Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor
Abstract
1. Introduction
2. Results and Discussion
2.1. Spectroscopic Studies
2.2. UV–Visible and Fluorescence Study

2.3. Stokes Shift
2.4. Detection Limit and Quantum Yield
2.5. Reuseability Study

2.6. Effect of pH

2.7. Anti-Interference Studies
2.8. Natural Water Samples Analysis by Spiking
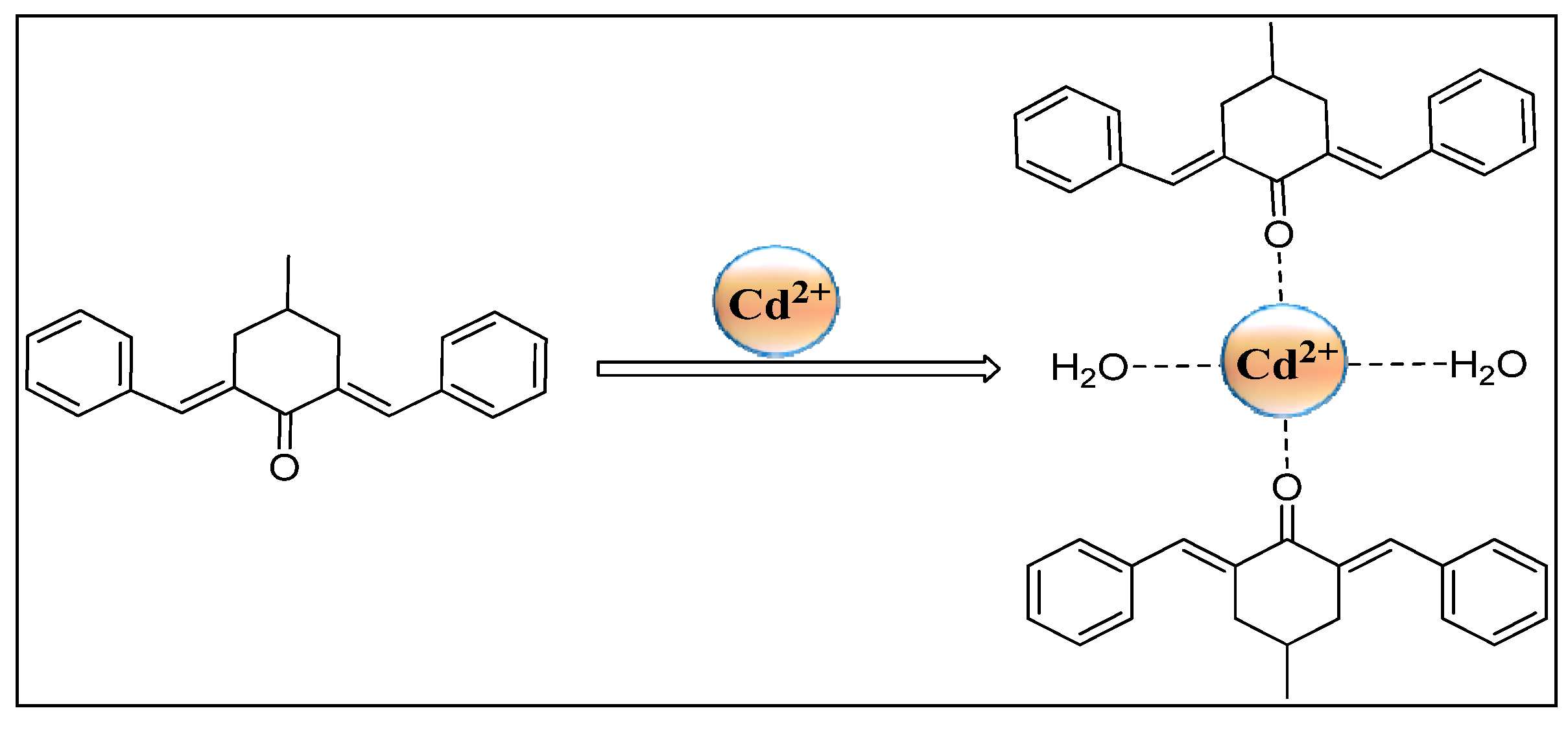
2.9. DFT Studies
2.10. Comparison with REPORTED Chemosensors
3. Materials and Methods
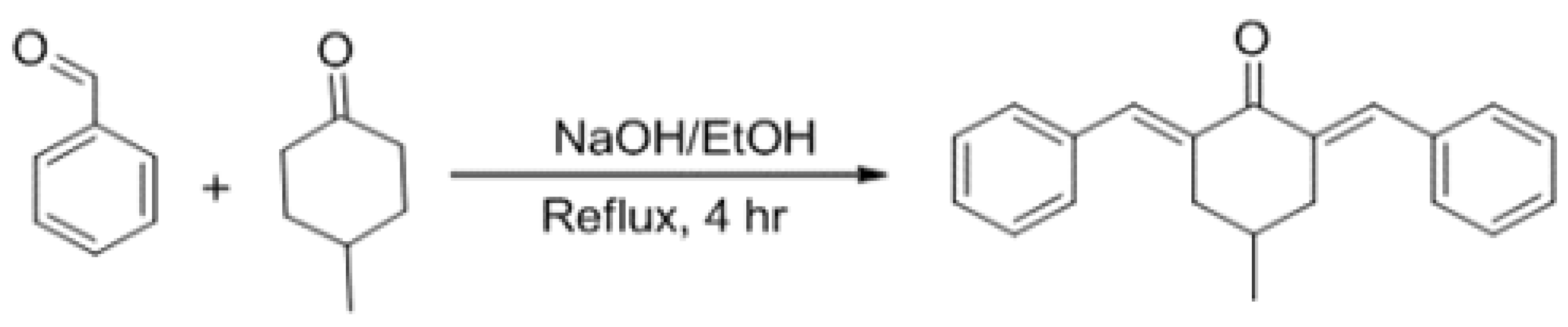
3.1. Synthesis of Chemosensor CM1
3.2. Solutions Preparation for Spectroscopic Measurements
3.3. General UV–Vis and Fluorescence Spectra Measurements
3.4. Excitation and Emission Spectra of Chemosensor CM1
3.5. Limit of Detection and Quantum Yield Calculation
3.6. Spiked Water Samples Analysis
3.7. Theoretical Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability Statement
References
- Wang, J.; Xia, T.; Zhang, X.; Zhang, Q.; Cui, Y.; Yang, Y.; Qian, G. A Turn-on Fluorescent Probe for Cd2+ Detection in Aqueous Environments Based on an Imine Functionalized Nanoscale Metal–Organic Framework. RSC Adv. 2017, 7, 54892–54897. [Google Scholar] [CrossRef]
- El-Saadani, Z.; Mingqi, W.; He, Z.; Hamukwaya, S.L.; Abdel Wahed, M.S.M.; Abu Khatita, A. Environmental Geochemistry and Fractionation of Cadmium Metal in Surficial Bottom Sediments and Water of the Nile River, Egypt. Toxics 2022, 10, 221. [Google Scholar] [CrossRef]
- Barone, G.; Storelli, A.; Garofalo, R.; Mallamaci, R.; Storelli, M.M. Residual Levels of Mercury, Cadmium, Lead and Arsenic in Some Commercially Key Species from Italian Coasts (Adriatic Sea): Focus on Human Health. Toxics 2022, 10, 223. [Google Scholar] [CrossRef]
- Smichowski, P.; Londonio, A. The Role of Analytical Techniques in the Determination of Metals and Metalloids in Dietary Supplements: A Review. Microchem. J. 2018, 136, 113–120. [Google Scholar] [CrossRef]
- He, D.; Zhu, Z.; Miao, X.; Zheng, H.; Li, X.; Belshaw, N.S.; Hu, S. Determination of Trace Cadmium in Geological Samples by Membrane Desolvation Inductively Coupled Plasma Mass Spectrometry. Microchem. J. 2019, 148, 561–567. [Google Scholar] [CrossRef]
