Constructing Molybdenum Phosphide@Cobalt Phosphide Heterostructure Nanoarrays on Nickel Foam as a Bifunctional Electrocatalyst for Enhanced Overall Water Splitting
Abstract
1. Introduction
2. Results and Discussion
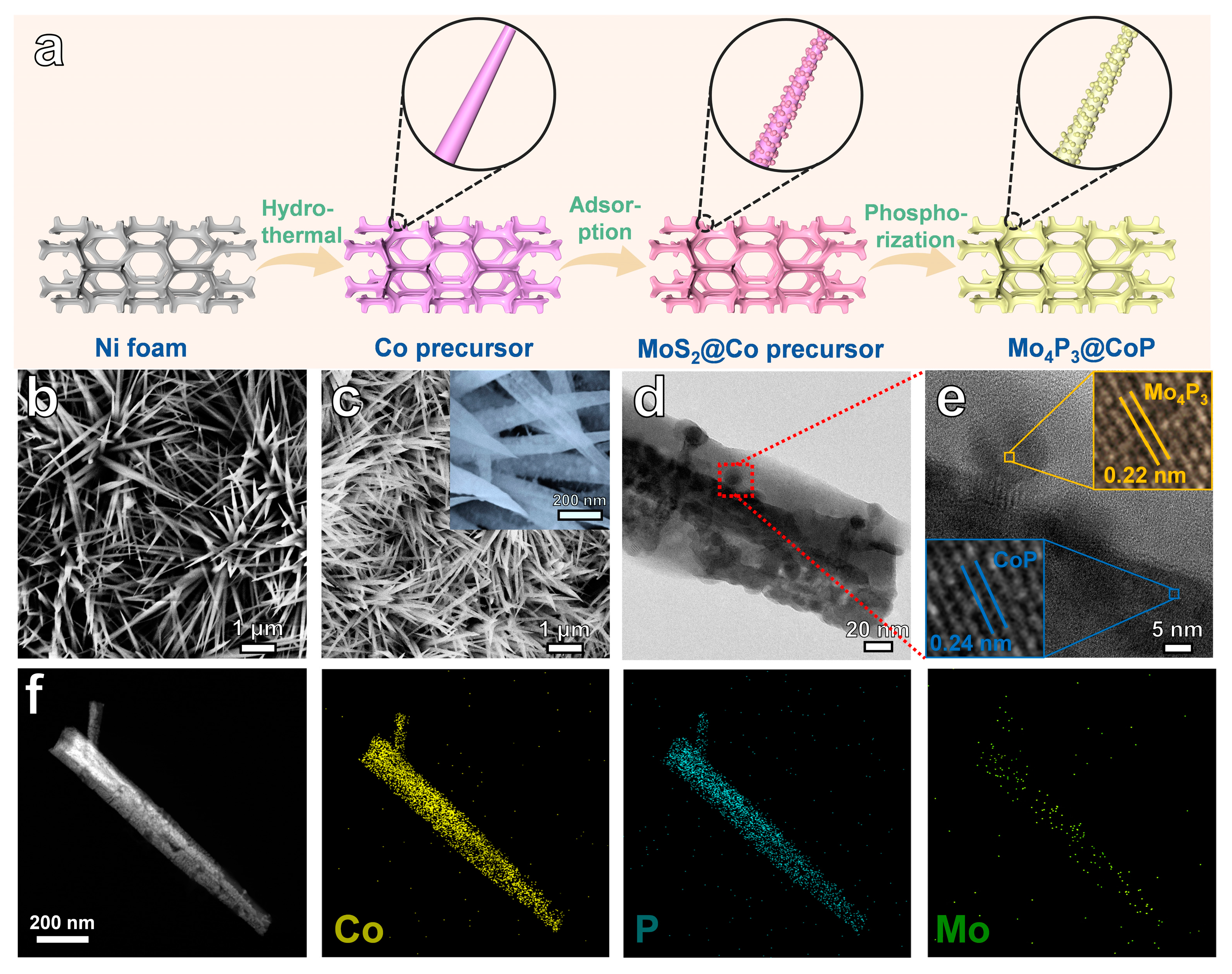
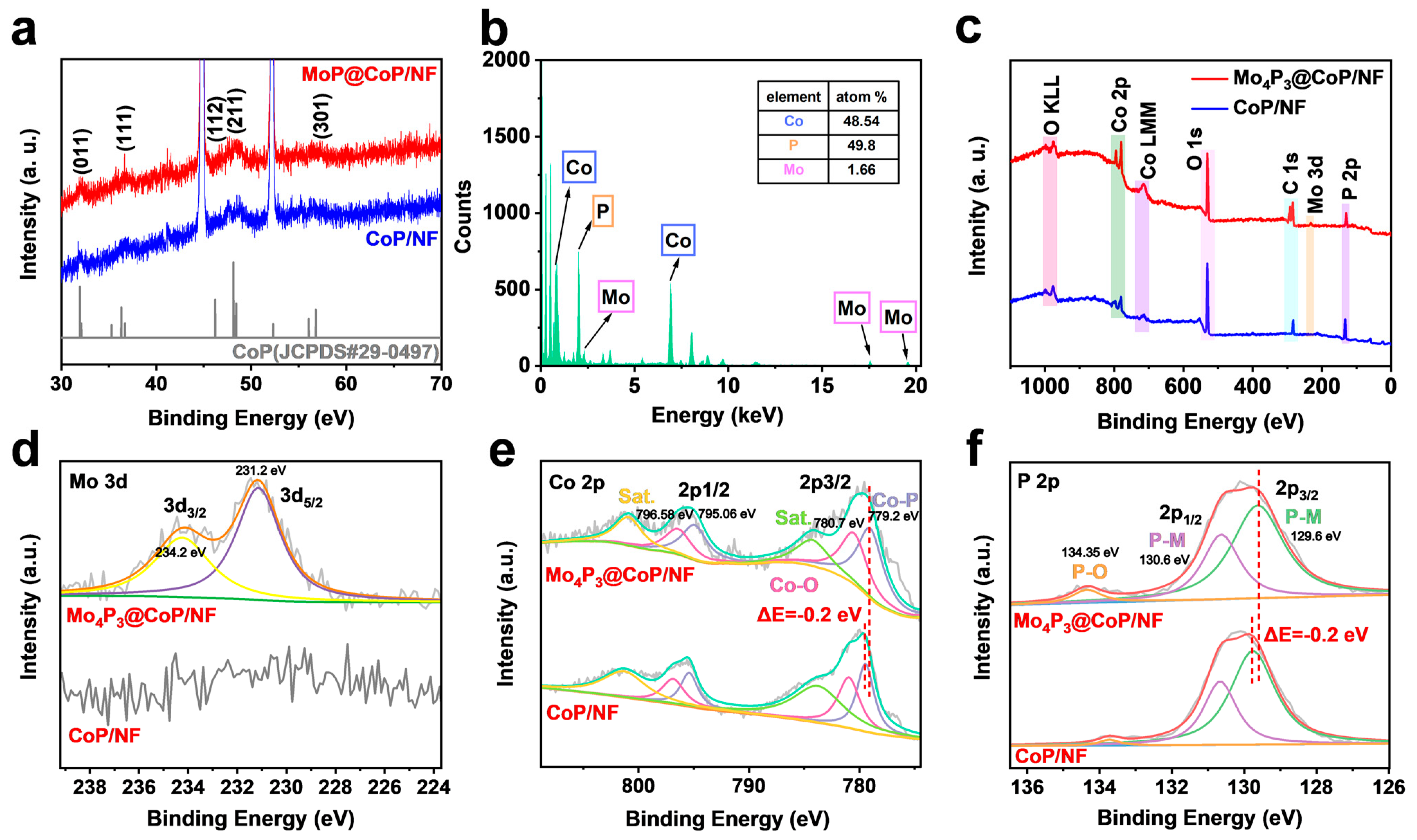
2.1. Characterization
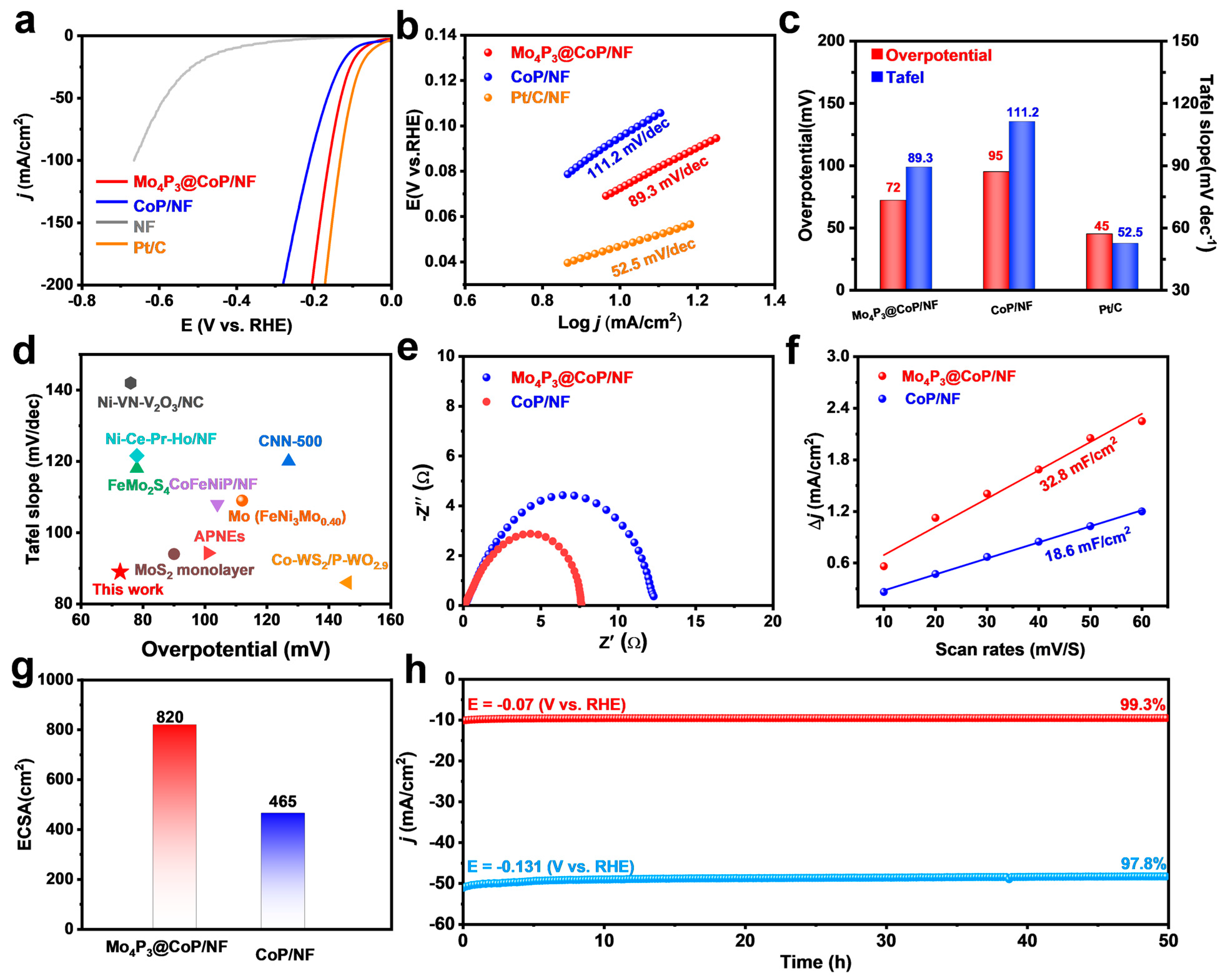
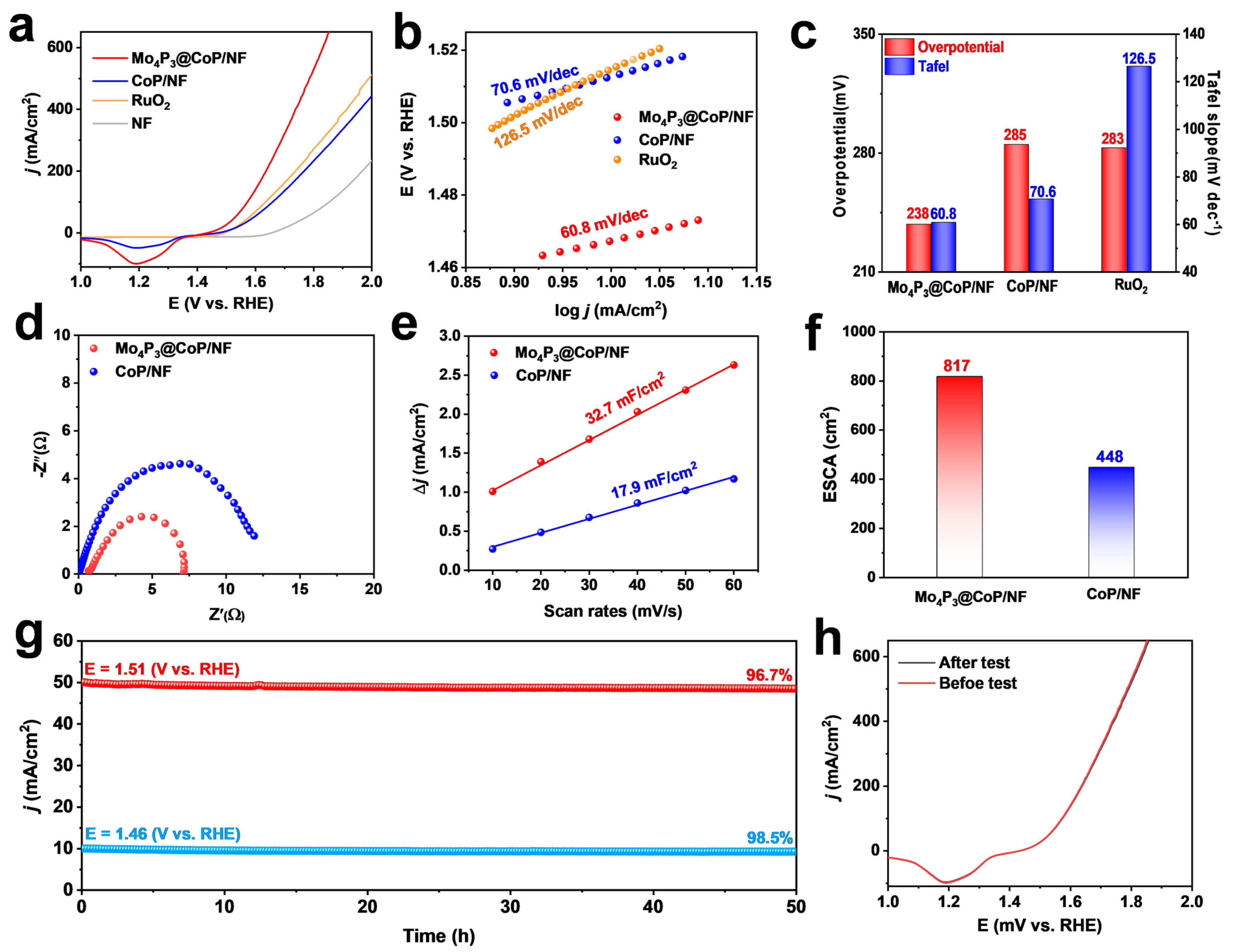
2.2. HER Performance
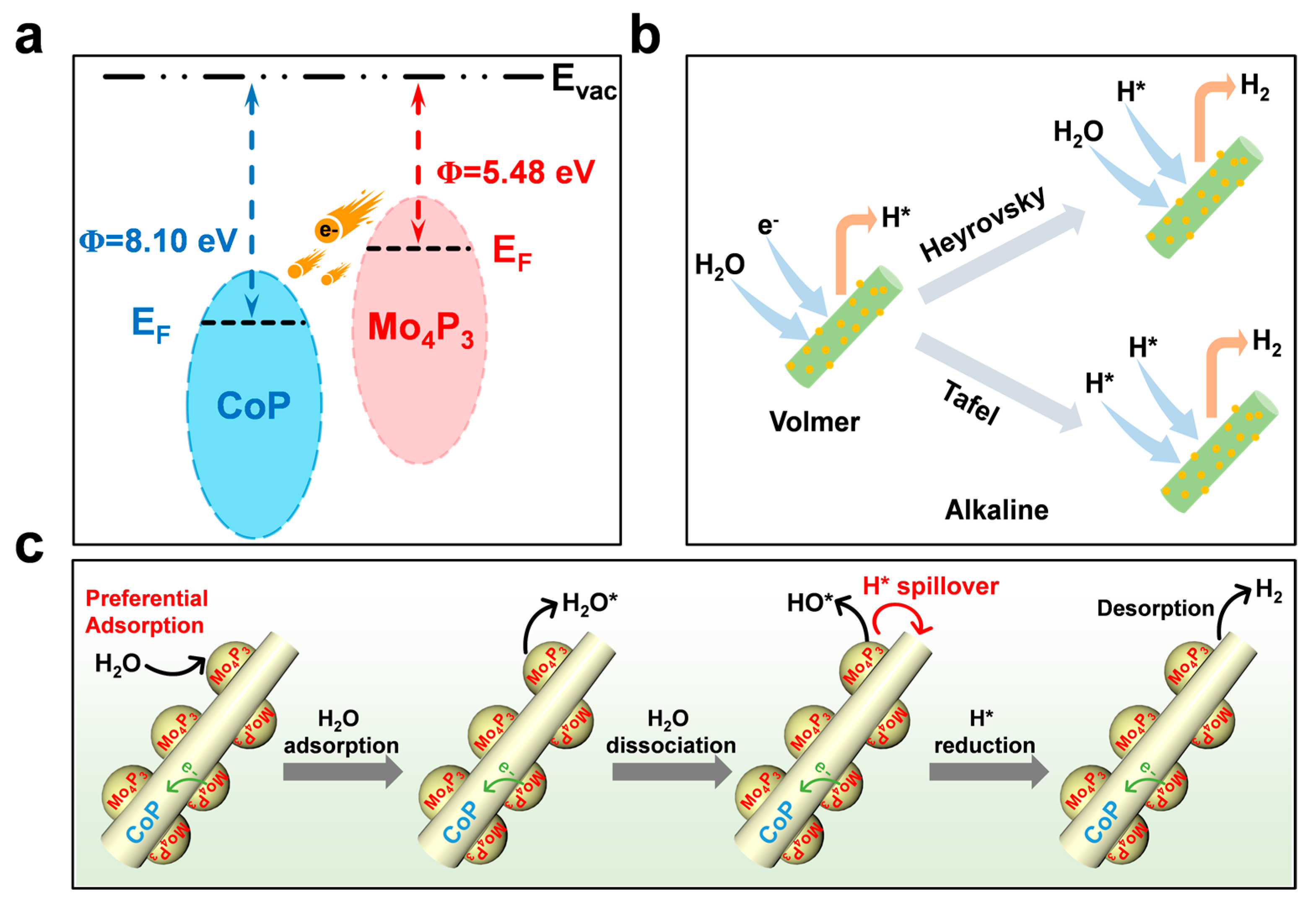
2.3. Mechanism Study
2.4. OER Performance
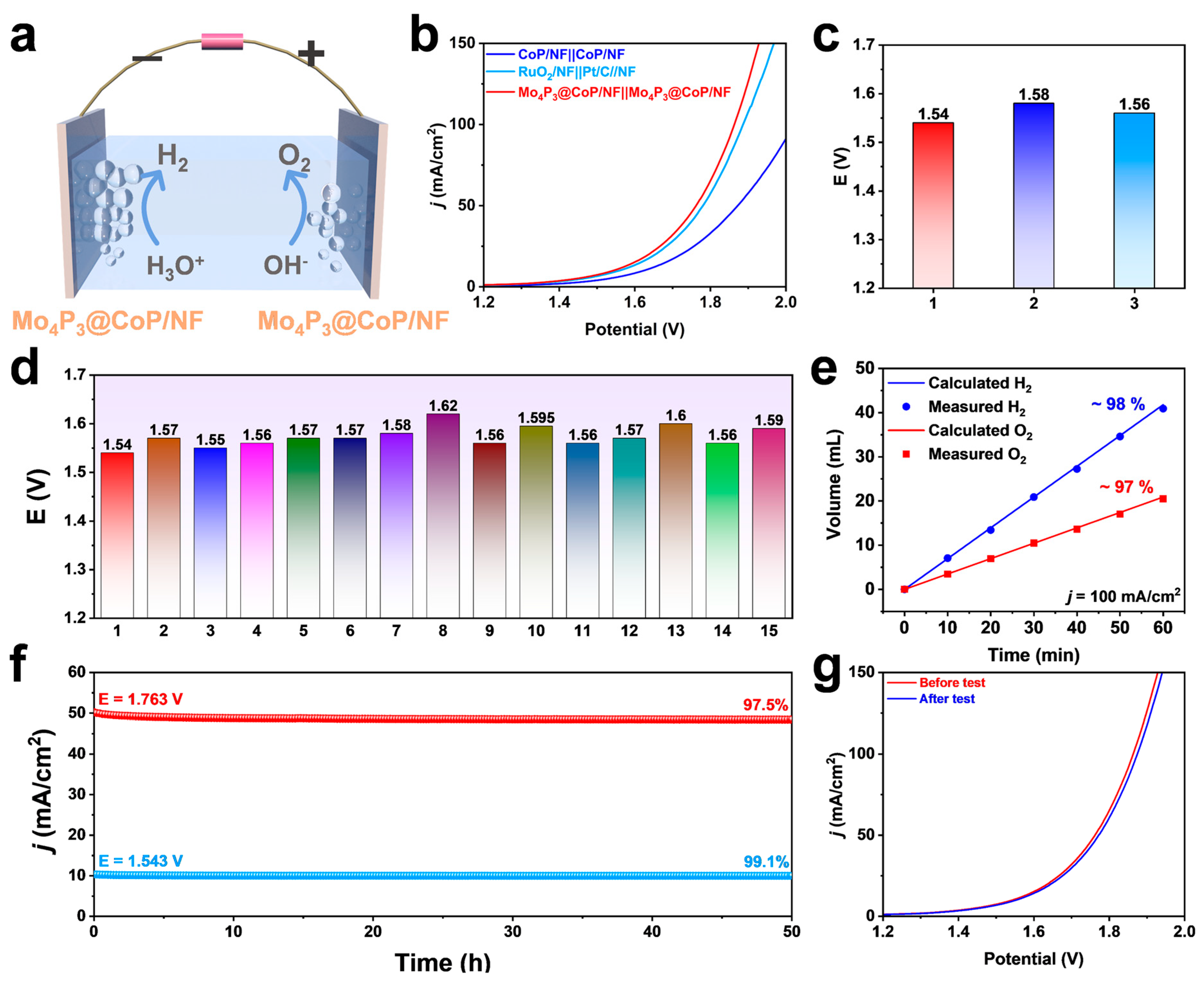
2.5. Overall Water-Splitting Performance
3. Materials and Methods
3.1. Synthesis of Co(CO3)0.5(OH)0.11·H2O Nanowires on Nickel Foam
3.2. Preparation of MoS2 NPs Dispersion
3.3. Preparation of MoS2@Co Precursor/NF
3.4. Preparation of Mo4P3@CoP/NF
3.5. Preparation of Pt/C and RuO2 Electrodes
3.6. Material Characterization
3.7. Electrochemical Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Samir De, B.; Singh, A.; Ji Dixit, R.; Khare, N.; Elias, A.; Basu, S. Hydrogen generation in additively manufactured membraneless microfluidic electrolysis cell: Performance evaluation and accelerated stress testing. Chem. Eng. J. 2023, 452, 139433. [Google Scholar] [CrossRef]
- Yan, D.; Mebrahtu, C.; Wang, S.; Palkovits, R. Innovative electrochemical strategies for hydrogen production: From electricity input to electricity output. Angew. Chem. Int. Ed. Engl. 2023, 135, e202214333. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. Progress in hydrogen production coupled with electrochemical oxidation of small molecules. Angew. Chem. Int. Ed. Engl. 2022, 61, e202213328. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.J.; Yang, H. Surface design strategy of catalysts for water electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, Q.; Xu, W.; Zhao, R.; Jiang, M.; Gao, Y.; Zhong, W.; Chen, K.; Chen, Y.; Li, X.; et al. Sowing single atom seeds: A versatile strategy for hyper-low noble metal loading to boost hydrogen evolution reaction. Adv. Energy Mater. 2023, 13, 2203955. [Google Scholar] [CrossRef]
- Pan, S.; Li, H.; Liu, D.; Huang, R.; Pan, X.; Ren, D.; Li, J.; Shakouri, M.; Zhang, Q.; Wang, M.; et al. Efficient and stable noble-metal-free catalyst for acidic water oxidation. Nat. Commun. 2022, 13, 2294. [Google Scholar] [CrossRef]
- Tajuddin, A.A.H.; Wakisaka, M.; Ohto, T.; Yu, Y.; Fukushima, H.; Tanimoto, H.; Li, X.; Misu, Y.; Jeong, S.; Fujita, J.I.; et al. Corrosion-resistant and high-entropic non-noble-metal electrodes for oxygen evolution in acidic media. Adv. Mater. 2023, 35, 2207466. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Gao, J.K.; Zhang, Q.C. Current progress in 2D metal-organic frameworks for electrocatalysis. Small Struct. 2022, 2200109. [Google Scholar] [CrossRef]
- Deng, Y.W.; Cao, Y.F.; Xia, Y.; Xi, X.Y.; Wang, Y.; Jiang, W.F.; Yang, D.; Dong, A.G.; Li, T.T. Self-templated synthesis of CoFeP@C cage-in-cage superlattices for enhanced electrocatalytic water splitting. Adv. Energy Mater. 2022, 12, 2202394. [Google Scholar] [CrossRef]
