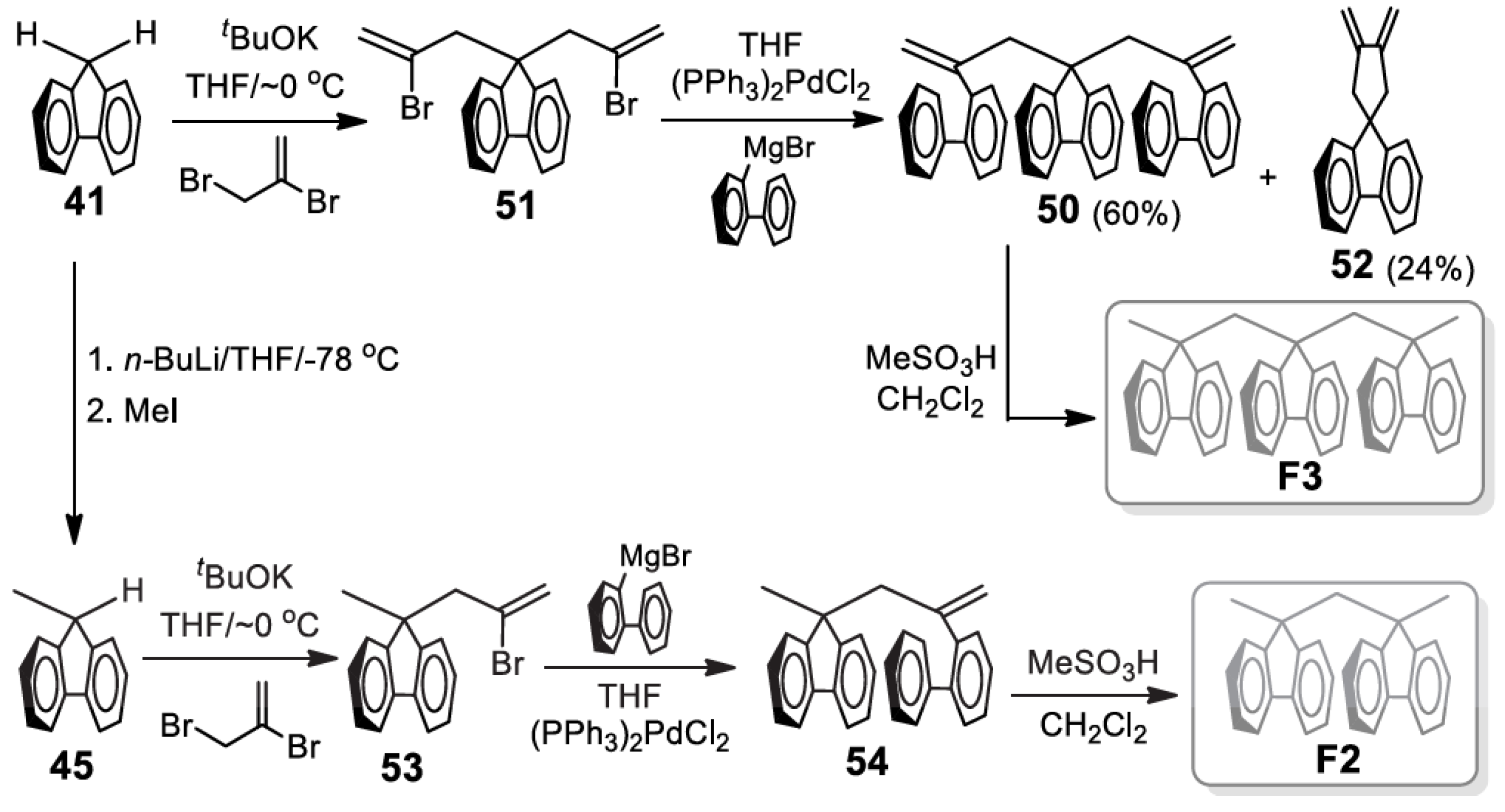
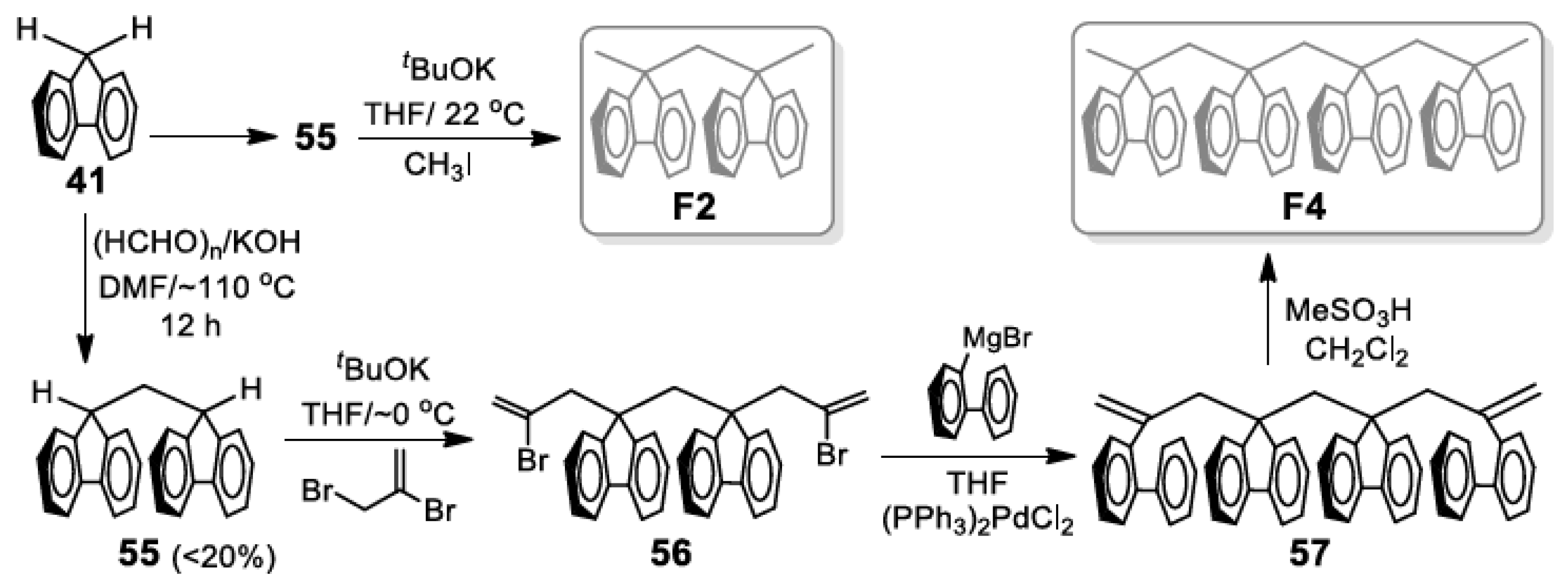
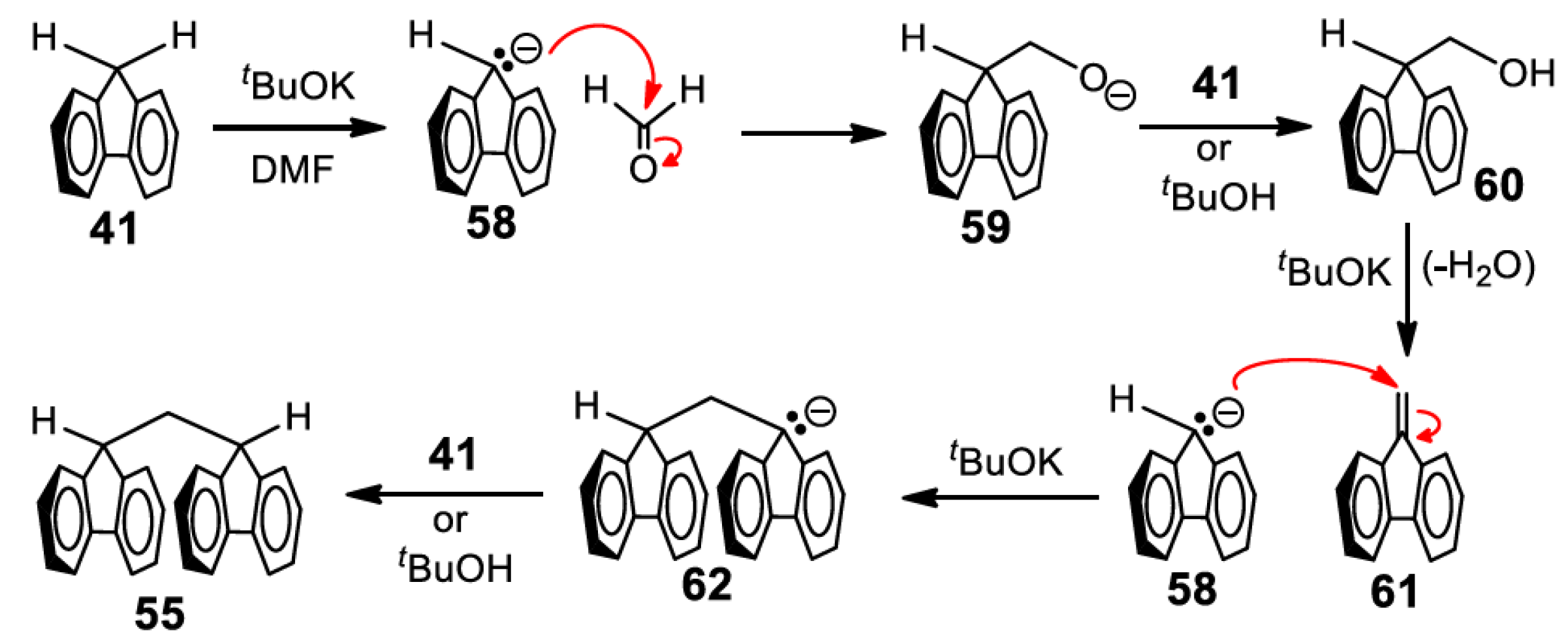
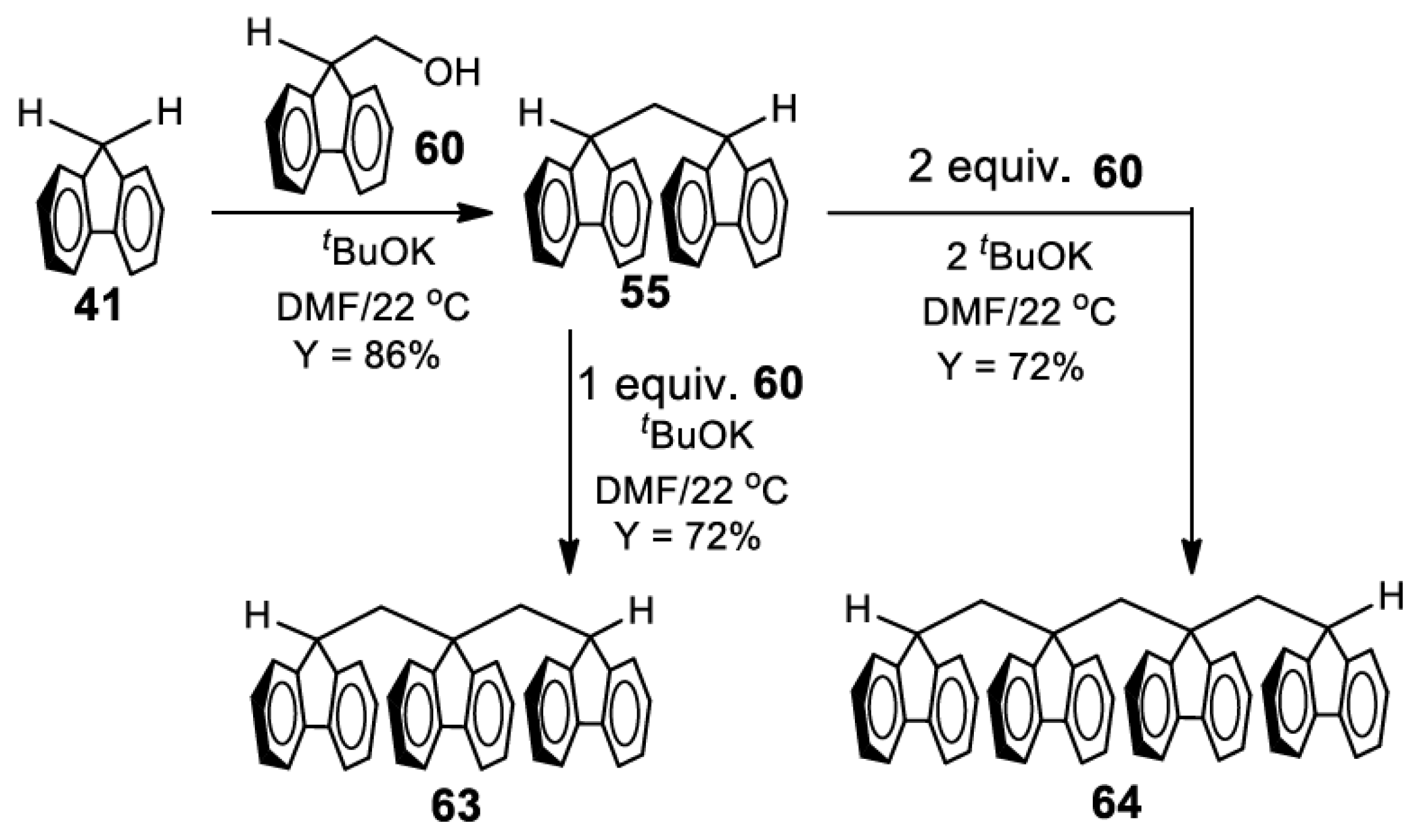
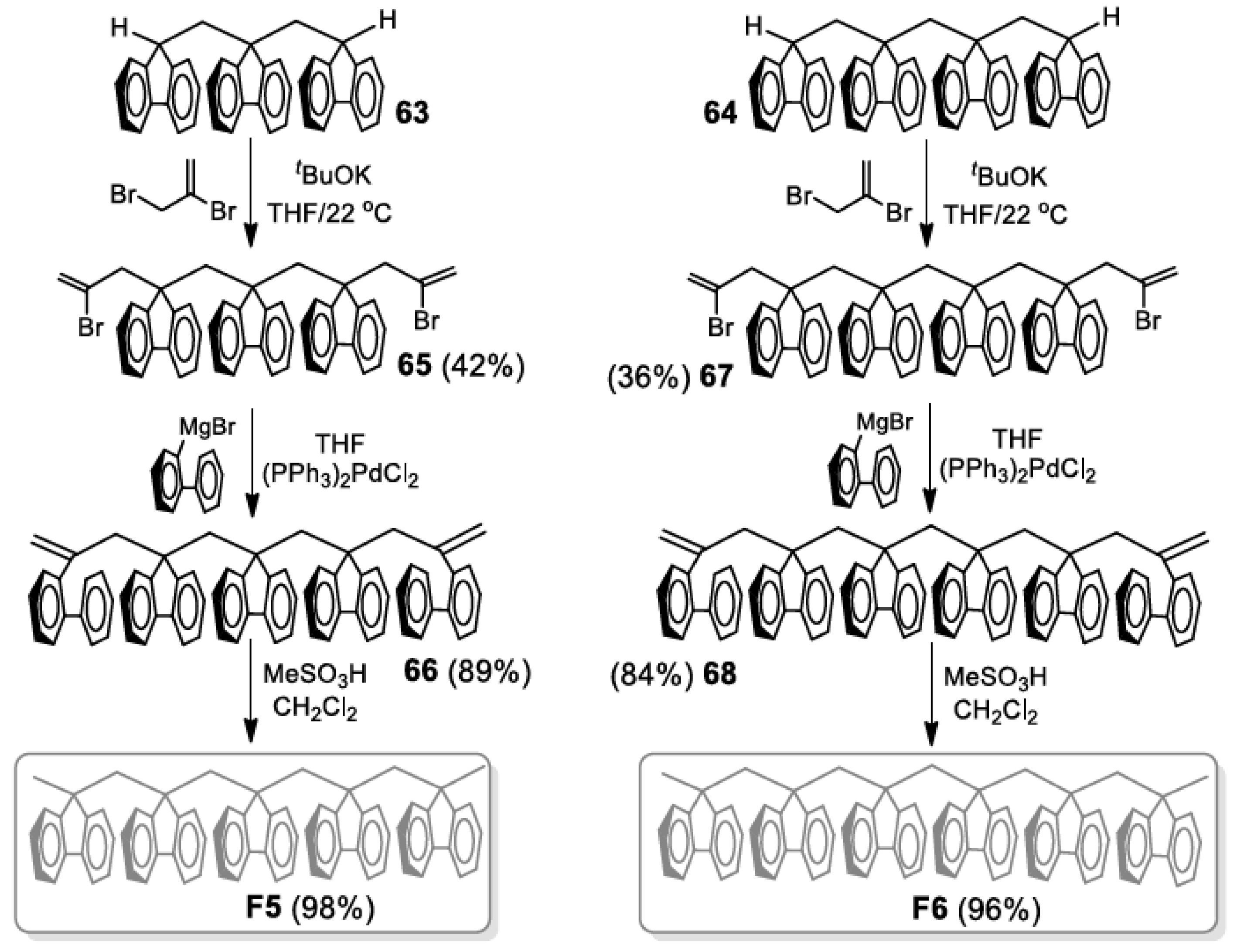
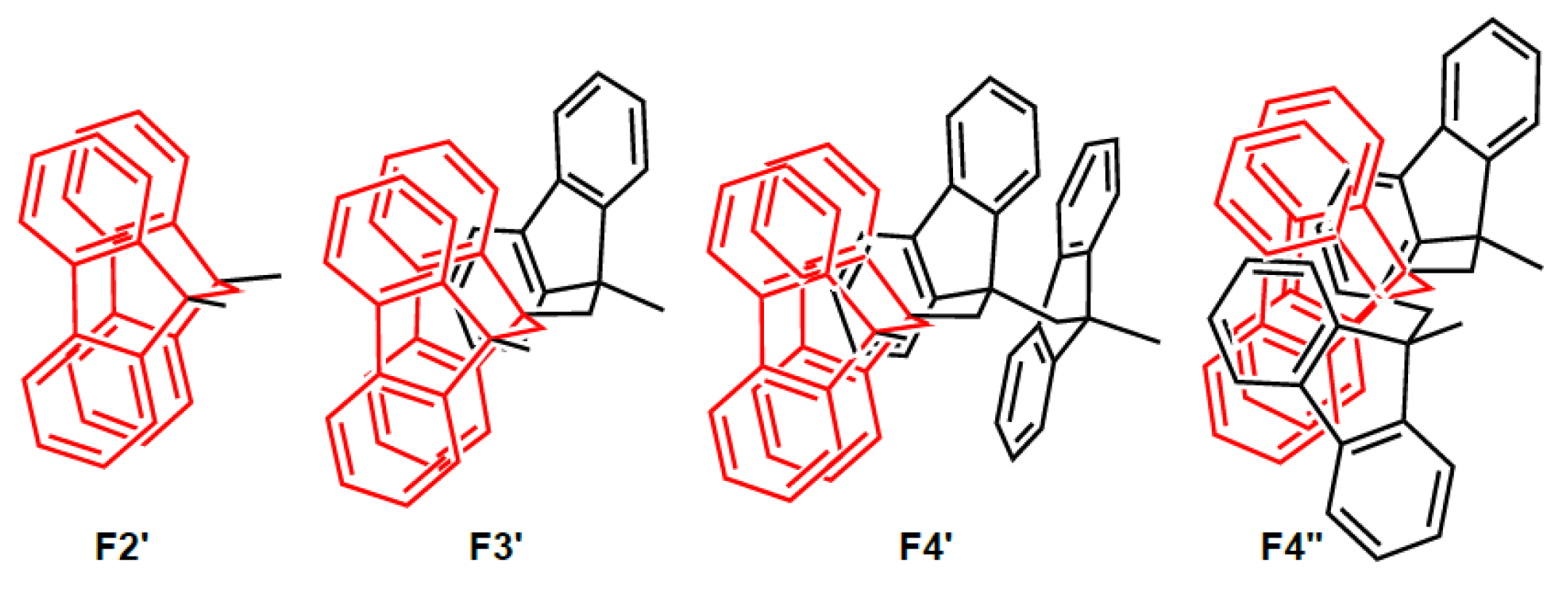
5. Development of Synthetic Strategies for the Preparation of π-Stacked Polyfluorene Oligomers
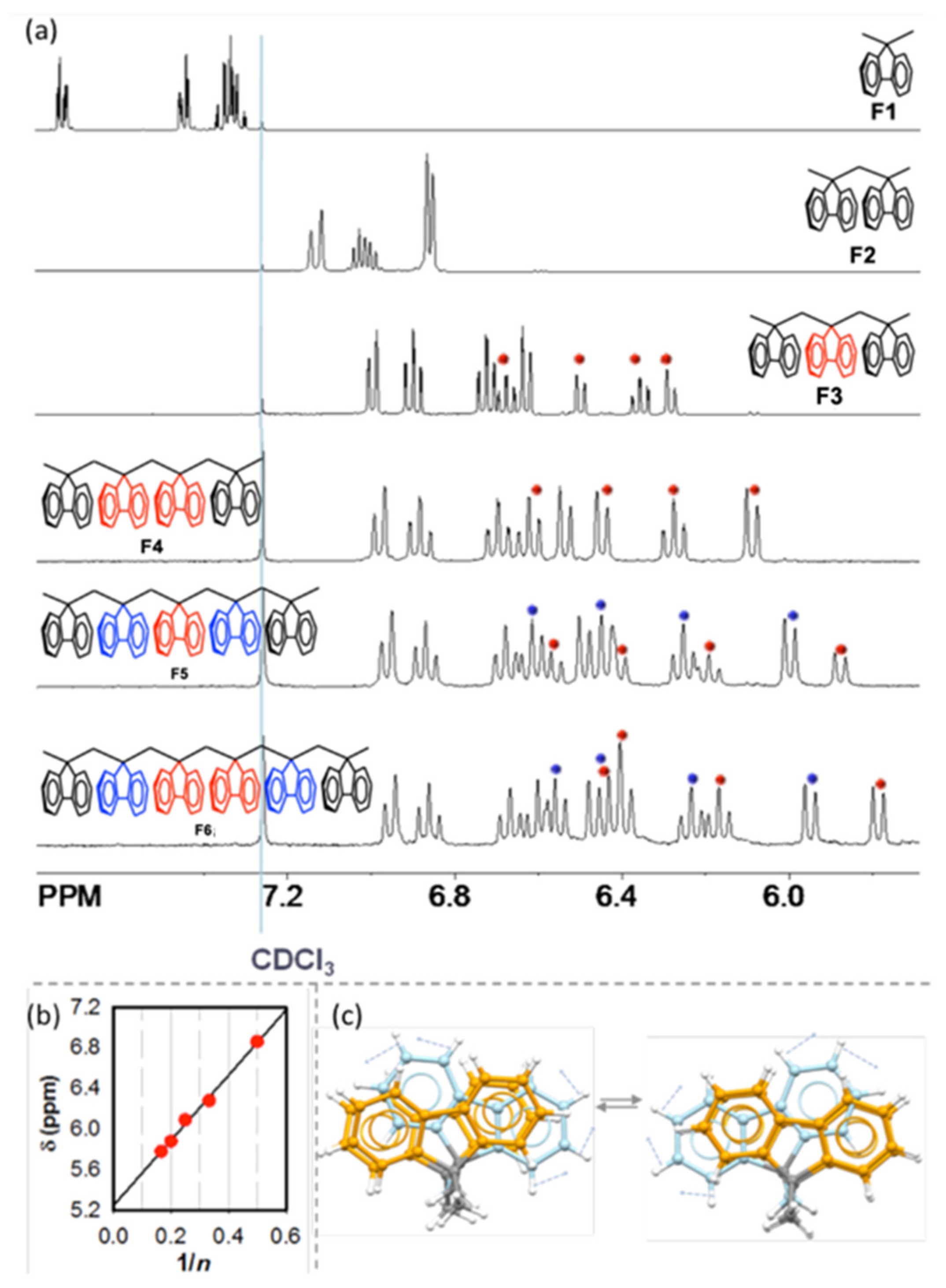
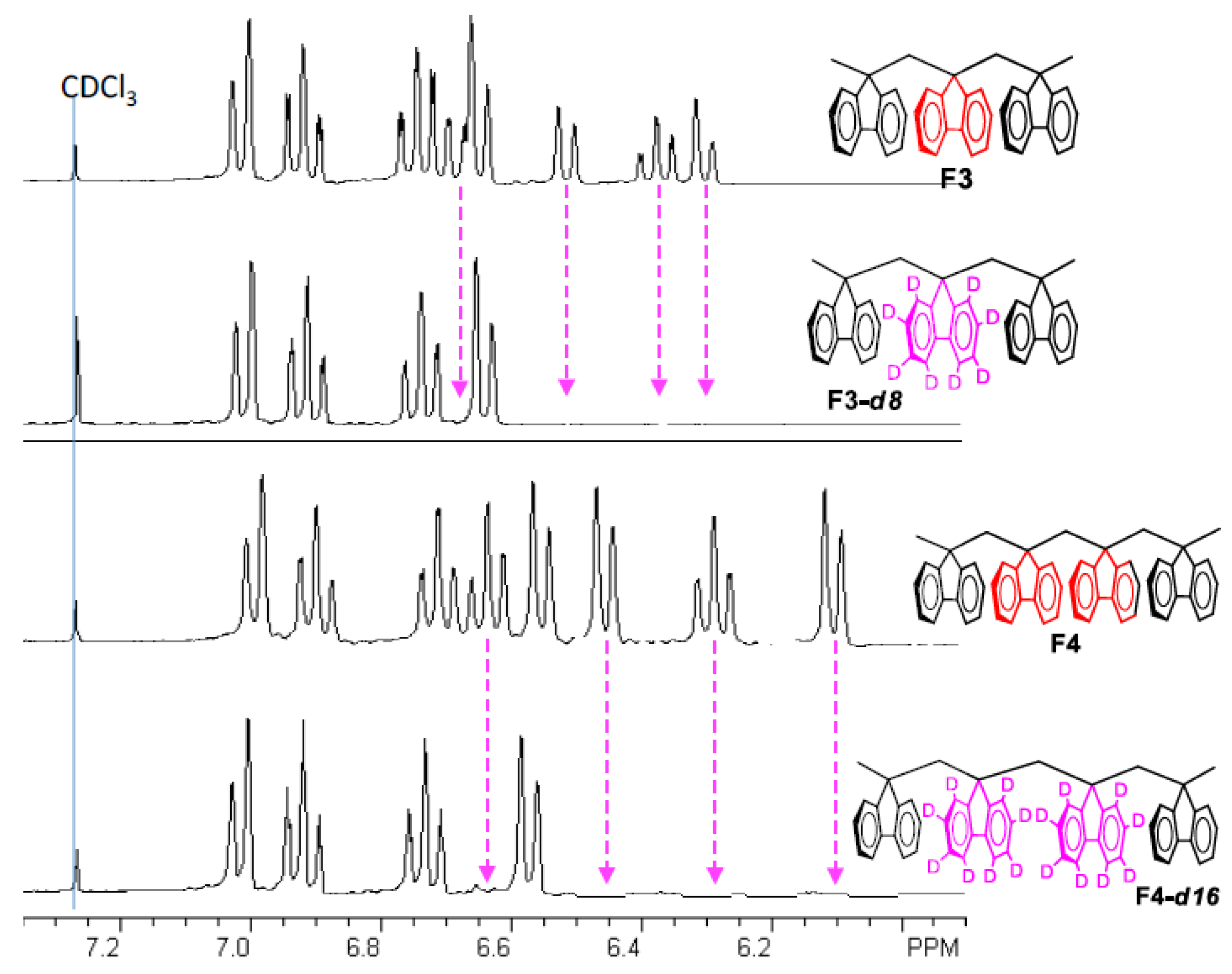
6. Demonstration of the Cofaciality of the Fluorene Rings in F1–F6 by 1H NMR Spectroscopy
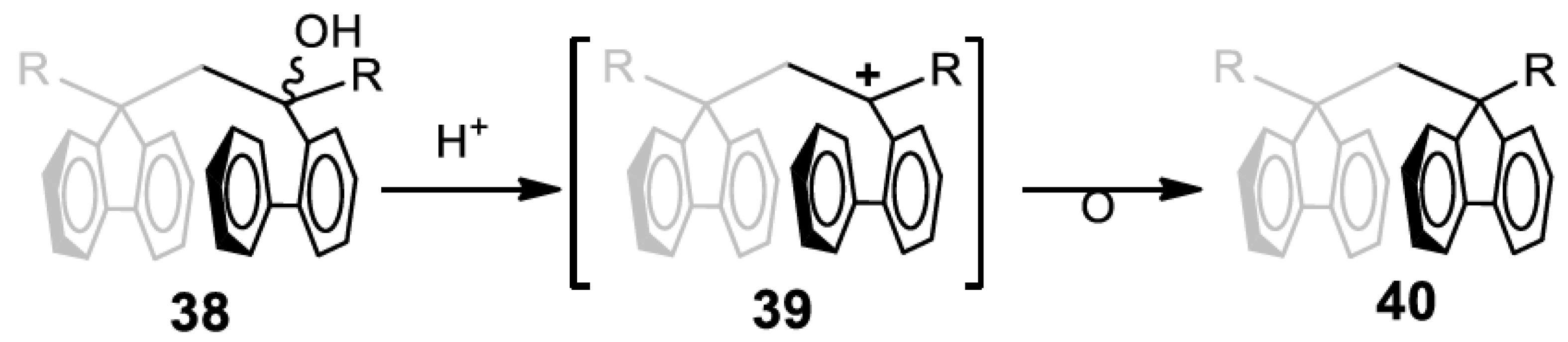
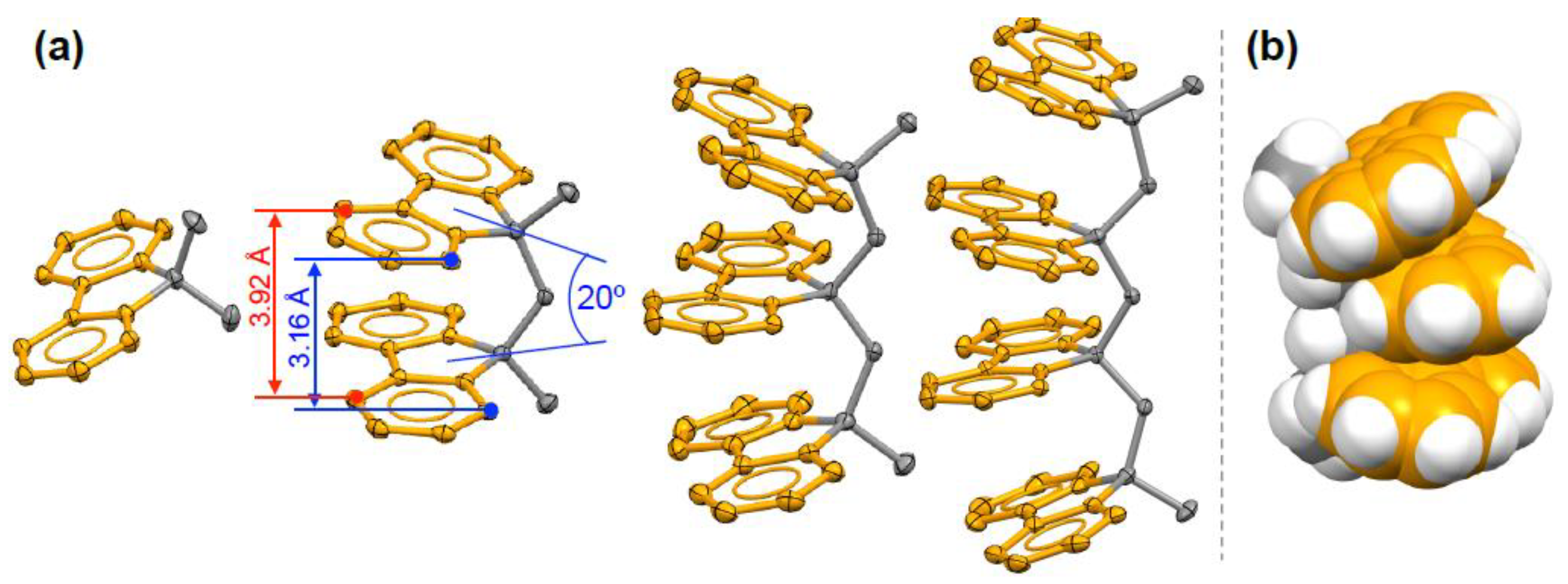
7. X-ray Crystallography of the π-Stacked Polyfluorenes
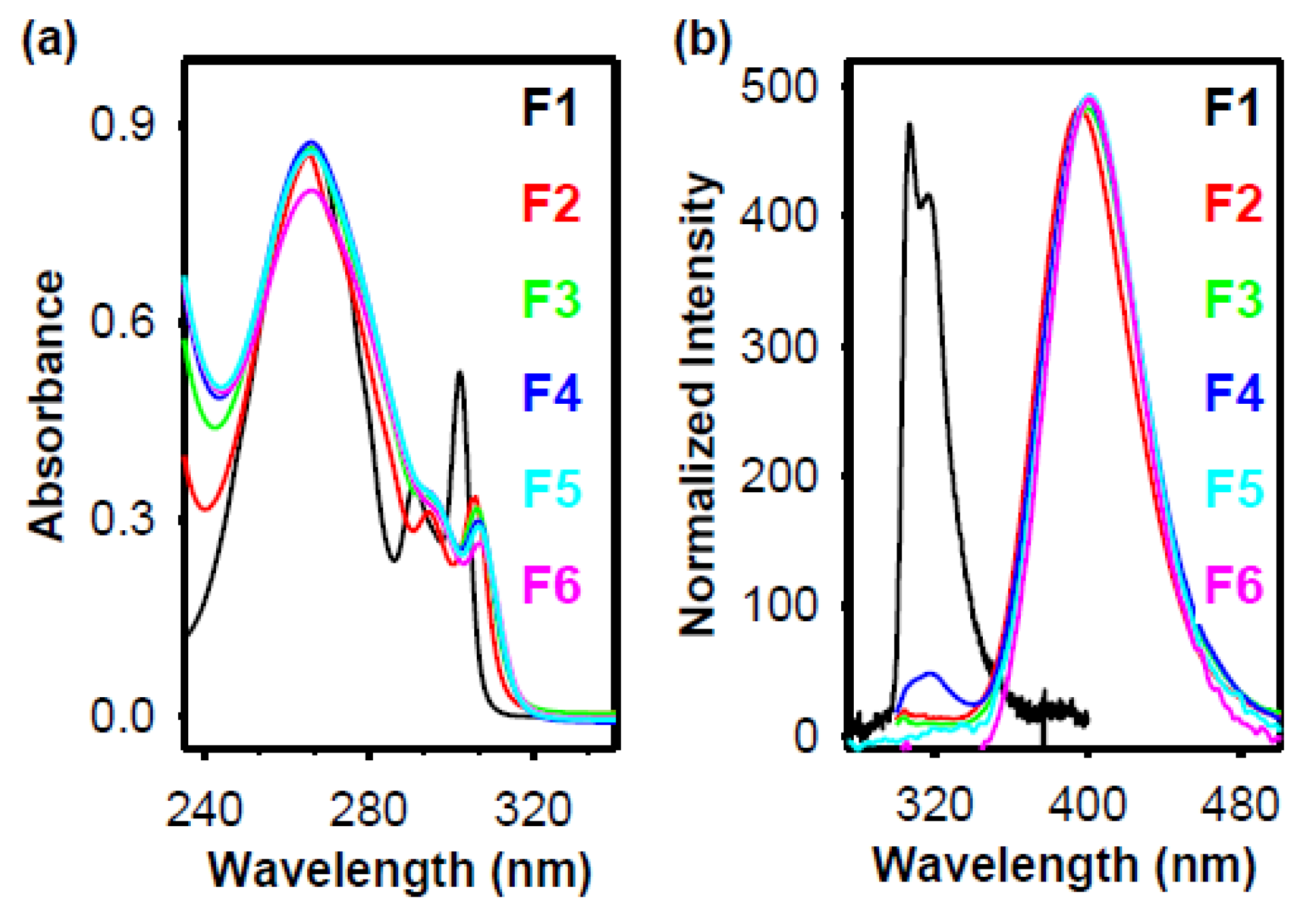
8. Electronic and Emission Spectroscopy of Polyfluorenes
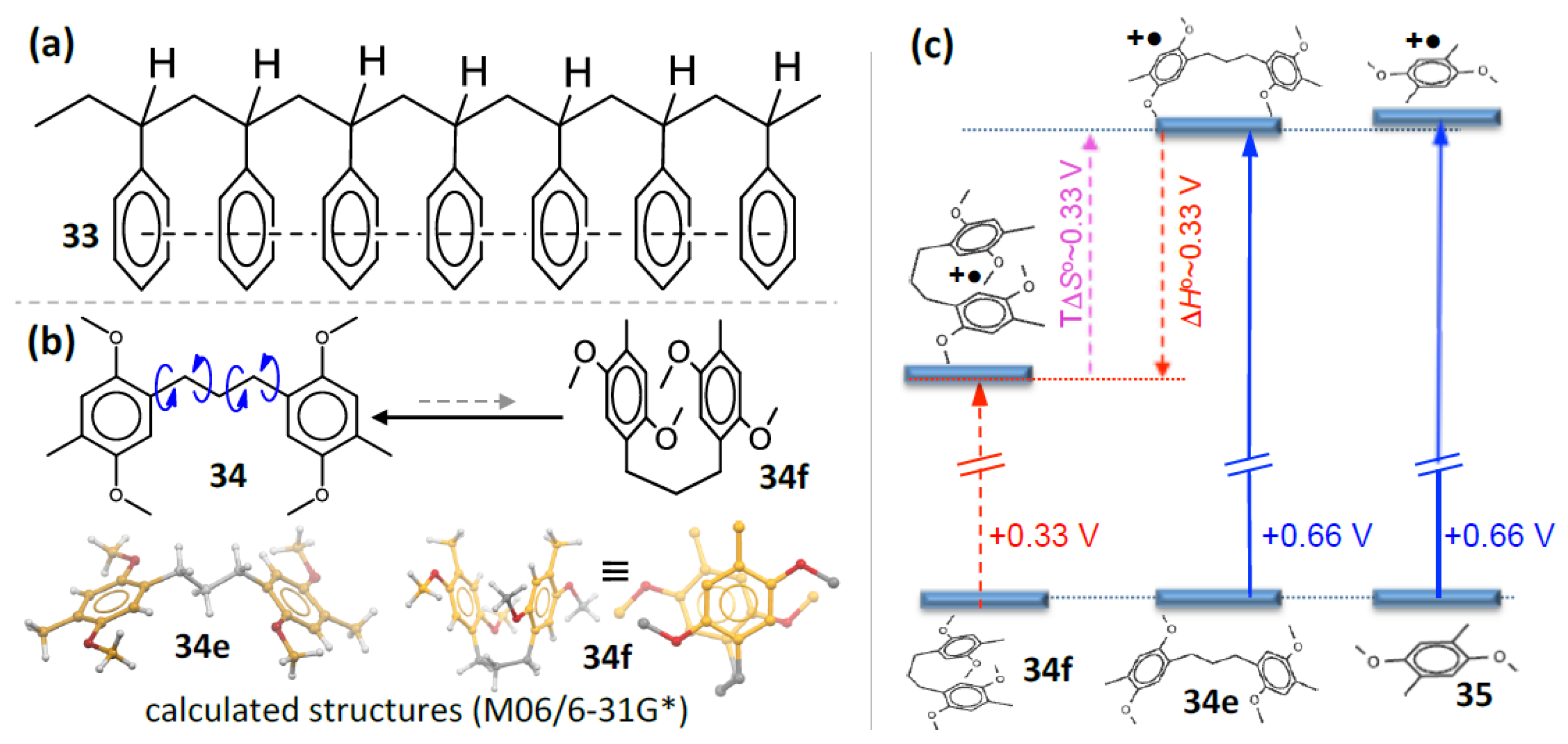
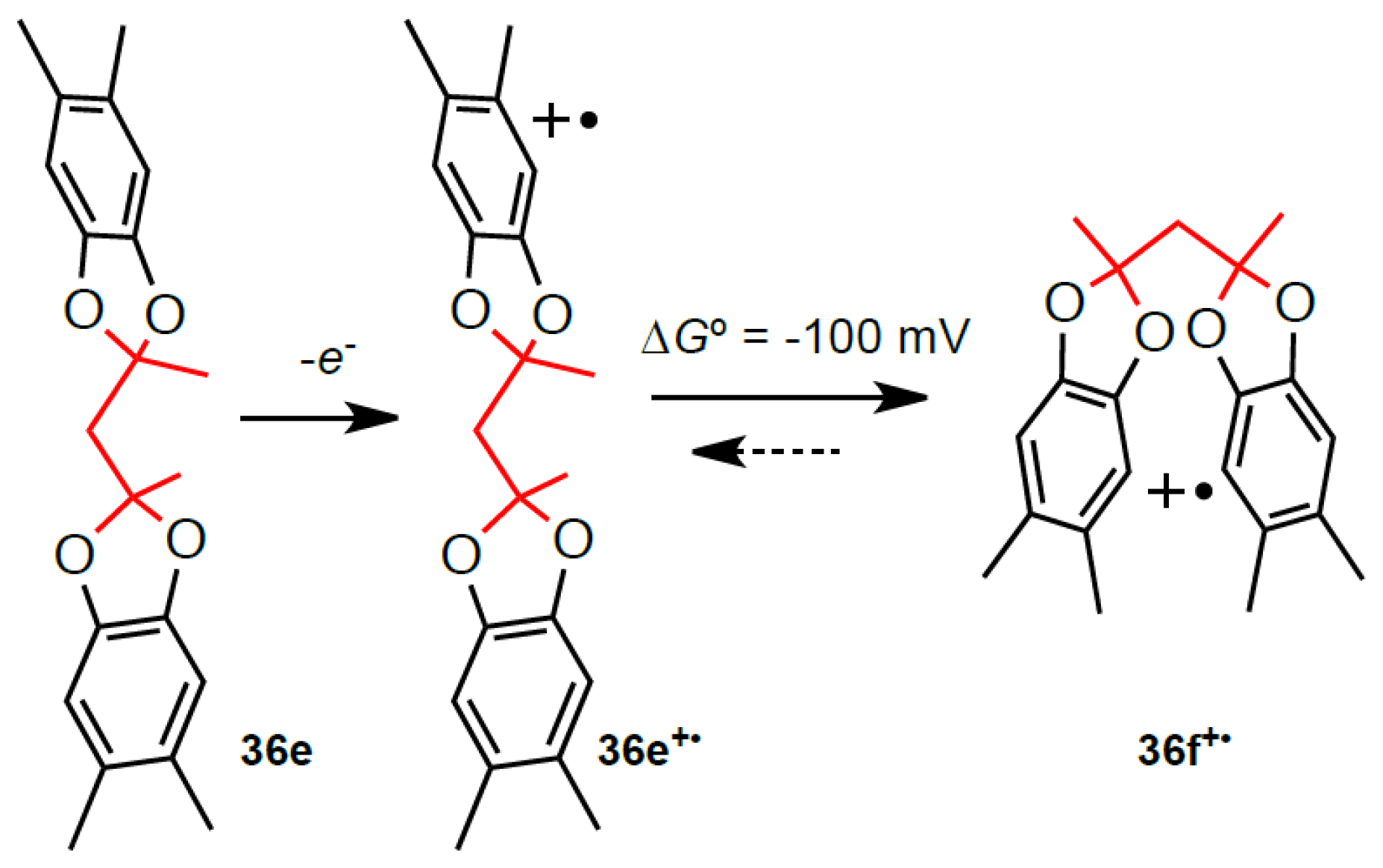
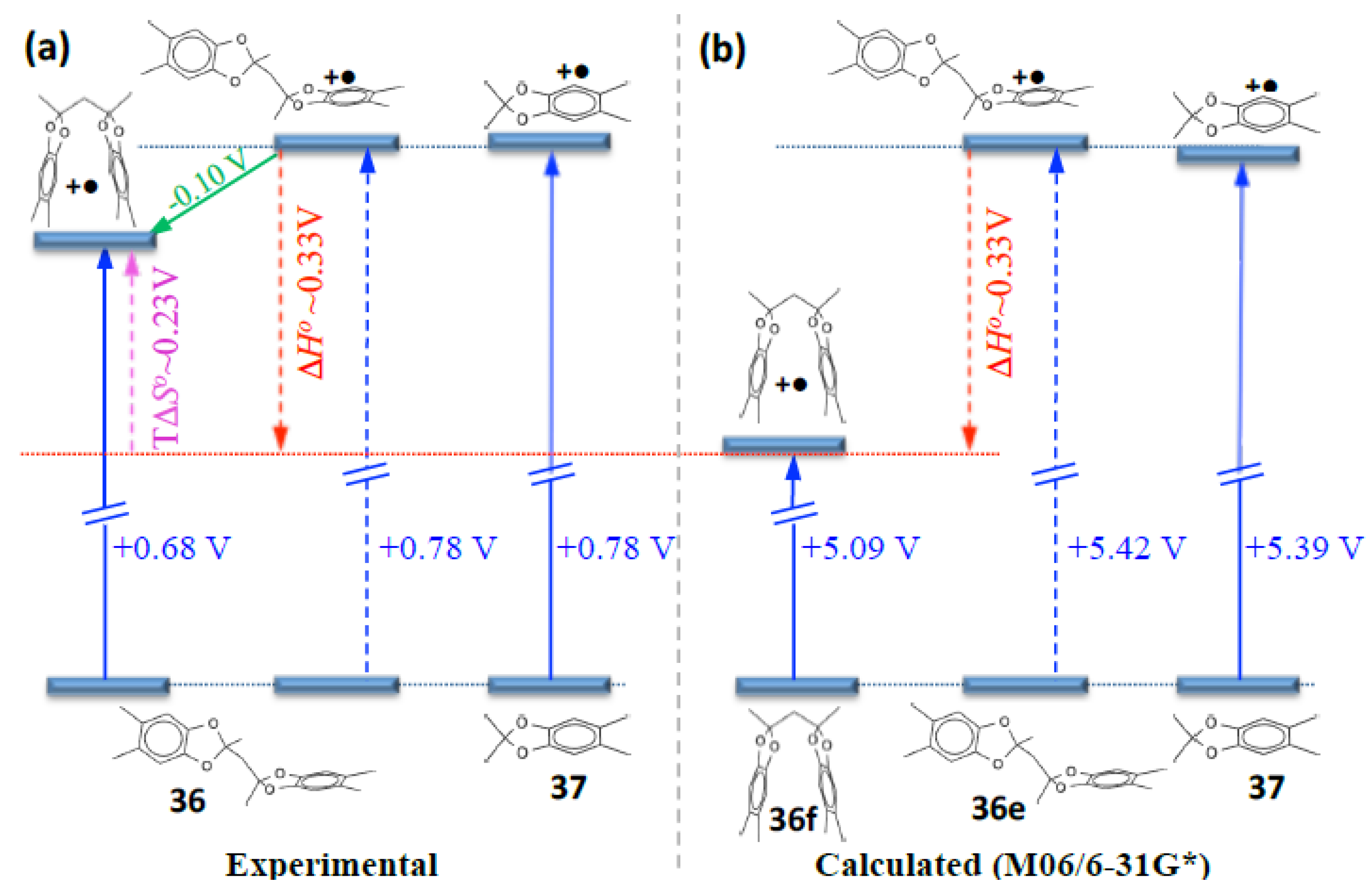
9. Electrochemistry/Photoelectron Spectroscopy of Polyfluorenes
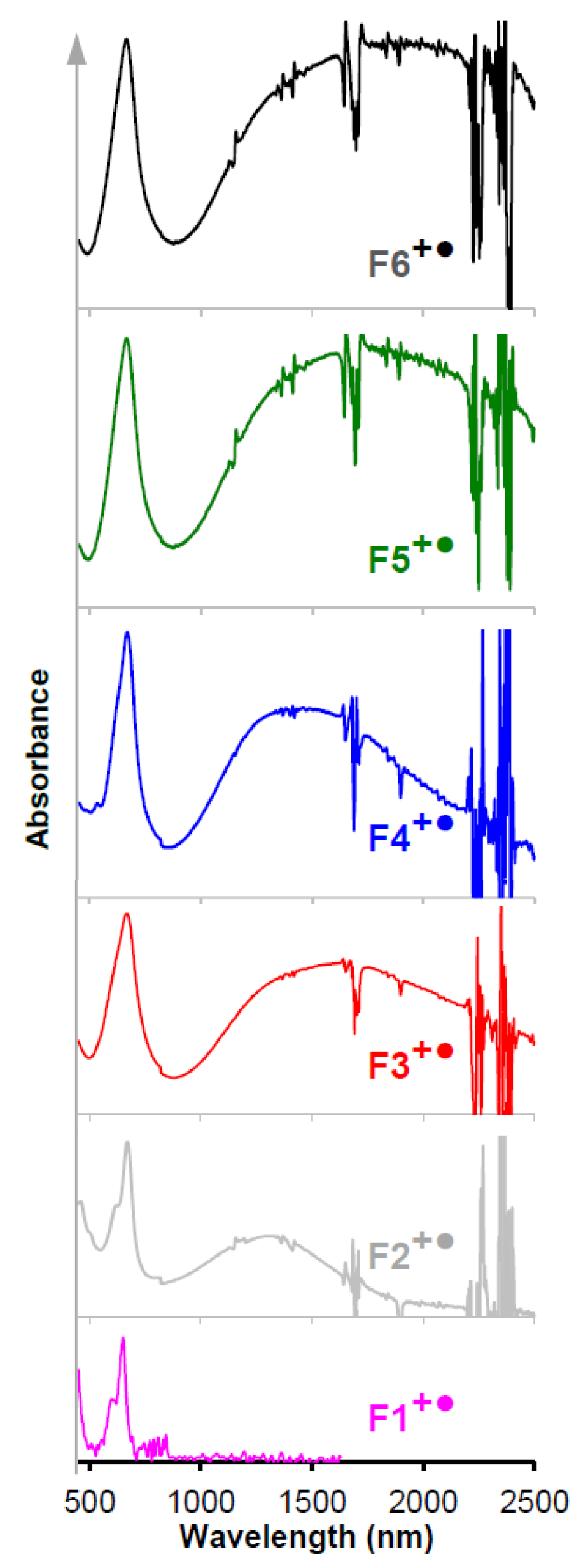
10. Generation and Spectroscopy of Polyfluorene Cation Radicals
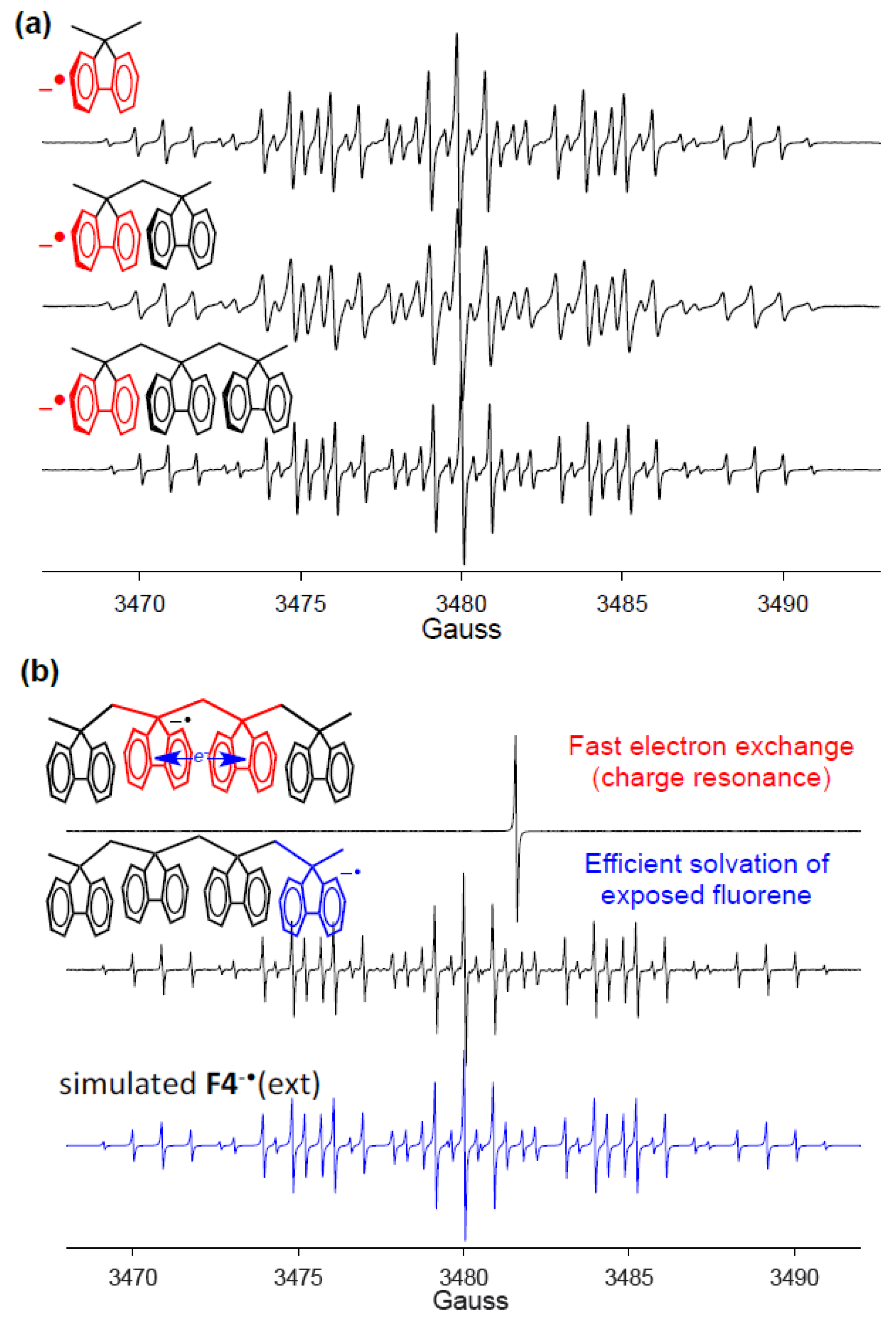
11. Generation of Polyfluorene Anion Radicals and Study of Charge Delocalization by EPR Spectroscopy
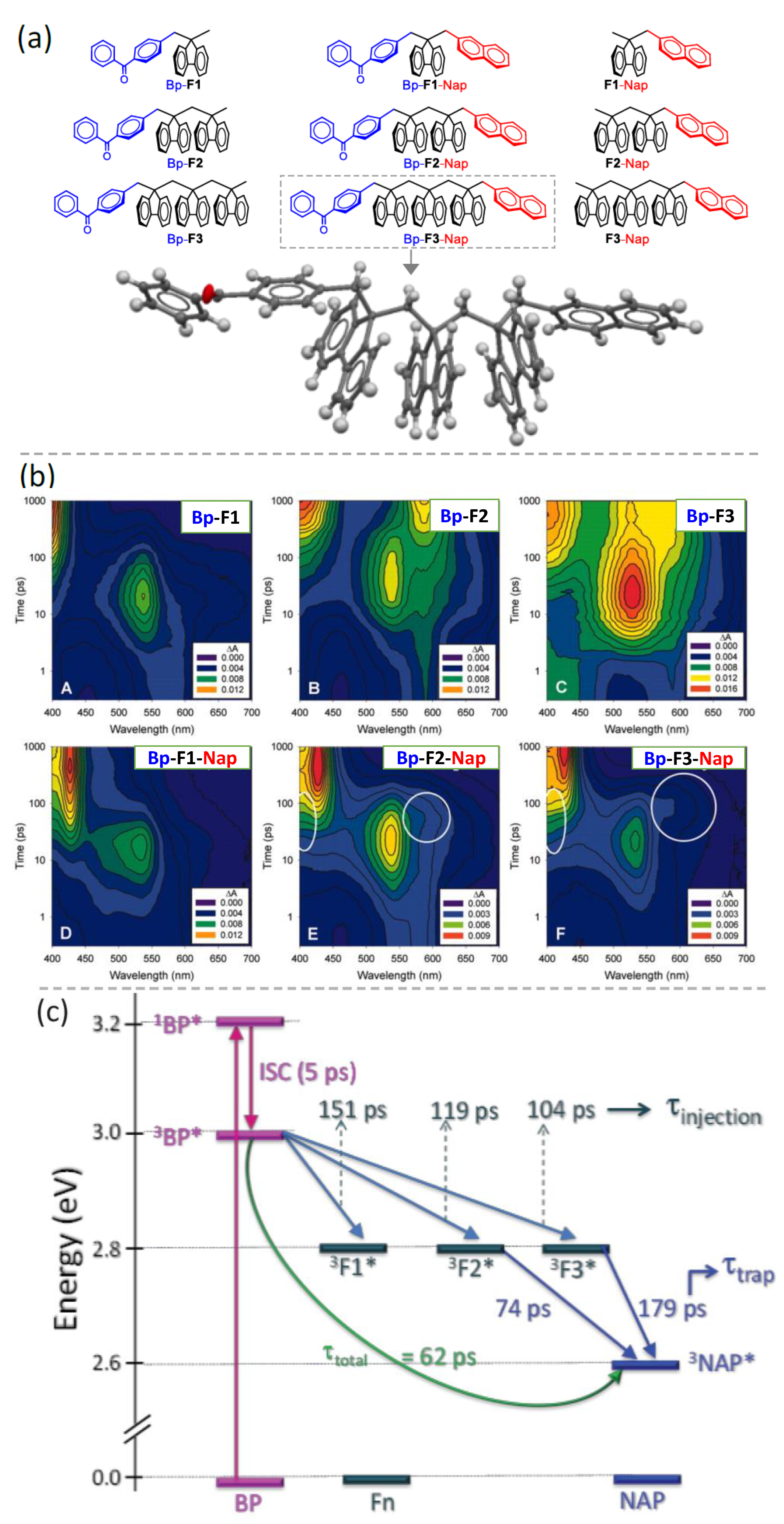
12. Donor–Spacer–Acceptor Triads with Polyfluorenes as Spacers and Demonstration of the Triplet Energy Transfer
13. Materials and Methods
13.1. Preparation of 9,9-Bis-(2-methyl-allyl)-9H-fluorene (42)
13.2. Preparation of 1-[9-(2-Oxo-propyl)-9H-fluoren-9-yl]propan-2-one (43)
13.3. Preparation of 2-Biphenyl-2-yl-1-[9-(2-biphenyl-2-yl-2-hydroxy-propyl)-9H-fluoren-9-yl]propan-2-ol (44)
13.4. Preparation of 9-Methyl-9H-fluorene (45)
13.5. Preparation of 2,3-Bis(9-methylfluorene)methylpropene (46)
13.6. Preparation of 1,3-Bis(9-methyl-9H-fluoren-9-yl)propan-2-one (47)
13.7. Preparation of 2-Biphenyl-2-yl-1,3-bis(9-methyl-9-H-fluoren-9-yl)propan-2-ol (48)
13.8. Preparation of 9-[(Fluoren-9-yl)-methyl]flourene (55)
13.9. Preparation of 9,9-Bis((9H-fluoren-9-yl)methyl)-9H-fluorene (63)