- Haribala; Hu, B.; Wang, C.; Gerilemandahu; Xu, X.; Zhang, S.; Bao, S.; Li, Y. Assessment of Radioactive Materials and Heavy Metals in the Surface Soil around Uranium Mining Area of Tongliao, China. Ecotoxicol. Env. Saf. 2016, 130, 185–192. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.; Kang, Y.; Lee, Y.; Yoon, Y. A Biosensor Platform for Metal Detection Based on Enhanced Green Fluorescent Protein. Sensors 2019, 19, E1846. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, H.; Ping, J. Simultaneous Determination of Cd(II) and Pb(II) Ions in Honey and Milk Samples Using a Single-Walled Carbon Nanohorns Modified Screen-Printed Electrochemical Sensor. Food Chem. 2019, 274, 8–15. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Xiao, Y.; Liu, W. Hydrothermal Synthesis of Carbon Dots Codoped with Nitrogen and Phosphorus as a Turn-on Fluorescent Probe for Cadmium(II). Microchim. Acta 2019, 186, 147. [Google Scholar] [CrossRef]
- Pan, Y.; Xiao, W.; Fan, M.; Chen, W.; Xu, S. Detection of Cd(II) Ions by a Self-Tuning Method Using Quantum Dot Fluorescence Sensing. Mater. Res. Express 2017, 4, 105008. [Google Scholar] [CrossRef]
- Petryayeva, E.; Algar, W.R.; Medintz, I.L. Quantum Dots in Bioanalysis: A Review of Applications across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013, 67, 215–252. [Google Scholar] [CrossRef]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent Sensors for Measuring Metal Ions in Living Systems. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Li, J.; Yim, D.; Jang, W.-D.; Yoon, J. Recent Progress in the Design and Applications of Fluorescence Probes Containing Crown Ethers. Chem. Soc. Rev. 2017, 46, 2437–2458. [Google Scholar] [CrossRef]
- Zhu, H.; Fan, J.; Wang, B.; Peng, X. Fluorescent, MRI, and Colorimetric Chemical Sensors for the First-Row d-Block Metal Ions. Chem. Soc. Rev. 2015, 44, 4337–4366. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, F.; Bai, Y.; Zhao, J.; Chen, X.; Ge, M.; Sun, W. Quinoline-Based Highly Selective and Sensitive Fluorescent Probe Specific for Cd2+ Detection in Mixed Aqueous Media. Tetrahedron Lett. 2017, 58, 3868–3874. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and Colorimetric Sensors for Detection of Lead, Cadmium, and Mercury Ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef]