- Lin, C.; Li, J.-L.; Li, X.; Yang, S.; Luo, W.; Zhang, Y.; Kim, S.H.; Kim, D.H.; Shinde, S.S.; Li, Y.F. In-situ reconstructed Ru atom array on α-MnO2 with enhanced performance for acidic water oxidation. Nat. Catal. 2021, 4, 1012–1023. [Google Scholar] [CrossRef]
- Ahmed, K.; Wang, Y.Y.; Bai, Y.; Sekar, K.; Li, W. A carbon nanowire-promoted Cu2O/TiO2 nanocomposite for enhanced photoelectrochemical performance. New J. Chem. 2022, 46, 15495–15503. [Google Scholar] [CrossRef]
- Bahuguna, G.; Cohen, A.; Harpak, N.; Filanovsky, B.; Patolsky, F. Single-step solid-state scalable transformation of Ni-based substrates to high-oxidation state nickel sulfide nanoplate arrays as exceptional bifunctional electrocatalyst for overall water splitting. Small Methods 2022, 6, 2200181. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.R.; Xiong, L.F.; Wang, B.; Zhu, D.L.; Hao, H.J.; Zhang, X.J.; Li, J.; Yang, S.C. Boosting the hydrogen evolution reaction of N-C@CoP through an N atom induced p-d orbital coupling. Chem. Eng. J. 2022, 446, 137132. [Google Scholar] [CrossRef]
- Zhou, P.Y.; Li, R.S.; Lv, J.J.; Huang, X.B.; Lu, Y.F.; Wang, G. Synthesis of CoP nanoarrays by morphological engineering for efficient electrochemical hydrogen production. Electrochim. Acta 2022, 426, 140768. [Google Scholar] [CrossRef]
- Xu, D.; Kang, Z.; Zhao, H.; Ji, Y.; Yao, W.; Ye, D.; Zhang, J. Coupling heterostructured CoP-NiCoP nanopin arrays with MXene (Ti3C2Tx) as an efficient bifunctional electrocatalyst for overall water splitting. J. Colloid Interface Sci. 2023, 639, 223–232. [Google Scholar] [CrossRef]
- Li, J.W.; Hu, Y.Z.; Huang, X.; Zhu, Y.; Wang, D.L. Bimetallic phosphide heterostructure coupled with ultrathin carbon layer boosting overall alkaline water and seawater splitting. Small 2023, 2206533. [Google Scholar] [CrossRef]
- Nallal, M.; Park, K.H.; Park, S.; Kim, J.; Shenoy, S.; Chuaicham, C.; Sasaki, K.; Sekar, K. Cu2O/reduced graphene oxide nanocomposites for electrocatalytic overall water splitting. ACS Appl. Nano Mater. 2022, 5, 17271–17280. [Google Scholar] [CrossRef]
- Zhang, L.L.; Lei, Y.T.; Xu, W.J.; Wang, D.; Zhao, Y.F.; Chen, W.X.; Xiang, X.; Pang, X.C.; Zhang, B.; Shang, H.S. Highly active and durable nitrogen-doped CoP/CeO2 nanowire heterostructures for overall water splitting. Chem. Eng. J. 2023, 460, 141119. [Google Scholar] [CrossRef]
- Chen, X.H.; Cheng, Y.M.; Wen, Y.Z.; Wang, Y.Y.; Yan, X.; Wei, J.; He, S.S.; Zhou, J. CoP/Fe-Co9S8 for highly efficient overall water splitting with surface reconstruction and self-termination. Adv. Sci. 2022, 9, 2204742. [Google Scholar] [CrossRef]
- Sun, Y.K.; Liu, T.; Li, Z.J.; Meng, A.L.; Li, G.C.; Wang, L.; Li, S.X. Morphology and interfacial charge regulation strategies constructing 3D flower-like Co@CoP2 heterostructure electrocatalyst for efficient overall water splitting. Chem. Eng. J. 2022, 433, 133684. [Google Scholar] [CrossRef]