13.10. Preparation of Bis(9-((9H-fluoren-9-yl)methyl)-9H-fluoren-9-yl)methane (64)
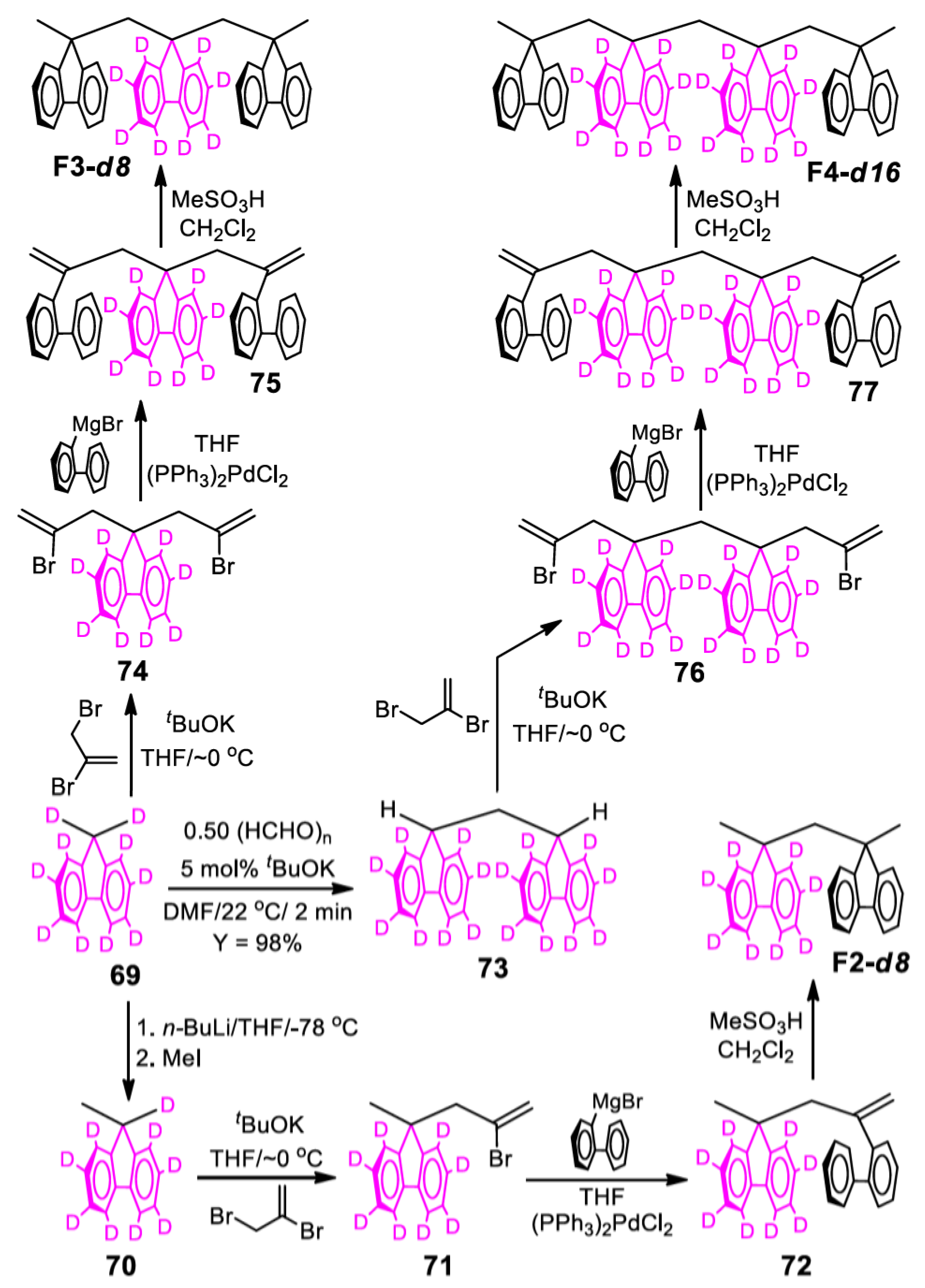
13.11. Preparation of 9-Methyl-9H-fluorene-d9 (70)
13.12. Preparation of 9-(2-Bromoallyl)-9-methyl-9H-fluorene-d8 (71)
13.13. Preparation of 9-(2-Biphenyl-2-yl-allyl)-9-methyl-9H-fluorene-d8 (72)
13.14. Preparation of Bis(9-methyl-9H-fluoren-9-yl)methane-d8 (F2-d8)
13.15. Preparation of Di(9H-fluoren-9-yl)methane-d16 (73)
13.16. Preparation of 9,9-Bis-(2-bromo-allyl)-9H-fluorene-d8 (74)
13.17. Preparation of 9,9-Bis(2-biphenyl-2-yl-allyl)-9H-fluorene-d8 (75)
13.18. Preparation of 9,9-Bis((9-methyl-9H-fluoren-9-yl)methyl)-9H-fluorene-d8 (F3-d8)
13.19. Preparation of 9-(2-Bromo-allyl)-9-[(9-(2-bromo-allyl)-fluoren-9-yl)methyl]fluorene-d16 (76)
13.20. Preparation of 9-(2-Biphenyl-2-yl-allyl)-9-[(9-(2-biphenyl-2-yl-allyl)fluoren-9-yl)methyl]fluorene-d16 (77)
13.21. Preparation of Bis(9-((9-methyl-9H-fluoren-9-yl)methyl)-9H-fluoren-9-yl)methane-d16 (F4-d16)
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shi, D.; Guo, Z.; Bedford, N. 12-DNA Nanotechnology. In Nanomaterials and Devices; Shi, D., Guo, Z., Bedford, N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2015; pp. 317–337. [Google Scholar]
- Vura-Weis, J.; Lewis, F.D.; Ratner, M.A.; Wasielewski, M.R. Base Pair Sequence and Hole Transfer through DNA: Rational Design of Molecular Wires. In Charge and Exciton Transport through Molecular Wires; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 133–156. [Google Scholar]
- Maiya, B.G.; Ramasarma, T. DNA, a molecular wire or not–The debate continues. Curr. Sci. 2001, 80, 1523–1530. [Google Scholar]
- Arnold, A.R.; Grodick, M.A.; Barton, J.K. DNA Charge Transport: From Chemical Principles to the Cell. Cell Chem. Biol. 2016, 23, 183–197. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Sevilla, M.D. Studies of Excess Electron and Hole Transfer in DNA at Low Temperatures. In Long-Range Charge Transfer in DNA II; Schuster, G.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 103–128. [Google Scholar]