- Qian, J.; Wang, K.; Wang, C.; Ren, C.; Liu, Q.; Hao, N.; Wang, K. Ratiometric Fluorescence Nanosensor for Selective and Visual Detection of Cadmium Ions Using Quencher Displacement-Induced Fluorescence Recovery of CdTe Quantum Dots-Based Hybrid Probe. Sens. Actuators B Chem. 2017, 241, 1153–1160. [Google Scholar] [CrossRef]
- Shi, C.; Huang, Z.; Wu, A.; Hu, Y.; Wang, N.; Zhang, Y.; Shu, W.; Yu, W. Recent Progress in Cadmium Fluorescent and Colorimetric Probes. RSC Adv. 2021, 11, 29632–29660. [Google Scholar] [CrossRef]
- Su, W.; Yuan, S.; Wang, E. A Rhodamine-Based Fluorescent Chemosensor for the Detection of Pb2+, Hg2+ and Cd2+. J. Fluoresc. 2017, 27, 1871–1875. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, X.; Zheng, M.; Yang, R.; Yang, H.; Jia, L.; Yang, M. A 4,5-Quinolimide-Based Fluorescent Sensor for the Turn-on Detection of Cd2+ with Live-Cell Imaging. Org. Biomol. Chem. 2017, 15, 2211–2216. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; He, W.; Yang, Z.; Gao, X.; Guo, Z. A Highly Sensitive Ratiometric Fluorescent Probe for Cd2+ Detection in Aqueous Solution and Living Cells. Chem. Commun. 2010, 46, 6138–6140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, X.; Wang, L.; Shang, Z.; Chao, J.; Jin, W. A New Acridine Derivative as a Highly Selective ‘off–on’ Fluorescence Chemosensor for Cd2+ in Aqueous Media. Sens. Actuators B Chem. 2011, 156, 126–131. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, T.; Zhu, W.; Xu, Y.; Qian, X. Highly Selective and Sensitive Near-Infrared Fluorescent Sensors for Cadmium in Aqueous Solution. Org. Lett. 2011, 13, 264–267. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.-J.; Li, M.; Lu, H.-L.; Xu, L.-H.; Xu, H.; Zang, S.-Q.; Tang, M.-S.; Hou, H.-W.; Mak, T.C.W. A Highly Sensitive C3-Symmetric Schiff-Base Fluorescent Probe for Cd2+. Inorg. Chem. 2014, 53, 12665–12667. [Google Scholar] [CrossRef] [PubMed]
- Khani, R.; Ghiamati, E.; Boroujerdi, R.; Rezaeifard, A.; Zaryabi, M.H. A New and Highly Selective Turn-on Fluorescent Sensor with Fast Response Time for the Monitoring of Cadmium Ions in Cosmetic, and Health Product Samples. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2016, 163, 120–126. [Google Scholar] [CrossRef]