- Shen, J.; Li, Q.; Cai, Z.Y.; Sun, X.L.; Liu, J.Q. Metal-organic framework-based self-supporting nanoparticle arrays for catalytic water splitting. ACS Appl. Nano Mater. 2023, 6, 1965–1974. [Google Scholar] [CrossRef]
- Honnappa, B.; Mohan, S.; Shanmugam, M.; Augustin, A.; Sagayaraj, P.J.J.; Chuaicham, C.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Transition metal quantum dots for the electrocatalytic hydrogen evolution reaction: Recent progresses and challenges. Energy Adv. 2022, 1, 738–760. [Google Scholar] [CrossRef]
- Lee, G.H.Y.; Jeong, M.; Kim, H.R.; Kwon, M.; Baek, S.; Oh, S.; Lee, M.H.Y.; Lee, D.J.; Joo, J.H. Controlled electrophoretic deposition strategy of binder-free CoFe2O4 nanoparticles as an enhanced electrocatalyst for the oxygen evolution reaction. ACS Appl. Mater. Interfaces 2022, 14, 48598–48608. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Wang, M.; Yan, J.; Chen, Y.; Liu, S. Unveiling the origin of Co3O4 quantum dots for photocatalytic overall water splitting. Small 2023, 2206695. [Google Scholar] [CrossRef]
- Liu, F.; Gao, Y.; Chi, X.; Zhu, Z.; Wang, X.; Guan, R. A PtS QDs/ZnIn2S4 heterojunction catalyst for efficient photocatalytic hydrogen production and reduction of p-nitrophenol. J. Environ. Chem. Eng. 2022, 10, 108840. [Google Scholar] [CrossRef]
- Jiang, J.; Xu, G.; Li, Y.; Wang, C.; Zhang, L. Interfacial engineering of metal–organic framework derived hierarchical CoP–Ni5P4 nanosheet arrays for overall water splitting. J. Mater. Chem. A 2023, 11, 1801–1809. [Google Scholar] [CrossRef]
- Mu, Y.; Zhang, Y.; Feng, Z.; Dong, X.; Jing, X.; Pei, X.; Zhao, Y.; Kou, Z.; Meng, C. Bifunctional electrocatalyst junction engineering: CoP nanoparticles in-situ anchored on Co3(Si2O5)2(OH)2 nanosheets for highly efficient water splitting. Chem. Eng. J. 2023, 460, 141709. [Google Scholar] [CrossRef]
- Ding, X.; Yu, J.; Huang, W.; Chen, D.; Lin, W.; Xie, Z. Modulation of the interfacial charge density on Fe2P–CoP by coupling CeO2 for accelerating alkaline electrocatalytic hydrogen evolution reaction and overall water splitting. Chem. Eng. J. 2023, 451, 138550. [Google Scholar] [CrossRef]
- Huang, H.; Cho, A.; Kim, S.; Jun, H.; Lee, A.; Han, J.W.; Lee, J. Structural design of amorphous CoMoPx with abundant active sites and synergistic catalysis effect for effective water splitting. Adv. Funct. Mater. 2020, 30, 2203889. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, J.; Wang, W.; Tsiakaras, P.; Li, Y. Oxygen vacancy and core-shell heterojunction engineering of anemone-like CoP@CoOOH bifunctional electrocatalyst for efficient overall water splitting. Small 2022, 18, 2106012. [Google Scholar] [CrossRef]
- Cai, Z.; Shen, J.; Zhang, M.; Cui, L.; Liu, W.; Liu, J. CuxO nanorod arrays shelled with CoNi layered double hydroxide nanosheets for enhanced oxygen evolution reaction under alkaline conditions. J. Colloid Interface Sci. 2023, 630, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Masoumi, Z.; Tayebi, M.; Kolaei, M.; Lee, B.-K. Improvement of surface light absorption of ZnO photoanode using a double heterojunction with α–Fe2O3/g–C3N4 composite to enhance photoelectrochemical water splitting. Appl. Surf. Sci. 2023, 608, 154915. [Google Scholar] [CrossRef]