- Porath, D.; Cuniberti, G.; Di Felice, R. Charge Transport in DNA-Based Devices. In Long-Range Charge Transfer in DNA II; Schuster, G.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 183–228. [Google Scholar]
- Lorenzo, E.R.; Olshansky, J.H.; Abia, D.S.D.; Krzyaniak, M.D.; Young, R.M.; Wasielewski, M.R. Interaction of Photogenerated Spin Qubit Pairs with a Third Electron Spin in DNA Hairpins. J. Am. Chem. Soc. 2021, 143, 4625–4632. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH). J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Moliton, A.; Hiorns, R.C. Review of electronic and optical properties of semiconducting π-conjugated polymers: Applications in optoelectronics. Polym. Int. 2004, 53, 1397–1412. [Google Scholar] [CrossRef]
- Facchetti, A. π-Conjugated Polymers for Organic Electronics and Photovoltaic Cell Applications. Chem. Mater. 2011, 23, 733–758. [Google Scholar] [CrossRef]
- Morisaki, Y.; Chujo, Y. Through-Space Conjugated Polymers Based on Cyclophanes. Angew. Chem. Int. Ed. 2006, 45, 6430–6437. [Google Scholar] [CrossRef]
- Nakano, T.; Yade, T. Synthesis, Structure, and Photophysical and Electrochemical Properties of a π-Stacked Polymer. J. Am. Chem. Soc. 2003, 125, 15474–15484. [Google Scholar] [CrossRef]
- Kanbayashi, N.; Kataoka, Y.; Okamura, T.-a.; Onitsuka, K. Stability Enhancement of a π-Stacked Helical Structure Using Substituents of an Amino Acid Side Chain: Helix Formation via a Nucleation–Elongation Mechanism. J. Am. Chem. Soc. 2022, 144, 6080–6090. [Google Scholar] [CrossRef]
- Bedi, A.; Carmieli, R.; Gidron, O. Radical cations of twisted acenes: Chiroptical properties and spin delocalization. Chem. Commun. 2019, 55, 6022–6025. [Google Scholar] [CrossRef]