- Liu, D.; Qi, J.; Liu, X.; Cui, Z.; Chang, H.; Chen, J.; Yang, G. 4-Amino-1,8-Naphthalimide-Based Fluorescent Cd2+ Sensor with High Selectivity against Zn2+ and Its Imaging in Living Cells. Sens. Actuators B Chem. 2014, 204, 655–658. [Google Scholar] [CrossRef]
- Mikata, Y.; Kizu, A.; Konno, H. TQPHEN (N,N,N′,N′-Tetrakis(2-Quinolylmethyl)-1,2-Phenylenediamine) Derivatives as Highly Selective Fluorescent Probes for Cd2+. Dalton Trans. 2014, 44, 104–109. [Google Scholar] [CrossRef]
- Shaily; Kumar, A.; Ahmed, N. A Coumarin–Chalcone Hybrid Used as a Selective and Sensitive Colorimetric and Turn-on Fluorometric Sensor for Cd2+ Detection. New J. Chem. 2017, 41, 14746–14753. [Google Scholar] [CrossRef]
- Cai, H.; Zou, J.; Lin, J.; Li, J.; Huang, Y.; Zhang, S.; Yuan, B.; Ma, J. Sodium Hydroxide-Enhanced Acetaminophen Elimination in Heat/Peroxymonosulfate System: Production of Singlet Oxygen and Hydroxyl Radical. Chem. Eng. J. 2022, 429, 132438. [Google Scholar] [CrossRef]
- Li, J.; Zou, J.; Zhang, S.; Cai, H.; Huang, Y.; Lin, J.; Li, Q.; Yuan, B.; Ma, J. Sodium Tetraborate Simultaneously Enhances the Degradation of Acetaminophen and Reduces the Formation Potential of Chlorinated By-Products with Heat-Activated Peroxymonosulfate Oxidation. Water Res. 2022, 224, 119095. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Lin, J.; Yang, H.; Wang, M.; Li, J.; Cao, W.; Yuan, B.; Ma, J. ABTS as Both Activator and Electron Shuttle to Activate Persulfate for Diclofenac Degradation: Formation and Contributions of ABTS•+, SO4•–, and •OH. Environ. Sci. Technol. 2022. [Google Scholar] [CrossRef]
- Bie, F.; Cao, H.; Yan, P.; Cui, H.; Shi, Y.; Ma, J.; Liu, X.; Han, Y. A Cyanobiphenyl-Based Ratiometric Fluorescent Sensor for Highly Selective and Sensitive Detection of Zn2+. Inorg. Chim. Acta 2020, 508, 119652. [Google Scholar] [CrossRef]
- Shaji, L.K.; Selva Kumar, R.; Jose, J.; Bhaskar, R.; Vetriarasu, V.; Bhat, S.G.; Ashok Kumar, S.K. Selective Chromogenic and Fluorogenic Signalling of Hg2+ Ions Using a Benzothiazole-Quinolinyl Acrylate Conjugate and Its Applications in the Environmental Water Samples and Living Cells. J. Photochem. Photobiol. A Chem. 2023, 434, 114220. [Google Scholar] [CrossRef]