- Zhai, T.; Niu, H.; Yan, Y.; Yang, S.; Lu, C.; Zhou, W. Rational hetero-interface design of Fe3N@Ni2Co-LDHs as high efficient electrocatalyst for oxygen evolution reaction. J. Alloy. Compd. 2021, 853, 157353. [Google Scholar] [CrossRef]
- Meng, T.; Qin, J.; Xu, D.; Cao, M. Atomic heterointerface-induced local charge distribution and enhanced water adsorption behavior in a cobalt phosphide electrocatalyst for self-powered highly efficient overall water splitting. ACS Appl. Mater. Interfaces 2019, 11, 9023–9032. [Google Scholar] [CrossRef]
- Yue, Q.; Wan, Y.; Sun, Z.; Wu, X.; Yuan, Y.; Du, P. MoP is a novel, noble-metal-free cocatalyst for enhanced photocatalytic hydrogen production from water under visible light. J. Mater. Chem. A 2015, 3, 16941–16947. [Google Scholar] [CrossRef]
- Xu, S.; Zhao, H.; Li, T.; Liang, J.; Lu, S.; Chen, G.; Gao, S.; Asiri, A.M.; Wu, Q.; Sun, X. Iron-based phosphides as electrocatalysts for the hydrogen evolution reaction: Recent advances and future prospects. J. Mater. Chem. A 2020, 8, 19729–19745. [Google Scholar] [CrossRef]
- Huang, C.-L.; Lin, Y.-G.; Chiang, C.-L.; Peng, C.-K.; Senthil Raja, D.; Hsieh, C.-T.; Chen, Y.-A.; Chang, S.-Q.; Yeh, Y.-X.; Lu, S.-Y. Atomic scale synergistic interactions lead to breakthrough catalysts for electrocatalytic water splitting. Appl. Catal. B Environ. 2023, 320, 122016. [Google Scholar] [CrossRef]
- Zheng, Y.; Shang, P.; Pei, F.; Ma, G.; Ye, Z.; Peng, X.; Li, D. Achieving an efficient hydrogen evolution reaction with a bicontinuous nanoporous PtNiMg alloy of ultralow noble-metal content at an ultrawide range of current densities. Chem. Eng. J. 2022, 433, 134571. [Google Scholar] [CrossRef]
- Pan, Y.D.; Abazari, R.; Wu, Y.H.; Gao, J.K.; Zhang, Q.C. Advances in metal-organic frameworks and their derivatives for diverse electrocatalytic applications. Electrochem. Commun. 2021, 126, 107024. [Google Scholar] [CrossRef]
- Li, J.; Tan, Y.; Zhang, M.; Gou, W.; Zhang, S.; Ma, Y.; Hu, J.; Qu, Y. Boosting electrocatalytic activity of Ru for acidic hydrogen evolution through hydrogen spillover strategy. ACS Energy Lett. 2022, 7, 1330–1337. [Google Scholar] [CrossRef]
- Song, L.; Zhang, X.; Zhu, S.; Xu, Y.; Wang, Y. Hydrogen spillover effect enhanced by carbon quantum dots activated MoS2. Carbon 2022, 199, 63–69. [Google Scholar] [CrossRef]
- Zhou, Y.-N.; Liu, X.; Yu, C.-J.; Dong, B.; Han, G.-Q.; Liu, H.-J.; Lv, R.-Q.; Liu, B.; Chai, Y.-M. Boosting hydrogen evolution through hydrogen spillover promoted by Co-based support effect. J. Mater. Chem. A 2023, 11, 6945. [Google Scholar] [CrossRef]
- Adamu, H.; Abba, S.I.; Anyin, P.B.; Sani, Y.; Yamani, Z.H.; Qamar, M. Tuning OER electrocatalysts toward LOM pathway through the lens of multi-descriptor feature selection by artificial intelligence-based approach. ACS Mater. Lett. 2022, 5, 299–320. [Google Scholar] [CrossRef]