- Bartholomew, G.P.; Bazan, G.C. Bichromophoric Paracyclophanes: Models for Interchromophore Delocalization. Acc. Chem. Res. 2001, 34, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Navale, T.S.; Thakur, K.; Vyas, V.S.; Wadumethrige, S.H.; Shukla, R.; Lindeman, S.V.; Rathore, R. Charge delocalization in self-assembled mixed-valence aromatic cation radicals. Langmuir 2012, 28, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Scott Lokey, R.; Iverson, B.L. Synthetic molecules that fold into a pleated secondary structure in solution. Nature 1995, 375, 303–305. [Google Scholar] [CrossRef]
- Hippius, C.; Schlosser, F.; Vysotsky, M.O.; Böhmer, V.; Würthner, F. Energy Transfer in Calixarene-Based Cofacial-Positioned Perylene Bisimide Arrays. J. Am. Chem. Soc. 2006, 128, 3870–3871. [Google Scholar] [CrossRef]
- Keshri, S.K.; Ishizuka, T.; Kojima, T.; Matsushita, Y.; Takeuchi, M. Long-Range Order in Supramolecular π Assemblies in Discrete Multidecker Naphthalenediimides. J. Am. Chem. Soc. 2021, 143, 3238–3244. [Google Scholar] [CrossRef] [PubMed]
- Rozenberg, V.; Sergeeva, E.; Hopf, H. Cyclophanes as templates in stereoselective synthesis. In Modern Cyclophane Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 435–462. [Google Scholar]
- Cram, D.J.; Allinger, N.L.; Steinberg, H. Macro Rings. VII. The Spectral Consequences of Bringing Two Benzene Rings Face to Face1. J. Am. Chem. Soc. 1954, 76, 6132–6141. [Google Scholar] [CrossRef]
- Sekine, Y.; Boekelheide, V. A study of the synthesis and properties of [26](1,2,3,4,5,6)cyclophane (superphane). J. Am. Chem. Soc. 1981, 103, 1777–1785. [Google Scholar] [CrossRef]
- Shibahara, M.; Watanabe, M.; Iwanaga, T.; Matsumoto, T.; Ideta, K.; Shinmyozu, T. Synthesis, structure, and transannular pi-pi interaction of three- and four-layered [3.3]paracyclophanes. J. Org. Chem. 2008, 73, 4433–4442. [Google Scholar] [CrossRef]
- Shibahara, M.; Watanabe, M.; Iwanaga, T.; Ideta, K.; Shinmyozu, T. Synthesis, Structure, and Transannular π−π Interaction of Multilayered [3.3]Metacyclophanes1. J. Org. Chem. 2007, 72, 2865–2877. [Google Scholar] [CrossRef]
- Mataka, S.; Shigaki, K.; Sawada, T.; Mitoma, Y.; Taniguchi, M.; Thiemann, T.; Ohga, K.; Egashira, N. Quadruple Decker [3.3][3.3][3.3]Orthocyclophane Acetal-An Orthocyclophane Ladder. Angew. Chem. Int. Ed. Engl. 1998, 37, 2532–2534. [Google Scholar] [CrossRef]