- Bhasin, A.K.K.; Chauhan, P.; Chaudhary, S. A Novel Coumarin-Tagged Ditopic Scaffold as a Selectively Sensitive Fluorogenic Receptor of Zinc (II) Ion. Sens. Actuators B Chem. 2021, 330, 129328. [Google Scholar] [CrossRef]
- Desai, M.L.; Basu, H.; Saha, S.; Singhal, R.K.; Kailasa, S.K. One Pot Synthesis of Fluorescent Gold Nanoclusters from Curcuma Longa Extract for Independent Detection of Cd2+, Zn2+ and Cu2+ Ions with High Sensitivity. J. Mol. Liq. 2020, 304, 112697. [Google Scholar] [CrossRef]
- Maity, A.; Ghosh, U.; Giri, D.; Mukherjee, D.; Maiti, T.K.; Patra, S.K. A Water-Soluble BODIPY Based ‘OFF/ON’ Fluorescent Probe for the Detection of Cd2+ Ions with High Selectivity and Sensitivity. Dalton Trans. 2019, 48, 2108–2117. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, D.; Chen, B. A Fluorescent Dansyl-Based Peptide Probe for Highly Selective and Sensitive Detect Cd2+ Ions and Its Application in Living Cell Imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 207, 276–283. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kumar, S.K.A.; Vijayakrishna, K.; Sivaramakrishna, A.; Paira, P.; Rao, C.V.S.B.; Sivaraman, N.; Sahoo, S.K. Bipyridine Bisphosphonate-Based Fluorescent Optical Sensor and Optode for Selective Detection of Zn2+ Ions and Its Applications. New J. Chem. 2018, 42, 8494–8502. [Google Scholar] [CrossRef]
- Ismail, B.A.; Nassar, D.A.; Abd El–Wahab, Z.H.; Ali, O.A.M. Synthesis, Characterization, Thermal, DFT Computational Studies and Anticancer Activity of Furfural-Type Schiff Base Complexes. J. Mol. Struct. 2021, 1227, 129393. [Google Scholar] [CrossRef]
- Lv, Y.; Wu, L.; Shen, W.; Wang, J.; Xuan, G.; Sun, X. A Porphyrin-Based Chemosensor for Colorimetric and Fluorometric Detection of Cadmium(II) with High Selectivity. J. Porphyr. Phthalocyanines 2015, 19, 769–774. [Google Scholar] [CrossRef]
- Pham, T.C.; Kim, Y.K.; Park, J.B.; Jeon, S.; Ahn, J.; Yim, Y.; Yoon, J.; Lee, S. A Selective Colorimetric and Fluorometric Chemosensor Based on Conjugated Polydiacetylenes for Cadmium Ion Detection. ChemPhotoChem 2019, 3, 1133–1137. [Google Scholar] [CrossRef]
- Kim, Y.K.; Pham, T.C.; Kim, J.; Bae, C.; Choi, Y.; Jo, M.H.; Lee, S. Polydiacetylenes Containing 2-Picolylamide Chemosensor for Colorimetric Detection of Cadmium Ions. Bull. Korean Chem. Soc. 2021, 42, 265–269. [Google Scholar] [CrossRef]