- Avci, O.N.; Sementa, L.; Fortunelli, A. Mechanisms of the Oxygen Evolution reaction on NiFe2O4 and CoFe2O4 inverse-spinel oxides. ACS Catal. 2022, 12, 9058–9073. [Google Scholar] [CrossRef]
- Li, Y.W.; Lu, M.T.; Wu, Y.H.; Ji, Q.H.; Xu, H.; Gao, J.K.; Qian, G.D.; Zhang, Q.C. Morphology regulation of metal-organic framework-derived nanostructures for efficient oxygen evolution electrocatalysis. J. Mater. Chem. A 2020, 8, 18215–18219. [Google Scholar] [CrossRef]
- Chen, M.P.; Liu, D.; Feng, J.X.; Zhou, P.F.; Qiao, L.L.; Feng, W.L.; Chen, Y.Y.; Ng, K.W.; Wang, S.P.; Ip, W.F.; et al. In-situ generation of Ni-CoOOH through deep reconstruction for durable alkaline water electrolysis. Chem. Eng. J. 2022, 443, 136432. [Google Scholar] [CrossRef]
- Wang, H.; Cui, M.; Fu, G.; Zhang, J.; Ding, X.; Azaceta, I.; Bugnet, M.; Kepaptsoglou, D.M.; Lazarov, V.K.; de la Peña O’Shea, V.A.; et al. Vertically aligned Ni/NiO nanocomposites with abundant oxygen deficient hetero-interfaces for enhanced overall water splitting. Sci. China Chem. 2022, 65, 1885–1894. [Google Scholar] [CrossRef]
- Yang, C.; Wu, Z.; Zhao, Z.; Gao, Y.; Ma, T.; He, C.; Wu, C.; Liu, X.; Luo, X.; Li, S.; et al. Electronic structure-dependent water-dissociation pathways of ruthenium-based catalysts in alkaline H2 -evolution. Small 2023, 19, 2206949. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Guo, S.; Xin, X.; Wang, Y.; Huang, W.; Wang, M.; Yang, B.; Jorge Sobrido, A.; Ghasemi, J.B.; et al. Gradient heating epitaxial growth gives well lattice-matched Mo2C-Mo2N heterointerfaces that boost both electrocatalytic hydrogen evolution and water vapor splitting. Angew. Chem. Int. Ed. Engl. 2022, 61, e202209703. [Google Scholar]
- Singh, T.I.; Maibam, A.; Cha, D.C.; Yoo, S.; Babarao, R.; Lee, S.U.; Lee, S. High-alkaline water-splitting activity of mesoporous 3D heterostructures: An amorphous-shell@crystalline-core nano-assembly of Co-Ni-phosphate ultrathin-nanosheets and V- doped cobalt-nitride nanowires. Adv. Sci. 2022, 9, 2201311. [Google Scholar] [CrossRef]
- Wang, C.; Jiu, H.; Zhang, L.; Song, W.; Zhang, Y.; Wei, H.; Xu, Q.; Che, S.; Guo, Z.; Qin, Y. Bifunctional CuCo2O4/CoOOH as a synergistic catalyst supported on nickel foam for alkaline overall water splitting. J. Alloys Compd. 2022, 929, 167367. [Google Scholar] [CrossRef]
- Yang, S.; Zhu, J.-Y.; Chen, X.-N.; Huang, M.-J.; Cai, S.-H.; Han, J.-Y.; Li, J.-S. Self-supported bimetallic phosphides with artificial heterointerfaces for enhanced electrochemical water splitting. Appl. Catal. B Environ. 2022, 304, 120914. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Nan, B.; Tang, T.; Niu, X.; Guan, J. Atomic manganese coordinated to nitrogen and sulfur for oxygen evolution. Nano Res. 2022, 15, 6019–6025. [Google Scholar] [CrossRef]
- Ghosh, S.; Mondal, A.; Tudu, G.; Ghosh, S.; Koppisetti, H.V.S.R.M.; Inta, H.R.; Saha, D.; Mahalingam, V. Efficient Electrochemical Reconstruction of a Cobalt- and Silver-Based Precatalytic Oxalate Framework for Boosting the Alkaline Water Oxidation Performance. ACS Sustain. Chem. Eng. 2022, 10, 7265–7276. [Google Scholar] [CrossRef]