- Grimme, W.; Kaemmerling, H.T.; Lex, J.; Gleiter, R.; Heinze, J.; Dietrich, M. ChemInform Abstract: Syn-Sesqui- and -Sesterbenzobicyclo(2.2.2)octene, Molecules with Stacked Benzene Rings. ChemInform 1991, 22, 1. [Google Scholar] [CrossRef]
- Kochi, J.K.; Rathore, R.; Maguères, P.L. Stable Dimeric Aromatic Cation−Radicals. Structural and Spectral Characterization of Through-Space Charge Delocalization. J. Org. Chem. 2000, 65, 6826–6836. [Google Scholar] [CrossRef] [PubMed]
- Chebny, V.J.; Navale, T.S.; Shukla, R.; Lindeman, S.V.; Rathore, R. X-ray Structural Characterization of Charge Delocalization onto the Three Equivalent Benzenoid Rings in Hexamethoxytriptycene Cation Radical. Org. Lett. 2009, 11, 2253–2256. [Google Scholar] [CrossRef] [PubMed]
- Kuo, A. Flourescence resulting from π-stacking in polystyrene solutions. CheM 2011, 1, 80–86. [Google Scholar] [CrossRef]
- Sun, D.; Lindeman, S.V.; Rathore, R.; Kochi, J.K. Intramolecular (electron) delocalization between aromatic donors and their tethered cation–radicals. Application of electrochemical and structural probes. J. Chem. Soc. Perkin Trans. 2001, 2, 1585–1594. [Google Scholar] [CrossRef]
- Tsuchida, A.; Tsujii, Y.; Ohoka, M.; Yamamoto, M. Laser photolysis studies on the intramolecular dimer radical cations formed in 1,3-dipyrenylpropanes. J. Phys. Chem. 1991, 95, 5797–5802. [Google Scholar] [CrossRef]
- Rathore, R.; Kochi, J.K. Vicinal-diaryl interactions in stilbenoid hydrocarbons as observed in the through-space charge delocalization of their cation radicals. Can. J. Chem. 1999, 77, 913–921. [Google Scholar] [CrossRef]
- Rathore, R.; Chebny, V.J.; Kopatz, E.J.; Guzei, I.A. Redox-induced transformation from an extended to a pi-stacked conformer in acyclic bis(catecholacetal)s of acetylacetone. Angew. Chem. Int. Ed. Engl. 2005, 44, 2771–2774. [Google Scholar] [CrossRef]