- Yadav, N.; Singh, A.K. Colorimetric and Fluorometric Detection of Heavy Metal Ions in Pure Aqueous Medium with Logic Gate Application. J. Electrochem. Soc. 2019, 166, B644. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, X.; Deng, M.; Zhu, T.; Edman, L.; Wang, J. Hydrophilic AgInZnS Quantum Dots as a Fluorescent Turn-on Probe for Cd2+ Detection. J. Alloys Compd. 2021, 864, 158109. [Google Scholar] [CrossRef]
- Farahani, Y.D.; Safarifard, V. Highly Selective Detection of Fe3+, Cd2+ and CH2Cl2 Based on a Fluorescent Zn-MOF with Azine-Decorated Pores. J. Solid State Chem. 2019, 275, 131–140. [Google Scholar] [CrossRef]
- Zhou, B.; Yang, X.-Y.; Wang, Y.-S.; Yi, J.-C.; Zeng, Z.; Zhang, H.; Chen, Y.-T.; Hu, X.-J.; Suo, Q.-L. Label-Free Fluorescent Aptasensor of Cd2+ Detection Based on the Conformational Switching of Aptamer Probe and SYBR Green I. Microchem. J. 2019, 144, 377–382. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, L.; Li, Z.; Li, J.; Ma, H.; Ning, L.; Li, N.; Liu, X. A Facile AIE Fluorescent Probe with Large Stokes Shift for the Detection of Cd2+ in Real Water Samples and Living Cells. J. Lumin. 2022, 243, 118672. [Google Scholar] [CrossRef]
- Carmona-Vargas, C.C.; Alves, L.d.C.; Brocksom, T.J.; De Oliveira, K.T. Combining Batch and Continuous Flow Setups in the End-to-End Synthesis of Naturally Occurring Curcuminoids. React. Chem. Eng. 2017, 2, 366–374. [Google Scholar] [CrossRef]
- Han, J.; Tang, X.; Wang, Y.; Liu, R.; Wang, L.; Ni, L. A Quinoline-Based Fluorescence “on-off-on” Probe for Relay Identification of Cu2+ and Cd2+ Ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 205, 597–602. [Google Scholar] [CrossRef]
- Khan, J.; Sadia, M.; Wadood Ali Shah, S.; Zahoor, M.; Alsharif, K.F.; Al-Joufi, F.A. Development of [(2E,6E)-2,6-Bis(4-(Dimethylamino)Benzylidene)Cyclohexanone] as Fluorescence-on Probe for Hg2+ Ion Detection: Computational Aided Experimental Studies. Arab. J. Chem. 2022, 15, 103710. [Google Scholar] [CrossRef]
- Mohammadi Ziarani, G.; Badiei, A.; Abbasi, A.; Farahani, Z. Cross-Aldol Condensation of Cycloalkanones and Aromatic Aldehydes in the Presence of Nanoporous Silica-Based Sulfonic Acid (SiO2-Pr-SO3H) under Solvent Free Conditions. Chin. J. Chem. 2009, 27, 1537–1542. [Google Scholar] [CrossRef]