- Hu, R.; Zhao, M.; Miao, H.; Liu, F.; Zou, J.; Zhang, C.; Wang, Q.; Tian, Z.; Zhang, Q.; Yuan, J. Rapidly reconstructing the active surface of cobalt-based perovskites for alkaline seawater splitting. Nanoscale 2022, 14, 10118–10124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Jiao, C.; Chen, H.; Wang, G.; Wu, W.; Zhuo, Z.; Mao, J. Axial Phosphate Coordination in Co Single Atoms Boosts Electrochemical Oxygen Evolution. Adv. Sci. 2023, 10, 2206107. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; An, L.; Yin, J.; Jin, J.; Yang, R.; Huang, B.; Hu, Y.; Zhao, Y.-Q.; Xi, P. Electronic engineering of amorphous Fe–Co–S sites in hetero-nanoframes for oxygen evolution and flexible Al–air batteries. J. Mater. Chem. A 2022, 10, 19757–19768. [Google Scholar] [CrossRef]
- Lv, J.Q.; Chen, X.; Chang, Y.; Li, Y.G.; Zang, H.Y. N, F Codoped FeOOH Nanosheets with Intercalated Carbonate Anions Rich in Oxygen Defects for Enhanced Alkaline Electrocatalytic Water Splitting. ACS Appl. Mater. Interfaces 2022, 14, 52877–52885. [Google Scholar] [CrossRef]
- Mei, Y.; Feng, Y.; Zhang, C.; Zhang, Y.; Qi, Q.; Hu, J. High-Entropy Alloy with Mo-Coordination as Efficient Electrocatalyst for Oxygen Evolution Reaction. ACS Catal. 2022, 12, 10808–10817. [Google Scholar] [CrossRef]
- Selvasundarasekar, S.S.; Bijoy, T.K.; Kumaravel, S.; Karmakar, A.; Madhu, R.; Bera, K.; Nagappan, S.; Dhandapani, H.N.; Mersal, G.A.M.; Ibrahim, M.M.; et al. Effective Formation of a Mn-ZIF-67 Nanofibrous Network via Electrospinning: An Active Electrocatalyst for OER in Alkaline Medium. ACS Appl. Mater. Interfaces 2022, 14, 46581–46594. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Z.; Yu, H.; Wen, H.; Yang, R.; Peng, S.; Sun, M.; Yu, L. Alkali treatment of layered double hydroxide nanosheets as highly efficient bifunctional electrocatalysts for overall water splitting. J. Colloid Interface Sci. 2023, 636, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Li, Y.; Li, F.; Li, T.; Wang, X.; Yin, Y.; Ma, L.; Schmidt, O.G.; Zhu, F. Direct Thermal Enhancement of Hydrogen Evolution Reaction of On-Chip Monolayer MoS2. ACS Nano 2022, 16, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Li, L.; Cui, W.; Zhang, Y.; Zhan, C.; Xiao, W. Aligned porous nickel electrodes fabricated via ice templating with submicron particles for hydrogen evolution in alkaline water electrolysis. J. Power Sources 2023, 556, 232441. [Google Scholar] [CrossRef]
- Wang, M.; Chen, Y.; Li, T. Controllable preparation of nickel phosphide using iron and cobalt as electrocatalyst for hydrogen evolution reaction in alkaline media. Mater. Today Chem. 2022, 24, 100914. [Google Scholar] [CrossRef]
- Rafei, M.; Wu, X.; Piñeiro Garcia, A.; Miranda la Hera, V.; Wågberg, T.; Gracia-Espino, E. Non-Stoichiometric NiFeMo Solid Solutions; Tuning the Hydrogen Adsorption Energy via Molybdenum Incorporation. Adv. Mater. Interfaces 2022, 9, 2201214. [Google Scholar] [CrossRef]