- Wang, D.; Talipov, M.R.; Ivanov, M.V.; Mirzaei, S.; Lindeman, S.V.; Cai, S.; Rathore, R.; Reid, S.A. Molecular Actuators in Action: Electron-Transfer-Induced Conformation Transformation in Cofacially Arrayed Polyfluorenes. J. Phys. Chem. Lett. 2018, 9, 4233–4238. [Google Scholar] [CrossRef]
- Rathore, R.; Abdelwahed, S.H.; Guzei, I.A. Synthesis, Structure, and Evaluation of the Effect of Multiple Stacking on the Electron-Donor Properties of π-Stacked Polyfluorenes. J. Am. Chem. Soc. 2003, 125, 8712–8713. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. The Role of Bidentate Fluorenylphosphines in Palladium-Catalyzed Cross-Coupling Reactions. Organometallics 2008, 27, 3924–3932. [Google Scholar] [CrossRef]
- Abdelwahed, S. Preliminary Studies Directed towards the Design and Synthesis of Electro-Active Fluorene and Calixarene Derivatives; Marquette University: Milwaukee, WI, USA, 2006; Available online: https://epublications.marquette.edu/dissertations_mu/1680 (accessed on 15 March 2023).
- Vura-Weis, J.; Abdelwahed, S.H.; Shukla, R.; Rathore, R.; Ratner, M.A.; Wasielewski, M.R. Crossover from Single-Step Tunneling to Multistep Hopping for Molecular Triplet Energy Transfer. Science 2010, 328, 1547–1550. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Abdelwahed, S.H.; Kiesewetter, M.K.; Reiter, R.C.; Stevenson, C.D. Intramolecular Electron Transfer in Cofacially π-Stacked Fluorenes: Evidence of Tunneling. J. Phys. Chem. B 2006, 110, 1536–1540. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Chang, J.; Abdelwahed, S.H.; Thakur, K.; Rathore, R.; Bard, A.J. Electrochemistry and Electrogenerated Chemiluminescence of π-Stacked Poly(fluorenemethylene) Oligomers. Multiple, Interacting Electron Transfers. J. Am. Chem. Soc. 2012, 134, 16265–16274. [Google Scholar] [CrossRef] [PubMed]
- Abzhanova, A.; Ivanova, L.V.; Wang, D.; Navale, T.S.; Abdelwahed, S.H.; Ivanov, M.V.; Lindeman, S.; Rathore, R.; Reid, S.A. Charge-transfer or excimeric state? Exploring the nature of the excited state in cofacially arrayed polyfluorene derivatives. J. Photochem. Photobiol. A Chem. 2019, 374, 125–130. [Google Scholar] [CrossRef]