- Tamang, N.; Ramamoorthy, G.; Joshi, M.; Choudury, A.R.; Siva Kumar, B.; Golakoti, N.R.; Doble, M. Diarylidenecyclopentanone Derivatives as Potent Anti-Inflammatory and Anticancer Agents. Med. Chem. Res. 2020, 29, 1579–1589. [Google Scholar] [CrossRef]
- Kubin, R.F.; Fletcher, A.N. Fluorescence Quantum Yields of Some Rhodamine Dyes. J. Lumin. 1982, 27, 455–462. [Google Scholar] [CrossRef]
- Delley, B. An All-electron Numerical Method for Solving the Local Density Functional for Polyatomic Molecules. J. Chem. Phys. 1990, 92, 508–517. [Google Scholar] [CrossRef]
- Delley, B. From Molecules to Solids with the DMol3 Approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A. Catalysts for Hydrogen Evolution from the [NiFe] Hydrogenase to the Ni2P(001) Surface: The Importance of Ensemble Effect. J. Am. Chem. Soc. 2005, 127, 14871–14878. [Google Scholar] [CrossRef]
- Grimme, S. Accurate Description of van Der Waals Complexes by Density Functional Theory Including Empirical Corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Mehta, P.K.; Hwang, G.W.; Park, J.; Lee, K.H. Highly sensitive ratiometric fluorescent detection of indium (III) using fluorescent probe based on phosphoserine as a receptor. Anal. Chem. 2018, 90, 11256–11264. [Google Scholar] [CrossRef]
- Wu, X.; Niu, Q.; Li, T. A novel urea-based “turn-on” fluorescent sensor for detection of Fe3+/F− ions with high selectivity and sensitivity. Sens. Actuators B Chem. 2016, 222, 714–720. [Google Scholar] [CrossRef]




| Type of Water Sample | Amount of Cd2+ Added (μM) | Amount of Cd2+ Found (μM) | % Recovery |
|---|---|---|---|
| Tap water | 2 | 2.7 | 94 |
| 4 | 4.6 | 96 | |
| 6 | 5.4 | 96 | |
| 8 | 7.5 | 97 | |
| 10 | 9.6 | 96 | |
| River water | 2 | 4.8 | 95 |
| 4 | 2.6 | 96 | |
| 6 | 4.5 | 97 | |
| 8 | 7.4 | 97 | |
| 10 | 9.4 | 96 | |
| Lake water | 2 | 2.6 | 95 |
| 4 | 3.4 | 95 | |
| 6 | 5.3 | 96 | |
| 8 | 7.6 | 97 | |
| 10 | 9.3 | 97 |
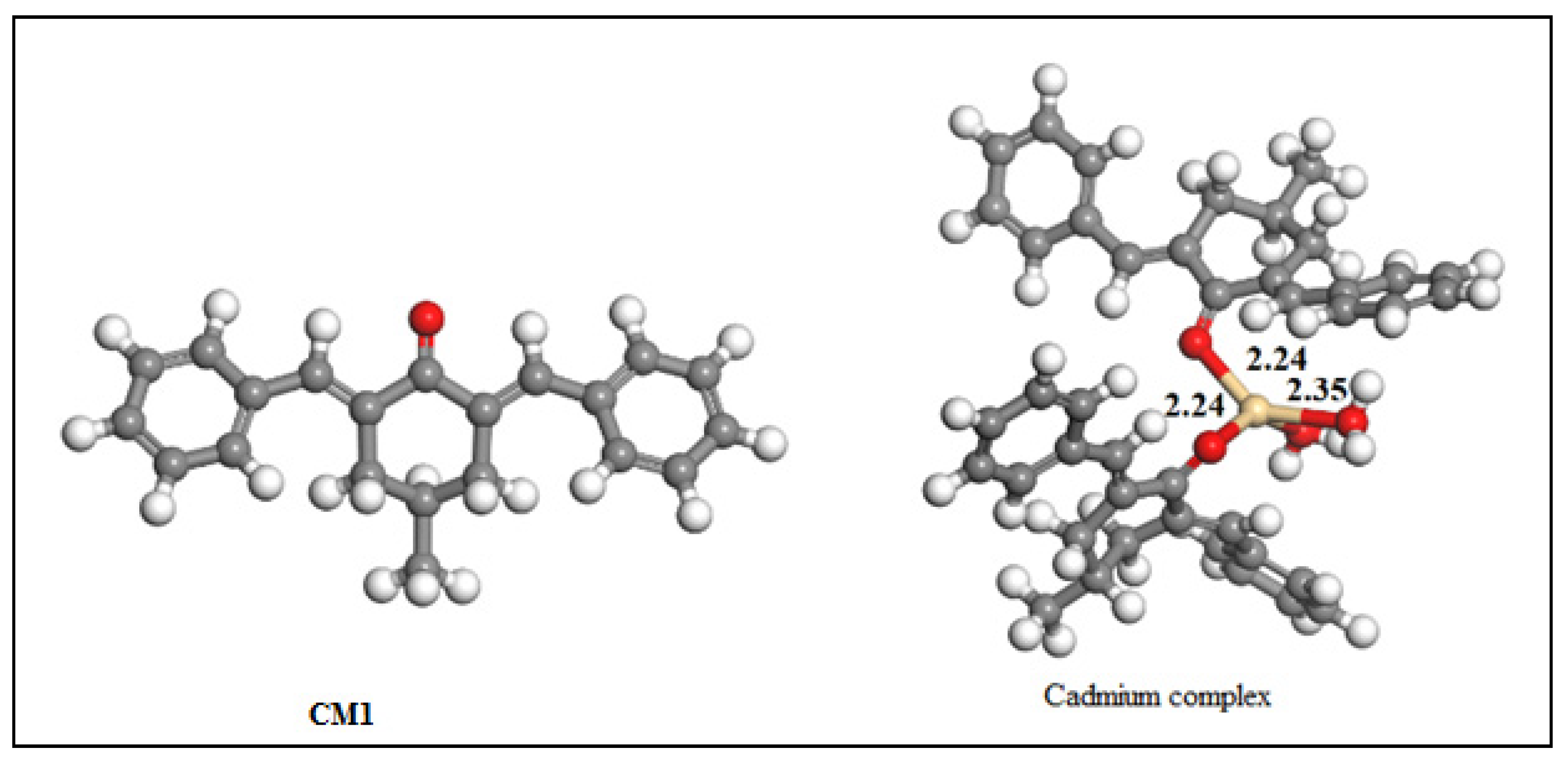
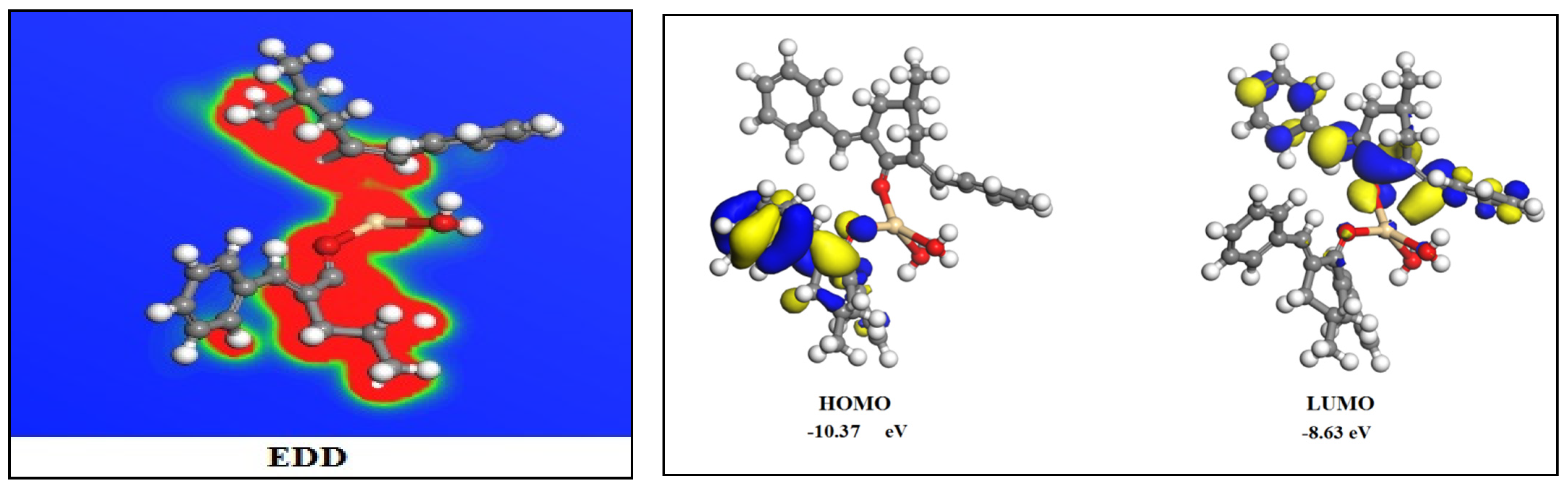
| Bond Length | Bond Angles | Metal CM1 Interaction Energy | HOMO Energy | LUMO Energy | Band Gap | Charges on CM1 | Charges after Complexation |
|---|---|---|---|---|---|---|---|