- Yan, Y.; Ma, Q.; Cui, F.; Zhang, J.; Cui, T. Carbon onions coated Ni/NiO nanoparticles as catalysts for alkaline hydrogen evolution reaction. Electrochim. Acta 2022, 430, 141090. [Google Scholar] [CrossRef]
- Tian, Z.Y.; Han, X.Q.; Du, J.; Li, Z.B.; Ma, Y.Y.; Han, Z.G. Bio-Inspired FeMo2S4 Microspheres as Bifunctional Electrocatalysts for Boosting Hydrogen Oxidation/Evolution Reactions in Alkaline Solution. ACS Appl. Mater. Interfaces 2023, 15, 11853–11865. [Google Scholar] [CrossRef]
- Liu, W.; Tan, W.; He, H.; Peng, Y.; Chen, Y.; Yang, Y. One–step electrodeposition of Ni–Ce–Pr–Ho/NF as an efficient electrocatalyst for hydrogen evolution reaction in alkaline medium. Energy 2022, 250, 123831. [Google Scholar] [CrossRef]
- Zou, H.-H.; Li, W.-Q.; Song, C.-H.; Cao, L.-M.; Zhang, X.-F.; Zhu, X.-Y.; Du, Z.-Y.; Zhang, J.; Zhong, S.-L.; He, C.-T. Disclosing the active integration structure and robustness of a pseudo-tri-component electrocatalyst toward alkaline hydrogen evolution. J. Energy Chem. 2022, 72, 210–216. [Google Scholar] [CrossRef]
- Wang, Y.; Yun, S.; Shi, J.; Zhang, Y.; Dang, J.; Dang, C.; Liu, Z.; Deng, Y.; Yang, T. Defect engineering tuning electron structure of biphasic tungsten-based chalcogenide heterostructure improves its catalytic activity for hydrogen evolution and triiodide reduction. J. Colloid Interface Sci. 2022, 625, 800–816. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Xing, Y.; Qin, Y.; Lin, J.; Qu, X.; Sun, B.; Du, S.; Shi, D.; Chen, C.; et al. Nickel-decorated RuO2 nanocrystals with rich oxygen vacancies for high-efficiency overall water splitting. J. Colloid Interface Sci. 2023, 630, 940–950. [Google Scholar] [CrossRef]
- Cui, Z.; Liang, X.; Wang, P.; Zhou, P.; Zhang, Q.; Wang, Z.; Zheng, Z.; Liu, Y.; Dai, Y.; Huang, B. In situ integration of Fe3N@Co4N@CoFe alloy nanoparticles as efficient and stable electrocatalyst for overall water splitting. Electrochim. Acta 2021, 395, 139218. [Google Scholar] [CrossRef]
- Guo, D.; Wan, Z.; Fang, G.; Zhu, M.; Xi, B. A Tandem Interfaced (Ni3S2-MoS2)@TiO2 Composite Fabricated by Atomic Layer Deposition as Efficient HER Electrocatalyst. Small 2022, 18, 2201896. [Google Scholar] [CrossRef]
- He, W.; Wang, L.; Zhang, H.; Gao, S.; Yu, W.; Yin, D.; Dong, X. SnO2@MoS2 heterostructures grown on nickel foam as highly efficient bifunctional electrocatalyst for overall water splitting in alkaline media. J. Alloy. Compd. 2023, 938, 168678. [Google Scholar] [CrossRef]
- Jiang, E.; Li, J.; Li, X.; Ali, A.; Wang, G.; Ma, S.; Shen, P.K.; Zhu, J. Mo4P3-Mo2C quantum dot heterostructures uniformly hosted on a heteroatom-doped 3D porous carbon sheet network as an efficient bifunctional electrocatalyst for overall water splitting. Chem. Eng. J. 2022, 431, 133719. [Google Scholar] [CrossRef]
- Li, L.; Chao, K.; Liu, X.; Zhou, S. Construction of La decorated CoMo4P3 composite and its highly efficient electrocatalytic activity for overall water splitting in alkaline media. J. Alloy. Compd. 2023, 941, 168952. [Google Scholar] [CrossRef]