- Kokkin, D.; Ivanov, M.; Loman, J.; Cai, J.-Z.; Uhler, B.; Reilly, N.; Rathore, R.; Reid, S.A. π-π stacking vs. C–H/π interaction: Excimer formation and charge resonance stabilization in van der Waals clusters of 9,9′-dimethylfluorene. J. Chem. Phys. 2018, 149, 134314. [Google Scholar] [CrossRef]
- Birks, J.B. Excimers. Rep. Prog. Phys. 1975, 38, 903. [Google Scholar] [CrossRef]
- Talipov, M.R.; Boddeda, A.; Lindeman, S.V.; Rathore, R. Does Koopmans’ Paradigm for 1-Electron Oxidation Always Hold? Breakdown of IP/Eox Relationship for p-Hydroquinone Ethers and the Role of Methoxy Group Rotation. J. Phys. Chem. Lett. 2015, 6, 3373–3378. [Google Scholar] [CrossRef]
- Wang, D.; Ivanov, M.V.; Kokkin, D.; Loman, J.; Cai, J.-Z.; Reid, S.A.; Rathore, R. The Role of Torsional Dynamics on Hole and Exciton Stabilization in π-Stacked Assemblies: Design of Rigid Torsionomers of a Cofacial Bifluorene. Angew. Chem. Int. Ed. 2018, 57, 8189–8193. [Google Scholar] [CrossRef]
- Banerjee, M.; Lindeman, S.V.; Rathore, R. Structural Characterization of Quaterphenyl Cation Radical: X-ray Crystallographic Evidence of Quinoidal Charge Delocalization in Poly-p-phenylene Cation Radicals. J. Am. Chem. Soc. 2007, 129, 8070–8071. [Google Scholar] [CrossRef]
- Rathore, R.; Lindeman, S.V.; Abdelwahed, S.H. Design, Synthesis, Electronic Properties, and X-ray Structural Characterization of Various Modified Electron-Rich Calixarene Derivatives and Their Conversion to Stable Cation Radical Salts. Molecules 2022, 27, 5994. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Kumar, A.S.; Lindeman, S.V.; Kochi, J.K. Preparation and Structures of Crystalline Aromatic Cation-Radical Salts. Triethyloxonium Hexachloroantimonate as a Novel (One-Electron) Oxidant. J. Org. Chem. 1998, 63, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Rathore, R.; Burns, C.L.; Deselnicu, M.I. Preparation of 1,4:5,8-Dimethano-1,2,3,4,5,6,7,8-Octahydro-9,10-Dimethoxyanthracenium Hexachloroantimonate (4+SbCl6−): A Highly Robust Radical-Cation Salt. Org. Synth. 2005, 82, 1–9. [Google Scholar] [CrossRef]