| 2.35 Å (Cd-Owater) | 103.08° (OCM-1Cd-OCM-1) | −14.3559 eV | −10.37eV | −8.63 eV | 1.74 | Owater = −0.309 | Owater = −0.208 |
| OCM−1 = −0.235 | OCM-1 = −0.215 | ||||||
| 2.24 Å Cd-OCM-1 | 99.24° (OCM-1-Cd-Owater) | Cd = 0.6352 |
| Chemosensors | Analytes | LOD (nM) | pH | Detection Matrixes | References |
|---|---|---|---|---|---|
| 5-(4-Aminophenyl)-10,15,20-triphenylporphyrin | Cd2+ | 73 | 6–8.5 | Aqueous media | [40] |
| Conjugated Polydiacetylenes | Cd2+ | 185 | 7.4 | Aqueous media | [41] |
| (E)-4-hydroxy-3-(3-(4-methoxyphenyl)acryloyl)-2H-chromen-2-one | Cd2+ | 58.4 | 7.0 | Mixed aqueous–organic media | [28] |
| 4-((pyridin-2-ylmethyl)carbamoyl)phenyl pentacosa-10,12-diynoate | Cd2+ | 2000 | NA | Aqueous media | [42] |
| Bis((indol-3-yl)methylene)oxalohydrazonamide | Hg2+, Cu2+, Cd2+ | 110 | NA | Aqueous media | [43] |
| ZnS quantum dots | Cd2+ | 37.8 | 5.6 to 11.5 | Aqueous media | [44] |
| Zn-based azine-functionalized TMU-16 MOF | Cd2+, Fe3+ | 500 | NA | Aqueous media | [45] |
| aptasensor | Cd2+ | 340 | 7.0 | Aqueous media | [46] |
| AIE fluorescent probe | Cd2+ | 500 | 8.0 | Aqueous media | [47] |
| 2,6-di((E)-benzylidene)-4-methylcyclohexan-1-one | Cd2+ | 19.25 | 7.0 | Aqueous media | Present work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadia, M.; Khan, J.; Khan, R.; Kamran, A.W.; Zahoor, M.; Ullah, R.; Bari, A.; Ali, E.A. Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor. Molecules 2023, 28, 3635. https://doi.org/10.3390/molecules28083635
Sadia M, Khan J, Khan R, Kamran AW, Zahoor M, Ullah R, Bari A, Ali EA. Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor. Molecules. 2023; 28(8):3635. https://doi.org/10.3390/molecules28083635
Chicago/Turabian StyleSadia, Maria, Jehangir Khan, Rizwan Khan, Abdul Waheed Kamran, Muhammad Zahoor, Riaz Ullah, Ahmed Bari, and Essam A. Ali. 2023. "Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor" Molecules 28, no. 8: 3635. https://doi.org/10.3390/molecules28083635
APA StyleSadia, M., Khan, J., Khan, R., Kamran, A. W., Zahoor, M., Ullah, R., Bari, A., & Ali, E. A. (2023). Rapid Detection of Cd2+ Ions in the Aqueous Medium Using a Highly Sensitive and Selective Turn-On Fluorescent Chemosensor. Molecules, 28(8), 3635. https://doi.org/10.3390/molecules28083635