- Li, W.; Chen, M.; Lu, Y.; Qi, P.; Liu, G.; Zhao, Y.; Wu, H.; Tang, Y. One-pot electrodeposition synthesis of NiFe-phosphate/phosphide hybrid nanosheet arrays for efficient water splitting. Appl. Surf. Sci. 2022, 598, 153717. [Google Scholar] [CrossRef]
- Li, X.; Duan, F.; Lu, X.; Gang, Y.; Zheng, W.; Lin, Y.; Chen, L.; Dan, Y.; Cheng, X. Surface engineering of flower-like Co-N-C on carbon paper for improved overall water splitting. J. Alloy. Compd. 2023, 935, 168128. [Google Scholar] [CrossRef]
- Liu, D.; Song, Z.; Cheng, S.; Wang, Y.; Saad, A.; Deng, S.; Shen, J.; Huang, X.; Cai, X.; Tsiakaras, P. Mesoporous IrNiTa metal glass ribbon as a superior self-standing bifunctional catalyst for water electrolysis. Chem. Eng. J. 2022, 431, 134210. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Z.; Wang, Z.; Singh, C.V.; Jiang, Q. Interface Engineering of Co/CoMoN/NF Heterostructures for High-Performance Electrochemical Overall Water Splitting. Adv. Sci. 2022, 9, 2105313. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, A.; Samanta, R.; Barman, S. MOF-Derived Porous Fe3O4/RuO2-C Composite for Efficient Alkaline Overall Water Splitting. ACS Appl. Energy Mater. 2022, 5, 6059–6069. [Google Scholar] [CrossRef]
- Tan, Q.; Xiao, R.; Yao, X.; Xiong, T.; Li, J.; Hu, Y.-W.; Huang, Y.; Balogun, M.S. Non-oxygen anion-regulated in situ cobalt based heterojunctions for active alkaline hydrogen evolution catalysis. Chem. Eng. J. 2022, 433, 133514. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H.; Bao, J.; Song, Y.; She, X.; Lv, G.; Deng, J.; Li, H.; Xu, H. Amorphized core–shell NiFeMo electrode for efficient bifunctional water splitting. Appl. Surf. Sci. 2023, 607, 154803. [Google Scholar] [CrossRef]
- Zahra, S.A.; Hakim, M.W.; Mansoor, M.A.; Rizwan, S. Two-dimensional double transition metal carbides as superior bifunctional electrocatalysts for overall water splitting. Electrochim. Acta 2022, 434, 141257. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Chen, H.; Zhang, B. Constructing Molybdenum Phosphide@Cobalt Phosphide Heterostructure Nanoarrays on Nickel Foam as a Bifunctional Electrocatalyst for Enhanced Overall Water Splitting. Molecules 2023, 28, 3647. https://doi.org/10.3390/molecules28093647
Huang Y, Chen H, Zhang B. Constructing Molybdenum Phosphide@Cobalt Phosphide Heterostructure Nanoarrays on Nickel Foam as a Bifunctional Electrocatalyst for Enhanced Overall Water Splitting. Molecules. 2023; 28(9):3647. https://doi.org/10.3390/molecules28093647
Chicago/Turabian StyleHuang, Yingchun, Hongming Chen, and Busheng Zhang. 2023. "Constructing Molybdenum Phosphide@Cobalt Phosphide Heterostructure Nanoarrays on Nickel Foam as a Bifunctional Electrocatalyst for Enhanced Overall Water Splitting" Molecules 28, no. 9: 3647. https://doi.org/10.3390/molecules28093647
APA StyleHuang, Y., Chen, H., & Zhang, B. (2023). Constructing Molybdenum Phosphide@Cobalt Phosphide Heterostructure Nanoarrays on Nickel Foam as a Bifunctional Electrocatalyst for Enhanced Overall Water Splitting. Molecules, 28(9), 3647. https://doi.org/10.3390/molecules28093647