- Talipov, M.R.; Ivanov, M.V.; Reid, S.A.; Rathore, R. Two’s Company, Three’s a Crowd: Exciton Localization in Cofacially Arrayed Polyfluorenes. J. Phys. Chem. Lett. 2016, 7, 2915–2920. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, C.D.; Kiesewetter, M.K.; Reiter, R.C.; Abdelwahed, S.H.; Rathore, R. Intramolecular C−H/C−D Exchange in Cofacially Stacked Polyfluorenes via Electron-Induced Bond Activation. J. Am. Chem. Soc. 2005, 127, 5282–5283. [Google Scholar] [CrossRef]





| Monomer | Eox (V) a | Cyclophane | Eox (V) a | D (V) | |
|---|---|---|---|---|---|
 23 | 0.66 |  | 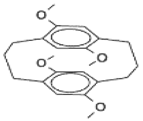 | 0.33 | 0.33 |
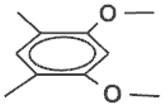 24 | 0.73 | 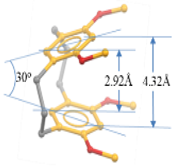 |  | 0.35 | 0.38 |
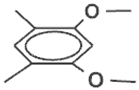 26 | 0.77 | 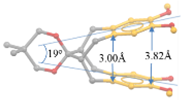 | 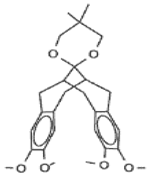 | 0.42 | 0.35 |
 27 | 0.82 | 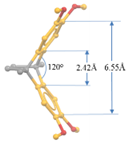 | 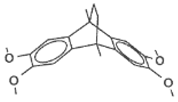 | 0.57 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathore, R.; Abdelwahed, S.H. Design and Synthesis of Cofacially-Arrayed Polyfluorene Wires for Electron and Energy Transfer Studies. Molecules 2023, 28, 3717. https://doi.org/10.3390/molecules28093717
Rathore R, Abdelwahed SH. Design and Synthesis of Cofacially-Arrayed Polyfluorene Wires for Electron and Energy Transfer Studies. Molecules. 2023; 28(9):3717. https://doi.org/10.3390/molecules28093717
Chicago/Turabian StyleRathore, Rajendra, and Sameh H. Abdelwahed. 2023. "Design and Synthesis of Cofacially-Arrayed Polyfluorene Wires for Electron and Energy Transfer Studies" Molecules 28, no. 9: 3717. https://doi.org/10.3390/molecules28093717
APA StyleRathore, R., & Abdelwahed, S. H. (2023). Design and Synthesis of Cofacially-Arrayed Polyfluorene Wires for Electron and Energy Transfer Studies. Molecules, 28(9), 3717. https://doi.org/10.3390/molecules28093717






